Abstract
The World Health Organization estimates that 10 million new cases of tuberculosis (TB) occurred worldwide in 2017, of which 600,000 were rifampicin or multidrug-resistant (RR/MDR) TB. Modelling estimates suggest that 32,000 new cases of MDR-TB occur in children annually, but only a fraction of these are correctly diagnosed and treated. Accurately diagnosing TB in children, who usually have paucibacillary disease, and implementing effective TB prevention and treatment programmes in resource-limited settings remain major challenges. In light of the underappreciated RR/MDR-TB burden in children, and the lack of paediatric data on newer drugs for TB prevention and treatment, we present an overview of new and repurposed TB drugs, describing the available evidence for safety and efficacy in children to assist clinical care and decision-making.
Keywords: bedaquiline, delamanid, drug-resistant tuberculosis, fluoroquinolones, MDR-TB treatment, preventive therapy, tuberculosis infection
Background
The World Health Organization (WHO) estimates that 10 million cases of tuberculosis (TB) occurred worldwide in 2017, of which 600,000 were rifampicin (RR) or multidrug-resistant (MDR) TB (defined as Mycobacterium tuberculosis with resistance to at least rifampicin and isoniazid).1 Mathematical modelling studies suggest that 25,000–32,000 new MDR-TB cases occur in children annually, but the major gap between reported cases and best estimates highlights the large number of children with unidentified MDR-TB.2,3 It is suspected that <5% of paediatric MDR-TB cases are identified globally each year, with few of them likely to receive optimal treatment.2,4 A recent study combining WHO MDR-TB treatment data and published case fatality ratio estimates for children indicates a mortality rate of 22% in children with untreated MDR-TB.4 TB has recently been identified as a previously unrecognized top 10 cause of under 5 mortality in TB endemic areas.5
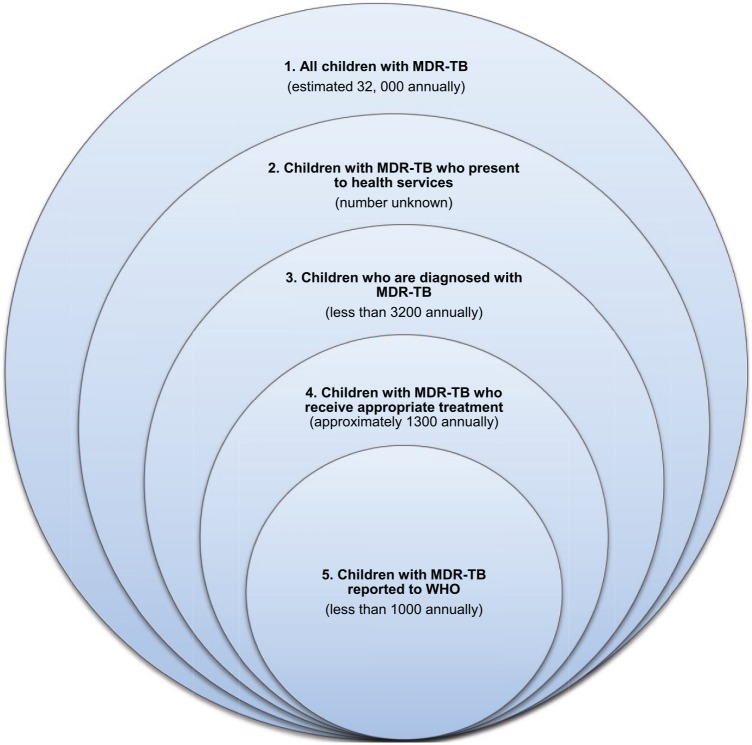
Using combined treatment data and estimates of household size and disease risk, it is estimated that carrying out household contact investigations around adult MDR-TB patients could find 12 times as many paediatric MDR-TB cases as are currently being identified.4 Children living in MDR-TB affected households experience a high risk of developing MDR-TB disease within 1–2 years of documented exposure.6 Figure 1 demonstrates the gaps that exist between the true incidence of MDR-TB in children, those accessing appropriate care and the numbers reported to WHO.
Figure 1.
Gaps in the detection, treatment and reporting of children with MDR-TB
1. Accurate MDR-TB incidence estimates are hampered by reliance on models that extrapolate child TB disease burden from adult TB data.
2. Access to health services are limited by inadequate service infrastructure, remoteness, poor community awareness of paediatric TB presentation and risks of death without diagnosis.
3. Barriers to MDR-TB diagnosis include: limited contact tracing, inadequate awareness and training of health personnel, difficult specimen collection and lack of laboratory diagnostic capacity.
4. Barriers to appropriate MDR-TB treatment include: poor access to optimal and child-friendly drug formulations and limited guidance on optimal clinical management.
5. Reporting to national and global TB programmes is hampered by dysfunctional health care and reporting systems, poor recording and data analysis at health facilities, particularly in the private sector, inaccurate attribution of MDR-TB cases to other conditions such as pneumonia, HIV or malnutrition; and previous exclusive reporting of sputum smear-positive cases excluded most children.
Diagnosing TB infection and disease
Infection with MDR strains of M. tuberculosis is estimated to occur in 2 million children worldwide.2 Data extrapolated from drug-susceptible TB suggests that the lifetime risk of progression from infection to disease is 4–10%.7,8 However certain populations such as immunosuppressed or malnourished people, those living with HIV, and adolescents are at higher risk of developing disease after exposure and infection.9 Young children are particularly vulnerable to disease progression following recent (within the past 12 months) TB infection. Children <5 years of age are generally regarded as the high-risk group (up to 20% develop disease following infection), but infants are at greatest risk (up to 50% disease progression) and are more likely to develop severe forms of disease, such as disseminated (miliary) TB or TB meningitis.10 Therefore, infants and children <5 years of age stand to benefit most from treatment of TB infection, also referred to as preventive therapy.
The diagnosis of MDR-TB infection requires active exclusion of disease with careful consideration of current symptoms, assessment of growth parameters, complete clinical examination, a chest radiograph and other tests (e.g. collection of respiratory specimens) as clinically indicated. Assessment of both anteroposterior (AP) and lateral chest radiograph views help to exclude intrathoracic lymphadenopathy. Tests of TB infection, the tuberculin skin test (TST) and an interferon gamma release assay (IGRA) are used in high-resource settings to guide preventive therapy. In resource-limited settings recent close/household exposure to an infectious TB case often serve as a proxy for TB infection.1 These children do not require further tests before initiation of preventive therapy if they are asymptomatic and thriving.11
Bacteriological confirmation of TB disease in children can be challenging, due to the paucibacillary nature of disease, difficult specimen collection and limited laboratory capacity in resource-limited settings. A bacteriological diagnosis is generally achieved in fewer than 40% of childhood TB cases,12–14 although yields are higher in children with advanced disease.13 Strengthening laboratory capacity with sensitive culture techniques and molecular diagnostics, such as Xpert MTB/RIF® (which is one of several molecular diagnostic methods) should improve diagnostic yields, but many cases will remain unconfirmed.15 Without microbiological confirmation (genotypic or phenotypic), diagnosis relies mostly on a thorough evaluation of risk factors for MDR-TB (Figure 2). Although microbiological confirmation should always be attempted, it is important to initiate MDR-TB treatment where there is high suspicion, given the potential for young children to deteriorate rapidly. It is therefore recommended that children with probable (clinical and radiological signs consistent with TB disease with confirmed close MDR-TB contact) MDR-TB and possible (clinical and radiological signs consistent with TB disease, but not improving after >2 months of adherent first-line therapy or close contact with a person who died from TB) be commenced on treatment. The decision to treat or monitor clinically is guided by the likelihood of clinical disease and the risk that an untreated child may develop severe disease versus the risk of exposing the child to potentially toxic drugs.
