Abstract
Fibrosis usually results from dysregulated wound repair and is characterized by excessive scar tissue. It is a complex process with unclear mechanisms. Accumulating evidence indicates that epigenetic alterations, including histone acetylation, play a pivotal role in this process. Histone acetylation is governed by histone acetyltransferases (HATs) and histone deacetylases (HDACs). HDACs are enzymes that remove the acetyl groups from both histone and nonhistone proteins. Aberrant HDAC activities are observed in fibrotic diseases, including cardiac and pulmonary fibrosis. HDAC inhibitors (HDACIs) are molecules that block HDAC functions. HDACIs have been studied extensively in a variety of tumors. Currently, there are four HDACIs approved by the US Food and Drug Administration for cancer treatment yet none for fibrotic diseases. Emerging evidence from in vitro and in vivo preclinical studies has presented beneficial effects of HDACIs in preventing or reversing fibrogenesis. In this review, we summarize the latest findings of the roles of HDACs in the pathogenesis of cardiac and pulmonary fibrosis and highlight the potential applications of HDACIs in these two fibrotic diseases.
Keywords: cardiac fibrosis, epigenetic, gene expression, HDAC, HDAC inhibitor, histone acetylation, myofibroblasts, pulmonary fibrosis
Introduction
When responding to injury, damaged tissue begins the repair process to recover the tissue’s original architecture and function. This injury–repair process is complex and must be well orchestrated. Dysregulation of this process would result in fibrosis and ultimately organ failure.1 Fibrosis can affect many organs, including the heart and lungs.2 Despite the different pathways in the different processes of organ fibrosis, there are some core signal pathways, such as transforming growth factor β1 (TGF-β1), which is activated in virtually all fibrotic process.3 TGF-β1 activates fibroblasts to become myofibroblasts.4 Myofibroblasts are the key effector cells, characterized by the production of extracellular matrix (ECM) and the expression of alpha-smooth muscle actin (α-SMA).4 The pathogenesis of fibrosis is unclear, for example, it is not completely understood why myofibroblasts are transient in the normal injury–repair process but are persistent in fibrotic diseases.5 Increasing evidence reveals that epigenetic mechanisms play a pivotal role in this process and indicates that epigenetic methods may provide great therapeutic opportunities for this disorder.
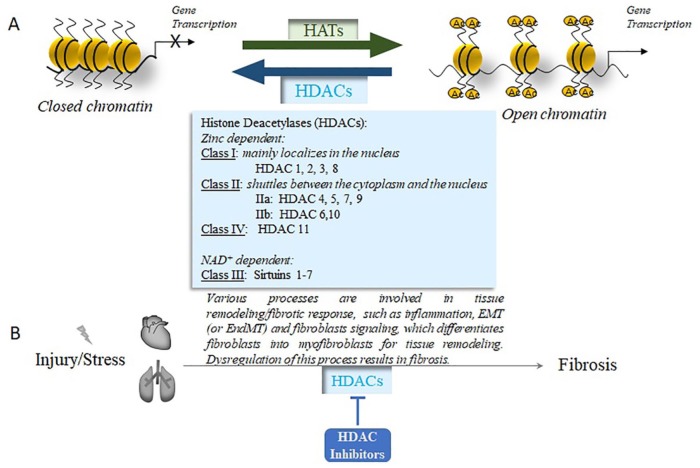
Epigenetic process refers to the inheritable alterations in phenotype without changes in gene sequence,6 which is critical in regulating gene expression. There are three major epigenetic modifications: DNA methylation, histone modifications, and microRNAs .7,8 Histone modification is a reversible process, which indicates the covalent posttranslational modification of histone proteins, including methylation, acetylation, phosphorylation, adenylation, ubiquitination, sumoylation, and ADP ribosylation.9 The most common and best-characterized histone modification is acetylation. The acetylation of histone is governed by histone acetyltransferases (HATs) and histone deacetylases (HDACs),10 which work together to control histone acetylation (Figure 1A). HATs catalyze the transfer of acetyl groups from acetyl-CoA to the lysine ε-amino groups on the N-terminal tails of histones, which weakens the interaction of the histone tail and DNA, promoting transcriptional activation. In contrast, HDACs remove the acetyl groups from acetylated histones, increase chromatin condensation, and suppress gene transcription.11
Figure 1.
A. Histone acetyltransferases and histone deacetylases control histone acetylation. HATs transfer the acetyl groups to the lysine residuals on histone proteins; this weakens the interaction of histone and DNA to promote gene transcription. HDACs remove the acetyl groups on histone proteins, increase chromatin condensation, and suppress gene transcription. There are four major classes of HDACs, as listed.
Ac, acetylated histone; HATs, histone acetyltransferases; HDACs, histone deacetylases.
B. HDAC inhibitors target various processes in which HDACs are involved in cardiac and pulmonary fibrosis. Cardiac or pulmonary injury/stress can trigger the remodeling of the heart or lung tissues. This involves many processes, such as inflammation, epithelial to mesenchymal transition (EMT), or endothelial to mesenchymal transition (EndMT). EMT (or EndMT) provides additional fibroblasts for the remodeling process. The fibroblasts, regardless of their origins, differentiate into myofibroblasts, the central effector cells of the remodeling process. Dysregulation of this remodeling process leads to cardiac or pulmonary fibrosis. Different HDACs are involved in this process, which could be blocked by various HDAC inhibitors to improve the resolution of fibrosis.
Interactions between HATs and HDACs control the acetylation status, and the HATs and HDACs are usually linked or physically associated with other proteins that have catalytic activities12 or are regulatory factors.13 It is critical to consider their roles in a cellular context–dependent manner and their interactions with other regulators.14 For example, inhibition of HAT p300 decreases the transcription of collagen and α-SMA in cardiac fibrosis by blocking the acetylated histone associated with the genes.15 On the other hand, inhibition of HDAC induces general histone acetylation yet decreases collagen expression by depleted association of acetylated histone at the collagen promoter region.16 The mechanisms of this change is not clear, but emerging data have shown that HDAC inhibition can induce histone deacetylation at a localized promoter to downregulate the target genes.16–19 In addition to histone, many transcriptional factors such as Sp1 and nuclear factor (NF)-kB are also targets of HDAC deacetylation.20,21 Inhibition of HDAC would also affect these proteins, which would affect the target gene transcription. A study in lymphoma cells observed increased transcriptional factor Sp1 acetylation by HDAC inhibition, which significantly decreased the binding of Sp1 to the Bcl-2 promoter region and contributed to Bcl-2 downregulation.19 Thus, extensive evaluations of associated proteins that are involved in the transcriptional control of a target gene are needed to determine gene expression change when inhibiting HDAC.
HDACs are involved in many cellular processes. Dysfunction of the HDACs is associated with various diseases, such as cancer, diabetes, and cardiac hypertrophy.22–24 HDAC inhibitors (HDACIs) are molecules that bind with HDACs and interfere/block their functions.25 HDACIs are involved in processes such as regulating gene expression and apoptosis, through acetylation of histone and nonhistone proteins.17,22 Currently, four HDACIs have been approved by the US Food and Drug Administration (FDA) for clinical use in hematologic tumors.26 There are many clinical trials in progress of HDACIs for a variety of tumors27 (Table 1). However, for many other diseases, such as fibrotic diseases, HDACIs are only in preclinical studies. In the past decade, a growing number of studies have demonstrated that HDACs are involved in the initiation and progression of fibrosis in multiple organs, including heart,28 lung,29 liver,30 and kidney.31 HDACIs have been shown to ameliorate various forms of fibrosis in animal models.16,32,33 In this review, we focus on the latest findings of the roles of HDACs in the pathogenesis of cardiac and pulmonary fibrosis and highlight the potential applications and limitations of HDACIs in these two fibrotic diseases.
Table 1.
