Abstract
Despite recent advances, the prognosis of pulmonary hypertension (PH) remains poor. While the initial insult in PH implicates the pulmonary vasculature, the functional state, exercise capacity, and survival of such patients are closely linked to right ventricular (RV) function. In the current study, we sought to investigate the effects of maximum incremental exercise on the matching of RV contractility and afterload (i.e. right ventricular–pulmonary arterial [RV–PA] coupling) in patients with exercise PH (ePH) and pulmonary arterial hypertension (PAH). End-systolic elastance (Ees), pulmonary arterial elastance (Ea), and RV–PA coupling (Ees/Ea) were determined using single-beat pressure-volume loop analysis in 40 patients that underwent maximum invasive cardiopulmonary exercise testing. Eleven patients had ePH, nine had PAH, and 20 were age-matched controls. During exercise, the impaired exertional contractile reserve in PAH was associated with blunted stroke volume index (SVI) augmentation and reduced peak oxygen consumption (peak VO2 %predicted). Compared to PAH, ePH demonstrated increased RV contractility in response to increasing RV afterload during exercise; however, this was insufficient and resulted in reduced peak RV–PA coupling. The dynamic RV–PA uncoupling in ePH was associated with similarly blunted SVI augmentation and peak VO2 as PAH. In conclusion, dynamic rest-to-peak exercise RV–PA uncoupling during maximum exercise blunts SV increase and reduces exercise capacity in exercise PH and PAH. In ePH, the insufficient increase in RV contractility to compensate for increasing RV afterload during maximum exercise leads to deterioration of RV–PA coupling. These data provide evidence that even in the early stages of PH, RV function is compromised.
Keywords: pulmonary arterial hypertension, exercise pulmonary hypertension, right ventricular–pulmonary arterial coupling
Introduction
Pre-capillary pulmonary hypertension (PH) is characterized by the presence of a mean pulmonary arterial pressure (mPAP) >20 mmHg and elevated resting pulmonary vascular resistance (PVR) with normal left-sided filling pressures during resting supine right heart catheterization (RHC). Pulmonary arterial hypertension (PAH), characterized by advanced pre-capillary pulmonary vascular remodeling, is a progressive disease whereby increased right ventricular (RV) afterload leads to RV failure and death.1,2 Despite recent therapeutic advances, the prognosis of PAH remains poor.3 The lack of response to PAH-specific therapy, in part, reflects the delayed detection of the disease.4 Exercise pulmonary hypertension (ePH) is increasingly being recognized as an early, intermediary phase of pre-capillary PH5–10 that is associated with adverse long-term outcomes11,12 and is potentially treatable.13 Similar to PAH, patients with ePH have impaired pulmonary vascular reserve and a blunted CO response to exercise, the latter of which plays a central role in determining the exercise capacity in ePH.5 However, the relative contributions to the blunted CO response in ePH (i.e. increased RV afterload and/or reduced contractile reserve) are not completely characterized.
The aim of this study was to investigate the effects of maximal incremental exercise on RV contractility (Ees), RV afterload (arterial elastance, Ea), and RV–PA coupling (Ees/Ea) in patients with early (ePH) and late stage pre-capillary disease (PAH) and to compare their findings to patients with normal physiological limit to exercise. We hypothesize that RV–PA uncoupling occurs dynamically during maximum incremental cycling even in ePH patients and substantively impairs aerobic capacity.
Methods
Study population and design
We identified patients from the Brigham and Women’s Hospital Dyspnea Clinic (Boston, MA, USA) between March 2011 and September 2017 who underwent resting supine RHC followed by symptom limited upright invasive cardiopulmonary exercise testing (iCPET) as part of their clinically indicated evaluation for unexplained exercise intolerance.14 The study protocol was approved by Partners Healthcare Human Research Committee (2011P000272).
PAH was defined by resting supine RHC as mPAP >20 mmHg and pulmonary arterial wedge pressure (PAWP) ≤15 mmHg along with PVR > 3 Wood units (WU).15 ePH was defined by age-specific exercise pulmonary hemodynamic criteria for maximum upright exercise as follows: (1) peak mPAP >30 mmHg and peak PVR > 1.34 WU for patients aged ≤ 50 years; or (2) peak mPAP > 33 mmHg and peak PVR > 2.10 WU for patients aged > 50 years.16
Controls were individuals who exhibited a normal physiological limit to exercise defined by a maximum oxygen consumption (peak VO2) and cardiac output (CO) of ≥80% predicted, respectively. Controls were age-matched to the ePH and PAH groups.
Exclusion criteria included: (1) left heart disease defined by moderate/severe mitral and/or aortic valvular disease of left ventricular ejection fraction (LVEF) <0.5 on resting echocardiography, or post-capillary PH identified by a mPAP ≥ 25 mmHg and PAWP > 15 mmHg on resting RHC or PAWP ≤ 15 mmHg at rest but abnormally elevated during exercise with associated normal peak PVR for the patient’s age (i.e. peak PAWP > 19 mmHg and peak PVR < 1.35 WU for ages ≤ 50 years or PAWP > 17 mmHg and peak PVR ≤ 2.10 WU for ages > 50 years;16 (2) submaximal cardiopulmonary exercise testing defined by peak respiratory exchange ratio (RER) <1.05 and a peak heart rate <85% predicted along with a peak mixed-venous partial pressure of oxygen >27 mmHg; (3) relevant lung disease defined as the forced expiratory volume in the first second divided by the forced vital capacity (FEV1/FVC) < 70% predicted with associated FEV1 < 60% predicted, or a radiological diagnosis of lung fibrosis;17 (4) incomplete exercise hemodynamics; and (5) absent or uninterpretable RV pressure waveform tracing at rest and/or peak exercise.
