Abstract
In eukaryotic cells, the growth rate is strictly regulated for proper progression of the cell cycle. In the budding yeast Saccharomyces cerevisiae, it was previously shown that cell growth dramatically slows down when the cells start budding at the G1/S transition. However, the molecular mechanism for this G1/S-associated growth arrest is unclear. In this study, using exocytic secretion, cyclin-dependent kinase (CDK) assay, immunoprecipitation, and microscopy, we demonstrate that the exocyst subunit Exo84, which is known to be phosphorylated in mitosis, can also be phosphorylated directly by Cdk1 in the late G1 phase. Of note, we found that the Cdk1-mediated Exo84 phosphorylation impairs exocytic secretion in the late G1 phase. Using conditional cdc mutants and phosphodeficient and phosphomimetic exo84 mutants, we further observed that Cdk1-phosphoryated Exo84 inhibits the exocyst complex assembly, exocytic secretion, and cell growth, which may be important for proper execution of the G1/S-phase transition before commitment to a complete cell cycle. Our results suggest that the direct Cdk1-mediated regulation of the exocyst complex critically contributes to the coordination of cell growth and cell cycle progression.
Keywords: cell cycle, exocytosis, G1/S cyclin, cell growth, cyclin-dependent kinase (CDK), mitosis, membrane trafficking, cell wall remodeling, exocyst, exocytic secretion, G1/S transition, membrane tethering
Introduction
The growth rate of eukaryotic cells is tightly controlled at each stage of the cell cycle for accurate reproduction and size homeostasis (1–4). A reduction of growth speed at the late G1 phase was seen in mammalian cells (5), which is thought to help cells adapt to the increased energy demands for subsequent cell cycle processes (6). Similarly, cell growth of fission yeast also decreases during late G1 (7–9). In budding yeast, cell polarity is established by G1 cyclin/cyclin-dependent kinase (CDK)3 complexes, and the cell growth rate is drastically reduced at late G1 phase (10–13).
It was reported that exocytosis is involved in cell growth during cell cycle progression (14, 15), and cell surface expansion is completed through polarized exocytosis in which post-Golgi secretory vesicles carrying proteins and membranes are docked to the growth site of plasma membrane and then fused with each other (16). The exocyst complex, consisting of Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84, is essential for polarized exocytic secretion and cell surface expansion in eukaryotic cells (17–22). The dynamic assembly and disassembly of the octameric protein complex link the post-Golgi secretory vesicles to the plasma membrane in a process called “tethering” in the late stage of the exocytosis process (17, 21, 23–27). The exocyst subunits have been shown to be direct targets of small GTPases and protein kinases that spatially or temporally regulate exocytosis for many physiological processes such as polarized cell growth, epithelium formation, neuronal branching, and cell migration (17, 18, 20, 28–31). However, little is known about how exocytosis is coordinated with cell growth arrest during cell cycle progression.
The CDKs are master regulators of the cell cycle and play central roles in growth control during cell cycle progression (2, 6, 32). Goranov et al. (11) showed that cells arrested at the time of late G1 phase or in metaphase exhibited lower growth rates, and Cdk1 activity negatively regulated cell growth. We have shown that Exo84 is phosphorylated by Clb2–Cdk1, which disrupts exocyst assembly, inhibits exocytic secretion, and arrests cell growth at metaphase (33). However, whether Exo84 is also phosphorylated by Cdk1 in late G1 and contributes to cell growth arrest is unknown. In this study, we demonstrate that Cdk1 is a regulator of exocytosis at late G1 phase during cell cycle progression in the budding yeast Saccharomyces cerevisiae. When bound to the late G1 cyclin Cln2, Cdk1 inhibits exocytosis before the G1/S transition by direct phosphorylation of the exocyst component Exo84. Cdk1-mediated phosphorylation of Exo84 disrupts the assembly of the exocyst complex in late G1 phase, thereby blocking exocytosis. Our study provides a molecular mechanism by which exocytosis and cell growth are controlled during cell cycle progression.
Results
Exocytic secretion is inhibited at late G1 phase
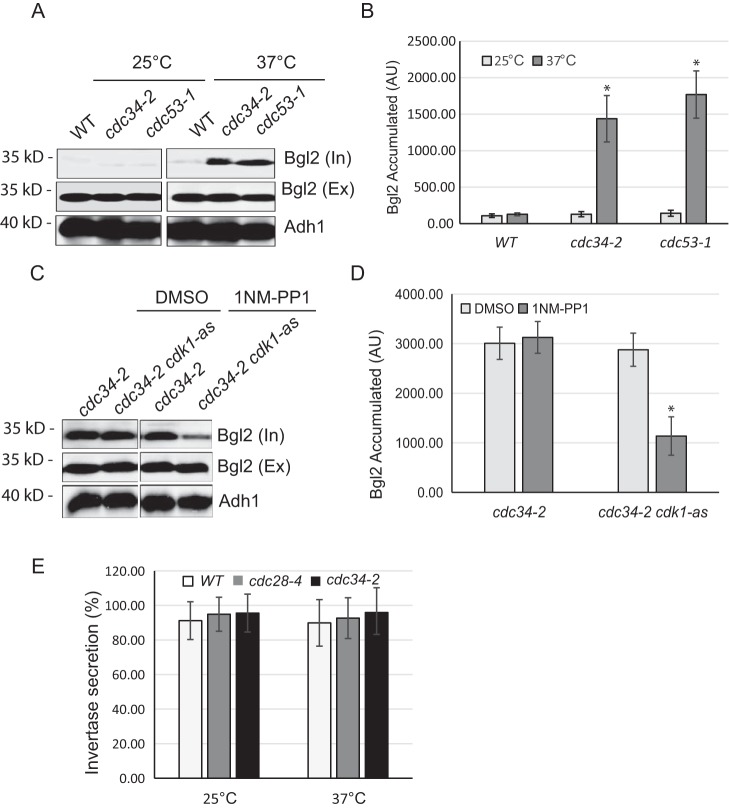
We have reported that cell growth and exocytic secretion are reduced at metaphase (33). To examine whether exocytosis is also regulated in late G1 phase, we monitored the secretion of an endo-β-1,3-glucanase, Bgl2, a cell wall–remodeling enzyme and a commonly used exocytosis marker, in cdc34-2 and cdc53-1 mutants. Cdc34 and Cdc53 are two catalytic subunits of Skp1/Cul1/F-box (SCF) ubiquitin-protein ligase complex that regulates cell cycle progression by targeting key substrates for degradation and is required for the G1/S transition (34). When transferred to restrictive temperature (37 °C), cdc34-2 and cdc53-1 cells arrest in late G1 phase with high Cln1/2–Cdk1 activity (35–37). As shown in Fig. 1, A and B, the internal Bgl2 levels were elevated in cdc34-2 and cdc53-1 mutant cells but not in WT cells, indicating that Bgl2 secretion was inhibited in these two mutants. This result is consistent with the observation that cell growth is largely reduced in late G1 phase (11). In addition to the Bgl2 secretion assay, we also examined the secretion of the cdc mutants using invertase, which marks a smaller branch of the exocytic routes (38). As expected, neither cdc34-2 nor cdc53-1 mutant cells had invertase secretion block (Fig. 1C). This result is consistent with previous observation that exocytic secretion of invertase is not compromised during cell cycle progression (33, 39).
Figure 1.