HDACIs in clinical trials.
| HDACI | Class | HDAC Target | Clinical Usage | Phase | Reference |
|---|---|---|---|---|---|
| Vorinostat (SAHA, Zolinza®) | Hydroxamates | Class I, II, and IV | CTCL | Phase II | Duvic et al.34 |
| Soft tissue sarcomas | Phase II | Schmitt et al.35 | |||
| Sickle cell disease | Phase I/II | Okam et al.36 | |||
| Melanoma | Phase II | Haas et al.37 | |||
| Gastrointestinal cancer | Phase I | Doi et al.38 | |||
| Follicular and mantle cell lymphoma | Phase I | Watanabe et al.39 | |||
| Prostate cancer | Phase II | Bradley et al.40 | |||
| Glioblastoma multiforme | Phase II | Galanis et al.41 | |||
| HIV infection | Phase II | Elliott et al.42 | |||
| Panobinostat (LBH589, Farydac®) | Hydroxamates | Class I and II | MDS or AML | Phase 3 | Bug et al.43 |
| Metastatic melanoma | Phase I | Ibrahim et al.44 | |||
| Neuroendocrine tumors | Phase II | Jin et al.45 | |||
| Solid tumors | Phase I | Jones et al.46 | |||
| HIV infection | Phase I/II | Olesen et al.47 | |||
| Belinostat (Beleodaq™, PXD101) | Hydroxamates | Class I and II | Lymphoma | Phase II | Puvvada et al.48 |
| PTCL | Phase II | O’Connor et al.49; Foss et al.50 | |||
| Liver cancer | Phase I/II | Wang et al.51; Yeo et al.52 | |||
| Ovarian cancer. | Phase II | Dizon et al.53 | |||
| Givinostat (ITF2357) | Hydroxamates | Class I and II | Duchenne muscular dystrophy | Phase I/II | Bettica et al.54 |
| Polycythemia vera | Phase II | Finazzi et al.55 | |||
| Myeloproliferative diseases | Phase IIA | Rambaldi et al.56 | |||
| Romidepsin (FK228, Istodax®) | Cyclic tetrapeptide | Class I and II | PTCL | Phase II | Shustov et al.57 |
| Non–small-cell lung cancer | Phase I | Gerber et al.58 | |||
| HIV infection | Phase I | Sogaard et al.59 | |||
| Entinostat (MS-275) | Benzamides | Class I | Metastatic colorectal cancer | Phase II | Azad et al.60 |
| Breast cancer | Phase II | Connolly et al.61 | |||
| Hodgkin lymphoma | Phase II | Batlevi et al.62 | |||
| Myeloid neoplasm | Phase II | Prebet et al.63; Fandy et al.64 | |||
| Valproic acid (VPA) | Short-chain fatty acids | Class I | Gastric cancer | Phase II | Fushida et al.65 |
| Non-small cell lung cancer | Phase I | Chu et al.66 | |||
| Rectal cancer | Phase I/II | Avallone et al.67 |
AML, acute myeloid leukemia; CTCL, cutaneous T-cell lymphoma; HIV, human immunodeficiency virus; MDS, myelodysplastic syndrome; PTCL, peripheral T-cell lymphoma.
Cardiac and pulmonary fibrosis
Cardiac fibrosis is the final stage of various cardiovascular diseases including hypertension, myocardial infarction (MI), and ischemic, dilated, and hypertrophic cardiomyopathy.68 Excess deposition of ECM, such as collagen and fibronectin, impairs cardiac systolic and diastolic functions, disrupts electrical conduction, and predisposes to fatal arrhythmias, heart failure, even sudden death.69,70 Although our knowledge of cardiac fibrosis has advanced tremendously over the past decade, there are still no viable therapies to reverse this disorder. Similarly, a variety of lung injuries and diseases result in pulmonary fibrosis.71 The most common and pernicious form of pulmonary fibrosis is idiopathic pulmonary fibrosis (IPF).72 IPF is a chronic progressive disease with high mortality and unknown etiology.73 The mechanisms governing fibrosis are not well understood, but studies show that epigenetic mechanisms are involved in the pathogenesis of cardiac and pulmonary fibrosis.74,75 HDACs participate in the process of cardiac and pulmonary fibrosis.76–78 HDACIs are reported to improve the resolution of these two kinds of fibrosis in preclinical studies .17,79 We reviewed the role of DNA methylation in organ fibrosis80; here, we focus on HDAC family members in the process of cardiac and pulmonary fibrosis.
HDACs, HDACIs, and related clinical/preclinical studies
HDACs
Eighteen HDACs have been identified in mammals (Figure 1A) and are classified into four groups based on their homology to yeast proteins.81 Class I includes HDAC 1, 2, 3, and 8, which exist in the nucleus. Class II has HDAC 4, 5, 6, 7, 9, and 10; these enzymes shuttle between the cytoplasm and nucleus, which further divide into two subgroups as Class IIa (HDAC 4, 5, 7, and 9) and Class IIb (HDAC 6, 10). HDAC11 is the sole member of Class IV, which shares the catalytic domain of both class I and II HDACs. These three classes of HDACs are all zinc-dependent enzymes, usually called classical HDACs.82 However, Class III HDAC, including sirtuins 1–7, is nicotinamide adenine dinucleotide (NAD+) dependent. Sirtuins are most commonly correlated with aging.83 We focus on the classical HDACs and their inhibitors in this review.
In addition to histone proteins, HDACs also deacetylate many nonhistone proteins to control diverse cellular processes.84 As we mentioned earlier, some transcriptional factors are targets of acetylation, whose activities can be affected by HDAC or HDACI.85 It is important to note that acetylation and deacetylation occur not only in the nucleus but also in the cytoplasm, and they influence the functions of target substrates.84
HDACIs
In general, the zinc-dependent HDACIs have three pharmacophores: a Zn2+-binding group, a polar connection unit, and a surface binding or cap group.86 Based on the structure of the Zn2+-binding group, the HDACIs are divided into four structurally distinct groups: (a) hydroxamic acids, (b) short-chain fatty acids (SCFAs), (c) benzamides, and (d) cyclic peptides.87 The group of SCFAs is further categorized into four classes according to their distinguished structures: linear compounds, cyclic tetrapeptides, cyclic depsipeptides, and miscellaneous HDACIs.87,88
The first discovered HDACI was trichrostatin A (TSA), identified from nature sources.89 Since then, numerous natural HDACI products have been obtained from microbes, dietary plants, and medicinal plants.90 HDACIs have emerged as a new class of anticancer drugs; a series of HDACIs have been synthesized and the number of HDACIs is constantly being updated.
HDACIs target histone and nonhistone proteins. Many acetylated nonhistone proteins are deacetylated by HDACs84 and can be modulated by HDACIs.91 These nonhistone proteins include structure proteins (such as α-tubulin), chaperone proteins (such as Hsp90), DNA binding nuclear receptors (such as androgen receptor), transcriptional factors (such as p53), signaling mediators, and enzymes.92 Therefore, HDACIs not only modulate the histone but also nonhistone protein acetylation statuses and affect their functions. For example, Quisinostat, a novel second-generation HDACI, increases p53 acetylation at K382/K373 sites, upregulates p21, and results in G1 phase arrest.93 Panobinostat is a pan-HDACI that is approved for cancer treatment; its cytotoxicity is linked to α-tubulin acetylation.94
Panobinostat is one of the four US FDA-approved HDACIs for clinical use, all of which are anticancer drugs. Panobinostat (Farydac®, Novartis) is a hydroxamic acid, a Class I and II HDACI, and was approved for treating multiple myeloma in 2015.95 Belinostat (Beleodaq™, Spectrum) is a pan-HDACI that was approved for treating patients with relapsed or refractory peripheral T-cell lymphoma (PTCL) in 2014.96 Romidepsin (Istodax®, Celgene) obtained FDA approval for treating cutaneous T-cell lymphoma in 200997 and then in 2011 was granted approval for treating PTCL.98 Vorinostat (Zolinza®, Merck, also known as SAHA, suberoylanilide hydroxamic acid) was the first approved pan-HDACI for the treatment of T-cell lymphoma in 2006.99
In addition to these HDACIs approved for clinical use, there are many clinical trials of HDACIs in other diseases34–67 (Table 1). SAHA was used in a phase II clinical trial to treat human immunodeficiency virus (HIV)–infected individuals42 and in phase I/II clinical trials of sickle cell disease36 with promising results. Givinostat (ITF2357), the hydroxamic acid HDACI, exhibited impressive therapeutic benefit and an excellent safety profile in a study in systemic-onset juvenile idiopathic arthritis.100 Although there is no approved clinical trial on HDACI in fibrotic diseases, many HDACIs are under extensive investigation in preclinical fibrotic models, including cardiac and pulmonary fibrosis101–103 (Figure 1B). The preclinical studies of HDACIs in cardiac and pulmonary fibrosis are summarized in Table 2.
Table 2.
