Abstract
Background:
The effect of statins on oxidative stress markers, such as malondialdehyde (MDA), is still a matter of debate. We sought to address this issue by conducting a systematic review and meta-analysis of published data on the effect of statin treatment on systemic MDA concentrations.
Methods:
A literature search was conducted on MEDLINE/PubMed, ISI Web of Sciences and Scopus. Data were pooled using a random-effects model.
Results:
A total of 35 studies assessing MDA concentrations before and after statin treatment in 1512 participants (mean age 53.6 years, 48.7% males) were identified. Extreme between-study heterogeneity was observed (I2 = 96.0%, p < 0.001). Pooled standardized mean difference (SMD) showed a significant reduction in plasma MDA concentrations after treatment (SMD = −1.47 µmol/l, 95% confidence interval = −1.89 to −1.05 μmol/l; p < 0.001). Similarly, a subgroup analysis of 10 studies that also included a placebo group showed a significant reduction in plasma MDA concentrations with statins (−1.03 μmol/l, 95% confidence interval = −1.52 to −0.29 μmol/l; p = 0.036).
Conclusions:
This systematic review and meta-analysis showed that statin treatment significantly reduces systemic MDA concentrations. However, the results should be interpreted with caution because of extreme between-study heterogeneity, which warrants further intervention studies.
Keywords: malondialdehyde, meta-analysis, oxidative stress, random-effects model, statin, systematic review, thiobarbituric acid reactive substances
Introduction
Hypercholesterolaemia is a leading risk factor for atherosclerotic cardiovascular disease (CVD) and its clinical manifestations such as peripheral vascular disease, ischaemic stroke and myocardial infarction.1,2 Hypercholesterolaemia, as well as other CVD risk factors, particularly hypertension, diabetes, and cigarette smoking, impair endothelial function thus promoting or accelerating the atherosclerotic process in the vessel wall.3 Due to their inhibition of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, the rate-limiting enzyme in the mevalonate pathway through which cells synthesize cholesterol, statins are widely prescribed for hypercholesterolaemia and have an established role in primary and secondary CVD prevention.4,5 The main action of statins in reducing cardiovascular morbidity and mortality is through their established low-density lipoprotein (LDL) lowering effects;6 however, this class of drugs might also exert beneficial effects that are not related to LDL lowering (‘pleiotropic’ effects).7 Therefore, it has been postulated that statins may have additional anti-atherogenic effects, including enhanced endothelial function, inhibition of vascular inflammation, reduction of vascular and myocardial remodelling, and stabilization of the atherosclerotic plaque.8 The reduction of oxidative stress (OS) might mediate these pleiotropic effects, which often occur very early in the course statin treatment.9
OS is considered an important contributing factor in the pathophysiology of atherosclerosis and CVD.10 Reactive oxygen species (ROS) are involved in virtually all disease stages, from endothelial dysfunction to atheromatous plaque formation and rupture. Furthermore, the involvement of LDL oxidation (by free radicals or cellular enzymes) in the development of atherosclerotic lesions has been well documented.11–13 The harmful effects of OS are due to the direct damage of lipids, thiols, DNA, and protein pools. In particular, increased concentrations of lipid peroxidation products stimulate the synthesis of proinflammatory cytokines and the adhesion of monocytes to endothelial surface.14 These effects are augmented in hypercholesterolaemia due to the concomitant presence of elevated OS and lipid concentrations.
The effects of statins on OS have been extensively studied both in vitro and in cellular systems.15–17 Statin therapy appears to increase glutathione reductase activity and to induce the heme-oxygenase 1 system.16 Moreover, the antioxidant effect exerted by some statins seems to be related to their ability to inhibit oxidant enzymes such as nicotinamide adenine dinucleotide phosphate (NAD[P]H) oxidase, and upregulate antioxidant enzymes such as catalase.17 Since malondialdehyde (MDA), measured as thiobarbituric acid reactive substances (TBARS), is the biomarker most frequently assessed to characterize the systemic lipid peroxidation status in several conditions, several studies have investigated the effect of statins on systemic MDA concentrations to evaluate their in vivo effect on OS. We sought to comprehensively assess this topic, and to provide a treatment effect size, by conducting a systematic review and meta-analysis of published studies of statin treatment on plasma MDA concentrations.
Materials and methods
Search strategy, eligibility criteria, and study selection
A systematic search of publications in the electronic databases PubMed, Web of Science, and Scopus, from inception to September 2018, was conducted using the following terms and their combination: ‘malondialdehyde’ or ‘MDA’ and ‘statins’. Abstracts were screened independently by two investigators to establish relevance. If relevant, the two investigators independently reviewed the full articles. Eligibility criteria were: (1) assessment of MDA plasma or serum concentration in humans at baseline and after statin treatment, (2) English language, and (3) full-text publications. Exclusion criteria were: (1) assessment of MDA in LDL or erythrocytes, and (2) duration of statin therapy <1 week. The references of the retrieved articles and reviews were also searched to identify additional studies. Any disagreement between the reviewers was resolved by a third investigator. We used the Newcastle–Ottawa Scale (NOS) to assess the quality of each study.18 The NOS evaluated the following components: selection of the cohort, comparability of cohorts on the basis of the design or analysis, how the exposure was ascertained, and how the outcomes of interest were assessed. NOS scores of 1–3, 4–6, 7–9 indicated low, intermediate and high quality, respectively.
The following information was extracted from each paper and entered in an MS Excel spreadsheet: article title, first author, year of the study, place of the study, NOS score, presence of a placebo group, sample matrix, assay type, total sample size, age, sex distribution, MDA concentrations at baseline, MDA concentrations post-treatment, disease type, type and dose of statin, and therapy duration.