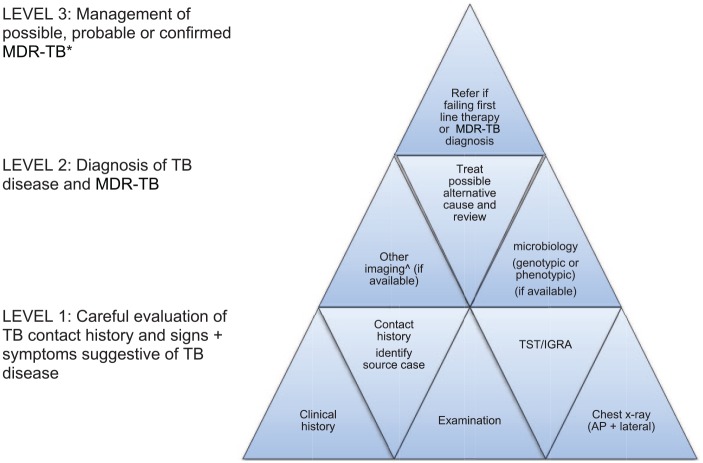
Figure 2.
A matrix approach to diagnose MDR-TB in children†.
AP, anteroposterior; IGRA, interferon gamma release assay; MDR-TB, multidrug resistant tuberculosis; TST, tuberculin skin test.
†Start at level 1 and obtain as much information as possible to fill the pieces. The more level 1 and 2 pieces that fit together, the more secure the MDR-TB diagnosis; concept adapted from Seddon J. International Child TB training course, Desmond Tutu TB centre Stellenbosch University, 2018.
*Provide treatment based on the drug susceptibility testing results of the likely source case, if no guidance is available from the child’s own specimens. The decision to treat should balance the likelihood of active disease and disease progression risk against the toxicity, cost and inconvenience of treatment. The following definitions apply.
Possible MDR-TB: TB symptoms and/or signs and/or radiology and child not improving after 2–3 months of first-line treatment or close contact with a patient who died from TB or failed TB treatment.
Probable MDR-TB: TB symptoms and/or signs and/or radiology with documented recent exposure to an infectious MDR-TB case.
Confirmed MDR-TB: MDR-TB strain isolated from a child.
^Computed tomography (CT), magnetic resonance imaging (MRI) and/or positron emission tomography (PET) depending on clinical symptoms and signs, as well as the cost, availability and radiation exposure of different modalities.
Treatment of MDR-TB infection
Although there is convincing evidence for isoniazid (INH) and/or rifamycins as effective preventive therapy options following infection with drug-susceptible TB strains there is uncertainty regarding optimal preventive therapy for MDR-TB infection.16–18
Newer generation fluoroquinolones
Fluoroquinolone-based regimens for MDR-TB infection have shown promise with prospective observational studies during outbreaks in the Federated States of Micronesia demonstrating no MDR-TB disease in the 104 adults and children who undertook preventive therapy. Of the 104 who initiated treatment 93 (89%) completed 12 months of preventive therapy. Furthermore, of the 26 contacts aged <12 years, completion rates of preventive therapy were higher at 25 (96%). MDR-TB disease developed in 3/15 (20%) of contacts who refused preventive therapy.19 South African studies in children using a 6-month fluoroquinolone-based regimen reported good adherence with a three-drug regimen (ofloxacin, ethambutol and high-dose INH), which was well tolerated. Only few children 6/186 (3%) developed TB during 219 patient years of observation time.20 Further support for MDR-TB preventive therapy in children comes from a 30-month follow-up study demonstrating significant MDR-TB transmission to childhood contacts in a TB endemic setting. An alarming 93/119 (78%) of children who completed follow up were either infected or developed disease.21
Historical concerns about the safety of fluoroquinolone use in children have largely been extrapolated from arthropathy observed in animal studies.22 In addition, the European Medicines Agency (EMA) recently issued a warning against the potential disabling side effects of fluoroquinolones. The US Food and Drug administration (FDA) have also highlighted the remote risk of aortic rupture.23,24 However, these adverse effects are mostly reported in adults with underlying vascular disease and have not been observed in children. There is now a significant body of evidence demonstrating the safety of fluoroquinolone use in children, including those <5 years of age receiving longer-term (6 months) treatment.20,25 As the most important bactericidal drug in the MDR-TB regimen, fluoroquinolones are well tolerated in children with MDR-TB even with treatment durations beyond 12 months.25 Levofloxacin has only been associated with self-limiting musculoskeletal ailments and no reports of corrected QT-interval (QTc) prolongation in children.26
Expert consensus based on current evidence, which includes 10 observational studies showing excellent efficacy as MDR-TB preventive therapy, supports the use of fluoroquinolone-based preventive therapy in high-risk child contacts.27 The most recent WHO guidance recommends that MDR-TB preventive therapy be considered on a case-by-case basis with levofloxacin (15-20 mg/kg/day) or moxifloxacin (10–15 mg/kg/day) the preferred drugs for 6 months.28 In Table 1, there is increasing evidence from field experience and observational studies, we are still awaiting efficacy, safety and tolerability data from randomised controlled trials. The V-QUIN trial in Vietnam and the TB-CHAMP (tuberculosis child and adolescent multidrug resistant preventive therapy) trial in South Africa, will both compare levofloxacin with placebo among infected household MDR-TB contacts and the international PHOENix (protecting households on exposure to newly diagnosed index multidrug resistant tuberculosis patients) trial will compare delamanid with standard dose INH in fluoroquinolone-resistant cases (Table 2). Preventive therapy also offers communal benefit by serving to prevent future TB transmission from infected individuals and can lead to dramatic reductions in TB disease burden if combined with active case finding strategies. However, this proactive strategy has only rarely been implemented by TB programmes.29,30
Table 1.
Proposed regimens for MDR-TB preventive therapy*.
| Drug/regimen** | Dose and duration of treatment |
|---|---|
|
Single drug regimen
|
|
| FQN alone |
Lfx: 15–20 mg/kg/day (max 750 mg) OR Mfx: 10–15 mg/kg/day (max 400 mg) Duration: 6–12 months# |
|
Two-drug regimen
|
|
| FQN and EMB |
FQN as above + EMB: 15–25 mg/kg/day (max
1 g) Duration: 6–12 months# |
| FQN and ETH |
FQN as above + ETH: 15–20 mg/kg/day (max
1 g) Duration: 6–12 months# |
| FQN and high dose INH |
FQN as above + INH: 15–20 mg/kg/day (max
450 mg) Duration: 6–12 months# |
|
Three-drug regimen
|
|
| FQN and ETH and high dose INH | FQN as above + ETH as above + INH as aboveDuration: 6–12 months#
|
EMB, ethambutol; ETH, ethionamide; FQN, fluoroquinolone; INH, isoniazid; MDR, multidrug-resistant (resistance to isoniazid and rifampicin); Mfx, moxifloxacin; Lfx, levofloxacin.