HDACIs used in cardiac and pulmonary fibrosis.
| Disease | Cell or animal model | HDACI | Mechanism | Reference |
|---|---|---|---|---|
| Cardiac fibrosis | Spontaneously HP rats | Valproic acid | Mineralocorticoid receptor acetylation | Kang et al.104 |
| Pressure overload induced by abdominal aortic constriction | Valproic acid | Inhibit sympathetic outflow | Liu et al.105 | |
| Ang II–induced cardiac fibrosis rats, myocardial pericytes | Valproic acid | HDAC 4–dependent phosphorylation of ERK | Zhang et al.106 | |
| Left anterior descending coronary artery ligation MI mice | Valproic acid, tributyrin | Regulate histone H4 acetylation and atrial natriuretic peptide mRNA expression | Lee et al.107 | |
| Left anterior descending coronary artery ligation MI mice | Trichostatin A | Through c-kit signaling | Zhang et al.108 | |
| Isoproterenol induced HF rats | MPT0E014 | Decrease TGF-β and Ang II type I receptor | Kao et al.109 | |
| Left anterior descending coronary artery occlusion–induced MI | Mocetinostat | Reduce Akt/GSK3b signaling; increase apoptosis | Nural-Guvener et al.110 | |
| Coronary artery occlusion–induced MI rats, cardiac fibroblasts | Mocetinostat | Attenuate interleukin-6/Stat3 signaling | Nural-Guvener et al.111 | |
| Ang II and aortic banding–induced cardiac hypertrophy mice | Trichostatin A Valproic acid SK-7041 | Inhibit Class I HDAC | Kee et al.112 | |
| Ang II–induced cardiac fibrosis | MGCD0103 | Control differentiation of bone marrow-derived fibrocytes | Williams et al.113 | |
| Isoproterenol-induced cardiac fibrosis rats | Tubacin | Elevate RASSF1A expression | Tao et al.114 | |
| Streptozotocin-induced diabetes mice | Sodium butyrate | Activate glucose transporters 1 acetylation and p38 phosphorylation | Chen et al.115 | |
| Type 1 diabetes OVE26 mice | RGFP966 | Inhibit DUSP5/ERK1/2 pathway | Xu et al.116 | |
| Deoxycorticosterone acetate–salt HP rats | SAHA | Decrease inflammatory cytokines | Iyer et al.117 | |
| Aortic constriction cardiac–induced HP mice | Trichostatin A | Suppress NF-κB target genes | Ooi et al.118 | |
| Left descending coronary artery ligation–induced MI | Valproic acid | Through Foxm1 pathway | Tian et al.119 | |
| Left descending coronary artery ligation–induced MI | Givinostat | Decrease EMT and inflammation | Milan et al.120 | |
| Primary cardiac fibroblasts | Trichostatin A MGCD0103 Apicidin | Block cell cycle progression | Schuetze et al.121 | |
| Atrial fibrosis | HOPX mice | Trichostatin A | Normalize connexin40 remodelling | Liu et al.32 |
| HOPX mice, dogs with sustained atrial fibrillation | CI-994 | Seki et al.122 | ||
| Deoxycorticosterone acetate–induced HP rats | CG200745 | Decrease collagen 1, collagen 3 connective tissue growth factor and fibronectin | Lee et al.123 | |
| Pulmonary fibrosis | Primary human lung fibroblasts | SAHA | Inhibit myofibroblast differentiation | Wang et al.124 |
| Bleomycin-induced pulmonary fibrosis mice, IPF fibroblasts | SAHA | Down-regulate collagen 3A1expressionIncreased lung fibroblast apoptosis | Zhang et al.16;Sanders et al.17 | |
| Lung fibroblasts | SAHA | Up-regulate cyclooxygenase-2 and prostaglandin E2 expression | Pasini et al.125 | |
| TGF-β1–induced EMT A549 cell | Valproic acid | Inhibit EMT, increase H3K27ac | Noguchi et al.126 | |
| Paraquat-induced pulmonary fibrosis, macrophages | Valproic acid | Enhance EMT, activate H3K4me3 and H3K9ac | Hu et al.127 | |
| TGF-β1 induced EMT A549 cell line, bleomycin induced pulmonary fibrosis | Trichostatin A | Restore surfactant protein-C expression via hyperacetylation of histone H4 | Ota et al.128 | |
| Bleomycin-induced pulmonary fibrosis | Trichostatin A | Inhibit HDAC2 expression | Ye et al.129 | |
| IPF and normal fibroblasts | Spiruchostatin A | Increase H3 acetylation and p21expression | Davies et al.130 | |
| Bleomycin-induced pulmonary fibrosis, lung fibroblasts | Tubastatin | Repress TGF-β–PI3K-Akt pathway | Saito et al.78 | |
| IPF lung tissue, normal human lung fibroblasts, bleomycin-induced pulmonary fibrosis | NCC170 | Ameliorate TGF-β1–induced loss of H3K27ac at the PPAR-γ gene enhancer | Saito et al.131 | |
| IPF lung tissue, primary IPF fibroblasts | LBH589 Valproic acid | Decrease ECM synthesis associated gene expression | Korfei et al.103 | |
| IPF lung fibroblasts, bleomycin-induced fibrosis mice | Trichostatin A | Restore Fas-mediated apoptosis | Huang et al.132 |
Ang II, angiotensin II; DUSP5, dual specific phosphatase 5; EMT, epithelial-to-mesenchymal; HDAC, histone deacetylases; HDACI, histone deacetylation inhibitor; HF, heart failure; HOPX, overexpressing homeodomain-only protein; HP, hypertension; IPF, idiopathic pulmonary fibrosis; MI, myocardial infarction; RASSF1ARas-association domain family protein 1A; SAHA, suberoylanilide hydroxamic acid; TGF-β, transforming growth factor β.
HDACs and HDACIs in cardiac fibrosis
Accumulating evidence has demonstrated that HDACs are dysregulated in cardiac fibrosis.133,134 Myocardial hypertrophy is both a pathological cause and a result of cardiac fibrosis. Initially, Class IIa HDACs were thought to act as endogenous inhibitors of cardiac hypertrophy.135 The first connection between HDACs and cardiac remodeling was the finding that Class IIa HDACs interact with myocyte enhancer factor 2 (MEF2), a key regulator of myocardial hypertrophy.136 Subsequent studies found that MEF2 interacts with HDAC4, HDAC5, and HDAC9 of Class IIa; overexpression of these HDACs decreases MEF2 expression and attenuates myocardial hypertrophy.135 However, recent studies elucidated that Class IIa HDACs also exert profibrotic functions.28,133,137,138 For example, cardiomyocyte-specific HDAC4 overexpression promotes cardiac hypertrophy and exacerbates interstitial fibrosis in the model of MI in 6-month-old mice.133 In another model of cardiac hypertrophy and fibrosis with natriuretic peptide receptor-A knockout mice, the HDAC7 expression is upregulated in parallel with an increase in TGF-β1 and collagen I.28
In addition to Class IIa involvement in cardiac remodeling, genetic studies have suggested that Class I and IIb HDACs are also involved.76,134 Downregulation of HDAC1 and HDAC2 by gallic acid attenuated cardiac fibrosis in rat primary cardiac fibroblasts and in hypertensive mice.134 HDAC3 was upregulated in an experimental model of heart failure; suppression of HDAC3 improved cardiac function and limited ventricular fibrosis.139 HDAC6 activity was increased in deoxycorticosterone acetate–salt hypertensive rats and spontaneous hypertensive rats,76,140 and HDAC6 contributed to cardiac dysfunction and skeletal muscle wasting.141
With the involvement of HDACs in the tissue remodeling process, many studies explored whether HDACIs could be used to correct the pathological remodeling in cardiac fibrosis. One of the major challenges in fibrotic disease is the increased myofibroblasts, the major effector cells of fibrosis.4 Many preclinical studies have demonstrated that pan or selective HDACIs could reverse myofibroblasts activation, blunt myocardial hypertrophy, and preserve cardiac function in different myocardial hypertrophic and heart failure animal models.104,105,107,110 SAHA was reported to improve the sarcoendoplasmic reticulum Ca2+-ATPase activity in cardiac myocytes.142 Some other pan-HDACIs also demonstrated their efficacy in cardiac fibrosis. Valproic acid (VPA)-attenuated cardiac hypertrophy and fibrosis in spontaneously hypertensive rats by affecting the mineralocorticoid receptor acetylation104; it also prevented right ventricular hypertrophy in the rat.143 In a study of a rat model of pressure overload, VPA inhibited sympathetic outflow and cardiac remodeling.105 VPA was reported to ameliorate Ang II-induced pericyte-myofibroblast transdifferentiation and cardiac fibrosis through HDAC4-dependent phosphorylation of ERK.106 VPA and tributyrin attenuated ventricular remodeling after infarction, likely through histone H4 acetylation and atrial natriuretic peptide mRNA expression in the cardiomyocytes.107 A recent study demonstrated that VPA attenuated atrial remodeling and delayed the onset of atrial fibrillation in transgenic mice.144 Other pan-HDACIs, such as MPT0E014, showed antifibrotic activities by decreasing TGF-β and Ang II type I receptor expressions in isoproterenol-induced dilated cardiomyopathy,109 whereas TSA promoted myocardial repair and prevented cardiac remodeling via c-kit signaling.108 TSA was reported to reverse atrial fibrosis and reduce the incidence of arrhythmia, without affecting the level of Ang II.32 In a later study by the same group, the researchers used the same mice model, and a dog model of sustained atrial fibrosis demonstrated that Class I HDAC inhibitor CI-994 reduced atrial fibrillation and fibrosis.122 These results provide evidence that HDACIs may be a new therapeutic option for atrial fibrillation.
