Abstract
Lipocalin-type prostaglandin D synthase (L-PGDS; EC:5.3.99.2) is an enzyme with dual functional roles as a prostaglandin D2-synthesizing enzyme and as an extracellular transporter for diverse lipophilic compounds in the cerebrospinal fluid (CSF). Transport of hydrophobic endocannabinoids is mediated by serum albumin in the blood and intracellularly by the fatty acid binding proteins, but no analogous transport mechanism has yet been described in CSF. L-PGDS has been reported to promiscuously bind a wide variety of lipophilic ligands and is among the most abundant proteins found in the CSF. Here, we examine the binding of several classes of endogenous and synthetic ligands to L-PGDS. Endocannabinoids exhibited low affinity toward L-PGDS, while cannabinoid metabolites and synthetic cannabinoids displayed higher affinities for L-PGDS. These results indicate that L-PGDS is unlikely to function as a carrier for endocannabinoids in the CSF, but it may bind and transport a subset of cannabinoids.
Keywords: Cannabinoid, Endocannabinoid, Lipocalin, Lipocalin-type prostaglandin D synthase, N-Acylethanolamine, Prostaglandin
Introduction
The endocannabinoid system regulates diverse biological processes including cognition and pain (Fowler, Naidu, Lichtman, & Onnis, 2009; Jonsson, Holt, & Fowler, 2006; Maccarrone et al., 2015; Schlosburg, Kinsey, & Lichtman, 2009). The endocannabinoids N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) serve as endogenous ligands for cannabinoid receptors (Dalton, Bass, Van Horn, & Howlett, 2009; Pertwee et al., 2010). Endocannabinoid signaling is terminated by cellular uptake followed by intracellular hydrolysis (Glaser, Kaczocha, & Deutsch, 2005; McKinney & Cravatt, 2005). Because of their inherent hydrophobicity, endocannabinoids require transport through the aqueous cytosol to reach their catabolic enzymes. Intracellular endocannabinoid trafficking is facilitated by fatty acid binding proteins (FABP), a family of small cytosolic proteins that bind and transport hydrophobic lipids including the endocannabinoids (Huang et al., 2016; Kaczocha, Glaser, & Deutsch, 2009). Within the blood, endocannabinoids are primarily transported by binding to serum albumin for which they exhibit high affinity (Bojesen & Hansen, 2003), but lipo-proteins may also play a partial role (Bilgin, Bindila, Graessler, & Shevchenko, 2015; Ruiz, Sanchez, Correnti, Strong, & Ganfornina, 2013).
Endocannabinoids are also released by cells and are present in the human cerebrospinal fluid (CSF) (Azim et al., 2015; Koethe, Giuffrida, et al., 2009; Morgan et al., 2013; Nicholson et al., 2015). Brain endocannabinoids regulate neurological function, and CSF endocannabinoid levels are altered in patients with schizophrenia, chronic pain, and Parkinson’s disease (Azim et al., 2015; Giuffrida et al., 2004; Koethe, Giuffrida, et al., 2009; Morgan et al., 2013; Pisani et al., 2010; Sarchielli et al., 2007). Furthermore, endocannabinoids and the structurally related N-acylethanolamine (NAE) and oleoylethanolamide (OEA)—which activates the nuclear peroxisome proliferator-activated receptor alpha—regulate sleep (Murillo-Rodriguez et al., 2016; Pava, Makriyannis, & Lovinger, 2016; Soria-Gomez et al., 2010). Oleamide is a primary fatty acid amide that likewise regulates sleep, and both oleamide and OEA levels are elevated in the CSF after sleep deprivation (Cravatt et al., 1995; Koethe, Schreiber, et al., 2009). Given the fluctuations in endocannabinoid and the related bioactive lipid levels in the CSF of patients, coupled with their hydrophobicity, we hypothesized that the CSF possesses a protein(s) that binds to and transports endocannabinoids/NAE in a manner analogous to intracellular FABP and serum albumin.
