Abstract
RNase 7 is a skin-derived antimicrobial peptide expressed in various epithelial tissues. It is upregulated by stimulation with microbes and pro-inflammatory cytokines. Herein, we examined the expression levels of RNase 7 in tissues from normal and inflamed oral epithelia and salivary glands via immunohistochemistry. RNase 7 was expressed mainly in the surface layers of the parakeratinized and orthokeratinized oral epithelium. In addition, it was strongly and weakly expressed in oral lichen planus and radicular cysts, respectively. RNase 7 was constitutively expressed in salivary glands, particularly in the serous and duct cells. In the case of Sjogren’s syndrome, RNase 7 was strongly expressed in serous, ductal, and mucous cells in areas with lymphocytic infiltration. The localization patterns of RNase 7 were similar to those of other epithelial antimicrobial peptides, including beta-defensins. Thus, epithelial antimicrobial peptides may act against microbial infections in a coordinated manner in oral epithelia and salivary glands.
Keywords: RNase 7, normal oral epithelium, oral lichen planus, salivary gland
I. Introduction
Oral health status needs to be constantly maintained owing to the continuous exposure of the diverse components of the oral cavity to various microorganisms, including commensal pathogens [21]. This process is guided by the innate immune system, which augments the physical and chemical barriers within the oral epithelium [12, 15]. Rigid intercellular connections between epithelial cells in the stratified squamous epithelia act as a mechanical barrier, and antimicrobial peptides (AMPs) produced by these cells function as chemical barriers against pathogenic organisms [4]. Several AMPs have been detected in the oral epithelium [10].
RNase 7, an AMP with ribonuclease activity, belongs to the RNase A superfamily and was first identified in healthy skin [16]. It is abundantly expressed in keratinocytes derived from skin along with other types of epithelial AMPs such as human beta-defensins (hBD), psoriasin, cathelicidin (LL-37), and calprotectin [25]. AMPs are mainly localized in the upper spinous and stratum corneum of the stratified squamous epithelium in healthy skin [24, 29]. RNase 7 has been detected in oral epithelial cells [12, 20]; nonetheless, details about its localization in oral tissues have not been described in the literature so far. It is not known whether the localization patterns of RNase 7 in the oral mucosa are similar to those in skin. The expression levels of epithelial AMPs differ between oral mucosa and skin [22]; moreover, saliva has been shown to affect these levels in a skin model [19]. Thus, the localization pattern of RNase 7 in the oral epithelium may be different from that in skin.
Epithelial AMPs have been involved in various skin diseases, including inflammatory diseases [11]. The expression levels of several epithelial AMPs, such as hBD-2 and -3, psoriasin, LL-37, and calprotectin, are induced by microbial infection or inflammation [25]. These AMPs are generally increased in inflammatory diseases, but are found to be decreased in several inflammatory skin diseases such as atopic dermatitis and chronic skin ulcer [11]. The decrease in the induction of RNase 7 might not be associated with atopic dermatitis [22].
The expression of the AMPs (hBD-1, -2, -3 and LL-37) in human salivary glands has been investigated in several studies [27, 28]. However, RNase 7 has not been detected in human saliva, and it is not known whether it is expressed in the salivary gland. Numerous AMPs such as lysozyme, lactoferrin, and histatin can be detected in saliva [1], and are crucial for the maintenance of commensal bacteria. Thus, inducible epithelial AMPs, including RNase 7, may exert an antibacterial effect by acting against bacterial infection.
Although localizations of RNase 7 have been reported in normal and diseased skin epithelium, there is little information about the expression of this protein in normal or diseased oral epithelium. Therefore, in the present study, we examined the immunohistochemical localization of RNase 7 in oral tissues, including normal and inflamed oral epithelia as well as the salivary glands. We used immunity disorder-related and non-specific inflamed oral epithelial tissue samples obtained from patients with oral lichen planus and radicular cysts, respectively. Likewise, autoimmune disease associated with inflamed salivary gland tissues were used from patients with Sjogren’s syndrome.