In addition to pan-HDACIs, selective HDACIs have also demonstrated antifibrotic properties. The selective Class I HDACI, Mocetinostat, inhibited the upregulated HDAC1 and 2 in an animal model of congestive heart failure by reversing myofibroblast phenotype and increasing apoptosis.110 Mocetinostat was also reported to attenuate interleukin (IL)-6/Stat3 signaling and decrease interstitial fibrosis and scar size in ventricular tissue in a rat model of heart failure.111 In a study of cardiac hypertrophy, the Class I HDAC inhibitor SK-7041, similar to pan-HDACIs TSA and VPA, partially reversed pre-established cardiac hypertrophy.112 MGCD0103 is another selective class I HDACI that inhibited Ang II-induced cardiac fibrosis by controlling the differentiation of bone marrow-derived fibrocytes.113 Inhibition of Class I HDACs with Apicidin derivative was able to prevent cardiac hypertrophy and failure in preclinical studies.145 HDAC6 of the class II HDACs was upregulated in cardiac fibroblast activation and fibrosis.76,140 Inhibition of HDAC6 by siRNA or its inhibitor, Tubacin, attenuated TGF-β1-induced myofibroblast markers, elevated Ras-association domain family protein 1A, and reduced cardiac fibrosis.114
HDAC inhibition also has protective effects on the diabetic heart. Diabetes can induce severe cardiovascular complications including diabetic cardiomyopathy (DCM), a myocardial disorder without coronary artery disease by unclear mechanisms.115,116 Pan-HDACI sodium butyrate reduced interstitial collagen deposition and attenuated cardiac hypertrophy by mitigating apoptosis, increasing antioxidant SOD1, stimulating angiogenesis, and activating glucose transporters 1 acetylation and p38 phosphorylation in a streptozotocin-induced diabetic model.115 Selective inhibitor RGFP966 of HDAC3 prevented fibrosis and the development of DCM by blocking the elevated phosphorylated ERK1/2, and upregulating dual specific phosphatase 5 (DUSP5) through increased acetylated histone H3 at DUSP5 promoter region in diabetic hearts.116
In addition to the above-mentioned antifibrotic aspects of HDACIs, these inhibitors are reported to reduce inflammatory processes associated with fibrotic diseases. During fibrogenesis, including myocardial fibrogenesis, proinflammatory cytokines play an important role in fibroblast activation.146 HDACs are regulators of inflammation and immunity.147 Targeting proinflammatory cytokines exerts antifibrotic effects and improves cardiac function.117,119,120 SAHA was reported to decrease inflammatory cytokines, including IL-1α and vascular endothelial growth factor in deoxycorticosterone acetate–salt hypertensive rats.117 VPA and Givinostat exhibited similar effects as SAHA in spontaneously hypertensive rats and an acute MI mouse model, inhibited proinflammatory cytokines production.119,120 These results demonstrate that HDACs are involved in inflammatory-mediated cardiac fibrosis.
Many studies with HDACIs demonstrated that these compounds exert overlapping and divergent effects. HDACIs are classified into different groups based on their chemical structure.148 For example, TSA is a hydroxamic acid, MGCD0103 belongs to the aminobenzamides, while Apicidin is a cyclic peptide,148 but they all present a common mechanism to inhibit the proliferation of cardiac fibroblasts and increase the expression of anti-proliferative genes to block cardiac fibrosis.121 Yet these different HDACIs display different efficacy and effects, which should be carefully assessed for specifically targeted processes. A study in cardiac fibroblasts demonstrated that MGCD0103 but not TSA or Apicidin paradoxically increased fibrotic-related PAI-1 expression.121 MGCD0103 and Apicidin are highly selective inhibitors of Class I HDACs, while TSA is mainly a Class I and IIb HDAC inhibitor. The divergent effects of these HDACIs are likely due to the different complexes that are specifically engaged with HDAC1 or HDAC2 by these inhibitors.121
Many of these HDACIs are synthetic, but there are natural HDACIs, including caffeic acid and polyphenols, which have been shown to attenuate cardiac hypertrophy and myocardial fibrosis.149,150 It is worth mentioning that some natural compounds contain Resveratrol, the Class III HDAC sirtuins activator, could ameliorate cardiac fibrosis by activating sirtuin 3 and affecting the TGF-β/SMAD3 signaling pathway.151
HDACIs have been reported to be beneficial for cardiac fibrosis in preclinical studies. Given the multiplicity of the distinct HDACs, it is critical to identify the specific functions of each HDAC to develop more selective and targeted inhibitors. Extensive studies are needed to understand the HDAC-mediated profibrotic processes in cardiac fibrosis, as HDACs may not only associate with the acetylation process in the cells. For example, HDAC2 can be phosphorylated, and the phosphorylation of S394 on HDAC2 would induce the binding of Hsp70 and result in cardiac hypertrophy in mice.152 Epigenetic-based therapies are still limited in the cardiovascular field; more studies are needed to explore the usage of HDACIs in cardiac fibrosis, including its safety and long-term effects.
HDACs and HDACIs in pulmonary fibrosis
Pulmonary fibrosis is another devastating disease, with IPF as the most common form. Although there are newly approved drugs mainly to relieve symptoms,73 there is no effective treatment. In the past decade, the involvement of HDACs in the pathogenesis of pulmonary fibrosis has been documented. The activities of all Class I and II HDACs were reported to be significantly upregulated in IPF lung tissues.103 Immunohistochemistry demonstrated that nearly all of these HDACs had a strong induction in myofibroblasts at fibroblast foci and in abnormal bronchiolar basal cells at sites of aberrant re-epithelialization in IPF but not in the control lungs.103 Among these HDACs, HDAC4 demonstrates its importance in lung fibrosis. HDAC4 can modulate the production of ECM in lung myofibroblasts; knocking down HDAC4 attenuates α-SMA expression stimulated by TGF-β1 in normal human lung fibroblasts.153 HDAC4 is also involved in the early stress response, while HDAC2 is increased in the middle and late stages of bleomycin-induced lung fibrosis in mice.102 A study with IPF fibroblasts showed that aberrant protein phosphatase 2A/HDAC4 axis suppresses miR-29, causing a pathological increase in type I collagen expression.154 In addition to HDAC4, HDAC6 and HDAC8 expressions are reported to be altered in IPF.78,155
To target the dysregulated HDACs in pulmonary fibrosis, many studies used HDACIs to examine their effects in blocking the fibrotic process. Although studies involving HDACIs in pulmonary fibrosis are relatively limited compared with those in cardiac fibrosis, growing evidence highlights the antifibrotic effects of HDACIs in IPF. The most studied HDACI in IPF is SAHA, a nonselective HDAC Class I and II inhibitor. It has been reported that SAHA can inhibit the differentiation of TGF-β1-induced myofibroblasts124. It induces myofibroblasts apoptosis17 and downregulates type III collagen expression on the transcriptional and translational levels.16 SAHA also upregulates antifibrotic cyclooxygenase-2 (COX-2) protein expression and prostaglandin E2 (PGE2) production by posttranscriptional mechanisms.125 Both SAHA and LBH589 (Panobinostat) have been reported to restore COX-2 expression in IPF.125,156
In addition to SAHA, other HDACIs have also demonstrated beneficial effects in lung fibrosis studies. VPA partially inhibited TGF-β1-induced epithelial-to-mesenchymal transition (EMT) process, in which VPA blocked the decreased histone acetylation (especially H3K27 acetylation) by TGF-β1 in human alveolar epithelial cells.126 However, in a study in paraquat-induced lung fibrosis with macrophages, VPA aggravated the acute inflammation and worsened fibrosis partially through enhanced histone acetylation at the IL-6 promoter regions.127 On the other hand, TSA attenuated bleomycin-induced lung fibrosis in mice; it restored decreased SFTPC, a gene encoding surfactant protein-C, via increased histone H4 acetylation at its promoter region in alveolar epithelial type II cells.128 TSA upregulated the antifibrotic gene Thy-1 in rat lung fibroblasts29; and through inhibition of HDAC2 expression, it reduced lung fibrosis in rats.129
Studies on selective HDACIs also presented beneficial effects in pulmonary fibrosis. Spiruchostatin A (SpA), a selective HDACI, inhibited TGF-β1-induced α-SMA, collagen I, and collagen III expression.130 Tubastatin, an HDAC-6 inhibitor, repressed TGF-β1-induced collagen expression by diminishing Akt phosphorylation.78 However, this study demonstrated that in bleomycin-induced lung fibrosis in mice, Tubastatin-treated mice could be protected from lung fibrosis but not HDAC6 knockout mice. This indicates that Tubastatin may ameliorate lung fibrosis via an HDAC6-independent mechanism.78 Inhibition of HDAC8 with its selective inhibitor NCC170 repressed TGF-β1-induced fibroblast–myofibroblast differentiation partially by increasing H3K27 acetylation at PPAR-γ enhancer regions to upregulate PPAR-γ mRNA, an antifibrotic molecule.131 This inhibitor also ameliorated bleomycin-induced lung fibrosis in mice.131
In addition to blocking the well-documented increased α-SMA and collagen expression in myofibroblasts, HDACIs have been reported to induce apoptosis by altering the apoptosis-related gene expression and subsequently alleviating fibrosis,17,103,132 as increased apoptosis resistance is a major characteristic of IPF myofibroblasts.157 SAHA induces apoptosis in IPF primary fibroblasts, at least in part, by regulating apoptosis-related genes through epigenetic mechanisms.17 Other HDACIs, LBH589 or VPA, considerably reduced the genes associated with the profibrotic and apoptosis-resistant phenotype in IPF fibroblasts.103 Fibrotic fibroblasts with decreased Fas expression and increased resistance to Fas-mediated apoptosis are associated with increased HDAC 2 and 4; and TSA or SAHA can increase Fas expression in these cells and restore susceptibility to Fas-induced apoptosis.132 Pirfenidone is the first anti-fibrotic agent for IPF to slow its progression, a recent study compared the antifibrotic efficacy of Panobinostat and Pirfenidone in IPF fibroblasts, and concluded that both have antifibrotic effects, but Panobinostat also induced cell cycle arrest and apoptosis and thus was more efficient in inactivating IPF fibroblasts.158
HDACIs are powerful epigenetic regulators, and epigenetic mechanisms are involved in pulmonary fibrosis.75 Many epigenetic factors that are affected by environmental changes,159 including metabolic alterations160 and aging,161 contribute to pulmonary fibrosis. Histone modifications, especially histone acetylation, are only part of this complexed process. Inhibition of HDAC at in vitro and in vivo models of pulmonary fibrosis has shown promising results, but progress in determining the biological mechanisms and therapeutic options remains obscure. More in-depth studies in this area will empower us to develop better-targeted therapeutic methods to treat this fatal disease.