Lipocalin-type prostaglandin D synthase (L-PGDS; EC:5.3.99.2) is a secreted enzyme and is the second most abundant protein in human CSF (Hoffmann et al., 1993). L-PGDS converts prostaglandin H2 to prostaglandin D2, a metabolite that is also implicated in the regulation of sleep (Saper, Romanovsky, & Scammell, 2012; Zeitzer, 2013). In addition to its enzymatic function, L-PGDS binds to and serves as a carrier for hydrophobic ligands including retinoids, hemoglobin metabolites, thyroid hormones, gangliosides, and fatty acids (Kume et al., 2012; Mohri et al., 2006; Tanaka et al., 1997; Zhou et al., 2010). The large and unusually shaped binding cavity of L-PGDS confers an ability to bind a broad range of ligands, and upon ligand binding, the protein undergoes conformational changes to become more compact, aiding in higher-affinity binding (Inoue, Yagi, Urade, & Inui, 2009; Kumasaka et al., 2009; Shimamoto et al., 2007). L-PGDS belongs to the lipocalin family of proteins, which consist of a highly conserved fold that is characterized by orthogonal β-sheets that form the lipid ligand binding pocket (Flower, North, & Sansom, 2000). Interestingly, L-PGDS is the only known lipocalin that acts as both an enzyme and a lipid transporter (Hoffmann et al., 1993). Despite sharing low amino-acid sequence homology, FABP and lipocalins, including L-PGDS, share a similar overall structural fold and likewise bind to a diverse array of hydrophobic ligands (Elmes et al., 2015; Flower et al., 2000; Furuhashi & Hotamisligil, 2008). Given the role of endocannabinoids and related NAE in numerous physiological processes, their presence in CSF, and the structural similarity and ligand-binding promiscuity between FABP and L-PGDS, we hypothesized that L-PGDS may serve as an endocannabinoid/NAE binding protein in the CSF.
Materials and Methods
Protein Purification
Residues 29–190 of L-PGDS were amplified from a human brain cDNA library and cloned into a pTXB1 vector (the first 22 N-terminal residues of L-PGDS comprise a hydro-phobic signal sequence that is posttranslationally cleaved). The expression construct was transformed into BL21(DE3) Escherichia coli, and recombinant Δ1–28 L-PGDS was purified using the IMPACT purification system (New England Biolabs, Ipswich, UK) as described previously (Kaczocha, Vivieca, Sun, Glaser, & Deutsch, 2012). Residual endogenous bacterial lipids were removed by incubation in a column of hydroxypropyl-beaded dextran for 1 h at 37 °C. The final delipidated protein was concentrated to 10 mg/mL in phosphate buffered saline (PBS) + 150 mM NaCl and flash-frozen with liquid nitrogen.
Fluorescence Displacement Binding Assays
Fluorescent binding assays were performed in 96-well Cost-ar® assay plates (Corning Life Sciences, Kennebunk, ME, USA). 12-N-methyl-(7-nitrobenz-2-oxa-1,3-diazo)aminostearic acid (NBD)-stearate was purchased from Avanti Polar Lipids (Alabaster, AL, USA). 11-(dansylamino)undecanoic acid (DAUDA) and 1-anilinonaphthalene-8-sulfonic acid (ANS) were purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
Purified L-PGDS (3 μM) was incubated with DAUDA (500 nM) or ANS (500 nM) in 30 mM Tris–HCl and 100 mM NaCl buffer (pH 7.6). Competitor test compounds (0.1–250 μM) were then added to the wells, mixed, and the system was allowed to reach equilibrium by incubating in the dark at 25 °C for 20 min. All experimental conditions were tested in triplicate. Each independent assay included wells containing a strong competitive binder (retinoic acid or oleic acid [OLA], 10 μM) as a positive control for probe displacement and background readings (absence of protein in wells). Loss of fluorescence intensity was monitored with an F5 Filtermax Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) using excitation (ex.) and emission (em.) wavelengths appropriate for each respective probe (DAUDA ex./em. = 345/535 nm, NBD-stearate ex./em. = 465/535 nm, ANS ex./em. = 370/465 nm). Following background subtraction, the fluorescence intensity values were normalized and fitted to a one-site binding analysis using the GraphPad Prism software (Prism version 7.0 for Mac OS; Graphpad Software Inc., La Jolla, CA, USA) to determine the Ki of the tested compounds from the equation Ki = IC50/(1 + ([DAUDA]/Kd)).
Determination of Fluorescent Probe Binding Affinity
Recombinant L-PGDS (1 μM) was titrated with DAUDA (0–16 μM), ANS (0–25 μM), or NBD (0–25 μM). The raw fluorescence intensity at each data point was corrected by subtracting the signal from each respective probe concentration in the absence of protein. Kd and Bmax were then calculated by fitting the titration curve to the single-site saturation binding equation Y = [Bmax × X/(Kd + X)] using the GraphPad Prism software.
Intrinsic Tryptophan Fluorescence Quenching Assay
Compounds were dissolved in dimethyl sulfoxide (DMSO) to a stock concentration of 2 mM. Compound solutions (10 μL) were added to 990 μL 5 mM Tris–HCl (pH 8.0) containing Δ1–28 L-PGDS (1.5 μM). The system was allowed to equilibrate for 30 min at 25 °C, and the intrinsic tryptophan fluorescence of the protein was measured (ex./em. 282/340 nm; 5 nm slit width) using a FP-6200 spectrofluorimeter (JASCO, Tokyo, Japan). Effects on tryptophan fluorescence resulting from nonspecific interactions with each compound were corrected with N-acetyl-L-tryptophanamide (1.5 μM).