Invasive cardiopulmonary exercise testing
The RHC and iCPET techniques used have been described previously14,16,18 and the details of the iCPET technique and conventional hemodynamic measurements can be found in the online supplementary material.
Conventional right heart hemodynamic data
The conventional right heart hemodynamics measured were mPAP, PAWP, CO, PVR, total pulmonary resistance (TPR), stroke volume (SV), and PA compliance. PVR was calculated as (mPAP – PCWP/CO) and expressed in Wood units (WU). The TPR was calculated as mPAP/CO. The TPR was calculated as mPAP/CO and expressed as mmHg/L/min. SV was calculated as CO/heart rate. CO and SV were indexed for body surface area to obtain both cardiac index (CI) and SV index (SVI). PA compliance was calculated as SV divided PA pulse pressure (stroke volume/systolic – diastolic PAP) and expressed as mL/mmHg. Chronotropic reserve was determined by the difference between peak exercise and resting heart rate (bpm). Chronotropic incompetence was defined by a peak heart rate adjusted for age, gender, and body size that is ≤85% predicted.
RV–PA coupling assessment
RV Ees was determined at rest and at peak exercise, using the single beat method (Fig. S1),19 as follows:
Where Pmax was calculated from non-linear extrapolation of the early and late isovolumic portions of the RV pressure curve, RV ESP represents the RV end-systolic pressure, and SVI represents the stroke volume index. The exertional contractile reserve was determined by the difference between rest and peak exercise Ees. The mPAP was used as a surrogate for the RV ESP. Ea was calculated as follows20:
RV–PA coupling was subsequently determined by the ratio between Ees and Ea:
Statistical analysis
Unless otherwise stated, values are presented as mean ± standard deviation (SD).
Comparisons of baseline characteristics, resting hemodynamics, CPET parameters, and load independent measures of RV function between the groups were performed using one-way ANOVA with Bonferroni post hoc correction for normally distributed data and Kruskal–Wallis for data not normally distributed. Comparison between rest-to-exercise response between patients in all three groups were performed using two-way repeated measures analysis of variance with Bonferroni post hoc correction. The effects of exercise between groups was evaluated using the P value comparing the delta change from rest-to-peak exercise of the hemodynamic variable measured. For two group comparisons, independent t-test was performed for normally distributed data and Wilcoxon Rank Sum test for data not normally distributed. The relationships of SVI augmentation (change from rest-to-peak exercise) and peak exercise oxygen consumption (VO2 %predicted) with peak exercise RV–PA coupling were examined using linear regression. To identify if peak RV–PA coupling predicts peak SVI, Pearson correlation was performed. Univariable analysis was performed to determine the predictors of peak RV–PA coupling and peak VO2 (% predicted) in ePH and PAH. Non-colinear variables (i.e. Pearson correlation r < 0.6) with a significant P value (P < 0.05) on univariable analysis were incorporated into bivariate models to identify independent predictors of peak RV–PA coupling and peak VO2 (% predicted). A P value < 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic and clinical characteristics
We identified 11 patients with ePH and nine patients with (resting) PAH based on the aforementioned inclusion, who additionally exhibited an interpretable RV pressure waveform (Fig. S2). Additionally, 20 age-matched controls were identified. Therefore, the study sample was made up of a total of 40 participants.
There was no statistical difference among controls, ePH, and PAH for age, body mass index (BMI), and baseline hemoglobin concentration. PAH had a higher percentage of women compared to ePH. The baseline characteristics and co-morbidities are summarized in Table 1.
Table 1.
Baseline characteristics and resting pulmonary hemodynamic data.