Limitations and side effects of HDACIs
HDACIs represent a new class of epigenetic drugs that have demonstrated promising results in preclinical studies. Histone and nonhistone proteins are the targets of HDACs, many of which are critical regulators of vital pathways.25 Even the same HDAC may have different roles at different stages of the same disease. For example, HDAC1 was reported to be oncosuppressive in the early stage while oncogenic in established tumor cells in a mouse tumor model.162 The inhibitors, even selective inhibitors of HDAC, could have many off-target effects. Undesired toxicity of certain HDACIs could lead to dysfunction of other important biological processes. For instance, selective Class I HDACIs showed potent anti-tumor effects, but inhibition of Class I HDACs, specifically HDAC3, caused impaired DNA damage response.163 Other major concerns include the cardiac toxicity by some HDACIs,164 and the cellular resistance to certain HDACIs.165 A study in phase I and II clinical trials of romidepsin in patients with cutaneous T-cell lymphoma, de novo resistance of the drug was found.165 The lack of response-predictive markers for cardiac and pulmonary fibrosis also delayed advances in establishing the valuable index for therapeutic efficacy. To overcome these limitations and side effects, a better designed, more selective, and better targeted HDACI would increase the potency against fibrotic diseases. For pulmonary fibrosis, localized administration of the drug (such as nasal delivery) would minimize the undesired whole-body side effects.
Conclusion
Studies in the past have shown that HDACs are critical in the pathogenesis of fibrosis by modulating acetylation of histone and nonhistone substrates. HDACs are found to be dysregulated in fibrotic diseases. Inhibition of HDACs in fibrotic models mitigates fibrotic status. HDACIs, especially selective inhibitors, would offer better understanding of how HDACs participate in the process of fibrogenesis. Inhibitors of HDACs would offer a novel category of therapeutic options in fibrosis. Extensive studies are needed to clarify the specific roles of each HDAC in the fibrotic process, which would form a mechanistic foundation for clinical usage. Many of the current HDACIs in clinical trials are broad-spectrum nonselective inhibitors and have more toxicity and unwanted side effects than selective HDACIs. Right now, most HDACIs either approved by the FDA or in clinical trials are mainly for cancer treatments. HDACIs for cardiac or pulmonary fibrosis are still mostly in preclinical stages. Better targeted, more specific HDACIs are needed to enhance the therapeutic efficacy and reduce toxicity. Furthermore, it is crucial to develop biomarkers for HDACI alone or in combination with other reagents to predict the response of individual patient to treatment. Elucidation and validation of the mechanisms of HDAC involved in the pathogenesis of fibrosis would provide a powerful tool in the treatment of fibrotic-related diseases, especially cardiac and pulmonary fibrosis.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the National Natural Science Foundation of China (grant number 81470256 to X.Z.) and US National Institutes of Health (grant number R01AG050567 to Y.Y.S.).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Yan Y Sanders  https://orcid.org/0000-0001-6575-9729
https://orcid.org/0000-0001-6575-9729
Contributor Information
Xing Lyu, Laboratory of Clinical Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Min Hu, Laboratory of Clinical Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Jieting Peng, Department of Geriatrics, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Xiangyu Zhang, Department of Geriatrics, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, China.
Yan Y Sanders, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Alabama at Birmingham, 901 19th Street South, BMRII Room 408, Birmingham, AL 35294, USA.
References
- 1. Rockey DC, Bell PD, Hill JA. Fibrosis–a common pathway to organ injury and failure. N Engl J Med 2015; 372: 1138–1149. [DOI] [PubMed] [Google Scholar]
- 2. Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007; 117: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gyorfi AH, Matei AE, Distler JHW. Targeting TGF-beta signaling for the treatment of fibrosis. Matrix Biol 2018; 68–69: 8–27. [DOI] [PubMed] [Google Scholar]
- 4. Kis K, Liu X, Hagood JS. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med 2011; 13: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 2003: 200: 500–503. [DOI] [PubMed] [Google Scholar]
- 6. Al Aboud NM, Jialal I. Genetics, epigenetic mechanism. Treasure Island, FL: StatPearls, 2019. [PubMed] [Google Scholar]
- 7. Rodriguez-Rodero S, Delgado-Alvarez E, Diaz-Naya L, et al. Epigenetic modulators of thyroid cancer. Endocrinol Diabetes Nutr 2017; 64: 44–56. [DOI] [PubMed] [Google Scholar]
- 8. Feinberg AP, Koldobskiy MA, Gondor A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet 2016; 17: 284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imhof A. Epigenetic regulators and histone modification. Brief Funct Genomic Proteomic 2006; 5: 222–227. [DOI] [PubMed] [Google Scholar]
- 10. Drazic A, Myklebust LM, Ree R, et al. The world of protein acetylation. Biochim Biophys Acta 2016; 1864: 1372–1401. [DOI] [PubMed] [Google Scholar]
- 11. Verdone L, Agricola E, Caserta M, et al. Histone acetylation in gene regulation. Brief Funct Genomic Proteomic 2006; 5: 209–221. [DOI] [PubMed] [Google Scholar]
- 12. Linares LK, Kiernan R, Triboulet R, et al. Intrinsic ubiquitination activity of PCAF controls the stability of the oncoprotein Hdm2. Nat Cell Biol 2007; 9: 331–338. [DOI] [PubMed] [Google Scholar]
- 13. Lee DY, Lee CI, Lin TE, et al. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci USA 2012; 109: 1967–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007; 26: 5310–5318. [DOI] [PubMed] [Google Scholar]
- 15. Rai R, Sun T, Ramirez V, et al. Acetyltransferase p300 inhibitor reverses hypertension-induced cardiac fibrosis. J Cell Mol Med 2019; 23: 3026–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Liu H, Hock T, et al. Histone deacetylase inhibition downregulates collagen 3A1 in fibrotic lung fibroblasts. Int J Mol Sci 2013; 14: 19605–19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanders YY, Hagood JS, Liu H, et al. Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. Eur Respir J 2014; 43: 1448–1458. [DOI] [PubMed] [Google Scholar]
- 18. Ferguson M, Henry PA, Currie RA. Histone deacetylase inhibition is associated with transcriptional repression of the Hmga2 gene. Nucleic Acids Res 2003; 31: 3123–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol 2005; 25: 1608–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryu H, Lee J, Olofsson BA, et al. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci USA 2003; 100: 4281–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiernan R, Bres V, Ng RW, et al. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem 2003; 278: 2758–2766. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Seto E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med 2016; 6: pii: a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eom GH, Kook H. Role of histone deacetylase 2 and its posttranslational modifications in cardiac hypertrophy. BMB Rep 2015; 48: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma S, Taliyan R. Histone deacetylase inhibitors: Future therapeutics for insulin resistance and type 2 diabetes. Pharmacol Res 2016; 113(Pt A): 320–326. [DOI] [PubMed] [Google Scholar]
- 25. Pang M, Zhuang S. Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther 2010, 335: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin HT, Li HQ, Liu F. Selective histone deacetylase small molecule inhibitors: recent progress and perspectives. Expert Opin Ther Pat 2017; 27: 621–636. [DOI] [PubMed] [Google Scholar]
- 27. Mai A, Massa S, Rotili D, et al. Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med Res Rev 2005; 25: 261–309. [DOI] [PubMed] [Google Scholar]
- 28. Ellmers LJ, Scott NJ, Piuhola J, et al. Npr1-regulated gene pathways contributing to cardiac hypertrophy and fibrosis. J Mol Endocrinol 2007; 38: 245–257. [DOI] [PubMed] [Google Scholar]
- 29. Sanders YY, Tollefsbol TO, Varisco BM, et al. Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. Am J Respir Cell Mol Biol 2011; 45: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qin L, Han YP. Epigenetic repression of matrix metalloproteinases in myofibroblastic hepatic stellate cells through histone deacetylases 4: implication in tissue fibrosis. Am J Pathol 2010; 177: 1915–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marumo T, Hishikawa K, Yoshikawa M, et al. Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am J Physiol Renal Physiol 2010; 298: F133–F141. [DOI] [PubMed] [Google Scholar]