Exocytosis is inhibited in late G1 phase. A, conditional cdc34-2 mutants exhibit Bgl2 secretion defects at the restrictive temperature. Internal (“In”) and external (“Ex”) pools of Bgl2 in WT, cdc34-2 (late G1 phase), and cdc53-1 (late G1 phase) cells were examined by Western blot analysis. Cells were grown at 25 °C or shifted to 37 °C for 2 h. Alcohol dehydrogenase (Adh1) levels were probed as a protein loading control. The conditional mutants and cell cycle arrest points are listed to the right. B, quantification of Bgl2 accumulation in A. n = 3. *, p < 0.01. C, Cdk1 is required for the reduction in Bgl2 secretion in metaphase-arrested cdc34-2 cells. cdc34-2 and cdc34-2 cdk1-as1 cells were grown to early log phase at 25 °C and shifted to 37 °C for 1.5 h. Cells were then treated with DMSO (mock) or 15 μm 1NM-PP1 for 30 min. Internal and external pools of Bgl2 in cdc34-2 and cdc34-2 cdk1-as1 cells were examined by Western blotting. Corresponding immunoblots of alcohol dehydrogenase serve as a protein loading control. D, quantification of Bgl2 accumulation in C. n = 3; *, p < 0.01. E, invertase secretion was not changed in cdc34-2 and cdc28-4 mutants. Cells were grown at 25 °C or shifted to 37 °C for 1.5 h, and secretion of invertase was examined. The percentage of external invertase (secreted) versus total invertase was measured. Error bars represent S.D. (n = 3). AU, arbitrary unit.
Because Cdk1 plays a central role in cell cycle progression, we speculated that Cdk1 may regulate the reduction of exocytic secretion in late G1 cells. To test this prediction, we assayed Bgl2 secretion in cdc34-2 cells that contain an analog-sensitive cdk1 allele (cdk1-as1), which encodes a mutant kinase that is specifically inhibited by 1NM-PP1 (40). We arrested cdc34-2 cdk1-as1 double-mutant cells in late G1 phase by shifting to 37 °C for 90 min and then added 1NM-PP1 to inhibit Cdk1. Within 15 min of 1NM-PP1 addition, the intracellular fraction of Bgl2 decreased significantly compared with DMSO-treated cells (Fig. 1, D and E). These data suggest that Cdk1 activity is critical for blocking exocytosis at late G1 phase. Alternatively, it is possible that Cdk1 inhibition alleviates the secretion defect by allowing late G1–arrested cdc34-2 cells to enter S phase.
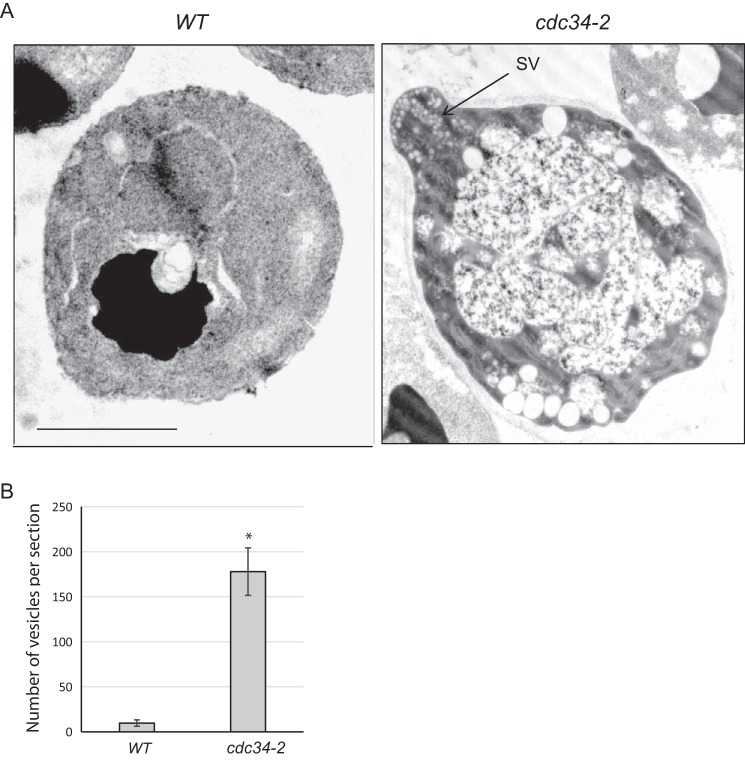
Secretory vesicles often accumulate in cells defective in exocytic secretion. Thus, we examined cdc34-2 cells via thin-section electron microscopy (EM) for secretory vesicle accumulation. Cells were grown at 25 °C to early log phase and then shifted to 37 °C for 90 min. Secretory vesicles were barely detectable in WT cells (Fig. 2, A and B). In contrast, there was clear accumulation of vesicles (179 ± 28 vesicles per section) in late G1–arrested cdc34-2 cells. The size of the accumulated vesicles (80–100 nm in diameter) was characteristic of post-Golgi secretory vesicles (41). These data suggest that exocytosis is blocked in late G1–arrested cells and are consistent with previous findings that yeast cell growth is inhibited when cells start to bud (11).
Figure 2.
Secretory vesicles accumulated in cdc34-2 mutant. A, cdc34-2 cells accumulate post-Golgi secretory vesicles at the restrictive temperature. WT and cdc34-2 cells were grown to early log phase at 25 °C and then shifted to 37 °C for 1.5 h and processed for thin-section EM. Post-Golgi secretory vesicles (typically 80–100 nm in diameter) accumulate in metaphase-arrested cdc34-2 cells. “SV” and the arrow indicate one of the many vesicles. Scale bar, 2.5 μm. B, quantification of vesicle number per EM section of WT and cdc34-2 cells. Error bars represent S.E. (n = 15). *, p < 0.01.
Exo84 is phosphorylated directly by Cdk1 in late G1 phase
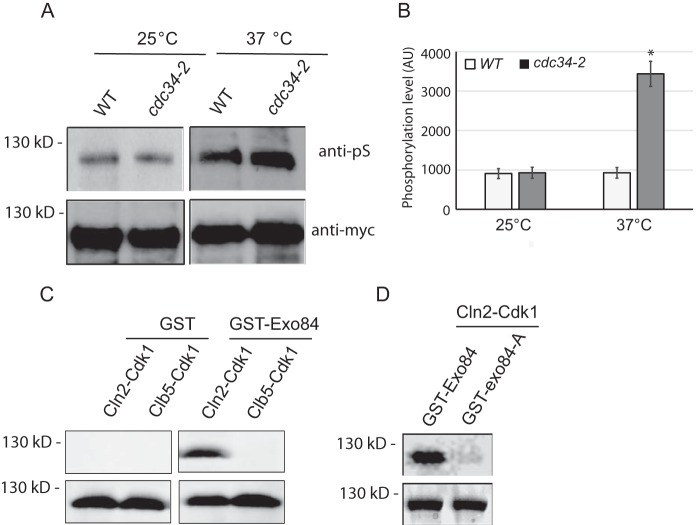
It has been shown that the exocyst subunit Exo84 can be phosphorylated by Clb2–Cdk1 at mitosis during cell cycle progression (33). However, previous data also indicate that Exo84 may also be a direct substrate of Cln2–Cdk1 in late G1 (33, 42). To confirm this result in vivo, we immunoprecipitated Exo84 from yeast and probed for Cdk1 phosphorylation using an antibody specific for Cdk1-phosphorylated peptides (Cell Signaling Technology, catalog number 2325) in cdc34-2 mutant arrested at late G1 phase at 37 °C. As shown in Fig. 3, A and B, phosphorylation of Exo84 in cdc34-2 mutant was similar to that in WT cells at 25 °C. At 37 °C, however, phosphorylation of Exo84 in cdc34-2 mutant was about 3 times more than that in WT cells. To confirm that Exo84 is a direct substrate of Cdk1 in late G1 phase, GST-tagged Exo84 was expressed and purified from Escherichia coli and incubated with Cln2–Cdk1 or Clb5–Cdk1 in the presence of [γ-32P]ATP. As shown in Fig. 3C, purified GST was not phosphorylated by any Cdk1 complexes, whereas GST-Exo84 was phosphorylated by Cln2–Cdk1 but not Clb5–Cdk1. This is consistent with previous reports (33, 42).
