Abstract
Background
Non-invasive stimulation of the vagus nerve has been proposed as a new neuromodulation therapy to treat primary headache disorders, as the vagus nerve is hypothesized to modulate the headache pain pathways in the brain. Vagus nerve stimulation can be performed by placing an electrode on the ear to stimulate the tragus nerve, which contains about 1% of the vagus fibers. Non-invasive vagus nerve stimulation (nVNS) conventionally refers to stimulation of the cervical branch of the vagus nerve, which is made up entirely of vagal nerve fibers. While used interchangeably, most of the research to date has been performed with nVNS or an implanted vagus nerve stimulation device. However, the exact mechanism of action of nVNS remains hypothetical and no clear overview of the effectiveness of nVNS in primary headache disorders is available.
Methods
In the present study, the clinical trials that investigated the effectiveness, tolerability and safety of nVNS in primary headache disorders were systematically reviewed. The second part of this study reviewed the central connections of the vagus nerve. Papers on the clinical use of nVNS and the anatomical investigations were included based on predefined criteria, evaluated, and results were reported in a narrative way.
Results
The first part of this review shows that nVNS in primary headache disorders is moderately effective, safe and well-tolerated. Regarding the anatomical review, it was reported that fibers from the vagus nerve intertwine with fibers from the trigeminal, facial, glossopharyngeal and hypoglossal nerves, mostly in the trigeminal spinal tract. Second, the four nuclei of the vagus nerve (nuclei of the solitary tract, nucleus ambiguus, spinal nucleus of the trigeminal nerve and dorsal motor nucleus (DMX)) show extensive interconnections. Third, the efferents from the vagal nuclei that receive sensory and visceral input (i.e. nuclei of the solitary tract and spinal nucleus of the trigeminal nerve) mainly course towards the main parts of the neural pain matrix directly or indirectly via other vagal nuclei.
Conclusion
The moderate effectiveness of nVNS in treating primary headache disorders can possibly be linked to the connections between the trigeminal and vagal systems as described in animals.
Keywords: Afferent pathways, anatomy, efferent pathways, headache disorders, histology, vagus nerve, vagus nerve stimulation
Introduction
Headache is a common symptom and, collectively, headache disorders have a prevalence of 49% (1). Approximately 95% of the general population have experienced headache during some stage in their life (2). In addition to having a significant impact on patients’ daily life due to pain and disability, the socioeconomic burden of headaches are considerable, with a cumulative burden of $14 billion per year (3). Although the pathophysiological mechanisms involved remain partially elusive, chronic headache is believed to have a neurogenic basis (4–11). For example, a recent fMRI study showed that areas within the hypothalamus and brainstem, including the trigeminal system, were involved in mediating migraine (12). Although various treatments are available for alleviating pain in headache, the results are often unsatisfactory (13–15). Therefore, experimental interventions that treat headache disorders more effectively are being investigated and show promising results. One of the options includes (non-invasive) vagus nerve stimulation (nVNS), which was found to inhibit nociceptive behavior in animals (16–21). In humans, multiple clinical trials have been conducted, showing the clinical relevance of nVNS in treating chronic headache disorders (22–25). However, the underlying mechanism supporting these effects is difficult to elucidate (26–29).
The vagus nerve plays a central role in autonomic, cardiovascular, respiratory, gastrointestinal, immune and endocrine systems (30). Four types of fibers within the vagus nerve have been described: a) The general somatic afferents (GSA); b) the general visceral afferents (GVA); c) the special visceral afferents (SVA); and d) the special visceral efferents (SVE). These afferents and efferents all terminate in the four vagus nuclei within the medulla: a) The nucleus of the solitary tract (NST); b) the nucleus ambiguus (NA); c) the trigeminal spinal nucleus (TSN); and d) the dorsal motor nucleus of the vagus nerve (DMX) (see Table 1) (29). Although the peripheral distribution of the vagus nerve has been well described before by others (for a review see Yuan and Silberstein (2016) and Berthoud and Neuhuber (2000) (29,30)), the subsequent central connections of the vagus nuclei remain opaquely described (31,32). Nevertheless, the central connections are thought to be of pivotal importance to increasing our understanding of nVNS in headache disorders (27–29). Therefore, this study aims to provide an overview of clinical research on nVNS as a treatment of primary headache disorders and to review the anatomical research on connections between the vagus nerve and other brain areas. Together, these overviews will contribute to our understanding of mechanisms involved in alleviating pain in primary headache disorders by use of nVNS.
Table 1.
Distribution of the types of afferents over the four vagus nuclei (adapted from Yuan and Silberstein 2016 (28)).
| Vagus nucleus | Type of afferent/efferent | Function |
|---|---|---|
| TSN | GSA | Sensory information from posterior external auditory meatus, tympanic membrane, dura in posterior fossa, hypopharynx, larynx and upper esophagus |
| NST, rostral part | SVA | Taste sensation from the epiglottis and pharynx |
| NST, caudal part | GVA | Visceral sensory information from hypopharynx, larynx, cardiopulmonary organs, organs of the digestive tract and aortic arch (baroreceptors and chemoreceptors) |
| DMX | GVE | Parasympathetic innervations of abdominal and thoracic organs |
| NA, branchiomotor part | SVE | Visceral motor control of various skeletal muscles of the pharynx, larynx and proximal esophagus |
| NA, external formation | GVE | a) Cardiac ganglia for cardiac inhibition b) Pulmonary ganglia for airway size and secretion regulation |
DMX: dorsal motor nucleus of the vagus nerve; GSA: general sensory afferent; GVA: general visceral afferents; GVE: general visceral efferents; NA: nucleus ambiguus; NST: nucleus of the solitary tract; SVA: special visceral afferents; SVE: special visceral efferents; TSN: trigeminal spinal nucleus.
Material and methods
Methodology for retrieval and assessment of nVNS trials
A systematic literature review concerning VNS in primary headache disorders was conducted by searching for literature until July 2018 on PubMed, Medline, EMBASE and Google Scholar. Search entry terms included: [Vagus Nerve Stimulation]; [Facial Neuralgia]; [Facial Pain]; [Trigeminal Nerve]; [Headache Disorders]; [Headache]; [Trigeminal Autonomic Cephalgia]; [Vagus Nerve]; [Migraine Disorders]; [Cluster Headache]; [Implantable Neurostimulators]; and/or [Electrodes, implanted]. Inclusion criteria for the review on nVNS in primary headache disorders were: a) Studies investigating the effect of nVNS of the cervical main branch of the vagus nerve to treat one or various primary headache disorders; b) study design being either a randomized-controlled trial or an observational study and c) studies that included only human subjects. Exclusion criteria were: a) Articles other than original research papers that did not fulfill the inclusion criteria (i.e. reviews, case reports, post-hoc analyses); b) articles written in other languages than English, Dutch, German or French; and c) articles that tested nVNS in animals. A total of 254 articles were identified by the searches. After removal of duplicates, 209 papers remained. Each paper was reviewed using the inclusion and exclusion criteria by at least two investigators (BD, MvD and/or NV) independently. When in doubt, another investigator (DH) was consulted. After exclusion, a total of 12 articles remained that met the inclusion criteria. From these papers, baseline characteristics, studied disorder, study design, primary and secondary outcomes and concluding remarks were extracted. The methodology, results and conclusions of each paper were assessed by two researchers independently (BD, MvD, NV, and/or DH). The studied population in the VNS review was described by means of the provided tables; primary and secondary outcomes were described in a narrative fashion. Two members of the team (DH and BD) independently reviewed each selected article for risk of bias using the Cochrane criteria checklist (33,34). When no agreement could be achieved between the two reviewers on the quality of the trial, a third reviewer (KV) was used in accordance with Cochrane methodology (33,34). Assessing the risk of bias was performed by the criteria presented in Table 2, following standardized instructions, which have been published before (34–37). Types of biases assessed by these criteria included: Selection bias (criteria 1, 2, 9), performance bias (criteria 3, 4, 10, 11), attrition bias (criteria 6, 7), detection (or measurement) bias (criteria 5, 12) and reporting bias (criterion 8).
Table 2.