Statistics
All quantitative data are expressed as means ± standard error (SE) from at least three independent experiments.
Results and Discussion
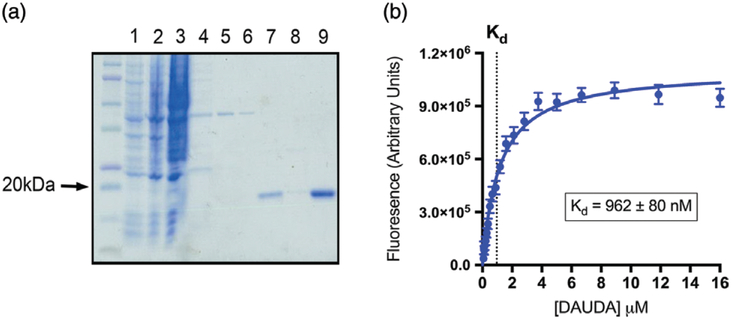
Human Δ1–28 L-PGDS was purified from E. coli, delipi-dated, and its purity confirmed by Coomassie staining (Fig. 1a). We employed fluorophore displacement assays to assess the binding affinities of ligands to Δ1–28 L-PGDS (Kume et al., 2012). Δ1–28 L-PGDS bound to the fluores-cent probe DAUDA with an affinity of 0.96 ± 0.08 μM (Fig. 1b), while binding to ANS was weaker (Kd = 3.4 ± 0.8 μM), and NBD-stearate did not exhibit appreciable binding. These probe affinities to L-PGDS are generally in agreement with the values found in the existing literature (Breustedt, Schonfeld, & Skerra, 2006). Consequently, we employed DAUDA for all subsequent binding studies unless otherwise stated.
Fig. 1.
Lipocalin-type prostaglandin D synthase (L-PGDS) protein purification and determination of 11-(dansylamino)undecanoic acid (DAUDA) binding affinity. (a) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of Δ1–28 L-PGDS protein purification. Unlabeled lane is protein ladder; lane 1, uninduced bacterial cell lysate; lane 2, isopropyl β-d-1-thiogalactopyranoside (IPTG)-induced bacterial cell lysate; lane 3, column effluent (flow through); lane 4, first wash; lane 5, second wash; lane 6, third wash; lane 7, pooled elution fractions; lane 8, fast protein liquid chromatography (FPLC)-purified and delipidated protein; lane 9, concentrated final product. (b) Saturation curve of DAUDA binding to Δ1–28 L-PGDS
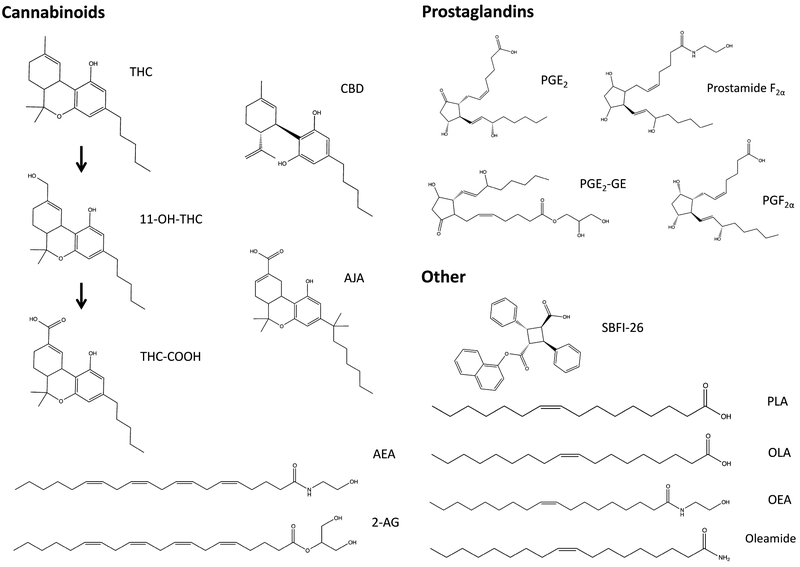
We examined the binding affinities of several classes of endogenous and synthetic ligands to L-PGDS (Fig. 2). The fatty acids OLA and palmitoleic acid (PLA) were selected because these fatty acids were previously shown to interact with L-PGDS (Kume et al., 2012; Zhou et al., 2010). We examined the binding of AEA, 2-AG, and their respective cyclooxygenase metabolites prostamide F2α and prostaglandin E2-glyceryl ester (PGE2-GE), which were compared to prostaglandin F2α (PGF2α) and prostaglandin E2 (PGE2). The cyclooxygenase metabolites were selected because of their involvement in pain and inflammation (Gatta et al., 2012; Hu, Bradshaw, Chen, Tan, & Walker, 2008). The NAE palmitoylethanolamide (PEA) and OEA, which activate the nuclear peroxisome proliferator-activated receptor alpha, and the primary fatty acid amide oleamide were selected because of their biological relevance and presence in the CSF. Furthermore, we examined the binding of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), the most abundant cannabinoids found in marijuana, which were recently shown to bind the brain-expressed FABP (Elmes et al., 2015). The binding of major THC metabolites and SBFI-26—a recently developed FABP inhibitor—were also explored (Berger et al., 2012).
