Short abstract
Vincristine, a vinca alkaloid, is widely used in many hematologic disorders and pediatric cancers, and acts by binding to the tubulin protein, to inhibit effective cell division. Vincristine-induced anemia has been observed, but its mechanism is not well understood. We describe a case involving serious vincristine-induced anemia in a patient with congenital spherocytosis and provide the explanation to the underlying mechanism. This report demonstrates that vincristine administration to patients with a red cell disorder may require additional clinical interventions to compensate for the vincristine-induced anemia.
Impact statement
Therapy with vincristine (VCR), a vinca alkaloid, is widely used in many hematologic disorders and pediatric cancers, and acts by binding to the tubulin protein, to inhibit effective cell division. Our paper indicates that treatment of patients with a red cell disorder may require additional clinical interventions to compensate for the VCR-induced anemia. Careful evaluation of other hematologic disorders is important in using a well-known oncology treatment. Whereas extreme concentrations of VCR were shown to alter red cell viability, data did not show negative effects in patients treated. Our data show that under conditions of increased hematopoiesis, hemoglobin in the circulation drops rapidly requiring transfusion. VCR administration to patients with a red cell disorder may require additional clinical interventions to compensate for the VCR-induced anemia.
Keywords: Vincristine, spherocytosis, anemia, oncology, transfusion
Introduction
Vincristine (VCR) is a vinca alkaloid widely used in many hematologic disorders and pediatric cancers, including acute lymphoblastic leukemia and lymphoma, Hodgkin lymphoma, Wilms’ tumor, sarcomas, and neuroblastoma. It works by binding to the tubulin protein, thereby inhibiting mitosis and preventing effective cell division.1 Common side effects are peripheral neuropathy and constipation. In contrast to most immunosuppressive or cytotoxic agents, hematopoietic toxicity is uncommon. While VCR-induced anemia has been observed in the clinical setting, its mechanism has not been well studied. Vinca alkaloids can alter the red cell membrane and induce changes similar to congenital spherocytosis. In fact, in the early studies of congenital spherocytosis, vinca alkaloid-induced membrane changes were used as a model to understand congenital spherocytosis.2,3 Anecdotal cases suggest VCR may increase hemolysis in congenital spherocytosis patients undergoing chemotherapy.4 Here, we describe a case involving serious VCR-induced anemia in a patient with congenital spherocytosis and an explanation regarding the mechanism.
Materials and methods
The patient was born at 39 weeks gestation by normal spontaneous vaginal delivery. At birth, a complete blood count was performed and she was noted to have multiple spherocytes on her peripheral smear. There was no ABO-RH incompatibility setup and the direct and indirect Coombs test was negative. The diagnosis of hereditary spherocytosis (HS) was suspected based on a strong family history, and this was confirmed on osmotic fragility testing and Eosin-5-maleimide binding test for band 3 protein. In the newborn nursery, she developed hyperbilirubinemia, requiring phototherapy for a total of seven days. At the time of discharge, she had a hemoglobin (Hb) count of 12 g/dL, with a reticulocyte count of 3.4%. She was followed closely during the newborn period and required two blood transfusions, the first at 3.5 weeks of age and the second at 7 weeks of age, when her Hb had dropped to 6.8 and 7.1 g/dL, respectively.
Thereafter, she did quite well clinically without any episodes of acute hemolytic or aplastic events. She did not require post neonatal transfusion therapy. Her serial CBCs demonstrated a compensated hemolytic process. Following the newborn period, her Hb averaged around 10 g/dL, with a chronic reticulocytosis of 6–8%. A mildly elevated serum bilirubin (average 1.7 mg/dL) was also consistent with ongoing red blood cell turnover, and she was supplemented with daily folic acid. She demonstrated normal growth with stable mild splenomegaly noted at 1–2 cm below the left costal margin. Unlike her brothers and father, she did not require splenectomy.
At six years of age, the patient presented to her pediatrician with facial swelling and prominent venous collaterals over the upper chest. She was referred to our center where a chest X-ray revealed a large anterior mediastinal mass; a computed tomography showed encasement of the left innominate and right brachiocephalic veins. Also noted were multiple calcified gallstones and moderate splenomegaly, consistent with the patient’s history of HS. A suprasternal biopsy revealed T-lymphoblastic lymphoma; bone marrow aspirates were negative for tumor involvement. At the time of diagnosis, the patient had a stable Hb (10 g/dL) and a prominent reticulocytosis (18%).
