Abstract
Mast cell activation (MCA) accompanies diverse physiologic and pathologic processes and is one of the more frequently encountered conditions in medicine. MCA-related symptoms are usually mild and often transient. In such cases, histamine receptor blockers and other mediator-targeting drugs can usually control MCA. In severe cases, a MCA syndrome (MCAS) may be diagnosed. However, overt MCAS is an unusual condition, and many patients referred because of suspected MCAS are diagnosed with other diseases (autoimmune, neoplastic, infectious) unrelated to MCA or suffer from MCA-related (e.g., allergic) disorders and/or co-morbidities without fulfilling criteria of an overt MCAS. These considerations are important as more and more patients are informed they may have MCA or even MCAS without completing a thorough medical evaluation. In fact, in several instances, symptoms are misinterpreted as MCA/MCAS, and other, clinically relevant conditions are not thoroughly pursued. The number of such referrals is increasing. In order to avoid such unnecessary referrals and to prevent misdiagnoses, we here propose a diagnostic algorithm through which a clinically relevant (systemic) MCA can be suspected and MCAS can subsequently be documented or excluded. In addition, the algorithm proposed should help guide the investigating care providers to consider the two principle diagnoses that may underlie MCAS, namely severe allergy and systemic mastocytosis accompanied by severe MCA. Although validation is required, we anticipate that this algorithm will facilitate the management of patients with suspected MCAS.
Keywords: Mast Cells, MCAS, Diagnostic Algorithm, Tryptase, KIT D816V
Introduction
Mast cells (MC) are multifunctional effector cells involved in innate and acquired immunity and attendant inflammatory reactions (1-5). In common with blood basophils, MC constitutively display high-affinity receptors for IgE (IgER), also known as FcεRI, and generate a number of inflammatory and vasoactive mediators (1-7). Many of these substances, including histamine, are stored in the metachromatic granules of MC and basophils. In the course of an allergic anaphylactic episode, allergen-induced cross-linking of IgER results in the sudden release of these preformed granule-derived mediators into the extracellular space (1-7). Basophils may participate in allergic and other inflammatory processes in the same way as MC (4,8,9). However, not all allergic episodes necessarily involve both cell lineages, even if the reaction is severe and systemic. Moreover, some of the mediators that provoke clinically relevant reactions are primarily synthesized and released by tissue MC (1-5). The ability of MC and basophils to secrete mediators of anaphylaxis in response to a specific agonist, often referred to as ‘releasability’, depends on several factors, including the underlying condition (disease), the number and type of involved receptors, the signaling molecules engaged, and the genetic background of the individual (9-13). The severity of a resulting reaction is also influenced by the numbers of MC (and basophils) involved in the event, the nature and number of IgE-reactive allergen(s), the type and amount of IgE, the presence of comorbidities, other patient-related factors (alcohol, nicotine, illegal substances), the type and amount of co-activating (priming) cytokines and chemokines, and the reactivity of organ systems to these mediators (14-19).
MC activation (MCA) can be documented in a number of physiologic and pathologic conditions. Acute MCA is thus encountered in IgE-mediated allergic reactions and in extreme instances may result in systemic anaphylaxis (1-3,5,18-20). Severe or even life-threatening MCA may develop when i) the burden of MC is high, ii) when MC are in a ‘hyper-activated’ state and iii) when comorbidities make the patient less tolerant to MCA events. When MC involvement is documented and the reaction is severe, a MCA syndrome (MCAS) may be diagnosed (21-28). In the past 50 years, clinical symptoms resulting from MCA have primarily been documented in the context of allergic diseases. More recently, however, MCA has also been considered in the context of mast cell neoplasms (21-23,26-28). In order to address its complex etiology, diagnostic criteria for MCAS have been set forth and MCAS variants have been delineated by a consensus group (26-28).
However, some consternation still remains over the diagnosis of MCAS, and many patients are referred because they believe they have MCAS or their doctors judged that the symptoms reported could be indicative of MCA or MCAS. In order to address this challenge our group has worked on a diagnostic algorithm for patients with suspected MCAS. This algorithm is presented here-in, together with associated criteria, assays and tools that will assist in the diagnosis of MCA and MCAS.
Consensus Criteria and Classification of MCAS
When MCA symptoms are severe and recurrent, the diagnosis MCAS must be considered. As per existing consensus criteria (27) the term MCAS applies when i) typical clinical signs of severe recurrent acute systemic MCA are present (especially in the form of clinical features and findings of anaphylaxis), ii) the involvement of MC can be demonstrated by biochemical analyses (preferably through an increase in tryptase following the 20%+2 formula as discussed below) and iii) the symptoms respond to treatment with MC stabilizing agents or drugs targeted against MC mediator production, secretion or receptor binding. All three criteria must be met to establish the diagnosis of MCAS (Table 1). Based on the underlying condition, patients with MCAS should then be further classified into i) primary MCAS where KIT-mutated, clonal (CD25+) MC are detected (with or without an underlying diagnosis of mastocytosis), ii) secondary MCAS where an underlying non-neoplastic disease, usually an IgE-dependent allergy or other hypersensitivity reaction is detected, and iii) idiopathic MCAS, where no KIT-mutated MC and no overt inflammatory disorders (that may explain MCA) are detected, and no trigger for a hypersensitivity reaction is found (Table 2) (27,28). In a considerable number of patients with MCAS, several factors act together to cause severe or even life-threatening anaphylaxis. For example, in patients with systemic mastocytosis (SM) and MCAS, an IgE-dependent allergy (e.g., against insect venom) is frequently documented. These patients suffer from a combination of primary and secondary MCAS, and, as a result, they are at high risk to develop recurrent life-threatening anaphylaxis (21-23). These patients require special attention and personalized therapy and are usually regarded as candidates for life-long immunotherapy and may additionally require omalizumab therapy and/or other pharmacologic intervention. Detailed knowledge about the etiology and the complexity of MCAS is thus important and forms the basis for establishing the exact diagnosis and developing an optimal treatment plan.
TABLE I.
