Abstract
Background:
Cervical spinal epidural abscess (CSEA) is a localized infection between the thecal sac and cervical spinal column which may result in neurological deficit and death if inadequately treated. Two treatment options exist: medical management and surgical intervention. Our objective was to analyze CSEA patient outcomes in order to determine the optimal method of treatment.
Methods:
An electronic literature search for relevant case series and retrospective reviews was conducted through June 2016. Data abstraction and study quality assessment were performed by two independent reviewers. A lack of available data led to a post hoc decision not to perform meta-analysis of the results; study findings were synthesized qualitatively.
Results:
927 studies were identified, of which 11 were included. Four studies were ranked as good quality, and seven ranked as fair quality. In total, data from 173 patients were included. Mean age was 55 years; 61.3% were male. Intravenous drug use was the most common risk factor for CSEA development. Staphylococcus aureus was the most commonly cultured pathogen. 140 patients underwent initial surgery, an additional 18 patients were surgically treated upon failure of medical management, and 15 patients were treated with antibiotics alone.
Conclusion:
The rates of medical management failure described in our review were much higher than those reported in the literature for thoracolumbar spinal epidural abscess patients, suggesting that CSEA patients may be at a greater risk for poor outcomes following nonoperative treatment. Thus, early surgery appears most viable for optimizing CSEA patient outcomes. Further research is needed in order to corroborate these recommendations.
Keywords: cervical spine, epidural abscess, surgery, treatment outcome
Introduction
Spinal epidural abscess (SEA) is a localized infection situated between the thecal sac of the spinal cord and the spinal ligaments and vertebrae. Back pain, fever, and neurological deficit are the classic triad of symptoms of SEA, although few patients show all three at presentation.1 Predisposing conditions of SEA include diabetes mellitus, human immunodeficiency virus (HIV), and alcohol abuse. The gold standard for SEA diagnosis is gadolinium-enhanced magnetic resonance imaging (MRI).2–4
Although a relatively rare condition, with a historical incidence of 0.2–2 cases per 10,000 hospital admissions,5 the incidence of SEA is on the rise, with recent estimates ranging from 2 to 12.5 per 10,000 hospital admissions.4,6–8 Multiple factors are thought to be responsible, including an aging population, rise in intravenous (IV) drug users, increased prevalence of medical comorbidities (e.g. diabetes mellitus), and greater rates of spinal surgery furthering iatrogenic spinal infection. Furthermore, improved medical imaging techniques and increased awareness of SEA have culminated in an escalation of diagnoses.4,6
Cervical spinal epidural abscess (CSEA) is a rare form of SEA. The relatively small epidural space in the cervical spine, when compared with the thoracic and lumbar spine, decreases its likelihood of infection.3,9 CSEA accounts for only 19% of all SEA.5 However, CSEA is arguably a more urgent condition, due to the fact that the smaller epidural space is less permissive of abscess and inflammation.2 CSEA may also affect breathing due to diaphragmatic innervation from C3, C4, and C5.2,9 This has led to reports of a poorer prognosis for CSEA than thoracolumbar SEA.10,11
Two main treatment options exist for SEA: medical management and surgical intervention. Generally, medical management is reserved for patients with significant comorbidities contraindicating surgery, patients having a significantly extended abscess, or patients having no neurological deficit or a neurological deficit lasting more than 48–72 h.7 Medical management may be accompanied by a computed tomography (CT)-guided percutaneous needle aspiration of the abscess.4,9,12 When surgical intervention is the chosen treatment regimen, its foremost goals include decompression of the epidural space and abscess drainage, achievement of spinal stability, and sampling of the abscess for pathogen identification.7,13
There is little consensus regarding the optimal treatment of SEA. While some studies indicate that surgery is the preferable method of treatment,8,14–16 others maintain that surgical intervention does not lead to significant clinical improvement.17–19 Arko and colleagues found no significant difference in the outcome of operative versus nonoperative management of SEA.7 Conversely, Suppiah and colleagues recommended that early surgery be the treatment of choice in cases of neurologically symptomatic SEA, although no differentiation in recommendation based on the affected spinal segment was made.20
The optimum management of CSEA is unclear. No systematic review or meta-analysis has been published exclusively analyzing CSEA data. Given the rising incidence of CSEA, it is important to determine the optimal treatment. The goal of our systematic review was to assess the neurological outcomes associated with operative versus nonoperative management of CSEA patients.
Methods
This systematic review conforms to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.21 Improvement of patients was assessed through various outcome measures; the primary outcome of interest was neurological improvement, while secondary outcome measures included radiological resolution of abscess, independence in ambulation, and adverse events (complications, recurrences, and deaths). An unpublished protocol was prepared for internal comment.
Search strategy
A literature search was performed of Medline, EMBASE and Cochrane Library databases through June 2015 using the search terms ‘epidural abscess’ and ‘extradural abscess’. Further refinement of the search results was accomplished using keywords ‘cervical’, ‘operative’, ‘antibiotic’, and their variations. In addition, an updated search of Medline via PubMed was completed through June 2016.
Identification of eligible studies
Results of the literature search were independently screened by two reviewers (AT and PG). Discrepancies between reviewers were resolved by discussion with a third reviewer (DMR) until consensus was reached.
Level 1 screening consisted of evaluation of all study information obtained through the literature search (e.g. date published, abstract, title), whereas level 2 screening consisted of evaluation of the studies’ full texts. All studies passing level 1 screening, as well as studies having insufficient information to determine eligibility during level 1 screening (e.g. no abstract available), proceeded to level 2 screening.
Studies were included for review based on the following inclusion criteria:
(1) study includes radiological diagnosis of CSEA,
(2) study deals with the effects of surgical treatment and/or antibiotic treatment of SEA,
(3) study includes any post-treatment outcome measures,
(4) study includes dose-specific antibiotic intervention,
(5) study includes at least five patients aged ⩾ 18 years with CSEA.
