Abstract
Although the burden of cancer is rapidly growing in Vietnam, there was no up-to-date review that describes cancer burden and control in Vietnam throughout the literature. By identifying various risk factors, means of prevention, and methods for early detection, this review seeks to systematically summarize the evidence for the future planning and management of cancer occurrence in Vietnam. Additionally, this report aims to identify improvements which are necessary for the treatment and palliative care of patients with cancer in Vietnam. We employed a hybrid approach including both a scoping review and narrative synthesis for this study. Information was identified, extracted, and charted from various sources, which include international and domestically published studies, in addition to gray literature. Our results illustrate that the burden of cancer in Vietnam has tripled in the past 30 years, and this situation could be partly explained by the growing prevalence of both old and new risk factors. Besides hepatitis B virus, various other important risk factors such as human papilloma virus, tobacco usage, physical inactivity, and improper diets are still not under control in Vietnam. There is presently a lack of national cancer screening programs, and the capacity of cancer care services could not maintain pace with the demands of a rapidly increasing Vietnamese population. Overall, policy frameworks for cancer control in Vietnam are in place, but there is still a lack of proper financing and governing models necessary to support a sustainable program. In conclusion, Cancer and its associated consequences are both persistent and emerging problems in Vietnam, and the results of cancer control programs are limited. A comprehensive and evidence-based approach toward the prevention and treatment of cancer should be the future direction for Vietnam.
Keywords: cancer, cancer control, cancer burden, prevention, early detection, screening, treatment, health systems, health policy, review, NCDs, Vietnam
Introduction
The International Agency for Research on Cancer (IARC) estimates that in 2018, there would be 18.1 million new cases and 9.6 million deaths linked to cancer worldwide.1 Unfortunately, the burden of cancer is disproportionate among nations, in which developing countries account for 57% of cases and 65% of deaths associated with cancer.2 However, these countries, including Vietnam, only received about 5% of the global cancer financial resources.3,4
In Vietnam, the estimated number of new cases in 2018 was 164 671 (0.17% of the population) and the estimated number of deaths in 2018 was 114 871 (0.12% of the population).1,5 Both of these statistics have tripled in the past 30 years.6 Nevertheless, the quality of national cancer registry data that were used as an input to calculate the statistics above has numerous deficiencies. For example, there is a lack of national data that describe both cancer survivorship and mortality.7,8
Although Vietnam proposed the National Cancer Control Programme (NCCP) in 2002,9 implemented the NCCP in 2008,7 and reasserted the commitment to this program in the national strategy for the prevention and control of noncommunicable diseases (NCDs),10 reports regarding cancer burden and control are still not well-documented in the literature. Therefore, to elucidate both the burden and control effort of cancer in Vietnam, we conducted a narrative review to summarize update-to-date evidence for the future planning and management of various risk factors, implementing effective prevention and early detection programs as well as the improvement in treatment and palliative care for patients with cancer in Vietnam.
Objectives
The primary objective of this article is to summarize and discuss both the burden of cancer and control efforts of this disease in Vietnam. We aimed to review available articles and gray literature on cancer burden and control. In addition, we identified issues for discussion which are necessary to strengthen health systems while disseminating new areas of interest for future research.
Methods
In this article, we used a hybrid of scoping review and narrative synthesis approach.11 Since there is a lack of review on cancer in Vietnam and the scope of the research question was quite broad, a scoping review in tandem with a narrative synthesis was deemed to be the most appropriate methodology for this study.11 The scoping review design would allow us to identify broad and diverse findings in numerous disciplines. A narrative synthesis would provide a context which would describe how it is necessary for different stakeholders and components to work together for a sustainable health system in Vietnam. The presented information for this study was identified, extracted, and charted from various sources, including international and domestically published studies, as well as gray literature. The review process and final review paper were prepared in accordance with the guidelines on scoping reviews as published by Peters et al12 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement13 (Supplemental PRISMA checklist).
We conducted a systematic search for peer-reviewed articles in the English language which were indexed in the MEDLINE database in October 2018. The present search strategy was developed to include studies with the following terms in the title/abstract: “Vietnam” OR “Viet Nam” AND “Cancer” together with “Burden” OR “Control” OR “Prevention” OR “Risk factors” OR “Early detection” OR “Screening” OR “Diagnosis” OR “Treatment” OR “Palliative care.” Eligible articles for this study were publications that reviewed or reported empirical data in relation to the previously mentioned topics in Vietnam. Papers that reported findings in Vietnamese from the context of other countries (eg, Vietnamese American) were excluded.
To identify related studies that were published domestically, we also searched the electronic databases at Hanoi Medical University Library, Hanoi University of Public Health Library, and Vietnam Oncology Journal collections at the National Cancer Hospital in October 2018. The same criteria that were noted above and the equivalent keywords in the Vietnamese language (gánh nặng ung thư, kiểm soát ung thư, phòng chống ung thư, dự phòng ung thư, chẩn đoán sớm ung thư, sàng lọc ung thư, tầm soát ung thư, chăm sóc giảm nhẹ ung thư, nguy cơ ung thư) were used in our search strategy.
Relevant gray literature was identified through an online search by employing the search engine Google, in addition to the websites of Vietnamese governmental agencies, international organizations, and nongovernmental organizations in Vietnam.
Key findings from documents in the Vietnamese language were summarized and translated into English. All relevant details from these documents, including titles, the year of publication, significant findings, and so forth, were extracted and charted using a standardized data extraction spreadsheet, which was provided by The University of Texas Health Science Center at Houston–School of Public Health Library,14 for further analysis. Using the data extraction sheets, we summarized information from the included documents and identified discrepancies and gaps in Vietnam’s cancer control effort. This process was completed by comparing recommendations from both the World Health Organization (WHO) and best practices initiatives in cancer prevention worldwide with the results from our review. Based on such information, we sought to recommend appropriate policy actions that could leverage Vietnam’s NCCP in the near future.
Results
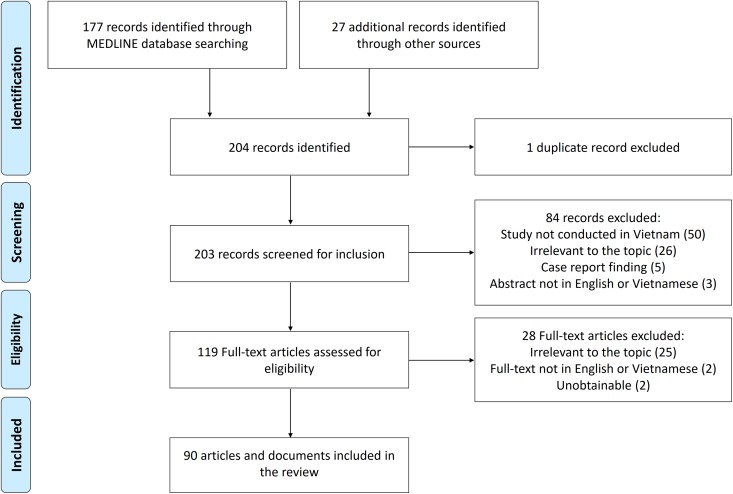
Our search strategy identified 177 English articles and 11 Vietnamese articles/research reports that met the inclusion criteria for this study. We also included 13 gray literature documents with information from international organizations and nongovernmental organizations in our review. Three more articles or documents were identified through bibliographies screening (Figure 1 and Supplemental Table 1). Our literature search did not identify any reviews that detailed this topic.
Figure 1.
PRISMA flowchart showing the selection process of articles.