Currently no guidance exists for MDR-TB infection with FQN resistance; high dose INH could be considered if the source case has a inhA mutation or low level INH resistance; trials using delamanid are being planned; **Choice of regimen based on drug susceptibility testing of likely source case.
Table 2.
Randomized controlled trials for MDR-TB preventive therapy in children.
| Study | Drugs used | Study population | Setting |
|---|---|---|---|
|
V-QUIN
phase III |
DRUG: levofloxacin versus
placebo DURATION: 24 weeks DOSE: weight banded 15–20 mg/kg (max 750 mg) daily |
All TST+ MDR-TB household contacts (adults + children^) |
Vietnam, multicentre |
| TB CHAMP¶phase III |
DRUG: levofloxacin† versus placebo DURATION: 24 weeksDOSE: weight banded15–20mg/kg (max 750mg) daily | Household MDR-TB contacts aged <55 years§ | South Africa – multicenter |
| PHOENix**phase III | DRUG: delamanid versus isoniazid*
DURATION: 26 weeks DOSE: ⩾30 kg 200 mg daily >2.5 kg to ⩽30 kg weight banded |
Adults and children deemed high-risk MDR-TB household contacts |
International#, multicentre |
HHC, high risk household contacts include those with HIV and non HIV immunosuppression, young children <5 years of age, and any age with proven latent TB infection; HIV, human immunodeficiency virus; MDR, multidrug-resistant (resistance to isoniazid and rifampicin); TST, tuberculin skin test.
This is the only study powered for efficacy in children; †Using a novel paediatric dispersible formulation.
Enrolment of children regardless of TST or interferon-gamma release assay (IGRA) status.
Given with daily pyridoxine (vitamin B6).
Children <15 years will only be enrolled towards the end of the trial.
MDR-TB with fluoroquinolone resistance - in planning stage #Inclusion of 27 international sites in 12 countries planned: Botswana, Brazil, Haiti, Kenya, India, Peru, Phillipines, South Africa, Tanzania, Thailand, Uganda, Zimbabwe.
A new 100 mg dispersible taste-masked levofloxacin formulation is more palatable and practical than the adult formulation.32 Emerging pharmacokinetic data demonstrates better bioavailability and significantly higher exposures compared with the same dose of crushed adult 250 mg tablets. Weight banded dosing with the dispersible levofloxacin tablet has been proposed, using doses of 15–20 mg/kg to achieve adult target exposures.33 WHO has endorsed both the moxifloxacin 100 mg and levofloxacin 100 mg dispersible tablet. Unlike levofloxacin, which is primarily excreted through the kidneys, moxifloxacin is 50% metabolized in the liver and the remainder eliminated unchanged in the faeces and urine.34 As such there is increased propensity for drug–drug interactions between moxifloxacin and other drugs (e.g. antiretroviral agents and rifampicin) that are metabolized by the liver.35,36 On balance of current evidence and given the high risk of progression from infection to severe disease in children <5 years, we recommend MDR-TB infected children be offered fluoroquinolone preventive therapy. This should be done in parallel with close monitoring for adverse effects, adherence and disease progression throughout the treatment duration and at least 1 year after MDR-TB exposure. Results from ongoing randomised controlled clinical trials will help to refine these preliminary recommendations.
Treatment of MDR-TB disease in children
Although children with MDR-TB generally have better treatment outcomes than adults, global treatment success rates for MDR-TB remain unacceptably low at just under 80%.37,38 The lack of paediatric data on the efficacy and safety of new and repurposed drugs used in MDR-TB is largely due to children being excluded from the first clinical trials with delayed inclusion in subsequent trials. Only recently have adolescents, and to some extent younger children, been included in MDR-TB drug trials.39 Urgent pharmacokinetic and pharmacodynamic data on second-line agents in young children are still awaited, but there is a renewed commitment to collect this data and studies are ongoing.40 We present the current evidence for the use of new and repurposed drugs to help clinicians make decisions about how to treat MDR-TB in children.
Bedaquiline
Bedaquiline (Sirturo®; Janssen pharmaceuticals) is a diarylquinoline that interferes with mycobacterial ATP synthase and was the first new drug approved to treat MDR-TB using a new mechanism of action.41 Bedaquiline has been associated with higher cure rates and reduced loss to follow up in adults with MDR-TB. When added to the standard MDR-TB regimen (five second-line anti-TB drugs) for 24 weeks and compared with placebo, bedaquiline resulted in faster time to culture conversion by 40 days and increased the rate of culture conversion by 20% at 24 weeks.41 Since receiving conditional approval for the treatment of MDR-TB by the FDA in 2012, it rapidly received subsequent approvals from the European Union, South Africa and India. Preliminary evidence from the STREAM (Evaluation of a Standardised Treatment Regimen of Anti-tuberculosis Drugs for Patients with Multidrug-resistant Tuberculosis) trial suggests that a shortened 9-month modified regimen for MDR-TB containing bedaquiline, which also includes levofloxacin, clofazimine, ethambutol, pyrazinamide and 4 months of isoniazid and prothionamide, is comparable with the standard WHO approved MDR-TB regimen of 18–24 months.28 Results from the next stage of the STREAM trial and the use of even shorter-course MDR-TB regimens are expected in 2021.42 Owing to good efficacy and tolerability, as well as oral bioavailability, bedaquiline has been recognized as a preferred (group A) drug in the most recent (2019) WHO MDR-TB treatment guidelines.43
Safety and dosing in children
Bedaquiline is currently approved by WHO for use in children >6 years of age and >15 kg in weight, and should be used routinely in place of injectable agents for MDR-TB.44 Dosing and safety data in younger children (<6 years of age) require urgent prioritization.45 An injectable-free MDR-TB regimen where an aminoglycoside could be substituted for bedaquiline is required to avoid the ototoxicity experienced by 25% of children who receive an injectable as part of their MDR-TB treatment regimen.46 For children <6 years of age or weighing <15 kg, there is currently no pharmacokinetic and safety data to guide dosing. Clinical trials (Jansen C211) evaluating the safety, tolerability and pharmacokinetic profile of bedaquiline in combination with other second-line agents are currently enrolling HIV-uninfected children as young as 2 years. Dosing recommendations from this trial are not expected until 2025.42 Although water-dispersible bedaquiline tablets are used in this clinical study, they are not yet commercially available. Recent data demonstrates that 100 mg bedaquiline tablets suspended in water has the same bioavailability as tablets swallowed whole or crushed. This suggests that the currently available formulation can be used, while awaiting availability of dispersible formulation(s).47 In the absence of formal dosing recommendations for children <6 years of age or <15 kg we recommend consultation with an expert if there is no alternative option available.
Adverse effects and drug–drug interactions
Initial concern regarding the potential toxic effects of bedaquiline was raised following unexpected late deaths in the first clinical trial.41 A subsequent systematic review showed ~10% of patients treated with bedaquiline experienced QTc prolongation (>450 ms) and 1% discontinued treatment for this reason. QTc prolongation appeared to correlate with drug exposure and peak at 16–18 weeks of therapy.48 A large observational study of bedaquiline containing regimens in 428 culture-confirmed MDR-TB cases showed that nausea (30%), peripheral neuropathy (20%) and otovestibular toxicity (20%) were more common than cardiotoxicity (10%).49 Of the 33 patients who died in the study, 19 had QTc information available of which only one had a QTc >500 ms. To date, there have been no reports of new or unexpected cardiac events in adolescents or children, although numbers remain small and close monitoring is required in both the research and clinical setting (Table 3).