Studies were excluded from review based on the following exclusion criteria:
(1) study conducted before 1980,
(2) study not published in English or French,
(3) study only available as abstract or conference proceeding,
(4) study not conducted in humans (i.e. in vitro or animal study),
(5) study consists of only basic science, biomechanics, or cadaver research,
(6) study deals with only Mycobacterium tuberculosis or Brucella species as cultured pathogens,
(7) study deals with only immunosuppressed patients,
(8) inability to extract cervical patients from total patient cohort,
(9) inability to differentiate surgically treated group from medically treated group in terms of outcome measures,
(10) inability to differentiate pediatric patients from nonpediatric patients.
Studies dealing with only M. tuberculosis or Brucella species as cultured pathogens were excluded, as their patient populations were not deemed to be representative of the total CSEA patient cohort. Furthermore, studies including nonextricable pediatric patients as well as studies including only immunosuppressed patients were excluded because patient characteristics (e.g. risk factors, adverse events) and indications for management of these patients differ from those of the general adult CSEA population.
Data abstraction
Data were independently abstracted from each study by two reviewers (AT and LZ). Discrepancies between reviewers were resolved by discussion with a third reviewer (DMR) until consensus was reached.
The following data were abstracted from each study:
(1) date of publication,
(2) total number of CSEA patients,
(3) patient characteristics (sex, age, and risk factors for the development of CSEA),
(4) method of diagnosis of CSEA,
(5) symptoms at presentation,
(6) total number of CSEA patients treated by antibiotics alone (nonoperative management),
(7) total number of CSEA patients treated by antibiotics and surgery (operative management),
(8) total number of crossover CSEA patients (patients who, having failed medical management, proceeded to surgery),
(9) number of patients with neurological deficit prior to treatment,
(10) number of patients with neurological deficit following treatment,
(11) cultured pathogens,
(12) anatomic location of epidural abscess (ventral, dorsal, or circumferential),
(13) changes in infectious parameters,
(14) outcome measures,
(15) adverse events (complications, recurrences, and deaths).
For the purpose of this systematic review, all abscesses were classified at their most rostral level. Therefore, cervicothoracic epidural abscesses were included in CSEAs. In addition, percutaneous needle aspiration of the abscess was not considered to be a surgical procedure.
Quality assessment
Quality assessment was completed independently by two reviewers, AT and LZ, using the National Heart, Lung, and Blood Institute’s Quality Assessment Tool for Case Series Studies22 in order to assess risk of bias in the included studies. All discrepancies between the reviewers’ evaluation was resolved by discussion between them until consensus was reached. Studies meeting six to nine out of the assessment tool’s nine criteria were rated as good; three to five were rated as fair; and none to two were rated as poor.
Data analysis
Meta-analysis was planned to assess the association of the type of treatment received (operative versus nonoperative) and timing of surgical intervention (early versus delayed) with post-treatment neurological outcome (improvement/stability versus deterioration) and the failure rate of medical management converting to surgical intervention. However, there was a lack of available data from identified studies due to an absence of investigation, reporting, or sample size to allow for meaningful meta-analysis. Therefore, we made a post hoc decision to not perform meta-analysis. Individual study findings were synthesized narratively.
Results
Search results and study characteristics
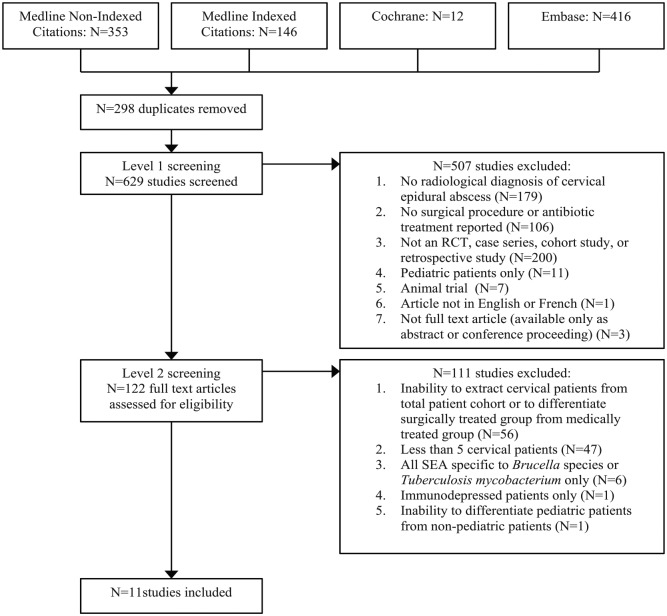
The literature search retrieved a total of 927 records, yielding 629 studies for level 1 screening after removal of duplicates. Of these studies, 507 were excluded and the full texts of the remaining 122 studies were assessed for level 2 screening. A further 111 studies were excluded based on their full texts, resulting in 11 studies included for narrative synthesis. No studies revealed in our search were published prior to 1980 despite the publication date restriction. The included studies all reported findings from single-center retrospective case series. Figure 1 details the study identification and selection process.
Figure 1.
Literature search and study selection process flowchart.
RCT, randomized controlled trial; SEA, spinal epidural abscess.
Following quality assessment, four studies were found to be of good quality; the remaining seven studies were assessed as having fair quality. Table 1 provides a summary of the quality assessment of each study.
Table 1.
Quality assessment summary of referenced studies.
| Reference | Was the study question or objective clearly stated? | Was the study population clearly and fully described, including a case definition? | Were the cases consecutive? | Were the subjects comparable? | Was the intervention clearly described? | Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants? | Was the length of follow up adequate? | Were the statistical methods well described? | Were the results well described? | Quality ranking |
|---|---|---|---|---|---|---|---|---|---|---|
| Alton et al.23 | Y | N | Y | Y | Y | Y | NR | Y | Y | Good |
| Bostrom et al.24 | Y | N | Y | Y | Y | Y | N/A | N/A | Y | Good |
| Ghobrial et al.25 | Y | N | Y | Y | Y | Y | NR | N/A | Y | Good |
| Klekamp and Samii26 | Y | N | Y | Y | Y | N | N/A | N | Y | Fair |
| Lindner et al.27 | N | N | N | Y | Y | N | N/A | N/A | Y | Fair |
| McGee-Collet and Johnston28 | Y | N | Y | N | Y | N | NR | N/A | N | Fair |
| Mondorf et al.29 | Y | Y | Y | Y | Y | Y | N/A | N/A | Y | Good |
| Muzii et al.30 | Y | N | Y | Y | Y | N | N/A | N/A | N | Fair |
| Piccolo et al.31 | Y | N | Y | Y | Y | N | N/A | N/A | N | Fair |
| Rigamonti et al.32 | Y | N | Y | Y | Y | N | N/A | N/A | Y | Fair |
| Wang et al.33 | Y | N | Y | Y | Y | N | N/A | N/A | Y | Fair |
N, no; N/A, not applicable; NR, not reported; Y, yes.