Fig. 2.
Chemical structures of the tested compounds
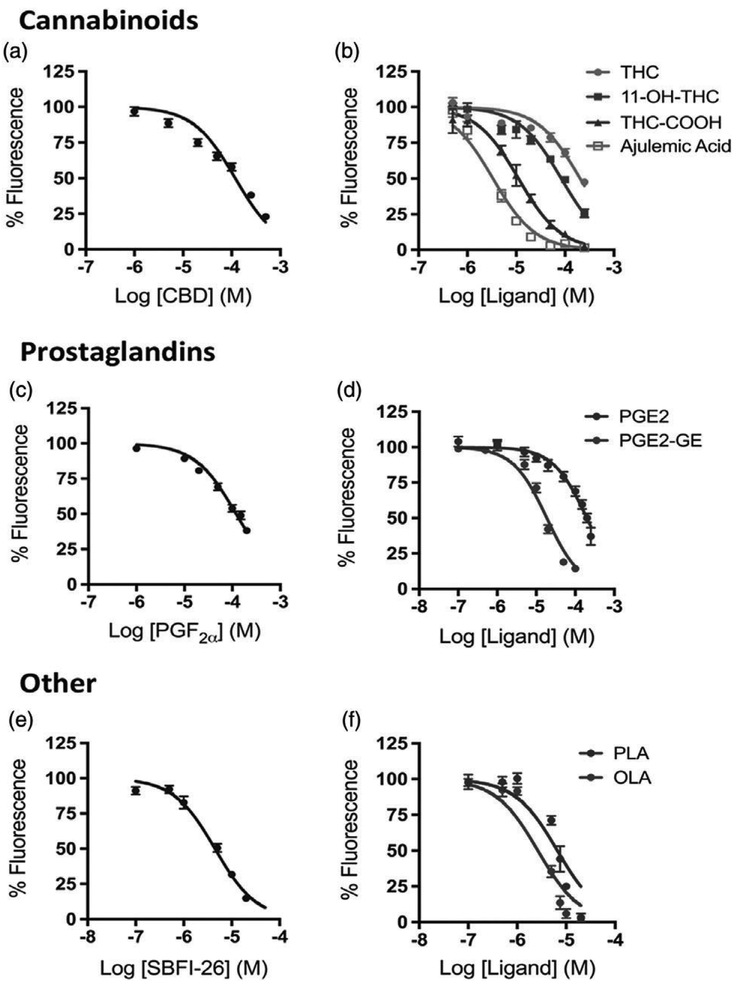
OLA and PLA bound Δ1–28 L-PGDS with Ki values of 1.8 ± 0.9 and 3.7 ± 0.5 μM, respectively (Fig. 3f and Table 1). In contrast, AEA and 2-AG did not bind to Δ1–28 L-PGDS as evidenced by a lack of DAUDA displacement (Table 1). To rule out the possibility that the inability of endocannabinoids to displace DAUDA from L-PGDS may have been inherent to the probe, we repeated the experiments using ANS and similarly observed a lack of affinity of AEA for Δ1–28 L-PGDS. PGF 2α weakly bound Δ 1–28 L-PGDS (Ki = 81 ± 9 μM); however, prostamide F2α, the cyclooxygenase metabolite of AEA, was not found to bind with any appreciable affinity (Fig. 3c and Table 1). In contrast, PGE2-GE, the cyclooxygenase metabolite of 2-AG, demonstrated a moderate affinity for Δ1–28 L-PGDS (Ki = 11.2 ± 1.2 μM) (Fig. 3f and Table 1). Interestingly, the cyclooxygenase metabolite of arachidonic acid, PGE2, displayed much weaker binding (Ki = 128 ± 16 μM) than PGE2-GE (Fig. 3d and Table 1). PEA and OEA did not display any appreciable binding to Δ1–28 L-PGDS.
Fig. 3.
