Abstract
The 2013 AVMA Guidelines for the Euthanasia of Animals recommends a chamber volume displacement rate of 10% to 30% per minute (v/min) when euthanizing small laboratory rodents with CO2. Group euthanasia of mice is a common practice, and grouping strangers is often avoided to minimize distress; however, emotional contagion, which occurs between familiar animals but not strangers, has not been studied in the context of group CO2 euthanasia. This study examined cagemate- and stranger-grouped mice exposed to 10%, 30%, or 50% v/min CO2 to determine whether emotional contagion plays a role in this context and whether that role is influenced by CO2 flow rate. Videos of adult male C57BL/6J mice exposed to different CO2 flow rates were scored for durations of dyspnea, ataxia, and consciousness as well as the numbers of face pawing and jump behaviors. Blood was collected at time of unconsciousness and assayed for ACTH. Cagemates experienced significantly longer durations of conscious dyspnea and ataxia with 10% v/min CO2 compared with 30% and 50% v/min. Similarly, strangers experienced significantly longer duration of conscious dyspnea with 10% v/min CO2 compared with 30% and 50% v/min and significantly longer duration of ataxia with 10% compared with 50% v/min. Cagemates showed significantly more jumps with 10% v/min CO2 compared with 30% and 50% v/min, whereas jumping was unaffected by CO2 flow rate in strangers. We conclude that more potential for distress exists when cagemate and stranger mice are exposed to a 10% v/min CO2 flow rate and that emotional contagion may contribute to distress in cagemates at this flow rate. Therefore, we propose that 30% v/min CO2 should be used for euthanasia of mice, and that 50% v/min should also be considered humane.
Providing euthanasia for research animals is important because of the moral and ethical imperatives to maintain high standards of animal welfare. As outlined by the fundamental concept of the ‘3 Rs’ (replacement, reduction, refinement),42 an essential component of improving animal welfare is the refinement of procedures to minimize pain and distress. Mice are a widely used research animal, and most research protocols involving mice include euthanasia as an experimental endpoint; therefore, optimizing mouse euthanasia practices is a powerful form of refinement.
Moreover, unless a departure from this practice is justified for scientific reasons, providing humane euthanasia to laboratory animals is federally mandated by the Animal Welfare Act.3 For Public Health Service Assured institutions, this requirement is also mandated by the Public Health Service Policy on Humane Care and Use of Laboratory Animals39 and the Guide for the Care and Use of Laboratory Animals.21 These documents cite the AVMA Guidelines for the Euthanasia of Animals2 (AVMA Guidelines) as the source of recommendations regarding humane methods of euthanasia for veterinary species. The AVMA Guidelines were last updated in 2013 and define euthanasia as “ending the life of an individual animal in a way that minimizes or eliminates pain and distress.”2
With this definition in mind, it is important to recognize whether pain or distress occur during euthanasia. Stressors such as pain, aversion, and social threat (as well as many nonharmful stimuli) cause the biologic response of stress, which becomes distress when other biologic functions are negatively affected and interfere with an animal's wellbeing.2,12 Definitively measuring distress in animals is difficult, but some indicators of stressors such as pain may be recognized more easily. For example, indicators of pain may include attempts to escape the painful stimulus and rubbing or licking at the painful area and, as with any stressor, activation of the stress response.12 Although the presence of stressors and stress are not synonymous with distress, distress cannot exist without stress; therefore, when a euthanasia method results in evidence of pain or stress, there is also potential for distress associated with the procedure.
With the goal to minimize pain and distress, some considerations when assessing the appropriateness of a euthanasia method include: the ability to induce unconsciousness and death with minimal pain and distress; time required to induce unconsciousness; reliability and irreversibility; appropriateness for the age and species of animal; ease of administration; ease of acquiring and maintaining equipment; and safety and emotional effects for personnel. The 2013 AVMA Guidelines consider CO2 inhalation to be acceptable with conditions for the euthanasia of small laboratory rodents.2 CO2 exposure is a very common method of euthanasia for laboratory mice and possesses many desirable qualities. CO2 acts relatively quickly, is reliable, simple to administer, relatively inexpensive, and safe for operators. However, CO2 is known to have the potential to cause pain (due to the formation of carbonic acid on the mucous membranes) and distress (due to dyspnea) in rodents, and these effects are mediated by the concentration20,26,29,34,37 and flow rate (displacement of chamber volume per minute, v/min) at which the gas is administered.20,36 In addition, CO2 is thought to cause fear in mice, given that the amygdala, which controls fear behavior, is sensitive to hypercarbia and acidosis.50 Exposure to CO2 has been used and validated as a model of panic disorder in mice.45 Mice may display pain or aversion behaviors such as pawing at the face14,49 or jumping to attempt escape from the CO2 chamber,14,45,47 which in addition to being signs of potential distress in the animals, can be emotionally difficult for operators to observe.4 Therefore, continuing to work to minimize the potential for pain and distress associated with CO2 euthanasia in mice is warranted.
The AVMA recommends a 10% to 30% v/min flow rate for CO2 euthanasia in small rodents,2 which is a change from the previous recommendation of a 20% v/min or higher CO2 flow rate.1 The purpose of the 2013 recommendation is to provide a suggested flow rate so that animals will become unconscious before the CO2 concentration in the chamber reaches a level that induces pain. In addition, the 2013 AVMA Guidelines deem prefilled CO2 chambers unacceptable, with the general position that a slower but gentler death is preferable to a faster, more distressful death. The 2013 recommendation is based on studies that were mainly performed with rats. Briefly, CO2 exposure was known to have the potential to produce a stress response in rats, in part due to hypoxia.9,14,31 Aversion tests in rats and mice and approach–avoidance tests in rats showed CO2 is aversive at concentrations of 15% and higher.26,27,38 CO2 does not cause pain until concentrations of approximately 40% are reached,2 and in rats, for example, 10% v/min CO2 produced unconsciousness at 21% CO2,10 and 17.25% v/min produced unconsciousness at 33% CO2.37
In the years since the 2013 AVMA Guidelines were released, a recent review article8 and studies focusing on CO2 euthanasia in mice have raised concern that slow CO2 flow rates, such as those within the recommended range, are more distressful than higher flow rates in this species. Slower flow rates (10% to 20% v/min) have been found to result in more pawing at the face and jumping14 as well as more time spent in conscious dyspnea14,32 than when higher (30% v/min or more) flow rates are used.