Of the included studies, six compared the outcome of nonoperative groups with operative groups in the treatment of CSEA, although for each of these studies, the nonoperative group accounts for only one third or less of the total patient cohort. The remaining five studies concerned only operative management.
Method of radiologic diagnosis of CSEA was reported in 10 of the 11 included studies. In all cases, radiologic diagnosis was made using independent or combined imaging involving MRI, CT, or myelography, with or without X-ray.
Outcome measures used in included studies are highly variable, with no two studies employing the same parameters. Three of the included studies used recognized spinal cord injury assessment scales; two used outcome classification scales defined within the study; and the remaining six studies employed loosely defined or nonsystematic measurement parameters, such as independence in ambulation, neurological improvement and sequelae, and return to normal function. Of the included studies, only two defined neurologic improvement for each patient in terms of the American Spinal Injury Association (ASIA) Impairment Scale (AIS), with one providing a comprehensive AIS grade, and the other reporting ASIA motor scores. The study by Wang and colleagues33 reported ASIA results but did not differentiate between cervical, thoracic, or lumbar SEA patients, and thus we were unable to extract CSEA patient data (Table 4).
Table 4.
Post-treatment outcomes of all patient groups, by referenced study.
| Reference | Patients undergoing nonoperative management (including failed), n | Patients undergoing operative management, n | Crossover patients (failed nonoperative management), n | Patients with neurological deficit prior to treatment, n | Patients with neurological deficit following treatment, n | Outcome measures | Outcome of nonoperative management group | Outcome of operative management group | Outcome of crossover group | Statistically significant conclusions regarding optimal treatment of CSEA if comparison operative versus nonoperative management |
|---|---|---|---|---|---|---|---|---|---|---|
| Alton et al.23 | 24 | 38 | 18 | 39 | 51 | ASIA motor scores at presentation and post-treatment | Presenting motor scores: 86.5 (SD
20.2) Post-treatment motor scores: 88.8 (SD 19.78). Average motor score increase: 2.3 (SD 4.4) |
Presenting motor scores: 72.4 (SD
30.2) Post-treatment motor scores: 84.3 (SD 27.5) Average motor score increase: 11.9 (SD 19.5; p < 0.01) |
Presenting motor scores: 99.2 (SD 3.3) Preoperative motor scores: 59.4 (SD 36.7) Postoperative motor scores: 83.3 (SD 24.6) Average motor score increase between pre- and postoperative: 23.9 Average motor decrease between presentation and postoperative: 15.9 |
Early surgery is preferable to both medical failure and delayed surgery, resulting in improved post-treatment motor scores |
| Bostrom et al.24 | 3 (all CT-guided punctures) | 6 | 0 | 8 | 2 + 1 death | Frankel grade pre- and post-treatment | Frankel grade at admission: D in 2 patients (66.66%) E in 1 patient (33.33%) Frankel grade at outcome: E in all 3 patients (100%) |
Frankel grade at admission: C in 2 patients (40%) B in in 2 patients (40%) A in 1 patient (20%) Frankel grade at outcome: E in 3 patients (60%) D in 1 patient (20%) Death in 1 patient (20%) |
N/A | No conclusions for CSEA |
| Ghobrial et al.25 | 0 | 40 | 0 | 31 | 23 | AIS grade at presentation and post-treatment | N/A | Improvement by 1 AIS grade in 16 patients
(40%) Deterioration by 1 AIS grade in 1 patient (2.5%) No change in AIS grade in 23 patients (57.5%) |
N/A | N/A |
| Klekamp and Samii26 | 1 (moribund patient having septicemia) | 6 | 0 | 7 | 5 + 2 deaths | Postoperative improvement and independence in ambulation | Neurological status pretreatment: severe
deficit Neurological status post-treatment: death |
Postoperative improvement with independent ambulation in 2
patients No postoperative improvement with ambulation requiring assistance in 3 patients Death in 1 patient |
N/A | No conclusions |
| Lindner et al.27 | 2 | 3 | 0 | 5 | 3 | Gait, independence in ambulation, and neurologic sequelae | Complete neurological resolution in 2 patients (1 patient remained incontinent) | Complete neurological resolution in 1
patient Remaining ataxic gait, tremors in gait, and disturbed bladder in 1 patient Tetraplegia in 1 patient |
N/A | No conclusions |
| McGee-Collet and Johnston28 | 1 (patient had percutaneous needle aspiration of abscess) | 4 | 0 | 3 at presentation, 5 at surgery | NR | Mobility and return to normal function | Clinical status pretreatment: paraparesis Clinical status post-treatment: mobile |
Preoperative clinical status: paraparesis in 3;
radiculopathy in 1 Clinical status postoperative: normal in 2; mobile with frame in 1; mobile with assistance in 1 |
N/A | No conclusions |
| Mondorf et al.29 | 0 | 5 | 0 | 4 | 4 | Independence in ambulation, neurologic sequelae, and radiological resolution | N/A | Improvement of plegia of right upper extremity and
resolution of incontinence in 1 patient Resolution of neurological symptoms in 1 patient Improvement of tetraparesis without ability to ambulate in 1 patient Improvement of tetraparesis and ambulation with assistance in 1 patient Improvement of tetraparesis in 1 patient |
N/A | N/A |
| Muzii et al.30 | 0 | 8 | 0 | 8 | 2 | Neurologic recovery and sequelae, radiological resolution, regression of pain | N/A | Full neurological recovery in 6 patients, moderate paraparesis (ambulation with assistance) in 1 patient, mild paraparesis in 1 patient | N/A | N/A |
| Piccolo et al.31 | 0 | 5 | 0 | 5 | 5 | Improvement in neurologic status | N/A | Improvement in 3 patients (60%), although patients did not return to normal clinical status. Death in 2 patients (40%) | N/A | N/A |
| Rigamonti et al.32 | 2 | 20 | 0 | 20 | 9 | Good, fair, and poor outcome | Neurological deficit pretreatment: none in 1, moderate in 1
Outcome: good in 2 |
Neurological deficit pretreatment: severe in 10, moderate in
9, none in 1 Outcome: good in 11, fair in 5, poor in 4 (death in 3) |
N/A | In certain cases, surgery can be avoided without negative outcome for the patient |
| Wang et al.33 | 0 | 5 | 0 | NR | NR | Significant or poor improvement | N/A | Significant improvement in 3 Poor improvement in 2 |
N/A | N/A |
AIS, ASIA Impairment Scale; ASIA, American Spinal Injury Association; CSEA, cervical spinal epidural abscess; CT, computed tomography; N/A, not applicable; NR, not reported; SD, standard deviation.