Figure 3.
Exo84 is phosphorylated by Cdk1 at late G1 phase. A, Exo84-myc was immunoprecipitated from the WT and cdc34-2 mutant cells at the permissive or restrictive temperature and then probed for Cdk1 phosphorylation by immunoblotting. B, quantification of Exo84 phosphorylation level in A. Error bars represent S.D. (n = 3). *, p < 0.01. C, Exo84 is phosphorylated by Cdk1 in vitro. GST and GST-Exo84 were purified from E. coli and incubated with Cln2–Cdk1 or Clb5–Cdk1 in the presence of [γ-32P]ATP. Exo84 phosphorylation was detected by autoradiography (top). Corresponding Coomassie Blue–stained gels are shown on the bottom. D, in vitro Cln2–Cdk1 kinase assays with recombinant Exo84-A, which lacks the five Cdk1 phosphorylation sites. The phosphorylation of Exo84-A by Cln2–Cdk1 is barely detectable. AU, arbitrary unit.
To confirm that in vitro phosphorylation occurs on Cdk1 consensus sites, we mutated the five confirmed phosphorylation sites of Exo84 (Thr28, Ser31, Ser291, Ser482, and Ser716) to nonphosphorylatable alanine residues and used the resultant “Exo84-A” protein as substrate for in vitro Cdk1 kinase assays. Exo84 Thr28, Ser31, Ser291, Ser482, and Ser716 were selected because previous MS analysis established that those residues were phosphorylated in vivo (43–45). As expected, Cln2–Cdk phosphorylation of Exo84-A was greatly diminished compared with unmutated Exo84 (Fig. 3D). Collectively, these data indicate that Exo84 is a substrate of Cln–Cdk1 and are consistent with the emerging model that Cdk1 inhibits exocytosis in late G1 phase.
Exocyst assembly is inhibited in late G1 phase
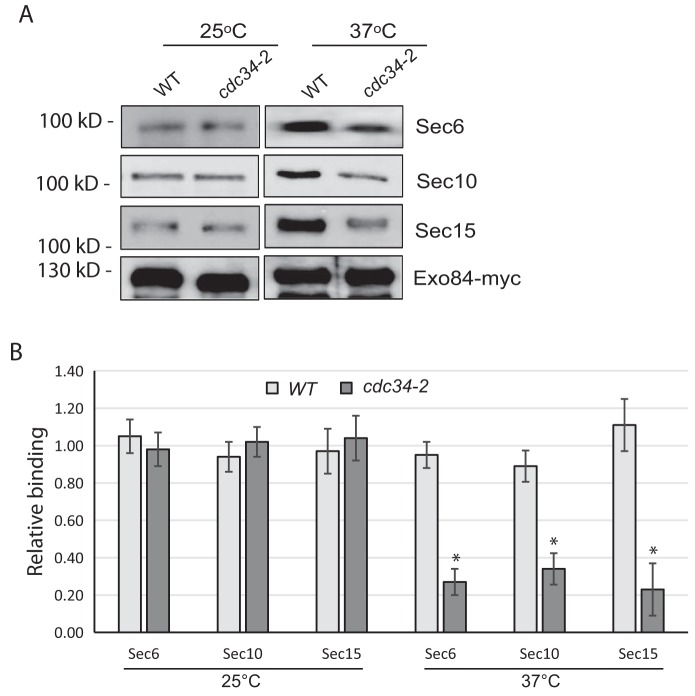
Cdk1 activity has been reported to inhibit exocyst assembly, leading to reduction of exocytic secretion (15, 33). To determine whether the assembly of the exocyst complex is inhibited in late G1 phase, in which the Cdk1–Cln2 is most active, we immunoprecipitated Exo84 from WT cells and cdc34-2 mutant cells arrested in late G1 phase at 37 °C and probed for associated exocyst components by Western blotting. As shown in Fig. 4, A and B, Exo84 bound less to Sec6, Sec10, and Sec15 in late G1 phase–arrested cells than those in WT cells, suggesting that exocyst assembly is blocked in late G1 phase. Our previous data also showed that the treatment of the cdk1-as cells with 1NM-PP1 led to an increase of Exo84 binding to other exocyst subunits (33). Taken together, these data suggest that Cdk1 activity in late G1 phase blocks exocyst assembly.
Figure 4.
Exocyst assembly is inhibited in late G1 phase. A, the cdc34-2 mutant exhibits exocyst assembly defects at the restrictive temperature. The WT and cdc34-2 mutant cells were grown at 25 °C and shifted to 37 °C for 2 h. Exo84-myc immunoprecipitation was performed using these cells. The levels of binding of Sec6, Sec10, and Sec15 decreased in the cdc34-2 mutant. B, quantification of the amounts of the exocyst coimmunoprecipitated with Exo84-myc. The levels of binding for Sec6, Sec10, and Sec15 decreased in the cdc34-2 mutant. Error bars represent S.D. *, p < 0.01; n = 3.
Exo84 phosphorylation is required for secretion reduction in cdc34-2 mutant cells
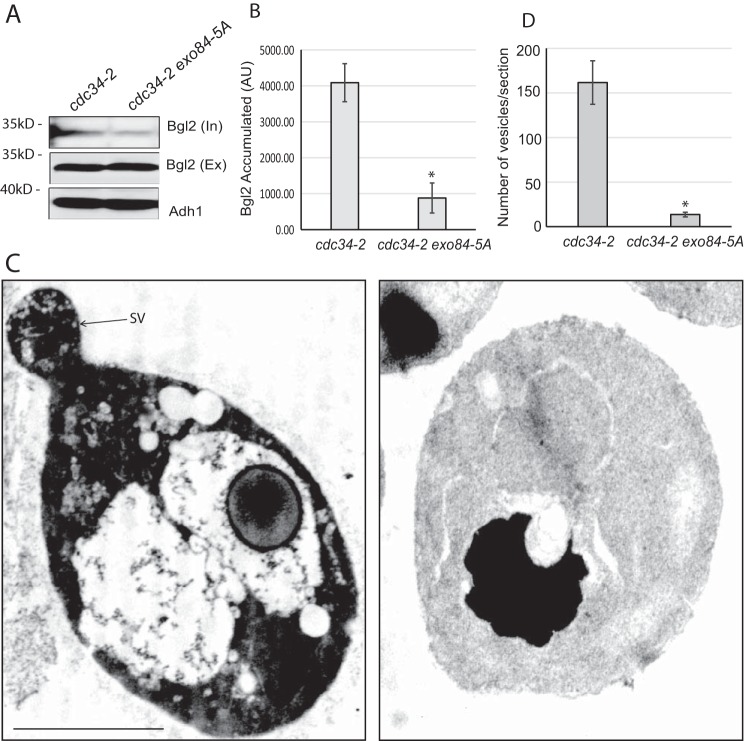
To more closely examine the effect of nonphosphorylatable Exo84 (Exo84-A) on exocytic secretion, we generated strains in which the Exo84 phosphomutant was expressed in the cdc34-2 mutant background. As shown in Fig. 5, A and B, the Bgl2 secretion defect was significantly reduced in cdc34-2 exo84-A double-mutant cells compared with the cdc34-2 single mutant, indicating that phosphorylation-deficient Exo84-A expression partially rescues the cdc34-2 secretion defect. These results suggest that Exo84 phosphorylation by Cdk1 is required for secretion reduction in late G1 phase.