Burden of Cancer
To estimate the burden of cancer, Vietnam established its first cancer registry in Hanoi in 1984.7 Since then, 6 cancer registries were established in Hanoi, Hai Phong, Thai Nguyen, Ho Chi Minh, and Can Tho until the year 2010,15 and 3 more were recently developed to improve the geographical representation of these registries.7 The Vietnam National Cancer Institute reported that most of the statistics acquired as inputs for their database were hospital based, and the quality of data varied among various regions in Vietnam.7 The Global Initiative for Cancer Registry Development (GICR) Partners Task Force reported that the Hanoi and Ho Chi Minh City registry had the best overall practices and data quality in the country which covers 45 and 30 hospitals, respectively, in their catchment areas.16
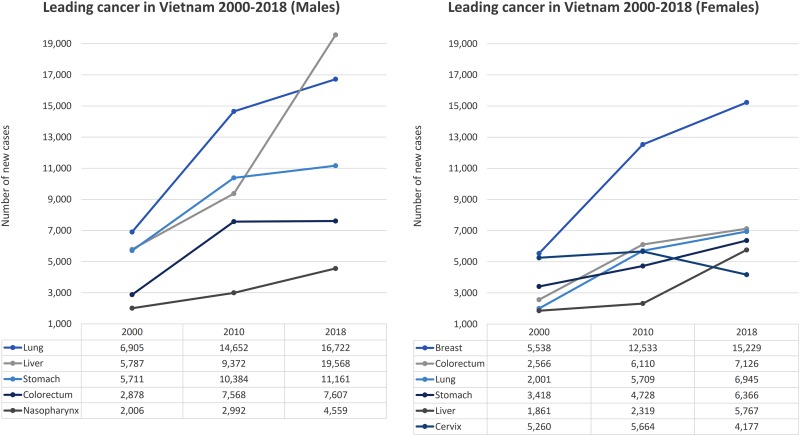
Based on both the Vietnamese cancer registry data and mathematical modeling, the IARC estimated a total of 164 671 new cases and 114 871 cancer deaths occurred in Vietnam during 2018.1,5 Both of these statistics had tripled since 1990, when there were 52 700 new cases and 37 700 deaths associated with cancer.6 The IARC also stated in the 2018 report that there were 300 033 people currently living with cancer in Vietnam (current 5-year prevalent cases).5 A detailed overview regarding the incidence, prevalence, and mortality of all cancer types can be retrieved from the Global Cancer Observatory–Vietnam Population fact sheets 2018.5 Regardless of sex, liver cancer remained the most common cancer and the number one killer of patients with cancer (25 335 new cases; 25 404 deaths), followed by lung (23 667 new cases; 20 710 deaths), stomach (17 527 new cases; 15 065 deaths), and breast (15 229 cases; 6103 deaths).5 The current leading cancers were the liver, lung, stomach, colorectum, and nasopharynx in males, while cancers of the breast, colorectum, lung, stomach, and liver were more typical in females5 (Table 1). The ranking of common cancers in males seems to be stable with the domination of the liver, lung, and stomach cancers5,17,18 (Figure 2). In females, breast, colorectum, and lung cancers were the most common types observed in recent years (2010-2018).5,17 Although the overall cancer incidence was on the rise, the national registry data suggest that the incidence of cervical and oral cancer is in decline.5,17,19,20
Table 1.
Leading Cancer Incidence in Vietnam, 2000 to 2018.
| Rank | Males | Females | ||||
|---|---|---|---|---|---|---|
| 2000a | 2010a | 2018b | 2000a | 2010a | 2018b | |
| 1 | Lung | Lung | Liver | Breast | Breast | Breast |
| 2 | Liver | Stomach | Lung | Cervix | Colorectum | Colorectum |
| 3 | Stomach | Liver | Stomach | Stomach | Lung | Lung |
| 4 | Colorectum | Colorectum | Colorectum | Colorectum | Cervix | Stomach |
| 5 | Nasopharynx | Esophagus | Nasopharynx | Lung | Stomach | Liver |
Figure 2.
Trend of leading cancer incidence in Vietnam, 2000 to 2018.
The Vietnam Burden of Disease and Injury Study in 2008 estimated that cancer was responsible for 22% of the total years of life lost (YLL)—nearly 1.5 million years—and ranked only lower than cardiovascular diseases (27% of total YLL).21,22 The same study also calculated that cancers ranked third among the leading causes of disease burden in Vietnam, which accounted for 14% of total disability-adjusted life years (DALYs)—nearly 1.7 million years.21,22 One article also suggested that colorectal cancer in Vietnam seems to appear at an earlier age in relation to the Asia Pacific consensus for recommended screening age—50 years old.23 The proportion of early-onset (younger than 50 years) colorectal cancer cases in this study was 28%.23
Among all patients with cancer, only 20% to 30% of cases appeared at early stages,7,9 and 1-year incidence of mortality was up to 25%.24 Other studies also revealed that the 5-year survival rate of patients with breast cancer in Vietnam was lower when compared to other countries with similar distributions at the stage of diagnosis.25 In addition, Vietnamese children with lymphoblastic leukemia also experienced poor relapse-free survival rates compared to Caucasian children.26
The cost of cancer treatment in Vietnam is dependent on the stage of diagnosis, access to public or private hospitals, and range of treatment options (US$11.7-US$11 400, regarding breast or cervical cancer).7,27,28 The ACTION (ASEAN Costs in Oncology) prospective cohort study reported that, in Vietnam, the 1-year incidence of financial catastrophe was 68%, which was the highest in the ASEAN region.24 This study identified that 37.4% of the households with patients with cancer were pushed into significant poverty due to high treatment costs.29
A cross-sectional study on the quality of life among patients with breast cancer using the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30 illustrated that the mean global health status score of these patients was estimated to be 58.6 of 100 points.30 The authors also identified several factors that were associated with quality of life, such as age, level of education, body mass index, treatment, and the stage of cancer.30 Patients with cancer also experience other comorbidities that can be associated with their disease, such as depression. The overall prevalence of serious depression among patients with cancer in Vietnam was 28%, which is 10 to 15 times higher than the general population relative to studies conducted in the same region.31
Important Risk Factors
Hereditary and genetic factors
Our review identified 4 studies that assessed several hereditary and genetic risk factors in the Vietnamese population. These studies include GSTA1 genotype and gastric cancer;32 hsa-miR-122 and hepatitis B virus (HBV)–related hepatocellular carcinoma (HCC),33 RB1 gene mutation, and retinoblastoma.34 The fourth study reported an association between family history and an increased risk of early-onset colorectal cancer among patients in Ho Chi Minh City.23 The Vietnam National Cancer Institute also stated that hereditary and genetic risk factors for cancer onset were not well-documented in Vietnam due to the lack of research capability in cancer biology.7
Infection
Infection with HBV and infection with human papilloma virus (HPV) remain significant risk factors for cancer incidence in Vietnam. Although universal HBV vaccination of infants is presently in effect in Vietnam, HBV-related liver disease burden was expected to increase.35 The chronic HBV prevalence in Vietnam grew from 6.4 million cases in 1990 to 8.4 million in 2005 and was expected to decrease to 8.0 million by 2025.35 However, HBV-related HCC incidence was predicted to increase from 9400 in 1990 to 25 000 in 2025.35 Another cross-sectional study illustrated that 68.2% of participants in the rural areas of Vietnam had evidence of HBV exposure.36 Risk factors for HBV identified by this study were being 60 years of age or older, hospital admissions, history of acupuncture, household contact with a person living with liver disease, reusing syringes, and sharing razors.36
In Vietnam, the HPV vaccine was not included in the national vaccination program at the time of this review, and individuals are obligated to pay out of pocket for the vaccine. The National Action Plan on Prevention and Control of Cervical Cancer set a goal that at least 25% of all girls and women would receive the HPV vaccine by 2025.37 Among the Vietnamese population, HPV DNA was detected in 97% cases of invasive cervical cancer, and the most common types were HPV 16 (50%), HPV 18 (35.4%), and HPV 52 (6.2%).38 Other cross-sectional studies and reviews reported that the prevalence of HPV infections among Vietnamese women ranged from 3.1% to 10.9% depending on the location of the study, and HPV type 16 and 18 were also the most common followed by type 58.39-45 In many provinces such as Hanoi and Can Tho, approximately 90% of the infection cases were caused by high-risk HPV strains (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, and 82).40,41 Human papilloma virus infection was associated with sexual habits, in addition to the presence of herpes simplex virus 2 antibodies, early age at one’s first sexual encounter, the number of lifetime sexual partners, history of childbirth, and usage of oral contraceptives.43,44 In a study by Vu and colleagues, the group found that only about one-third of women have ever heard about HPV, the HPV vaccine, and were aware that HPV is a risk factor for cervical cancer.39 The level of awareness of participants in this study also differed vastly across geographical areas.39
Regarding other infections in relation to cancer, the prevalence of Kaposi sarcoma–associated herpesvirus (KSHV) among women in Hanoi was around 11.3% and in Ho Chi Minh City was 15.5%.46 Unfortunately, this cross-sectional study did not test for HIV infection among the participants in the previously mentioned regions.46 In this study, the prevalence of KSHV was slightly increased in patients with a higher age in areas with a high prevalence of KSHV and it decreased in patients who have attained a higher degree of education.46 Moreover, Opisthorchis viverrini and Clonorchis sinensis liver flukes, which were linked to cholangiocarcinoma and HCC by the IARC,47,48 are also common in Vietnam with nearly a million cases.47 Clonorchis sinensis is widespread in northern Vietnam (prevalence ranged from 5% to 26%), while O viverrini is endemic in the central and southern Vietnam.47 Both of these parasites were found to be linked to the habit of eating raw or undercooked fish.47
Behavioral risk factors