In addition to CO2 flow rate, the factor of social stress is an important consideration during euthanasia procedures. Culling large numbers of mice is often necessary in research facilities, and grouping mice in CO2 chambers (group euthanasia) to facilitate this process is a common practice. The caveat with this practice is that mice, especially males, display increased agonistic behaviors (for example, fighting) when exposed to unknown conspecifics (strangers).28,40 Furthermore, regular disruption of cage group composition has been used and verified as a model of chronic stress in mice.44,46 For these reasons, combining mice that are strangers for group euthanasia is generally thought to add a level of stress to the euthanasia process. However, the AVMA Guidelines do not provide specific recommendations for group euthanasia in mice beyond stating that if animals are combined, they be of the same species and in compatible cohorts and that chambers not be overloaded.2 In attempts to minimize the stress associated with CO2 euthanasia, some facilities may choose to avoid grouping stranger mice—only euthanizing cagemates in groups—which will limit the efficiency with which euthanasia of large cohorts can be performed.
A previous study found that group-CO2-euthanized stranger mice had significantly lower serum catecholamine levels than individually euthanized mice, regardless of CO2 flow rate.14 This finding conflicts with the idea that grouping stranger mice increases the stress associated with CO2 exposure. One possible explanation for why grouping strangers may not, in fact, exacerbate stress could involve the concept of emotional contagion (a primitive form of empathy); however, to our knowledge, studies have not been performed to assess stress in stranger- compared with cagemate-grouped mice during CO2 euthanasia.
Empathy is the ability to experience and understand the cause of another's emotional state and requires self-other awareness.17 Several distinct empathy-related behaviors and responses are described, including emotional contagion, which is an evolutionary precursor to empathy.17 This primitive response allows animals to automatically perceive and share another's affective state, even without the ability to recognize or understand the cause of the emotion in the other.17 Emotional contagion of pain has been demonstrated in humans and animals, including mice, as evidenced by pain behaviors in subjects observing conspecifics in pain.16,23,30 This response is modulated by familiarity, where groups of familiar animals (and people) display emotional contagion and strangers do not.16,23,30 For example, when given the same noxious stimulus, paired familiar mice display increased pain behavior compared with individual animals or pairs of strangers.23,30 If this phenomenon occurs in the context of CO2 euthanasia, mice in cagemate groups can, in fact, be expected to exhibit exacerbated pain responses compared with strangers.
Stress influences pain sensitivity, either inhibiting or exacerbating pain perception depending on the nature and chronicity of the stressor.11,12,15 Stress-induced analgesia—largely mediated by the endogenous opioid system—generally occurs when stressors are robust and acute and is thought to be a survival mechanism toward imminent danger (part of the ‘fight-or-flight’ response).11,12,15 Stress-induced analgesia is considered to explain why stranger-paired mice do not display emotional contagion,23,30 consistent with how pretreating with naloxone or glucocorticoid inhibitors before delivering a noxious stimulus allows stranger pairs to display increased pain behavior compared with individual animals (that is, allows strangers to display emotional contagion).30 In this case, analgesia-inducing stress is likely due to acute social stress. Male mice given a noxious stimulus exhibit reduced pain responses when in the presence of an intact stranger male compared with when alone or paired with a castrated male.24 Because potential conspecific aggression is highest between sexually mature males,40 this finding suggests that the apparent stress-induced analgesia in paired strangers is related to level of perceived social threat. If grouped stranger mice experience an element of this social stress-induced analgesia in the context of CO2 euthanasia, stranger groups can be expected to exhibit reduced pain responses compared with cagemates.
In the current study, we explored the possible role of emotional contagion in the context of group euthanasia by analyzing behavioral and biochemical markers of pain and potential distress in stranger and cagemate mice exposed to 10%, 30%, or 50% v/min CO2. Conscious dyspnea and ataxia were used as markers of potential distress,6,14,32 pawing at the face was used as a marker of pain,14,41,49 jumping was interpreted as escape attempts and used as a marker of aversion,14,45,47 and plasma ACTH was used as a marker of stress.6,7,43 We hypothesized that lower CO2 flow rates will result in longer duration of consciousness and increased behavioral and biochemical markers of potential distress in both strangers and cagemates. In addition, according to the concept of emotional contagion, we hypothesized that cagemates will show increased behavioral and biochemical markers of pain and potential distress compared with strangers.
Materials and Methods
Animals.
All animal care and use for this study was performed in accordance with a protocol reviewed and approved by the IACUC at The Jackson Laboratory, an AAALAC-accredited animal program.