Figure 5.
Phosphorylation of Exo84 negatively regulates exocytic secretion in cdc34-2 mutant. A, the phosphodeficient exo84-A mutant rescued the Bgl2 secretion defect of the cdc34-2 cells. The cdc34-2 and cdc34-2 exo84-A mutants were grown at 25 °C and then shifted to 37 °C for 1.5 h. Internal (In) and external (Ex) pools of Bgl2 were examined by Western blot analysis. Alcohol dehydrogenase (Adh1) was probed as a protein loading control. B, quantification of Bgl2 accumulation in the cdc34-2 and cdc34-2 exo84-A mutants at 37 °C. Error bars represent S.D. *, p < 0.01; n = 3. C, cells were fixed with permanganate and processed for thin-section EM. The cdc34-2 cells accumulated a large amount of post-Golgi secretory vesicles at the restrictive temperature, whereas cdc34-2 exo84-A double mutant did not. SV and the arrow indicate one of the vesicles. Scale bar, 2.5 μm. D, quantification of accumulated vesicles in the cdc34-2 and cdc34-2 exo84-A mutants at 37 °C. Error bars represent S.E. *, p < 0.01; n = 15. AU, arbitrary unit.
Exo84 phosphorylation inhibits cell growth and polarization of Sec4 in sec3 mutant cells
Exocytic secretion defects often lead to inhibition of cell growth. However, our previous data showed that the growth of exo84-A and exo84-E mutant cells were only slightly changed compared with WT cells (33). To determine whether phosphorylation of Exo84 by Cdk1 affects cell growth, we examined the growth rate of the sec3Δ strain carrying either exo84-A or exo84-E mutant. Sec3 is the only nonessential gene among exocyst subunits, and it was reported that the sec3Δ strain is deficient in apical growth at the beginning of the cell cycle and is unable to generate pointed projections when cells were arrested in late G1 phase (46). To explore the role of Exo84 phosphorylation in cell growth and cell cycle progression, the original copy of Exo84 in sec3Δ strain was replaced with a plasmid carrying either exo84-A or exo84-E mutant gene, and the cell growth of these mutant cells was examined using serial dilution experiments. As shown in Fig. 6A, exo84-A can rescue the growth of sec3Δ mutant at 25 °C, whereas exo84-E was synthetically lethal with sec3Δ mutant. A similar result was also observed for the sec3-2 temperature-sensitive mutant strain at restrictive temperature. We found that exo84-A can rescue the growth of sec3-2 at 37 °C, whereas exo84-E was synthetically lethal with sec3-2 (Fig. 6B). These results indicate that phosphorylation of Exo84 inhibits cell growth.
Figure 6.
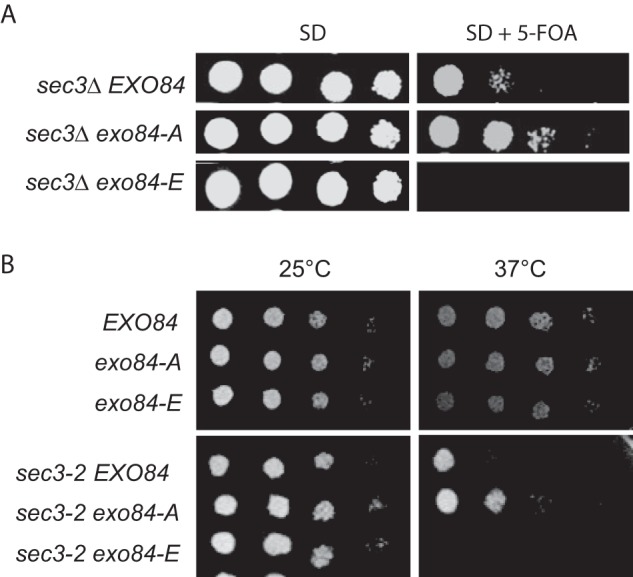
Cell growth of sec3 exo84-ps mutant. A, growth of exo84-ps mutants in the sec3Δ background strain. Various exo84-ps mutants expressed under the EXO84 promoter in a CEN plasmid were introduced into exo84Δ strain supplemented with a EXO84 balancer plasmid (CEN, URA3). The cells were serially diluted onto synthetic defined (SD) plates with or without 5-fluoroorotic acid (5-FOA) and incubated for 3 days at 25 °C. exo84-A rescued the growth defect of sec3Δ at 25 °C, whereas the sec3Δ exo84-E mutant was lethal at 25 °C. B, growth of the WT (EXO84), exo84-A, exo84-E, sec3-2, sec3-2 exo84-A, and sec3-2 exo84-E cells after serial dilution on plates at 25 and 37 °C. The exo84-E cells grew slightly slower compared with WT and exo84-A cells at 37 °C. The sec3-2 exo84-A cells grew better than the sec3-2 single mutant, whereas sec3-2 exo84-E cells displayed severe growth defects at 34 °C.
Sec4 is a marker protein of post-Golgi secretory vesicles (26, 46). Using Sec4 as a marker, we next examined whether phosphorylation of Exo84 regulates targeting of secretory vesicles to the bud tip in cells lacking SEC3. Sec4 is normally concentrated in a small spot at the bud tip (46, 47). As shown in Fig. 7, sec3Δ cells showed a much broader distribution of Sec4 than WT cells as expected (46), whereas the distribution area of Sec4 in sec3Δ was reduced by exo84-A. Our finding demonstrates that phosphorylation of Exo84 negatively regulates the spatial regulation of secretion in sec3Δ strains. Considering that Sec3 is required for apical growth at the beginning of the cell cycle (46), these data suggest that phosphorylation of Exo84 by Cdk1 plays an important role in G1/S transition during cell cycle progression.
Figure 7.
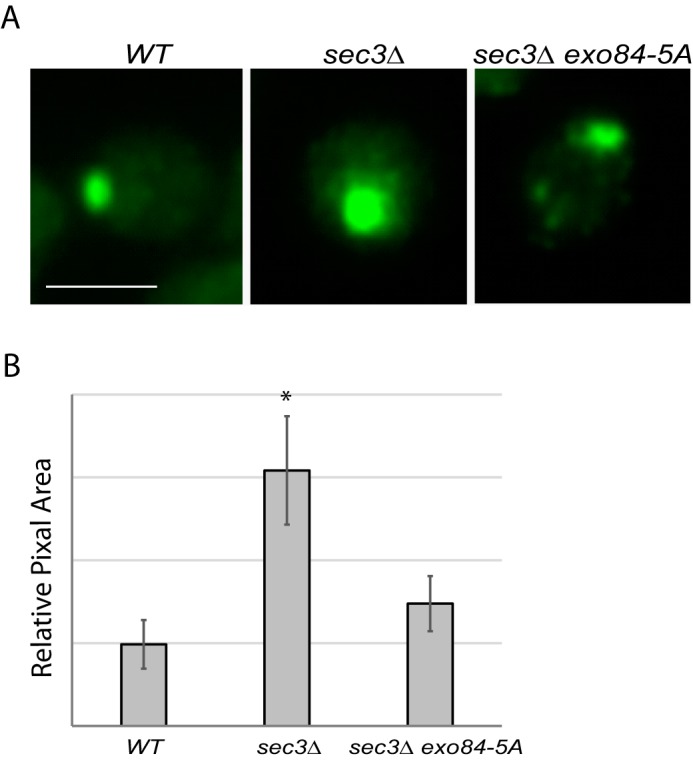
Defect of apical growth in sec3 exo84-A cells during early bud growth. Shown is immunofluorescence of Sec4 in WT, sec3Δ EXO84, and sec3Δ exo84-A cells. Corresponding differential interference contrast is shown in the bottom panels. A, Sec4 is concentrated at incipient bud sites. Scale bar, 5 μm. B, the Sec4-positive area in yeast buds was measured using NIH ImageJ. n = 3. Error bars represent S.D. *, p < 0.05.