II. Materials and Methods
Specimen collection
This study was conducted at the Department of Oral Medicine and Pathology, Health Sciences University of Hokkaido. Samples were collected from patients irrespective of gender and age. Forty-eight samples of normal oral epithelium (tongue, 15; hard palate, 6; attached gingiva, 5; lips, 12; and buccal mucosa, 10) from the healthy margins of excised fibrous polyps, 20 samples from buccal mucosa of individuals with oral lichen planus, 25 from radicular cysts, 15 from non-inflamed salivary gland tissues (minor salivary glands, 5; parotid, 5; and sublingual glands, 5) adjacent to excised mucous cysts or pleomorphic adenomas, and 8 from inflamed salivary gland tissues in the labial glands of Sjogren’s syndrome patients were collected. The paraffin-embedded blocks were cut into sections of 4 μm thickness. Histological examinations were done by using Harri’s haematoxylin and eosin (H/E) staining method.
Ethics
Approval for the study was obtained from the ethics review board of the Institute of Personalized Medical Science, Health Sciences University of Hokkaido (2012-005). All the patients were informed and consents were taken during collection of the samples.
Immunohistochemistry (IHC)
Paraffin-embedded sections (thickness, 4 μm) were deparaffinized in xylene, rehydrated in 100%, 95%, and 70% ethanol for 5 min each, and rinsed with demineralized water. Antigen binding sites were exposed using the heat-induced antigen retrieval method in a pressure cooker containing citrate buffer (pH, 6.0). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. The primary antibody (anti-Ribonuclease 7 antibody [CL0224]; dilution, 1:50; catalog number ab 154143; Abcam Cambridge, U.K) was added and the sections were incubated for one hour at 37°C. After washing with PBS, 100 μL of secondary antibody (horse radish peroxide-labelled polymer anti-mouse antibody; Dako EnVision + System, Dako North America, Inc. 6392 Via Real Carpinteria, CA 93013 USA) was added to the sections, which were then incubated for 30 min at 37°C. The sections were visualized with DAB (3,3-diaminobenzidine; code K3468; Dako North America, Inc. 6392 Via Real Carpinteria, CA 93013 USA) for 3 min at room temperature, followed by immersion in distilled water to stop overstaining. Subsequently, they were counterstained with hematoxylin, dehydrated, and mounted in malinol. Normal rabbit serum was used instead of primary antibody for the negative controls. The sections were observed under a light microscope (Olympus BX 50, Olympus Corporation, Japan) and the expression levels of RNase 7 were evaluated with respect to the intensity of staining showing brown color. The findings were interpreted using the following scores: negative, no reactivity; weakly positive, low intensity of brown color; positive, immunoreactivity showing brown-colored staining of moderate intensity; and strongly positive, strong intensity of brown-colored staining.
III. Results
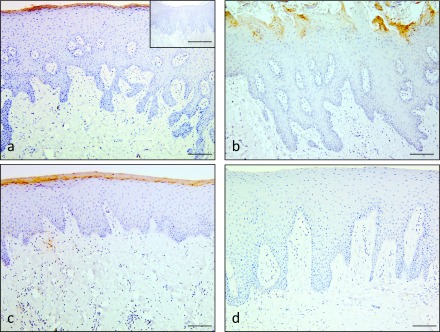
The superficial layers of all the hard palate (Fig. 1a), dorsum of tongue (Fig. 1b), and attached gingiva (Fig. 1c) showed positive staining for RNase 7. No immunohistochemical staining was observed in the negative control (insets in Fig. 1a). No positive staining was observed in the non-keratinized epithelial tissues of the buccal and lips mucosa (Fig. 1d; Table 1).
Fig. 1.
Immunohistochemical staining for RNase 7 in normal oral epithelium. Positive staining for RNase 7 was observed in the surface layers of hard palate (a), tongue (b), and attached gingiva (c). No immunoreaction was detected in the normal epithelium of buccal mucosa (d). Negative control showed negative staining (inset of a). Bars = 100 μm (a–d); 100 μm (insets of a).
Table 1. .
Localization of RNase 7 in normal oral epithelium
| Tissue types | Location | Grading |
|---|---|---|
| Parakeratinized | ||
| Palate (hard) | aSurface layers of parakeratinized epithelium | + (positive) |
| Tongue (dorsum) | aSurface layers of parakeratinized epithelium | + (positive) |
| Attached gingiva | aSurface layers of parakeratinized epithelium | + (positive) |
| Non-keratinized | ||
| Buccal mucosa | bnone | − (negative) |
| Lip mucosa | bnone | − (negative) |
a: Positive staining in surface layers of parakeratinized epithelium in 6/6 of hard palate, 15/15 of tongue and 5/5 of attached gingiva specimens.
b: No positive staining seen in buccal and lip mucosa.