Table 3.
Overview of new and repurposed TB drugs used in children.
| Drug | Dose | Duration | Indication | Use in TB meningitis | Drug–drug interactions (with ARV agents) |
Recommended monitoring |
|---|---|---|---|---|---|---|
|
Bedaquiline
(100 mg tablet) Group A |
6 mg/kg/day for 14 days then 3–4 mg/kg three times
/week OR weight-based dosing a (<15 kg consult an expert) |
6 months | Confirmed or probable MDR-TB; in children >6 years of age and >15 kg weight | Highly protein bound; predicted to have poor CSF penetration; uncertain value | Efavirenz: Reduced BDQ levels Lopinavir/ritonavir: Increased BDQ levels |
Baseline and monthly - ECGc - Electrolytes and liver functions |
|
Linezolid
(600 mg tablet / 20 mg/ml suspension / 150 mg tablets not yet available) Group A |
⩾16 years: 10–12 mg/kg/day ⩽16 years: 15 mg/kg/day OR weight-based dosing a |
As long as tolerated (usually not well tolerated beyond 8 weeks) |
Confirmed or probable MDR-TB; requires close monitoring of adverse effects | Excellent CNS penetration; highly recommended in MDR-TB meningitis | NRTIs: increased risk for adverse effects | Baseline and monthly - Full blood count (every 2 weeks for first 2 months) - Peripheral and optic neuropathy evaluatione: Rare adverse effects: lactic acidosis and pancreatitis |
|
Clofazimine
(50 or 100 mg gelcaps) Group B |
2–5 mg/kg/day (alternate days if gelcaps cannot be split) OR weight-based dosing a |
Entire treatment course or as long as tolerated | Confirmed or probable MDR-TB | Poor CSF penetration; uncertain value | None documented | Baselinef and monthly - ECGc |
|
Delamanid
b
(50 mg tablet) Group C |
3–4 mg/kg/day OR weight-based dosinga (<6 kg consult an expert) |
6 months | Confirmed or probable MDR-TB; currently preferred over bedaquiline in children <6 years of aged | Low CSF penetration, reasonable brain tissue penetration in animal studies; further research required; uncertain value | None documented | Baseline and monthly - ECGc - Electrolytes and liver functions (albumin) |
ARV, antiretroviral agent; BD, twice daily; BDQ, bedaquiline; CNS, central nervous system; CSF, cerebrospinal fluid; ECG, electrocardiogram; MDR, multidrug-resistant; NRTI, nucleoside reverse transcriptase inhibitor; TB, tuberculosis.
For weight-based dosing refer to ‘Management of multi-drug resistant tuberculosis in children: A field Guide 4th Edition’.
Available via the Global TB Consilium (tbconsilium@gmail.com) or Sentinel Project on Paediatric Drug Resistant TB (tbsentinelproject@gmail.com).
More frequent monitoring may be required if used with other QTc prolonging medications particularly moxifloxacin.
No pharmacokinetic data exists in this age group for bedaquiline (outcome of ongoing studies awaited); may be considered if access to delamanid is limited.
Monitor visual acuity and colour vision (as age appropriate). Corticosteroids may be considered if optic neuritis is confirmed.
Counsel patients and families about skin colour changes.
Bedaquiline is highly protein bound, extensively distributed to the tissues and has a long terminal half-life of 4–5 months. It is mainly metabolised by the cytochrome P450 enzymatic pathway.50 Widely used antiretroviral therapy (ART) such as ritonavir (inhibitor of CYP3A4) and efavirenz can affect enzymes involved in bedaquiline metabolism. Concomitant lopinavir–ritonavir (LPV/r) and bedaquiline administration result in threefold higher bedaquiline exposures, whereas efavirenz results in a 50% reduction of bedaquiline exposure.51,52 The clinical implications of the drug–drug interaction between LPV/r and bedaquiline are unclear but may enhance bedaquiline toxicity, most notably QTc prolongation. Studies in adults suggest that coadministration of efavirenz and bedaquiline should be avoided.52 Current WHO guidance for individuals on efavirenz or LPV/r containing ART regimens and bedaquiline is to change ART to nevirapine, which has minimal effect on bedaquiline concentrations. In these instances, close monitoring of hepatotoxicity with nevirapine and bedaquiline is recommended as nevirapine has a more severe side-effect profile.53 Preliminary data from the PRAXIS (Promoting Engagement in the Drug Resistant TB/HIV Care Continuum in South Africa) study suggests reduced antiretroviral adherence when twice daily nevirapine containing antiretroviral regimens are used, raising concerns about potential HIV treatment failure and emphasizing the need for increased treatment adherence support.53 Pharmacokinetic studies evaluating augmented dosing of bedaquiline with efavirenz and dose reduction of bedaquiline with LPV/r are urgently required. Bedaquiline with LPV/r should only be considered where other drug options are limited and used cautiously in a setting where close monitoring (monthly monitoring for QTc interval prolongation) and expertise is available.54 Owing to QTc prolongation, moxifloxacin should preferably not be used with bedaquiline whereas close safety monitoring is recommended if bedaquiline is used with clofazimine. Levofloxacin, which has shown to cause fewer drug–drug interactions and less QTc prolongation, is preferred in instances where a fluoroquinolone is used in bedaquiline-containing regimens.55,56
Delamanid
Delamanid (Deltyba®, Otsuka Pharmaceuticals) is a nitroimidazole agent that has potent bactericidal activity against replicating intracellular M. tuberculosis bacilli, by inhibiting synthesis of mycolic acids.57 An exploratory placebo-controlled, multinational clinical trial demonstrated a 50% increase in sputum culture conversion at 2 months in those receiving 100 mg or 200 mg delamanid twice daily combined with a WHO optimized background regimen.58 An extension of this trial using longer durations of delamanid and 24-month follow up demonstrated that the addition of 6 months of delamanid reduced mortality (from 8% to 1%) and was associated with higher favourable treatment outcomes compared with 2 months of delamanid therapy.59 Following strong evidence for benefit, delamanid received conditional market approval in the European Union, Japan and South Korea. In 2014, the WHO recommended delamanid for the treatment of MDR-TB in adults, and subsequently provided provisional guidance on its use in children in 2016.60 However a more recent phase III randomized controlled trial failed to confirm the efficacy benefit of delamanid. Although safety conclusions were supported, the demonstrated benefit of delamanid added to an optimized background regimen was small and limited to a reduction in time to culture conversion by 6 days.60 As such, current WHO guidelines only recognize delamanid as a Group C drug, although it may have particular value in children <6 years of age in whom bedaquiline use is not recommended.