Discussion
Membrane growth and surface expansion of eukaryotic cells are tightly controlled during cell cycle progression and mainly rely on the tight regulation of polarized exocytic secretion, by which proteins and lipids from post-Golgi are incorporated into the plasma membrane. Although several aspects of the cell cycle such as DNA replication and chromosomal segregation have been intensively studied, the molecular mechanisms controlling cell membrane growth are still unclear (3, 6). An important feature of late G1 phase before the G1/S transition of budding yeast cells is growth reduction, which is critical to control the cell size and to help dividing cells adapt to the energy demands required for cell cycle progression (6, 7, 9, 11, 16, 48–53).
Cell growth and morphogenesis have been carefully investigated in budding yeast for each stage of the cell cycle, providing solid evidence for the coordination of the cell cycle and membrane growth mechanisms by Cdk1 (11, 54). It has been shown that budding yeast cells arrested in early G1 phase grow faster than those in other stages, whereas cells arrested in late G1 phase right before G1/S transition and metaphase grow slower than those in other stages (11, 55). Although we have shown that phosphorylation of Exo84 by Cdk1 is required for growth reduction and secretion at metaphase (33), whether it is involved in the growth inhibition in late G1 phase is unknown. In this study, we confirmed that Exo84 is a direct substrate of Cln–Cdk1 in late G1 phase, and phosphorylation of Exo84 inhibits exocyst assembly and exocytic secretion, which may contribute to G1/S transition during cell cycle progression.
It has been reported that cell membrane growth depends on exocytic secretion (11, 55). But how exocytic secretion is coordinated with cell cycle progression is unclear. We have found that the exocyst complex subunit Exo84 is a direct substrate of Clb2–Cdk1 in mitosis stage (33). Phosphorylation of Exo84 disrupts exocyst assembly, which leads to a secretion defect and cell growth arrest at metaphase. Previous data suggest that Exo84 can be phosphorylated by Cln2–Cdk1 and Clb2–Cdk1 in vitro at late G1 phase and mitosis phase, respectively (33, 42), which coincides with the cell growth reduction when cells start budding or entering mitosis stage. Because phosphorylation of Exo84 plays a critical role in cell growth arrest at metaphase, we hypothesized that it may also be involved in cell growth reduction when cells start to bud, that is the late G1 phase before G1/S transition. We showed that Cln–Cdk1 phosphorylates Exo84 in vivo and in vitro, and phosphorylation site–mutated exo84 mutant cannot be phosphorylated by Cln2–Cdk1 in vitro, confirming the prediction that Exo84 is a direct substrate of Cdk1 in late G1 phase.
It would be very interesting to determine which CDK consensus site(s) play a critical role for Exo84 phosphorylation. However, we found that mutation of any one of these five sites (T28P, S31PAK, S291P, S482PNK, and S716P) did not affect the phosphorylation of Exo84 in vitro (Fig. S1). In our study, we tested five consensus Cdk1 sites in Exo84, but it also contains three additional putative full-consensus Cdk1 sites (T22PAK, T474PGR, and T496PGR), although these sites were not confirmed by MS. These three putative full-consensus sites may compensate the phosphorylation of Exo84 when one of the five sites tested in this study is mutated. In addition, previous studies have shown that CDKs can phosphorylate proteins at sites lacking the proline +1 of consensus CDK target sites (56–58), and mutating all or most of the consensus CDK phosphorylation sites on a single substrate such as Swe1 (58), Slk19 (59), Cdc24 (12), Boi1 (54), and Rga2 (60) had no significant consequences. Taken together, it seems that other consensus Cdk1 sites or nonconsensus Cdk1 sites can compensate the Exo84 phosphorylation by Cdk1 when one Cdk1 site is mutated.
Similar to the phosphorylation at metaphase, phosphorylation of Exo84 in late G1 phase disrupts exocyst assembly (Fig. 4), which further leads to the reduction of exocytic secretion and cell growth (Figs. 5–7). To investigate whether phosphorylation of Exo84 is involved in cell growth arrest in late G1 phase and affects G1/S transition, we compared the distribution of Sec4, the exocytic vesicle marker protein, in the bud tip of WT, sec3Δ, and sec3Δ exo84-A cells arrested at late G1 and found that distribution area of Sec4 in sec3Δ bud tip was reduced by exo84-A (Fig. 7), indicating that phosphorylation of Exo84 negatively regulates the spatial secretion in the sec3Δ strain.
At present, Exo84 is the only protein in the exocytic secretory pathway found to be regulated directly by Cdk1. Although Sec3 was able to be phosphorylated by Cdk1 in vitro (61), we found that Sec3 cannot be phosphorylated by Cdk1 in vivo.4 The result that Exo84 phosphorylation by Cdk1 in late G1 and metaphase negatively regulates exocytic secretion correlates with the fact that cell growth are arrested in these two stages (6). These data support a model in which phosphorylation of Exo84 by Cdk1 negatively regulates cell membrane growth in late G1 and early mitosis during cell cycle progression in budding yeast. As the exocyst complex is evolutionarily conserved in eukaryotic cells, we speculate that this mechanism also operates in other eukaryotes, and it would be interesting to investigate whether Exo84 is phosphorylated in mammalian cells arrested in late G1 and metaphase and whether Exo84 dephosphorylation and subsequent exocyst assembly/activation contribute to the plasma membrane expansion that has been observed during cell cycle progression (9, 16). Our study demonstrates how cell cycle progression and membrane trafficking are coordinated and provides a molecular mechanism for cell growth inhibition in late G1 phase.
Materials and methods
Yeast strains, plasmids, and procedures
Standard methods were used for yeast growth and genetic manipulations as described previously (62). All strains used in this study are listed in Tables S1. Yeast transformation was performed based on the lithium acetate method (63).
Yeast strains containing mutations of the CDK consensus sites (as described in Table S1) were constructed as follows. The balancer plasmid pG279, which contains Exo84 ORF in p416TEF (URA3, CEN) plasmid (64), was transformed into the parental yeast strain, and chromosomal Exo84 was then replaced by KanMX6 according to the procedure described previously (65) and confirmed by PCR. The CEN plasmid containing WT EXO84, exo84-A, or exo84-E under control of the endogenous EXO84 promoter and terminator was transformed into this strain. The yeast transformants were counterselected for pG279 in medium containing 1 mg/ml 5-fluoroorotic acid. All plasmids used in this study are listed in Tables S2. The CDK sites in Exo84 were mutated using a multimutagenesis kit (Stratagene).