In the Vietnamese male population, smoking was responsible for 28% of all-cause deaths in adults and 85% of lung cancer death.49,50 The prevalence of tobacco smoking in males was reduced from 56.1% (2002)7,10,51 to 47.4% (2010)7,10,51 and to 45.3% (2015).7,10,51 However, this reduction was not as high as expected.51 Exposure to secondhand smoking is notably high and ranges from 48% to 70% in homes and outdoor or public places.52
Other case–control studies in the Vietnamese population identified that alcohol use and physical inactivity were linked to an increased risk for breast cancer (odds ratio [OR]: 1.85 and 2.2, respectively).53,54 Diets high in carotenoids were associated with a decreased prostate cancer risk (OR for lycopene: 0.46, tomatoes: 0.39, and carrots: 0.35).55 Conversely, roasted meats, bread, and biscuit consumption were associated with increased stomach and colorectal cancer risk (OR: 1.63, 1.4, and 1.6, respectively).56 In 2015, the Vietnam national survey on the risk factors of NCDs (STEPS) in 2015 estimated that 77.3% of males and 11% of females were current alcohol drinkers (43.8% overall) and that there was an increasing trend in alcohol comsumption.57 The STEPS survey also reported that the prevalence of insufficient physical activities was 20.2% in men and 35.7% in women (28.1% overall).57 A recent review of NCDs and risk factors pointed out a distinct change in Vietnamese lifestyle, in which the general population changed their dietary pattern by increasing the intake of both foods with rich quantities of proteins and fats between the years 1981 and 2010.52 This review and the STEPS survey also indicate that there is a high consumption of salt (9.4 to 22 g/person/d), sugar-sweetened beverages (more than 900 million liters/year for the whole population), and low consumption of fruit and vegetables (57.2%-80% of the population with less than 5 serving/day) in typical diets.52,57 Other hazard factors such as betel quid chewing, which was associated with an increased risk of oral cancer, was shown to be in decline among the general Vietnamese population.58
Regarding other physiological and metabolic risk factors, we did not identify any study that assessed the association of these risk factors and cancer in Vietnam. However, the Vietnamese population is presently experiencing an increased prevalence of hypertension (15% in 2002 to 20% in 2015) and overweight/obesity (2.3% in 1993 to 15% in 2015) among adults.52,57 In addition, the prevalence of diabetes mellitus and elevated blood cholesterol among adults in 2015 was 4.1% and 32%, respectively.52,57
Environmental risk factors
We identified 4 case–control studies that assessed several environmental risk factors that are involved with cancer risk. The most recent article, which was published in 2017, collected data from 195 patients with breast cancer and 254 controls; the authors reported that elevated blood cadmium level was associated with increased breast cancer risk.59 In another case–control study with 152 male cases of HCC and 241 controls, the authors identified an association between the use of organophosphate-based pesticides and increased HCC risk.60 However, 2 smaller studies, which included only 21 and 87 cancer cases, did not find the association between organochlorines with choriocarcinoma and breast cancer.61,62
Screening and Early Detection Programs
One of the first recorded screening programs that we identified for this study was for cervical cancer. In 1996, the burden of cervical cancer in Vietnam was shown to be associated with troop movements during the Vietnam War.63 In general, women whose husbands were enrolled in military services presented an increased risk for cervical cancer, though this was dependent on the geographical areas (OR: 2.6-3.9).63 Based on that finding, the Viet/American cervical cancer prevention project established a Papanicolaou screening service and recorded a decrease in cervical cancer incidence from 29.2 in 100 000 in 1998 to 16 in 100 000 in 2003.64 Presently, there is still a lack of national screening and early detection programs, which are available to the entire population. Limited resources only allow for some pilot screening programs to be conducted on a small scale, which covers about 2% of the target population.8 The shortage of trained personnel, lack of appropriate diagnostic equipment, and the lack of appropriate funding mechanisms from the national health insurance are major factors which prevent the scale-up of cancer screening in Vietnam.65 Since there is no nationwide screening program, Vietnamese people are expected to actively seek screening services at health-care units independently without reimbursement from the national health insurance system.65 A cross-sectional study on female workers in Ho Chi Minh City in 2016 observed that 35.2% of the participants never had a Pap smear test, and only 28.3% reported having a Pap smear test within the past 3 years.66 The STEPS survey also indicated that only 24.9% of women between the ages of 18 and 69 and 31.5% of those aged 30 to 49 had ever had cervical cancer screening.57
From 2008 to 2015, pilot screening programs for cervical, breast, oral, and colorectal cancers65 were implemented with the support of various domestic and international partners.7,8,67,68 During the period between 2008 and 2010 alone, nearly 100 000 women aged 30 to 54 years received cervical cancer (with Pap smear test) and breast cancer (with breast examination) screenings, and 9634 individuals received oral cancer (with visual inspection) and colorectal cancer (with Fecal Occult Blood Test) screenings throughout Vietnam.7,8,67 Support from the International Atomic Energy Agency in 2015 to 2016 allowed for 24 000 more women in Hanoi and Can Tho access to cervical and breast cancer screening services by employing both visual inspections with acetic acid (VIA) techniques and breast examinations.7 The Vietnam National Cancer Institute reported that the most significant drawbacks of the previously mentioned screening programs were the cost and technical difficulties of subsequence follow-up; this is a limitation as health-care teams from central/provincial hospitals had to come to their local communities to organize such programs regularly.7 Despite these difficulties, cervical cancer screening programs in Vietnam and other developing countries showed that VIA and cryotherapy is a feasible approach to identify cancer cases in low-resource settings.69 Screenings for prostate cancer using prostate-specific antigen tests were also considered, but the benefits of such mass screening programs have not been proven.70
Diagnosis and Treatment Capacity
At the time of this review, the health-care services in Vietnam can be described as disease-specific and vertically organized.71 Most of the cancer treatments were provided at provincial or national hospitals, and the 2 largest national cancer centers are “K Hospital”—The National Cancer Hospital, located in Hanoi, and Ho Chi Minh City Cancer Hospital, located in Ho Chi Minh City. In 2016, there were 3 comprehensive cancer centers and 45 oncology departments located at general hospitals throughout Vietnam.7 The Vietnam Ministry of Health recommends that cancer surgery should be conducted at provincial/national hospitals, but benign tumors could be surgically removed at district-level hospitals.72 However, a report from 2010 illustrated that 10 of 63 provincial hospitals throughout Vietnam were unable to provide services for patients with cancer.15 The number of health staff with sufficient knowledge and skills related to the treatment of cancer was also inadequate.65 With the current amount of limited resources, the present health-care system could only meet 30% to 40% of the demand for cancer services in Vietnam.65
The most significant problem regarding cancer diagnosis in Vietnam and many other developing countries is access to quality pathology services. Currently, the Vietnamese health-care system has only recorded pathological results from 48% of patients, who were diagnosed with cancer and/or received cancer treatment,68 and 9 of 63 provincial hospitals in Vietnam lack a pathology department.15 Many modern techniques for immunohistochemistry and molecular analysis are only available at a small number of comprehensive cancer centers,7 and the accuracy of pathology testing at the centers that host these facilities is still limited.7 A recent report from a team of hematopathologists at the University of Minnesota Medical School demonstrated that the exact/complete diagnostic concordance in comparison to their tests was 50% or less for all 3 hospitals that the team visited while in Vietnam.73
Regarding the accessibility of radiation therapy, the Vietnam National Cancer Institute reported that in 2016, there were 36 linear accelerators (an increase from 13 linear accelerators in 2010), which could deliver 3-dimensional conformal radiation therapy, in Vietnam.7 In addition, it was noted that advanced techniques, such as intensity-modulated radiation therapy, were limited in Vietnam.7 In several cancer centers, older radiation techniques such as cobalt-60 external radiotherapy machines were still in use due to its low cost.7 Nevertheless, the 2016 report of Vietnam National Cancer Institute stated that many provinces did not have radiotherapy treatment facilities.7
In terms of accessibility to cancer drugs, the QuintilesIMS Institute reported that 42 new cancer drugs were launched during the period 2011 to 2015, but only one of these drugs was available in Vietnam’s pharmaceutical market.74 This fact is not only limited to cancer drugs as several essential drugs for NCDs management were also found to be unavailable for distribution at community health stations.65