Safety and dosing in children
Otsuka is leading a paediatric development program for delamanid with full enrolment in a safety, efficacy and pharmacokinetic study, but study outcomes are only expected in 2020.42 Interim WHO policy guidance in 2016 followed initial pharmacokinetic and safety data in children and detailed reporting from the delamanid compassionate use programme of 19 children with bacteriologically confirmed pulmonary MDR-TB. Delamanid drug exposures at doses ranging from 1.5 to 3.8 mg/kg administered to paediatric participants were comparable to the recommended adult dose of 100 mg twice daily.60
Preliminary data has shown delamanid to be safe in children aged as young as 3 years.61,62 Experts recommend that delamanid should replace injectable agents in children with RR/MDR-TB as young as 3 years at 3–4 mg/kg/dose, with consideration in children <3 years once safety and dosing data become available.45 A paediatric dispersible formulation of delamanid is being evaluated. Currently paediatric administration of delamanid requires suspending adult tablets in water, but bioequivalence studies are lacking. It is recommended that expert advice be sought through the Global TB Consilium (tbconsilium@gmail.com), where clinical guidance is provided within 48 h, or through the Sentinel Project on Paediatric Drug Resistant TB (tbsentinelproject@gmail.com) if delamanid is considered in < 3 years of age or <7 kg.
Adverse effects and drug–drug interactions
Delamanid is largely metabolized by albumin rather than cytochrome P450 enzymes. It neither inhibits nor induces P450 enzymes, making it an attractive option to minimise drug-drug interactions.57 There are no known drug–drug interactions with antiretroviral drugs.63 The primary safety concern is QTc prolongation. Preliminary paediatric data from Otsuka revealed a significant temporal trend with QTc interval increasing over the first month of delamanid exposure before plateauing and returning to baseline.60 Prolonged QTc is exacerbated by hypoalbuminaemia, hypokalaemia and other QTc prolonging medications.64 Although QTc prolongation was reported more frequently in adults receiving delamanid, there were no clinical events due to QTc prolongation even when used in combination with bedaquiline.65 In adults the most common adverse events reported were nausea, vomiting and upper abdominal pain followed by headache and insomnia. To date, delamanid has not been associated with any severe adverse events in children.59
Linezolid
Linezolid (Zyvox®, Pfizer; multiple generics also available) is a member of the oxazolidinone antibiotic class for the treatment of drug-resistant, Gram-positive bacterial infections. It also exhibits in vitro high activity against M. tuberculosis including MDR and extensively drug-resistant (XDR; MDR with added resistance to an injectable and a fluoroquinolone) strains.66 A prospective, randomised trial of linezolid added to a background regimen in patients with chronic XDR-TB demonstrated high efficacy, with 80% of patients showing sputum culture conversion by 4 months.67 A systematic review and meta-analysis further supports the efficacy of linezolid, reporting a pooled proportion of 97% of adult patients with culture conversion using doses of ⩾600 mg/day, and 68% with treatment success defined as at least 5 negative cultures in the last 12 months of therapy or completion of therapy without evidence of treatment failure.68 Paediatric data, although more limited, is promising with observational data showing good efficacy. Children treated for MDR-TB have similar high rates of culture conversion with over 80% having successful long-term outcomes.69 In many of these studies, good outcomes occur despite extensive disease, substantial drug resistance and failed treatment with other second-line agents. Importantly, linezolid has excellent cerebrospinal fluid (CSF) penetration and has been shown to contribute to CSF sterilization, making it a crucial agent in MDR-TB meningitis regimens.70 WHO recommends that linezolid be included as a core agent in the treatment of children with MDR-TB, using a regimen of at least four active agents.
Safety and dosing in children
Linezolid is well absorbed in both the oral suspension and tablet formulation, with bioavailability approaching 100%.71 Based on published pharmacokinetics, a dose of 10 mg/kg in children 3 months to <12 years will approximate peak concentrations reached with an adult dose of 600 mg. However, because of increased clearance, the exposure of a 10 mg/kg/dose in this age group will approximate to 300 mg in adults.69 Twice-daily dosing would be expected to provide similar coverage as a 600 mg adult dose. Dosages of 10–12 mg/kg once daily for children who weigh ⩾16 kg and 15 mg/kg once daily for children weighing <16 kg (not exceeding 600 mg daily) are now recommended, with potential twice-daily dosing in children with extensive disease or TB meningitis, at least initially.44,55 The most recent pharmacokinetic study would suggest that these doses offer optimal efficacy whilst minimizing concentration-related toxicity.72 However there remains uncertainty about the optimal dose and duration of linezolid in children to balance efficacy benefit with toxicity concerns.73 Further study is required, with ongoing close observation for adverse effects when used for a prolonged period of time. The availability of linezolid suspension is limited by prohibitive cost, such that dosing currently requires crushing of adult 600 mg tablets which can be problematic.
Adverse effects and drug–drug interactions
The use of linezolid has been limited by concerns with its toxicity profile, especially high rates of bone marrow suppression and peripheral neuropathy with prolonged use. Toxicity is concentration dependent and children tend to suffer fewer linezolid related adverse effects than adults, but peripheral neuropathy may be difficult to detect and can be irreversible.74 In children the most common adverse effects tend to be gastrointestinal disturbance (diarrhoea 9% and vomiting 4%), which rarely require alteration or discontinuation of the drug. With MDR-TB treatment, observations are that around 50% of children develop an adverse event of whom one quarter require dose reduction and 10% discontinuation.75 Grade 3 and 4 adverse events occur more commonly after ⩾60 days, and are more common in HIV-infected children on ARV therapy as multiple medications administered together have overlapping adverse event profiles.33,76 Children receiving linezolid should have close monitoring with full blood counts every 2 weeks for the first 2 months and then monthly, with evaluation for neuropathy at each visit and a low threshold for interruption or discontinuation. In addition close monitoring should be performed for children on nucleoside reverse-transcriptase inhibitors (NRTIs) given the potential for both linezolid and NRTIs to inhibit mitochondrial protein synthesis.75 Monitoring for mitochondrial toxicity includes evaluation for myopathy, lactic acidosis and metabolic disorders such as lipoatrophy, insulin resistance and dyslipidaemia particularly if linezolid is used for long durations.
Clofazimine
Clofazimine (Lamprene®, patent expired) is a fat soluble riminophenazine that has been used for many years to treat leprosy, but has also shown potential as a sterilising drug to treat MDR-TB. Its novel mode of action includes intracellular activity and membrane destabilization by increasing reactive oxidant species, thereby promoting the killing of antibiotic-tolerant M. tuberculosis persister organisms.77 A systematic review identified observational studies of patients with MDR-TB, with 65% experiencing either cure or treatment completion.78 Conclusions from the review were limited by significant study heterogeneity due to variable treatment regimens and durations. A subsequent randomised controlled trial of adult patients who received clofazimine for 21 months had earlier sputum culture conversion, earlier pulmonary cavity closure and higher treatment success (74% versus 54%) compared with a clofazimine-free regimen.77 No paediatric studies have been performed, but clofazimine is known to be effective and well tolerated in children with leprosy caused by Mycobacterium leprae.79
Safety and dosing
The pharmacokinetics of clofazimine in children has not been studied and to date there are no planned trials to evaluate this Group B drug. The current WHO recommendation is a dose of 2–5 mg/kg per day (maximum dose, 100 mg daily) for children. Clofazimine comes in 50- and 100-mg gelcaps which cannot be divided, making dosing difficult in younger children. Owing to the long half-life of clofazimine, lower doses on alternate days could be considered in younger children. Clofazimine should be given for the entire duration of therapy as long as it is tolerated.