Statistical analysis
Standardized mean differences (SMDs) were used to generate forest plots of continuous data and to evaluate differences in MDA concentrations before and after statin treatment. A p value <0.05 was considered statistically significant, and 95% confidence intervals (CIs) were reported. Heterogeneity of SMD across studies was tested by using the Q statistic (significance level at p < 0.10). The I2 statistic, a quantitative measure of inconsistency across studies, was also calculated (I2<25%, no heterogeneity; I2 between 25% and 50%, moderate heterogeneity; I2 between 50% and 75%, large heterogeneity; and I2>75%, extreme heterogeneity).19–20 Statistical heterogeneity was defined as an I2 statistic value ⩾50%.20 In analyses in which heterogeneity was high, a random-effects model was applied. Sensitivity analysis was conducted to investigate the influence of an individual study on the overall risk estimate, by sequentially excluding one study in each turn.21 To evaluate the presence of potential publication bias, the associations between study size and magnitude of effect were analysed by means of Begg’s adjusted rank correlation test and Egger’s regression asymmetry test at the p < 0.05 level of significance.22–23 We also performed the Duval and Tweedie ‘trim-and-fill’ procedure24 to further assess the possible effect of publication bias. This method considers the possibility of hypothetical ‘missing’ studies that might exist and recalculates a pooled SMD that incorporates the hypothetical missing studies as though they actually existed. Statistical analyses were performed using MedCalc for MS Windows, version 15.4 64 bit (MedCalc Software, Ostend, Belgium) and Stata 14 (STATA Corp., College Station, TX, USA). The reporting of the present meta-analysis adhered to the PRISMA guidance.25
Results
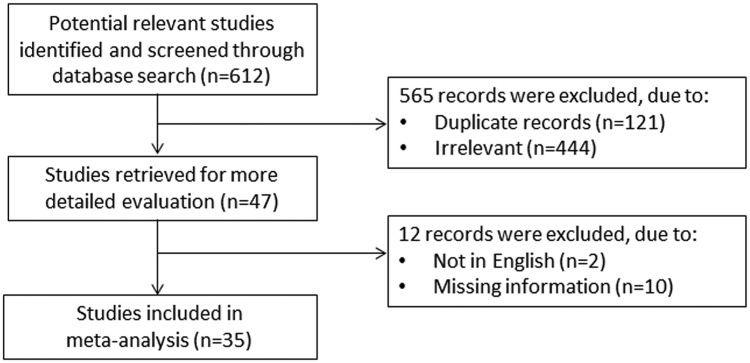
A flow chart describing the screening process is presented in Figure 1 (details of the full electronic search strategy in PubMed are described in Supplementary File 1). We initially identified 612 studies. A total of 565 studies were excluded after the first screening because they were either duplicates or irrelevant. After a full-text revision of 47 articles, a further 12 studies were excluded either because of missing information or because they did not meet the inclusion criteria (2 papers were not in English, 4 papers measured MDA in LDL, 3 papers did not provide post-treatment MDA concentrations, 2 papers provided only graphical data, and 1 paper did not provide the units of measurement for MDA). Thus, 35 studies were included in the meta-analysis.26–59 The characteristics of the retrieved studies are presented in Table 1.
Figure 1.
Flow chart of study selection.
Table 1.
Summary of the studies included in the meta-analysis.
| First author year, country | NOS (stars) | Placebo group | Sample matrix | Assay type | n | Age mean or median (years) | Sex (M/F) | MDA before mean ±SD (μmol/l) | MDA after mean ±SD (μmol/l) | Disease | Therapy type | Therapy duration (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang and colleagues26 Belgium | 9 | yes | p | Colorimetric | 20 | 43 | 16/4 | 2.4±0.6 | 2.5±0.9 | T1DM | Pravastatin 20 mg/day | 12 |
| Nishikawa and colleagues27 Japan | 8 | no | s | NR | 38 | 58.7 | 20/18 | 0.569±0.139 | 0.500±0.097 | ESRD | Simvastatin 5 mg/day | 24 |
| Chang and colleagues28 Korea | 9 | yes | p | Colorimetric | 31 | 63 | 8/23 | 3.15±1.27 | 3.47±1.17 | ESRD | Simvastatin 20 mg/day | 8 |
| Harangi and colleagues29 Hungary | 8 | no | s | Colorimetric | 13 | 53.6 | 4/9 | 1.00±0.26 | 0.79±0.20 | Hyperlipidaemia | Atorvastatin 10 mg/day | 52 |
| Koh and colleagues31
South Korea |
9 | yes | p | ELISA | 32 | 62 | 13/19 | 1.94±0.71 | 1.58±0.75 | CAD | Simvastatin 20 mg/day + diet | 14 |
| Kural and colleagues32 Turkey | 8 | no | s | Colorimetric | 40 | 53.5 | 21/19 | 5.96±2.25 | 3.81±1.00 | Hypercholesterolemia and hyperlipidaemia | Atorvastatin 10 mg/day | 10 |
| Koh and colleagues30 South Korea | 8 | no | p | ELISA | 50 | 59 | 22/28 | 1.36±0.57 | 1.17±0.49 | Hypercholesterolaemia | Simvastatin 20 mg/day | 8 |
| Koh and colleagues30 South Korea | 8 | no | p | ELISA | 50 | 59 | 22/28 | 1.45±0.64 | 1.01±0.49 | Hypercholesterolaemia | Simvastatin 20 mg/day + ramipril 10 mg/day | 8 |
| Manuel-y-Keenoy and colleagues33 Belgium | 8 | no | p | Colorimetric | 11 | 59 | 9/2 | 1.17±0.31 | 0.80±0.21 | T1DM | Atorvastatin 20 mg/day | 26 |
| Manuel-y-Keenoy and colleagues33 Belgium | 8 | no | p | Colorimetric | 11 | 59 | 10/1 | 1.27±0.39 | 0.79±0.16 | T1DM | Atorvastatin 20 mg/day + α-tocopherol 750 IU | 26 |
| Skrha 200434 Czech Republic | 8 | no | p | Colorimetric | 20 | 57 | 12/8 | 2.56±0.45 | 2.39±0.50 | T2DM | Simvastatin 20 mg/day | 12 |
| Karatzis and colleagues35 Greece | 9 | yes | s | Colorimetric | 20 | 62.1 | 20/0 | 3.89±1.99 | 2.67±1.30 | Unstable angina | Pravastatin 40 mg/day | 1.5 |
| Save and colleagues36 India | 8 | no | s | Colorimetric | 100 | 51.3 | 29/71 | 6.5±1.1 | 5.3±0.9 | Hyperlipidaemia T2DM | Atorvastatin 10 mg/day | 24 |
| Molcányiová and colleagues39 Slovakia | 8 | no | p | Colorimetric | 42 | 42 | 12/30 | 2.00±0.48 | 1.88±0.44 | Hypercholesterolaemia | Simvastatin 20 mg/day | 8 |
| Koh and colleagues38 South Korea | 8 | no | p | ELISA | 50 | 59 | 30/20 | 0.85±0.41 | 0.72±0.31 | T2DM | Simvastatin 20 mg/day | 8 |