| Controls (n = 20) | ePH (n = 11) | PAH (n = 9) | |
|---|---|---|---|
| Characteristics | |||
| Age (years) | 63 ± 9 | 66 ± 13 | 63 ± 12 |
| Female (n (%)) | 8 (40) | 2 (18) | 6 (66)* |
| BMI (kg/m2) | 28 ± 5 | 32 ± 5 | 27 ± 5 |
| Hemoglobin (g/dL) | 14.9 ± 1.7 | 14.1 ± 1.4 | 14.5 ± 2.2 |
| Co-morbidities (n (%)) | |||
| Hypertension | 13 (65) | 7 (63) | 6 (67) |
| Hyperlipidemia | 7 (35) | 6 (55) | 7 (78) |
| Diabetes | 1 (5) | 2 (18) | 2 (22) |
| Coronary artery disease | 2 (10) | 3 (27) | 1 (11) |
| Medications (n (%)) | |||
| Beta adrenergic receptor blocker | 3 (15) | 3 (27) | 4 (44) |
| Calcium channel receptor blocker | 2 (10) | 1 (9) | 0 |
| ACE inhibitor or ARB | 8 (40) | 2 (18) | 4 (44) |
| Diuretics | 5 (25) | 2 (18) | 7 (77)*,† |
| Ambrisentan | 0 | 0 | 1 (11) |
| Tadalafil | 0 | 0 | 1 (11) |
| Pulmonary function testing | |||
| FEV1 (% predicted) | 101 ± 13 | 83 ± 16† | 83 ± 25† |
| FVC (% predicted) | 101 ± 14 | 84 ± 15† | 80 ± 21† |
| FEV1 / FVC (% predicted) | 100 ± 8 | 98 ± 9 | 104 ± 9 |
| SaO2 (%) | 98 (97–98) | 97 (96–97)† | 96 (95–98) |
| Resting hemodynamics | |||
| Heart rate (bpm) | 72 ± 10 | 70 ± 10 | 74 ± 15 |
| Mean RAP (mmHg) | 2 ± 3 | 4 ± 3 | 4 ± 4 |
| Cardiac output (L/min) | 5.0 ± 0.9 | 5.6 ± 1.3 | 4.9 ± 0.9 |
| SV index (mL/m2) | 34 ± 7 | 38 ± 8 | 36 ± 6 |
| Cardiac index (L/min2) | 2.4 ± 0.6 | 2.6 ± 0.5 | 2.8 ± 0.6 |
| mPAP (mmHg) | 13 (12–15) | 18 (17–19)† | 35 (30–42)*,† |
| PAWP (mmHg) | 5 ± 3 | 8 ± 3 | 8 ± 3 |
| PVR (WU) | 1.4 (1.2–1.8) | 1.7 (1.4–2.4) | 6.1 (4.8–6.9)*,† |
| TPR (WU) | 2.5 (2.0–3.6) | 3.0 (2.9–3.8)† | 7.6 (6.9–8.6)*,† |
| PA compliance (mL/mmHg) | 6.9 (5.4–9.0) | 5.5 (4.7–6.8)† | 1.9 (1.5–3.2)† |
Data presented as n (%) or mean ± SD unless otherwise stated.
P < 0.005 when comparing ePH vs. PAH.
P < 0.005 compared to controls.
ePH, exercise pulmonary hypertension; PAH, resting pulmonary arterial hypertension; BMI, body mass index; CPET, cardio pulmonary exercise test; CCB, calcium channel blocker; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SaO2, peripheral oxygen saturation; bpm, beats per minute; SV, stroke volume; VD/VT, dead space ventilation; RAP, right atrial pressure; PAWP, pulmonary artery wedge pressure; mPAP, mean pulmonary arterial pressure; PA, pulmonary artery; PVR, pulmonary vascular resistance; TPR, total pulmonary resistance.
There was no difference in resting values of heart rate, SVI, CI, and right atrial pressure between ePH and PAH. At rest, the PVR and TPR were higher while the PA compliance was lower in PAH compared to ePH. PA compliance was decreased in ePH and PAH in relation to controls but there was no difference between the two.
Peak exercise hemodynamics, ventilation, and gas exchange parameters variables
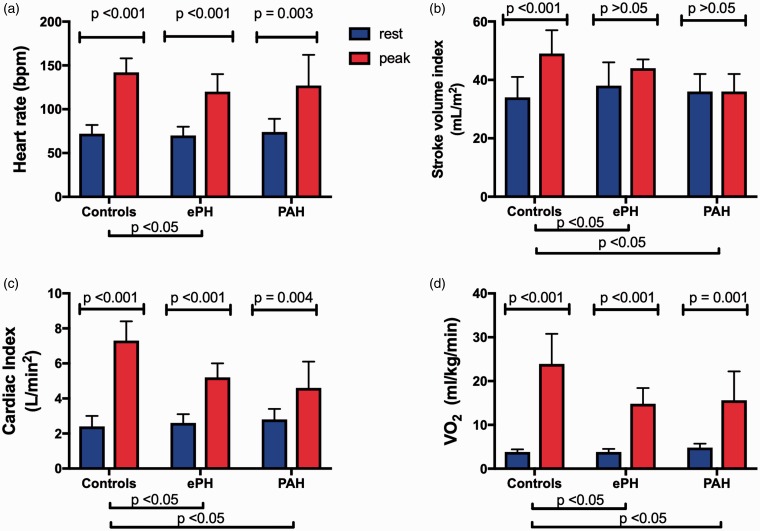
The maximum iCPET and peak exercise hemodynamic data are summarized in Table 2. The rest-to-peak exercise hemodynamic patterns for each study group are presented in Fig. 1.
Table 2.