The study population comprised 48 experimentally naïve male C57BL/6J mice (Mus musculus; age, 6 to 10 wk; The Jackson Laboratory, Sacramento, CA). Animals represented unsold inventory and were scheduled to be culled. No animals were purchased or bred specifically for this project; instead, animals were used opportunistically as a form of animal reduction. Animals were bred inhouse, weaned into their respective groups, and remained in their groups until time of use. All animals were negative for the following pathogens and opportunistic organisms: Ectromelia virus, Theiler mouse encephalomyelitis virus, Hantaan virus, K virus, lactate dehydrogenase-elevating virus, lymphocytic choriomeningitis virus, murine adenovirus, murine cytomegalovirus, murine hepatitis virus, murine minute virus, murine parvovirus, murine thymic virus, murine norovirus, pneumonia virus of mice, polyoma virus, reovirus 3, rotavirus, Sendai virus, Bordetella spp., cilia-associated respiratory bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Corynebacterium bovis, Pasteurella pneumotropica, Helicobacter spp., β-hemolytic Streptococcus spp., Klebsiella spp., Pneumocystis murina, Pseudomonas spp., Proteus mirabilis, Staphylococcus aureus, Streptococcus pneumoniae, Mycoplasma pulmonis, Salmonella spp., Streptobacillus moniliformis, Encephalitozoon cuniculi, fleas, fur mites, lice, follicle mites, pinworms, roundworms and other helminths, tapeworms, and opportunistic protozoa (for example, Giardia, Spironucleus). Mice were housed in groups of 10 on individually ventilated racks (Thoren Caging Systems, Hazleton, PA) supplied with 60 air changes hourly. Mice were housed in steam-sterilized polysulfone caging (Thoren Caging Systems) on bedding composed of steam-sterilized aspen wood chips (Nepco, East Providence, RI) and shavings (PJ Murphy, Montville, NJ); provided steam-sterilized rodent chow (LabDiet JL 6% 5K0Q, Purina, St Louis, MO) without restriction; and received steam-sterilized, filtered, and acidified water. Mice were kept under a 14:10-h light:dark cycle.
CO2 exposure and behavior analysis.
Study groups consisted of 6 groups of 8 mice each: cagemates exposed to 10%, 30%, or 50% v/min CO2, and strangers exposed to 10%, 30%, or 50% v/min CO2. Because no animals were purchased or bred specifically for this project, animal numbers were based on numbers of available mice scheduled for culling. Given previous research20,37 and inhouse CO2 euthanasia studies in which groups of 6 to 12 animals yielded statistically significant results, we determined that a group size of 8 would be sufficient to yield meaningful data.
CO2 exposure was performed by using the JAX Euthanasia System with CO2 flow rates of 10%, 30%, or 50% v/min. This system was designed and validated inhouse for mouse euthanasia at The Jackson Laboratory (data not shown). A programmable logic controller (Barry-Wehmiller Design Group, Roseville, CA) is used to automatically set the desired flow rate for CO2 in a standardized, empty euthanasia chamber (model no. 2, Weaning Cage, Thoren Caging Systems) that measures 112.9 in.2. The flow rate is regulated by a mass flow controller (Sierra Instruments, Monterey, CA), and CO2 is directed into the top of the euthanasia chamber. Weep holes are drilled into the euthanasia chamber lid to allow room air to escape as CO2 fills the chamber. 100% compressed CO2 gas was used. The chamber was overturned, cleaned with 70% ethanol and allowed to dry between groups of mice.
Five cages of 10 mice were removed from their home ventilated rack, placed in a designated procedure area, and allowed to acclimate for approximately 3 h prior to CO2 exposure. Euthanasia took place during a 3-h window on a single day, to minimize circadian effects. Animals were exposed to CO in groups of 4 mice each, for a total 8 mice per study group. For cagemate groups, we placed 4 mice from one of the original cages of 10 into the euthanasia chamber and began CO2 exposure at the indicated flow rate. The second round of cagemate euthanasia was repeated at that flow rate, with 4 mice removed from another original cage of 10 (that is, cagemate mice were euthanized in partial housing groups). All cagemate euthanasia groups were formed in this manner. For stranger groups, we placed 4 mice from 4 different original cages into the euthanasia chamber and began CO2 exposure at the indicated flow rate. All stranger euthanasia groups were formed in this manner. Both cagemate and stranger mice were placed into the euthanasia chamber immediately before CO2 exposure began (that is, strangers were introduced to each other at the time of euthanasia and were not housed together for any appreciable amount of time prior to euthanasia).
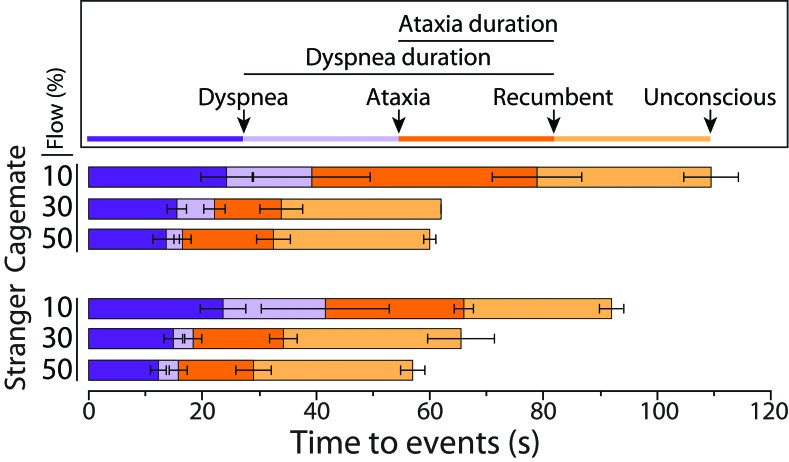
CO2 exposure trials were video recorded by using a high-definition video camera (model CX560V, Sony, Tokyo, Japan). Videos were scored for durations of ataxia, dyspnea, recumbency, and consciousness and the numbers of observed jumps (interpreted as escape attempts) and paws at the face (interpreted as pain response). A single-blinded observer scored all videos, with each mouse scored individually for all observations except unconsciousness, which was scored per cage. The longitudinal events measured are depicted in Figure 1, and the definitions of mouse behaviors used to record observations are in Figure 2.