| Koh and colleagues38 South Korea | 8 | no | p | ELISA | 50 | 59 | 30/20 | 0.83±0.36 | 0.60±0.31 | T2DM | Simvastatin 20 mg/day + ramipril 10 mg/day | 8 |
| Abou-Raya and colleagues37 Egypt | 9 | yes | p | NR | 20 | 59.8 | 5/15 | 5.95±3.70 | 4.91±3.50 | SSc | Atorvastatin 40 mg/day | 26 |
| Castro and colleagues40 Chile | 9 | yes | P | Colorimetric | 38 | 58 | 31/7 | 1.18±0.43 | 1.03±0.43 | CHF | Atorvastatin 20 mg/day | 8 |
| Usharani and colleagues41 India | 9 | yes | s | Colorimetric | 23 | 50 | 12/11 | 3.46±0.51 | 2.16±0.17 | T2DM | Atorvastatin 10 mg/day | 8 |
| Nagila and colleagues42 Thailand | 8 | no | s | Colorimetric | 22 | 57.7 | NR | 17.6±2.7 | 14.8±2.9 | Hypercholesterolaemia | Atorvastatin 10 mg/day | 3 |
| Abdin and colleagues15 Egypt | 8 | no | s | Colorimetric | 22 | 35–75 | NR | 7.65±3.64 | 4.75±2.30 | T2DM | Atorvastatin 10/20 mg/day | 12 |
| Li and colleagues43 China | 8 | no | p | Colorimetric | 80 | 54 | 45/35 | 5.23±0.13 | 4.06±0.14 | CAD | Simvastatin 20 mg/day | 12 |
| Li and colleagues43 | 8 | no | p | Colorimetric | 84 | 56 | 51/33 | 5.42±0.15 | 3.82±0.12 | CAD | Atorvastatin 10 mg/day | 12 |
| Su and colleagues44 China | 8 | no | p | HPLC | 75 | 55 | 39/36 | 8.01±0.73 | 4.49±0.58 | T2DM | Simvastatin 40 mg/day | 12 |
| Su and colleagues44 China | 8 | no | p | HPLC | 76 | 56 | 43/33 | 8.04±0.93 | 4.22±0.45 | T2DM | Atorvastatin 10 mg/day | 12 |
| El-Barbary and colleagues45 Egypt | 8 | no | s | Colorimetric | 15 | 54.8 | 3/12 | 4.42±0.81 | 2.25±0.32 | RA | Atorvastatin 40 mg/day + prednisone 10 mg/day + MTX 0.2 mg/kg/week |
26 |
| Koh and colleagues46 South Korea | 9 | yes | p | ELISA | 42 | 53 | 22/20 | 1.26±0.45 | 1.07±0.32 | Hypertension | Atorvastatin 20 mg/day | 8 |
| Koh and colleagues46 South Korea | 8 | yes | p | ELISA | 42 | 53 | 22/20 | 1.30±0.45 | 1.02±0.32 | Hypertension | Atorvastatin 20 mg/day + amlodipine 10 mg/day | 8 |
| Samy and colleagues47 Egypt | 9 | no | s | Colori-metric | 25 | 35–62 | 11/14 | 3.03±0.23 | 2.77±0.41 | NAFLD | Atorvastatin 40 mg/day | 34 |
| Sathyapalan and colleagues48 United Kingdom | 8 | yes | s | HPLC | 19 | 26.6 | NR | 0.29±0.04 | 0.23±0.03 | PCOS | Atorvastatin 20 mg/day | 12 |
| Lazich and colleagues49 USA | 8 | no | s | ELISA | 20 | 55.1 | 14/6 | 8.48±2.89§ | 7.21±2.35§ | Metabolic syndrome | Simvastatin 40 mg/day | 26 |
| Lazich and colleagues49 USA | 8 | no | s | ELISA | 23 | 58.7 | 9/14 | 9.22±2.01§ | 7.00±2.72§ | Metabolic syndrome | Simvastatin 40 mg/day + rosiglitazone 4 mg/day | 26 |
| Zinellu and colleagues51 Italy | 8 | no | p | CE | 10 | 63 | 8/2 | 0.248±0.095 | 0.220±0.129 | CKD (III–IV) | Simvastatin 40 mg/day | 52 |
| Zinellu and colleagues51 Italy | 8 | no | p | CE | 20 | 59 | 14/6 | 0.203±0.163 | 0.154±0.118 | CKD (III–IV) | Simvastatin 20–40 mg/day + ezetimibe 10 mg/day | 52 |
| Murrow and colleagues50 USA | 8 | no | p | Colorimetric | 17 | 54.2 | 8/9 | 3.7±1.5 | 2.1±0.8 | Hyperlipidaemia metabolic syndrome T2DM | Atorvastatin 10 mg/day | 12 |
| Murrow and colleagues50 USA | 8 | no | p | Colorimetric | 19 | 51.4 | 5/14 | 3.1±1.0 | 2.5±1.0 | Hyperlipidaemia metabolic syndrome T2DM | Pravastatin 80 mg/day | 12 |
| Andrade and colleagues52 Brazil | 9 | no | p | Colorimetric | 25 | 19–58 | 0/25 | 16.0±9.8§ | 9.5±4.1§ | Obesity | Simvastatin 20 mg/day | 6.5 |
| Scheffer and colleagues53 Netherlands | 8 | no | p | HPLC | 12 | 40 | 6/6 | 7.7±3.3 | 8.4±3.1 | T1DM or T2DM obesity hypertension | Atorvastatin 10 mg/day | 12 |
| Scheffer and colleagues53 Netherlands | 8 | no | p | HPLC | 18 | 45.3 | 6/12 | 7.6±2.8 | 7.1±2.8 | T1DM or T2DM obesity hypertension | Simvastatin 40 mg/day | 12 |
| Villela and colleagues54 Brazil | 8 | no | p | Colorimetric | 25 | 19–58 | 0/25 | 12.3±0.9 | 8.5±0.7 | Obesity | Simvastatin 20 mg/day | 6 |
| Çiftçi and colleagues55 Turkey | 8 | no | p | HPLC | 30 | 40–60 | 10/20 | 0.09±0.02 | 0.06±0.03 | Hypercholesterolaemia | Atorvastatin 10 mg/day | 12 |
| Mirjanic-Azaric and colleagues56 Slovenia | 8 | no | p | HPLC | 44 | 61.5 | 12/32 | 1.39±0.34 | 1.68±0.51 | Stable angina | Atorvastatin 20 mg/day | 10 |
| Villegas-Rivera and colleagues57 Mexico | 9 | yes | p | Colorimetric | 25 | 55 | 10/15 | 0.99±0.75 | 0.52±0.50 | T2DM | Simvastatin 20 mg/day + ezetimibe 10 mg/day | 16 |
| Villegas-Rivera and colleagues57 Mexico | 9 | yes | p | Colorimetric | 25 | 54 | 12/13 | 0.82±0.75 | 0.53±0.50 | T2DM | Rosuvastatin 20 mg/day | 16 |
| Yildiz and colleagues58 Turkey | 8 | no | s | HPLC | 18 | 38.3 | 9/9 | 2.83±0.22 | 1.10±0.39 | Kidney transplant | Fluvastatin 80 mg/day | 4 |
| Rasmussen and colleagues59 Denmark | 9 | yes | p | HPLC | 20 | 25.2 | 20/0 | 0.160±0.042 | 0.160±0.041 | Healthy | Simvastatin 40 mg/day | 2 |
CAD, coronary artery disease; CE, capillary electrophoresis; CHF, congestive heart failure; CKD, chronic kidney disease; ELISA, enzyme-linked immunosorbent assay; ESRD, end-stage renal disease; F, female; HPLC, high-performance liquid chromatography; NOS, Newcastle–Ottawa quality assessment scale for case-control studies; M, male; MDA, malondialdehyde; MTX, methotrexate; NAFLD, nonalcoholic fatty liver disease; NR, not reported; PCOS, polycystic ovary syndrome; RA, rheumatoid arthritis; SD, standard deviation; SSc, systemic sclerosis; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
nmol/l.