Consensus criteria for MCAS*
| Criterion A: Typical clinical signs of severe, recurrent (episodic) systemic MCA are present (often in form of anaphylaxis) (definition of systemic: involving at least 2 organ systems) |
| Criterion B: Involvement of MC is documented by biochemical studies: preferred marker: increase in serum tryptase level from the individual’s baseline to plus 20% + 2 ng/ml† |
| Criterion C: Response of symptoms to therapy with MC-stabilizing agents, drugs directed against MC mediator production or drugs blocking mediator release or effects of MC-derived mediators‡ |
The consensus criteria for MCAS were first published in Valen et al.27. All 3 MCAS criteria (A + B + C) must be fulfilled to call a condition MCAS.
Other MC-derived markers of MCA (histamine and histamine metabolites, PGD2 metabolites, and heparin) have also been proposed, but are less specific compared with tryptase.
Example: histamine receptor blockers.
TABLE II.
Recognized variants of MCAS and diagnostic features
| Variant of MCAS | Main diagnostic features |
|---|---|
| Primary MCAS (Clonal MCAS)* | The KIT D816V mutation is detected and MCs aberrantly display CD25 in most cases (a) with confirmed mastocytosis (CM or SM)† (b) with only 2 minor SM criteria |
| Secondary MCAS | An IgE-mediated allergy, another hypersensitivity reaction, or another immunologic disease that can induce MCA, and thus MCAS, is diagnosed, but no neoplastic MC or KIT D816V is found‡ |
| Idiopathic MCAS | Criteria to diagnose MCAS are met, but no related reactive disease, no IgE-dependent allergy, and no neoplastic/clonal MCs are found‡ |
The terms clonal MCAS and monoclonal MCAS (MMCAS) can be used synonymously with the term primary MCAS.
Most of the patients suffer from CM or SM. However, in some cases, only 2 minor SM criteria are detected and criteria for SM and CM are not fulfilled.
No KIT mutation at codon 816 is detected, and flow cytometry (if performed) will not detect a clonal population of CD25+MCs.
Symptoms Produced by Systemic MCA
Symptoms of MCA are among the most frequently recorded and treated symptoms in the daily practice of applied medicine. MCA-related symptoms range from mild to severe, and may at times be life-threatening, especially in patients with mastocytosis and concomitant allergy. MCA symptoms are caused by several different vasoactive and pro-inflammatory mediators released from MC when these cells are activated by an allergen via IgER cross-linking or other mechanisms (1-5,29-31). As a result, the severity of MCA correlates with the extent of mediator release from MC during an anaphylactic reaction. Well-recognized symptoms of systemic MCA include, among others, acute urticaria, flushing, abdominal cramping, diarrhea, hypotensive syncope or near syncope and tachycardia (Table 3) (1-3,26-28,31). Although none of these symptoms are completely specific for MCA, one or more are typically detected in these patients. The likelihood of MCA increases when two or more of such symptoms are documented, and the likelihood is even higher when the symptoms respond to agents blocking mediator effects, mediator production or mediator secretion. Indeed, the response to such drugs is helpful in practice and is therefore a criterion of MCAS (26-28). Another important aspect is that several different mediators may be involved in MCA-related symptomatology (1-6,26-31) (Table 4). In fact, depending on the organ and pathology involved, certain MC products may act as critical inducers of MCA, and sometimes treatment may need adjustment because of the effects of such mediators. Likewise, vascular instability may not only be triggered by histamine but also by prostaglandins (PG) and/or leukotrienes (LT) derived from activated MC in the same patient, so that the reaction can only be managed when administering histamine receptor (HR) blockers and PG/LT synthesis inhibitors and/or receptor blockers (32). Other potentially relevant mediators associated with activation of MC are platelet activating factor (PAF), tryptases and various cytokines (20,31,33-36) (Table 4).
TABLE III.
Clinical symptoms typically associated with local or systemic MCA
| Acute episodic symptom | Typical for MCA | MCAS more likely |
|---|---|---|
| Urticaria | ++ | + |
| Flushing | + | +/− |
| Pruritus | + | +/− |
| Angioedema | + | + |
| Nasal Congestion | +/− | − |
| Nasal Pruritus | +/− | − |
| Wheezing | + | +/− |
| Throat Swelling | +/− | +/− |
| Hoarseness | +/− | − |
| Headache | +/− | − |
| Hypotensive syncope | +/− | − |
| Tachycardia | +/− | − |
| Abdominal cramping | +/− | +/− |
| Diarrhea | +/− | +/− |
++, Higher specificity; +, moderate specificity; +/−, low specificity; −, not considered to be indicative of MCAS (as single symptom).
Note. To count as indication of MCA, these symptoms need to be episodic and recurrent and cannot be explained by other known disorders or conditions (other than MCA).
TABLE IV.
Clinical effects of MC mediators produced and released during MCA*
| Symptomatology of MCA | Relevant involved mediators† |
|---|---|
| Vascular instability, hypotension, tachycardia, syncopy, anaphylaxis* | Histamine, LTC4, LTE4, PGD2, VEGF, PAF, TNF-α |
| Enhanced vasopermeability, edema formation in various organs | Histamine, VEGF, LTC4, LTE4, PAF |
| Headache and nausea | Histamine |
| Fever and chills‡ | TNF-α |
| Urticaria, pruritus, flushing | Histamine, VEGF |
| Bronchoconstriction | Histamine, PGD2, LTC4, LTD4, PAF |
| Mucus secretion | Histamine, proteases, PGD2, LTC4 |
| Nasal congestion, wheezing | Histamine |
| Gastric hypersecretion | Histamine |
| Abdominal pain and cramping | Histamine, LTC4, PAF |
| Diarrhea | Histamine |
PAF, Platelet-activating factor; VEGF, vascular endothelial growth factor (=vascular permeability factor).
Clinical symptoms recorded in patients with MCA and MCAS. In patients with MCAS, more than 1 symptom is typically recorded, and in most patients, hypotension and signs of anaphylaxis are found.
Some of the clinically most relevant MC-derived mediators are listed. In patients with MCAS, histamine and arachidonic acid derivatives may play a central role. The impact of the other MC-derived mediators, such as PAF, remains at present unknown.
In about 1% of all patients with MCA, severe hypotension is associated with fever.