Invasive cardiopulmonary exercise data.
| Controls (n = 20) | ePH (n = 11) | PAH (n = 9) | |
|---|---|---|---|
| Maximum invasive CPET data | |||
| Peak work (W) | 163 ± 60 | 92 ± 33* | 72 ± 48* |
| Peak VO2 (%predicted) | 104 ± 16 | 76 ± 15* | 72 ± 19* |
| Peak VO2 (mL/kg/min) | 24.0 ± 6.9 | 14.8 ± 3.6* | 15.6 ± 6.5* |
| VO2 at AT (%predicted) | 55 ± 14 | 46 ± 9 | 38 ± 10* |
| Peak heart rate (bpm) | 142 ± 16 | 120 ± 20* | 127 ± 35 |
| Peak heart rate (%predicted) | 90 ± 8 | 78 ± 10* | 81 ± 20 |
| Peak SaO2 (%) | 98 (97–98) | 96 (94–98)* | 91 (82–94)*,† |
| VE/VCO2 slope | 33 ± 7 | 32 ± 5 | 49 ± 7* |
| Peak DO2 (mL/min) | 3100 ± 790 | 2010 ± 497* | 1469 ± 611* |
| Peak DO2 (mL/kg/min) | 35.7 ± 7.0 | 22.4 ± 5.4* | 21.2 ± 9.3* |
| Peak CaO2 (mL/dL) | 20.4 ± 2.4 | 18.5 ± 1.8 | 17.9 ± 3.2 |
| Peak PA-aO2 (mmHg) | 16 ± 11 | 33 ± 14* | 58 ± 14*,† |
| Peak VD/VT | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1* |
| Peak Ca-vO2 | 14.6 ± 2.0 | 12.0 ± 1.4* | 11.8 ± 2.5* |
| Peak Ca-vO2 corrected for Hgb | 97.4 ± 9.9 | 86.0 ± 11.9* | 81.2 ± 14.0* |
| Peak exercise hemodynamics | |||
| Mean RAP (mmHg) | 6 ± 3 | 8 ± 4 | 9 ± 4* |
| Cardiac output (L/min) | 13.9 ± 3.2 | 10.8 ± 1.8* | 8.0 ± 2.5* |
| Stroke volume index (mL/m2) | 48.9 ± 8.1 | 43.7 ± 3.2* | 36.1 ± 5.6*,† |
| Cardiac index (L/min2) | 7.3 ± 1.1 | 5.2 ± 0.8* | 4.6 ± 1.5* |
| PAWP (mmHg) | 11 ± 4 | 13 ± 3 | 13 ± 4 |
| mPAP (mmHg) | 26 (20–29) | 35 (34–37)* | 59 (58–67)*,† |
| PVR (WU) | 0.9 (0.7–1.0) | 2.2 (1.6–2.4)* | 5.6 (5.3–8.0)*,† |
| TPR (WU) | 1.6 (1.3–2.0) | 3.3 (2.6–3.8)* | 7.0 (6.8–11.0)*,† |
| PA compliance (mL/mmHg) | 3.3 (3.1–4.3) | 2.8 (2.4–3.0)* | 1.3 (1.1–1.4)* |
Data presented as n (%) and mean ± standard deviation unless otherwise stated.
P < 0.005 compared to controls.
P < 0.005 when comparing ePH vs. PAH.
ePH, exercise pulmonary hypertension; PAH, resting pulmonary arterial hypertension; BMI, body mass index; CPET, cardio pulmonary exercise test; V02MAX, maximal oxygen consumption; AT, anaerobic threshold; bpm, beats per minute; SaO2, peripheral oxygen saturation; RAP, right atrial pressure; mPAP, mean pulmonary arterial pressure; Ve/VCO2, ventilator efficiency; CaO2, arterial oxygen content; DO2, oxygen delivery; PA-aO2, alveolar arterial oxygen difference; Ca-vO2, arterial-mixed venous oxygen content difference; O2, oxygen; PA, pulmonary arterial; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; TPR, total pulmonary resistance.
Fig. 1.
(a) Heart rate increased significantly in all three groups with ePH demonstrating impaired chronotropic reserve compared to controls and PAH. (b) Stroke volume index (SVI) significantly increased during exercise in controls only, with a blunted increase in SVI augmentation (rest-to-exercise response) seen in ePH and PAH. (c) Cardiac index (CI) increased significantly in all three groups, with a blunted increase in CI augmentation seen in ePH and PAH. (d) Oxygen consumption (VO2) (mL/kg/min) increased significantly in all three groups, with a blunted increase in VO2 (mL/kg/min) augmentation seen in PAH and ePH. Data are presented as mean ± standard deviation with the blue bars representing data at rest and red bars representing data at peak exercise.
ePH and PAH demonstrated similar reduction in exercise capacity as evident by their reduced peak VO2. This reduction in peak VO2 was driven by a reduced peak CI and reduced peak systemic oxygen extraction (Ca-vO2 difference) when compared to controls. With exercise, both PAH and ePH had similarly blunted SVI augmentation. ePH and PAH demonstrated similar degree of chronotropic incompetence. The impaired chronotropic reserve in ePH was found irrespective of B-adrenergic blocker use (Table S1). Although there was progressive widening of the peak alveolar-arterial gradient comparing ePH to PAH, peak exercise CaO2 was not different.
At peak exercise, PVR and TPR for ePH was intermediate between PAH and controls. ePH and PAH patients demonstrated similarly reduced peak PA compliance compared to controls (Table 2).