Adverse effects and drug–drug interactions
Clofazimine has an excessively long half-life of 10–70 days in humans, with a propensity to cause tissue accumulation and crystallization.80 These pharmacokinetic properties of the drug lead to unwelcome adverse events such as skin discolouration. Rates of reversible skin discolouration are high (~90%) and can lead to social stigmatization but rarely result in discontinuation with adequate counseling.77 A new rimino-phenazine, TBI-166, which produces less skin discolouration is being explored in phase I trials. Cardiotoxicity has been described in adults with electrolyte abnormalities, and those on concomitant bedaquiline, delamanid and moxifloxacin.81,82 Although there are ongoing safety concerns regarding QTc prolongation serious life-threatening arrhythmias have yet to be reported. In the absence of clear safety data in children, we recommend that children have a monthly ECG if clofazimine is used with other QTc prolonging drugs. There are no drug-drug interactions between clofazimine and antiretrovirals.
Building a MDR-TB treatment regimen for children
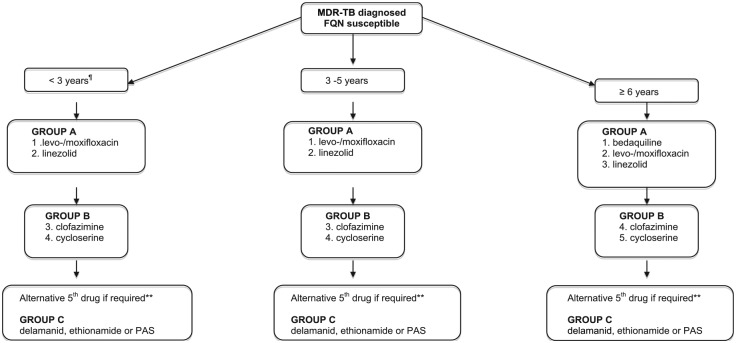
Building a treatment regimen for MDR-TB disease in children can be complex, but most recent WHO guideline provide guidance regarding the prioritisation of second-line agents into Group A (levofloxacin/moxifloxacin, bedaquiline, linezolid), B (clofazimine, cycloserine/terizidone) and C [ethambutol, delamanid, pyrazinamide, imipenem-cilastatin, meropenem, amikacin, eth/prothionamide, para-aminosalicylic (PAS) acid] drugs. It is recommended that bedaquiline be prioritised for the treatment of children ⩾6 years and delaminid for those aged 3–5 years.28 As with adults, regimens should consist of at least 4 drugs to which the organism is likely to be susceptible (based on drug susceptibility results from the child or the likely source case), and a 5th drug for the first several months in cases of severe disease, or with planned discontinuation of linezolid after 8 weeks. At least 3 agents should be included for the remainder of treatment after bedaquiline is ceased at 6 months. The regimen should avoid injectable agents if possible, given that up to 25% of children develop irreversible hearing loss. Figure 3 provides an overview of treatment options in different age groups in children with fluoroquinolone-susceptible MDR-TB.
Figure 3.
Building a treatment regimen for MDR-TB (fluoroquinolone susceptible) in children.
MDR-TB with fluoroquinolone resistance is associated with a 20% reduction in treatment success and poorer clinical outcomes compared to MDR-TB alone.83 In children >6 years of age with limited treatment options bedaquiline and delamanid combination therapy may be considered if there is adequate expertise and monitoring capacity. A small number of case reports and a clinical cohort of 28 adults from various settings reported few serious adverse events directly attributed to the combination of bedaquiline and delamanid,84,85 but treatment success rates were low (only 46%) in this select group.86 Data in children is currently lacking and use of bedaquiline and delamanid in combination should be carefully monitored and reported to inform future decision-making.61,87 Building a MDR-TB treatment regimen for children <6 years of age with fluoroquinolone resistance is a particular challenge as they are without the option of two Group A drugs. However delamanid may be regarded as a bedaquiline replacement in this age group, until bedaquiline safety and pharmacokinetic data becomes available. Ethionamide remains a potent second-line drug with good CSF penetration (if there is proven susceptibility) whereas PAS presents another oral option. For clinicians involved in the care of children with MDR-TB the recently published ‘Field guide for the management of MDR-TB in children’, developed by The Sentinel Project for Pediatric Drug-Resistant Tuberculosis, provides an important practical resource.44
Conclusion
Recent advances in the development of new and repurposed drugs for the treatment of MDR-TB infection and disease in children are promising, but a lot still remains to be done. Although child-friendly drug formulations for drug-susceptible and drug-resistant TB are now available through the Global Drug Facility (GDF), major barriers still include inadequate diagnostic and treatment access in resource-limited settings. There is also an urgent need for better pharmacokinetic and safety data in all relevant age groups. Tackling childhood TB will require better integration and strong partnerships between government and child health agencies, with an emphasis of TB prevention programs and political will to ensure that children are not left behind.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Huynh J  https://orcid.org/0000-0002-9247-4267
https://orcid.org/0000-0002-9247-4267
Contributor Information
Julie Huynh, Department of Infectious Diseases and Microbiology, The Children’s Hospital Westmead, New South Wales, 2145, Australia; Discipline of Child and Adolescent Health, University of Sydney, The Children’s Hospital Westmead, Westmead, New South Wales, 2145, Australia.
Ben J. Marais, Department of Infectious Diseases and Microbiology, The Children’s Hospital Westmead, New South Wales, Australia Discipline of Child and Adolescent Health, University of Sydney, The Children’s Hospital Westmead, New South Wales, Australia; Marie Bashir Institute for Infectious Diseases and Biosecurity, University of Sydney, Sydney, Australia.
References
- 1. World Health Organisation. Global tuberculosis report, https://www.who.int/tb/publications/global_report/en/ (2018, accessed 10 January 2019).
- 2. Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis 2016; 16: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 3. Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet 2014; 383: 1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jenkins HE, Yuen C.M. The burden of multidrug-resistant tuberculosis in children. Int J Tuberc Lung Dis 2018; 22: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dodd PJ, Yuen CM, Sismanidis C, et al. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017; 5: e898–e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah NS, Yuen CM, Heo M, et al. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2014; 58: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horsburgh CR, Jr., O’Donnell M, Chamblee S, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med 2010; 182: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Styblo K. The relationship between the risk of tuberculous infection and the risk of developing infectious tuberculosis. Bull IUAT 1985; 60: 117–119. [Google Scholar]
- 9. Narasimhan P, Wood J, Macintyre CR, et al. Risk factors for tuberculosis. Pulm Med 2013; 2013: 828939. [Google Scholar]
- 10. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8: 392–402. [PubMed] [Google Scholar]
- 11. Kruk A, Gie RP, Schaaf HS, et al. Symptom-based screening of child tuberculosis contacts: improved feasibility in resource-limited settings. Pediatrics 2008; 121: e1646–1652. [DOI] [PubMed] [Google Scholar]
- 12. Chiang SS, Swanson DS, Starke JR. New diagnostics for childhood tuberculosis. Infect Dis Clin North Am 2015; 29: 477–502. [DOI] [PubMed] [Google Scholar]
- 13. Marais BJ, Hesseling AC, Gie RP, et al. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis 2006; 42: e69–71. [DOI] [PubMed] [Google Scholar]
- 14. Zar H, Hanslo D, Apolles P, et al. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005; 365: 130–134. [DOI] [PubMed] [Google Scholar]
- 15. Brent A.J, Mugo D, Musyimi R, et al. Bacteriological diagnosis of childhood TB: a prospective observational study. Sci Rep 2017; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diallo T, Adjobimey M, Ruslami R, et al. Safety and Side Effects of Rifampin versus Isoniazid in Children. N Engl J Med 2018; 379: 454–463. [DOI] [PubMed] [Google Scholar]
- 17. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis 2005; 40: 670–676. [DOI] [PubMed] [Google Scholar]
- 18. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med 2018; 379: 440–453. [DOI] [PubMed] [Google Scholar]
- 19. Bamrah S, Brostrom R, Dorina F, et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis 2014; 18: 912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seddon JA, Hesseling AC, Finlayson H, et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis 2013; 57: 1676–1684. [DOI] [PubMed] [Google Scholar]
- 21. Schaaf HS, Gie RP, Kennedy M, et al. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics 2002; 109: 765–771. [DOI] [PubMed] [Google Scholar]
- 22. Burkhardt JE, Walterspiel JN, Schaad UB. Quinolone arthropathy in animals versus children. Clin Infect Dis 1997; 25: 1196–1204. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency. Quinolone and fluoroquinolone Article-31 referral - Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics, https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (2019, accessed 7 April 2019).