Quality assessment of the individual trials.
| Internal validity |
Score | Quality | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors (Ref) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Goadsby et al. (2014) (38) | − | − | − | − | − | + | + | + | − | + | + | + | 6 | Moderate |
| Kinfe et al. (2015) (39) | − | − | − | − | − | + | + | + | + | + | + | + | 7 | Moderate |
| Barbanti et al. (2015) (40) | − | − | − | − | − | + | + | + | + | + | + | + | 7 | Moderate |
| Grazzi et al. (2015) (42) | − | − | − | − | − | + | + | + | + | + | + | + | 7 | Moderate |
| Silberstein et al. (2016) (43) | + | + | + | + | + | − | + | + | + | + | + | + | 11 | High |
| Grazzi et al. (2017) (22) | − | − | − | − | − | − | + | + | + | + | − | + | 5 | Low |
| Tassorelli et al. (2018)* (41) | + | + | + | + | + | + | + | + | + | + | + | + | 12 | High |
| Nesbitt et al. (2015) (44) | − | − | − | − | − | + | + | + | − | + | + | + | 6 | Moderate |
| Gaul et al. (2016) (45) | + | + | − | − | − | + | + | + | + | + | + | + | 9 | Moderate |
| Silberstein et al. (2016) (46) | + | + | + | + | + | + | + | + | + | + | + | + | 12 | High |
| Goadsby et al. (2017) (23) | + | + | + | + | + | + | + | + | + | + | + | + | 12 | High |
| Trimboli et al. (2017) (21) | − | − | − | − | − | − | + | + | + | + | − | + | 5 | Low |
| Tso et al. (2017) (47) | − | − | − | − | − | − | + | + | + | − | + | + | 5 | Low |
1. Was the method of randomization adequate?
2. Was the treatment allocation concealed?
3. Was the patient blinded to the intervention?
4. Was the care provider blinded to the intervention?
5. Was the outcome assessor blinded to the intervention?
6. Was the dropout rate described and acceptable?
7. Were all randomized participants analyzed in the group to which they were allocated?
8. Are reports of the study free of suggestion of selective outcome reporting?
9. Were the groups similar at baseline regarding the most important prognostic indicators?
10. Were co-interventions avoided or similar?
11. Was the compliance acceptable in all groups?
12. Was the timing of the outcome assessment similar in all groups?
+, criterion achieved; −, criterion not achieved; ∗, assessors initially disagreed.
High: > 75% of the criteria have been fulfilled (≥ 10/12). Where they have not been fulfilled, the conclusions of the study or review are thought very unlikely to have been altered.
Moderate: 50–75% of the criteria have been fulfilled (6–9/12). Those criteria that have not been fulfilled or not adequately described are thought unlikely to have altered the conclusions.
Low: Less than 50% of the checklist criteria were fulfilled (< 6/12). The conclusions of the study are thought likely or very likely to alter had those criteria been fulfilled.
Methodology for retrieval and assessment of anatomical papers
Regarding the anatomical review, various anatomical atlases and textbooks (31,32,38–42) were used to create a preliminary overview of vagus nerve anatomy and central connectivity. Thereafter, cross-referencing was carried out in order to enrich the anatomical results. PubMed, Medline, EMBASE and Google Scholar were searched using: [Vagus Nerve]; [Dorsal Motor Nucleus]; [Ambiguus Nucleus]; [Trigeminal Spinal Nucleus]; [Nucleus of the Solitary Tract]; [Anatomy]; [Neural Pathways]; [Afferent Pathways]; and [Efferent Pathways]. Medical subject headings were used to enrich the results. All searches were conducted until July 2018. Inclusion criteria were: a) Anatomy of the central portion of the vagus nerve was discussed; b) studies implemented techniques like tracing studies, dissections or neuroimaging; and c) investigation was carried out in either humans or animals. Exclusion criteria were: a) Articles were other than original research papers (i.e. reviews); b) articles were written in other languages than English, Dutch, German or French; c) articles investigated the peripheral portion of the vagus nerve; and d) articles investigated the anatomy by use of computational modeling. Each paper was reviewed using the inclusion and exclusion criteria by two investigators (BD, MvD and/or NV) independently. When in doubt, a third investigator (DH) was consulted. A total of 507 articles were retrieved, 358 of which remained after the removal of duplicates. Finally, 94 anatomical papers could be included based on the inclusion and exclusion criteria. Each paper was assessed by two researchers independently (BD, MvD, NV and/or DH). Findings were grouped per vagus nucleus, afferent pathway or efferent pathway and presented narratively and summarized in schematic figures.
Results
nVNS in primary headache disorders
Twelve clinical trials were included, representing a total of 866 patients who underwent the treatments as defined per protocol. Sex of the patients was reported in 836 cases (373 males; 44.6%). Primary headache disorders that were treated with nVNS constituted migraine (n = 469), cluster headache (n = 376), and other trigeminal autonomic cephalgias, including hemicrania continua (n = 13), paroxysmal hemicrania (n = 6) and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA) (n = 2). In episodic migraine, it was found that nVNS elicited a significant pain reduction (22,43–47). One paper described a significant reduction of the number of headache days per month (44), although the number of headache days or tolerability of the attacks were not influenced by nVNS according to another paper (48). nVNS also has been described as capable of reducing the intake of rescue medication in cases of migraine attack (23). In cluster headaches, nVNS improved the mean overall condition of the patients (22,49), significantly reduced weekly attack frequency (50) and induced significantly higher pain-free rates with active nVNS compared with sham nVNS (24,50,51). In hemicrania continua, the majority of patients reported reduced severity of continuous pain (22,52). A more modest, but similar trend was observed in patients suffering from paroxysmal hemicrania (52). Neither of the patients suffering from SUNA benefited from nVNS therapy (22). The quality assessment of the individual trials is provided in Table 2. An extensive overview of the included papers is given in Supplemental Tables 1 and 2.