MCA may also develop with chronic and/or a less severe symptomatology (Table 5). In such patients, the symptoms are often less specific and include headache, nausea and non-specific gastrointestinal complaints (31). It is important to state that these symptoms alone are not regarded criteria of severe systemic MCA or MCAS. Nevertheless such symptoms may possibly relate to local MCA, and thus the administration of anti-mediator-type drugs or MC-stabilizing agents may be considered. However, it is of utmost importance to be aware that there are a number of diseases and conditions in the differential diagnoses that must be taken into account in such cases, including psychiatric, cardiovascular, infectious, endocrinologic, gastrointestinal, toxic, and oncologic disorders. In some patients no definitive organic diagnosis will be made during the initial evaluation and follow-up will be necessary to watch for the evolution of a diagnosable disorder.
TABLE V.
Classification of mast cell activation (MCA) and related conditions
| (a) According to organ involvement and severity |
| Systemic MCA* |
| Mild or moderate systemic MCA (MCAS criteria not fulfilled) |
| Severe systemic MCA = MCAS (MCAS criteria fulfilled) |
| Local MCA (mild/moderate or severe) (MCAS criteria not fulfilled) |
| (b) According to underlying condition |
| Primary (clonal) MCA |
| Cutaneous mastocytosis (CM) |
| Systemic mastocytosis (SM) |
| 1-2 minor SM criteria recorded but no SM can be diagnosed |
| IgE-dependent allergy (or atopy) |
| Organ-specific variants |
| IgE-independent hypersensitivity reactions |
| Other conditions |
| Reactive conditions (inflammation) |
| Toxic tissue damage (intoxication) |
| Physical, neurologic, and others |
| (c) According to frequency and symptom-free interval |
| Episodic recurrent |
| With a known trigger (eg, allergen) |
| Without a known trigger |
| Chronic persistent |
Systemic MCA involves 2 or more organ systems.
All in all, MCA can be divided into severe and less severe types, into acute, episodic and chronic forms, and into systemic and local variants (Table 5). However, severe MCA fulfilling MCAS consensus criteria is almost always associated with the occurrence of acute severe recurrent symptoms affecting more than one organ or tissue, often with severe hypotension and anaphylaxis (26-28). In the absence of such a symptomatology, the diagnosis MCAS is unlikely (Figure 1). There are also frequently reported symptoms (by patients with suspected MCAS) that are not necessarily related to MCA, such as joint hypermobility, sleep disruption, erythromelalgia, burning hands, odor aversion, dysautonomia, obesity, sweating and anxiety. It may also be that a number of these patients suffer from psychological or psychiatric problems rather than MCA and which may require special attention and appropriate management.
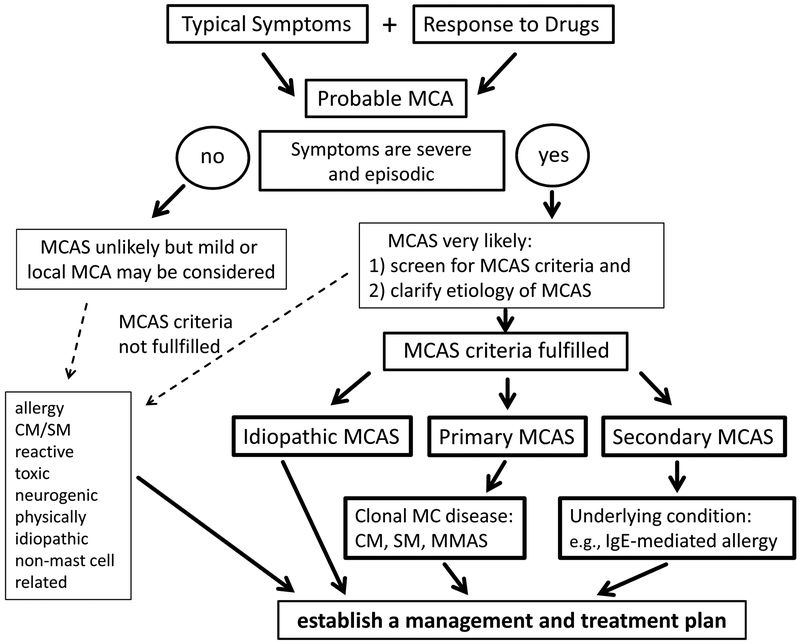
Figure 1.
Proposed algorithm for patients with suspected mast cell activation syndrome (MCAS) After the patient has been clinically stabilized, the physician examines potential etiologies and asks for MCAS criteria. When the symptoms are severe and episodic, the likelihood of MCAS is quite high. MCAS consensus criteria are then applied to confirm mast cell involvement. MCAS criteria can also be applied when the symptoms are less severe and/or atypical. However, in most of these patients, MCAS criteria are not fulfilled. In a next step, the underlying etiology is examined. At this phase of the work-up, it is important to screen for multiple underlying disorders, as in MCAS patients, more than one such underlying disease may be present (e.g., mastocytosis and allergy). With regard to mastocytosis, typical indicators are a persistently elevated serum tryptase level and detection of KIT D816V in peripheral blood cells. According to the underlying condition, MCAS is classified into primary (clonal) MCAS, secondary MCAS (usually with an IgE-dependent allergy) and idiopathic MCAS. In patients with clonal MCAS, the final diagnosis may be CM, SM or monoclonal MCAS (=MMAS) defined by two (but not more) SM criteria. In a final step, the management and treatment plan is established. MCA, mast cell activation.
Laboratory Assessments in Patients with suspected MCA
MCA is associated with the release of preformed and newly generated mediators and their effects on target cells (26-28,31). In severe systemic reactions, increased levels of MC-derived mediators should be measurable in biological fluids. Some mediators, like tryptase, are more specific than others for MC and thereby considered as the most precise parameters for the demonstration and documentation of MCA (27,28,37-40). However, the sensitivity of the tryptase algorithm decreases with decreasing clinical severity and with delayed blood draws after resolution of clinical symptoms.
Other mediators, potentially more sensitive than tryptase, are less specific because they are also synthesized and released by other cell types. For example, histamine is produced, stored and released not only by MC but also by blood basophils (which contain amounts comparable to those in MC) and histamine-secreting carcinoid tumors. By contrast, MC contain more than 100-fold higher levels of tryptase than basophils (41,42). Even immature (leukemic) basophils express relatively low amounts of tryptase (43). Therefore, in a routine evaluation, a rapid substantial increase in serum tryptase levels above the individual’s baseline is considered specific for MC involvement and MCA, and thus can be employed as a MCAS criterion. In the following paragraph, a practical guide for the measurement of tryptase in the MCA/MCAS-context is provided:
First, MC involvement should be confirmed by measuring an event-related, transient, increase in serum tryptase. This ‘event-related’ increase in tryptase is best captured in a 1-4 hour post-event interval during which tryptase remains elevated, and the resulting enzyme level must then be compared to the individual’s baseline tryptase. If no previous baseline level is available, the baseline level must be assessed at least 24-48 hours after complete resolution of all signs and symptoms (27,37-39).