Resting and peak exercise effects on load-independent RV function
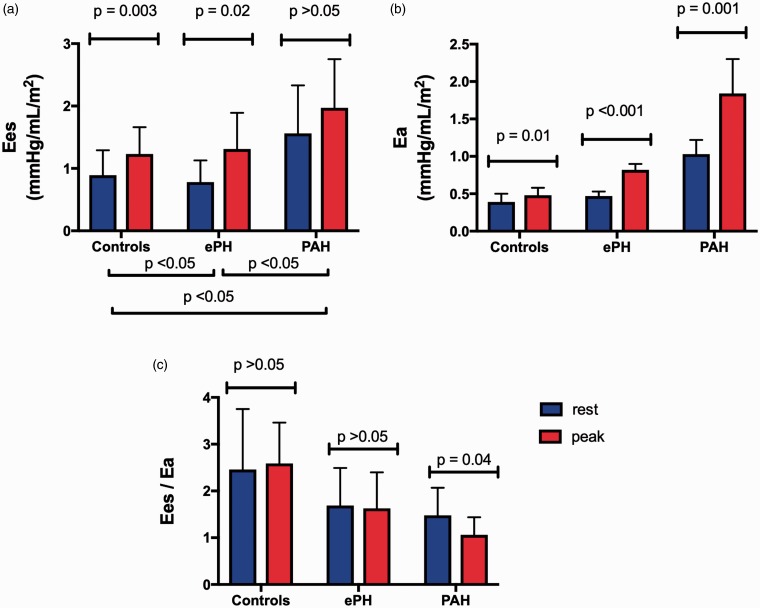
Resting and peak exercise load-independent RV function is presented in Table 3. The rest-to-peak exercise effects are presented in Fig. 2. Resting RV Ees and Ea were higher in PAH compared to ePH and controls. There was no difference in resting Ees and Ea between ePH and controls. Additionally, there was no difference in resting RV-PA coupling ratio among the groups.
Table 3.
Load-independent right ventricular function data.
| Controls (n = 20) | ePH (n = 11) | PAH (n = 9) | |
|---|---|---|---|
| Rest Ees (mmHg/mL/m2) | 0.89 ± 0.40 | 0.78 ± 0.35 | 1.56 ± 0.77*,† |
| Peak Ees (mmHg/mL/m2) | 1.23 ± 0.43 | 1.31 ± 0.58 | 1.97 ± 0.78*,† |
| Contractile reserve (mmHg/mL/m2) | 0.35 ± 0.46 | 0.53 ± 0.63 | 0.41 ± 0.87 |
| Rest Ea (mmHg/mL/m2) | 0.39 ± 0.11 | 0.47 ± 0.06 | 1.03 ± 0.19*,† |
| Peak Ea (mmHg/mL/m2) | 0.48 ± 0.10 | 0.82 ± 0.08* | 1.84 ± 0.46*,† |
| Rest Ees/Ea | 2.46 ± 1.29 | 1.69 ± 0.80 | 1.48 ± 0.59 |
| Peak Ees/Ea | 2.59 ± 0.87 | 1.63 ± 0.77* | 1.06 ± 0.38* |
Data presented as n (%) and mean ± standard deviation unless otherwise stated.
P < 0.005 compared to controls.
P < 0.005 when comparing ePH vs. PAH.
ePH, exercise pulmonary hypertension; PAH, resting pulmonary arterial hypertension; Ees, end-systolic elastance (right ventricular contractility); Ea, arterial elastance (right ventricular afterload); Ees/Ea, right ventricular–pulmonary arterial coupling.
Fig. 2.
(a) End-systolic elastance (Ees) significantly increased in controls and ePH but not in PAH suggesting an impaired exertional contractile reserve in PAH. (b) Arterial elastance (Ea) increased significantly in all three groups with the greatest increase seen in PAH. (c) There was significant decrease in RV-PA coupling (Ees/Ea) from rest-to-peak exercise in PAH. Data are presented as mean ± standard deviation with the blue bars representing data at rest and red bars representing data at peak exercise.
At peak exercise, Ea was significantly increased in PAH compared to ePH and controls. Peak exercise Ea was greater in ePH compared to controls. Additionally, peak exercise RV–PA coupling ratio was reduced in PAH and ePH compared to controls. Only PAH showed impaired exertional contractile reserve.
Independent predictors of RV–PA coupling and peak VO2
The univariate and bivariate analysis for predicting RV-PA coupling and peak VO2 (%predicted) in ePH and PAH are presented in the online supplementary material. Maximum heart rate (%predicted) and resting Ees were the only predictors of peak VO2 (%predicted) (ß coefficient 7.72 and −7.76, respectively) (Table S2). Resting PVR, peak Ees, and RV contractile reserve emerged as independent predictors of peak RV–PA coupling (ß coefficient –0.34, 0.32, and 0.38, respectively). On bivariate analysis, RV contractile reserve emerged as an independent predictor of peak RV–PA coupling (ß coefficient 0.30) in a model that also included resting PVR (Table S3).
Discussion
This is the first study to demonstrate dynamic RV–PA uncoupling in ePH and its adverse effect on simultaneously measured maximum exercise capacity. We showed that the depressed peak VO2 and SVI is evident even in ePH and is associated with dynamic rest-to-peak exercise RV–PA uncoupling. Patients with PAH exhibited a similar but more severe pathophysiology.