- 24. U.S. Food and Drug Administration. Drug Safety Communication: FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients, https://www.fda.gov/Drugs/DrugSafety/ucm628753.htm (2019, accessed 7 April 2019).
- 25. Seddon JA, Hesseling AC, Godfrey-Faussett P, et al. High treatment success in children treated for multidrug-resistant tuberculosis: an observational cohort study. Thorax 2014; 69: 458–464. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Prats AJ, Draper HR, Finlayson H, et al. Clinical and cardiac safety of long-term levofloxacin in children treated for multidrug-resistant tuberculosis. Clin Infect Dis 2018; 67: 1777–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seddon JA, Fred D, Amanullah F, et al. Post-exposure management of multidrug-resistant tuberculosis contacts: evidence-based recommendations, http://sentinel-project.org/2015/11/09/hms-center-for-global-health-delivery-dubai-publishes-1st-policy-brief/ (2015, accessed 10 January 2019).
- 28. World Health Organisation. WHO consolidated guidelines on drug-resistant tuberculosis treatment, https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?ua=1 (2019, accessed 10 April 2019). [PubMed]
- 29. Fox GJ, Nhung NV, Sy DN, et al. Household-contact investigation for detection of tuberculosis in Vietnam. N Engl J Med 2018; 378: 221–229. [DOI] [PubMed] [Google Scholar]
- 30. Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet 2015; 386: 2344–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis 2017; 64: 1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Purchase SE, Garcia-Prats AJ, De PK, et al. Acceptability of a novel levofloxacin dispersible tablet formulation in young children exposed to multidrug-resistant tuberculosis. Pediatr Infect Dis J, 38(6): 608–610, 2018. [DOI] [PubMed] [Google Scholar]
- 33. Garcia-Prats AJ, Purchase SE, Osman M, et al. Pharmacokinetics, safety, and dosing of novel pediatric levofloxacin dispersible tablets in children with multidrug-resistant tuberculosis exposure. Antimicrob Agents Chemother 2019; 63: e01865–01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schaaf HS, Garcia-Prats AJ, McKenna L, et al. Challenges of using new and repurposed drugs for the treatment of multidrug-resistant tuberculosis in children. Expert Rev Clin Pharmacol 2018; 11: 233–244. [DOI] [PubMed] [Google Scholar]
- 35. Thee S, Garcia-Prats AJ, Draper HR, et al. Pharmacokinetics and safety of moxifloxacin in children with multidrug-resistant tuberculosis. Clin Infect Dis 2015; 60: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nijland HM, Ruslami R, Suroto AJ, et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007; 45: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 37. Harausz EP, Garcia-Prats AJ, Law S, et al. Treatment and outcomes in children with multidrug-resistant tuberculosis: A systematic review and individual patient data meta-analysis. PLoS Med 2018; 15: e1002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chiang SS, Starke JR, Miller AC, et al. Baseline Predictors of Treatment Outcomes in Children With Multidrug-Resistant Tuberculosis: A Retrospective Cohort Study. Clin Infect Dis 2016; 63: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 39. Becerra MC, Swaminathan S. Commentary: a targets framework: dismantling the invisibility trap for children with drug-resistant tuberculosis. J Public Health Policy 2014; 35: 425–454. [DOI] [PubMed] [Google Scholar]
- 40. Nachman S, Ahmed A, Amanullah F, et al. Towards early inclusion of children in tuberculosis drugs trials: a consensus statement. Lancet Infect Dis 2015; 15: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014; 371: 723–732. [DOI] [PubMed] [Google Scholar]
- 42. Resist-TB. Drug resistant TB clinical trials progress report, http://www.resisttb.org/?page_id=1602 (2018, accessed 20 January 2019).
- 43. World Health Organisation. Rapid communication. Key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). https://www.who.int/tb/publications/2018/rapid_communications_MDR/en/ (2018, accessed 20 January 2019).
- 44. The Sentinel Project for Pediatric Drug-Resistant Tuberculosis. Management of multidrug-resistant tuberculosis in children: a field guide, http://sentinel-project.org/wp-content/uploads/2019/02/Updated_DRTB-Field-Guide-2019-V3.pdf (2019, accessed 10 April 2019).