- 32. Liu F, Levin MD, Petrenko NB, et al. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol 2008; 45: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pang M, Kothapally J, Mao H, et al. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 2009; 297: F996–F1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 2007; 109: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmitt T, Mayer-Steinacker R, Mayer F, et al. Vorinostat in refractory soft tissue sarcomas - Results of a multi-centre phase II trial of the German Soft Tissue Sarcoma and Bone Tumour Working Group (AIO). Eur J Cancer 2016; 64: 74–82. [DOI] [PubMed] [Google Scholar]
- 36. Okam MM, Esrick EB, Mandell E, et al. Phase 1/2 trial of vorinostat in patients with sickle cell disease who have not benefitted from hydroxyurea. Blood 2015; 125: 3668–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haas NB, Quirt I, Hotte S, et al. Phase II trial of vorinostat in advanced melanoma. Invest New Drugs 2014; 32: 526–534. [DOI] [PubMed] [Google Scholar]
- 38. Doi T, Hamaguchi T, Shirao K, et al. Evaluation of safety, pharmacokinetics, and efficacy of vorinostat, a histone deacetylase inhibitor, in the treatment of gastrointestinal (GI) cancer in a phase I clinical trial. Int J Clin Oncol 2013; 18: 87–95. [DOI] [PubMed] [Google Scholar]
- 39. Watanabe T, Kato H, Kobayashi Y, et al. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci 2010; 101: 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bradley D, Rathkopf D, Dunn R, et al. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer 2009; 115: 5541–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol 2009; 27: 2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elliott JH, Wightman F, Solomon A, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014; 10: e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bug G, Burchert A, Wagner EM, et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia 2017; 31: 2523–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ibrahim N, Buchbinder EI, Granter SR, et al. A phase I trial of panobinostat (LBH589) in patients with metastatic melanoma. Cancer Med 2016; 5: 3041–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin N, Lubner SJ, Mulkerin DL, et al. A phase II trial of a histone deacetylase inhibitor panobinostat in patients with low-grade neuroendocrine tumors. Oncologist 2016; 21: 785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones SF, Bendell JC, Infante JR, et al. A phase I study of panobinostat in combination with gemcitabine in the treatment of solid tumors. Clin Adv Hematol Oncol 2011; 9: 225–230. [PubMed] [Google Scholar]
- 47. Olesen R, Vigano S, Rasmussen TA, et al. Innate immune activity correlates with CD4 T cell-associated HIV-1 DNA decline during latency-reversing treatment with Panobinostat. J Virol 2015; 89: 10176–10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Puvvada SD, Guillen-Rodriguez JM, Rivera XI, et al. A phase II exploratory study of PXD-101 (Belinostat) followed by Zevalin in patients with relapsed aggressive high-risk lymphoma. Oncology 2017; 93: 401-405. [DOI] [PubMed] [Google Scholar]
- 49. O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol 2015; 33: 2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Foss F, Advani R, Duvic M, et al. A phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol 2015; 168: 811–819. [DOI] [PubMed] [Google Scholar]
- 51. Wang LZ, Ramirez J, Yeo W, et al. Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients. PLoS One 2013; 8: e54522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeo W, Chung HC, Chan SL, et al. Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: a multicenter phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group. J Clin Oncol 2012; 30: 3361–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dizon DS, Damstrup L, Finkler NJ, et al. Phase II activity of belinostat (PXD-101), carboplatin, and paclitaxel in women with previously treated ovarian cancer. Int J Gynecol Cancer 2012; 22: 979–986. [DOI] [PubMed] [Google Scholar]
- 54. Bettica P, Petrini S, D’Oria V, et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord 2016; 26: 643–649. [DOI] [PubMed] [Google Scholar]
- 55. Finazzi G, Vannucchi AM, Martinelli V, et al. A phase II study of Givinostat in combination with hydroxycarbamide in patients with polycythaemia vera unresponsive to hydroxycarbamide monotherapy. Br J Haematol 2013; 161: 688–694. [DOI] [PubMed] [Google Scholar]
- 56. Rambaldi A, Dellacasa CM, Finazzi G, et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol 2010; 150: 446–55. [DOI] [PubMed] [Google Scholar]
- 57. Shustov A, Coiffier B, Horwitz S, et al. Romidepsin is effective and well tolerated in older patients with peripheral T-cell lymphoma: analysis of two phase II trials. Leuk Lymphoma 2017; 58: 2335–2341. [DOI] [PubMed] [Google Scholar]
- 58. Gerber DE, Boothman DA, Fattah FJ, et al. Phase 1 study of romidepsin plus erlotinib in advanced non-small cell lung cancer. Lung Cancer 2015; 90: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sogaard OS, Graversen ME, Leth S, et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 2015; 11: e1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Azad NS, El-Khoueiry A, Yin J, et al. Combination epigenetic therapy in metastatic colorectal cancer (mCRC) with subcutaneous 5-azacitidine and entinostat: a phase 2 consortium/stand up 2 cancer study. Oncotarget 2017; 8: 35326–35338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Connolly RM, Li H, Jankowitz RC, et al. Combination epigenetic therapy in advanced breast cancer with 5-azacitidine and entinostat: a phase II national cancer institute/stand up to cancer study. Clin Cancer Res 2017; 23: 2691–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Batlevi CL, Kasamon Y, Bociek RG, et al. ENGAGE- 501: phase II study of entinostat (SNDX-275) in relapsed and refractory Hodgkin lymphoma. Haematologica 2016; 101: 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prebet T, Sun Z, Ketterling RP, et al. , Eastern Cooperative Oncology Group and North American Leukemia intergroup. Azacitidine with or without Entinostat for the treatment of therapy-related myeloid neoplasm: further results of the E1905 North American Leukemia Intergroup study. Br J Haematol 2016; 172: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fandy TE, Herman JG, Kerns P, et al. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood 2009; 114: 2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fushida S, Kinoshita J, Kaji M, et al. Paclitaxel plus valproic acid versus paclitaxel alone as second- or third-line therapy for advanced gastric cancer: a randomized Phase II trial. Drug Des Devel Ther 2016; 10: 2353–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chu BF, Karpenko MJ, Liu Z, et al. Phase I study of 5-aza-2’-deoxycytidine in combination with valproic acid in non-small-cell lung cancer. Cancer Chemother Pharmacol 2013; 71: 115–121. [DOI] [PubMed] [Google Scholar]
- 67. Avallone A, Piccirillo MC, Delrio P, et al. Phase 1/2 study of valproic acid and short-course radiotherapy plus capecitabine as preoperative treatment in low-moderate risk rectal cancer-V-shoRT-R3 (Valproic acid–short Radiotherapy–rectum 3rd trial). BMC Cancer 2014; 14: 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weber KT, Sun Y, Bhattacharya SK, et al. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 2013; 10: 15–26. [DOI] [PubMed] [Google Scholar]
- 69. Ma ZG, Yuan YP, Wu HM, et al. Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci 2018; 14: 1645–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fan X, Xie J, Tian J. Reducing cardiac fibrosis: Na/K-ATPase signaling complex as a novel target. Cardiovasc Pharm Open Access 2017; 6: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thannickal VJ, Toews GB, White ES, et al. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004; 55: 395–417. [DOI] [PubMed] [Google Scholar]
- 72. Barratt SL, Creamer A, Hayton C, et al. Idiopathic Pulmonary Fibrosis (IPF): an overview. J Clin Med 2018; 7: pii: E201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sgalla G, Iovene B, Calvello M, et al. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res 2018; 19: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grimaldi V, De Pascale MR, Zullo A, et al. Evidence of epigenetic tags in cardiac fibrosis. J Cardiol 2017; 69: 401–408. [DOI] [PubMed] [Google Scholar]