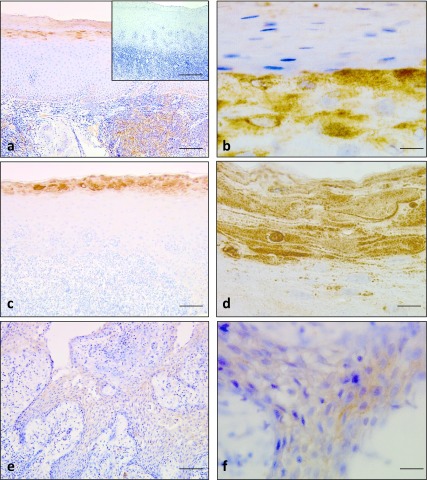
Expression of RNase 7 was observed in the inflamed oral epithelia of oral lichen planus. The surface layers in the orthokeratinized/parakeratinized epithelia demonstrated strong immunoreactions for RNase 7 (Fig. 2a, 2c). The granular cell layers in orthokeratinized epithelium were also positively stained with RNase 7; the staining was detected in the cytoplasm of the cells in these layers (Fig. 2b). In the parakeratinized epithelium, positive staining was observed in the pyknotic nuclei of the parakeratinized cells. (Fig. 2d). The positive dots were often seen beneath the surface layers of the parakeratinized epithelium (Fig. 2d). No immunoreactions for RNase 7 were noted in the negative controls (inset in Fig. 2a; Table 2)
Fig. 2.
Immunohistochemical staining for RNase 7 in oral lichen planus and radicular cyst. Intense immunostaining was observed in the surface layers and granular layers of oral lichen planus (a) and (b). Negative control showed negative staining (inset of a). Strong immunoreactions were observed in the surface layers, pyknotic nuclei and positive dots for RNase 7 (c) and (d). Faint staining for RNase 7 in the epithelial lining of the radicular cyst (e) and (f). Bars = 100 μm (a, c, e); 100 μm insets of (a); 10 μm (b, d & f).
Table 2. .
Expression of RNase 7 in inflamed oral epithelia from oral lichen planus and radicular cyst
| Tissue types | Location | Grading |
|---|---|---|
| Oral lichen planus | aSurface layers and granular layers of orthokeratinized epithelium | + ~ ++ (positive ~ strongly positive) |
| aSurface layers and pyknotic nuclei of parakeratinized epithelium | + ~ ++ (positive ~ strongly positive) | |
| Radicular cyst | bSurface of lining epithelium | − ~ ± (weakly positive) |
a: Out of 20 cases of oral lichen planus, 8 showed strongly positive staining in surface layers and granular layers of orthokeratinized epithelium. 2 cases showed weak intensity and 10 cases showed strong intensity of staining in surface layers and pyknotic nuclei of parakeratinized epithelium.
b: Only 3/25 cases of radicular cyst showed weak intensity of positivity in the lining epithelium.
The inflamed oral epithelia obtained from radicular cysts showed weakly positive staining for RNase 7 in the lining epithelium (Fig. 2e; Table 2).
In the non-inflamed salivary glands, RNase 7 was expressed in the serous cells (Fig. 3a, 3b) and duct cells (Fig. 3c, 3d), whereas no positive staining was noted in the mucous cells (Fig. 3c, 3d). Positive staining for RNase 7 was observed in the serous demilunes and weak expression in the ductal cells of the labial glands (Fig. 3e, 3f; Table 3). No immunoreactions for the protein were noted in the negative controls (inset in Fig. 3a). Sebaceous glands are sometimes observed in the salivary glands [13]. We found some sebaceous glands in the non-inflamed salivary glands.
Fig. 3.
Immunohistochemical staining for RNase 7 in non-inflamed salivary glands. Positive immunostaining was observed in the serous cells of parotid glands (a) and (b). Negative control showed negative staining (insets of a). Weak staining of RNase 7 in duct cells of sublingual glands in (c) and (d). Positive immunoreaction was observed in serous demilune and faint staining in duct cells of labial glands (e) and (f). Bars = 100 μm (a, c & e); 100 μm insets of (a); 10 μm (b, d & f).