The other important consideration is: what minimal increase in serum tryptase is required to establish it as indicative of severe systemic MCA (MCAS criterion). Here the consensus proposal is that a minimal increase of the acute serum tryptase level to greater than plus 20% of baseline plus 2 ng/mL absolute tryptase strongly supports the diagnosis of MCAS (27). Example: if the baseline tryptase level is 5 ng/mL, an increase >8 ng/mL suggests MCA. This approach has recently been validated in the context of mastocytosis (PV, KB and LBS, unpublished data) and non-mastocytosis conditions (44). When the post-event baseline tryptase level remains elevated (>20 ng/ml) a number of underlying disorders have to be considered, including hereditary alpha tryptasemia, systemic mastocytosis (SM) and non-MC-lineage myeloid neoplasms (Supplemental Table S1).
Additional mediators, when rising from baseline, may also serve as markers of MCA or even MCAS. These include, among others, histamine (plasma, urine), histamine metabolites (urine) and the 24-hour urine PGD2 metabolite, 11β-prostaglandin F2α or the LTC4 metabolite, LTE4, level (urine) (27,31,32,45-50). However, as noted, these mediators are less specific for MCA compared to tryptase. Moreover, no data are available to establish what minimal increase of these mediators would count as a reliable indicator (and thus criterion) of systemic MCA. It is suggested that an event-related increase in 2 or more of the plasma or urinary histamine-metabolites or PGD2-metabolites, or LTE4 of at least 50% from baseline (e.g., from 50 to at least 75) could function as an indication of MCA. Another possibility is a determination of a level 2-fold above the upper limit of normal. Measurement of such additional mediators may indeed be helpful in the evaluation of patients with suspected MCA/MCAS, and should therefore be considered, especially when the serum tryptase test is not available or did not produce a convincing result or when no blood (but only urine) could be collected during the event. This is important because other MC mediators are sometimes also relevant clinically, as they can provoke MCA and may lead to adjustments of (individualized) therapeutic approaches (32). It is also worth noting that PGD2 is primarily synthesized in MC but not in blood basophils.
Cell-based assays have also been proposed to evaluate MC and basophil activation. Reliable and established parameters of basophil- and MC activation include analysis of cell surface levels of CD63 and/or CD203c (51-55). Both proteins (antigens) increase on the surface of FcεR cross-linked MC and basophils (51-55). However, whereas basophils are easily accessible for (repeated) investigations, MC are not, unless a tissue biopsy specimen is available. In addition, many of the activation-linked surface antigens, including CD63 and CD203c are upregulated on neoplastic resting MC in SM (55). Therefore, MC typing is not recommended as a screening approach to define or quantify MCA. Rather, MC typing is recommended as a diagnostic approach to diagnose or exclude SM in patients with MCA or MCAS (56-58).
Proposed Diagnostic Algorithm for Patients with Suspected MCAS
When a patient is critically ill and/or presents with moderate to severe hypotension, it is essential to clinically stabilize the patient before exploring etiology. At the same time, the physical examination may reveal the presence of typical skin lesions of mastocytosis (59). In other cases, the patient or the relatives may inform the emergency team about a known diagnosis of mastocytosis or allergy.
After stabilization, the etiology must be clarified: in a first step, symptoms should be classified as ‘probably MCA-related’ based on clinical features, elimination of other etiologies and response to certain drugs (Figure 1). In a second step, it is important to define i) the severity of the reaction, ii) whether the reaction is systemic and concurrently involves two or more of the following organ systems: cardiovascular, skin, pulmonary, or gastrointestinal, iii) whether the symptoms are recurrent (case history), and iv) whether the reaction may be related to MCA with certainty (or at least with high probability) which is usually done by taking an acute blood sample within 1-4 hours after onset of the reaction and comparing it to the baseline serum tryptase level (26-28). If the reaction is not severe the likelihood of MCAS is less likely (Figure 1).
In most patients with severe MCA, the symptomatology is compatible with anaphylaxis and involves two or more organ systems. Reporting that such episodes are recurrent increases the likelihood of MCAS (Figure 1). A diagnosis of MCAS is further supported by demonstrating an event-related increase in tryptase and a sustained prophylactic response to MC-stabilizing drugs or drugs directed against MC mediators. The diagnosis of anaphylaxis does not require these MCAS criteria.
In a final step, after having confirmed the presence of MCA using consensus criteria, the diagnosis of MCAS can be made (Figure 1). For many MCAS patients, an IgE-dependent allergy is known or will be detected (Figure 1). In others, underlying mastocytosis may be found. When neither is the case, the patient may still suffer from mastocytosis. Indirect signs for the presence of an underlying (occult) SM include an elevated baseline serum tryptase level detected well after complete resolution of all symptoms or a D816V KIT mutation in circulating blood leukocytes, blood count abnormalities (e.g., eosinophilia), symptoms suggestive of SM such as unexplained osteoporosis, gastrointestinal symptoms (diarrhea, abdominal cramps, malabsorption), or an elevated REMA or NIHCAS score (60). A bone marrow investigation may confirm the presence of SM in these patients (Figure 1) (61).
In other patients, clonal KIT-mutated (CD25+) MC are detected, but only one or two minor SM criteria and no major SM criterion are found (21,22,26-28). These patients as well as those with cutaneous mastocytosis (CM) or SM are both classified as primary (clonal) MCAS (Table 2). If an IgE-dependent allergy or other underlying reactive disease (e.g., IgE-dependent or other hypersensitivity disorder) is present in the absence of clonal MC, the diagnosis is secondary MCAS (Table 2, Figure 2). If no evidence for primary or secondary MCAS is found, the patient is classified as having idiopathic MCAS (Table 2, Figure 1) (26-28).
What if symptoms suggest MCA but MCAS criteria are not met?