A total of 1512 participants (mean age 53.6 years, 48.7% males) with various CVD risk factors were evaluated before and after statin administration. The number of participants in these studies ranged from 10 to 100. The included studies, published between 1995 and 2016, were conducted in Europe (n = 11), Africa (n = 4), America (n = 6), and Asia (n = 14). The statin used was atorvastatin in 16 studies, simvastatin in 15 studies, pravastatin in 2 studies, and fluvastatin and rosuvastatin in 1 study, in doses ranging between 5–80 mg/day. Duration of therapy ranged between 10 days and 12 months. No significant post- versus pre-treatment differences in MDA concentrations were reported in 10 of the 35 retrieved studies.
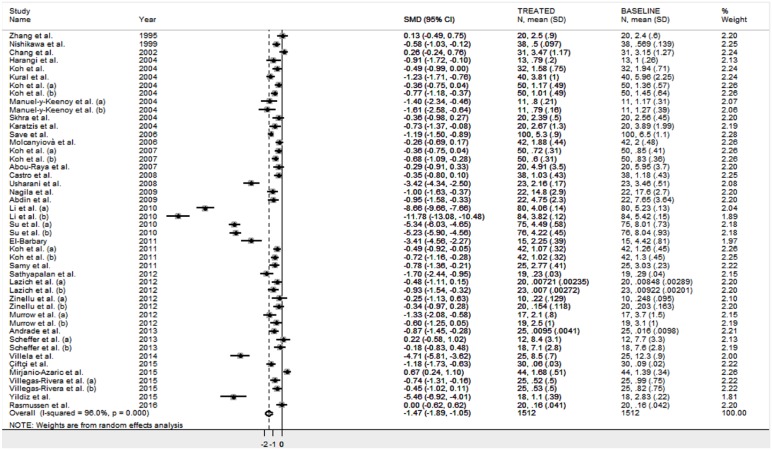
The forest plot for MDA concentrations in patients treated with statin is shown in Figure 2. Substantial heterogeneity between studies was observed (I2 = 96.0%, p < 0.001). Thus, random-effects models were used. Overall, pooled results showed that MDA concentrations were significantly lower after statin treatment (SMD = −1.47 μmol/l, 95% CI: −1.89 to −1.05 μmol/l; Z = 6.84, p < 0.001). Subgroup analysis revealed a similar impact on MDA concentrations of both hydrophilic (rosuvastatin, pravastatin and fluvastatin; n = 102; SMD: −1.22 μmol/l, 95% CI: −2.31 to −1.33, Z = 2.20, p = 0.028), and hydrophobic statins (simvastatin and atorvastatin; n = 1,410; SMD: −1.50 μmol/l, 95% CI: −1.95 to −1.04, Z = 6.46, p < 0.0001).
Figure 2.
Forest plot of studies examining MDA concentrations at baseline and after statin treatment.
CI, confidence interval; MDA, malondialdehyde; SD, standard deviation.
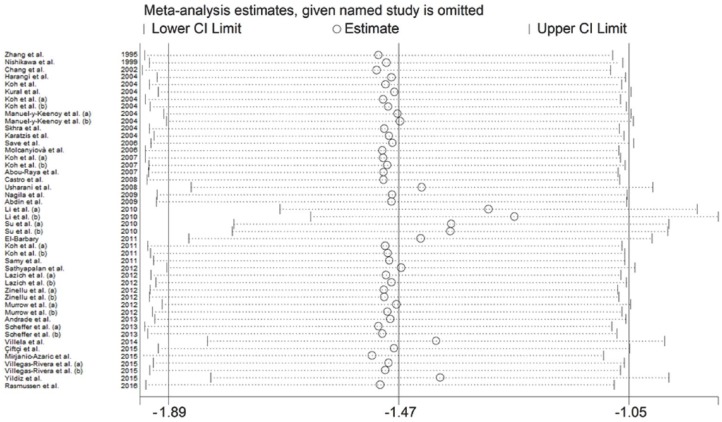
Results stability was evaluated through sensitivity analysis (Figure 3). The corresponding pooled SMD values were not substantially altered when single studies were sequentially removed, with effect size ranging between −1.89 and −1.05 μmol/l.
Figure 3.
Sensitivity analysis of the association between MDA and statin treatment. The influence of individual studies on the overall SMD is shown. The middle vertical axis indicates the overall SMD and the two vertical axes indicate the 95% CIs. Hollow circles represent the pooled SMD when the remaining study is omitted from the meta-analysis. The two ends of each broken line represent the 95% CI.
CI, confidence interval; MDA, malondialdehyde; SD, standard deviation; SMD, standardized mean difference.
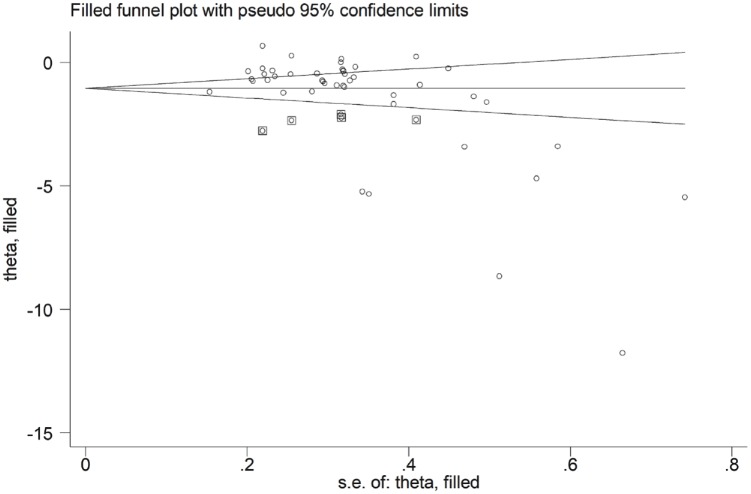
The Begg’s (p < 0.001) and Egger’s tests (p < 0.001) showed a high publication bias. When the trim-and-fill method was used to correct the asymmetry in the funnel plot, five potential missing studies were required in the left side to ensure symmetry (Figure 4). The adjusted SMD was further increased (−1.56 μmol/l, 95% CI: −1.96 to −1.16 μmol/l; p < 0.0001).