Table 3. .
Expression of RNase 7 in different areas of the salivary gland
| Tissue types | Location | Grading |
|---|---|---|
| Major salivary gland | ||
| Parotid gland | aserous cells | ± ~ + (weakly positive ~ positive) |
| aduct cells | ± (weakly positive) | |
| asebaceous gland | + ~ ++ (positive ~ strongly positive) | |
| Sublingual gland | bduct cells | ± (weakly positive) |
| Minor salivary gland | ||
| Labial gland | cserous demilune | ± ~ + (weakly positive ~ positive) |
| cduct cells | ± (weakly positive) |
a: In parotid gland, 5/5 cases demonstrated positive staining in serous and duct cells. Sebaceous gland present in parotid gland was strongly positive.
b: 4/5 cases of sublingual gland showed weak intensity of staining in duct cells.
c: 5/5 cases of minor salivary gland demonstrated positive staining in serous demilune and weakly positive staining in ductal cells of labial gland.
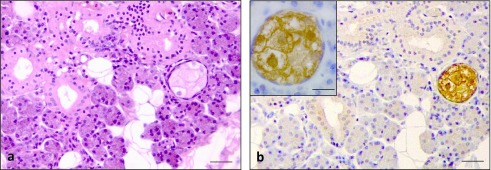
The sebaceous glands showed a pale staining of cytoplasm with shrunken or absence of nuclei in the mature sebaceous cells. Immature sebaceous cells at the periphery of sebaceous gland showed rounded nuclei (Fig. 4a). Strong staining for RNase 7 was observed in the cytoplasm of sebaceous glands (Fig. 4b; Table 3).
Fig. 4.
H/E staining and immunohistochemical staining for RNase 7 in sebaceous glands often observed in non-inflamed salivary glands. H/E staining showed a pale staining of cytoplasm with shrunken or absence of nuclei in the mature sebaceous cell at the center of sebaceous gland. Immature sebaceous cells at the periphery of sebaceous gland showed rounded nuclei (a). Intense immunoreaction for RNase 7 in the cytoplasm of the sebaceous glands (b) & inset of (b). Bars = 100 μm (a & b); 10 μm insets of (b).
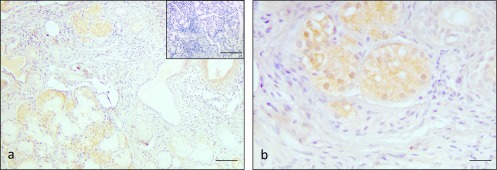
In the inflamed salivary glands obtained from the labial glands of patients with Sjogren’s syndrome, RNase 7 was strongly expressed in the mucous and ductal cells (Fig. 5a, 5b). The expression levels of RNase 7 were higher in the inflamed salivary glands when compared with the non-inflamed salivary glands (Table 4). No immunoreactions were noted in the negative controls (inset in Fig. 5a).
Fig. 5.
Immunohistochemical staining for RNase 7 in inflamed salivary glands of Sjogren’s syndrome. Intense immunostaining was observed in the serous demilune, mucous cells and duct cells of the inflammatory area (a) and (b). Negative control showed negative staining (insets of a). Bars = 100 μm (a); 100 μm insets; 20 μm (b).
Table 4. .
Expression of RNase 7 in inflamed areas of the salivary gland
| Tissue type | Location | Grading |
|---|---|---|
| Sjogren’s syndrome | aserous demilune | + ~ ++ (positive ~ strongly positive) |
| amucous cells | + ~ ++ (positive ~ strongly positive) | |
| aduct cells | + ~ ++ (positive ~ strongly positive) |
a: In Sjogren’s syndrome, 8/8 cases showed strongly positive staining of RNase 7 in serous demilune, mucous cells and duct cells.