Figure 1.
Time to events throughout exposure to CO2. The mean of longitudinal events from time 0 to unconsciousness are shown. The duration of dyspnea is a composite variable defined as the onset of dyspnea until the time of recumbency. The duration of ataxia is defined as the onset of ataxia until the time of recumbency. Values (duration [in s] from time 0) are given as means (bars, 1 SD) for each event (defined in Figure 2).
Figure 2.
Definitions of scored observations in mouse CO2 exposure videos.
Pilot studies (data not shown) were conducted to determine the average time to reach unconsciousness after recumbency, to minimize the number of times needed to test for response to noxious stimulus. Once all mice in the euthanasia chamber reached recumbency, we waited 10 s (determined according to pilot data) before testing response to a noxious stimulus generated by clamping the base of one animal's tail with 9-in. intestinal Doyen hemostatic forceps (Skylar Surgical Instruments, West Chester, PA) inserted through a weep hole in the chamber lid. In all tests, the hemostats were clamped to the 1st of the 3 notches, to provide a standardized level of pressure during noxious stimulus testing. If a mouse responded to the noxious stimulus with purposeful movement, we waited 10 s prior to clamping again. Clamping was alternated among the animals within the group, with the time to unconsciousness recorded when 2 successive mice did not respond to the noxious stimulus.
Blood collection and analysis.
Immediately after mice reached unconsciousness, they were decapitated by using sharp scissors. Whole blood from the body trunk was collected in EDTA tubes and processed into plasma through centrifugation. Plasma was stored at –80 °C until it was shipped on dry ice for testing. Plasma ACTH was measured by using ELISA (The Jackson Laboratory, Bar Harbor, ME).
Blood samples that yielded insufficient plasma were unsuitable for statistical analysis of ACTH levels, but each study group yielded at least 6 usable plasma samples.
Statistics.
Two-way ANOVA followed by Tukey (for comparing flow rates within stranger and cagemate groups) or Sidak (for comparing strangers and cagemates within each flow rate) multiple-comparisons tests were performed by using Prism version 6 (GraphPad Software, La Jolla, CA). Reported P values were adjusted for multiple comparisons according to the model. To understand the clumping structure of the data, we used unsupervised hierarchical clustering which was performed in JMP version 14.0 (SAS Institute, Cary, NC). The input variables were scaled, and the Ward method was used to calculate the distances between the clusters. P values less than 0.05 were considered significant.
Results
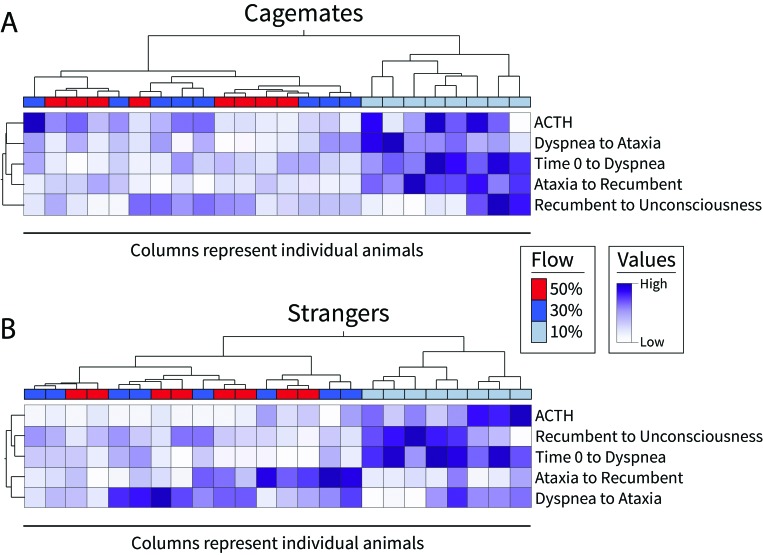
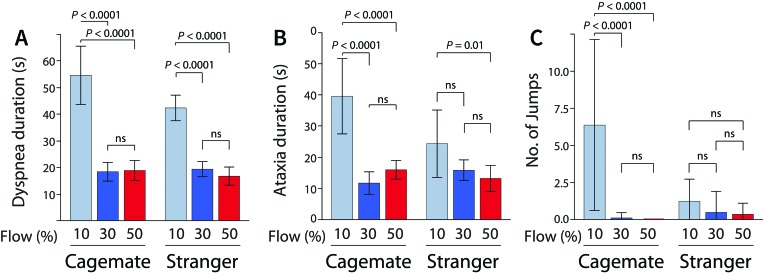
Regarding potential distress at different CO2 flow rates, 10% v/min resulted in the longest time to unconsciousness in both cagemate and stranger mice (Figures 1 and 3). As such, we found that 10% v/min results in a significantly longer mean duration of conscious dyspnea (cagemates, 54.6 s; strangers, 42.4 s) compared with 30% v/min (cagemates, 18.4 s, P < 0.0001; strangers, 19.4 s, P < 0.0001) and 50% v/min (cagemates, 18.9 s, P < 0.0001; strangers, 16.8 s, P < 0.0001), with no significant difference between 30% and 50% v/min (Figure 4 A). Similarly, in cagemates, 10% v/min CO2 results in significantly longer mean duration of ataxia (39.6 s) compared with 30% v/min (11.8 s, P < 0.0001) and 50% v/min (16.0 s, P < 0.0001), and in strangers, 10% v/min results in significantly longer mean duration of ataxia (24.4 s) compared with 50% v/min (13.3 s, P = 0.01; Figure 4 B). Furthermore, cagemate mice demonstrated significantly more jumps at 10% v/min (mean, 6.4) compared with 30% v/min (0.1, P < 0.0001) or 50% (0.0, P < 0.0001; Figure 4 C). In contrast to cagemates, jump behavior by strangers was unaffected by CO2 flow rate (Figure 4 C). Although in cagemates mean plasma ACTH levels decreased numerically as CO2 flow rate increased (Figure 3), these changes did not reach statistical significance.