Patient characteristics
In total, 173 CSEA patients from 11 retrospective case series were included in this review. The mean patient age was 55 years (range: 18–86 years) and 61.3% of patients were male. Patient characteristics for each referenced study’s respective CSEA patient cohort are available in Table 2. Pooled patient characteristics are presented in Table 3.
Table 2.
Clinical patient characteristics, by referenced study.
| Reference | Total number of CSEA patients | Number of male patients | Number of female patients | Mean age of patients in years (range) | Cultured pathogen (patients affected %) | Risk factors/comorbidities (patients affected %) | Infectious parameters at presentation | Complications/recurrences/deaths |
|---|---|---|---|---|---|---|---|---|
| Alton et al.23 | 62 | 41 | 21 | 23 (22–85) |
MSSA: 38.6%; MRSA: 32.3% Streptococcus milleri: 4.8%; Group B Streptococcus 3.2%; Staphylococcus epidermis, Streptococcus anginosus, and Clostridium glabrata: 1.6% each Pathogen unknown: 16.3% |
IVDU: 53% Hepatitis: 31% DM: 21% Renal: 6.5% Immune compromise: 6.5% Liver: 4.8% Obesity: 3.2% |
Mean WBC and ESR were elevated in all groups | None |
| Bostrom et al.24 | 9 | NR | NR | ⩾18 (32 – 86) |
NR | NR | NR | 1 death |
| Ghobrial et al.25 | 40 | 30 | 10 | 53 (23–74) |
MSSA: 57.5% MRSA: 12.5% Pseudomonas: 5% Polymicrobial infection: 5% Beta-hemolytic Streptococcus species, Klebsiella, and Escherichia coli: 2.5% each Cultures negative: 12.5% |
Overweight: 35% IVDU: 25% Tobacco: 23% DM: 15% Malignancy: 15% Obese: 13% Prior remote abscess: 10% Alcoholism: 8% Neck radiation: 8% Endocarditis: 5% Neck surgery: 3% |
NR | 6% 90-day complication rate, no mortality |
| Klekamp and Samii26 | 7 | 5 | 2 | 64 (51–74) |
Staphylococcus aureus:
42.9% Pseudomonas aeruginosa: 14.3% Streptococci species: 14.3% Cultures negative: 28.9% |
Alcoholism: 43% DM: 29% Nephritis: 29% GI ulcer: 14% PE: 14% Malignancy: 14% Peridural catheter: 14% |
Elevated ESR in 100% patients | 2 deaths |
| Lindner et al.27 | 5 | 2 | 3 | 63 (58–69) |
S. aureus: 100% | Paravertebral injections: 40% Peridural injections: 20% Permanent catheter: 20% Thrombophlebitis: 20% DM: 20% |
Elevated ESR and WBC in 100% patients | Spinal arachnoiditis: 20% |
| McGee-Collet and Johnston28 | 5 | 2 | 3 | 55 (49–64) |
S. aureus: 60% P. aeruginosa: 20% Mycobacterium tuberculosis: 20% |
Tarsal osteomyelitis: 20% Sinusitis: 20% Pulmonary TB: 20% Furuncle: 20% |
NR | No mortality |
| Mondorf et al.29 | 5 | 3 | 2 | 68 (58–77) |
S. aureus: 100% | NR | NR | No mortality |
| Muzii et al.30 | 8 | 8 | 0 | 54 (27–66) |
S. aureus: 50% Streptococcus viridians:12.5% Streptococcus intermedius (S. milleri group): 12.5% S. epidermis and M. tuberculosis: 12.5% Streptococcus sanguinis and M. tuberculosis: 12.5% |
Pyogenic arthritis: 25% Hepatitis C: 25% Drug abuse: 25% Dental infection: 50% Catheter infection: 13% Pharyngeal abscess: 13% DM: 13% Sarcoidosis: 13% Orchiepididymitis: 13% Malignancy: 13% |
NR | NR |
| Piccolo et al.31 | 5 | 1 | 4 | 66 (64–69) |
S. aureus: 40% No bacteriological data available: 60% |
DM: 40% Hepatitis: 40% |
NR | 2 deaths due to sepsis |
| Rigamonti et al.32 | 22 | 14 | 8 | 48 (18–73) |
MSSA: 59.1% MRSA: 13.6% P. aeruginosa: 13.6% Group A Streptococcus, E. coli, S. epidermis, Enterococcus, and Acinetobacter: 4.5% Pathogen unknown: 9.1% |
IVDU: 54.6% Trauma: 36.4% DM: 27.3% Prior Sx: 22.7% HIV: 9.1% Obesity: 4.5% End-stage renal disease: 4.5% Nerve block: 4.5% |
Elevated WBC in 77% patients ESR elevated above 20 in 100% of patients for whom it was measured |
3 deaths |
| Wang et al.33 | 5 | NR | NR | ⩾18 | NR | NR | NR | NR |
CSEA, cervical spinal epidural abscess; DM, diabetes mellitus; ESR, erythrocyte sedimentation rate; GI, gastrointestinal; HIV, human immunodeficiency virus; IVDU, intravenous drug use; MSSA, methicillin-sensitive Staphylococcus aureus; MSRA, methicillin-resistant Staphylococcus aureus; NR, not reported; PE, pulmonary embolism; WBC, white blood cell count.