Dynamic RV–PA uncoupling during maximum incremental exercise
The principle finding of this study is that ePH patients are able to increase RV contractility in response to increasing RV afterload during exercise, but this is insufficient and results in deterioration in RV–PA coupling at peak exercise (Fig. 2). This is associated with a blunted SVI response and depressed peak VO2. We have previously shown that in patients with ePH and PAH, a plateau mPAP versus VO2 relationship is encountered, indicating a blunted CO response with exercise.6 Our current study suggests that dynamic RV–PA uncoupling during maximum incremental exercise likely accounts for the observed plateau mPAP versus VO2 relationship.
In PAH, both impaired RV contractile reserve and increasing RV afterload contributed to RV–PA uncoupling at peak exercise and a reduced peak SVI. In contrast, ePH patients were able to maintain a greater peak SVI when compared to PAH because of a preserved exertional contractile reserve. However, this increase in RV contractility was insufficient because in the face of increasing RV afterload, ePH patients were unable to improve their RV–PA coupling at peak exercise (Fig. 2 and Table 3). Impaired exertional contractile reserve in PAH 20 has been previously described and is attributed to downregulation and desensitization of B-adrenergic receptors from sympathetic overstimulation.21–25
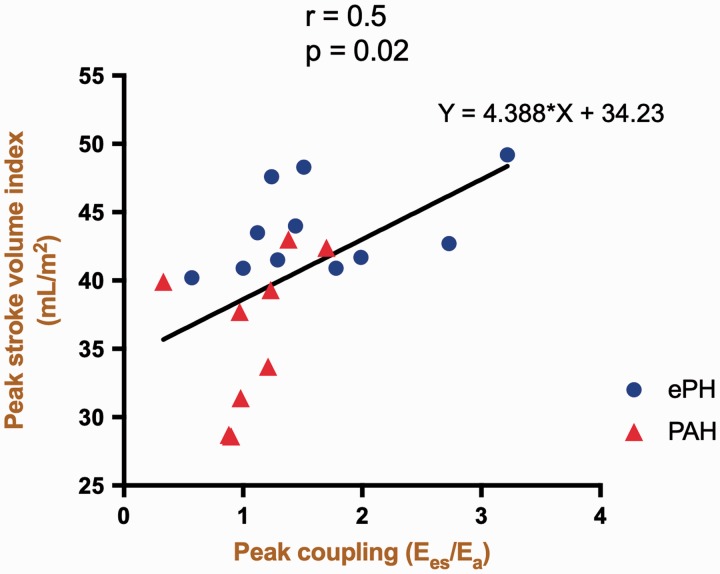
ePH and PAH demonstrated an increase in Ea during exercise compared to controls with the greatest increase seen in PAH (Fig. 2). In PAH, the ensuing impaired exertional contractile reserve (i.e. impaired rest-to-peak augmentation of RV contractility) in the face of dynamic RV afterload increase, culminated in deterioration of peak RV–PA coupling. Consequently, the SVI augmentation and therefore peak SVI was significantly reduced in PAH compared to ePH (Fig. 3). In ePH, the increase in RV contractility was insufficient because the peak RV–PA coupling ratio was reduced compared to controls. Consequently, ePH patients experienced reduced SVI augmentation during exercise with resulting blunted peak SVI.
Fig. 3.
Peak stroke volume index (SVI) is associated with peak right ventricular–pulmonary arterial (RV–PA) uncoupling. A scatter plot of change in peak SVI vs. peak RV–PA coupling is depicted. Peak RV–PA coupling is a strong determinant of SVI at peak exercise.
In the current study, the resting RV–PA coupling ratio in our controls was greater than that reported by Spruijt et al. using similar method to determine RV Ees (Pmax – mPAP/SVI).20 We found that while our resting Pmax value approximates that of Spruijt et al., our Ees and therefore RV–PA coupling (Ees/Ea) were greater at rest. This difference is probably related to the different CPET methodology (upright versus supine cycle ergometer testing). While supine, venous return and therefore SV is higher at rest compared to upright position. The transition from a supine to upright position decreases left ventricular end-diastolic volume and pressure as well as SV.26 In our study, all participants underwent upright exercise testing. Consequently, reduced RV preload resulted in a lower RV SV. This resulted in greater calculated Ees and therefore Ees/Ea at rest.
Similar to Spruijt et al.,20 but different from the study by Hsu et al.,27 our PAH cohort demonstrated impaired exertional contractile reserve. The latter study also demonstrated preserved RV–PA coupling in idiopathic PAH during supine exercise at 25 W workload using multi-beat pressure volume analysis. As pointed out by the authors, the single beat method may underestimate Ees in PH or with dynamic changes in RV function. However, compared to the study by Hsu et al., our PAH patients were subjected to maximum upright exercise. Furthermore, for the first time, we linked impaired RV contractile reserve along with dynamic RV–PA uncoupling during maximum upright exercise in PAH to a blunted CI and SVI response with consequent depressed maximum exercise capacity. Hence, we suspect that the greater workload imposed by our CPET protocol is more likely to elicit dynamic RV dysfunction in PAH.
Mechanism of reduced exercise capacity in ePH and PAH
In the absence of a pulmonary mechanical limit, reduced peak VO2 is a function of a blunted CO response and/or impaired systemic oxygen extraction (abnormal Ca-vO2 difference). In both ePH and PAH, the depressed peak VO2 was driven by a reduced cardiac index and impaired systemic oxygen extraction at peak exercise.