Binding curves for active compounds. Displacement of 11-(dansylamino)undecanoic acid (DAUDA) from Δ1–28 lipocalin-type prostaglandin D synthase (L-PGDS) by (a) cannabidiol (CBD), (b) Δ9-tetrahydrocannabinol (THC), THC metabolites (11-OH-THC and THC-COOH), and ajulemic acid (AJA), (c) prostaglandin F2α (PGF2α), (d) prostaglandin E2 (PGE2) and prostaglandin E2-glyceryl ester (PGE2-GE), (e) SBFI-26, and (f) fatty acids oleic acid (OLA) and palmitoleic acid (PLA). Displacement curve data presented as a mean ± SE from at least three independent assays. Solid lines represent optimal curve-fitting to a one-site competition model
Table 1.
In vitro affinities of select compounds for Δ1–28 lipocalin-type prostaglandin D synthase (L-PGDS) as determined by fluorescence displacement binding assay and intrinsic tryptophan fluorescence quenching assay
| Chemical class | Compound | Ki (μM)a | Quenching (%)a,b |
|---|---|---|---|
| Prostaglandins | Prostaglandin F2α (PGF2α) | 81 ± 9 | 2.7 ± 1.7 |
| Prostamide F2α | >200 | 0.1 ± 2.6 | |
| Prostaglandin E2 (PGE2) | 128 ± 16 | 6.5 ± 1.5 | |
| Prostaglandin E2-glyceryl ester (PGE2-GE) | 11.2 ± 1.2 | 15.1 ± 1.7 | |
| Phytocannabinoids | Cannabidiol (CBD) | 77.9 ± 3.9 | 7.2 ± 2.1 |
| Δ9-Tetrahydrocannabinol (THC) | 175 ± 26 | 14.0 ± 2.2 | |
| THC metabolites | 1l-Hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) | 61 ± 14 | 9.7 ± 1.3 |
| 1l-Nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH) | 7.8 ± 1.7 | 15.6 ± 1.3 | |
| Synthetic THC-COOH analog | Ajulemic acid (AJA) | 2.3 ± 0.2 | 21.9 ± 2.9 |
| Endocannabinoids | N-Arachidonoylethanolamine/anandamide (AEA) | >200 | 3.1 ± 3.3 |
| 2-Arachidonoylglycerol (2-AG) | >200 | −0.8 ± 3.0 | |
| NAE | Palmitoylethanolamide (PEA) | >200 | 1.3 ± 4.7 |
| Oleoylethanolamide (OEA) | >200 | −0.9 ± 2.1 | |
| Fatty acid amide | Oleamide | >200 | 3.6 ± 2.0 |
| Fatty acids | Oleic acid (OLA) | 1.8 ± 0.9 | 15.1 ± 3.7 |
| Palmitoleic acid (PLA) | 3.7 ± 0.5 | 11.9 ± 2.3 | |
| Synthetic FABP inhibitor | SBFI-26 | 3.0 ± 0.5 | 33.3 ± 2.2 |
Values represent mean ± SE from at least three independent assays.
Data expressed as % fluorescence reduction relative to vehicle-treated Δ1–28 L-PGDS.
The phytocannabinoids THC and CBD both weakly interacted with Δ1–28 L-PGDS (Ki = 175 ± 26 and 77.9 ± 3.9 μM, respectively) (Fig. 3a, b). Following con-sumption of marijuana, the cytochrome P450 system hydroxylates THC to its primary metabolite 11-hydroxy-Δ9-THC (11-OH-THC), which in turn is further oxidized to the secondary metabolite 11-nor-9-carboxy-Δ9-THC (THC-COOH) (Wall & Perez-Reyes, 1981). Intriguingly, 11-OH-THC and THC-COOH exhibited much higher affinities for L-PGDS than the parent compound (Ki = 61 ± 14 and 7.8 ± 1.7 M, respectively) (Fig. 3b). Similarly, ajulemic acid (AJA), a nonpsychoactive synthetic derivative of THC-COOH used to treat inflammatory pain, displayed strong affinity for Δ1–28 L-PGDS (Ki = 2.2 0.3 μM) (Fig. 3b) (Mitchell, Aslan, Safaei, & Vaughan, 2005). The FABP inhibitor SBFI-26 bound to L-PGDS with high affinity, similar to the fatty acids (Ki = 3.0 ± 0.5 μM) (Fig. 3e and Table 1).