Figure 4.
Funnel plot of studies investigating MDA concentrations (at baseline and after statin treatment) after trimming and filling. Dummy studies and genuine studies are represented by enclosed circles and free circles, respectively.
CI, confidence interval; MDA, malondialdehyde; se, standard error.
To identify the sources of heterogeneity and publication bias, we investigated the effects of different study characteristics, including disease phenotype, sample size, publication year, continent where the study was conducted (Europe, Africa, Asia, and America), age, sex, body mass index (BMI), biological sample (plasma or serum) and assay type used [spectrophotometric, enzyme-linked immunosorbent (ELISA), high-performance liquid chromatography (HPLC), or capillary electrophoresis], total cholesterol concentrations, statin type (atorvastatin, simvastatin, pravastatin, fluvastatin or rosuvastatin), statin class (hydrophilic or hydrophobic) and therapy duration through univariate meta-regression. In our analyses, disease phenotype (t = 0.43, p = 0.669), publication year (t = −1.06, p = 0.297), age (t = 0.40, p = 0.688), sex (t = 0.15, p = 0.884), BMI (t = 0.47, p = 0.646), biological sample (t = −1.15, p = 0.883), assay type used (t = −1.28, p = 0.207), statin type (t = 0.34, p = 0.732), statin class (t = 0.16, p = 0.887) and therapy duration (t = 0.85, p = 0.401) were not associated with the SMD. Conversely, the continent where the study was conducted [t = −2.50, p = 0.016, Figure 5(a)], total cholesterol concentrations [t = 2.91, p = 0.006, Figure 5(b)] and sample size [t = −3.85, p < 0.0001, Figure 5(c)] were associated with the SMD.
Figure 5.
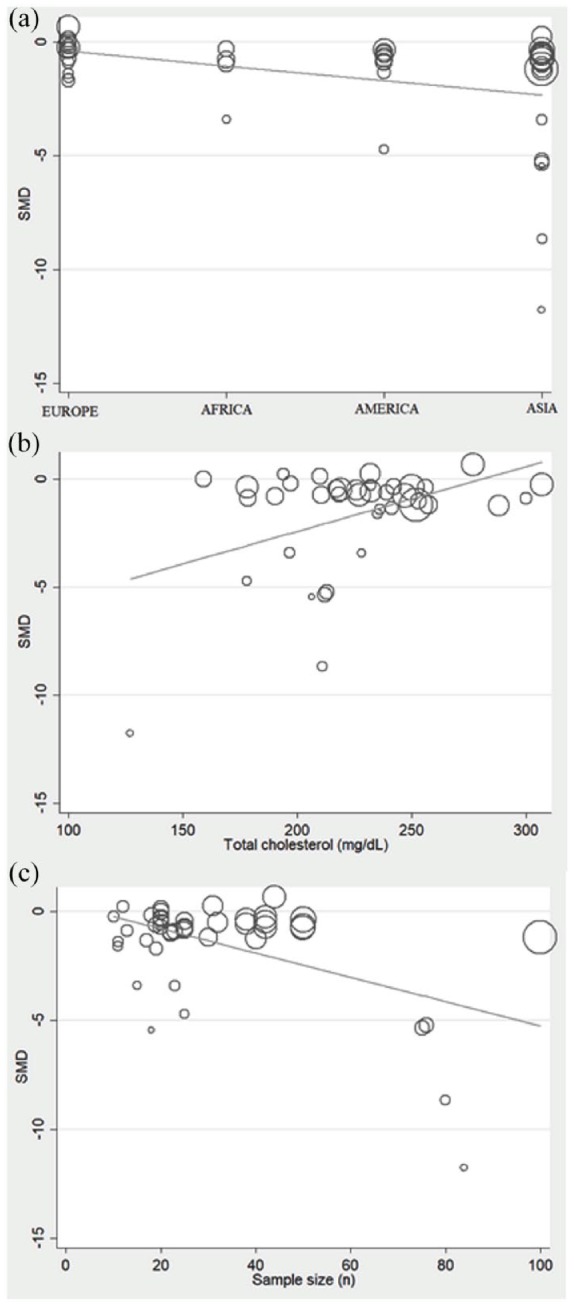
Meta-regression analysis showing correlations between SMD and continent where the study was conducted (a), total cholesterol concentrations (b), and study sample size (c).
SMD, standardized mean difference.
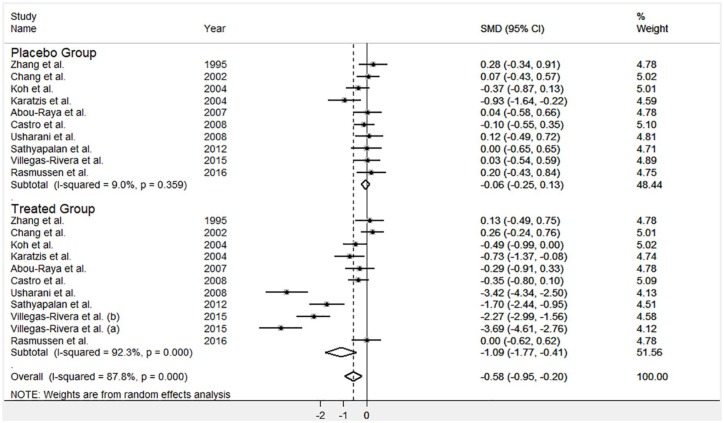
Overall, 10 out of the 35 studies included in the meta-analysis also compared the effect of statin treatment versus placebo. As reported in Figure 6, the statin treated group showed a reduction in MDA concentrations (−1.03 μmol/l; 95% CI = −1.52 to −0.29 μmol/l) that was significant in meta-regression analysis (p = 0.036). However, once again, extreme heterogeneity was observed (I2 = 92.3%, p < 0.0001).
Figure 6.
Forest plot for circulating MDA concentrations in studies comparing statins with placebo.
CI, confidence interval; MDA, malondialdehyde; SMD, standardized mean difference.