Figure 3.
Mice in the 10% v/min group have a different structure of the observed stages of exposure to CO2 and the level of ACTH. Heat map showing scaled values for the various stages of exposure to CO2 and the plasma level of ACTH (pg/mL). The flow rate of each animal is depicted as a horizontal bar at the top of the heatmap. Mice (shown as columns) and variables (shown as rows) were clustered by using unsupervised hierarchical clustering (Ward method).
Figure 4.
Flow rate influences the potential distress level during euthanasia. (A) The duration of dyspnea is significantly greater in the 10% v/min group compared with the 30% or 50% v/min groups for both cagemates and strangers. (B) Similarly, in both cagemate and stranger groups, mice exposed to 10% v/min CO2 experienced a longer duration of ataxia compared with other flow rate groups. (C) The number of observed jumps (interpreted as escape attempts) was greater in the 10% v/min group (compared with 30% and 50% v/min groups) only in paired familiar mice. P values were calculated by using 2-way ANOVA followed by Tukey multiple comparison testing. Error bars, 1 SD; ns, not significant.
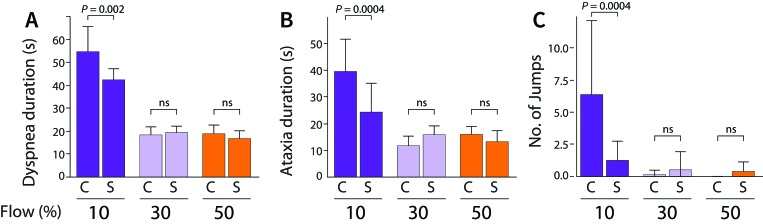
When we compared markers of potential distress between cagemates and strangers, within the 10% CO2 flow rate, cagemates experienced significantly longer mean durations of dyspnea (cagemates, 54.6 s; strangers, 42.4 s; P = 0.002) and ataxia (cagemates, 39.6 s; strangers, 24.4 s; P = 0.0004) and more jumps (cagemates, 6.4; strangers, 1.2; P = 0.0004; Figure 5). Neither plasma ACTH levels nor face pawing differed significantly between any of the study groups (data not shown).
Figure 5.
Group composition influences the potential distress level during euthanasia at 10% v/min CO2. (A) The duration of dyspnea is significantly higher in cagemates (C) compared with strangers (S) at 10% v/min. (B) Similarly, the duration of ataxia is significantly higher in cagemates compared with strangers at 10% v/min. (C) The number of observed jumps (interpreted as escape attempts) were higher in cagemates compared with strangers at 10% v/min. P values were calculated using 2-way ANOVA followed by Sidak's multiple comparison test. The error bars show standard deviations of the mean.
Discussion
Unsurprisingly, our results indicate that, in mice, more potential for distress exists at 10% v/min CO2 than at higher flow rates (30% or 50%): at 10% v/min, both strangers and cagemates experienced longer durations of conscious dyspnea and ataxia. These results agree with previous studies5,14,32 that found the duration of dyspnea in mice undergoing CO2 euthanasia was inversely related to CO2 flow rate. As further evidence that 10% v/min CO2 is potentially distressful to mice, cagemates in our study displayed significantly more jumps at 10% v/min than at higher flow rates.
A previous study found that CO2 flow rate had no effect on behavioral or physiologic responses in mice;6 however, the authors evaluated individually euthanized mice, which may explain why they did not see the flow rate-mediated differences we observe in our cagemates. Indeed, the results of the cited study6 are similar to what we saw in our stranger groups. We interpret our finding of exacerbated escape (jumping) responses at 10% v/min CO2 in cagemates—but not strangers—as supportive of a role for emotional contagion in the context of group CO2 euthanasia in mice.
In our current study, jumping is the behavioral indicator of potential distress that yielded the most interesting results. Jumping behavior, interpreted as escape attempts, has been observed in mice experiencing acutely aversive conditions19 and is often observed in studies of rodent averseness to CO2.14,45,47 However, another interpretation in the context of CO2 euthanasia could be that mice are experiencing the excitement stage of anesthesia. Supporting the idea that jumping behavior is a valid measure of stress, one study45 showed that corticosterone levels in mice 30 min after exposure to CO2 were positively correlated to number of jumps during the exposure, and furthermore, showed that this jumping behavior was likely panic-related, given that it was significantly reduced by pretreatment with an anxiolytic, either fluoxetine or alprazolam. The authors concluded that CO2 exposure is an acceptable model for panic disorder in mice.45
If jumping is a behavioral response to CO2 exposure indicating panic, one might expect jumping behavior regardless of CO2 flow rate. In our study, we saw that cagemates jumped significantly more at 10% v/min CO2 compared with higher flow rates, whereas the number of jumps in strangers was low regardless of flow rate. This discrepancy might be explained in that jumping is a sign of aversion, and CO2 may be aversive to mice for various reasons, including evoked feelings of anxiety or pain. In a previous study,45 the CO2 concentration was increased to 20% over 2 min and then maintained at 20% CO2 for an additional 5 min.; the authors saw jumping behavior in the mice only after the first 2 min of exposure. In our study, although cagemates exposed at 10% v/min CO2 flow rate showed the longest duration of consciousness among our study groups, all mice became unconsciousness within 2 min (mean, 109.5 s). Prolonged conscious CO2 exposure may induce jumping due to a panic reaction, consistent with previous findings.45 In our current study, the pattern of jumping we observed is consistent with emotional contagion of pain, where paired familiar animals show exacerbated pain responses compared with paired strangers; therefore, our results suggest that cagemates exposed to 10% v/min CO2 exhibit jumping due to exacerbated pain perception at that flow rate.