Whole-cell extract preparation
Yeast extracts were prepared by using glass beads and vortexing as described previously (66). The hot-SDS protein extraction protocol was used with slight modification (67). 50 A600 units of yeast cells were collected by centrifugation, washed once with water, and then suspended in 1000 μl of cold distilled water followed by the addition of 1000 μl of 0.2 m NaOH. Samples were mixed and incubated for 5 min at room temperature. Cells were collected by centrifugation, resuspended in 1000 μl of SDS sample buffer (0.06 m Tris-HCl, pH 8.6, 5% glycerol, 1% SDS, and 10 mm β-mercaptoethanol), and boiled for 5 min. Samples were centrifuged, and protein concentrations were determined using the DC protein assay kit (Bio-Rad).
Secretion assays
Analyses of the secretion of Bgl2 and invertase were carried out as described previously (68). 20 ml of yeast cells were grown to early log phase (A600 of 0.6–1.0) at 25 °C. NaN3 and NaF were added directly to the culture at a final concentration of 10 mm each. 10 A600 units of cells were collected and washed with cell wash buffer (20 mm Tris-HCl, pH 7.5, 10 mm NaN3, and 10 mm NaF). The cells were resuspended in 300 μl of spheroplast solution buffer (50 mm Tris-HCl, pH 7.5, 1.4 m sorbitol, 10 mm NaN3, 10 mm NaF, 30 mm 2-mercaptoethanol, and 0.2 mg/ml Zymolyase) and incubated at 37 °C in a water bath for 30 min. The spheroplasts were pelleted gently by centrifugation at 2000 rpm for 5 min at 4 °C. 100 μl of supernatant were carefully transferred to a new tube and mixed with 20 μl of 6× SDS loading buffer (300 mm Tris-HCl, pH 6.8, 600 mm DTT, 12% SDS, 0.6% bromphenol blue, and 60% glycerol). This is the external pool. The remaining supernatant was removed, and the pellet (spheroplasts) was washed once with 1 ml of spheroplast wash buffer (50 mm Tris-HCl, pH 7.5, 1.4 m sorbitol, 10 mm NaN3, and 10 mm NaF) to remove residue external pool. The spheroplasts were dissolved in 300 μl of lysate buffer (20 mm Tris-HCl, pH 7.5, 100 mm NaCl, 2 mm MgCl2, 0.5% Triton X-100, and 1× protease inhibitor mixture (Roche Applied Science)) by leaving on ice for 10 min. The cell debris was removed after the sample was centrifuged at 13,000 rpm for 5 min at 4 °C. 100 μl of supernatant (lysates) were transferred to a new tube and mixed with 20 μl of 6× SDS loading buffer. This is the internal pool. The internal pool and external pool samples were boiled at 95 °C for 5 min and loaded for 12% SDS-PAGE. Bgl2 was detected by Western blotting with an anti-Bgl2 rabbit polyclonal antibody (1:2000). For temperature-sensitive mutants, the cells were grown at 25 °C or shifted to 37 °C for 90 min before being processed for the Bgl2 assay.
Invertase secretion was examined as described previously (41). 20 ml of yeast cells were grown to early log phase (A600 of 0.6–1.0) at 25 °C. 1 A600 unit if cells was transferred to a new tube and washed with 1 ml of ice-cold 1 mm NaN3. The cells were then resuspended in 1 ml of YP plus glucose medium (1% Bacto yeast extract, 2% Bacto peptone, and 0.1% glucose) and incubated at 25 °C for 1 h to induce invertase expression. After 1 h of incubation, the cells were collected and washed once with 1 ml of 10 mm NaN3. The cells were resuspended in 1 ml of 10 mm NaN3 and kept on ice. The external invertase was measured directly on the whole intact cells, whereas the internal invertase was measured after preparation of lysates. 0.5 ml of cells was removed and mixed with 0.5 ml of 2× spheroplast mixture (2.8 m sorbitol, 0.1 m Tris-HCl, pH 7.5, 10 mm NaN3, 0.4% 2-mercaptoethanol, and 10 μg/ml Zymolyase-100T). The cells were incubated in a water bath at 37 °C for 45 min. The spheroplasts were collected, and the supernatant was removed carefully without disturbing the pellet. The spheroplasts were dissolved at 4 °C in 0.5 ml of 0.5% Triton X-100. The invertase assay was performed in 13 × 100-mm test tubes. 20 μl of sample were placed in the tube, and 80 μl of 50 mm sodium acetate, pH 5.1, were added. Then 25 μl of 0.5 m sucrose were added, and the tube was incubated at 37 °C for 30 min. 150 μl of 0.2 m K2HPO4 were added, and the tube was placed on ice to stop the reaction. The sample was boiled for 3 min and put on ice. 1 ml of assay mixture was added (0.1 m KPi buffer, pH 7.0, 10 units/ml glucose oxidase, 2.5 μg/ml peroxidase, 150 μg/ml o-dianisidine, and 20 μm N-ethylmaleimide), and the sample was incubated at 37 °C for 30 min. 1 ml of 6 n HCl was added into the tube, and the value of A540 was measured by a spectrophotometer (SmartSpec 3000, Bio-Rad). For temperature-sensitive mutants, the cells were grown at 25 °C or shifted to 37 °C for 90 min and then grown in low-glucose medium (0.1% glucose) at the same temperature for 1 h.
Immunoprecipitation and detection of Exo84 phosphorylation
For immunoprecipitation of Exo84-myc and Exo84, total proteins were extracted by using a glass-bead method (66). A mouse anti-myc antibody (9E10) or rabbit anti-Exo84 antibody was used for immunoprecipitation as described below. Typically, 50 A600 units of a mid-log-phase yeast culture were harvested, and total protein was extracted using the NaOH-SDS method (67). Cell lysates were used for immunoprecipitation using protein G beads and anti-myc antibody (9E10) in immunoprecipitation buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 10% glycerol, 1× protease inhibitor (Roche Applied Science), and 1× phosphatase inhibitor I (Roche Applied Science)) at 4 °C for 4 h with rotation. Beads were washed with washing buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, and 10% glycerol) three times. The beads were mixed with 30 μl of 1× SDS loading buffer and incubated at 95 °C for 5 min. Protein samples were then cooled on ice for several minutes and separated by 10% SDS-PAGE. Western blotting was performed using the phospho-CDK substrate antibody (catalog number 2325, Cell Signaling Technology) and anti-Exo84 antibody as described previously (33).
In vitro Cdk1 kinase assays
50-μl reactions were prepared in kinase assay buffer (50 mm HEPES, pH 7.5, 2 mm MgCl2, 0.05% Tween 20, 2.5% glycerol, 1 mm DTT, and 1 mm ATP) containing 7 nm Cln2–Cdk1 or Clb5–Cdk1, 1 μg of GST or 2 μg of GST-Exo84 fusion protein, and 10 μCi of [γ-32P]ATP. Reactions were incubated at room temperature for 60 min and terminated by the addition of 10 μl of 6× sample buffer. Samples were boiled for 5 min and resolved by 10% SDS-PAGE. The gel was stained with Coomassie Blue and dried on a filter paper, and 32P radioactive signal was analyzed by a phosphorimaging system (Storm 860 molecular imager).