Anatomical characteristics of the vagal central connections
Central connections of the NST
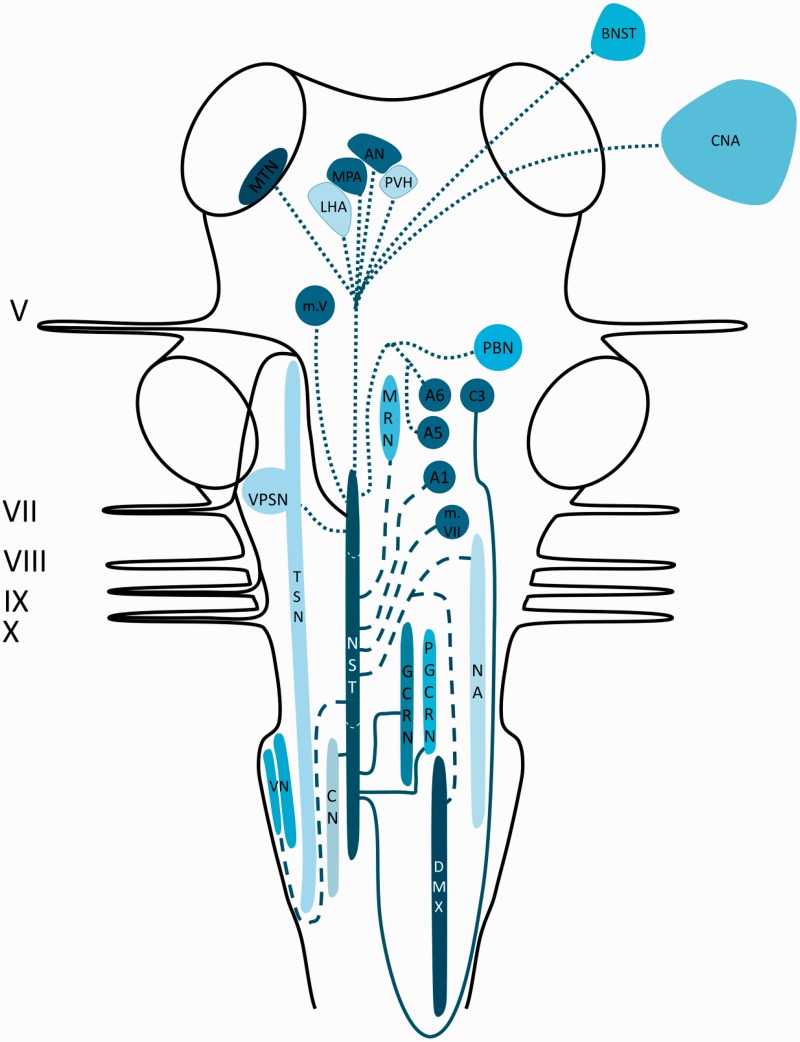
The NST has been reported to receive afferents from the vagus nerve, the facial nerve, the trigeminal nerve and the glossopharyngeal nerve (53–55). Labeled fibers of the vagus nerve could be traced bilaterally to the NST and ipsilaterally to the external cuneate nucleus. However, the majority of the fibers that headed to the external cuneate nucleus continued and entered the solitary tract (56,57). Projections from the NST terminated in the vagal preganglionic motor neurons of the DMX and the NA (58,59). A prominent projection traversed towards the dorsomedial reticular formation and several medullary nuclei (i.e. the dorsal medullary raphe nuclei (60), the gigantocellular reticular nucleus and the paragigantocellular nucleus (60,61)). From the NST, fibers were observed to terminate in the dorsal or ventral part of the dorsal raphe nuclei, the locus coeruleus (A6 noradrenergic cell groups) (61), the A1 and A5 noradrenergic cell groups (61–63), the dorsal medial medulla (C3 adrenergic cell groups) (57), the lateral paragigantocellular reticular nucleus (C1 adrenergic cell groups) (61) and the medial and lateral parabrachial nuclei (58,59,64,65). Together with the parabrachial nuclei, the A1 and A5 catecholamine cell groups were reported to receive input from the NST and together they are believed to play a role as relay nuclei for sensory and gustatory information, which is processed in different areas of the forebrain. From the caudal part of the NST, projections arise that travel to the vagal preganglionic neurons in the DMX (66–68) and the NA (58,59,63,69), the main sites of vagal motor neurons. Each area that receives a direct projection from the NST, with the exception of the motor nuclei of the cranial nerves V, VII, X and XII, projects back to the DMX. In addition, projections have been described to the motor nuclei of the trigeminal, the facial and the hypoglossal nerves. These motor nuclei have an important role in the motor functions of the ingestive behavior (63,65). Furthermore, the trigeminal sensory nucleus complex (including the principal sensory nucleus and the TSN) has connections with the NST (57). Ipsilateral vestibular afferents were labeled and could be traced via two different routes: a) Fibers that traveled within the medial vestibular nucleus before entering the NST (this route is known as the lateral pathway), and b) fibers that traveled within the nucleus prepositus hypoglossi to its caudal part, whereupon they entered the NST (this route is known as the medial pathway). Besides, labeled fibers from the caudal part of the medial vestibular nucleus and the inferior vestibular nucleus could be traced bilaterally to the NST (70–72). Another tract originating from the NST occupies a region known as the intermediate reticular zone as it courses towards the intermediolateral nucleus (64). Finally, there is a group of projections that ascend from the NST to thalamic, hypothalamus and other limbic regions (e.g. the midline thalamic nuclei (73,74), the bed nucleus of the stria terminalis (59), the central nucleus of the amygdala (59), lateral hypothalamic area (75), paraventricular hypothalamic nucleus (59,63,76), the arcuate nucleus (73) and the medial preoptic area (75,77)). The aforementioned connections are depicted in Figure 1.
Figure 1.
Schematic overview of the central projections of the NST. Latin numbers indicate cranial nerves or their corresponding nuclei; color of the tract is consistent with the color of the nucleus/area it sprouts from.
AN: arcuate nucleus; A1: noradrenergic cell group in the adjacent to the lateral reticular nucleus and the medullary reticular formation; A5: noradrenergic cell group in the adjacent to the superior olivary complex in the pontine tegmentum; A6: noradrenergic cell group that together forms the locus coeruleus; BNST: bed nucleus of stria terminalis; CN: cuneate nucleus; CNA: central nucleus of amygdala; C3: adrenergic cell group in the dorsal midline of the rostral medulla; DMX: dorsal motor of X; GCRN: gigantocellular reticular nucleus; LHA: lateral hypothalamic area; MPA: medial preoptic area; MRN: medullary raphe nuclei; MTN: Midline thalamic nuclei; m.V: motor nucleus of V; m.VII: motor nucleus of VII; NA: nucleus ambiguus; NST: nucleus of the solitary tract; PBN: parabrachial nucleus; PGCRN: para-gigantocellular reticular nucleus; PVH: paraventricular hypothalamic nucleus; TSN: trigeminal spinal nucleus; VN: vestibular nuclei; VPSN: ventral principal sensory nucleus of V.
Central connections of the NA
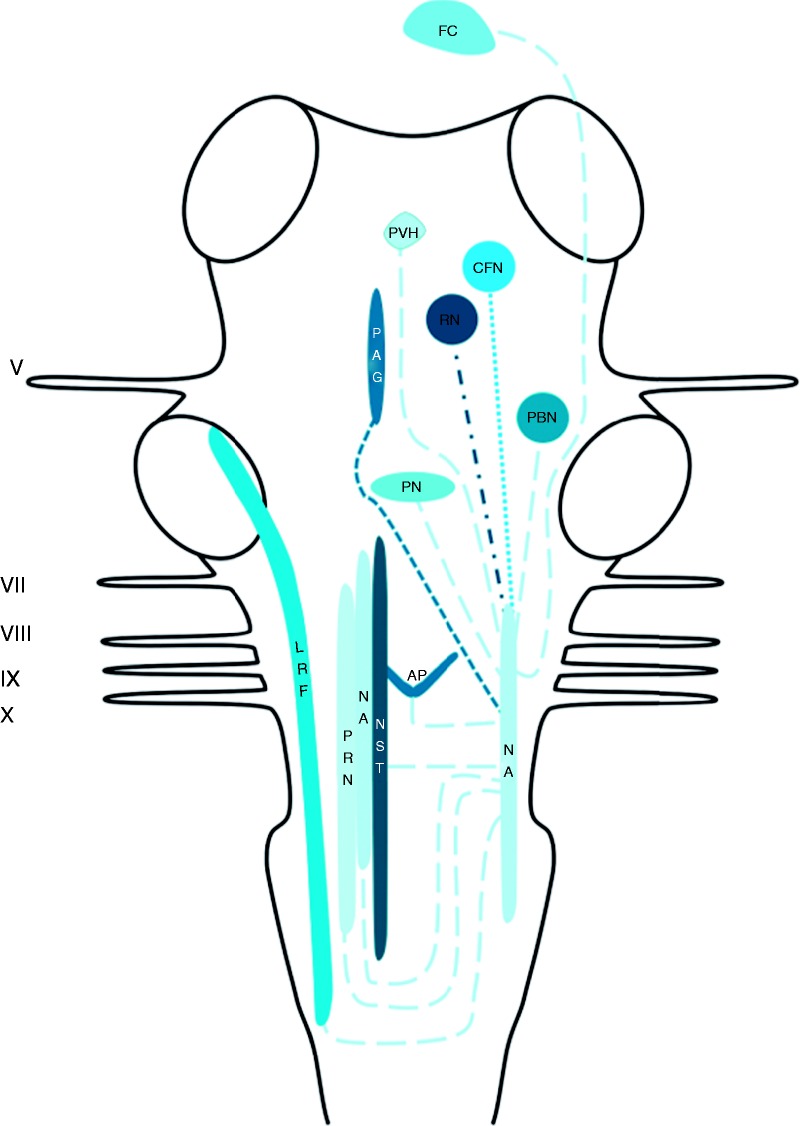
Afferents were reported to terminate in the NA originate from the periaqueductal grey (PAG), the cuneiform nuclei, the red nucleus and the frontal cortex (78,79). Efferent projections to the area postrema, the contralateral NA and the lateral reticular formation were found (78–81). Furthermore, connections to the medial parts of the medulla oblongata and the parabrachial nuclei were described as well (78,79). The aforementioned connections are depicted in Figure 2.