Interestingly, our data suggest that emotional contagion in the context of group CO2 euthanasia may be mediated by CO2 flow rate. We observe that cagemate animals displayed increased escape behavior (jumping) compared with strangers at a low (10% v/min) CO2 flow rate but not at higher rates (30% or 50% v/min). Evidence for the emotional contagion effect at 10% v/min CO2 suggests that euthanasia is aversive (possibly due to exacerbated pain perception) at this flow rate. Conversely, we did not find evidence that a CO2 flow rate of 30% or 50% v/min is aversive or painful for mice.
Indeed, our results suggest that aversion (increased escape attempts)—possibly due to exacerbated pain perception—is associated with a low CO2 flow rate in familiar groups but not in stranger groups. This notion is consistent with the concept of emotional contagion in familiar groups; this effect is blocked by social stress-induced analgesia in stranger groups. In addition, a previous study14 found that serum catecholamine levels were lower in CO2-euthanized stranger groups compared with individual mice; this result is consistent with social-stress–induced analgesia, given that grouped stranger mice likely experience more social stress than individual animals.
Because differences in ACTH levels between cagemates and strangers did not reach significance in this study, one interpretation of our data is that, at the tested CO2 flow rates, euthanizing mice in stranger groups was no more stressful than euthanizing cagemates. However, it is important to remember that the analgesic effect present in stranger groups has been shown to be due to stress;23,24,30 therefore, grouping stranger mice together for euthanasia indeed is likely creating social stress. To develop recommendations regarding group composition for CO2 euthanasia, further studies are needed to determine the contributions of pain compared with social stress to the overall potential distress that grouped mice might experience during CO2 euthanasia.
Of course, when combining stranger mice for euthanasia, every precaution should be taken to avoid injuries due to fighting associated with the procedure. Studies of aggression in mice have shown that agonistic behavior, such as attacks, begin within minutes of introducing strangers.5,28,40 Microenvironment is another important consideration, given that mice may be more aggressive toward conspecifics when in their home cages as compared with when placed in a new environment.5,18 We took stranger mice from their home cages and combined them directly in a clean euthanasia chamber immediately before starting CO2 exposure and did not observe any fighting behavior during our experiments.
Caveats and limitations in our current study include the interpretation of unconsciousness, animal numbers, our use of ACTH as the sole physiologic indicator of stress, and the single sex and strain of mouse evaluated. It is difficult to determine the exact time point during euthanasia when a mouse reaches unconsciousness, yet this determination is important when assessing animal welfare. For example, dyspnea may occur after unconsciousness is reached, but only conscious dyspnea and its associated anxiety influences animal welfare. Mice can still show purposeful movement and respond to stimuli after the righting reflex is lost and recumbency achieved;33 therefore, we used lack of response to a noxious stimulus as the definition of unconsciousness in our current study. Although this approach may overestimate the duration of consciousness, such overestimation is preferable to underestimation when assessing animal welfare.
Our study groups each consisted of 2 subgroups of 4 mice each for a total of 8 mice per study group. Although we measured most parameters in individual mice, unconsciousness was determined on a cage-level basis (that is, 2 per study group). Using more cages within each test treatment would allow for more robust comparisons to ensure that findings are consistent across groups. In addition, using more cages of mice may allow more subtle patterns in the data to emerge. Further studies in this area are needed to ensure reproducibility of results before general recommendations regarding group composition during CO2 euthanasia in mice can be made.
We used plasma ACTH as our sole physiologic measure of stress. It might have been informative to compare ACTH levels with catecholamine and corticosterone levels, but the latter 2 parameters are less likely to reliably reflect stress in the context of CO2 euthanasia. Catecholamine levels can be influenced by hypercapnia,43 and indeed, a previous study14 found that catecholamine levels did not differ between CO2 flow rates. One possible interpretation of this finding is that catecholamine levels indicated hypercapnia rather than stress. Corticosteroids (for example, corticosterone) are not as useful as ACTH in indicating acute stress, because corticosteroid levels take minutes to rise after a stressor,45 whereas ACTH levels take seconds.6,7,22,43,48 Furthermore, corticosteroid levels may not correlate to degree of stress as well as do ACTH levels, because corticosteroid concentrations start to plateau even with small increases in ACTH.6,7,22 Given that CO2 euthanasia is acutely and possibly highly stressful, we chose ACTH at the time of unconsciousness as the most appropriate parameter to measure and did not necessarily expect a correlation between ACTH and catecholamine or corticosterone levels at the time point of interest.
The findings of our current study cannot necessarily be applied to mice universally, because we evaluated male C57BL/6J mice only, and sex- and strain-associated differences might influence emotional contagion. For example, one study35 found that male C57BL/6J mice are sociable and have a preference for social novelty. Indeed, another group13 found that C57BL/6J mice responded significantly more to distress cues from conspecifics than did BALB/cJ mice, suggesting that the relatively gregarious C57BL/6J may be more prone to emotional contagion than other strains. Another study25 found that female but not male mice approached familiar same-sex conspecifics in pain more frequently than they approached affected strangers, demonstrating that sex may be another factor in empathy-related behaviors in mice. In addition, sex- and strain-associated differences might modulate susceptibility to stress and stress-induced alteration of pain perception.11,15 For example, previous studies23,24 found stress-induced analgesia occurred in paired male but not female mice. Therefore, although outside the scope of the current study, further studies are warranted to investigate possible strain- and sex-related variability in emotional contagion and stress-induced analgesia during group CO2 euthanasia in mice.