Intrinsic tryptophan fluorescence quenching assays were employed as a secondary means of assessing relative in vitro affinities. Each compound was screened at 20 μM, and changes in intrinsic Δ1–28 L-PGDS fluorescence was monitored relative to the vehicle-treated protein (Table 1). As expected, all-trans retinoic acid, a known high-affinity L-PGDS ligand (Kd = 290 [notdef] 30 nM), displayed potent fluorescence quenching in this assay (71.1 ± 1.4%) (Breustedt et al., 2006). These data support and validate the results obtained from displacement assays, with lower Ki affinity values generally being predictive of higher quenching, and all compounds that were unable to displace DAUDA exhibited little to no (<4%) quenching.
This study was the first to thoroughly examine the binding of a variety of endogenous bioactive lipids, phytocannabinoids, and synthetic ligands to L-PGDS. Δ1–28 L-PGDS displayed the highest affinity for lipids bearing free carboxylate moieties while low or no affinity for ligands lacking this functional group, consistent with structural data demonstrating electrostatic interactions between the carboxylate groups of ligands and residues lining the binding cavity of L-PGDS (Lim et al., 2013). Contrary to our hypothesis, Δ1–28 L-PGDS displayed weak or no affinity for a variety of endogenous ligands including the endocannabinoids and NAE, suggesting that other proteins in the CSF may bind and transport these lipids. Indeed, serum albumin is present in CSF, and recent work indicates that apolipoprotein D binds to AEA (Huhmer, Biringer, Amato, Fonteh, & Harrington, 2006; Ruiz et al., 2013), potentially suggesting that these proteins facilitate CSF endocannabinoid transport. However, it is noteworthy that the levels of endocannabinoids in the CSF are orders of magnitude lower than those found in serum (Azim et al., 2015; Jumpertz, Guijarro, Pratley, Piomelli, & Krakoff, 2011), raising the possibility that these lipids may be present at sufficiently low concentrations to permit sufficient solubility in CSF. Additionally, endogenous L-PGDS is highly glycosylated and may potentially display an altered ligand-binding profile than our data suggests due to inherent limitations of the bacterially expressed and truncated recombinant protein (Hoffmann, Nimtz, Wurster, & Conradt, 1994). Our study expands the repertoire of ligands that bind to L-PGDS and further indicates that this protein may serve as a carrier for a broad range of lipids in CSF; however, further investigations will be needed to validate any physiological implications from this work.
Acknowledgements
We would like to thank Dr Dale Deutsch for the use of his facilities and equipment. This work was supported by National Institute on Drug Abuse (NIDA) grants F31DA042545 (M.E.), DA035923 and DA035949 (M.K.).
Abbreviations
- 2-AG
2-arachidonoylglycerol
- 11-OH-THC
11-hydroxy-Δ9-tetrahydrocannabinol
- AEA
N-arachidonoylethanolamine/anandamide
- AJA
ajulemic acid
- ANS
1-anilinonaphthalene-8-sulfonic acid
- CBD
cannabidiol
- CSF
cerebrospinal fluid
- DAUDA
11-(dansylamino)undecanoic acid
- FABP
fatty acid binding protein
- L-PGDS
lipocalin-type prostaglandin D synthase
- NAE
N-acylethanolamine
- NBD
12-N-methyl-(7-nitrobenz-2-oxa-1,3-diazo) aminostearic acid
- OEA
oleoylethanolamide
- OLA
oleic acid
- PEA
palmitoylethanolamide
- PGE2
prostaglandin E2
- PGE2-GE
prostaglandin E2-glyceryl ester
- PGF2α
prostaglandin F2α
- PLA
palmitoleic acid
- THC
Δ9-tetrahydrocannabinol
- THC-COOH
11-nor-9-carboxy-Δ9-tetrahydrocannabinol
Footnotes
Conflicts of Interest The authors declare that they have no conflicts of interest.