Figure 2.
Schematic overview of the central projections of the NA. Latin numbers indicate cranial nerves or their corresponding nuclei; color of the tract is consistent with the color of the nucleus/area it sprouts from.
AP: area postrema; CFN: cuneiform nucleus; LHA: lateral hypothalamic area; LRF: lateral reticular formation; NA: nucleus ambiguus; NST: nucleus of the solitary tract; PAG: periaqueductal grey; PBN: parabrachial nucleus; PN: pontine nuclei; PRN: paramedian reticular nucleus; PVH: paraventricular hypothalamic nucleus; RN: red nucleus.
Central connections of the TSN
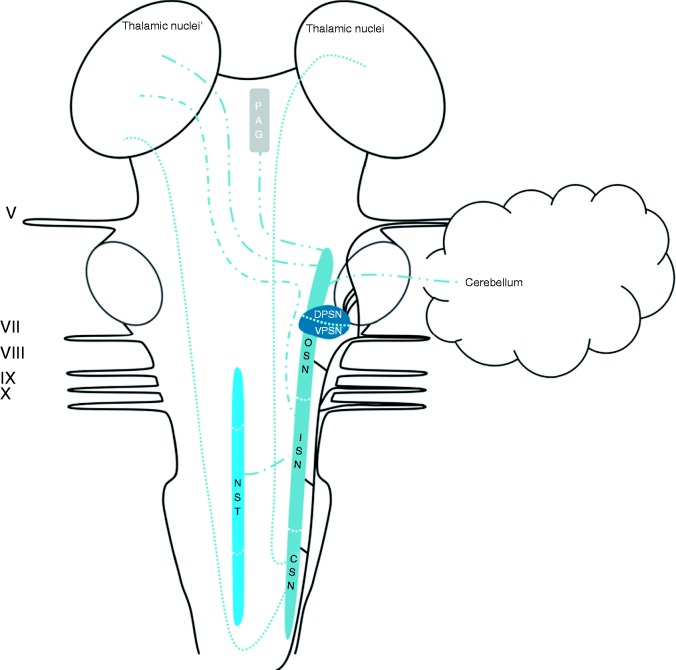
The trigeminal connections have been described in depth in a recent review by Henssen et al. (2016) (82). Afferents of the trigeminal, vagus, facial and glossopharyngeal nerves contribute to the trigeminal spinal tract and the TSN (53–55). The TSN can be subdivided into three subnuclei: The oral part, the interpolar part and the caudal part. The efferents from the oral part of the TSN project mainly to the contralateral thalamic subnuclei, the ventral posteromedial nucleus (VPM) in particular, via the ventral trigeminothalamic tract. However, the oral part of the TSN was also observed to have projections to lamina III and IV of the medullary dorsal horn (83). These connections were found to contribute to a minor ascending tract that terminates partially in the PAG. This tract is named the intranuclear pathway and it receives most of its input from the interpolar and caudal parts of the TSN (83–85). The interpolar part of the TSN also projects via the ventral trigeminothalamic tract to the contralateral VPM85. Lastly, the interpolar part of the TSN56, together with the caudal part of the TSN and the principal sensory nucleus, is known to have connections with the NST (54,57). The caudal part is the most investigated subnucleus of the TSN. The caudal part of the TSN was found to project to the contralateral VPM mainly, although a bilateral projection to the mediodorsal nucleus of the thalamus was also reported. Furthermore, connections between the principal sensory nucleus of the trigeminal nerve and the caudal part of the TSN were observed (86). The caudal part of the TSN was furthermore connected to the VPM and dorsomedial region of the thalamus in a bilateral fashion and to the contralateral posterior nucleus of the thalamus (87,88). As stated, the TSN contributes to the intranuclear pathway and therefore has extensive connections with the PAG and the NST (54,57,85). The aforementioned connections are depicted in Figure 3.
Figure 3.
Schematic overview of the central projections of the TSN. Latin numbers indicate cranial nerves or their corresponding nuclei; color of the tract is consistent with the color of the nucleus/area it sprouts from.
CSN: caudal subnucleus of the trigeminal spinal nucleus; DPSN: dorsal part of the principal sensory nucleus; ISN: interpolar subnucleus of the trigeminal spinal nucleus; NST: nucleus of the solitary tract; OSN: oral subnucleus of the trigeminal spinal nucleus; PAG: periaqueductal grey; VPSN: ventral part of the principal sensory nucleus.
Central connections of the DMX
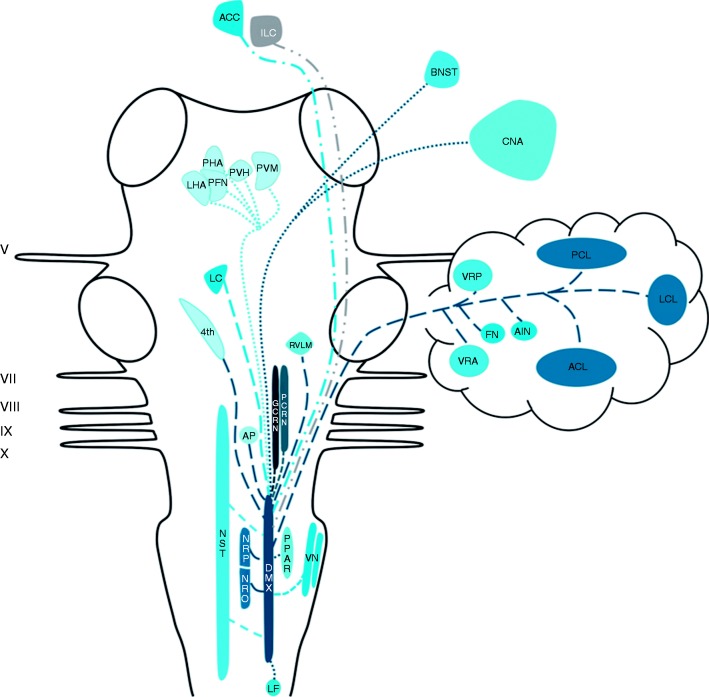
Rogers et al. (1980) reviewed afferent projections to the DMX in the late 20th century and described three major sources of afferent input to the DMX: The NST, the magnocellular paraventricular nucleus and several medullary nuclei (89). Projections of the NST to the DMX have been described by others as well (90,91). The paraventricular nucleus, the magnocellular neurons in particular, has been reported as a sprouting zone of a substantial amount of fibers that terminate in the DMX in rats (92). Concerning the medullary nuclei, the nucleus reticularis and the nucleus gigantocellularis have been shown to project to the DMX (89). Additionally, thyrotropin-releasing hormone-immunoreactive neurons residing in the nucleus raphe pallidus, nucleus raphe obscurus and the parapyramidal region of the ventral medulla have been suggested to project to the DMX and NST in rats (93). Furthermore, a fourth, minor, afferent pathway has been described by Berk and Finkelstein. They found that, in the pigeon, a minority of afferents of the nucleus periventricularis magnocellularis of the hypothalamus project to the ventral parvocellular subnucleus of the DMX (94). Zardetto-Smith et al. also found evidence in 1988 for a dynorphinergic innervation of the DMX by the perifornical nucleus, another hypothalamic area believed to be involved in the general arousal associated with emotive behaviours, as dynorphin has been implicated in mediating a wide variety of behavioral and autonomic-related processes including pain modulation (95). A fifth pathway was described by Ter Horst et al. in 1991, as they found projections of the locus coeruleus to the DMX in medial and rostral levels (96). In addition, a sixth projection system has been described. The ventral descending pathway, sprouting from the infralimbic cortex in the rat, projects to several autonomic cell groups in the brainstem, including the DMX (97). Furthermore, in 1996 Ruggiero et al. found that vestibular afferents reside in the NST and DMX (72). Earlier, in 1994, Balaban et al. studied central connectivity of the vestibular nuclei and found that some axons ended near somata of DMX neurons (70). Finally, studies conducted by Chiba et al. in 2001 and Kuipers et al. in 2006 found that the anterior cingulate cortex projects to the DMX in a bilateral fashion (98,99). Studies that investigated the morphological characteristics of the efferents from the DMX show that, together with the red nucleus, the DMX projects to the contralateral hemicord via the dorsal part of the lateral funiculus (100). In 1982, Zheng et al. investigated the DMX efferents to the cerebellum in 70 cats, who had been injected with horseradish peroxidase in all cerebellar cortical lobules and cerebellar nuclei. Injections in the anterior cerebellar lobe resulted in labeled neurons situated in the caudal half of the DMX. The cerebellar posterior lobe received input from the rostral half of the DMX, while the medial DMX projects to the vermal regions of the cerebellum in both the anterior and posterior lobe. In a few cases, labeling of the fastigial and anterior interposed nucleus was found (101). Gastric motoneurons of the DMX possess numerous dendrites that penetrate discrete regions of the adjacent NST. The same study showed that a small number of DMX neurons penetrated the ependyma of the fourth ventricle (80). Moreover, the DMX has been shown to interact with the area postrema via both afferents (80,102,103) and efferents (80,104). The central nucleus of the amygdala in the rat sends a considerable projection to, and receives projections from, the parabrachial nucleus and the dorsal vagal complex (DVC; constituents of the DVC are the NST and the DMX) (105). In other animals, this amygdalofugal pathway has also been described (106,107). Also, the posterior hypothalamic area and mostly the lateral hypothalamic area in rats have been shown to project to the DVC (95,108–110). Manaker and Fogarty conducted a study in 1995 that described raphespinal and reticulospinal neurons projecting to the DVC, while focusing primarily on NST afferents (111). Finally, the rostroventrolateral medulla, a brain structure known to be involved in cardiovascular regulation, has restricted terminal fields in the DVC (96). The aforementioned connections are depicted in Figure 4.