Although 70% to 80% of Vietnamese patients with cancer were diagnosed at the terminal stage,7,8 palliative care units only exist in Hanoi, Ho Chi Minh City, and 3 other provincial hospitals.7 There is also a complete lack of hospice care service for patients with cancer in Vietnam.7 On the other hand, opioids for pain relief have been readily available since 2008 with the introduction of the Revised Opioid Prescribing Regulations.7,75 Physicians are now able to prescribe opiates for up to 30 days and adjust the dosage according to patients’ needs.7 However, opiates are not presently listed among the essential drugs for NCD management at community health stations.65 The prevalence of moderate to severe pain among patients with cancer is very high (50% in 2006), and we did not identify any study which assesses the impact of the 2008 revision of Opioid Prescribing Regulations for cancer pain.76 Another author also suggested that the development of comprehensive programs, which simultaneously address insomnia, dyspnea, and cough, could be a viable approach to fatigue in patients with cancer.77
National Policies Relating to Cancer Control
The NCCP9 was one of 5 programs within the national strategy for the prevention and control of NCDs.10 During 2012 to 2015, the cancer control plan aimed to raise community awareness on both the prevention and early detection of cancer, increase the number of early diagnosed cancer cases, and reduce mortality rates of breast, cervical, oral, and colorectal cancer.9,10 However, as of 2015, community awareness did not improve, and 79% of patients were still diagnosed at late stages of cancer.10 There were also insignificant quantities of data to properly assess the changes in the mortality rate.10 The new national strategy for the prevention and control of NCDs during the years 2015 to 2025 set several specific objectives to control the growing cancer burden in Vietnam. These goals include diagnosing 40% of individuals with common cancers at an earlier stage and reaching a 20% relative reduction in premature (aged <70) mortality rate due to cancer, cardiovascular diseases, diabetes, and chronic obstructive pulmonary disease compared to 2015.10 A specific plan for cervical cancer prevention and control, which is being implemented between 2016 and 2025, was also approved.37 Moreover, during 2012 to 2018, the Ministry of Health also developed 3 national guidelines for the diagnosis and treatment of non-small cell lung cancers, colorectal cancer, and liver cancer.72
Although HBV was included in the national vaccination program, there is no plan at the present to adopt and cover the HPV in the vaccination program or the cervical cancer prevention and control action plan of 2016 to 2025.10,37 Several studies showed that HPV vaccination against types 16 and 18 would be cost-effective for Vietnam if the cost per individual was less than US$25.78,79 Vaccination combined with cervical cancer screening, in this case, would be the most favorable program for Vietnam.78 However, if the cost for each vaccination per person is greater than 100 USD, cervical cancer screening alone would be the most cost-effective solution for Vietnam.78
In reference to financing cancer care in Vietnam, 80% of cancer services costs (examination and treatment) were covered for most patients with health insurance, and the remaining costs were paid out of pocket.80 However, only 50% of the costs for certain expensive or specialized drugs, such as trastuzumab, was reimbursed.80 National health insurance also did not cover many essential preventive services, such as tobacco cessation counseling, nutritional counseling, and cancer screening.65 Moreover, NCDs programs, including programs for cancer, only received 2.5% to 3.5% of the total national health annual spending, and funding for these health-targeted programs was declining despite their emerging burden.10,52
Discussion
Our review demonstrates that data regarding cancer burden and control in Vietnam are still deficient. Most of the studies we reviewed had nominal sample sizes and were localized, which decreased the generalizability of the presented results. Nevertheless, there was no properly designed population-based cohort which reports data on cancer in Vietnam. Besides some small clinical studies that include patient follow-up, most of the articles were designed as cross-sectional or case–control studies. In general, government investments in population-based cohort studies with multidisciplinary approaches for assessing cancer and other NCDs are genuinely needed. Moreover, the national registry data require significant improvements as only 9 of 63 provinces have their own hospital-based or provincial registries.7 Therefore, the quality of incidence and prevalence estimation was relatively poor. In addition, the current registry does not account for or provide any data on cancer survivorship, mortality, and quality of life.7,16 The WHO and GICR in tandem have recommended that Vietnam should choose between developing additional cancer incidence registries or bolstering the quality of the current sites.16,71
Nevertheless, funding for these previously mentioned cancer prevention programs as a part of NCD-targeted program, accounting for only 2.5% to 3.5% of the national health budget, has been in decline during recent years.10,52 The lack of funding could hinder the efforts to bolster cancer research and data quality in Vietnam. In a report detailing national cancer control strategies among Asian countries, The Economist Intelligence Unit has emphasized the “need for more and better data, and evidence-based policy”; this statement is essential as few countries in their report possess a well-built cancer registry with mortality data.81 Therefore, we believe that without public investment in research, effective data collection, and prevention programs, Vietnam’s policy-level interventions may not be able to deliver the expected results and achieve a powerful impact.
Burden of Cancer
Our results have suggested that the burden of cancer in Vietnam has been rising rapidly for the past 30 years. The increased incidence and mortality could be partly explained by the growing prevalence of old and new risk factors as well as improvements in data collection of the death and cancer registry. In 2015, the Economist Intelligence Unit estimated that the proportion of deaths attributable to cancer in Vietnam was 18%, which is similar to several countries within the regions such as Thailand (17%) and Malaysia (15%), but this percentage was higher compared to Indonesia (13%), Myanmar (11%), and India (7%).81 Moreover, 70% to 80% of cancer cases in Vietnam were diagnosed at either stage 3 or 4,7,9 compared to 57% average of the ASEAN region.82 However, the ACTION study reported that the 1-year incidence of mortality in Vietnam (25%) appears to be similar to Thailand (25%) and lower than Indonesia (36%), Philippines (36%), and Myanmar (45%),24 but the 1-year incidence of financial catastrophe in Vietnam was the highest in the ASEAN region (68%).24 Another compelling finding from the ACTION study was that approximately 7.5 million DALYs were lost in the ASEAN region which can be attributed to cancer in 2008.24,83 Surprisingly, 1.7 million cancer DALYs were recorded in Vietnam in the same year, which was equivalent to 22.67% of all DALYs lost to cancer in ASEAN21,22—one of the highest among nations in ASEAN.83 Nevertheless, the results of this study should be carefully interpreted as there were several considerable biases. For example, the variation in the proportion of patients who completed a 1-year follow-up ranged from 28% to 88% (Vietnam: 78%).24 As ACTION was one of the few studies that reported the burden of cancer in Vietnam, we emphasize that there is an urgent need for the production of high-quality data and research activities to strengthen Vietnam’s cancer control effort.
Primary Prevention: Controlling Important Risk Factors
In tandem with a variety of Asian countries in recent years, Vietnam has achieved more than 90% coverage in the universal infant HBV vaccination program,81,84 which is one of the “Best Buys” interventions recommended by the WHO.85 However, the high prevalence of chronic HBV (68.2%) in the general population is likely to lead to an increase in HBV-related HCC incidence in the upcoming years,35 as HCC may also be driven by hepatitis C, liver fluke, and alcohol drinking. A similar situation can be observed in South Korea where HBV vaccination has been implemented since 1985, but the incidence of HCC is still twice as high as compared to the global average.81 We hope that maintaining the coverage for HBV vaccine would help to decrease HCC burden as demonstrated in Taiwan and other similar countries.81 Another promising target for primary prevention would be HPV vaccination since it has been observed that nearly 100% of cases of cervical cancer were attributed to HPV infection.86 The most common types of HPV in Vietnam were HPV 16 and HPV 18, which could be prevented effectively by vaccination.39-44 However, the program would only be cost-effective if the costs per individual was less than US$25,78,79 which is a significant financial obstacle for Vietnam. In the ASEAN region besides Malaysia and Singapore, no countries have implemented a national HPV vaccination program,81,87 Even Thailand, a country with a relatively good NCCP, still considered HPV vaccination as not cost-effective at this point.81 At present, there are 2 possible solutions for Vietnam and other developing countries: (1) negotiate and purchase HPV vaccines at a competitive price from the GAVI Alliance and/or (2) wait until a cheaper generic version of the vaccine is produced and available in the market.