References
- Azim S, Nicholson J, Rebecchi MJ, Galbavy W, Feng T, Reinsel R, … Kaczocha M (2015) Endocannabinoids and acute pain after total knee arthroplasty. Pain, 156:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC, … Deutsch DG (2012) Targeting fatty acid binding protein (FABP) anandamide transporters—A novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One, 7:e50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin M, Bindila L, Graessler J, & Shevchenko A (2015) Quantitative profiling of endocannabinoids in lipoproteins by LC-MS/MS. Analytical and Bioanalytical Chemistry, 407: 5125–5131. [DOI] [PubMed] [Google Scholar]
- Bojesen IN, & Hansen HS (2003) Binding of anandamide to bovine serum albumin. Journal of Lipid Research, 44:1790–1794. [DOI] [PubMed] [Google Scholar]
- Breustedt DA, Schonfeld DL, & Skerra A (2006) Comparative ligand-binding analysis of ten human lipocalins. Biochimica et Biophysica Acta, 1764:161–173. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, & Lerner RA (1995) Chemical characterization of a family of brain lipids that induce sleep. Science, 268:1506–1509. [DOI] [PubMed] [Google Scholar]
- Dalton GD, Bass CE, Van Horn CG, & Howlett AC (2009) Signal transduction via cannabinoid receptors .CNS & Neurological Disorders Drug Targets, 8:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, … Deutsch DG (2015) Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). The Journal of Biological Chemistry, 290:8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower DR, North AC, & Sansom CE (2000) The lipocalin protein family: Structural and sequence overview. Biochimica et Biophysica Acta, 1482:9–24. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Naidu PS, Lichtman A, & Onnis V (2009) The case for the development of novel analgesic agents targeting both fatty acid amide hydrolase and either cyclooxygenase or TRPV1. British Journal of Pharmacology, 156:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, & Hotamisligil GS (2008) Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nature Reviews. Drug Discovery, 7:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta L, Piscitelli F, Giordano C, Boccella S, Lichtman A, Maione S, & Di Marzo V (2012) Discovery of prostamide F2alpha and its role in inflammatory pain and dorsal horn nociceptive neuron hyperexcitability. PLoS One, 7:e31111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, … Piomelli D (2004) Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology, 29: 2108–2114. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Kaczocha M, & Deutsch DG (2005) Anandamide transport: A critical review. Life Sciences, 77:1584–1604. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Conradt HS, Gross G, Nimtz M, Lottspeich F, & Wurster U (1993) Purification and chemical characterization of beta-trace protein from human cerebrospinal fluid: Its identification as prostaglandin D synthase. Journal of Neurochemistry, 61: 451–456. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Nimtz M, Wurster U, & Conradt HS (1994) Carbohydrate structures of beta-trace protein from human cerebrospinal fluid: Evidence for “brain-type” N-glycosylation. Journal of Neuro-chemistry, 63:2185–2196. [DOI] [PubMed] [Google Scholar]
- Hu SS, Bradshaw HB, Chen JS, Tan B, & Walker JM (2008) Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. British Journal of Pharmacology, 153:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, McIntosh AL, Martin GG, Landrock D, Chung S, Landrock KK, … Schroeder F (2016) FABP1: A novel hepatic endocannabinoid and cannabinoid binding protein. Biochemistry, 55:5243–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhmer AF, Biringer RG, Amato H, Fonteh AN, & Harrington MG (2006) Protein analysis in human cerebrospinal fluid: Physiological aspects, current progress and future challenges. Disease Markers, 22:3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Yagi N, Urade Y, & Inui T (2009) Compact packing of lipocalin-type prostaglandin D synthase induced by binding of lipophilic ligands. Journal of Biochemistry, 145:169–175. [DOI] [PubMed] [Google Scholar]
- Jonsson KO, Holt S, & Fowler CJ (2006) The endocannabinoid system: Current pharmacological research and therapeutic possibilities. Basic & Clinical Pharmacology & Toxicology, 98:124–134. [DOI] [PubMed] [Google Scholar]
- Jumpertz R, Guijarro A, Pratley RE, Piomelli D, & Krakoff J (2011) Central and peripheral endocannabinoids and cognate acylethanolamides in humans: Association with race, adiposity, and energy expenditure. The Journal of Clinical Endocrinology and Metabolism, 96:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, & Deutsch DG (2009) Identification of intracellular carriers for the endocannabinoid anandamide. Proceedings of the National Academy of Sciences of the United States of America, 106:6375–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Vivieca S, Sun J, Glaser ST, & Deutsch DG (2012) Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. The Journal of Biological Chemistry, 287:3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe D, Giuffrida A, Schreiber D, Hellmich M, Schultze-Lutter F, Ruhrmann S, … Leweke FM (2009) Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. The British Journal of Psychiatry, 194:371–372. [DOI] [PubMed] [Google Scholar]