In PAH and ePH, reduced peak CI was the result of decreased peak SVI and chronotropic incompetence (Table 2). The blunted CI augmentation (rest-to-peak) in PAH is associated primarily with a depressed SVI increase during exercise. In ePH, the blunted CI augmentation is linked to a blunted increase in SVI and impaired chronotropic reserve during exercise (Fig. 1), irrespective of beta-blocker use.
ePH and PAH had similarly impaired systemic oxygen extraction as demonstrated by the blunted Ca-vO2 difference at peak exercise along with preserved peak exercise CaO2 (Table 2). This is consistent with prior reports implicating impaired peak systemic oxygen extraction as a cause of aerobic limitation in ePH 28 and PAH.29 Impaired systemic oxygen extraction in patients with pulmonary vascular disease has been attributed to systemic arteriolar endothelial dysfunction, intrinsic mitochondrial dysfunction, and capillary rarefaction.30–33
Study limitations
Our study sample was small despite or institution performing > 300 iCPETs/year. This is because we do not routinely subject patients with PAH to maximum incremental exercise testing and also because not all RV tracings were interpretable during upright exercise.
Our normal controls were derived from iCPET evaluation for unexplained exertional dyspnea and therefore the controls may not be representative of a completely healthy population. However, the controls were selected based on a preserved peak exercise capacity defined by a normal cardiac limit to exercise (peak VO2 and peak CO ≥ 80% predicted). Therefore, they represent a studied population with a normal physiologic response to exercise and reflect “symptomatic normal” individuals. Exercise hemodynamics varies considerably according to age.34,35 Hence, for the ePH definition, we used age-related upper limits of normal for mPAP and PVR. We previously demonstrated that that there is a 93% concordance between the ePH definition used in the current study and the alternative proposed criteria that is defined by a peak mPAP > 30 mmHg and peak TPR > 3 WU irrespective of age.28
The gold standard to determine RV–PA coupling is by multi-beat RV pressure–volume analysis using a conductance catheter.27 However, this method requires repeated pre-load alteration typically with Valsalva maneuver and is therefore not feasible in patients undergoing maximum upright incremental exercise testing.
The single beat method was initially developed for the left ventricle19 and has since been applied in several clinical studies in PAH.36,37 There are number of assumptions that must be considered when using the single beat method, including the use of mPAP as a surrogate for the RV end-systolic pressure.20,38 On multi-beat pressure–volume analysis in patients with resting PH, the RV end-systolic pressure rather than mPAP most closely approximates the RV end-systolic elastance.39 However, we have previously shown that in healthy adults there is a flow-related systolic pressure gradient that develops during upright incremental exercise leading to a significant increase in RV systolic pressure when compared to the PA systolic pressure.40 Using the RV systolic pressure during exercise as a surrogate for the RV end-systolic pressure will lead to a spuriously low Ees among our controls. We therefore used the mPAP as a surrogate for the RV end-systolic pressure as was previously described to determine dynamic RV–PA coupling using the single beat methodology.20
Approximately 27% of ePH patients were on beta-adrenergic receptor blocker therapy. We speculate that it was initiated as empiric therapy for unexplained dyspnea and could have influenced the results of this study. However, all of the load independent parameters of RV function, including the RV contractile reserve and peak RV–PA coupling were not different among patients who received beta-adrenergic receptor blocker therapy and those who did not (Table S1). We recognize that other disease states such as mitral valve disease and heart failure with preserved ejection fraction (HFpEF) can cause dynamic RV–PA uncoupling during exercise. We therefore specifically excluded patients with HFpEF and mitral valve disease from the current study.
Conclusions
Dynamic rest-to-peak exercise RV–PA uncoupling depresses the normal SV response and is associated with reduced maximum exercise capacity in pre-capillary PH. Unlike patients with advanced disease, ePH patients are able to increase RV contractility in response to increasing RV afterload during exercise, but this is insufficient and results in deterioration in RV–PA coupling at peak exercise. These findings suggest that even in early stage disease, RV function is already compromised and supports the potential role of dynamic RV–PA coupling as an index for early detection of PH.