Vietnam should also prioritize on controlling behavioral risk factors, such as tobacco use, alcohol drinking, unhealthy diets, and physical inactivity,85 all of which are prevalent throughout Vietnam.51,52,57 Some studies in Vietnam revealed the association between these risk factors with breast cancer53,54 and prostate cancer55; however, these findings were limited by both the sample size and study design. The lack of convincing evidence may slow down the policy development process in Vietnam. It is known that industries could make use of the lack of empirical evidence in the country to push back against many policy initiatives.88,89 Regarding Vietnam’s options, the WHO has identified a set of cost-effective and feasible NCDs “Best Buys,” such as tax increases for tobacco and alcohol and increasing public awareness on the benefits of healthy diets and physical activity. Among that list, the most significant issue for Vietnam was tobacco. As our review illustrated, approximately 50% of males in Vietnam are smokers, and the reduction in smoking prevalence was insignificant in recent years.51 In the region, the progress also seemed to weaken from 2006 to 2012 as the decline in smoking rates in many countries was lower than anticipated, and the smoking prevalence was shown to have increased in China, Thailand, and Indonesia.81 Furthermore, rapid economic development has led to a modification in the pattern of dietary habits, and the population has shifted toward a Western lifestyle, which includes decreased physical activity, in Vietnam and many ASEAN countries.52,57,81 However, the evidence of an effective intervention is still lacking. To summarize, we believe that without a firm commitment to the “Best Buys” strategy and effective public health solutions, Vietnam may not be able to curve the rising tide of behavioral risk factors in the population.
Finally, pollution has become a looming problem in Vietnam and Asian countries as a result of rapid development. In addition, there have been reports of numerous so-called “cancer villages” in Vietnam, China, and other countries.81 However, we were able to only identify 2 case–control studies with proper design and statistical power, which assessed blood cadmium levels and organophosphate pesticides as potential risk factors for cancer.59,60 The other 2 studies, which investigated organochlorines, were all conducted in the early 1990s and suffered from small sample sizes in addition to information bias since the authors did not employ a subjective measurement for the exposures.61,62 This situation again stresses the necessity for a proper mechanism from the government to fund a prospective cohort study in the future.
Secondary Prevention: Improving Screening and Early Detection
Although breast and cervical cancer screenings are recommended to Vietnam by the WHO,71,85 our review indicates that there is no national screening program implemented in Vietnam. While many countries in Asia, such as Taiwan, Singapore, Thailand, and China, have implemented their population cancer screening programs,90 the pilot screening programs for cervical, breast, oral, and colorectal cancers in Vietnam were only able to reach 2% of the target population.7,8 The feasibility of all cancer screening programs mentioned above also requires further evidence to prove their efficacy as only one study reported that the combination of VIA and cryotherapy could be a feasible approach toward cervical cancer screening in Vietnam.69 In the near future, low-cost HPV DNA testing in addition to self-sampled vaginal specimens could be another promising solution for Vietnam and many other developing countries.91
The major drawbacks to secondary prevention were the organization and funding mechanisms required for maintaining the screening programs. First of all, the programs were centrally operated by health-care providers who work at central/provincial hospitals,7 while the WHO recommends the screening procedure to be included as a part of the primary health-care package.71 Moreover, there is no centralized registry at the time of this review that allowed easy tracking and follow-up of individuals with positive screening results. This inefficiency prevents screening programs from broadening coverage within the population. Second, cancer screening and other preventive NCDs services, in general, are not covered by the national health insurance system.52,65 The lack of insurance coverage could be one of the primary reasons for the low coverage of cervical cancer screening.57,66 In a recommendation provided for the Vietnam NCD prevention and control program, the WHO stated that the Vietnamese government should review and implement a mechanism that allows national health insurance to cover essential cancer prevention services at primary health-care centers.71 Such funding would also help to relieve the shortage of trained personnel and diagnostic equipment that hinders pilot screening programs.65
Tertiary Prevention: Scaling Up Cancer Care Services
When conducting this review, it was challenging to accurately capture a clear picture of the effectiveness of cancer tertiary care in Vietnam. This difficulty arises as official reports on the medical practice of health professionals, survivorship, in addition to patient satisfaction and quality of life, were found to be absent in the literature. Thus, the cost-effectiveness of a range of interventions would be taxing to assess and interpret in the long run.
In Vietnam, the government is obligated to reserve 30% of the annual health budget for preventive medicine, while the remaining 70% of the budget are supposed to be directed toward tertiary care.92,93 With such an enormous investment, cancer care in Vietnam has experienced tremendous improvement during the past few years as the number of radiotherapy units and the oncology departments increased rapidly throughout the country.7,8,15 However, such a significant emphasis on cancer treatment in Vietnam, Myanmar, and Malaysia was considered to be not cost-effective in the long term. This is due to the fact that tertiary care facilities would become quickly overwhelmed with late-stage patients while prevention and screening programs are still poorly developed.81 Although the national comprehensive cancer centers are very well equipped, numerous provinces in Vietnam still lack a functional cancer service center (10 of 63), pathology departments (9t of 63), and radiotherapy units.7,15 Primary health-care centers also lack the required number of health staff with sufficient oncology knowledge and skills to maintain such facilities.65 The Ministry of Health even reported that the capacity of the current system could only accommodate for 30% to 40% of the cancer service needs in Vietnam.65 This dilemma illustrates that the Vietnamese government is currently facing a difficult challenge, as coverage for curative services must be improved while preventive services also require increased investment. However, pursuing expensive curative care investments is not a cost-effective or long-term solution for cancer care.81 Vietnam should at least maintain or even extend the preventive medicine budget beyond 30% of the annual health spending because many of the preventive measures at present were found to be significantly cost-effective and considered to be “Best Buys” by the WHO.85,94
Regarding cancer outcomes, 70% to 80% of Vietnamese patients with cancer appeared for treatment at late stages of the illness7,8 and experienced poor survival rates.24-26 In addition, the coverage of palliative care was found to be severely inadequate in Vietnam.7 Our review has also illustrated that palliative care was a component that lagged behind many other areas of NCCP in Asia,81 even for developed countries, such as Japan and South Korea.90 Moreover, palliative care services in Vietnam are organized in larger hospitals located in urban areas.7 In contrast, more than 90% of Vietnamese patients prefer to die at home as they wish to spend their last hours with loved ones in a familiar place.7 Therefore, the Vietnam National Cancer Institute is in the process of developing a community-based palliative care model by providing training for community health centers and provincial hospitals, especially for morphine uses and psychosocial support.7 One of the bottlenecks for this approach was the lack of opioids and other essential drugs for cancer/NCDs management at community health stations.65 Therefore, together with the improvement in the knowledge and skill sets required for health providers at the local level, we also recommend that access to pain relief and other essential drugs at community health stations must be improved in order to create a successful community-based palliative care model in Vietnam.
Last but not least, Vietnamese patients with cancer experienced not only poor outcomes but also the highest rate of financial catastrophe in the ASEAN region.24 Although around 80% of cancer services were covered by health insurance, high treatment costs pushed 37.4% of the households with patients with cancer into poverty.29 This discrepancy could be partly explained by the high out-of-pocket payment as health insurance only covered 50% of the costs for certain expensive and specialized drugs, which were required for effective cancer treatment.80 To improve social security for patients with cancer, the Ministry of Health and national health insurance agencies should conduct a comprehensive health economic analysis to recalculate the necessary payment for cancer services. The policymakers should also explore alternative options to increase the pooling of fund for health insurance by raising taxes on harmful substances, such as tobacco, alcohol, sugar-sweetened beverages, and so forth.
National Policy: Toward a Better Cancer Control Program
Overall, our review demonstrates that Vietnam is lagging behind in the fight against cancer. The NCCP has been implemented in Vietnam for the past 20 years, but the key indicators of the NCCP show that the results are limited: Cancer awareness stays relatively the same, late stages diagnosis of cancer is still dominant, and there is presently no national mortality data available.10 A significant reason for these unfortunate results is the lack of public investment in the program. Our review found that the funding for NCCP together with 4 other NCDs programs was declining and only accounted for less than 3.5% of government health budget.10,52 Therefore, the NCCP plan may appear to be well written on paper but ultimately remains impractical due to funding constraints.81 This situation was not only limited to Vietnam as many countries in the region, including Myanmar, Malaysia, and Indonesia, also did not have costings and provision of the national budget to support NCCP.81
Wealthy countries have more resources at their disposal to control cancer, so their NCCPs are more successful compared to other nations with low resources.81 However, how resources are deployed and how the programs are managed also impact the outcomes of NCCPs, even among developed countries.81 A prime example for Vietnam would be Thailand, where the comprehensive NCCP with limited resources achieved far better outcomes in comparison to countries with a higher gross domestic product, such as China and Malaysia.81 First, Thailand had 16 provincial cancer registries and aimed to cover every province in the near future.90 The analysis of cancer data was the foundation for all cancer control policies, which reflected the comprehensive and evidence-based approach of Thai legislators.81 Second, Thailand’s NCCP provided the foundation for all services across the cancer control continuum rather than focusing on treatment; the NCCP also emphasizes on cancer data collecting and capacity building.81,95 Finally, the Thailand Ministry of Health was also involved with other ministries and government agencies in the process for developing health policies as well as working with/providing funding for nongovernmental organizations, especially to raise the awareness of cancer control in the community.81,95 All of these 3 approaches were in line with the Economist Intelligence Unit’s recommendations regarding cancer control plans in Asia: “A need for more and better data, and evidence-based policy, “A need for a more holistic approach to cancer care,” and “A need to engage more with those outside the health system.”81 The final recommendation for NCCPs in Asia and Vietnam, in particular, was “A need to consider appropriate legal foundations,” which could provide stable budgets to leverage the requirements for data collection and usage through the country.81
Limitations
Our review comes with several limitations. First of all, because there is no central database for domestic scientific studies, our review did not have the capacity to cover all articles which were published in Vietnamese. Second, many Vietnamese gray literature documents were not in electronic form and could not be found via our online search. Therefore, our ability to create a comprehensive overview of cancer burden and control effort in Vietnam could be limited.