IV. Discussion
The present study is the first to report the distribution of RNase 7 in different sites within normal and inflamed oral epithelia and salivary glands. Normal oral epithelium in the dorsum of the tongue, attached gingiva, and hard palate demonstrated positive staining for the peptide, primarily in the surface layers of the epithelium. Expression of RNase 7 in the surface layers of normal oral epithelium was similar to that of healthy skin [24]. In the buccal mucosa, no immunopositive staining for RNase 7 was seen. Localization of RNase7 in the surface layers of the parakeratinized epithelium was similar to that of hBD-1, -2, and -3 [3, 9]. Since the keratinized layer acts as a barrier against the penetration of tracers [26], RNase 7 may be deposited in these layers. Although expression of RNase 7 at both mRNA and protein levels has been shown in the keratinocytes [16, 17], there are some possible reasons why no immunopositive for RNase 7 in non-keratinized oral epithelium. In a previous study, we were able to detect the mRNA level of hBD-2 in non-keratinized oral epithelium via in-situ hybridization, but not the hBD-2 peptide by immunohistochemistry [3], and speculated that the peptide may have diffused out without being deposited in the keratinized layers due to its low molecular weight. The molecular weight of RNase 7 is low at 14.5 kD [6], and possibly released through cellular transport from the non-keratinized oral epithelium. The expression levels of hBDs increase following keratinocyte differentiation [3, 17]. Since the immunohistochemical expression pattern of RNase 7 is similar to those of the hBDs, less differentiation may indicate lower levels of RNase 7. In fact, the mRNA and protein expression levels of RNase 7 were higher in differentiating epidermal keratinocytes when compared to proliferating keratinocytes in cultured epidermal keratinocytes [5]. Low levels of RNase 7 expression may not be detected via immunohistochemistry. Further investigations are required to clarify the phenomenon.
The keratinized layer, owing to the presence of beta-defensins and RNase 7, may have more defensive mechanisms against bacterial infection on the surface of the oral epithelium. We also observed localization of RNase 7 in inflamed oral epithelium using samples from oral lichen planus and radicular cyst. The keratinized layer in oral lichen planus showed strongly positive staining for RNase 7 as expected; and positive staining was also observed in the granular layers of orthokeratinized epithelium in some of the lichen planus specimens. The granular layers are not prominent in parakeratinized epithelia; nonetheless, dispersed keratohyalin granules can be detected beneath the surface layers of this epithelium [14]. Therefore, we speculated that the positively-stained dots in this epithelium in the current study may represent the dispersed keratohyalin granules. RNase 7 expression was observed in a few of the radicular cyst specimens. The expression of RNase 7 in the spinous layers may be attributed to inflammatory stimulations [6]. Inflammation in lichen planus and radicular cysts are caused by T cell-specific stimulations and bacterial infections, respectively. The frequency of positive reactions in the spinous layers of lichen planus was higher than that in the radicular cysts. Similar results pertaining to the expression of hBD-2 have been reported in lichen planus and radicular cyst [2]. Several cytokines including interleukin (IL)-6, IL-17, IFN-γ, and TNF-α are increased in T cell-specific inflammatory conditions [8]. RNase 7 expression levels are upregulated by stimulation with IFN-γ, and TNF-α [21]. T cell-specific stimulation may induce the expression of both RNase 7 and hBD-2 to a greater degree than bacterial infections do; however, further investigations are required to prove this theory.
We observed the expression of RNase 7 in non-inflamed salivary glands. RNase 7 was detected in certain parts of normal salivary glands (no inflammation), indicating that it may be constitutively expressed in salivary glands. To the best of our knowledge, this is the first study to demonstrate the localization of this peptide in salivary gland tissues. Our findings are consistent with a previous study where mRNA expression of RNase 7 was reported in salivary glands [16]. AMPs including hBD-1, -2, and -3, lysozyme, lactoferrin, and cathelicidin were detected in the labial glands [1, 27]. Localizations of hBD and cathelicidin have been observed in serous acini and intralobular ducts, whereas lysozyme and lactoferrin localizations were noted in serous acini and demiluni cells. The localization profile of RNase 7 is similar to that of hBD, cathelicidin, lysozyme and lactoferrin. No AMPs have been detected in the mucous acini. In one study, salivary mucins were shown to inhibit the activity of LL-37 [7], whereas in another study, hBD-1 expression was masked by salivary mucins [23], which could result in the inhibition of the immunoreactivities of AMPs in mucous acini. In addition to acini, sebaceous glands are sometimes observed in the normal salivary glands [13]. We found positive staining for RNase 7 in the cytoplasm of the sebaceous glands. These findings are consistent with a previous study that shows the positive staining for sebaceous glands in the skin [18]. The RNase 7 expressed by the sebaceous glands may contribute to innate immunity of the skin. It is not known how small number of sebaceous glands contribute to it in the salivary glands. Further investigations need to clarify this phenomenon.