She commenced chemotherapy on the Children’s Oncology Group protocol AALL0434 and four days after her first doses of chemotherapy, she required a packed red blood cell (PRBC) transfusion for a Hb count of 5.6 g/dL. Other blood cell lines at this time were within normal limits, with a white blood cell count of 9.7k/mm3 and platelets of 283k/mm3; total bilirubin was 2.1 mg/dL and the reticulocyte count four days prior had been 18%. Over the ensuing months, she received required transfusions every couple of weeks as she progressed through the standard phases of therapy. By the start of maintenance eight months later, she had received 14 PRBC transfusions, far more than the average patient receiving this therapy. The intermittent drops in hemoglobin did not seem to correlate with any one particular agent, and her clinical and imaging studies demonstrated a stable, mildly enlarged spleen.
A clear pattern of VCR-induced anemia began to emerge once the patient started the maintenance phase of therapy. She was noted to have Hb values in the range of 8–9 g/dL at each monthly clinic visit, when she received intravenous VCR, commenced a five-day steroid pulse, and received intermittent intrathecal methotrexate. By 3–4 days later, her Hb dropped to the point of requiring transfusion. Her other blood cell lines were minimally affected; total white blood cell and platelet count did not change significantly during the four days after VCR treatment or thereafter.
Blood was collected in Ethylenediaminetetraacetic acid (EDTA) or Acid citrate dextrose (ACD) at different time points during treatment. Routine Complete Blood Count (CBC) and blood chemistry measurements were performed in the clinical laboratory at Benioff Children’s Hospital Oakland (BCHO). Analysis with the Advia 120 hematology analyzer (Siemens, Laguna Hills, CA) and assessment of the osmotic deformability profile5,6 were conducted on selected samples at the red cell laboratory at Children's Hospital Oakland Research Institute (CHORI).
Results
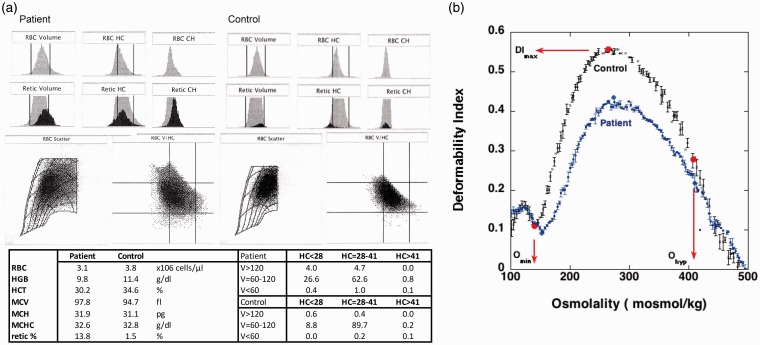
Figure 1 shows a typical hematology assessment on the day before VCR treatment. The decreased RBC count and Hb level, and increased reticulocyte count are typical for a red cell disorder with a decreased RBC survival. The histograms of volume and RBC hemoglobin concentration indicate a heterogeneous RBC and reticulocyte population (Figure 1(a)). The RBC matrix shows a shift in the density profile of the RBC population as compared to normal.
Figure 1.
(a) Routine hematology analysis of the patient (top) and typical density profile of a patient sample (right) and control (left) before VCN treatment. The distribution of volume/hemoglobin concentration (V/HC) in both RBC and reticulocytes is strikingly different between patient and control, indicating a highly diverse RBC population. The percentage of cells in each of the nine RBC volume and hemoglobin concentration matrix sections is indicated. (b) Osmotic deformability profile shows a typical shift for hereditary spherocytosis with a decrease in maximum deformability (DImax) and increase in the minimum at low osmolality (Omin) and a slight decreased deformability in high osmolarity (Ohyp). (A color version of this figure is available in the online journal.)
The osmotic deformability profile shown in Figure 1(b) shows a typical shift for HS with a decrease in maximum deformability (DImax), an increase in the minimum deformability at low osmolality (Omin), and a slight decreased deformability at high osmolality (Ohyp). Labeling with Eosin-5-maleimide and microscopic evaluation as well as review of the family history (not shown) confirmed HS.