- 45. Seddon JA, Schaaf HS, Marais BJ, et al. Time to act on injectable-free regimens for children with multidrug-resistant tuberculosis. Lancet Respir Med 2018; 6: 662–664. [DOI] [PubMed] [Google Scholar]
- 46. Seddon JA, Thee S, Jacobs K, et al. Hearing loss in children treated for multidrug-resistant tuberculosis. J Infect 2013; 66: 320–329. [DOI] [PubMed] [Google Scholar]
- 47. Svensson EM, du Bois J, Kitshoff R, et al. Relative bioavailability of bedaquiline tablets suspended in water: implications for dosing in children. Br J Clin Pharmacol. Epub ahead of print 27 June 2018. DOI: 10.1111/bcp.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pontali E, Sotgiu G, Tiberi S, et al. Cardiac safety of bedaquiline: a systematic and critical analysis of the evidence. Eur Respir J 2017; 50. [DOI] [PubMed] [Google Scholar]
- 49. Borisov SE, Dheda K, Enwerem M, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J 2017; 49. [DOI] [PubMed] [Google Scholar]
- 50. Svensson EM, Dooley KE, Karlsson MO. Impact of lopinavir-ritonavir or nevirapine on bedaquiline exposures and potential implications for patients with tuberculosis-HIV coinfection. Antimicrob Agents Chemother 2014; 58: 6406–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brill MJ, Svensson EM, Pandie M, et al. Confirming model-predicted pharmacokinetic interactions between bedaquiline and lopinavir/ritonavir or nevirapine in patients with HIV and drug-resistant tuberculosis. Int J Antimicrob Agents 2017; 49: 212–217. [DOI] [PubMed] [Google Scholar]
- 52. Svensson EM, Aweeka F, Park JG, et al. Model-based estimates of the effects of efavirenz on bedaquiline pharmacokinetics and suggested dose adjustments for patients coinfected with HIV and tuberculosis. Antimicrob Agents Chemother 2013; 57: 2780–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O’Donnell MR, Padayatchi N, Daftary A, et al. Antiretroviral switching and bedaquiline treatment of drug-resistant tuberculosis HIV co-infection. Lancet HIV 2019; 6: e201–e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schaaf HS, Garcia-Prats AJ, McKenna L, et al. Challenges of using new and repurposed drugs for the treatment of multidrug-resistant tuberculosis in children. Expert Rev Clin Pharmacol 2018; 11: 233–244. [DOI] [PubMed] [Google Scholar]
- 55. Harausz EP, Garcia-Prats AJ, Seddon JA, et al. New and repurposed drugs for pediatric multidrug-resistant tuberculosis. Practice-based recommendations. Am J Respir Crit Care Med 2017; 195: 1300–1310. [DOI] [PubMed] [Google Scholar]
- 56. Denti P, Garcia-Prats AJ, Draper HR, et al. Levofloxacin population pharmacokinetics in south african children treated for multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2018; 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Y, Matsumoto M, Ishida H, et al. Delamanid: from discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB). Tuberculosis (Edinb) 2018; 111: 20–30. [DOI] [PubMed] [Google Scholar]
- 58. Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012; 366: 2151–2160. [DOI] [PubMed] [Google Scholar]
- 59. Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J 2013; 41: 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. World Health Organisation. The use of delamanid in the treatment of multidrug-resistant tuberculosis in children and adolescents: interim policy guidance, http://apps.who.int/iris/bitstream/handle/10665/250614/9789241549899-eng.pdf;jsessionid=79A71A0B84C9703812B4B15FD9B9B38F?sequence=1 (2016, accessed 20 January 2019). [PubMed]
- 61. Tadolini M, Garcia-Prats AJ, D’Ambrosio L, et al. Compassionate use of new drugs in children and adolescents with multidrug-resistant and extensively drug-resistant tuberculosis: early experiences and challenges. Eur Respir J 2016: ERJ-00705–02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hafkin J, Frias M, De Leon A, et al. Long-term safey, tolerability, and pharmacokinetics of delamanid in pediatric MDR-TB patients ages 12–17 years. In: 46th Union World Conference on Lung Health, Capetown, South Africa, 2015. [Google Scholar]
- 63. Mallikaarjun S, Wells C, Petersen C, et al. Delamanid coadministered with antiretroviral drugs or antituberculosis drugs shows no clinically relevant drug-drug interactions in healthy subjects. Antimicrob Agents Chemother 2016; 60: 5976–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lewis JM, Sloan DJ. The role of delamanid in the treatment of drug-resistant tuberculosis. Ther Clin Risk Manag 2015; 11: 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lachatre M, Rioux C, Le Du D, et al. Bedaquiline plus delamanid for XDR tuberculosis. Lancet Infect Dis 2016; 16: 294. [DOI] [PubMed] [Google Scholar]
- 66. Diekema DJ, Jones RN. Oxazolidinone antibiotics. Lancet 2001; 358: 1975–1982. [DOI] [PubMed] [Google Scholar]
- 67. Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012; 367: 1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cox H, Ford N. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2012; 16: 447–454. [DOI] [PubMed] [Google Scholar]
- 69. Garcia-Prats AJ, Rose PC, Hesseling AC, et al. Linezolid for the treatment of drug-resistant tuberculosis in children: a review and recommendations. Tuberculosis (Edinb) 2014; 94: 93–104. [DOI] [PubMed] [Google Scholar]
- 70. Sun F, Ruan Q, Wang J, et al. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis Antimicrob Agents Chemother 2014; 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tan TQ. Update on the use of linezolid: a pediatric perspective. Pediatr Infect Dis J 2004; 23: 955–956. [DOI] [PubMed] [Google Scholar]
- 72. Srivastava S, Deshpande D, Pasipanodya J, et al. Optimal clinical doses of Faropenem, Linezolid, and Moxifloxacin in children with disseminated tuberculosis: Goldilocks Clin Infect Dis 2016; 63: S102–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Garcia-Prats AJ, Schaaf HS, Draper HR, et al. Pharmacokinetics, optimal dosing, and safety of linezolid in children with multidrug-resistant tuberculosis: combined data from two prospective observational studies. PLoS Med 2019; 16: e1002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chiappini E, Conti C, Galli L, et al. Clinical efficacy and tolerability of linezolid in pediatric patients: a systematic review. Clin Ther 2010; 32: 66–88. [DOI] [PubMed] [Google Scholar]
- 75. Rose PC, Hallbauer UM, Seddon JA, et al. Linezolid-containing regimens for the treatment of drug-resistant tuberculosis in South African children. Int J Tuberc Lung Dis 2012; 16: 1588–1593. [DOI] [PubMed] [Google Scholar]
- 76. Kjollerstrom P, Brito MJ, Gouveia C, et al. Linezolid in the treatment of multidrug-resistant/extensively drug-resistant tuberculosis in paediatric patients: experience of a paediatric infectious diseases unit. Scand J Infect Dis 2011; 43: 556–559. [DOI] [PubMed] [Google Scholar]
- 77. Tang S, Yao L, Hao X, et al. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis 2015; 60: 1361–1367. [DOI] [PubMed] [Google Scholar]
- 78. Gopal M, Padayatchi N, Metcalfe JZ, et al. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis 2013; 17: 1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kroger A, Pannikar V, Htoon M, et al. International open trial of uniform multi-drug therapy regimen for 6 months for all types of leprosy patients: rationale, design and preliminary results. Trop Med Int Health 2008; 13: 594–602. [DOI] [PubMed] [Google Scholar]
- 80. Lu Y, Zheng M, Wang B, et al. Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob Agents Chemother 2011; 55: 5185–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wallis RS. Cardiac safety of extensively drug-resistant tuberculosis regimens including bedaquiline, delamanid and clofazimine. Eur Respir J 2016; 48: 1526–1527. [DOI] [PubMed] [Google Scholar]
- 82. Yoon HY, Jo KW, Nam GB, et al. Clinical significance of QT-prolonging drug use in patients with MDR-TB or NTM disease. Int J Tuberc Lung Dis 2017; 21: 996–1001. [DOI] [PubMed] [Google Scholar]
- 83. Falzon D, Gandhi N, Migliori GB, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013; 42: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ferlazzo G, Mohr E, Laxmeshwar C, et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect Dis 2018; 18: 536–544. [DOI] [PubMed] [Google Scholar]
- 85. Maryandyshev A, Pontali E, Tiberi S, et al. Bedaquiline and delamanid combination treatment of 5 patients with pulmonary extensively drug-resistant tuberculosis. Emerg Infect Dis 2017; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mohr E, Ferlazzo G, Hewison C, et al. Bedaquiline and delamanid in combination for treatment of drug-resistant tuberculosis. Lancet Infect Dis 2019; 19: 470. [DOI] [PubMed] [Google Scholar]
- 87. Tadolini M, Tiberi S, Migliori GB. Combining bedaquiline and delamanid to treat multidrug-resistant tuberculosis. Lancet Infect Dis 2018; 18: 480–481. [DOI] [PubMed] [Google Scholar]