Supplemental Material
Supplemental Material for Dynamic right ventricular–pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension by Inderjit Singh, Farbod N. Rahaghi, Robert Naeije, Rudolf K.F. Oliveira, Rebecca R. Vanderpool, Aaron B. Waxman and David M. Systrom in Pulmonary Circulation
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006; 174: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 3.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 4.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira RK, Urbina MF, Maran BA, et al. Functional impact of exercise pulmonary hypertension with borderline resting pulmonary arterial pressure. Pulm Circ 2017; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolle JJ, Waxman AB, Van Horn TL, et al. Exercise-induced pulmonary arterial hypertension. Circulation 2008; 118: 2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira RK, Waxman AB, Agarwal M, et al. Pulmonary haemodynamics during recovery from maximum incremental cycling exercise. Eur Respir J 2016; 48: 158–167. [DOI] [PubMed] [Google Scholar]
- 8.Steen V, Chou M, Shanmugam V, et al. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008; 134: 146–151. [DOI] [PubMed] [Google Scholar]
- 9.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. [DOI] [PubMed] [Google Scholar]
- 10.Saggar R, Khanna D, Furst DE, et al. Exercise-induced pulmonary hypertension associated with systemic sclerosis: four distinct entities. Arthritis Rheum 2010; 62: 3741–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamm A, Saxer S, Lichtblau M, et al. Exercise pulmonary haemodynamics predict outcome in patients with systemic sclerosis. Eur Respir J 2016; 48: 1658–1667. [DOI] [PubMed] [Google Scholar]
- 12.Fowler RM, Maiorana AJ, Jenkins SC, et al. Implications of exercise-induced pulmonary arterial hypertension. Med Sci Sports Exerc 2011; 43: 983–989. [DOI] [PubMed] [Google Scholar]
- 13.Segrera SA, Lawler L, Opotowsky AR, et al. Open label study of ambrisentan in patients with exercise pulmonary hypertension. Pulm Circ 2017; 7: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maron BA, Cockrill BA, Waxman AB, et al. The invasive cardiopulmonary exercise test. Circulation 2013; 127: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira RK, Agarwal M, Tracy JA, et al. Age-related upper limits of normal for maximum upright exercise pulmonary haemodynamics. Eur Respir J 2016; 47: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 17.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–116. [DOI] [PubMed] [Google Scholar]
- 18.Berry NC, Manyoo A, Oldham WM, et al. Protocol for exercise hemodynamic assessment: performing an invasive cardiopulmonary exercise test in clinical practice. Pulm Circ 2015; 5: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunagawa K, Yamada A, Senda Y, et al. Estimation of the hydromotive source pressure from ejecting beats of the left ventricle. IEEE Trans Biomed Eng 1980; 27: 299–305. [DOI] [PubMed] [Google Scholar]
- 20.Spruijt OA, de Man FS, Groepenhoff H, et al. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 2015; 191: 1050–1057. [DOI] [PubMed] [Google Scholar]
- 21.Piao L, Fang YH, Parikh KS, et al. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation 2012; 126: 2859–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristow MR, Minobe W, Rasmussen R, et al. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 1992; 89: 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014; 115: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nootens M, Kaufmann E, Rector T, et al. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol 1995; 26: 1581–1585. [DOI] [PubMed] [Google Scholar]
- 25.Velez-Roa S, Ciarka A, Najem B, et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004; 110: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 26.Poliner LR, Dehmer GJ, Lewis SE, et al. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation 1980; 62: 528–534. [DOI] [PubMed] [Google Scholar]
- 27.Hsu S, Houston BA, Tampakakis E, et al. Right Ventricular Functional Reserve in Pulmonary Arterial Hypertension. Circulation 2016; 133: 2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira RKF, Faria-Urbina M, Maron BA, et al. Functional impact of exercise pulmonary hypertension in patients with borderline resting pulmonary arterial pressure. Pulm Circ 2017; 7: 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolle J, Waxman A, Systrom D. Impaired systemic oxygen extraction at maximum exercise in pulmonary hypertension. Med Sci Sports Exerc 2008; 40: 3–8. [DOI] [PubMed] [Google Scholar]
- 30.Tran DL, Lau EMT, Celermajer DS, et al. Pathophysiology of exercise intolerance in pulmonary arterial hypertension. Respirology 2018; 23: 148–159. [DOI] [PubMed] [Google Scholar]
- 31.Potus F, Malenfant S, Graydon C, et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med 2014; 190: 318–328. [DOI] [PubMed] [Google Scholar]
- 32.Faria-Urbina M, Oliveira RKF, Segrera SA, et al. Impaired systemic oxygen extraction in treated exercise pulmonary hypertension: a new engine in an old car? Pulm Circ 2018; 8: 2045893218755325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batt J, Ahmed SS, Correa J, et al. Skeletal muscle dysfunction in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2014; 50: 74–86. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs G, Berghold A, Scheidl S, et al. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. [DOI] [PubMed] [Google Scholar]
- 35.Wolsk E, Bakkestrom R, Thomsen JH, et al. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail 2017; 5: 337–346. [DOI] [PubMed] [Google Scholar]
- 36.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017; 69: 236–243. [DOI] [PubMed] [Google Scholar]
- 37.Tabima DM, Philip JL, Chesler NC. Right ventricular-pulmonary vascular interactions. Physiology (Bethesda) 2017; 32: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemla D, Hebert JL, Coirault C, et al. Matching dicrotic notch and mean pulmonary artery pressures: implications for effective arterial elastance. Am J Physiol 1996; 271: H1287–1295. [DOI] [PubMed] [Google Scholar]
- 39.Metkus TS, Mullin CJ, Grandin EW, et al. Heart rate dependence of the pulmonary resistance x compliance (RC) time and impact on right ventricular load. PLoS One 2016; 11: e0166463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright SP, Opotowsky AR, Buchan TA, et al. Flow-related right ventricular - pulmonary arterial pressure gradients during exercise. Cardiovasc Res 2019; 115: 222–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Dynamic right ventricular–pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension by Inderjit Singh, Farbod N. Rahaghi, Robert Naeije, Rudolf K.F. Oliveira, Rebecca R. Vanderpool, Aaron B. Waxman and David M. Systrom in Pulmonary Circulation