Conclusion
Given the summary of evidence in this review, it is conclusive that cancer and its associated consequences are both persistent and emerging problem in Vietnam. In addition, the NCCP results are also limited regarding crucial indicators of success. The current situation requires urgent evidence-based control policies on both prevention and the continuity of care for patients with cancer. A holistic approach to cancer services with the participation of various stakeholders, which could be more complicated than working within the health system alone, would be the best direction for Vietnam.
Serious and transparent public investment in data collection and capacity building is needed as high-quality data and further research findings are essential to provide comprehensive insights into the complex situation of cancer in Vietnam.
Supplemental Material
Supplemental_Material for Cancers in Vietnam—Burden and Control Efforts: A Narrative Scoping Review by Tung Pham, Linh Bui, Giang Kim, Dong Hoang, Thuan Tran and Minh Hoang in Cancer Control
Acknowledgments
The authors thank the Vietnam National Institute for Cancer Control and National Cancer Hospital for providing technical support to this review. The authors also thank Jonathan Frank Josephs-Spaulding for assistance with the language editing and comments that greatly improved the manuscript.
Authors’ Note: This review was not a study conducted on animal and human and was exempt from the institutional review board’s review.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was supported by Hanoi University of Public Health and Hanoi Medical University, Vietnam.
ORCID iD: Tung Pham  https://orcid.org/0000-0002-7242-8976
https://orcid.org/0000-0002-7242-8976
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA a Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3. Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376(9747):1186–1193. doi:10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 4. Prager GW, Braga S, Bystricky B, et al. Global cancer control: responding to the growing burden, rising costs and inequalities in access. ESMO Open. 2018;3(2):e000285 doi:10.1136/esmoopen-2017-000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Agency for Research on Cancer (IARC). Global Cancer Observatory—Vietnam Population fact sheets. http://gco.iarc.fr/today/data/factsheets/populations/704-viet-nam-fact-sheets.pdf. Accessed October 26, 2018.
- 6. Anh PTH, Duc NB. The situation with cancer control in Vietnam. Jpn J Clin Oncol. 2002;32(suppl):S92–S97. [DOI] [PubMed] [Google Scholar]
- 7. Van Thuan T, Tuan Anh P, Van Tu D, Thi Thanh Huong T. Cancer control in Vietnam: where are we? | Cancer control. Cancel Control. 2016. http://www.cancercontrol.info/cc2016/cancer-control-in-vietnam-where-we-are/. Accessed June 10, 2018.
- 8. Van Thuan T, Dieu B, Thi Hoai Nga N. The status and future of cancer control in Vietnam. Vietnam Med J. 2018;472(suppl):1–8. [Google Scholar]
- 9. World Health Organization. Vietnam—Overview: National Strategy for Cancer Control (2010 and 2020). 2006. http://www.who.int/cancer/modules/Viet%20Nam.pdf. Accessed July 19, 2018.
- 10. Mo H. National Strategy for the Prevention and Control of Noncommunicable Diseases 2015-2025. Hanoi, Vietnam; 2015:117 http://vncdc.gov.vn/files/document/2016/4/chien-luoc-quoc-gia-phong-chong-benh-khong-lay-nhiem.pdf. Accessed October 28, 2018. [Google Scholar]
- 11. Khalil H, Peters M, Godfrey CM, McInerney P, Soares CB, Parker D. An evidence-based approach to scoping reviews. Worldviews Evid Based Nurs. 2016;13(2):118–123. doi:10.1111/wvn.12144. [DOI] [PubMed] [Google Scholar]
- 12. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–146. doi:10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-SCR): checklist and explanation. Ann Internal Medicine. 2018;169(7):467 doi:10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 14. Hooper A. Lib Guides: Systematic Review Resources: Systematic Review Overview. //libguides.sph.uth.tmc.edu/c.php?g=543382&p=5375107. Accessed April 17, 2019.
- 15. Nguyen BD. Results of the national cancer program 2008–2010. Vietnam Oncol J. 2010;(1):21–26. [Google Scholar]
- 16. Mery L, Torode J, Pearlman P. Global Initiative for Cancer Registry Development Partners Task Force Visit to Vietnam. GICR; 2017.
- 17. Nguyen BD, Dieu B, Thuan TV, et al. The cancer incidence of Vietnam in 2010 with reported data of 6 areas from 2004–2008. Vietnam Oncol J. 2010(1):73–80. [Google Scholar]
- 18. Are C, Meyer B, Stack A, et al. Global trends in the burden of liver cancer. J Surg Oncol. 2017;115(5):591–602. doi:10.1002/jso.24518. [DOI] [PubMed] [Google Scholar]
- 19. Vuong DA, Velasco-Garrido M, Lai TD, Busse R. Temporal trends of cancer incidence in Vietnam. Asian Pac J Cancer Prev. 1993-2007;11(3):739–745. [PubMed] [Google Scholar]
- 20. Dong HV, Lee AH, Nga NH, Quang N, Chuyen VL, Binns CW. Epidemiology and prevention of prostate cancer in Vietnam. Asian Pac J Cancer Prev. 2014;15(22):9747–9751. doi:10.7314/APJCP.2014.15.22.9747. [DOI] [PubMed] [Google Scholar]
- 21. Nhung NTT, Long TK, Linh BN, Vos T, Anh ND, Huong NT. Viet Nam Burden of Disease and Injury Study. Hanoi, Vietnam: Medical Publishing House; 2011. [Google Scholar]
- 22. Nhung NTT, Long TK, Linh BN, Vos T, Huong NT, Anh ND. Estimation of Vietnam national burden of disease 2008. Asia Pac J Public Health. 2014;26(5):527–535. doi:10.1177/1010539513510556. [DOI] [PubMed] [Google Scholar]
- 23. Quach DT, Nguyen OT. Clinical, endoscopic and pathological characteristics of early-onset colorectal cancer in Vietnamese. Asian Pac J Cancer Prev. 2012;13(5):1767–1770. doi:10.7314/APJCP.2012.13.5.1767. [DOI] [PubMed] [Google Scholar]
- 24. The ACTION Study Group. Policy and priorities for national cancer control planning in low- and middle-income countries: lessons from the Association of Southeast Asian Nations (ASEAN) costs in oncology prospective cohort study. Eur J Cancer. 2017;74:26–37. doi:10.1016/j.ejca.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 25. Lan NH, Laohasiriwong W, Stewart JF. Survival probability and prognostic factors for breast cancer patients in Vietnam. Global Health Action. 2013;6(1):18860 doi:10.3402/gha.v6i0.18860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vu Hoang PT, Ambroise J, Dang Chi VL, et al. Comparison of long-term outcome between white and Vietnamese children treated for acute lymphoblastic leukemia according to the Fralle 2000 Protocol. J Pediatr Hematol Oncol. 2014;36(7):534–540. doi:10.1097/MPH.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 27. Van Minh H, My NTT, Jit M. Cervical cancer treatment costs and cost-effectiveness analysis of human papillomavirus vaccination in Vietnam: a PRIME modeling study. BMC Health Serv Res. 2017;17(1). doi:10.1186/s12913-017-2297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoang Lan N, Laohasiriwong W, Frederick Stewart J, Dinh Tung N, Coyte PC. Cost of treatment for breast cancer in central Vietnam. Glob Health Action. 2013;6(1):18872 doi:10.3402/gha.v6i0.18872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoang VM, Pham CP, Vu QM, et al. Household financial burden and poverty impacts of cancer treatment in Vietnam. BioMed Res Int. 2017;2017:1–8. doi:10.1155/2017/9350147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen TH, Binh TTT, Anh NQ. Quality of life of breast cancer patients measured by QLQ-C30 and related factors in Oncology hospitals in Vietnam. Vietnam J Prev Med. 2017;27(5):102. [Google Scholar]