In a reasonable number of cases, signs and symptoms of MCA will be detected, but the criteria of MCAS will not be fulfilled. One such cohort of patients are those that present with severe recurrent symptoms and a diagnostic increase in serum tryptase levels, but where treatment with conventional drugs does not lead to a major improvement of symptoms. In these cases, a provisional diagnosis of “possibly MCAS” may be established and further treatment should be introduced.
In other patients, severe symptoms may be recorded, but tryptase levels increase only slightly. In these patients, it is reasonable to determine the levels of additional relevant mediators such as PGD2, if the test is available. Whenever a major increase in the PGD2 metabolite level is found and the symptoms respond to cyclooxygenase inhibitors, the diagnosis of MCAS may also be considered (27) although the cell source may be ambiguous, particularly if both tryptase and histamine or histamine metabolite levels are normal. In such patients, the symptoms must be severe and the increase in mediator levels must be substantial.
However, as noted, there are also patients in whom the symptoms are less severe and/or restricted to one organ system or even a local organ site. In these patients, it may still be reasonable to ask for MCAS criteria (Figure 1). However, in most of these cases, it will be determined that they are suffering from either an unrelated disease (Table 5) or from a less severe form of MCA that does not meet MCAS criteria. These may include patients suffering from less severe allergic reactions or patients with mastocytosis with mild mediator symptomatology. Others may be suffering from food-intolerance, drug side effects, toxin exposure, an autoimmune disease, psychiatric factors or other, less severe, reactive conditions associated with MCA (62).
A special situation is when mastocytosis with MCA does not fulfill MCAS criteria. The consensus group has recommended that in cases with mastocytosis (irrespective of the variant) any form of MCA requiring continuous mediator-targeted therapy should be marked by the diagnostic label ‘SY’ that appears as a subscript in the final diagnosis (27,63). These patients include those who have MCAS and those who do not have an overt MCAS but suffer from MCA-related symptoms requiring therapy. As an example: in a patient with indolent SM (ISM) requiring continuous histamine receptor-targeting agents and glucocorticosteroids to control MCA, the final diagnosis should be ISMSY even if the criteria of MCAS are not met (or were not documented).
Another special situation is hereditary alpha-tryptasemia, an autosomal dominant condition defined by germline replications (usually duplications or triplications) of the TPSAB1 gene encoding alpha-tryptase (64-67). In affected family members, symptoms of MCA, if present, may be chronic and/or acute, and other symptoms and findings, including dysautonomia, chronic pain, and connective tissue abnormalities such as joint hypermobility, may also be observed (64-67).
There are also patients from families where a slightly elevated tryptase is measured but the genetic (molecular) background remains undefined. It is important to note in this regard that an elevated basal serum tryptase level per se is neither an indication for MCA or MCAS nor is it an ‘a priori’ risk factor for the occurrence of MCA or MCAS. Rather, an elevated basal tryptase level is not only found in patients with mastocytosis or hereditary alpha-tryptasemia (where the risk for anaphylaxis may be increased) but also in patients with myeloid non-MC-lineage neoplasms (Supplemental Table S1) (68-71). In addition, elevated basal tryptase levels are detectable in patients with end stage kidney disease and some parasitic infections (Supplemental Table S1).
Disorders Underlying MCA and MCAS: Final Diagnosis
A number of pathologic conditions can be associated with systemic MCA, including allergic reactions, mastocytosis, auto-immune disorders, infectious diseases (e.g., infections involving the skin or Helicobacter pylori+ gastritis) and intoxications. The most frequent underlying cause is an IgE-dependent allergy. By contrast, only a few patients will have mastocytosis, which is a rare disease compared to IgE-dependent allergies. However, the prevalence of MCAS is rather high among mastocytosis patients. The highest prevalence of MCAS appears in patients who suffer from both an IgE-dependent allergy and mastocytosis. The population requiring special attention consists of patients suffering from primary MCAS and insect venom allergy. The induction of long-term tolerance to an insect venom allergen is reduced in primary MCAS and severe or even fatal reactions after discontinuation of immunotherapy have been described (71,72). Based on available data, it seems likely that patients with primary MCAS and insect venom allergy are only protected while under continuous venom immunotherapy which is therefore a recommended approach.
When an IgE-dependent allergy is suspected, a detailed diagnostic evaluation for allergies and an appropriate management plan should be initiated. Similarly, when a clonal MC disease has been identified, the disorder needs to be staged (e.g., CM variants and SM variants). A key diagnostic parameter is a mutational analysis of KIT. In most adults with SM, the D816V KIT mutation will be detected. Using a highly-sensitive allele-specific qPCR, the mutation can also be identified in the peripheral blood of most patients with SM (73-78).
However, in some adults and more commonly in children, other KIT point mutations are found (76,80,81). Pediatric patients most commonly have CM. The prevalence of MCAS in pediatric patients is unknown, but both cutaneous and systemic reactions have been reported (82).
Differential Diagnoses to MCA and MCAS
A number of differential diagnoses have to be taken into account in patients with suspected MCAS (26-28) (Supplemental Table S1). In those patients who have severe hypotension and shock resembling anaphylaxis, differential diagnoses (to both, anaphylaxis and MCAS) include, among other, cardiovascular and cerebrovascular disorders, acute endocrinologic emergencies, severe infections (septicemia), acute dehydration, drug overdose, exposure to environmental toxins, somatoform disorders and acute psychiatric events. In other patients, no signs of a severe systemic reaction (and no hypotension) are recorded but the physician is of the opinion that MCAS has to be ruled out. The differential diagnoses in such cases then relate to organ-specific local events, such as acute diarrhea (gastrointestinal diseases or infections), skin rash (cutaneous diseases), or neurological symptoms (neurological or psychiatric diseases). In a reasonable number of patients, the etiology will remain unclear until all relevant laboratory parameters have been collected. Importantly, acute serum tryptase levels are not known to increase in conditions unrelated to MCA. And, as mentioned, it is also important to recognize that elevated basal serum tryptase levels may be detected in several different conditions (even in healthy individuals) and thus an elevated basal tryptase level alone is not diagnostic of MCA or MCAS.