Conclusions from the current study are that 10% v/min CO2 carries greater potential for distress than 30% or 50% v/min, in light of the longer durations of conscious dyspnea and ataxia in both cagemates and strangers and the increased jump behavior (suggesting emotional contagion of pain) in cagemates at the 10% v/min flow rate. Therefore, we recommend avoiding 10% v/min CO2 when euthanizing mice. Within the range recommended in the 2013 AVMA Guidelines, 30% v/min CO2 carries less potential for distress and should be used for group euthanasia of mice; however, a 50% v/min flow rate should be considered equally humane. A general recommendation regarding group composition during CO2 euthanasia cannot yet be made, although we obtained evidence that social modulation of pain is relevant in the context of CO2 euthanasia and should be considered with flow rate. Further studies are needed to ensure reproducibility, to investigate possible sex- and strain-associated differences in emotional contagion, and to elucidate the contributions of pain compared with social stress to the overall potential distress experienced by mice in the context of group CO2 euthanasia.
Acknowledgments
We thank Fironica Lao and Gavin Oliver for their assistance in gathering data in this study.
References
- 1.American Veterinary Medical Association. 2007. AVMA guidelines on euthanasia. [Cited 2 August 2018]. Available at: https://grants.nih.gov/grants/olaw/Euthanasia2007.pdf
- 2.American Veterinary Medical Association. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 2 August 2018]. Available from: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
- 3.Animal Welfare Act as Amended. 2008. 7 USC §2143.
- 4.Baker BA, Hickman DL. 2018. Bias in rating of rodent distress during anesthesia induction for anesthesia compared with euthanasia. J Am Assoc Lab Anim Sci 57:143–156. [PMC free article] [PubMed] [Google Scholar]
- 5.Bisazza A. 1978. Development of aggressive behavior in the mouse (Mus musculus): effects of different environmental conditions. Boll Zool 45:353–357. 10.1080/11250007809440142. [DOI] [Google Scholar]
- 6.Boivin GP, Bottomley MA, Dudley ES, Schiml PA, Wyatt CN, Grobe N. 2016. Physiological, behavioral, and histological responses of male C57BL/6N mice to different CO2 chamber replacement rates. J Am Assoc Lab Anim Sci 55:451–461. [PMC free article] [PubMed] [Google Scholar]
- 7.Boivin GP, Bottomley MA, Schiml PA, Goss L, Grobe N. 2017. Physiologic, behavioral, and histologic responses to various euthanasia methods in C57BL/6NTac male mice. J Am Assoc Lab Anim Sci 56:69–78. [PMC free article] [PubMed] [Google Scholar]
- 8.Boivin GP, Hickman DL, Creamer-Hente MA, Pritchett-Corning KR, Bratcher NA. 2017. Review of CO2 as a euthanasia agent for laboratory rats and mice. J Am Assoc Lab Anim Sci 56:491–499. [PMC free article] [PubMed] [Google Scholar]
- 9.Borovsky V, Herman M, Dunphy G, Caplea A, Ely D. 1998. CO2 asphyxia increases plasma norepinephrine in rats via sympathetic nerves. Am J Physiol 274:R19–R22. [DOI] [PubMed] [Google Scholar]
- 10.Burkholder TH, Niel L, Weed JL, Brinster LR, Bacher JD, Foltz CJ. 2010. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J Am Assoc Lab Anim Sci 49:448–453. [PMC free article] [PubMed] [Google Scholar]
- 11.Butler RK, Finn DP. 2009. Stress-induced analgesia. Prog Neurobiol 88:184–202. 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Carstens E, Moberg GP. 2000. Recognizing pain and distress in laboratory animals. ILAR J 41:62–71. 10.1093/ilar.41.2.62. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Panksepp JB, Lahvis GP. 2009. Empathy is moderated by genetic background in mice. PLoS One 4:1–14. 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creamer-Hente MA, Lao FK, Dragos ZP, Waterman LL. 2018. Sex- and strain-related differences in the stress response of mice to CO2 euthanasia. J Am Assoc Lab Anim Sci 57:513–519. 10.30802/AALAS-JAALAS-18-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferdousi M, Finn DP. 2018. Stress-induced modulation of pain: role of the endogenous opioid system. Prog Brain Res 239:121–177. 10.1016/bs.pbr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brüne M. 2014. Emotional contagion in mice: the role of familiarity. Behav Brain Res 263:16–21. 10.1016/j.bbr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Liencres C, Shamay-Tsoory SG, Brüne M. 2013. Towards a neuroscience of empathy: ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci Biobehav Rev 37:1537–1548. 10.1016/j.neubiorev.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Gray S, Hurst JL. 1995. The effects of cage cleaning on aggression within groups of male laboratory mice. Anim Behav 49:821–826. 10.1016/0003-3472(95)80213-4. [DOI] [Google Scholar]
- 19.Harikai N, Sugawara T, Tomogane K, Mizuno K, Tashiro S. 2004. Acute heat stress induces jumping escape behavior in mice. Physiol Behav 83:373–376. 10.1016/j.physbeh.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Hickman DL, Fitz SD, Bernabe CS, Caliman IF, Haulcomb MM, Federici LM, Shekhar A, Johnson PL. 2016. Evaluation of low versus high volume per minute displacement CO2 methods of euthanasia in the induction and duration of panic-associated behavior and physiology. Animals (Basel) 6:1–18. 10.3390/ani6080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 22.Keller-Wood ME, Shinsako J, Dallman MF. 1983. Integral as well as proportional adrenal responses to ACTH. Am J Physiol 245:R53–R59. [DOI] [PubMed] [Google Scholar]