Discussion
We found a statistically significant reduction of MDA concentrations in patients treated with statins both when considered as a single class and when categorized based on their physicochemical properties, that is, hydrophobic statins (simvastatin and atorvastatin) that might be dispersed at low concentrations throughout human tissues, and hydrophilic statins (pravastatin, rosuvastatin and fluvastatin) that primarily target the liver and are found in the circulation. The observed SMD values are also likely to be biologically significant in terms of CVD risk reduction as previous studies have reported mean differences in MDA concentrations of 1.59 μmol/l, between healthy controls and patients with ischaemic heart disease, and of 0.37 μmol/l, between healthy controls and patients with ischaemic stroke, respectively.60,61 However, these results must be interpreted with some caution due to the relatively high heterogeneity observed between the studies. The latter also accounts for the fact that 10 of selected studies did not found significant differences in MDA after statin treatment while 16 studies reported a decrease greater than 30%. Clinical differences between patients studied, type and dose of statin and therapy duration may account for the high heterogeneity observed. The absence of a common and well-standardized method for pre-analytical sample treatment and for MDA detection may also increase heterogeneity. Furthermore, different biological responses to specific statins could not be excluded.
Several lines of evidence suggest that statins exhibit a lipid-independent antioxidant activity. For example, simvastatin significantly attenuates the increase in plasma concentrations of F2-isoprostanes and MDA associated with experimental hypercholesterolaemia, in absence of any lipid lowering effect,62 and fluvastatin reduces superoxide anion (O2−) concentrations both in vitro and in vivo.63,64 Atorvastatin upregulates catalase expression both in rat vascular smooth muscle cells in vitro and in normocholesterolaemic hypertensive rats in vivo.65 Moreover, both simvastatin and rosuvastatin reduce the concentrations of oxidized LDL in vivo.66 Similarly, fluvastatin and lovastatin reduce the susceptibility to LDL oxidation in patients with hypercholesterolaemia. This phenomenon seems partly due to a direct binding of the drugs to the phospholipid fraction of LDL.67 By contrast, it has been reported that ROS may be generated during statin metabolism, leading to increased OS, tissue injury and toxicity.68 A recent study revealed that simvastatin, lovastatin and atorvastatin caused a significant increase in ROS formation when freshly isolated rat hepatocytes were treated with statins.69 Another study found that atorvastatin led to an increase in the liver mitochondrial concentrations of ROS in rats.70 Moreover, it has been reported that 8-week treatment with atorvastatin induced an increase in ROS in hepatic and renal tissues, along with significant renal tubular injury and liver damage.71 These different, and often opposed, biological effects may potentially account for the heterogeneity of the observed results.
To overcome heterogeneity, we tried to identify homogenous subgroups of participants. MDA can be determined either in serum or in plasma. In our systematic review, we found that the sample matrix was serum in 13 studies and plasma in the remaining 22. The use of different sample matrices may result, in fact, in different MDA concentrations and contribute to heterogeneity. However, heterogeneity remained high even after accounting for the sample matrix used (serum or plasma). Similarly, accounting for the assay type used for MDA detection (ELISA: 5 studies; colorimetric: 20 studies; HPLC or capillary electrophoresis: 8 studies; not reported: 2 studies), statin used, statin type, disease phenotype or therapy duration did not reduce heterogeneity.
In the meta-regression analysis, statin type, therapy duration and disease phenotype did not influence the effect size. Conversely, the continental where the study was conducted, baseline total cholesterol concentrations, and sample size were significantly associated with the SMD, suggesting an important effect on both heterogeneity and publication bias. In regard to the latter, Begg’s adjusted rank correlation test and Egger’s regression asymmetry test showed the presence of a significant publication bias in the selected studies. However, the addition of five potential missing studies by trim-and-fill method, to correct the asymmetry in the funnel plot, further increased the SMD of MDA concentrations after statin treatment (from −1.47 to −1.56 μmol/l), suggesting that the pooled difference, without correction, is underestimated.
Finally, when analysing a subgroup of 10 studies that compared the effect of statin treatment with that of placebo, we found a similar significant decrease in MDA concentrations (1.03 μmol/l) with statin therapy. However, once again, a high heterogeneity in the statin group was observed. Interestingly, the homogeneity observed in the placebo group suggest that technical aspects, such as sample treatment and MDA assay, might have a relatively small impact on the observed heterogeneity.
This meta-analysis had several limitations. The eligible studies investigated relatively small groups (between 10 and 100 patients) and had different population characteristics, study design, statins used and their dose. Furthermore, the effect of smoking, an important determinant of OS, could not be considered because of the lack of available data. In order to address these issues, we used a more conservative random-effects model and performed sensitivity analysis and trim-and-fill method to correct publication bias.
In summary, the available evidence shows that statin treatment exerts significant lowering effects, from a statistical and, possibly, also a biological point of view, on the systemic concentrations of MDA, a marker of OS. This suggests an additional mechanism for the beneficial effects of this drug class on CVD risk. However, the marked heterogeneity observed in our analyses warrants further observational or interventional studies to confirm, or refute, these findings and to identify possible differences in the magnitude of MDA lowering between specific statins.
Supplemental Material
Supplemental material, Supplementary_file_1 for Effect of statin treatment on circulating malondialdehyde concentrations: a systematic review and meta-analysis by Angelo Zinellu, Panagiotis Paliogiannis, Maria Franca Usai, Ciriaco Carru and Arduino A. Mangoni in Therapeutic Advances in Chronic Disease
Acknowledgments
A Visiting Professorship awarded to Professor Mangoni by the Department of Biomedical Sciences, University of Sassari (Italy), facilitated this work.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Arduino A. Mangoni  https://orcid.org/0000-0001-8699-1412
https://orcid.org/0000-0001-8699-1412
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Angelo Zinellu, Department of Biomedical Sciences, University of Sassari, Sassari, Italy.
Panagiotis Paliogiannis, Department of Biomedical Sciences, University of Sassari, Sassari, Italy.
Maria Franca Usai, Department of Chemistry and Pharmacy, University of Sassari, Sassari, Italy.
Ciriaco Carru, Department of Biomedical Sciences, University of Sassari, Sassari, Italy.
Arduino A. Mangoni, Discipline of Clinical Pharmacology, College of Medicine and Public Health, Flinders University and Flinders Medical Centre, Bedford Park, SA 5042, Australia.