Summary and Future Perspectives
MCAS is a well-defined condition that occurs primarily in patients with IgE-dependent allergies and/or mastocytosis, but may also occur in a number of other conditions. In few cases, no underlying cause or disease will be found, leading to the diagnosis ‘idiopathic MCAS’. Diagnostic MCAS criteria include typical clinical symptoms, often with hypotension, an event-related, substantial increase in serum tryptase levels and response of clinical symptoms to MC-stabilizing drugs or drugs counteracting the effects of MC-derived mediators. When patients with suspected MCAS are referred, it is helpful to follow a diagnostic algorithm that is able to help differentiate between true MCAS, other MCA-related disorders, and unrelated conditions where MC are not involved. A key diagnostic checkpoint is vascular instability (hypotension) combined with other typical signs of MCA, which is almost always seen in MCAS. In a next step, serum tryptase levels are measured. When the event-related increase in tryptase, compared to symptom-free intervals, exceeds a certain threshold (20% from baseline plus 2 ng/ml) the diagnosis MCAS is quite likely. It is also standard to measure other MC-related parameters such as urinary histamine- and/or PGD2 metabolites. However, a selective increase of these mediators (in the absence of an increase in tryptase) may be found associated with chronic MCA or a less severe form of MCA but not with MCAS. In a next step, the response of the symptoms to MC-stabilizing and/or anti-mediator-type drugs confirms the presence of MCAS. In a final step, the patient is examined for the presence of underlying disorders, such as mastocytosis and IgE-dependent allergy. In this final phase, the MCAS is classified into primary MCAS, secondary MCAS and idiopathic MCAS. The algorithm provided in the current article is designed to assist in the evaluation and management of patients with suspected MCA and MCAS. In addition, our proposed algorithm should support the preparation and conduct of clinical studies on MCAS.
Supplementary Material
Acknowledgments
Support
This work was supported in part by the Austrian Science Funds (FWF), projects F4701 and F4704 and the Division of Intramural Research, NIAID.
List of Abbreviations:
- BM
Bone marrow
- CM
Cutaneous mastocytosis
- IgE
Immunoglobulin E
- ISM
Indolent systemic mastocytosis
- MC
Mast cells
- MCA
Mast cell activation
- MCAS
Mast cell activation syndrome
- SM
Systemic mastocytosis
- VIP
Vasoactive intestinal peptide
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
LBS receives a portion of the royalties from Thermo Fisher Scientific for the tryptase assay that are received by Virginia Commonwealth University. All other authors have no conflict of interest to declare in this project.
References
- 1.Schwartz LB. Mast cells and basophils. Clin Allergy Immunol 2002;16:3–42. [PubMed] [Google Scholar]
- 2.Metcalfe DD. Mast cells and mastocytosis. Blood 2008;112:946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol 2010;40:1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falcone FH, Knol EF, Gibbs BF. The role of basophils in the pathogenesis of allergic disease. Clin Exp Allergy 2011;41:939–47. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012;18:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol 1999;17:931–72. [DOI] [PubMed] [Google Scholar]
- 7.Nadler MJ, Matthews SA, Turner H, Kinet JP. Signal transduction by the high-affinity immunoglobulin E receptor Fc epsilon RI: coupling form to function. Adv Immunol 2000;76:325–55. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs BF. Human basophils as effectors and immunomodulators of allergic inflammation and innate immunity. Clin Exp Med 2005;5:43–9. [DOI] [PubMed] [Google Scholar]
- 9.Marone G, Spadaro G, Patella V, Genovese A. The clinical relevance of basophil releasability. J Allergy Clin Immunol 1994;94:1293–1303. [DOI] [PubMed] [Google Scholar]
- 10.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Multiple defects in Fc epsilon RI signaling in Syk-deficient nonreleaser basophils and IL-3-induced recovery of Syk expression and secretion. J Immunol 2000;165:5913–20. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald SM, Vonakis BM. Association of the Src homology 2 domain-containing inositol 5' phosphatase (SHIP) to releasability in human basophils. Mol Immunol 2002;38:1323–27. [DOI] [PubMed] [Google Scholar]
- 12.Okayama Y, Kashiwakura JI, Matsuda A, Sasaki-Sakamoto T, Nunomura S, Yokoi N, et al. The interaction between Lyn and FcεRIβ is indispensable for FcεRI-mediated human mast cell activation. Allergy 2012;67:1241–49. [DOI] [PubMed] [Google Scholar]
- 13.Havard S, Scola AM, Kay LJ, Ishmael SS, MacGlashan DW Jr, Peachell PT. Characterization of syk expression in human lung mast cells: relationship with function. Clin Exp Allergy 2011;41:378–88. [DOI] [PubMed] [Google Scholar]
- 14.Valent P, Besemer J, Muhm M, Majdic O, Lechner K, Bettelheim P. Interleukin 3 activates human blood basophils via high-affinity binding sites. Proc Natl Acad Sci (USA) 1989;86:5542–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischoff SC, Dahinden CA. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med 1992;175:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperr WR, Czerwenka K, Mundigler G, Müller MR, Semper H, Klappacher G, et al. Specific activation of human mast cells by the ligand for c-kit: comparison between lung, uterus and heart mast cells. Int Arch Allergy Immunol 1993;102:170–5. [DOI] [PubMed] [Google Scholar]
- 17.Komai-Koma M, Brombacher F, Pushparaj PN, Arendse B, McSharry C, Alexander J, et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy 2012;67:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol 2008;8:310–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol 2009;124:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalesnikoff J, Galli SJ. Anaphylaxis: mechanisms of mast cell activation. Chem Immunol Allergy 2010;95:45–66. [DOI] [PubMed] [Google Scholar]