- 31. Yen NTK, Weiss B, Trung LT. Caseness rates and risk factors for depression among Vietnamese cancer patients. Asian J Psychiatr. 2016;23:95–98. doi:10.1016/j.ajp.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen TV, Janssen MJR, van Oijen MGH, et al. Genetic polymorphisms in GSTA1, GSTP1, GSTT1, and GSTM1 and gastric cancer risk in a Vietnamese population. Oncol Res. 2009;18(7):349–355. doi:10.3727/096504010X12626118080064. [DOI] [PubMed] [Google Scholar]
- 33. Quoc NB, Phuong NDN, Ngan TK, Linh NTM, Cuong PH, Chau NNB. Expression of plasma hsa-miR122 in HBV-related hepatocellular carcinoma (HCC) in Vietnamese patients. Microrna. 2018;7(2):92–99. doi:10.2174/2211536607666180427165114. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen HH, Nguyen HTT, Vu NP, et al. Mutational screening of germline RB1 gene in Vietnamese patients with retinoblastoma reveals three novel mutations. Mol Vis. 2018;24:231–238. [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen VTT, Law MG, Dore GJ. An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025: an enormous HBV-related liver disease burden projected by 2025. Liver Int. 2008;28(4):525–531. doi:10.1111/j.1478-3231.2007.01646.x. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen VTT, McLaws ML, Dore GJ. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22(12):2093–2100. doi:10.1111/j.1440-1746.2007.05010.x. [DOI] [PubMed] [Google Scholar]
- 37. Vietnam Ministry of Health. National Action Plan on Prevention and Control of Cervical Cancer 2016–2025. Hanoi, Vietnam: Vietnam Ministry of Health; 2016. http://mch.moh.gov.vn/van-ban/van-ban-phap-quy/van-banbieu-mau/-Ke-hoach-hanh-dong-quoc-gia-ve-du-phong-va-kiem-soat-ung-thu-co-tu-cung-giai-doan-2016-2025-804.html. [Google Scholar]
- 38. Quek SC, Lim BK, Domingo E, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical intraepithelial neoplasia across 5 countries in Asia. Int J Gynecol Cancer. 2013;23(1):148–156. doi:10.1097/IGC.0b013e31827670fd. [DOI] [PubMed] [Google Scholar]
- 39. Vu LT, Bui D, Le HT. Prevalence of cervical infection with HPV type 16 and 18 in Vietnam: implications for vaccine campaign. BMC Cancer. 2013;13:53 doi:10.1186/1471-2407-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vu LTH, Bui D. Prevalence of cervical human papilloma virus infection among married women in Vietnam, 2011. Asian Pac J Cancer Prev. 2012;13(1):37–40. doi:10.7314/APJCP.2012.13.1.037. [DOI] [PubMed] [Google Scholar]
- 41. Vu L, Le H, Luong O, Tran H, Nguyen N, Luu H. Prevalence of cervical human papillomavirus infection among married women in Hanoi, Vietnam, 2010. Asia Pac J Public Health. 2012;24(2):385–390. doi:10.1177/1010539510393727. [DOI] [PubMed] [Google Scholar]
- 42. Domingo EJ, Noviani R, Noor MRM, et al. Epidemiology and prevention of cervical cancer in Indonesia, Malaysia, the Philippines, Thailand and Vietnam. Vaccine. 2008;26:(suppl 12):M7M71–M79. doi:10.1016/j.vaccine.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 43. Anh PTH, Hieu NT, Herrero R, et al. Human papillomavirus infection among women in South and North Vietnam: HPV in Women in Vietnam. Int J Cancer. 2003;104(2):213–220. doi:10.1002/ijc.10936. [DOI] [PubMed] [Google Scholar]
- 44. Vu LTH, Le HTT. Cervical human papilloma virus infection among the general female population in Vietnam: a situation analysis. Asian Pac J Cancer Prev. 2011;12(2):561–566. [PubMed] [Google Scholar]
- 45. Van SN, Khac MN, Dimberg J, Matussek A, Henningsson AJ. Prevalence of cervical infection and genotype distribution of human papilloma virus among females in Da Nang, Vietnam. Anticancer Res. 2017;37(3):1243–1247. doi:10.21873/anticanres.11440. [DOI] [PubMed] [Google Scholar]
- 46. de Sanjose S, Mbisa G, Perez-Alvarez S, et al. Geographic variation in the prevalence of Kaposi sarcoma–associated herpesvirus and risk factors for transmission. J Infect Dis. 2009;199(10):1449–1456. doi:10.1086/598523. [DOI] [PubMed] [Google Scholar]
- 47. Shin HR, Oh JK, Masuyer E, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101(3):579–585. doi:10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. IARC Working Group on the Evaluation of Carcinogenic Risk to. Opisthorchis viverrini and Clonorchis sinensis. International Agency for Research on Cancer; 2012. https://www.ncbi.nlm.nih.gov/books/NBK304354/. Accessed November 2, 2018. [Google Scholar]
- 49. Norman RE, Vos T, Barendregt JJ, et al. Mortality attributable to smoking in Vietnamese men in 2008. Prev Med. 2013;57(3):232–237. doi:10.1016/j.ypmed.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 50. Ng N, Winkler V, Minh HV, Tesfaye F, Wall S, Becher H. Predicting lung cancer death in Africa and Asia: differences with WHO estimates. Cancer Causes Control. 2009;20(5):721–730. doi:10.1007/s10552-008-9285-8. [DOI] [PubMed] [Google Scholar]
- 51. Van Minh H, Giang KB, Ngoc NB, et al. Prevalence of tobacco smoking in Vietnam: findings from the Global Adult Tobacco Survey 2015. Int J Public Health. 2017;62(suppl 1):121–129. doi:10.1007/s00038-017-0955-8. [DOI] [PubMed] [Google Scholar]
- 52. Nguyen TT, Hoang MV. Non-communicable diseases, food and nutrition in Vietnam from 1975 to 2015: the burden and national response. Asia Pac J Clin Nutr. 2018;27(1):19–28. doi:10.6133/apjcn.032017.13. [DOI] [PubMed] [Google Scholar]
- 53. Nichols HB, Trentham-Dietz A, Love RR, et al. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomark Prev. 2005;14(1):41–47. [PubMed] [Google Scholar]
- 54. Trieu PDY, Mello-Thoms C, Peat JK, Do TD, Brennan PC. Risk factors of female breast cancer in Vietnam: a case–control study. Cancer Res Treat. 2017;49(4):990–1000. doi:10.4143/crt.2016.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van Hoang D, Pham N, Lee A, Tran D, Binns C. Dietary carotenoid intakes and prostate cancer risk: a case–control study from Vietnam. Nutrients. 2018;10(1):70 doi:10.3390/nu10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ngoan LT, Thu NT, Lua NT, et al. Cooking temperature, heat-generated-carcinogens, and the risk of stomach and colorectal cancers. Asian Pac J Cancer Prev. 2009;10(1):83–86. [PubMed] [Google Scholar]
- 57. Vietnam Ministry of Health. Vietnam National Survey on the Risk Factors of NCDs (STEPS) 2015 Report. Hanoi, Vietnam: Vietnam Ministry of Health; 2016. http://www.who.int/ncds/surveillance/steps/VietNam_2015_STEPS_Report.pdf. Accessed November 1, 2018. [Google Scholar]
- 58. Reichart PA, Nguyen XH. Betel quid chewing, oral cancer and other oral mucosal diseases in Vietnam: a review: betel quid chewing in Vietnam. J Oral Pathol Med. 2008;37(9):511–514. doi:10.1111/j.1600-0714.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 59. Dang TH, Thanh DT, Huong NT, et al. Investigation of the Potential Association Between Endocrine Disrupting Chemicals (EDCs) and Breast Cancer Risk in Viet Nam. Hanoi, Vietnam: Hanoi University of Public Health; 2017:111 http://opac.huph.edu.vn/opac/wpDetail.aspx?Id=4793. [Google Scholar]
- 60. Cordier S, Le TB, Verger P, et al. Viral infections and chemical exposures as risk factors for hepatocellular carcinoma in Vietnam. Int J Cancer. 1993;55(2):196–201. [DOI] [PubMed] [Google Scholar]
- 61. Schecter A, Toniolo P, Dai LC, Thuy LT, Wolff MS. Blood levels of DDT and breast cancer risk among women living in the north of Vietnam. Arch Environ Contam Toxicol. 1997;33(4):453–456. [DOI] [PubMed] [Google Scholar]
- 62. Ha MC, Cordier DS, Bard D, et al. Agent orange and the risk of gestational trophoblastic disease in Vietnam. Arch Environ Health Int J. 1996;51(5):368–374. doi:10.1080/00039896.1996.9934424. [DOI] [PubMed] [Google Scholar]