- 75. Tzouvelekis A, Kaminski N. Epigenetics in idiopathic pulmonary fibrosis. Biochem Cell Biol 2015; 93: 159–170. [DOI] [PubMed] [Google Scholar]
- 76. Kee HJ, Bae EH, Park S, et al. HDAC inhibition suppresses cardiac hypertrophy and fibrosis in DOCA-salt hypertensive rats via regulation of HDAC6/HDAC8 enzyme activity. Kidney Blood Press Res 2013; 37: 229–239. [DOI] [PubMed] [Google Scholar]
- 77. Montgomery RL, Davis CA, Potthoff MJ, et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 2007; 21: 1790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saito S, Zhuang Y, Shan B, et al. Tubastatin ameliorates pulmonary fibrosis by targeting the TGFbeta-PI3K-Akt pathway. PLoS One 2017; 12: e0186615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kong Y, Tannous P, Lu G, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation 2006; 113: 2579–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang X, Hu M, Lyu X, et al. DNA methylation regulated gene expression in organ fibrosis. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 2389–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. de Ruijter AJ, van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 2003; 370(Pt 3): 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gallinari P, Di Marco S, Jones P, et al. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res 2007; 17: 195–211. [DOI] [PubMed] [Google Scholar]
- 83. O’Callaghan C, Vassilopoulos A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 2017; 16: 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 2014; 6: a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Glozak MA, Sengupta N, Zhang X, et al. Acetylation and deacetylation of non-histone proteins. Gene 2005; 363: 15–23. [DOI] [PubMed] [Google Scholar]
- 86. Zwergel C, Valente S, Jacob C, et al. Emerging approaches for histone deacetylase inhibitor drug discovery. Expert Opin Drug Discov 2015; 10: 599–613. [DOI] [PubMed] [Google Scholar]
- 87. Elvir L, Duclot F, Wang Z, et al. Epigenetic regulation of motivated behaviors by histone deacetylase inhibitors. Neurosci Biobehav Rev. Epub ahead of print 8 October 2017. DOI: 10.1016/j.neubiorev.2017.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tan S, Liu ZP. Natural products as zinc-dependent histone deacetylase inhibitors. ChemMedChem 2015; 10: 441–450. [DOI] [PubMed] [Google Scholar]
- 89. Yoshida M, Kijima M, Akita M, et al. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 1990; 265: 17174–17179. [PubMed] [Google Scholar]
- 90. Licciardi PV, Kwa FA, Ververis K, et al. Influence of natural and synthetic histone deacetylase inhibitors on chromatin. Antioxid Redox Signal 2012; 17: 340–354. [DOI] [PubMed] [Google Scholar]
- 91. Ganai SA. Histone deacetylase inhibitors modulating non-epigenetic players: the novel mechanism for small molecule based therapeutic intervention. Curr Drug Targets 2018; 19: 593–601. [DOI] [PubMed] [Google Scholar]
- 92. Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 2007; 5: 981–989. [DOI] [PubMed] [Google Scholar]
- 93. Bao L, Diao H, Dong N, et al. Histone deacetylase inhibitor induces cell apoptosis and cycle arrest in lung cancer cells via mitochondrial injury and p53 up-acetylation. Cell Biol Toxicol 2016; 32: 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dias JNR, Aguiar SI, Pereira DM, et al. The histone deacetylase inhibitor panobinostat is a potent antitumor agent in canine diffuse large B-cell lymphoma. Oncotarget 2018; 9: 28586–28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Panobinostat approved for multiple myeloma. Cancer Discov 2015; 5: OF4. [DOI] [PubMed] [Google Scholar]
- 96. Beleodaq approved for rare lymphomas. Cancer Discov 2014; 4: 978. [DOI] [PubMed] [Google Scholar]
- 97. Grant C, Rahman F, Piekarz R, et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther 2010; 10: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Harrison SJ, Bishton M, Bates SE, et al. A focus on the preclinical development and clinical status of the histone deacetylase inhibitor, romidepsin (depsipeptide, Istodax((R))). Epigenomics 2012; 4: 571–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 2007; 25: 84–90. [DOI] [PubMed] [Google Scholar]
- 100. Vojinovic J, Damjanov N, D’Urzo C, et al. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 2011; 63: 1452–1458. [DOI] [PubMed] [Google Scholar]
- 101. Choi SY, Piao ZH, Jin L, et al. Piceatannol attenuates renal fibrosis induced by unilateral ureteral obstruction via downregulation of histone deacetylase 4/5 or p38-MAPK signaling. PLoS One 2016; 11: e0167340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li M, Zheng Y, Yuan H, et al. Effects of dynamic changes in histone acetylation and deacetylase activity on pulmonary fibrosis. Int Immunopharmacol 2017; 52: 272–280. [DOI] [PubMed] [Google Scholar]
- 103. Korfei M, Skwarna S, Henneke I, et al. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax 2015; 70: 1022–1032. [DOI] [PubMed] [Google Scholar]
- 104. Kang SH, Seok YM, Song MJ, et al. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol Pharmacol 2015; 87: 782–791. [DOI] [PubMed] [Google Scholar]
- 105. Liu Y, Li S, Zhang Z, et al. Effects of valproic acid on sympathetic activity and left ventricularmyocardial remodelling in rats during pressure overload. Turk J Med Sci 2017; 47: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 106. Zhang Y, Gao F, Tang Y, et al. Valproic acid regulates Ang II-induced pericyte-myofibroblast trans-differentiation via MAPK/ERK pathway. Am J Transl Res 2018; 10: 1976–1989. [PMC free article] [PubMed] [Google Scholar]
- 107. Lee TM, Lin MS, Chang NC. Inhibition of histone deacetylase on ventricular remodeling in infarcted rats. Am J Physiol Heart Circ Physiol 2007; 293: H968–H977. [DOI] [PubMed] [Google Scholar]
- 108. Zhang L, Chen B, Zhao Y, et al. Inhibition of histone deacetylase-induced myocardial repair is mediated by c-kit in infarcted hearts. J Biol Chem 2012; 287: 39338–39348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kao YH, Liou JP, Chung CC, et al. Histone deacetylase inhibition improved cardiac functions with direct antifibrotic activity in heart failure. Int J Cardiol 2013; 168: 4178–483. [DOI] [PubMed] [Google Scholar]
- 110. Nural-Guvener HF, Zakharova L, Nimlos J, et al. HDAC class I inhibitor, Mocetinostat, reverses cardiac fibrosis in heart failure and diminishes CD90+ cardiac myofibroblast activation. Fibrogenesis Tissue Repair 2014; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nural-Guvener H, Zakharova L, Feehery L, et al. Anti-fibrotic effects of class I HDAC inhibitor, mocetinostat is associated with IL-6/Stat3 signaling in ischemic heart failure. Int J Mol Sci 2015; 16: 11482–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kee HJ, Sohn IS, Nam KI, et al. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 2006; 113: 51–59. [DOI] [PubMed] [Google Scholar]
- 113. Williams SM, Golden-Mason L, Ferguson BS, et al. Class I HDACs regulate angiotensin II-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol Cell Cardiol 2014; 67: 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tao H, Yang JJ, Hu W, et al. HDAC6 promotes cardiac fibrosis progression through suppressing RASSF1A expression. Cardiology 2016; 133: 18–26. [DOI] [PubMed] [Google Scholar]
- 115. Chen Y, Du J, Zhao YT, et al. Histone deacetylase (HDAC) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc Diabetol 2015; 14: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xu Z, Tong Q, Zhang Z, et al. Inhibition of HDAC3 prevents diabetic cardiomyopathy in OVE26 mice via epigenetic regulation of DUSP5-ERK1/2 pathway. Clin Sci (Lond) 2017; 131: 1841–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Iyer A, Fenning A, Lim J, et al. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br J Pharmacol 2010; 159: 1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ooi JY, Tuano NK, Rafehi H, et al. HDAC inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes. Epigenetics 2015; 10: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tian S, Lei I, Gao W, et al. HDAC inhibitor valproic acid protects heart function through Foxm1 pathway after acute myocardial infarction. EBioMedicine. Epub 11 December 2018. DOI: 10.1016/j.ebiom.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Milan M, Pace V, Maiullari F, et al. Givinostat reduces adverse cardiac remodeling through regulating fibroblasts activation. Cell Death Dis 2018; 9: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schuetze KB, Stratton MS, Blakeslee WW, et al. Overlapping and divergent actions of structurally distinct histone deacetylase inhibitors in cardiac fibroblasts. J Pharmacol Exp Ther 2017; 361: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Seki M, LaCanna R, Powers JC, et al. Class I histone deacetylase inhibition for the treatment of sustained atrial fibrillation. J Pharmacol Exp Ther 2016; 358: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lee E, Song MJ, Lee HA, et al. Histone deacetylase inhibitor, CG200745, attenuates cardiac hypertrophy and fibrosis in DOCA-induced hypertensive rats. Korean J Physiol Pharmacol 2016; 20: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang Z, Chen C, Finger SN, et al. Suberoylanilide hydroxamic acid: a potential epigenetic therapeutic agent for lung fibrosis? Eur Respir J 2009; 34: 145–155. [DOI] [PubMed] [Google Scholar]