Figure 4.
Schematic overview of the central projections of the DMX. Latin numbers indicate cranial nerves or their corresponding nuclei; color of the tract is consistent with the color of the nucleus/area it sprouts from.
ACC: anterior cingulate cortex; ACL: anterior cerebellar lobe; AIN: anterior interposite nucleus; AP: area postrema; BNST: bed nucleus of stria terminalis; CNA: central nucleus of amygdala; DMX: dorsal motor of X; FN: fastigial nucleus; GCRN: gigantocellular reticular nucleus; LC: locus coeruleus; LCL: lateral cerebellar lobe; LF: lateral funiculus; LHA: lateral hypothalamic area; ILC: infralimbic cortex; NRO: nucleus raphe obscurus; NRP: nucleus raphe pallidus; NST: nucleus of the solitary tract; PCL: posterior cerebellar lobe; PCRN: parvocellular reticular nucleus; PFN: perifornical nucleus; PHA: posterior hypothalamic area; PPAR: parapyramidal region; PVH: paraventricular hypothalamic nucleus; PVM: nucleus periventricularis magnocellularis; RVLM: rostral ventrolateral medulla; VN: vestibular nuclei; VRA: vermal region in the anterior lobe; VRP: vermal region in the posterior lobe; 4th: ependyma of the fourth ventricle.
Discussion
The present review synthesized evidence suggesting that nVNS can be beneficial in treating primary headache disorders, migraine and episodic cluster headaches in particular. Two level one studies on episodic and chronic migraine showed effectiveness of nVNS as an acute and prophylactic treatment option (46,48). In episodic cluster headache, on the other hand, only the effectiveness of the acute treatment of nVNS was reported in two level one studies (24,51). In addition, results suggest that nVNS is a well-tolerated and safe therapy. Nevertheless, the exact neural underpinnings of nVNS remain largely elusive, though it has been suggested that modulation of the central connections of the vagus nerve are of importance in alleviating pain in primary headache disorders. Therefore, the second part of this study reviewed the central connections of the vagus nerve. However, no original research papers on this topic were found that included humans. In animals, extensive interconnections between the different vagal nuclei have been described. Also, the TSN and NST, the vagal nuclei that receive sensory and visceral afferents, are the main sprouting areas for fibers that course towards main parts of the neural pain matrix directly or indirectly via other vagal nuclei. Moreover, it has been described that vagal nerve fibers intertwine with fibers from the trigeminal, facial, glossopharyngeal and hypoglossal nerves within the trigeminal spinal tract, forming a trigeminovagal complex.
Clinical relevance of findings
Several studies investigated the possible mechanisms of action of nVNS in both animals and humans (53–55,57,85,112–116). The results of the study of Nonis et al. showed that nVNS in humans was capable of activating vagal afferents as measured by electrophysiological recordings, indicating that efficacy of nVNS can be monitored in future trials (112). An extensive body of literature provides evidence for the anti-inflammatory effect of VNS (113), which may also play a role in the mechanisms by which (n)VNS ameliorates pain (114). Another mechanism of action includes modulation of the trigeminal system by nVNS. In a rat model of migraine-like headaches, nVNS affected the levels of extracellular glutamate in the caudal part of the TSN (115). Another study investigated the firing rate of trigeminocervical neurons in rats treated with nVNS. They suggested that nVNS may inhibit the firing rates of trigeminocervical neurons directly or indirectly, although the exact physiological mechanisms involved remain elusive (116). Based on animal research, direct and indirect connections of the trigeminal and vagal nerves at the level of the brainstem are suggested to be part of the pathways involved in headache pain (53–55,57,85). It is therefore hypothesized that inhibition of the firing pattern of the trigeminal neurons occurs due to the existence of these reciprocal connections between the TSN and the NST, which may serve as the main targets for afferent fiber tracts from the vagus nerve (117,118). This was also in agreement with the study of Frangos et al., which showed that, next to other brain regions, the NTS and STN were respectively activated and deactivated by nVNS (119).
Our review further contributes to elucidating the working mechanism of nVNS in primary headache disorders by synthesizing the evidence of the presence of a trigeminovagal complex in the brainstem of a wide range of animals. However, the existence of an anatomically distinguishable trigeminovagal complex in humans remains uncertain. Future research should focus on imaging human neuroanatomy, especially of the trigeminovagal complex. New, high-resolution imaging techniques could contribute to revealing such neuroanatomical pathways in humans. For example, diffusion magnetic resonance imaging (dMRI) and tractography have become well-known methods to study white matter anatomy (120). To study white matter architecture in even greater detail, an innovative technique called polarized light imaging microscopy (121) might be a valuable asset to study the hypothesized trigeminovagal complex in humans.
Strengths and limitations
This systematic review of the clinical studies on nVNS reveals four level one studies in primary headache disorders which are supplemented by numerous prospective and retrospective reviews. There is heterogeneity in applied methodologies, disease states, duration of headache and outcome measures. These aspects make it more difficult to perform a single meta-analysis on the results. One particular problem concerned the higher than expected sham results with the sham device in both the cluster headache and migraine studies. This suggests the possibility of a placebo effect (46,122,123). Alternatively, we now know that in several of these studies the sham was later determined to be active stimulation, and thus there was no true inactive device or sham stimulation (124). The methods that we have used in this review strengthen our conclusion. Another strength of this study concerns the combination of a systematic review on nVNS in primary headache disorders with an anatomical review. By combining both reviews, we aimed to contribute to the elucidation of the underpinnings involved in this clinical phenomenon. Therefore, based on the reviewed evidence, we put forth two hypotheses. First, we hypothesize the existence of a trigeminovagal complex in humans. Second, we hypothesize the involvement of this complex in nVNS to treat primary headache disorders. Furthermore, the combination of anatomical evidence, and the suggestions that come forth from the clinical trials, can be regarded as a strength of this paper. However, the complete absence of original research on the central connections of the vagus nerve in humans uncovers a remarkable imperfection in knowledge of human neuroanatomy.
Conclusion
Based on the results from several clinical trials reviewed in this paper, moderate effectiveness of nVNS in primary headache disorders is suggested, although the heterogeneity of the included studies precludes the drawing of a sound recommendation. Furthermore, the hypothesized trigeminovagal complex in humans, similar to an anatomical feature in the brainstems of various animal species, could further elucidate the neural underpinnings of nVNS in alleviating pain in primary headache disorders, migraine and cluster headaches in particular.