- 63. Huynh MLD, Raab SS, Suba EJ. Association between war and cervical cancer among Vietnamese women. Int J Cancer. 2004;110(5):775–777. doi:10.1002/ijc.20164. [DOI] [PubMed] [Google Scholar]
- 64. Suba EJ, Raab SS. Lessons learned from successful Papanicolaou cytology cervical cancer prevention in the socialist republic of Vietnam. Boon M, ed. Diagn Cytopathol. 2012;40(4):355–366. doi:10.1002/dc.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vietnam Ministry of Health. Vietnam Joint Annual Health Review 2014. Strengthening Prevention and Control of Non-Communicable Disease. Hanoi, Vietnam: Vietnam Ministry of Health; 2015:328 www.jahr.org.vn/downloads/JAHR2014/JAHR%202014_EN_full.pdf. Accessed November 1, 2018. [Google Scholar]
- 66. Lai TTH, Thuy TTT. Cervical cancer screening rate and its related factors among work women at two companies in Gò Vấp district, Ho Chi Minh City. Ho Chi Minh City Medical Journal. 2016;20(suppl 5):51–56. [Google Scholar]
- 67. Bui D, Thuan TV, Nga NTH, et al. The screening results for early detection of breast and cervical cancer in some cities and provinces from 2008 to 2010. Vietnam Oncol J. 2010;(1):152–155. [Google Scholar]
- 68. Bui D. Results of the national cancer program 2011–2014. Vietnam Oncol J. 2014;(2):21–28. [Google Scholar]
- 69. Paul P, Winkler JL, Bartolini RM, et al. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18(12):1278–1284. doi:10.1634/theoncologist.2013-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vu Le C, Dao OQ, Khac Tran LN. Mass screening of prostate cancer in Vietnam: current status and our opinions. Urol Oncol. 2010;28(6):673–676. doi:10.1016/j.urolonc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 71. Catherine H. Vietnam Noncommunicable Disease Prevention and Control Programme 2002–2010. Geneva, Switzerland: WHO Press; 2011:73 http://www.wpro.who.int/vietnam/topics/chronic_diseases/vietnam_noncommunicable_disease_prevention_and_control_program_2002_2010_imple_review.pdf. Accessed November 1, 2018. [Google Scholar]
- 72. Vietnam Ministry of Health. Vietnam Decisions and Guidelines for Cancer Diagnosis and Treatment (Decree 5250, 2549, and 4825/QĐ-BYT). Hanoi, Vietnam: Vietnam Ministry of Health; 2012. [Google Scholar]
- 73. Dayton V, Nguyen CK, Van TT, et al. Evaluation of opportunities to improve hematopathology diagnosis for Vietnam pathologists. Am J Clin Pathol. 2017;148(6):529–537. doi:10.1093/ajcp/aqx108. [DOI] [PubMed] [Google Scholar]
- 74. QuintilesIMS Institute. Global Oncology Trends 2017. https://www.communityoncology.org/wp-content/uploads/2017/06/QIIHI_Oncology_Trend_Report_2017_Advances_Complexity_Cost.pdf. Accessed November 1, 2018.
- 75. Krakauer EL, Ngoc NTM, Green K, Van Kham L, Khue LN. Vietnam: integrating palliative care into HIV/AIDS and cancer care. J Pain Symptom Manage. 2007;33(5):578–583. doi:10.1016/j.jpainsymman.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 76. Reyes-Gibby CC, Ba Duc N, Phi Yen N, et al. Status of cancer pain in Hanoi, Vietnam: a hospital-wide survey in a tertiary cancer treatment center. J Pain Symptom Manage. 2006;31(5):431–439. doi:10.1016/j.jpainsymman.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 77. Long NH, Thanasilp S, Thato R. A causal model for fatigue in lung cancer patients receiving chemotherapy. Eur J Oncol Nurs. 2016;21:242–247. doi:10.1016/j.ejon.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 78. Kim JJ, Kobus KE, Diaz M, O’Shea M, Van Minh H, Goldie SJ. Exploring the cost-effectiveness of HPV vaccination in Vietnam: insights for evidence-based cervical cancer prevention policy. Vaccine. 2008;26(32):4015–4024. doi:10.1016/j.vaccine.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 79. Goldie SJ, Diaz M, Kim SY, Levin CE, Van Minh H, Kim JJ. Mathematical models of cervical cancer prevention in the Asia Pacific region. Vaccine. 2008;26: M17–M29. doi:10.1016/j.vaccine.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 80. Vietnam Health Insurance Agency. Vietnam Health Insurance Agency Decree 62/2009/NĐ-CP: Detailed Regulations and Implementation Guidelines of Articles Related to the Vietnam Law on Health Insurance. Hanoi, Vietnam: Vietnam Health Insurance Agency; 2009. [Google Scholar]
- 81. The Economist Intelligence Unit. Controlling Cancer: The State of National Cancer Control Plans in Asia. Perspectives from The Economist Intelligence Unit (EIU). 2015. https://eiuperspectives.economist.com/healthcare/controlling-cancer. Accessed November 21, 2018.
- 82. The George Institute for Global Health. Policy Roundtable on ASEAN Member States Readiness in Cancer Control – “Turning Action Results into Policy Actions.” Bali, Indonesia; 2015. https://www.georgeinstitute.org/sites/default/files/action-study-bali-rountable-meeting-report.pdf. Accessed November 20, 2018. [Google Scholar]
- 83. Kimman M, Norman R, Jan S, Kingston D, Woodward M. The burden of cancer in member countries of the association of Southeast Asian Nations (ASEAN). Asian Pac J Cancer Prev. 2012;13(2):411–420. doi:10.7314/APJCP.2012.13.2.411. [DOI] [PubMed] [Google Scholar]
- 84. World Health Organization. Hepatitis B 3rd Dose (HepB3) Immunization Coverage. World Health Organization; http://www.who.int/gho/immunization/hepatitis/en/. Accessed November 23, 2018. [Google Scholar]
- 85. World Health Organization. Tackling NCDs: “Best Buys” and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases. World Health Organization; 2017. http://apps.who.int/iris/handle/10665/259232. Accessed November 9, 2018. [Google Scholar]
- 86. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi:10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. doi:10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 88. Buse K, Hawkes S. Health post-2015: evidence and power. Lancet. 2014;383(9918):678–679. doi:10.1016/S0140-6736(13)61945-5. [DOI] [PubMed] [Google Scholar]
- 89. Shiffman J. Knowledge, moral claims and the exercise of power in global health. Int J Health Policy Manage. 2014;3(6):297–299. doi:0.15171/ijhpm.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moore MA. Cancer control programs in East Asia: evidence from the international literature. J Prev Med Public Health. 2014;47(4):183–200. doi:10.3961/jpmph.2014.47.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gravitt PE, Belinson JL, Salmeron J, Shah KV. Looking ahead: a case for HPV testing of self-sampled vaginal specimens as a cervical cancer screening strategy. Int J Cancer. 2011;129(3):517–527. doi:10.1002/ijc.25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Somanathan A, Tandon A, Dao HL, Hurt KL, Fuenzalida-Puelma HL. Moving toward Universal Coverage of Social Health Insurance in Vietnam: Assessment and Options. Washington, D.C: The World Bank; 2014. [Google Scholar]
- 93. Vietnam Health Insurance Agency. Resolution No. 18/2008/NQ-QH12. Hanoi, Vietnam: Vietnam National Assembly; 2008. [Google Scholar]
- 94. Van Minh H, Lan Huong D, Bao Giang K, Byass P. Economic aspects of chronic diseases in Vietnam. Glob Health Action. 2009;2(1):1965 doi:10.3402/gha.v2i0.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Thailand Ministry of Health. National Cancer Control Programmes 2013–2017. Thailand: Thailand Ministry of Health; 2013. http://www.nci.go.th/en/File_download/D_index/NCCP_2556-2560.pdf. Accessed November 23, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Material for Cancers in Vietnam—Burden and Control Efforts: A Narrative Scoping Review by Tung Pham, Linh Bui, Giang Kim, Dong Hoang, Thuan Tran and Minh Hoang in Cancer Control