- 21.Akin C, Scott LM, Kocabas CN, Kushnir-Sukhov N, Brittain E, Noel P, Metcalfe DD. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with "idiopathic" anaphylaxis. Blood 2007;110:2331–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonneck K, Florian S, Müllauer L, Wimazal F, Födinger M, Sperr WR, Valent P. Diagnostic and subdiagnostic accumulation of mast cells in the bone marrow of patients with anaphylaxis: Monoclonal mast cell activation syndrome. Int Arch Allergy Immunol 2007;142:158–64. [DOI] [PubMed] [Google Scholar]
- 23.Bonadonna P, Perbellini O, Passalacqua G, Caruso B, Colarossi S, Dal Fior D, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol 2009;123:680–86. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton MJ, Hornick JL, Akin C, Castells MC, Greenberger NJ. Mast cell activation syndrome: a newly recognized disorder with systemic clinical manifestations. J Allergy Clin Immunol 2011;128:147–152.e2. [DOI] [PubMed] [Google Scholar]
- 25.Valent P, Horny HP, Triggiani M, Arock M. Clinical and laboratory parameters of mast cell activation as basis for the formulation of diagnostic criteria. Int Arch Allergy Immunol 2011;156:119–27. [DOI] [PubMed] [Google Scholar]
- 26.Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: Proposed diagnostic criteria. J Allergy Clin Immunol 2010;126:1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol 2012;157:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valent P Mast cell activation syndromes: definition and classification. Allergy 2013;68:417–24. [DOI] [PubMed] [Google Scholar]
- 29.Serafin WE, Austen KF. Mediators of immediate hypersensitivity reactions. N Engl J Med 1987;317:30–34. [DOI] [PubMed] [Google Scholar]
- 30.Holgate ST, Robinson C, Church MK. The contribution of mast cell mediators to acute allergic reactions in human skin and airways. Allergy 1988;43(S5):22–31. [DOI] [PubMed] [Google Scholar]
- 31.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med 2015;373:163–72. [DOI] [PubMed] [Google Scholar]
- 32.Butterfield JH, Weiler CR. Prevention of mast cell activation disorder-associated clinical sequelae of excessive prostaglandin D(2) production. Int Arch Allergy Immunol 2008;147:338–43. [DOI] [PubMed] [Google Scholar]
- 33.Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol Today 1990;11:458–64. [DOI] [PubMed] [Google Scholar]
- 34.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med 2008;358:28–35. [DOI] [PubMed] [Google Scholar]
- 35.Vadas P, Perelman B, Liss G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol 2013;131:144–49. [DOI] [PubMed] [Google Scholar]
- 36.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 2018;282:121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med 1987;316:1622–26. [DOI] [PubMed] [Google Scholar]
- 38.Shanmugam G, Schwartz LB, Khan DA. Prolonged elevation of serum tryptase in idiopathic anaphylaxis. J Allergy Clin Immunol 2006;117:950–51 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–63. [DOI] [PubMed] [Google Scholar]
- 40.Ferrer M, Nuñez-Córdoba JM, Luquin E, Grattan CE, De la Borbolla JM, Sanz ML, Schwartz LB. Serum total tryptase levels are increased in patients with active chronic urticaria. Clin Exp Allergy 2010;40:1760–66. [DOI] [PubMed] [Google Scholar]
- 41.Castells MC, Irani AM, Schwartz LB. Evaluation of human peripheral blood leukocytes for mast cell tryptase. J Immunol 1987;138:2184–89. [PubMed] [Google Scholar]
- 42.Jogie-Brahim S, Min HK, Fukuoka Y, Xia HZ, Schwartz LB. Expression of alpha-tryptase and beta-tryptase by human basophils. J Allergy Clin Immunol. 2004;113:1086–92. [DOI] [PubMed] [Google Scholar]
- 43.Samorapoompichit P, Kiener HP, Schernthaner GH, Jordan JH, Agis H, Wimazal F, et al. Detection of tryptase in cytoplasmic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood 2001;98:2580–83. [DOI] [PubMed] [Google Scholar]
- 44.Baretto RL, Beck S, Heslegrave J, Melchior C, Mohamed O, Ekbote A. Validation of international consensus equation for acute serum total tryptase in mast cell activation: a perioperative perspective. Allergy 2017;72:2031–34. [DOI] [PubMed] [Google Scholar]
- 45.Lin RY, Schwartz LB, Curry A, Pesola GR, Knight RJ, Lee HS, et al. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J Allergy Clin Immunol 2000;106:65–71. [DOI] [PubMed] [Google Scholar]
- 46.Keyzer JJ, de Monchy JG, van Doormaal JJ, van Voorst Vader PC. Improved diagnosis of mastocytosis by measurement of urinary histamine metabolites. N Engl J Med 1983;309:1603–5. [DOI] [PubMed] [Google Scholar]
- 47.Watkins J, Wild G. Improved diagnosis of anaphylactoid reactions by measurement of serum tryptase and urinary methylhistamine. Ann Fr Anesth Reanim 1993;12:169–72. [DOI] [PubMed] [Google Scholar]
- 48.Awad JA, Morrow JD, Roberts LJ. Detection of the major urinary metabolite of prostaglandin D2 in the circulation: demonstration of elevated levels in patients with disorders of systemic mast cell activation. J Allergy Clin Immunol 1994;93:817–24. [DOI] [PubMed] [Google Scholar]
- 49.Ono E, Taniguchi M, Mita H, Akiyama K. Salicylamide-induced anaphylaxis: increased urinary leukotriene E4 and prostaglandin D2 metabolite. Allergy 2008;63:480–82. [DOI] [PubMed] [Google Scholar]
- 50.Ravi A, Butterfield J, Weiler CR. Mast cell activation syndrome: improved identification by combined determinations of serum tryptase and 24-hour urine 11β-prostaglandin2α. J Allergy Clin Immunol Pract 2014;2:775–78. [DOI] [PubMed] [Google Scholar]
- 51.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol 1991;88:328–38. [DOI] [PubMed] [Google Scholar]
- 52.Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, et al. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol 2002;110:102–9. [DOI] [PubMed] [Google Scholar]
- 53.Bühring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol 2004;133:317–29. [DOI] [PubMed] [Google Scholar]
- 54.Valent P, Hauswirth AW, Natter S, Sperr WR, Bühring HJ, Valenta R. Assays for measuring in vitro basophil activation induced by recombinant allergens. Methods 2004;32:265–70. [DOI] [PubMed] [Google Scholar]