Article highlights
nVNS is a moderately effective, safe and well-tolerated therapy for migraine and cluster headache.
In animals, connections between the trigeminal and vagus systems were found at the level of the brainstem. These connections could contribute to the neural underpinnings of nVNS in primary headache disorders.
The existence of a trigeminovagal complex remains elusive in humans.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Staats is an employee of both National Spine and Pain Centers and ElectroCore.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material, Supplemental Material1 for Vagus nerve stimulation for primary headache disorders: An anatomical review to explain a clinical phenomenon by Dylan Jozef Hendrik Augustinus Henssen, Berend Derks, Mats van Doorn, Niels Verhoogt, Anne-Marie Van Cappellen van Walsum, Peter Staats and Kris Vissers in Cephalalgia
Supplemental Material
Supplemental material, Supplemental Material2 for Vagus nerve stimulation for primary headache disorders: An anatomical review to explain a clinical phenomenon by Dylan Jozef Hendrik Augustinus Henssen, Berend Derks, Mats van Doorn, Niels Verhoogt, Anne-Marie Van Cappellen van Walsum, Peter Staats and Kris Vissers in Cephalalgia
References
- 1.Ahmed F. Headache disorders: Differentiating and managing the common subtypes. Br J Pain 2012; 6: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007; 27: 193–210. [DOI] [PubMed] [Google Scholar]
- 3.Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: Disability and economic costs. Arch Intern Med 1999; 159: 813–818. [DOI] [PubMed] [Google Scholar]
- 4.Holle D, Obermann M, Katsarava Z. The electrophysiology of cluster headache. Curr Pain Headache Rep 2009; 13: 155–159. [DOI] [PubMed] [Google Scholar]
- 5.Mendizabal JE, Umana E, Zweifler RM. Cluster headache: Horton’s cephalalgia revisited. South Med J 1998; 91: 606–617. [DOI] [PubMed] [Google Scholar]
- 6.Cutrer FM, Charles A. The neurogenic basis of migraine. Headache 2008; 48: 1411–1414. [DOI] [PubMed] [Google Scholar]
- 7.Solstrand Dahlberg L, Linnman CN, Lee D, et al. Responsivity of periaqueductal gray connectivity is related to headache frequency in episodic migraine. Front Neurol 2018; 9: 61–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrosini A, Rossi P, De Pasqua V, et al. Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain: J Neurol 2003; 126: 2009–2015. [DOI] [PubMed] [Google Scholar]
- 9.Ambrosini A, Schoenen J. The electrophysiology of migraine. Curr Opin Neurol 2003; 16: 327–331. [DOI] [PubMed] [Google Scholar]
- 10.Akerman S, Romero-Reyes M, Holland PR. Current and novel insights into the neurophysiology of migraine and its implications for therapeutics. Pharmacol Ther 2017; 172: 151–170. [DOI] [PubMed] [Google Scholar]
- 11.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014; 8: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulte LH, Allers A, May A. Hypothalamus as a mediator of chronic migraine: Evidence from high-resolution fMRI. Neurology 2017; 88: 2011–2016. [DOI] [PubMed] [Google Scholar]
- 13.Rizzoli P, Mullally WJ. Headache. Am J Med 2018; 131: 17–24. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-de-Las-Penas C, Cuadrado ML. Physical therapy for headaches. Cephalalgia 2016; 36: 1134–1142. [DOI] [PubMed] [Google Scholar]
- 15.Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014; 19: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aicher SA, Lewis SJ, Randich A. Antinociception produced by electrical stimulation of vagal afferents: Independence of cervical and subdiaphragmatic branches. Brain Res 1991; 542: 63–70. [DOI] [PubMed] [Google Scholar]
- 17.Chandler MJ, Hobbs SF, Bolser DC, et al. Effects of vagal afferent stimulation on cervical spinothalamic tract neurons in monkeys. Pain 1991; 44: 81–87. [DOI] [PubMed] [Google Scholar]
- 18.Randich A, Ren K, Gebhart GF. Electrical stimulation of cervical vagal afferents. II. Central relays for behavioral antinociception and arterial blood pressure decreases. J Neurophysiol 1990; 64: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 19.Ren K, Randich A, Gebhart GF. Vagal afferent modulation of spinal nociceptive transmission in the rat. J Neurophysiol 1989; 62: 401–415. [DOI] [PubMed] [Google Scholar]
- 20.Thurston CL, Randich A. Quantitative characterization and spinal substrates of antinociception produced by electrical stimulation of the subdiaphragmatic vagus in rats. Pain 1991; 44: 201–209. [DOI] [PubMed] [Google Scholar]
- 21.Thurston CL, Randich A. Effects of vagal afferent stimulation on ON and OFF cells in the rostroventral medulla: Relationships to nociception and arterial blood pressure. J Neurophysiol 1992; 67: 180–196. [DOI] [PubMed] [Google Scholar]
- 22.Trimboli M, Al-Kaisy A, Andreou AP, et al. Non-invasive vagus nerve stimulation for the management of refractory primary chronic headaches: A real-world experience. Cephalalgia 2018; 38: 1276–1285. [DOI] [PubMed] [Google Scholar]
- 23.Grazzi L, Egeo G, Liebler E, et al. Non-invasive vagus nerve stimulation (nVNS) as symptomatic treatment of migraine in young patients: a preliminary safety study. Neurol Sci 2017; 38: 197–199. [DOI] [PubMed] [Google Scholar]
- 24.Goadsby PJ, de Coo IF, Silver N, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A randomized, double-blind, sham-controlled ACT2 study. Cephalalgia 2018; 38: 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lainez MJ, Jensen R. Noninvasive neuromodulation in cluster headache. Curr Opin Neurol 2015; 28: 271–276. [DOI] [PubMed] [Google Scholar]
- 26.Lenaerts ME, Oommen KJ, Couch JR, et al. Can vagus nerve stimulation help migraine? Cephalalgia 2008; 28: 392–395. [DOI] [PubMed] [Google Scholar]
- 27.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache 2016; 56: 259–266. [DOI] [PubMed] [Google Scholar]
- 28.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part III. Headache 2016; 56: 479–490. [DOI] [PubMed] [Google Scholar]
- 29.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part I. Headache 2016; 56: 71–78. [DOI] [PubMed] [Google Scholar]
- 30.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85: 1–17. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwenhuys R, Voogd J, Van Huijzen C. The human central nervous system: A synopsis and atlas, 4th ed. Berlin, Germany: Springer-Verlag Berlin Heidelberg, 2007, pp. 987–987. [Google Scholar]
- 32.Mai JK, Paxinos G. The human nervous system, 2nd ed. San Diego, CA: Academic Press, 2011, pp. 1366–1366. [Google Scholar]
- 33.Furlan AD, Pennick V, Bombardier C, et al. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009; 34: 1929–1941. [DOI] [PubMed] [Google Scholar]
- 34.Furlan AD, Malmivaara A, Chou R, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine (Phila Pa 1976) 2015; 40: 1660–1673. [DOI] [PubMed] [Google Scholar]
- 35.van Tulder M, Furlan A, Bombardier C, et al. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976) 2003; 28: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 36.Boutron I, Moher D, Tugwell P, et al. A checklist to evaluate a report of a nonpharmacological trial (CLEAR NPT) was developed using consensus. J Clin Epidemiol 2005; 58: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 37.Deeks J, Higgins J, Altman D, et al. Cochrane handbook for systematic reviews of interventions version 5.1. 0 (updated March 2011). The Cochrane Collaboration 2011. http://handbook-5-1.cochrane.org/. [Google Scholar]
- 38.Mai JK, Majtanik M, Paxinos G. Atlas of the human brain, 4th ed. San Diego, CA: Academic Press, 2015, pp. 456–456. [Google Scholar]
- 39.Ten Donkelaar JH. Clinical neuroanatomy: Brain circuitry and its disorders, 1st ed. Berlin, Germany: Springer-Verlag Berlin Heidelberg, 2011, pp. 834–834. [Google Scholar]