Table 3.
Pooled patient data.
| Characteristic | Patients affected, n |
|---|---|
| CSEA patients, n | 173 |
| Males, n (%) | 106 (61.3) |
| Females, n (%) | 53 (30.6) |
| Mean age, years (range) | 55 (18–86) |
| Patients undergoing initial operative management, n (%) | 140 (80.9) |
| Patients undergoing initial nonoperative management, n (%) | 33 (19.1) |
| Patients undergoing delayed operative management after failing initial nonoperative management, n (%) | 18 (10.4) |
| Patients with neurological deficit prior to treatment, n (%) | 132 out of 168 reported (78.6) |
| Patients with neurological deficit following to treatment, n (%) | 97 out of 163 reported (59.5) |
| Anatomic abscess position, n (%) | Ventral in 48 of 129 reported (37.2) Dorsal in 42 of 129 reported (32.6) Circumferential in 39 of 129 reported (30.2) |
| Cultured pathogens, n = 159 (%) |
Staphylococcus aureus: 94
(59.12) [MSSA: 47 out of 72 reported (65.28); MRSA: 25 out of 72 reported (34.72)] Pseudomonas aeruginosa: 5 (3.14% of 159 pts reported) Streptococcus milleri: 3 (1.89) Staphylococcus epidermis: 3 (1.89) Mycobacterium tuberculosis: 3 (1.89) Group B Streptococcus: 2 (1.26) Pseudomonas: 2, species not reported (1.26) Escherichia coli: 2 (1.26) Streptococcus species: 1 (0.63) Streptococcus anginosus: 1 (0.63) Clostridium glabrata: 1 (0.63) Beta-hemolytic Streptococcus species: 1 (0.63) Streptococcus viridians: 1 (0.63) Streptococcus intermedius: 1 (0.63) Streptococcus sanguinis: 1 (0.63) Group A Streptococcus: 1 (0.63) Klebsiella: 1, species not reported (0.63) Enterococcus: 1, species not reported (0.63) Acinetobacter: 1, species not reported (0.63) Negative cultures: 37 (23.27) Not reported: 14 |
| Risk factors and comorbidities (%) | IV drug use: 55 (36.67% of 150 patients
reported) Diabetes mellitus: 31 (20.67% of 150 patients reported) Hepatitis: 23 (15.33), including hepatitis C: 2 (1.33) Tobacco use: 9 (6) Prior trauma: 8 (5.33) Malignancy: 7 (4.67) Alcohol abuse: 6 (4) Prior surgery: 6 (4), including prior neck surgery: 1 (0.67) BMI 25–29.99: 14 (9.33) Obesity (BMI ⩾ 30): 8 (5.33) End-stage renal disease: 5 (3.33) Treatment of dental infections: 4 (2.67) Immune compromise: 4 (2.67) HIV: 2 (1.33) Liver disease: 3 (2) Neck radiation: 3 (2) Prior remote abscess: 4 (2.67) Pharyngeal abscess: 1 (0.67) Prior endocarditis: 2 (1.33) Pyogenic arthritis of the knee: 2 (1.33) Drug abuse: 2 (1.33) Nephritis: 2 (1.33) GI ulcer: 1 (0.67) Pulmonary embolism: 1 (0.67) Nerve block: 1 (0.67) Tarsal osteomyelitis: 1 (0.67) Sinusitis: 1 (0.67) Pulmonary TB: 1 (0.67) Furuncle: 1 (0.67) Sarcoidosis with steroid therapy: 1 (0.67) Tubercular orchiepididymitis: 1 (0.67) Peridural catheter: 1 (0.67) Permanent catheter: external jugular vein: 1 (0.67) Deep peridural injections: 1 (0.67) Deep paravertebral injections to neck: 2 (1.33) IV infusions leading to thrombophlebitis of arm: 1 (0.67) Hyperpharyngeal tumor with radiotherapy: 1 (0.67) Subclavian venous catheter infection: 1 (0.67) Not reported for 23 (includes 4 patients with multiple medical comorbidities) |
| Presenting symptoms/number of patients in whom presence or absence of this symptom is reported (%) | Neurological deficit: 132/168 (78.6) Neck or back pain: 69/112 (61.6) Constitutional symptoms (including fever, malaise, and weight loss): 39/112 (34.8) Urinary or sphincter incontinence/disturbed micturition or bladder: 16/112 (14.3) Shoulder or extremity pain: 12/112 (10.7) Altered mental status: 4/112 (3.57) Pain: 4/112 (3.57) Headache: 2/112 (1.79) Meningism: 2/112 (1.79) Dysphagia: 1/112 (0.892) Sudden hearing loss: 1/112 (0.892) |
BMI, body mass index; CSEA, cervical spinal epidural abscess; GI, gastrointestinal; HIV, human immunodeficiency virus; IV, intravenous; MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; TB, tuberculosis.
Anatomic position of abscess was reported in 129 CSEA patients. Of these, 37.2% had a ventral abscess, 32.6% had a dorsal abscess, and the remaining 30.2% had circumferential abscesses. A majority of patients (78.6%) presented with neurological deficit. Other common presenting symptoms for CSEA included neck or back pain in 61.6% of patients, constitutional symptoms (including fever, malaise, and weight loss) in 34.8%, and incontinence or disturbed micturition/bladder in 14.3%. All percentages were calculated based on the number of included patients for which presenting symptoms were reported and CSEA patient data was differentiable from the total SEA patient cohort.
Risk factors and comorbidities
Of the 173 CSEA patients, risk factors and medical comorbidities were reported for only 150 patients and are summarized in Table 3. In the remaining 23 patients, risk factors and comorbidities were not reported, or we were unable to extract the relevant data regarding CSEA patients from the studies’ total SEA patient cohort.