Microscopy
Fluorescence microscopy was conducted with a Leica CTR6000 fluorescence microscope equipped with a Plan Apo 100×, 1.40 oil immersion objective. Images were taken using LAS AF 1.5.1 acquisition software. For EM, cells were collected by vacuum filtration using a 0.45-μm nitrocellulose membrane and fixed for 1 h at room temperature in 0.1 m cacodylate, pH 7.4, 3% formaldehyde, 1 mm MgCl2, and 1 mm CaCl2. The cells were spheroplasted and fixed with 1% glutaraldehyde (in PBS, pH 7.4) at 4 °C overnight. The spheroplasts were washed in 0.1 m cacodylate buffer and postfixed twice with ice-cold 0.5% OsO4 and 0.8% potassium for 10 min each. After dehydration and embedding in Spurr's epoxy resin (Polysciences, Inc.), thin sections were cut and transferred onto 600 mesh uncoated copper grids (Ernest F. Fullam, Inc.) and poststained with uranyl acetate and lead citrate. Cells were observed on a transmission electron microscope (Model 1010, JEOL) at 100,000× magnification.
Author contributions
Y. D., Q. G., T. Z., Y. M., and D. S. investigation; Y. D. writing-original draft; G. L. and Y. L. supervision; G. L. and Y. L. writing-review and editing; Y. L. resources; Y. L. funding acquisition; Y. L. project administration.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grant 31570819. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Fig. S1 and Tables S1 and S2.
Y. Duan, Q. Guo, T. Zhang, Y. Meng, D. Sun, G. Luo, and Y. Liu, unpublished data.
- CDK
- cyclin-dependent kinase
- 1NM-PP1
- 4-amino-1-tert-butyl-3-(1′-naphthylmethyl)pyrazolo[3,4-d]pyrimidine
- GST
- glutathione S-transferase.
References
- 1. Soma S., Yang K., Morales M. I., and Polymenis M. (2014) Multiple metabolic requirements for size homeostasis and initiation of division in Saccharomyces cerevisiae. Microb. Cell 1, 256–266 10.15698/mic2014.08.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garmendia-Torres C., Tassy O., Matifas A., Molina N., and Charvin G. (2018) Multiple inputs ensure yeast cell size homeostasis during cell cycle progression. Elife 7, e34025 10.7554/eLife.34025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jorgensen P., and Tyers M. (2004) How cells coordinate growth and division. Curr. Biol. 14, R1014–R1027 10.1016/j.cub.2004.11.027 [DOI] [PubMed] [Google Scholar]
- 4. Björklund M. (2019) Cell size homeostasis: Metabolic control of growth and cell division. Biochim. Biophys. Acta Mol. Cell Res. 1866, 409–417 10.1016/j.bbamcr.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 5. Son S., Tzur A., Weng Y., Jorgensen P., Kim J., Kirschner M. W., and Manalis S. R. (2012) Direct observation of mammalian cell growth and size regulation. Nat. Methods 9, 910–912 10.1038/nmeth.2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goranov A. I., and Amon A. (2010) Growth and division—not a one-way road. Curr. Opin. Cell Biol. 22, 795–800 10.1016/j.ceb.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchison J. M., and Nurse P. (1985) Growth in cell length in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 75, 357–376 [DOI] [PubMed] [Google Scholar]
- 8. Mitchison J. M. (2003) Growth during the cell cycle. Int. Rev. Cytol. 226, 165–258 10.1016/S0074-7696(03)01004-0 [DOI] [PubMed] [Google Scholar]
- 9. Tzur A., Kafri R., LeBleu V. S., Lahav G., and Kirschner M. W. (2009) Cell growth and size homeostasis in proliferating animal cells. Science 325, 167–171 10.1126/science.1174294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moseley J. B., and Nurse P. (2009) Cdk1 and cell morphology: connections and directions. Curr. Opin. Cell Biol. 21, 82–88 10.1016/j.ceb.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 11. Goranov A. I., Cook M., Ricicova M., Ben-Ari G., Gonzalez C., Hansen C., Tyers M., and Amon A. (2009) The rate of cell growth is governed by cell cycle stage. Genes Dev. 23, 1408–1422 10.1101/gad.1777309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gulli M. P., Jaquenoud M., Shimada Y., Niederhäuser G., Wiget P., and Peter M. (2000) Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell 6, 1155–1167 10.1016/S1097-2765(00)00113-1 [DOI] [PubMed] [Google Scholar]
- 13. Howell A. S., and Lew D. J. (2012) Morphogenesis and the cell cycle. Genetics 190, 51–77 10.1534/genetics.111.128314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erickson C. A., and Trinkaus J. P. (1976) Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp. Cell Res. 99, 375–384 10.1016/0014-4827(76)90595-4 [DOI] [PubMed] [Google Scholar]
- 15. McCusker D., and Kellogg D. R. (2012) Plasma membrane growth during the cell cycle: unsolved mysteries and recent progress. Curr. Opin. Cell Biol. 24, 845–851 10.1016/j.ceb.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boucrot E., and Kirchhausen T. (2007) Endosomal recycling controls plasma membrane area during mitosis. Proc. Natl. Acad. Sci. U.S.A. 104, 7939–7944 10.1073/pnas.0702511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He B., and Guo W. (2009) The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21, 537–542 10.1016/j.ceb.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J., and Guo W. (2012) The exocyst complex in exocytosis and cell migration. Protoplasma 249, 587–597 10.1007/s00709-011-0330-1 [DOI] [PubMed] [Google Scholar]
- 19. Heider M. R., and Munson M. (2012) Exorcising the exocyst complex. Traffic 13, 898–907 10.1111/j.1600-0854.2012.01353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu B., and Guo W. (2015) The exocyst at a glance. J. Cell Sci. 128, 2957–2964 10.1242/jcs.156398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo G., Zhang J., and Guo W. (2014) The role of Sec3p in secretory vesicle targeting and exocyst complex assembly. Mol. Biol. Cell 25, 3813–3822 10.1091/mbc.e14-04-0907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y., Zhang T., Sun D., and Luo G. (2019) The Cdc42 effectors Gic1 and Gic2 regulate polarized post-Golgi secretion. Cell Biosci. 9, 33 10.1186/s13578-019-0295-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo W., Sacher M., Barrowman J., Ferro-Novick S., and Novick P. (2000) Protein complexes in transport vesicle targeting. Trends Cell Biol. 10, 251–255 10.1016/S0962-8924(00)01754-2 [DOI] [PubMed] [Google Scholar]
- 24. Whyte J. R., and Munro S. (2002) Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627–2637 [DOI] [PubMed] [Google Scholar]
- 25. Hsu S. C., TerBush D., Abraham M., and Guo W. (2004) The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 233, 243–265 10.1016/S0074-7696(04)33006-8 [DOI] [PubMed] [Google Scholar]
- 26. Boyd C., Hughes T., Pypaert M., and Novick P. (2004) Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 167, 889–901 10.1083/jcb.200408124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Munson M., and Novick P. (2006) The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 13, 577–581 10.1038/nsmb1097 [DOI] [PubMed] [Google Scholar]
- 28. Nelson W. J., and Yeaman C. (2001) Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 11, 483–486 10.1016/S0962-8924(01)02145-6 [DOI] [PubMed] [Google Scholar]
- 29. Bryant D. M., and Mostov K. E. (2008) From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887–901 10.1038/nrm2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall A., and Lalli G. (2010) Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect. Biol. 2, a001818 10.1101/cshperspect.a001818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polgar N., and Fogelgren B. (2018) Regulation of cell polarity by exocyst-mediated trafficking. Cold Spring Harb. Perspect. Biol. 10, a031401 10.1101/cshperspect.a031401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rancati G., and Li R. (2007) Polarized cell growth: double grip by CDK1. Curr. Biol. 17, R600–R603 10.1016/j.cub.2007.05.065 [DOI] [PubMed] [Google Scholar]