- 125. Pasini A, Brand OJ, Jenkins G, et al. Suberanilohydroxamic acid prevents TGF-beta1-induced COX-2 repression in human lung fibroblasts post-transcriptionally by TIA-1 downregulation. Biochim Biophys Acta Gene Regul Mech 2018; 1861: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Noguchi S, Eitoku M, Moriya S, et al. Regulation of gene expression by sodium valproate in epithelial-to-mesenchymal transition. Lung 2015; 193: 691–700. [DOI] [PubMed] [Google Scholar]
- 127. Hu L, Yu Y, Huang H, et al. Epigenetic regulation of interleukin 6 by histone acetylation in macrophages and its role in paraquat-induced pulmonary fibrosis. Front Immunol 2016; 7: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ota C, Yamada M, Fujino N, et al. Histone deacetylase inhibitor restores surfactant protein-C expression in alveolar-epithelial type II cells and attenuates bleomycin-induced pulmonary fibrosis in vivo. Exp Lung Res 2015; 41: 422–434. [DOI] [PubMed] [Google Scholar]
- 129. Ye Q, Li Y, Jiang H, et al. Prevention of pulmonary fibrosis via trichostatin A (TSA) in bleomycin induced Rrats. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31: 219–226. [PubMed] [Google Scholar]
- 130. Davies ER, Haitchi HM, Thatcher TH, et al. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. Am J Respir Cell Mol Biol 2012; 46: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Saito S, Zhuang Y, Suzuki T, et al. HDAC8 inhibition ameliorates pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. Epub ahead of print 25 October 2018. DOI: 10.1152/ajplung.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Huang SK, Scruggs AM, Donaghy J, et al. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis 2013; 4:e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhang LX, Du J, Zhao YT, et al. Transgenic overexpression of active HDAC4 in the heart attenuates cardiac function and exacerbates remodeling in infarcted myocardium. J Appl Physiol (1985) 2018; 125:1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jin L, Lin MQ, Piao ZH, et al. Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with NG-nitro-L-arginine methyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J Hypertens 2017; 35: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 135. Zhang CL, McKinsey TA, Chang S, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 2002; 110: 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci 2002; 27: 40–47. [DOI] [PubMed] [Google Scholar]
- 137. Backs J, Song K, Bezprozvannaya S, et al. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 2006; 116: 1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vega RB, Harrison BC, Meadows E, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol 2004; 24: 8374–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sharifi-Sanjani M, Shoushtari AH, Quiroz M, et al. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc 2014; 3: e000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lemon DD, Horn TR, Cavasin MA, et al. Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. J Mol Cell Cardiol 2011; 51: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Demos-Davies KM, Ferguson BS, Cavasin MA, et al. HDAC6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-II signaling. Am J Physiol Heart Circ Physiol 2014; 307: H252–H258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Meraviglia V, Bocchi L, Sacchetto R, et al. HDAC inhibition improves the sarcoendoplasmic teticulum Ca(2+)-ATPase activity in cardiac myocytes. Int J Mol Sci 2018; 19: pii: E419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cho YK, Eom GH, Kee HJ, et al. Sodium valproate, a histone deacetylase inhibitor, but not captopril, prevents right ventricular hypertrophy in rats. Circ J 2010; 74: 760–770. [DOI] [PubMed] [Google Scholar]
- 144. Scholz B, Schulte JS, Hamer S, et al. HDAC (Histone Deacetylase) inhibitor valproic acid attenuates atrial remodeling and delays the onset of atrial fibrillation in mice. Circ Arrhythm Electrophysiol 2019; 12: e007071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gallo P, Latronico MV, Gallo P, et al. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res 2008; 80: 416–424. [DOI] [PubMed] [Google Scholar]
- 146. Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 2014; 102: 258–269. [DOI] [PubMed] [Google Scholar]
- 147. Shakespear MR, Halili MA, Irvine KM, et al. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol 2011; 32: 335–343. [DOI] [PubMed] [Google Scholar]
- 148. West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 2014; 124: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ren J, Zhang N, Liao H, et al. Caffeic acid phenethyl ester attenuates pathological cardiac hypertrophy by regulation of MEK/ERK signaling pathway in vivo and vitro. Life Sci 2017; 181: 53–61. [DOI] [PubMed] [Google Scholar]
- 150. Akinwumi BC, Raj P, Lee DI, et al. Disparate effects of stilbenoid polyphenols on hypertrophic cardiomyocytes in vitro vs. in the spontaneously hypertensive heart failure rat. Molecules 2017; 22: pii: E204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chen T, Li J, Liu J, et al. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-beta/Smad3 pathway. Am J Physiol Heart Circ Physiol 2015; 308: H424–H434. [DOI] [PubMed] [Google Scholar]
- 152. Yoon S, Kim M, Min HK, et al. Inhibition of heat shock protein 70 blocks the development of cardiac hypertrophy by modulating the phosphorylation of histone deacetylase 2. Cardiovasc Res. Epub ahead of print 28 December 2018. DOI: 10.1093/cvr/cvy317. [DOI] [PubMed] [Google Scholar]
- 153. Guo W, Shan B, Klingsberg RC, et al. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 2009; 297: L864–L870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Khalil W, Xia H, Bodempudi V, et al. Pathologic regulation of collagen I by an aberrant protein phosphatase 2A/histone deacetylase C4/microRNA-29 signal axis in idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 2015; 53: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Saito S, Zhuang Y, Suzuki T, et al. HDAC8 inhibition ameliorates pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2019; 316: L175–L186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Coward WR, Watts K, Feghali-Bostwick CA, et al. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol 2009; 29: 4325–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 2007; 257: 143–179. [DOI] [PubMed] [Google Scholar]
- 158. Korfei M, Stelmaszek D, MacKenzie B, et al. Comparison of the antifibrotic effects of the pan-histone deacetylase-inhibitor panobinostat versus the IPF-drug pirfenidone in fibroblasts from patients with idiopathic pulmonary fibrosis. PLoS One 2018; 13: e0207915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Macneal K, Schwartz DA. The genetic and environmental causes of pulmonary fibrosis. Proc Am Thorac Soc 2012; 9: 120–125. [DOI] [PubMed] [Google Scholar]
- 160. Bai L, Bernard K, Tang X, et al. Glutaminolysis epigenetically regulates anti-apoptotic gene expression in IPF fibroblasts. Am J Respir Cell Mol Biol 2018. DOI: 10.1165/rcmb.2018-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sanders YY, Liu H, Liu G, et al. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic Biol Med 2015, 79, 197–205. [DOI] [PubMed] [Google Scholar]
- 162. Santoro F, Botrugno OA, Dal Zuffo R, et al. A dual role for Hdac1: oncosuppressor in tumorigenesis, oncogene in tumor maintenance. Blood 2013; 121: 3459–3468. [DOI] [PubMed] [Google Scholar]
- 163. Khabele D. The therapeutic potential of class I selective histone deacetylase inhibitors in ovarian cancer. Front Oncol 2014; 4: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Shultz MD, Cao X, Chen CH, et al. Optimization of the in vitro cardiac safety of hydroxamate-based histone deacetylase inhibitors. J Med Chem 2011; 54: 4752–4772. [DOI] [PubMed] [Google Scholar]
- 165. Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 2009; 27: 5410–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]