- 23.Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. 2006. Social modulation of pain as evidence for empathy in mice. Science 312:1967–1970. 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 24.Langford DJ, Tuttle AH, Briscoe C, Harvey-Lewis C, Baran I, Gleeson P, Fischer DB, Buonora M, Sternberg WF, Mogil JS. 2011. Varying perceived social threat modulates pain behavior in male mice. J Pain 12:125–132. 10.1016/j.jpain.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Langford DJ, Tuttle AH, Brown K, Deschenes S, Fischer DB, Mutso A, Root KC, Sotocinal SG, Stern MA, Mogil JS, Sternberg WF. 2010. Social approach to pain in laboratory mice. Soc Neurosci 5:163–170. 10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- 26.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Aversion to gaseous euthanasia agents in rats and mice. Comp Med 52:249–257. [PubMed] [Google Scholar]
- 27.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Degrees of aversion shown by rats and mice to different concentrations of inhalational anaesthetics. Vet Rec 150:808–815. 10.1136/vr.150.26.808. [DOI] [PubMed] [Google Scholar]
- 28.Mackintosh JH, Grant EC. 1966. The effect of olfactory stimuli on the agonistic behavior of laboratory mice. Z Tierpsychol 23:584–587. 10.1111/j.1439-0310.1966.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 29.Makowska IJ, Vickers L, Mancell J, Weary DM. 2009. Evaluating methods of gas euthanasia for laboratory mice. Appl Anim Behav Sci 121:230–235. 10.1016/j.applanim.2009.10.001. [DOI] [Google Scholar]
- 30.Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, Slepian PM, Trost Z, Bartz JA, Sapolsky RM, Sternberg WF, Levitin DJ, Mogil JS. 2015. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr Biol 25:326–332. 10.1016/j.cub.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Marotta SF, Sithichoke N, Garcy AM, Yu M. 1976. Adrenocortical responses of rats to acute hypoxic and hypercapnic stresses after treatment with aminergic agents. Neuroendocrinology 20:182–192. 10.1159/000122482. [DOI] [PubMed] [Google Scholar]
- 32.Moody CM, Chua B, Weary DM. 2014. The effect of carbon dioxide flow rate on the euthanasia of laboratory mice. Lab Anim 48:298–304. 10.1177/0023677214546509. [DOI] [PubMed] [Google Scholar]
- 33.Moody CM, Makowska IJ, Weary DM. 2015. Testing 3 measures of mouse insensibility following induction with isoflurane or carbon dioxide gas for a more humane euthanasia. Appl Anim Behav Sci 163:183–187. 10.1016/j.applanim.2014.11.010. [DOI] [Google Scholar]
- 34.Moody CM, Weary DM. 2014. Mouse aversion to isoflurane versus carbon dioxide gas. Appl Anim Behav Sci 158:95–101. 10.1016/j.applanim.2014.04.011. [DOI] [Google Scholar]
- 35.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. 2004. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 3:303–314. 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 36.Niel L, Stewart SA, Weary DM. 2008. Effect of flow rate on aversion to gradual-fill carbon dioxide exposure in rats. Appl Anim Behav Sci 109:77–84. 10.1016/j.applanim.2007.02.004. [DOI] [Google Scholar]
- 37.Niel L, Weary DM. 2006. Behavioural responses of rats to gradual-fill CO2 euthanasia and reduced oxygen concentrations. Appl Anim Behav Sci 100:295–308. 10.1016/j.applanim.2005.12.001. [DOI] [Google Scholar]
- 38.Niel L, Weary DM. 2007. Rats avoid exposure to carbon dioxide and argon. Appl Anim Behav Sci 107:100–109. 10.1016/j.applanim.2006.08.002. [DOI] [Google Scholar]
- 39.Office of Laboratory Animal Welfare. 2015. Public health service policy on humane care and use of laboratory animals. Bethesda (MD): National Institutes of Health. [Google Scholar]
- 40.Poole TB, Morgan HD. 1975. Aggressive behaviour of male mice (Mus musculus) towards familiar and unfamiliar opponents. Anim Behav 23:470–479. 10.1016/0003-3472(75)90096-2. [DOI] [PubMed] [Google Scholar]
- 41.Raff H, Roarty TP. 1988. Renin, ACTH, and aldosterone during acute hypercapnia and hypoxia in conscious rats. Am J Physiol 254:R431–R435. [DOI] [PubMed] [Google Scholar]
- 42.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London: Methuen. [Google Scholar]
- 43.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, Greetfeld M, Uhr M, Holsboer F, Müller MB. 2007. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology 32:417–429. 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Spiacci A, Jr, Vilela-Costa HH, Sant'Ana AB, Fernandes GG, Frias AT, da Silva GSF, Antunes-Rodrigues J, Zangrossi H., Jr 2018. Panic-like escape response elicited in mice by exposure to CO2, but not hypoxia. Prog Neuropsychopharmacol Biol Psychiatry 81:178–186. 10.1016/j.pnpbp.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Müller MB, Schmidt MV. 2008. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav 53:386–394. 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Thomas AA, Flecknell PA, Golledge HDR. 2012. Combining nitrous oxide with CO2 decreases the time to loss of consciousness during euthanasia in mice—refinement of animal welfare? PLoS One 7:1–8. 10.1371/journal.pone.0032290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. 2005. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–E828. 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 49.Valentine H, Williams WO, Maurer KJ. 2012. Sedation or inhalant anesthesia before euthanasia with CO2 does not reduce behavioral or physiologic signs of pain and stress in mice. J Am Assoc Lab Anim Sci 51:50–57. [PMC free article] [PubMed] [Google Scholar]
- 50.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, Welsh MJ, Wemmie JA. 2009. The amygdala is a chemosensor that detects CO2 and acidosis to elicit fear behavior. Cell 139:1012–1021. 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]