- 40.Olszewski J, Baxter D. Cytoarchitecture of the human brain stem. Cytoarchitecture of the human brain stem, 3rd ed. Munich, Germany: Karger Publishers, 1954, pp. 290–290. [Google Scholar]
- 41.Naidich TP, Duvernoy HM, Delman BN, et al. Duvernoy’s atlas of the human brain stem and cerebellum: High-field MRI, surface anatomy, internal structure, vascularization and 3 D sectional anatomy, 1st ed. Wien, Austria: Springer-Verlag Wien, 2009, pp. 876–876. [Google Scholar]
- 42.Haines DE. Neuroanatomy: An atlas of structures, sections, and systems, 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2004, pp. 310–310. [Google Scholar]
- 43.Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: An open-label pilot study. Cephalalgia 2014; 34: 986–993. [DOI] [PubMed] [Google Scholar]
- 44.Kinfe TM, Pintea B, Muhammad S, et al. Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: A prospective observational cohort study. J Headache Pain 2015; 16: 101–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbanti P, Grazzi L, Egeo G, et al. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: An open-label study. J Headache Pain 2015; 16: 61–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tassorelli C, Grazzi L, de Tommaso M, et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study. Neurology 2018; 91: e364–e373. DOI: 10.1212/WNL.0000000000005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grazzi L, Padovan A, Barbanti P. Role of neurostimulation in migraine. Neurol Sci 2015; 36: S121–S123. [DOI] [PubMed] [Google Scholar]
- 48.Silberstein SD, Calhoun AH, Lipton RB, et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology 2016; 87: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nesbitt AD, Marin JC, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology 2015; 84: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 50.Gaul C, Diener HC, Silver N, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): A randomised controlled study. Cephalalgia 2016; 36: 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the ACute Treatment of cluster headache: Findings from the randomized, double-blind, sham-controlled ACT1 study. Headache 2016; 56: 1317–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tso AR, Marin J, Goadsby PJ. Noninvasive vagus nerve stimulation for treatment of indomethacin-sensitive headaches. JAMA Neurol 2017; 74: 1266–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gobel S, Purvis MB. Anatomical studies of the organization of the spinal V nucleus: The deep bundles and the spinal V tract. Brain Res 1972; 48: 27–44. [DOI] [PubMed] [Google Scholar]
- 54.Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: An autoradiographic study in the rat. J Auton Nerv Syst 1982; 6: 303–322. [DOI] [PubMed] [Google Scholar]
- 55.Rustioni A, Baan JW, Verdonk-Karlsen S. Afferents from the facial, vago-glossopharyngeal and second cervical nerves to the substantia gelatinosa of the rat. Brain Res 1972; 37: 137–140. [DOI] [PubMed] [Google Scholar]
- 56.Ciriello J, Hrycyshyn AW, Calaresu FR. Glossopharyngeal and vagal afferent projections to the brain stem of the cat: A horseradish peroxidase study. J Auton Nerv Syst 1981; 4: 63–79. [DOI] [PubMed] [Google Scholar]
- 57.Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold’s nerve (the auricular branch of the vagus nerve): A transganglionic HRP study in the cat. Brain Res 1984; 292: 199–205. [DOI] [PubMed] [Google Scholar]
- 58.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 1978; 3: 207–218. [DOI] [PubMed] [Google Scholar]
- 59.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res 1978; 153: 1–26. [DOI] [PubMed] [Google Scholar]
- 60.Ruggiero DA, Underwood MD, Mann JJ, et al. The human nucleus of the solitary tract: Visceral pathways revealed with an “in vitro” postmortem tracing method. J Auton Nerv Syst 2000; 79: 181–190. [DOI] [PubMed] [Google Scholar]
- 61.Peyron C, Luppi PH, Fort P, et al. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol 1996; 364: 402–413. [DOI] [PubMed] [Google Scholar]
- 62.Dahlstroem A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl 1964; S232: 1–55. [PubMed] [Google Scholar]
- 63.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 1982; 257: 275–325. [DOI] [PubMed] [Google Scholar]
- 64.Loewy AD, Burton H. Nuclei of the solitary tract: Efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol 1978; 181: 421–449. [DOI] [PubMed] [Google Scholar]
- 65.Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol 1973; 150: 217–237. [DOI] [PubMed] [Google Scholar]
- 66.Beckstead RM, Norgren R. An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal, and vagal nerves in the monkey. J Comp Neurol 1979; 184: 455–472. [DOI] [PubMed] [Google Scholar]
- 67.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 1982; 211: 248–265. [DOI] [PubMed] [Google Scholar]
- 68.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol 1980; 193: 435–465. [DOI] [PubMed] [Google Scholar]
- 69.Morest DK. Experimental study of the projections of the nucleus of the tractus solitarius and the area postrema in the cat. J Comp Neurol 1967; 130: 277–300. [DOI] [PubMed] [Google Scholar]
- 70.Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: Potential substrates for vestibulo-autonomic interactions. Exper Brain Res 1994; 98: 200–212. [DOI] [PubMed] [Google Scholar]
- 71.Yates BJ, Grelot L, Kerman IA, et al. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol 1994; 267: R974–R983. [DOI] [PubMed] [Google Scholar]
- 72.Ruggiero DA, Mtui EP, Otake K, et al. Vestibular afferents to the dorsal vagal complex: Substrate for vestibular-autonomic interactions in the rat. Brain Res 1996; 743: 294–302. [DOI] [PubMed] [Google Scholar]
- 73.Sawchenko PE. Central connections of the sensory and motor nuclei of the vagus nerve. J Auton Nerv Syst 1983; 9: 13–26. [DOI] [PubMed] [Google Scholar]
- 74.Otake K, Reis DJ, Ruggiero DA. Afferents to the midline thalamus issue collaterals to the nucleus tractus solitarii: An anatomical basis for thalamic and visceral reflex integration. J Neuroscience 1994; 14: 5694–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayward JN. Functional and morphological aspects of hypothalamic neurons. Physiol Rev 1977; 57: 574–658. [DOI] [PubMed] [Google Scholar]
- 76.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science 1981; 214: 685–687. [DOI] [PubMed] [Google Scholar]
- 77.Swanson LW, Mogenson GJ. Neural mechanisms for the functional coupling of autonomic, endocrine and somatomotor responses in adaptive behavior. Brain Res 1981; 228: 1–34. [DOI] [PubMed] [Google Scholar]
- 78.Rubsamen R, Betz M. Control of echolocation pulses by neurons of the nucleus ambiguus in the rufous horseshoe bat, Rhinolophus rouxi. I. Single unit recordings in the ventral motor nucleus of the laryngeal nerves in spontaneously vocalizing bats. J Comp Physiol A 1986; 159: 675–687. [DOI] [PubMed] [Google Scholar]
- 79.Rubsamen R, Schweizer H. Control of echolocation pulses by neurons of the nucleus ambiguus in the rufous horseshoe bat, Rhinolophus rouxi. II. Afferent and efferent connections of the motor nucleus of the laryngeal nerves. J Comp Physiol A 1986; 159: 689–699. [DOI] [PubMed] [Google Scholar]
- 80.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 1985; 238: 473–488. [DOI] [PubMed] [Google Scholar]
- 81.Davies RO, Kalia M. Carotid sinus nerve projections to the brain stem in the cat. Brain Res Bull 1981; 6: 531–541. [DOI] [PubMed] [Google Scholar]
- 82.Henssen DJ, Kurt E, Kozicz T, et al. New insights in trigeminal anatomy: A double orofacial tract for nociceptive input. Front Neuroanat 2016; 10: 53–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panneton WM, Burton H. Origin of ascending intratrigeminal pathways in the cat. Brain Res 1982; 236: 463–470. [DOI] [PubMed] [Google Scholar]
- 84.Dubner R, Sessle BJ, Storey AT. The neurological basis of oral and facial function, 1st ed. New York, NY: Plenum Press, 1978, pp. 483–483. [Google Scholar]