In Sjogren’s syndrome, RNase 7 was strongly expressed in mucous cells (in areas with lymphocytic infiltration) and also in the ductal and serous demilune. RNase 7 expression can be induced by stimulation of inflammatory cytokines [16]; thus, lymphocytic infiltration may have stimulated the expression of RNase 7 in the mucous cells in the current study.
In conclusion, this study demonstrated the localization of RNase 7 peptide within different sites in normal and inflamed oral epithelia and salivary glands. The localization patterns of RNase 7 were similar to that of other epithelial AMPs, including beta-defensins. The epithelial AMPs may act against microbial infections in a coordinated manner in the oral epithelia and salivary glands. Nevertheless, further studies investigating these cooperative mechanisms are warranted.
V. Conflicts of Interest
The authors declare that there are no conflicts of interest.
VI. References
- 1.Abiko Y. and Saitoh M. (2007) Salivary defensins and their importance in oral health and disease. Curr. Pharm. Des. 13; 3065–3072. [DOI] [PubMed] [Google Scholar]
- 2.Abiko Y., Jinbu Y., Noguchi T., Nishimura M., Kusano K., Amaratunga P., Shibata T. and Kaku T. (2002) Upregulation of human beta-defensin 2 peptide expression in oral lichen planus, leukoplakia and candidiasis. An immunohistochemical study. Pathol. Res. Pract. 198; 537–542. [DOI] [PubMed] [Google Scholar]
- 3.Abiko Y., Nishimura M., Kusano K., Yamazaki M., Arakawa T., Takuma T. and Kaku T. (2003) Upregulated expression of human beta defensin-1 and -3 mRNA during differentiation of keratinocyte immortalized cell lines, HaCaT and PHK16-0b. J. Dermatol. Sci. 1; 225–228. [DOI] [PubMed] [Google Scholar]
- 4.Abiko Y., Saitoh M., Nishimura M., Yamazaki M., Sawamura D. and Kaku T. (2007) Role of beta-defensins in oral epithelial health and disease. Med. Mol. Morphol. 40; 179–184. [DOI] [PubMed] [Google Scholar]
- 5.Abtin A., Eckhart L., Mildner M., Ghannadan M., Harder J., Schroder J. M. and Tschachler E. (2009) Degradation by stratum corneum proteases prevents endogenous RNase inhibitor from blocking antimicrobial activities of RNase 5 and RNase 7. J. Invest. Dermatol. 129; 2193–2201. [DOI] [PubMed] [Google Scholar]
- 6.Becknell B. and Spencer J. D. (2016) A review of ribonuclease 7’s structure, regulation, and contributions to host defense. Int. J. Mol. Sci. 17; 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucki R., Namiot D. B., Namiot Z., Savage P. B. and Janmey P. A. (2008) Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J. Antimicrob. Chemother. 62; 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrozzo M. (2014) Understanding the pathobiology of oral lichen planus. Curr. Oral Health Rep. 1; 173–179. [Google Scholar]
- 9.Dale B. A., Kimball J. R., Krisanaprakornkit S., Roberts F., Robinovitch M., O’Neal R., Valore E. V., Ganz T., Anderson G. M. and Weinberg A. (2001) Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 36; 285–294. [DOI] [PubMed] [Google Scholar]
- 10.Diamond G., Beckloff N. and Ryan L. K. (2008) Host defense peptides in the oral cavity and the lung: similarities and differences. J. Dent. Res. 87; 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doss M., White M. R., Tecle T. and Hartshorn K. L. (2010) Human defensins and LL-37 in mucosal immunity. J. Leukoc. Biol. 87; 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhard J., Menzel N., Dommisch H., Winter J., Jepsen S. and Mutters R. (2008) The stage of native biofilm formation determines the gene expression of human β-defensin-2, psoriasin, ribonuclease 7 and inflammatory mediators: a novel approach for stimulation of keratinocytes with in situ formed biofilms. Oral Microbiol. Immunol. 23; 21–28. [DOI] [PubMed] [Google Scholar]