IV drug use was the most common risk factor, having affected 36.7% of patients for whom risk factors were reported. Diabetes mellitus and hepatitis were the second and third most common risk factors for the development of CSEA, having been reported in 20.7% and 15.3% of patients respectively. Iatrogenic risk factors affected 12.7% of patients, and included prior surgery, treatment of dental infections, neck radiation, use of a peridural catheter, use of a permanent catheter in the external jugular vein, deep peridural injections, deep paravertebral injections to the neck, IV infusions leading to thrombophlebitis of the arm, and a subclavian venous catheter infection.
Operative versus nonoperative management and outcomes
Of the included studies, 5 of 11 focused solely on the operative management of CSEA, accounting for 63 patients undergoing surgical treatment of the 173 CSEA patients. Of the six included studies that described both operative and medical management, 77 patients received initial surgical treatment, and an additional 18 patients failed medical management and received delayed surgery. Operative management of CSEA, either early or delayed, was by far more frequent than nonoperative management, accounting for 158 of 173 patients’ treatment (91.3%). Outcome results for each study are reported in Table 4.
Of the 15 patients treated with nonoperative management alone, three received a CT-guided puncture; 2 patients improved to an AIS E grade, and 1 remained at AIS E grade throughout treatment. One additional medically managed patient received percutaneous needle aspiration of the abscess; this patient exhibited paraparesis at presentation but was described as mobile post-treatment. When compared with the operative management group, patients selected for nonoperative management tended to present with less severe or no neurological deficit, or with significant comorbidities contraindicating surgery.
Cultured pathogens
Pathogen culture results were presented for 159 of 173 CSEA patients and are summarized in Table 3. In the remaining 14 patients, we were unable to extract the relevant data regarding CSEA patients from the total SEA patient cohort. Staphylococcus aureus was the most commonly cultured pathogen and was seen in 59.1% of patients for whom pathogens were reported. Of the studies reporting methicillin sensitivity, methicillin-sensitive S. aureus (MSSA) accounted for 65.3% of S. aureus cases. Pseudomonas aeruginosa was the second most common pathogen, affecting 3.1% of patients. Streptococcus milleri, Staphylococcus epidermis, and M. tuberculosis were cultured in 1.9% of patients each. Cultures were negative in 23.3% of patients.
Discussion
Our review results suggest that early operative management is advisable, even in the case of neurologically intact CSEA patients. This finding arises despite, and is contrary to, the noted selection bias caused by the recent shift to treat neurologically intact patients conservatively. This selection bias, documented in our analysis, represents not only a trend but a paradigm shift in SEA treatment, which has been recorded in the existing literature.7,13
Of the included studies comparing operative with nonoperative management for CSEA, only two provided conclusions regarding treatment recommendations. Rigamonti and colleagues32 recommended nonoperative management in the case of ‘selected patients’ with CSEA; two nonpediatric patients undergoing antibiotic treatment alone presented with either no neurological deficit or moderate neurological deficit, and both had good outcomes. Alton and colleagues23 recommended early surgical treatment of all incoming CSEA patients, including neurologically intact patients, based on their ASIA motor score results, with the suggested optimal time to the operating room being 24 h or less. Our finding regarding the importance of early surgery in CSEA treatment is primarily based on the study by Alton and coworkers.23 In comparison, in SEA patients, Patel and colleagues8 found that surgically managed and medically managed groups (including both successful and failed medical management) had statistically similar post-treatment ASIA motor scores. However, the surgical group showed a net improvement to obtain these motor scores, while their medical group deteriorated from originally higher pretreatment motor scores.
The most common presenting symptoms seen in our CSEA patient population were: neurological deficit; neck or back pain; constitutional symptoms including fever and malaise; and urinary or sphincter incontinence/disturbed micturition or bladder. Together, these four most prevalent symptoms mirror the results in the SEA literature.5,7 IV drug use was the most frequently reported risk factor, while diabetes mellitus and hepatitis were the most common medical comorbidities, once again mirroring previous SEA findings.7
Ventral anatomic location of abscess was more common than dorsal or circumferential abscesses in the CSEA patient population included for review. This is consistent with previous findings in the CSEA literature,2,23,34 and differs from the general SEA population, wherein dorsal SEAs are most common due to the larger epidural space and epidural fat in the dorsal region.13
Kim and colleagues35 sought to define risk factors for failure of medical management in SEA, indicating that neurologic impairment was the most significant risk factor, with patients age over 65 years, diabetes, and methicillin-resistant S. aureus (MRSA) also identified as risk factors. Even in the absence of these conditions, a risk of medical management failure of 17% was reported. In our systematic review, failure of medical management for CSEA was described in only one study, which did not find any significant correlation between identified risk factors and failure or success of conservative treatment. The investigators attributed this finding to the exceptionally small number of patients treated successfully with medical management (25%).23
The 75% medical failure rate among CSEA patients reported by Alton and colleagues23 was significantly higher than medical failure rates for SEA patients. Patel and colleagues8 reported a medical failure rate of 41%. Suppiah and coworkers20 reported a mean medical to surgical patient crossover rate of 28.15%. Stratton and colleagues36 found a pooled medical management failure rate of 29.3% in their recent systematic review and meta-analysis, although they highlighted the considerable heterogeneity in the literature regarding the definition of medical management failure, which hinders the comparison of treatment outcomes across studies. These results suggest that CSEA patients may be at greater risk for medical failure than the general SEA population, and therefore operative management should be considered to a greater extent in these patients. However, as only one of the case series included in this review reported medical management failure rate, further investigation is needed to confirm this comparative finding between CSEA and SEA.