- 85.Ikeda M, Matsushita M, Tanami T. Termination and cells of origin of the ascending intra-nuclear fibers in the spinal trigeminal nucleus of the cat. A study with the horseradish peroxidase technique. Neurosci Lett 1982; 31: 215–220. [DOI] [PubMed] [Google Scholar]
- 86.Ganchrow D. Intratrigeminal and thalamic projections of nucleus caudalis in the squirrel monkey (Saimiri sciureus): A degeneration and autoradiographic study. J Comp Neurol 1978; 178: 281–312. [DOI] [PubMed] [Google Scholar]
- 87.Burton H, Craig AD., Jr Distribution of trigeminothalamic projection cells in cat and monkey. Brain Res 1979; 161: 515–521. [DOI] [PubMed] [Google Scholar]
- 88.Kunzle H. Trigeminal projections to thalamus and subthalamus in the hedgehog tenrec. Neuroscience 1998; 86: 651–661. [DOI] [PubMed] [Google Scholar]
- 89.Rogers RC, Kita H, Butcher LL, et al. Afferent projections to the dorsal motor nucleus of the vagus. Brain Res Bull 1980; 5: 365–373. [DOI] [PubMed] [Google Scholar]
- 90.Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: An HRP and autoradiographic tracing study in the cat. J Comp Neurol 1987; 260: 98–126. [DOI] [PubMed] [Google Scholar]
- 91.Holstege G, Tan J, van Ham JJ, et al. Anatomical observations on the afferent projections to the retractor bulbi motoneuronal cell group and other pathways possibly related to the blink reflex in the cat. Brain Res 1986; 374: 321–334. [DOI] [PubMed] [Google Scholar]
- 92.van der Kooy D, Koda LY, McGinty JF, et al. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 1984; 224: 1–24. [DOI] [PubMed] [Google Scholar]
- 93.Lynn RB, Kreider MS, Miselis RR. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J Comp Neurol 1991; 311: 271–288. [DOI] [PubMed] [Google Scholar]
- 94.Berk ML, Finkelstein JA. Long descending projections of the hypothalamus in the pigeon, Columba livia. J Comp Neurol 1983; 220: 127–136. [DOI] [PubMed] [Google Scholar]
- 95.Zardetto-Smith AM, Moga MM, Magnuson DJ, et al. Lateral hypothalamic dynorphinergic efferents to the amygdala and brainstem in the rat. Peptides 1988; 9: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 96.Ter Horst GJ, Toes GJ, Van Willigen JD. Locus coeruleus projections to the dorsal motor vagus nucleus in the rat. Neuroscience 1991; 45: 153–160. [DOI] [PubMed] [Google Scholar]
- 97.Hurley KM, Herbert H, Moga MM, et al. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol 1991; 308: 249–276. [DOI] [PubMed] [Google Scholar]
- 98.Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res 2001; 888: 83–101. [DOI] [PubMed] [Google Scholar]
- 99.Kuipers R, Mensinga GM, Boers J, et al. Infralimbic cortex projects to all parts of the pontine and medullary lateral tegmental field in cat. Eur J Neurosci 2006; 23: 3014–3024. [DOI] [PubMed] [Google Scholar]
- 100.Cruce WL, Newman DB. Brain stem origins of spinal projections in the lizard Tupinambis nigropunctatus. J Comp Neurol 1981; 198: 185–207. [DOI] [PubMed] [Google Scholar]
- 101.Zheng ZH, Dietrichs E, Walberg F. Cerebellar afferent fibres from the dorsal motor vagal nucleus in the cat. Neurosci Lett 1982; 32: 113–118. [DOI] [PubMed] [Google Scholar]
- 102.Strominger NL, Knox AP, Carpenter DO. The connectivity of the area postrema in the ferret. Brain Res Bull 1994; 33: 33–47. [DOI] [PubMed] [Google Scholar]
- 103.Knox AP, Strominger NL, Battles AH, et al. The central connections of the vagus nerve in the ferret. Brain Res Bull 1994; 33: 49–63. [DOI] [PubMed] [Google Scholar]
- 104.Momose-Sato Y, Kinoshita M, Sato K. Development of vagal afferent projections circumflex to the obex in the embryonic chick brainstem visualized with voltage-sensitive dye recording. Neuroscience 2007; 148: 140–150. [DOI] [PubMed] [Google Scholar]
- 105.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: A combined retrograde transport-immunohistochemical study. Brain Res 1984; 303: 337–357. [DOI] [PubMed] [Google Scholar]
- 106.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res 1978; 32: 529–547. [DOI] [PubMed] [Google Scholar]
- 107.Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1981; 1: 1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hosoya Y, Matsushita M. Brainstem projections from the lateral hypothalamic area in the rat, as studied with autoradiography. Neurosci Lett 1981; 24: 111–116. [DOI] [PubMed] [Google Scholar]
- 109.Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol 1976; 169: 409–442. [DOI] [PubMed] [Google Scholar]
- 110.Veening JG, Lie ST, Posthuma P, et al. A topographical analysis of the origin of some efferent projections from the lateral hypothalamic area in the rat. Neuroscience 1987; 22: 537–551. [DOI] [PubMed] [Google Scholar]
- 111.Manaker S, Fogarty PF. Raphespinal and reticulospinal neurons project to the dorsal vagal complex in the rat. Exp Brain Res 1995; 106: 79–92. [DOI] [PubMed] [Google Scholar]
- 112.Nonis R, D’Ostilio K, Schoenen J, et al. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers. Cephalalgia 2017; 37: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007; 117: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simon B, Blake J. Mechanism of action of non-invasive cervical vagus nerve stimulation for the treatment of primary headaches. Am J Manag Care 2017; 23: S312–S316. [PubMed] [Google Scholar]
- 115.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: A model for recurrent headache. Headache 2007; 47: 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akerman S, Simon B, Romero-Reyes M. Vagus nerve stimulation suppresses acute noxious activation of trigeminocervical neurons in animal models of primary headache. Neurobiol Dis 2017; 102: 96–104. [DOI] [PubMed] [Google Scholar]
- 117.Lyubashina OA, Sokolov AY, Panteleev SS. Vagal afferent modulation of spinal trigeminal neuronal responses to dural electrical stimulation in rats. Neuroscience 2012; 222: 29–37. [DOI] [PubMed] [Google Scholar]
- 118.Zerari-Mailly F, Buisseret P, Buisseret-Delmas C, et al. Trigemino-solitarii-facial pathway in rats. J Comp Neurol 2005; 487: 176–189. [DOI] [PubMed] [Google Scholar]
- 119.Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul 2017; 10: 19–27. [DOI] [PubMed] [Google Scholar]
- 120.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008; 44: 1105–1132. [DOI] [PubMed] [Google Scholar]
- 121.Axer M, Amunts K, Grassel D, et al. A novel approach to the human connectome: Ultra-high resolution mapping of fiber tracts in the brain. Neuroimage 2011; 54: 1091–1101. [DOI] [PubMed] [Google Scholar]
- 122.Staats P, Hekmat H, Staats A. Suggestion/placebo effects on pain: Negative as well as positive. J Pain Symptom Manag 1998; 15: 235–243. [DOI] [PubMed] [Google Scholar]
- 123.Staats PS, Hekmat H and Staats AW. The psychological behaviorism theory of pain and the placebo: Its principles and results of research application. Adv Psychosom Med 2004; 25: 28–40. [DOI] [PubMed]
- 124.Schroeder CF, Möller N and May A. Vagus nerve stimulation modulates the cranial trigeminal-autonomic reflex – a comparison trial of different sham-conditions. In: MTIS 2018–2019. London, United Kingdom, 2018. http://www.mtis2018.org/abstracts/MTIS18–262_MTIS2018-019.PDF. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Vagus nerve stimulation for primary headache disorders: An anatomical review to explain a clinical phenomenon by Dylan Jozef Hendrik Augustinus Henssen, Berend Derks, Mats van Doorn, Niels Verhoogt, Anne-Marie Van Cappellen van Walsum, Peter Staats and Kris Vissers in Cephalalgia
Supplemental material, Supplemental Material2 for Vagus nerve stimulation for primary headache disorders: An anatomical review to explain a clinical phenomenon by Dylan Jozef Hendrik Augustinus Henssen, Berend Derks, Mats van Doorn, Niels Verhoogt, Anne-Marie Van Cappellen van Walsum, Peter Staats and Kris Vissers in Cephalalgia