- 55.Hauswirth AW, Escribano L, Prados A, Nuñez R, Mirkina I, Kneidinger M, et al. CD203c is overexpressed on neoplastic mast cells in systemic mastocytosis and is upregulated upon IgE receptor cross-linking. Int J Immunopathol Pharmacol 2008;21:797–806. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Twose I, González de Olano D, Sánchez-Muñoz L, Matito A, Esteban-López MI, Vega A, et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J Allergy Clin Immunol 2010;125:1269–1278.e2. [DOI] [PubMed] [Google Scholar]
- 57.Alvarez-Twose I, Zanotti R, González-de-Olano D, Bonadonna P, Vega A, Matito A, et al. on behalf of the Spanish Network on Mastocytosis (REMA) and the Italian Network on Mastocytosis (RIMA). Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J Allergy Clin Immunol 2014;133:520–28. [DOI] [PubMed] [Google Scholar]
- 58.Matito A, Alvarez-Twose I, Morgado JM, Sánchez-Muñoz L, Orfao A, Escribano L. Anaphylaxis as a clinical manifestation of clonal mast cell disorders. Curr Allergy Asthma Rep 2014;14:450. [DOI] [PubMed] [Google Scholar]
- 59.Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016;137:35–45. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez-Twose I, González-de-Olano D, Sánchez-Muñoz L, Matito A, Jara-Acevedo M, Teodosio C, et al. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int Arch Allergy Immunol 2012;157:275–80. [DOI] [PubMed] [Google Scholar]
- 61.Horny HP, Sotlar K, Valent P. Evaluation of mast cell activation syndromes: impact of pathology and immunohistology. Int Arch Allergy Immunol 2012;159:1–5. [DOI] [PubMed] [Google Scholar]
- 62.Valent P, Akin C, Bonadonna P, Hartmann K, Broesby-Olsen S, Brockow K, et al. Mast Cell Activation Syndrome: Importance of Consensus Criteria and Call for Research. J Allergy Clin Immunol 2018;142:1008–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest 2007;37:435–53. [DOI] [PubMed] [Google Scholar]
- 64.Lyons JJ, Sun G, Stone KD, Nelson C, Wisch L, O'Brien M, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol 2014;133:1471–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet 2016;48:1564–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyons JJ, Stotz SC, Chovanec J, Liu Y, Lewis KL, Nelson C, et al. A common haplotype containing functional CACNA1H variants is frequently coinherited with increased TPSAB1 copy number. Genet Med 2018;20:503–12. [DOI] [PubMed] [Google Scholar]
- 67.Sabato V, Chovanec J, Faber M, Milner JD, Ebo D, Lyons JJ. First Identification of an Inherited TPSAB1 Quintuplication in a Patient with Clonal Mast Cell Disease. J Clin Immunol. 2018;38:457–59. [DOI] [PubMed] [Google Scholar]
- 68.Sperr WR, Jordan JH, Baghestanian M, Kiener HP, Samorapoompichit P, Semper H, et al. Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood 2001;98:2200–9. [DOI] [PubMed] [Google Scholar]
- 69.Sperr WR, Stehberger B, Wimazal F, Baghestanian M, Schwartz LB, Kundi M, et al. Serum tryptase measurements in patients with myelodysplastic syndromes. Leuk Lymphoma 2002;43:1097–1105. [DOI] [PubMed] [Google Scholar]
- 70.Sperr WR, El-Samahi A, Kundi M, Girschikofsky M, Winkler S, Lutz D, et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: a novel diagnostic approach and screen marker in clinical haematology. Eur J Clin Invest. 2009;39:914–23. [DOI] [PubMed] [Google Scholar]
- 71.Valent P, Sperr WR, Sotlar K, Reiter A, Akin C, Gotlib J, et al. The serum tryptase test: an emerging robust biomarker in clinical hematology. Expert Rev Hematol. 2014;7:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oude Elberink JN1, de Monchy JG, Kors JW, van Doormaal JJ, Dubois AE. Fatal anaphylaxis after a yellow jacket sting, despite venom immunotherapy, in two patients with mastocytosis. J Allergy Clin Immunol 1997;99:153–54. [DOI] [PubMed] [Google Scholar]
- 73.Bonadonna P, Zanotti R, Pagani M, Bonifacio M, Scaffidi L, Olivieri E, et al. Anaphylactic reactions after discontinuation of hymenoptera venom immunotherapy: a clonal mast cell disorder should be suspected. J Allergy Clin Immunol Pract 2018;6:1368–72. [DOI] [PubMed] [Google Scholar]
- 74.Gülen T, Hägglund H, Sander B, Dahlén B, Nilsson G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin Exp Allergy 2014;44:1179–87. [DOI] [PubMed] [Google Scholar]
- 75.Broesby-Olsen S, Oropeza AR, Bindslev-Jensen C, Vestergaard H, Møller MB, Siebenhaar F, et al. ; Mastocytosis Centre Odense University Hospital (MastOUH); Odense Research Centre for Anaphylaxis. Recognizing mastocytosis in patients with anaphylaxis: value of KIT D816V mutation analysis of peripheral blood. J Allergy Clin Immunol 2015;135:262–64. [DOI] [PubMed] [Google Scholar]
- 76.Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia 2015;29:1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kristensen T, Vestergaard H, Bindslev-Jensen C, Mortz CG, Kjaer HF, Ollert M, et al. ; Mastocytosis Centre Odense University Hospital (MastOUH). Prospective evaluation of the diagnostic value of sensitive KIT D816V mutation analysis of blood in adults with suspected systemic mastocytosis. Allergy 2017;72:1737–43. [DOI] [PubMed] [Google Scholar]
- 78.Carter MC, Desai A, Komarow HD, Bai Y, Clayton ST, Clark AS, et al. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. J Allergy Clin Immunol 2018;141:180–88.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jara-Acevedo M, Teodosio C, Sanchez-Muñoz L, Álvarez-Twose I, Mayado A, Caldas C, et al. Detection of the KIT D816V mutation in peripheral blood of systemic mastocytosis: diagnostic implications. Mod Pathol 2015;28:1138–49. [DOI] [PubMed] [Google Scholar]
- 80.Bodemer C, Hermine O, Palmérini F, Yang Y, Grandpeix-Guyodo C, Leventhal PS, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol 2010;130:804–15. [DOI] [PubMed] [Google Scholar]
- 81.Méni C, Georgin-Lavialle S, Le Saché de Peufeilhoux L, Jais JP, Hadj Rabia S, et al. Pediatric mastocytosis: long term follow up of 53 patients with whole sequencing of KIT. A prospective study. Br J Dermatol 2018;179:925–32. [DOI] [PubMed] [Google Scholar]
- 82.Matito A, Carter M. Cutaneous and systemic mastocytosis in children: a risk factor for anaphylaxis? Curr Allergy Asthma Rep 2015;15:22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.