Our results are similar to those reported by Howie and colleagues37 in their systematic review on thoracic SEA, which highlights the faster onset of neurological deficit in thoracic and cervical SEA compared with lumbar SEA, and recommends immediate surgical decompression in the case of thoracic SEA presenting with neurological deficit. In addition, a recent systematic review on lumbar SEA reported that CSEA is more likely to cause paraparesis or paraplegia than SEA at any other spinal level.38
S. aureus was the most commonly cultured pathogen in our systematic review, affecting 59.1% of patients for whom positive cultures were obtained. In addition to our pooled sex ratios and mean patient age, our results are similar to a recent systematic review on SEA that included 1099 patients by Arko and coworkers,7 as well as an older meta-analysis on SEA in 915 patients.5
If conservative management is selected, close monitoring paired with a high degree of suspicion for deterioration should be maintained in order to allow for emergent surgery. A notable treatment option for all patients undergoing medical management is an adjuvant CT-guided puncture or percutaneous needle aspiration of the abscess, if dorsal. Although presenting the added risk of inadvertent iatrogenic seeding of the infection into the thecal sac,2 this led to a good outcome in all of our included patients having undergone these procedures,24,28 and its efficacy has been reported elsewhere.12,17 That being said, due to our small patient population having undergone these procedures, we cannot advocate it as a treatment of choice.
Due to the gravity of CSEA, randomized controlled trials (RCTs) in humans are unethical. Thus, our review is limited to a body of evidence that comprises retrospective case series, leading to a lack of uniformity across these studies and within their described patient cohorts. None of our included studies featured a clearly defined protocol with indications for choice of operative versus nonoperative management, or a standardized time to surgery or type of surgical decompression. Often, patient treatment decisions were made without both medical and surgical consultations upon patient presentation and were sometimes due to factors unrelated to the patient’s condition (e.g. operating room availability).
With retrospective studies, reporting bias can affect the data abstracted, due to the fact that patient comorbidities, risk factors, and presenting symptoms are based on what factors the attending physician chose to report at the time. This could contribute to under-reporting for these characteristics and may account for the lack of any significant risk factors for failure of medical management.
Selection bias may also have influenced our pooled results. Of our five included studies featuring operative management of CSEA only, two specifically excluded CSEA patients having undergone conservative management,24,29 with one solely describing polyetheretherketone (PEEK) cage cervical ventral fusion.29 In addition, one of our included studies concerned iatrogenic spinal infection only.27 Inclusion of patients with iatrogenic CSEA as well as CSEA caused by M. tuberculosis may have skewed our results in favor of early surgery. Due to these constraints, the CSEA patient populations included in our review may not be generalizable to the CSEA population as a whole.
Finally, high variability across outcome measures ensured an inefficient pooling of study results. AIS is the current gold standard for assessment of spinal cord injury, and thus its lack of pervasiveness as an outcome measure in the existing literature is indicative of the paucity of high-quality CSEA research. One of our included studies employed Frankel grades as an outcome measurement, a scale that is recognized as being outdated and limited due to its fundamentally subjective grading and failure to classify level of injury.39 The remaining included studies employ either self-defined outcome classification scales, or qualitative, nonsystematic outcome measures. These include highly subjective and incomprehensive parameters, such as ‘neurological improvement’, description of gait, and independence of ambulation, which are not necessarily indicative of patients’ neurological status.
Conclusion
Ours is the only systematic review on CSEA to date. On the basis of the available evidence, emergent surgical decompression and abscess evacuation is the best course of action for these patients whenever possible. This recommendation is limited by the lack of good quality research available on CSEA in the scientific literature and the small number of studies included in this review. Early surgery seems to be even more important in the CSEA population than that of thoracolumbar SEA. Even so, if medical management is chosen as a treatment modality, we recommend immediate surgical consultation paired with close monitoring for neurological deterioration, with emergent surgery remaining available. Future research in this area is needed in order to further corroborate and expand on these results. Although RCTs remain unethical, larger case series directly comparing medical with surgical management, with well-defined and objective outcome measures, are needed.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Anastasia Turner  https://orcid.org/0000-0002-4670-421X
https://orcid.org/0000-0002-4670-421X
Contributor Information
Anastasia Turner, Faculty of Medicine, University of Ottawa, 451 Smyth Road, Ottawa, ON K1H8L1, Canada.
Linlu Zhao, Ottawa Spine Collaborative Analytics Network, The Ottawa Hospital, Ottawa, ON, Canada.
Paul Gauthier, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada; Ottawa Spine Collaborative Analytics Network, The Ottawa Hospital, Ottawa, ON, Canada.
Suzan Chen, Ottawa Spine Collaborative Analytics Network, The Ottawa Hospital, Ottawa, ON, Canada; Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Darren M. Roffey, Ottawa Spine Collaborative Analytics Network, The Ottawa Hospital, Ottawa, ON, Canada Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Eugene K. Wai, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada Ottawa Spine Collaborative Analytics Network, The Ottawa Hospital, Ottawa, ON, Canada; Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada; Division of Orthopedic Surgery, University of Ottawa, The Ottawa Hospital, Ottawa, ON, Canada.