References
- 1. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016; 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 2. Rafieian-Kopaei M, Setorki M, Doudi M, et al. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med 2014; 5: 927–946. [PMC free article] [PubMed] [Google Scholar]
- 3. Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol 2003; 23: 729–736. [DOI] [PubMed] [Google Scholar]
- 4. Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol 2008; 52: 1769–1781. [DOI] [PubMed] [Google Scholar]
- 5. Reiner Ž. Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol 2013; 10: 453–464. [DOI] [PubMed] [Google Scholar]
- 6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016; 316: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 7. Oesterle A, Laufs U, Liao K. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017; 120: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou Q, Liao JK. Pleiotropic effects of statins. Basic research and clinical perspectives. Circ J 2010; 74: 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. John S, Schneider MP, Delles C, et al. Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am Heart J 2005; 149: 473. [DOI] [PubMed] [Google Scholar]
- 10. Ho E, Karimi Galougahi K, Liu CC, et al. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 2013; 1: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maiolino G, Rossitto G, Caielli P, et al. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediat Inflamm 2013; 2013: 714653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediat Inflamm 2013; 2013: 152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steinberg D, Parthasarathy S, Carew TE, et al. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989; 320: 915–924. [DOI] [PubMed] [Google Scholar]
- 14. Matsuura E, Atzeni F, Sarzi-Puttini P, et al. Is atherosclerosis an autoimmune disease? BMC Med 2014; 12: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdin AA, Hassanien MA, Ibrahim EA, et al. Modulating effect of atorvastatin on paraoxonase 1 activity in type 2 diabetic Egyptian patients with or without nephropathy. J Diabetes Complications 2010; 24: 325–333. [DOI] [PubMed] [Google Scholar]
- 16. Hsu M, Muchova L, Morioka I, et al. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Commun 2006; 343: 738–744. [DOI] [PubMed] [Google Scholar]
- 17. Wassmann S, Laufs U, Müller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 2002; 22: 300–305. [DOI] [PubMed] [Google Scholar]
- 18. Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2013, accessed 1 July 2019).
- 19. Bowden J, Tierney JF, Copas AJ, et al. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol 2011; 11: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 21. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin 1999; 47: 15–17. [Google Scholar]
- 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 23. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2011; 54: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 24. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang A, Vertommen J, Van Gaal L, et al. Effects of pravastatin on lipid levels, in vitro oxidizability of non-HDL lipoproteins and microalbuminuria in IDDM patients. Diabetes Res Clin Pract 1995; 29: 189–194. [DOI] [PubMed] [Google Scholar]
- 27. Nishikawa O, Mune M, Miyano M, et al. Effect of simvastatin on the lipid profile of hemodialysis patients. Kidney Int Suppl 1999; 71: S219–S221. [DOI] [PubMed] [Google Scholar]
- 28. Chang JW, Yang WS, Min WK, et al. Effects of simvastatin on high-sensitivity C-reactive protein and serum albumin in hemodialysis patients. Am J Kidney Dis 2002; 39: 1213–1217. [DOI] [PubMed] [Google Scholar]
- 29. Harangi M, Seres I, Varga Z, et al. Atorvastatin effect on high-density lipoprotein-associated paraoxonase activity and oxidative DNA damage. Eur J Clin Pharmacol 2004; 60: 685–691. [DOI] [PubMed] [Google Scholar]
- 30. Koh KK, Son JW, Ahn JY, et al. Simvastatin combined with ramipril treatment in hypercholesterolemic patients. Hypertension 2004; 44: 180–185. [DOI] [PubMed] [Google Scholar]
- 31. Koh KK, Son JW, Ahn JY, et al. Vascular effects of simvastatin combined with ramipril in hypercholesterolemic patients with coronary artery disease, compared with simvastatin alone: a randomized, double-blind, placebo-controlled, crossover study. Atherosclerosis 2004; 177: 147–153. [DOI] [PubMed] [Google Scholar]
- 32. Kural BV, Orem C, Uydu HA, et al. The effects of lipid-lowering therapy on paraoxonase activities and their relationships with the oxidant-antioxidant system in patients with dyslipidemia. Coron Artery Dis 2004; 15: 277–283. [DOI] [PubMed] [Google Scholar]
- 33. Manuel-Y-Keenoy B, Vinckx M, Vertommen J, et al. Impact of Vitamin E supplementation on lipoprotein peroxidation and composition in Type 1 diabetic patients treated with Atorvastatin. Atherosclerosis 2004; 175: 369–376. [DOI] [PubMed] [Google Scholar]
- 34. Skrha J, Stulc T, Hilgertová J, et al. Effect of simvastatin and fenofibrate on endothelium in Type 2 diabetes. Eur J Pharmacol 2004; 493: 183–189. [DOI] [PubMed] [Google Scholar]
- 35. Karatzis E, Lekakis J, Papamichael C, et al. Rapid effect of pravastatin on endothelial function and lipid peroxidation in unstable angina. Int J Cardiol 2005; 101: 65–70. [DOI] [PubMed] [Google Scholar]
- 36. Save V, Patil N, Moulik N, et al. Effect of atorvastatin on type 2 diabetic dyslipidemia. J Cardiovasc Pharmacol Ther 2006; 11: 262–270. [DOI] [PubMed] [Google Scholar]
- 37. Abou-Raya A, Abou-Raya S, Helmii M. Statins as immunomodulators in systemic sclerosis. Ann N Y Acad Sci 2007; 1110: 670–680. [DOI] [PubMed] [Google Scholar]
- 38. Koh KK, Quon MJ, Han SH, et al. Combined therapy with ramipril and simvastatin has beneficial additive effects on tissue factor activity and prothrombin fragment 1+2 in patients with type 2 diabetes. Atherosclerosis 2007; 194: 230–237. [DOI] [PubMed] [Google Scholar]
- 39. Molcányiová A, Stancáková A, Javorský M, et al. Beneficial effect of simvastatin treatment on LDL oxidation and antioxidant protection is more pronounced in combined hyperlipidemia than in hypercholesterolaemia. Pharmacol Res 2006; 54: 203–207. [DOI] [PubMed] [Google Scholar]
- 40. Castro PF, Miranda R, Verdejo HE, et al. Pleiotropic effects of atorvastatin in heart failure: role in oxidative stress, inflammation, endothelial function, and exercise capacity. J Heart Lung Transplant 2008; 27: 435–441. [DOI] [PubMed] [Google Scholar]
- 41. Usharani P, Mateen AA, Naidu MU, et al. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D 2008; 9: 243–250. [DOI] [PubMed] [Google Scholar]