- Koethe D, Schreiber D, Giuffrida A, Mauss C, Faulhaber J, Heydenreich B, … Leweke FM (2009) Sleep deprivation increases oleoylethanolamide in human cerebrospinal fluid. Journal of Neural Transmission, 116:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumasaka T, Aritake K, Ago H, Irikura D, Tsurumura T, Yamamoto M, … Hayaishi O (2009) Structural basis of the catalytic mechanism operating in open-closed conformers of lipocalin type prostaglandin D synthase. The Journal of Biological Chemistry, 284:22344–22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S, Lee YH, Miyamoto Y, Fukada H, Goto Y, & Inui T (2012) Systematic interaction analysis of human lipocalin-type prostaglandin D synthase with small lipophilic ligands. The Biochemical Journal, 446:279–289. [DOI] [PubMed] [Google Scholar]
- Lim SM, Chen D, Teo H, Roos A, Jansson AE, Nyman T, … Nordlund P (2013) Structural and dynamic insights into substrate binding and catalysis of human lipocalin prostaglandin D synthase. Journal of Lipid Research, 54:1630–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Bab I, Biro T, Cabral GA, Dey SK, Di Marzo V, … Zimmer A (2015) Endocannabinoid signaling at the periphery: 50 years after THC. Trends in Pharmacological Sciences, 36:277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney MK, & Cravatt BF (2005) Structure and function of fatty acid amide hydrolase. Annual Review of Biochemistry, 74: 411–432. [DOI] [PubMed] [Google Scholar]
- Mitchell VA, Aslan S, Safaei R, & Vaughan CW (2005) Effect of the cannabinoid ajulemic acid on rat models of neuropathic and inflammatory pain. Neuroscience Letters, 382:231–235. [DOI] [PubMed] [Google Scholar]
- Mohri I, Taniike M, Okazaki I, Kagitani-Shimono K, Aritake K, Kanekiyo T, … Suzuki K (2006) Lipocalin-type prostaglandin D synthase is up-regulated in oligodendrocytes in lysosomal storage diseases and binds gangliosides. Journal of Neurochemistry, 97:641–651. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, … Leweke FM (2013) Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. The British Journal of Psychiatry, 202:381–382. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Guzman K, Arankowsky-Sandoval G, Salas-Crisostomo M, Jimenez-Moreno R, & Arias-Carrion O (2016) Evidence that activation of nuclear peroxisome proliferator-activated receptor alpha (PPARalpha) modulates sleep homeostasis in rats. Brain Research Bulletin, 127:156–163. [DOI] [PubMed] [Google Scholar]
- Nicholson J, Azim S, Rebecchi MJ, Galbavy W, Feng T, Reinsel R, … Kaczocha M (2015) Leptin levels are negatively correlated with 2-arachidonoylglycerol in the cerebrospinal fluid of patients with osteoarthritis. PLoS One, 10:e0123132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Makriyannis A, & Lovinger DM (2016) Endocannabinoid signaling regulates sleep stability. PLoS One, 11:e0152473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, … Ross RA (2010) International union of basic and clinical pharmacology.LXXIX. Cannabinoid receptors and their ligands: Beyond CB and CB. Pharmacological Reviews, 62:588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani V, Moschella V, Bari M, Fezza F, Galati S, Bernardi G, … Maccarrone M (2010) Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Movement Disorders, 25:920–924. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Sanchez D, Correnti C, Strong RK, & Ganfornina MD (2013) Lipid-binding properties of human ApoD and Lazarillo-related lipocalins: Functional implications for cell differentiation. The FEBS Journal, 280:3928–3943. [DOI] [PubMed] [Google Scholar]
- Saper CB, Romanovsky AA, & Scammell TE (2012) Neural circuitry engaged by prostaglandins during the sickness syndrome. Nature Neuroscience, 15:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Pini LA, Coppola F, Rossi C, Baldi A, Mancini ML, & Calabresi P (2007) Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology, 32:1384–1390. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, & Lichtman AH (2009) Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. The AAPS Journal, 11:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto S, Yoshida T, Inui T, Gohda K, Kobayashi Y, Fujimori K, … Ohkubo T (2007) NMR solution structure of lipocalin-type prostaglandin D synthase: Evidence for partial overlapping of catalytic pocket and retinoic acid-binding pocket within the central cavity. The Journal of Biological Chemistry, 282: 31373–31379. [DOI] [PubMed] [Google Scholar]
- Soria-Gomez E, Guzman K, Pech-Rueda O, Montes-Rodriguez CJ, Cisneros M, & Prospero-Garcia O (2010) Oleoylethanolamide affects food intake and sleep-waking cycle through a hypothalamic modulation. Pharmacological Research, 61:379–384. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Urade Y, Kimura H, Eguchi N, Nishikawa A, & Hayaishi O (1997) Lipocalin-type prostaglandin D synthase (beta-trace) is a newly recognized type of retinoid transporter. The Journal of Biological Chemistry, 272:15789–15795. [DOI] [PubMed] [Google Scholar]
- Wall ME, & Perez-Reyes M (1981) The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man. Journal of Clinical Pharmacology, 21:178S–189S. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM (2013) Control of sleep and wakefulness in health and disease. Progress in Molecular Biology and Translational Science, 119:137–154. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shaw N, Li Y, Zhao Y, Zhang R, & Liu ZJ (2010) Structure–function analysis of human l-prostaglandin D synthase bound with fatty acid molecules. The FASEB Journal, 24: 4668–4677. [DOI] [PubMed] [Google Scholar]