References
- 1. Davis DP, Wold RM, Patel RJ, et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med 2004; 26: 285–291. [DOI] [PubMed] [Google Scholar]
- 2. Bagley CA, Dudukovich KJ, Wolinsky JP, et al. Surgical management of lumbosacral spinal epidural abscess. Operat Tech Neurosurg 2005; 7: 206–211. [Google Scholar]
- 3. Darouiche RO. Spinal epidural abscess. N Engl J Med 2006; 355: 2012–2020. [DOI] [PubMed] [Google Scholar]
- 4. Johnson KG. Spinal epidural abscess. Crit Care Nurs Clin North Am 2013; 25: 389–397. [DOI] [PubMed] [Google Scholar]
- 5. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev 2000; 23: 175–205. [DOI] [PubMed] [Google Scholar]
- 6. Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol 1999; 52: 189–197. [DOI] [PubMed] [Google Scholar]
- 7. Arko L, IV, Quach E, Nguyen V, et al. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus 2014; 37: E4. [DOI] [PubMed] [Google Scholar]
- 8. Patel AR, Alton TB, Bransford RJ, et al. Spinal epidural abscesses: risk factors, medical surgical management, a retrospective review of 128 cases. Spine J 2014; 14: 326–330. [DOI] [PubMed] [Google Scholar]
- 9. Al-Hourani K, Al-Aref R, Mesfin A. Upper cervical epidural abscess in clinical practice: diagnosis and management. Global Spine J 2016; 6: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lasker BR, Harter DH. Cervical epidural abscess. Neurology 1987; 37: 1747–1753. [DOI] [PubMed] [Google Scholar]
- 11. Soehle M, Wallenfang T. Spinal epidural abscess: clinical manifestations, prognostic factors, and outcomes. Neurosurgery 2002; 51: 79–87. [DOI] [PubMed] [Google Scholar]
- 12. Bluman EM, Palumbo MA, Lucas PR. Spinal epidural abscess in adults. J Am Acad Orthop Surg 2004; 2004:155–163. [DOI] [PubMed] [Google Scholar]
- 13. Pradilla G, Nagahama Y, Spivak AM, et al. Spinal epidural abscess: current diagnosis and management. Curr Infect Dis Rep 2010; 12: 484–491. [DOI] [PubMed] [Google Scholar]
- 14. Khanna RK, Malik GM, Rock JP, et al. Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery 1996; 39: 958–964. [DOI] [PubMed] [Google Scholar]
- 15. Curry WT, Jr, Hoh BL, Amin-Hanjani S, et al. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol 2005; 64: 364–371. [DOI] [PubMed] [Google Scholar]
- 16. Connor DE, Jr, Chittiboina P, Caldito G, et al. Comparison of operative and nonoperative management of spinal epidural abscess: a retrospective review of clinical and laboratory predictors of neurological outcome. J Neurosurg Spine 2013; 19:119–127. [DOI] [PubMed] [Google Scholar]
- 17. Siddiq F, Chowfin A, Tight R, et al. Medical vs surgical management of spinal epidural abscess. Arch Intern Med 2004; 164: 2409–2412. [DOI] [PubMed] [Google Scholar]
- 18. Karikari IO, Powers CJ, Reynolds RM, et al. Management of a spontaneous spinal epidural abscess: a single-center 10-year experience. Neurosurgery 2009; 65: 919–924. [DOI] [PubMed] [Google Scholar]
- 19. Adogwa O, Karikari IO, Carr KR, et al. Spontaneous spinal abscess in patients of 50 years of age and older: a 15-year institutional perspective and review of the literature: clinical article. J Neurosurg Spine 2014; 20: 344–349. [DOI] [PubMed] [Google Scholar]
- 20. Suppiah S, Meng Y, Fehlings MG, et al. How best to manage the spinal epidural abscess? A current systematic review. World Neurosurg 2016; 93: 20–28. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Heart, Lung, and Blood Institute. Study quality assessment tools, https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. (accessed 2016).
- 23. Alton TB, Patel AR, Bransford RJ, et al. Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J 2015; 15: 10–17. [DOI] [PubMed] [Google Scholar]
- 24. Boström A, Oertel M, Ryang Y, et al. Treatment strategies and outcome in patients with non-tuberculous spinal epidural abscess–a review of 46 cases. Minim Invasive Neurosurg 2008; 51: 36–42. [DOI] [PubMed] [Google Scholar]
- 25. Ghobrial GM, Viereck MJ, Margiotta PJ, et al. Surgical management in 40 consecutive patients with cervical spinal epidural abscesses. Spine 2015; 40: E949–4953. [DOI] [PubMed] [Google Scholar]
- 26. Klekamp J, Samii M. Extradural infections of the spine. Spinal Cord 1999; 37: 103–109. [DOI] [PubMed] [Google Scholar]
- 27. Lindner A, Warmuth-Metz M, Becker G, et al. Iatrogenic spinal epidural abscess: early diagnosis essential for good outcome. Eur J Med Res 1997; 2: 201–205. [PubMed] [Google Scholar]
- 28. McGee-Collett M, Johnston IH. Spinal epidural abscess: presentation and treatment. A report of 21 cases. Med J Aust 1991; 155: 14–17. [PubMed] [Google Scholar]
- 29. Mondorf Y, Gaab MR, Oertel JM. PEEK cage cervical ventral fusion in spondylodiscitis. Acta Neurochirurgica 2009; 151: 1537–1541. [DOI] [PubMed] [Google Scholar]
- 30. Muzii VF, Mariottini A, Zalaffi A, et al. Cervical spine epidural abscess: experience with microsurgical treatment in eight cases. J Neurosurg Spine 2006; 5: 392–397. [DOI] [PubMed] [Google Scholar]
- 31. Piccolo R, Passanisi M, Chiaramonte I, et al. Cervical spinal epidural abscesses. A report on five cases. J Neurosurg Sci 1999; 43: 63–67. [PubMed] [Google Scholar]
- 32. Rigamonti D, Liem L, Wolf AL, et al. Epidural abscess in the cervical spine. Mt Sinai J Med 1994; 61: 357–362. [PubMed] [Google Scholar]
- 33. Wang TC, Lu MS, Yang JT, et al. Motor function improvement in patients undergoing surgery for spinal epidural abscess. Neurosurgery 2010; 66: 910–916. [DOI] [PubMed] [Google Scholar]
- 34. Lu CH, Chang WN, Lui CC, et al. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg 2002; 104: 306–310. [DOI] [PubMed] [Google Scholar]
- 35. Kim SD, Melikian R, Ju KL, et al. Independent predictors of failure of nonoperative management of spinal epidural abscesses. Spine J 2014; 14: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 36. Stratton A, Gustafson K, Thomas K, et al. Incidence and risk factors for failed medical management of spinal epidural abscess: a systematic review and meta-analysis. J Neurosurg Spine 2017; 26: 81–89. [DOI] [PubMed] [Google Scholar]
- 37. Howie BA, Davidson IU, Tanenbaum JE, et al. Thoracic epidural abscesses: a systematic review. Global Spine J 2018; 8(Suppl. 4): 68S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Leeuw CN, Fann PR, Tanenbaum JE, et al. Lumbar epidural abscesses: a systematic review. J Emerg Med, 2018; 8(Suppl. 4): 85S–95S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Middendorp JJ, Goss B, Urquhart S, et al. Diagnosis and prognosis of traumatic spinal cord injury. Global Spine J 2011; 1: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]