- 42. Nagila A, Permpongpaiboon T, Tantrarongroj S, et al. Effect of atorvastatin on paraoxonase1 (PON1) and oxidative status. Pharmacol Rep 2009; 61: 892–898. [DOI] [PubMed] [Google Scholar]
- 43. Li J, Sun YM, Wang LF, et al. Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clin Cardiol 2010; 33: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Su Y, Xu Y, Sun YM, et al. Comparison of the effects of simvastatin versus atorvastatin on oxidative stress in patients with type 2 diabetes mellitus. J Cardiovasc Pharmacol 2010; 55: 21–25. [DOI] [PubMed] [Google Scholar]
- 45. El-Barbary AM, Hussein MS, Rageh EM, et al. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol 2011; 38: 229–235. [DOI] [PubMed] [Google Scholar]
- 46. Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of atorvastatin combined with amlodipine in patients with mild-to-moderate hypertension. Int J Cardiol 2011; 146: 319–325. [DOI] [PubMed] [Google Scholar]
- 47. Samy W, Hassanian MA. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab J Gastroenterol 2011; 12: 80–85. [DOI] [PubMed] [Google Scholar]
- 48. Sathyapalan T, Shepherd J, Coady AM, et al. Atorvastatin reduces malondialdehyde concentrations in patients with polycystic ovary syndrome. J Clin Endocrinol Metab 2012; 97: 3951–3955. [DOI] [PubMed] [Google Scholar]
- 49. Lazich I, Sarafidis P, de Guzman E, et al. Effects of combining simvastatin with rosiglitazone on inflammation, oxidant stress and ambulatory blood pressure in patients with the metabolic syndrome: the SIROCO study. Diabetes Obes Metab 2012; 14: 181–186. [DOI] [PubMed] [Google Scholar]
- 50. Murrow JR, Sher S, Ali S, et al. The differential effect of statins on oxidative stress and endothelial function: atorvastatin versus pravastatin. J Clin Lipidol 2012; 6: 42–49. [DOI] [PubMed] [Google Scholar]
- 51. Zinellu A, Sotgia S, Loriga G, et al. Oxidative stress improvement is associated with increased levels of taurine in CKD patients undergoing lipid-lowering therapy. Amino Acids 2012; 43: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 52. Andrade VL, Sertório JT, Eleuterio NM, et al. Simvastatin treatment increases nitrite levels in obese women: modulation by T(-786)C polymorphism of eNOS. Nitric Oxide 2013; 33: 83–87. [DOI] [PubMed] [Google Scholar]
- 53. Scheffer PG, Schindhelm RK, van Verschuer VM, et al. No effect of atorvastatin and simvastatin on oxidative stress in patients at high risk for cardiovascular disease. Neth J Med 2013; 71: 359–365. [PubMed] [Google Scholar]
- 54. Villela MP, Andrade VL, Eccard B, et al. Homocysteine and nitrite levels are modulated by MTHFR 677C>T polymorphism in obese women treated with simvastatin. Clin Exp Pharmacol Physiol 2014; 41: 744–747. [DOI] [PubMed] [Google Scholar]
- 55. Çiftçi AG, Ertorun İ, Akalin A, et al. The effects of atorvastatin on antioxidant/antiinflammatory properties of HDLs in hypercholesterolemics. Turk J Med Sci 2015; 45: 345–351. [DOI] [PubMed] [Google Scholar]
- 56. Mirjanic-Azaric B, Rizzo M, Jürgens G, et al. Atorvastatin treatment increases plasma bilirubin but not HMOX1 expression in stable angina patients. Scand J Clin Lab Invest 2015; 75: 382–389. [DOI] [PubMed] [Google Scholar]
- 57. Villegas-Rivera G, Román-Pintos LM, Cardona-Muñoz EG, et al. Effects of Ezetimibe/Simvastatin and Rosuvastatin on Oxidative Stress in Diabetic Neuropathy: a randomized, double-blind, placebo-controlled clinical trial. Oxid Med Cell Longev 2015; 2015: 756294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yildiz A, Gul CB, Ocak N, et al. Fluvastatin decreases oxidative stress in kidney transplant patients. Transplant Proc 2015; 47: 2870–2874. [DOI] [PubMed] [Google Scholar]
- 59. Rasmussen ST, Andersen JT, Nielsen TK, et al. Simvastatin and oxidative stress in humans: a randomized, double-blinded, placebo-controlled clinical trial. Redox Biol 2016; 9: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khan MA, Baseer A. Increased malondialdehyde levels in coronary heart disease. J Pak Med Assoc 2000; 50: 261–264. [PubMed] [Google Scholar]
- 61. Polidori MC, Cherubini A, Stahl W, et al. Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: relationship to early outcome. Free Radic Res 2002; 36: 265–268. [DOI] [PubMed] [Google Scholar]
- 62. Wilson SH, Simari RD, Best PJ, et al. Simvastatin preserves coronary endothelial function in hypercholesterolaemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol 2001; 21: 122–128. [DOI] [PubMed] [Google Scholar]
- 63. Yamamoto A, Hoshi K, Ichihara K. Fluvastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase, scavenges free radicals and inhibits lipid peroxidation in rat liver microsomes. Eur J Pharmacol 1998; 361: 143–149. [DOI] [PubMed] [Google Scholar]
- 64. Rikitake Y, Kawashima S, Takeshita S, et al. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 2001; 154: 87–96. [DOI] [PubMed] [Google Scholar]
- 65. Wassmann S, Laufs U, Müller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 2002; 22: 300–305. [DOI] [PubMed] [Google Scholar]
- 66. Moutzouri E, Liberopoulos EN, Tellis CC, et al. Comparison of the effect of simvastatin versus simvastatin/ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolaemia. Atherosclerosis 2013; 231: 8–14. [DOI] [PubMed] [Google Scholar]
- 67. Aviram M, Hussein O, Rosenblat M, et al. Interactions of platelets, macrophages, and lipoproteins in hypercholesterolaemia: antiatherogenic effects of HMG-CoA reductase inhibitor therapy. J Cardiovasc Pharmacol 1998; 31: 39–45. [DOI] [PubMed] [Google Scholar]
- 68. Liu A, Wu Q, Guo J, et al. Statins: adverse reactions, oxidative stress and metabolic interactions. Pharmacol Ther 2019; 195: 54–84. [DOI] [PubMed] [Google Scholar]
- 69. Abdoli N, Azarmi Y, Eghbal MA. Mitigation of statins-induced cytotoxicity and mitochondrial dysfunction by L-carnitine in freshly-isolated rat hepatocytes. Res Pharm Sci 2015; 10: 143–151. [PMC free article] [PubMed] [Google Scholar]
- 70. Wat E, Ng CF, Wong EC, et al. The hepatoprotective effect of the combination use of Fructus Schisandrae with statin–A preclinical evaluation. J Ethnopharmacol 2016; 178: 104–114. [DOI] [PubMed] [Google Scholar]
- 71. Pal S, Sarkar A, Pal PB, et al. Protective effect of arjunolic acid against atorvastatin induced hepatic and renal pathophysiology via MAPK, mitochondria and ER dependent pathways. Biochimie 2015; 112: 20–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_file_1 for Effect of statin treatment on circulating malondialdehyde concentrations: a systematic review and meta-analysis by Angelo Zinellu, Panagiotis Paliogiannis, Maria Franca Usai, Ciriaco Carru and Arduino A. Mangoni in Therapeutic Advances in Chronic Disease