- 33. Luo G., Zhang J., Luca F. C., and Guo W. (2013) Mitotic phosphorylation of Exo84 disrupts exocyst assembly and arrests cell growth. J. Cell Biol. 202, 97–111 10.1083/jcb.201211093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drury L. S., Perkins G., and Diffley J. F. (1997) The Cdc4/34/53 pathway targets Cdc6p for proteolysis in budding yeast. EMBO J. 16, 5966–5976 10.1093/emboj/16.19.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nugroho T. T., and Mendenhall M. D. (1994) An inhibitor of yeast cyclin-dependent protein kinase plays an important role in ensuring the genomic integrity of daughter cells. Mol. Cell. Biol. 14, 3320–3328 10.1128/MCB.14.5.3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwob E., Böhm T., Mendenhall M. D., and Nasmyth K. (1994) The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell 79, 233–244 10.1016/0092-8674(94)90193-7 [DOI] [PubMed] [Google Scholar]
- 37. Patton E. E., Willems A. R., Sa D., Kuras L., Thomas D., Craig K. L., and Tyers M. (1998) Cdc53 is a scaffold protein for multiple Cdc34/Skp1/F-box protein complexes that regulate cell division and methionine biosynthesis in yeast. Genes Dev. 12, 692–705 10.1101/gad.12.5.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harsay E., and Bretscher A. (1995) Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 131, 297–310 10.1083/jcb.131.2.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makarow M. (1988) Secretion of invertase in mitotic yeast cells. EMBO J. 7, 1475–1482 10.1002/j.1460-2075.1988.tb02965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bishop A. C., Ubersax J. A., Petsch D. T., Matheos D. P., Gray N. S., Blethrow J., Shimizu E., Tsien J. Z., Schultz P. G., Rose M. D., Wood J. L., Morgan D. O., and Shokat K. M. (2000) A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 10.1038/35030148 [DOI] [PubMed] [Google Scholar]
- 41. Novick P., Field C., and Schekman R. (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- 42. Kõivomägi M., Valk E., Venta R., Iofik A., Lepiku M., Morgan D. O., and Loog M. (2011) Dynamics of Cdk1 substrate specificity during the cell cycle. Mol. Cell 42, 610–623 10.1016/j.molcel.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smolka M. B., Albuquerque C. P., Chen S. H., and Zhou H. (2007) Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 10.1073/pnas.0701622104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Albuquerque C. P., Smolka M. B., Payne S. H., Bafna V., Eng J., and Zhou H. (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell. Proteomics 7, 1389–1396 10.1074/mcp.M700468-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holt L. J., Tuch B. B., Villén J., Johnson A. D., Gygi S. P., and Morgan D. O. (2009) Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682–1686 10.1126/science.1172867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiederkehr A., Du Y., Pypaert M., Ferro-Novick S., and Novick P. (2003) Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell 14, 4770–4782 10.1091/mbc.e03-04-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walch-Solimena C., Collins R. N., and Novick P. J. (1997) Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell Biol. 137, 1495–1509 10.1083/jcb.137.7.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lucocq J. M., and Warren G. (1987) Fragmentation and partitioning of the Golgi apparatus during mitosis in HeLa cells. EMBO J. 6, 3239–3246 10.1002/j.1460-2075.1987.tb02641.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leaf D. S., Roberts S. J., Gerhart J. C., and Moore H. P. (1990) The secretory pathway is blocked between the trans-Golgi and the plasma membrane during meiotic maturation in Xenopus oocytes. Dev. Biol. 141, 1–12 10.1016/0012-1606(90)90097-3 [DOI] [PubMed] [Google Scholar]
- 50. Kreiner T., and Moore H. P. (1990) Membrane traffic between secretory compartments is differentially affected during mitosis. Cell Regul. 1, 415–424 10.1091/mbc.1.5.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lowe M., Nakamura N., and Warren G. (1998) Golgi division and membrane traffic. Trends Cell Biol. 8, 40–44 10.1016/S0962-8924(97)01189-6 [DOI] [PubMed] [Google Scholar]
- 52. Seemann J., Pypaert M., Taguchi T., Malsam J., and Warren G. (2002) Partitioning of the matrix fraction of the Golgi apparatus during mitosis in animal cells. Science 295, 848–851 10.1126/science.1068064 [DOI] [PubMed] [Google Scholar]
- 53. Boucrot E., and Kirchhausen T. (2008) Mammalian cells change volume during mitosis. PLoS One 3, e1477 10.1371/journal.pone.0001477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCusker D., Denison C., Anderson S., Egelhofer T. A., Yates J. R 3rd, Gygi S. P., and Kellogg D. R. (2007) Cdk1 coordinates cell-surface growth with the cell cycle. Nat. Cell Biol. 9, 506–515 10.1038/ncb1568 [DOI] [PubMed] [Google Scholar]
- 55. Brennwald P. (2013) Membrane traffic: the exocyst meets the cell cycle. Curr. Biol. 23, R838–R840 10.1016/j.cub.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 56. Garrett S., Barton W. A., Knights R., Jin P., Morgan D. O., and Fisher R. P. (2001) Reciprocal activation by cyclin-dependent kinases 2 and 7 is directed by substrate specificity determinants outside the T loop. Mol. Cell. Biol. 21, 88–99 10.1128/MCB.21.1.88-99.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Murray A. W. (2004) Recycling the cell cycle: cyclins revisited. Cell 116, 221–234 10.1016/S0092-8674(03)01080-8 [DOI] [PubMed] [Google Scholar]
- 58. Harvey S. L., Charlet A., Haas W., Gygi S. P., and Kellogg D. R. (2005) Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 122, 407–420 10.1016/j.cell.2005.05.029 [DOI] [PubMed] [Google Scholar]
- 59. Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., Shah K., Shokat K. M., and Morgan D. O. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 10.1038/nature02062 [DOI] [PubMed] [Google Scholar]
- 60. Zheng X. D., Lee R. T., Wang Y. M., Lin Q. S., and Wang Y. (2007) Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J. 26, 3760–3769 10.1038/sj.emboj.7601814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Loog M., and Morgan D. O. (2005) Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434, 104–108 10.1038/nature03329 [DOI] [PubMed] [Google Scholar]
- 62. Sherman F. (2002) Getting started with yeast. Methods Enzymol. 350, 3–41 10.1016/S0076-6879(02)50954-X [DOI] [PubMed] [Google Scholar]
- 63. Gietz D., St Jean A., Woods R. A., and Schiestl R. H. (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20, 1425 10.1093/nar/20.6.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mumberg D., Müller R., and Funk M. (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119–122 10.1016/0378-1119(95)00037-7 [DOI] [PubMed] [Google Scholar]
- 65. Longtine M. S., McKenzie A. 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., and Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 66. Inagaki M., Schmelzle T., Yamaguchi K., Irie K., Hall M. N., and Matsumoto K. (1999) PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol. 19, 8344–8352 10.1128/MCB.19.12.8344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kushnirov V. V. (2000) Rapid and reliable protein extraction from yeast. Yeast 16, 857–860 [DOI] [PubMed] [Google Scholar]
- 68. Zhang X., Zajac A., Zhang J., Wang P., Li M., Murray J., TerBush D., and Guo W. (2005) The critical role of Exo84p in the organization and polarized localization of the exocyst complex. J. Biol. Chem. 280, 20356–20364 10.1074/jbc.M500511200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.