- 13.Ellis, G. L., Auclair, P. L., Gnepp, D. R. (ed) (1991) Surgical Pathology of the Salivary Gland. Major Problems in Pathology in the Series. 1 ed (Vol. 25), W.B. Saunders Co., Philadelphia, pp. 261–262. [Google Scholar]
- 14.Fiorellini J. P., Kao D. W. K, Kim D. M. and Uzel N. G. (2011) Anatomy of the periodontium. In “Carranza’s Clinical Periodontology”, 11th edition, Elsevier Saunders, St. Louis, Missouri, pp. 16. [Google Scholar]
- 15.Ganz T. (2004) Defensins: antimicrobial peptides of vertebrates. C. R. Biol. 327; 539–549. [DOI] [PubMed] [Google Scholar]
- 16.Harder J. and Schroder J. M. (2002) RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 277; 46779–46784. [DOI] [PubMed] [Google Scholar]
- 17.Harder J., Meyer-Hoffert U., Wehkamp K., Schwichtenberg L. and Schroder J. M. (2004) Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J. Invest. Dermatol. 123; 522–529. [DOI] [PubMed] [Google Scholar]
- 18.Koten B., Simanski M., Glaser R., Podschun R., Schroder J. M. and Harder J.. RNase 7 contributes to the cutaneous defense against Enterococcus faecium. PLoS One 4; e6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohanty T., Alberius P., Schmidtchen A., Reiss K., Schröder J. M. and Sorensen O. E. (2017) Saliva induces expression of antimicrobial peptides and promotes intracellular killing of bacteria in keratinocytes by epidermal growth factor receptor transactivation. Br. J. Dermatol. 176; 403–412. [DOI] [PubMed] [Google Scholar]
- 20.Neopane P., Yoshida K., Adhikari B. R., Harada F., Paudel D., Morikawa T., Onishi A., Hiraki D., Sato J., Nishimura M. and Abiko Y. (2017) Epithelium-derived antimicrobial peptide RNase 7 in oral health and disease: A mini-review. Dent. J. Health Sci. Univ. Hokkaido 36; 27–33. [Google Scholar]
- 21.Presland R. B. and Dale B. A. (2000) Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit. Rev. Oral Biol. Med. 11; 383–408. [DOI] [PubMed] [Google Scholar]
- 22.Rademacher F., Simanski M. and Harder J. (2016) RNase 7 in cutaneous defense. Int. J. Mol. Sci. 17; 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahasrabudhe K. S., Kimball J. R., Morton T. H., Weinberg A. and Dale B. A. (2000) Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. J. Dent. Res. 79; 1669–1674. [DOI] [PubMed] [Google Scholar]
- 24.Scola N., Gambichler T., Saklaoui H., Bechara F. G., Georgas D., Stucker M., Glaser R. and Kreuter A. (2012) The expression of antimicrobial peptides is significantly altered in cutaneous squamous cell carcinoma and precursor lesions. Br. J. Dermatol. 167; 591–597. [DOI] [PubMed] [Google Scholar]
- 25.Simanski M., Rademacher F., Schroder L., Schumacher H. M., Glaser R. and Harder J. (2013) IL-17A and IFN-γ synergistically induce RNase 7 expression via STAT3 in primary keratinocytes. PLoS One 8; e59531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squier C. A. (1991) The permeability of oral mucosa. Crit. Rev. Oral Biol. Med. 2; 13–32. [DOI] [PubMed] [Google Scholar]
- 27.Stoeckelhuber M., Loeffelbein D. J., Olzowy B., Schmitz C., Koerdt S. and Kesting M. R. (2016) Labial salivary glands in infants: histochemical analysis of cytoskeletal and antimicrobial proteins. J. Histochem. Cytochem. 64; 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svensson D., Aidoukovitch A., Anders E., Agerberth B., Andersson F., Ekblad E., Ericson D., Nebel D., Voss U. and Nilsson B. O. (2018) The host defense peptide LL-37 is detected in human parotid and submandibular/sublingual saliva and expressed in glandular neutrophils. Eur. J. Oral Sci. 126; 93–100. [DOI] [PubMed] [Google Scholar]
- 29.Wittersheim M., Cordes J., Meyer-Hoffert U., Harder J., Hedderich J. and Glaser R. (2013) Differential expression and in vivo secretion of the antimicrobial peptides psoriasin (S100A7), RNase 7, human beta-defensin-2 and -3 in healthy human skin. Exp. Dermatol. 22; 364–366. [DOI] [PubMed] [Google Scholar]