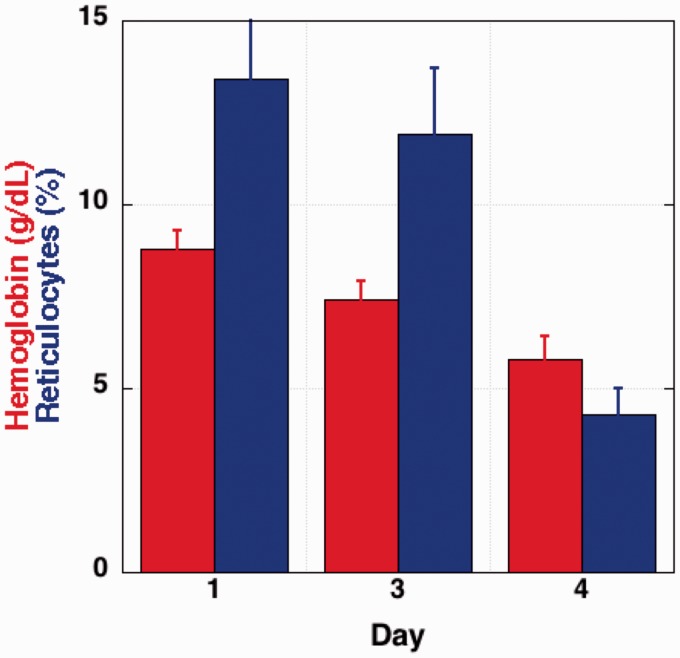
Figure 2 shows data for Hb and reticulocyte count collected during the one-year maintenance phase of therapy in which the patient received monthly intravenous VCR. The average data at the day of treatment (day 1) were similar to her baseline levels as shown for a typical sample in Figure 1. On day 4 after treatment, Hb had decreased from 8.8 ± 0.5 to 5.8 ± 0.6 g/dL and the retic count decreased from 13.4 ± 4 to 4.3 ± 0.7%. Transfusion at day 4 corrected Hb, and the reticulocyte count as well as Hb returned to maintenance therapy baseline levels.
Figure 2.
Hemoglobin and reticulocyte counts compiled during a one-year treatment with VCN. The average data at the day of treatment (day 1) as well as days after treatment are indicated. (A color version of this figure is available in the online journal.)
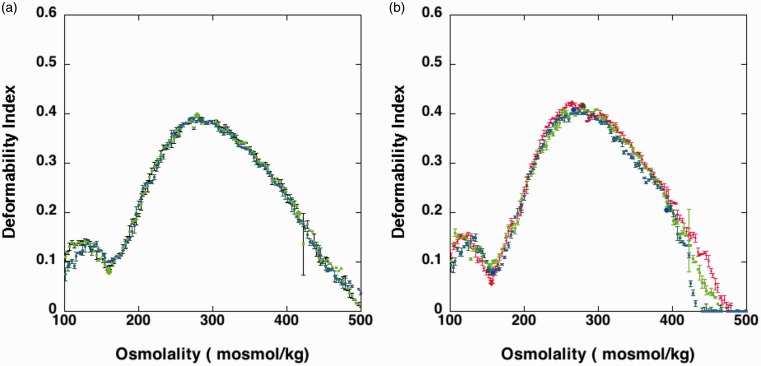
As vinblastine has been noted to denature spectrin in red cell membranes and induce spherocytosis,2,3 we hypothesized that VCR, another vinca alkaloid, could have a similar effect on RBCs. The level of VCR in her bloodstream at the time of dosing was estimated to be around 0.9 µg/mL, which is the equivalent of a final concentration of approximately 1 µM. Vinblastine was reported to increase RBC rigidity significantly at 10 µmoles/ml of RBC.3 The concentration relative to RBC of this reported incubation in vitro is roughly 2000 times the concentration in the circulation of the patient with a hematocrit of 20%. Blood samples taken before and 1 h after VCR treatment were analyzed by ektacytometry. Figure 3(a) shows that the curves obtained were virtually identical, indicating that the RBC deformability was not affected in the 1 h after dosing. Neither total blood count nor RBC density changed in this one-hour period immediately after treatment (not shown). A direct comparison with published data is hampered by the fact that the concentration in the circulation is a rough estimate and peripheral blood levels of VCR are likely to drop rapidly after dosing. To confirm the assessment that a change in RBC rigidity was unlikely the reason for the drop in Hb, blood collected from the patient was treated with 100 µM VCR for 1 hour at 37°C. The condition of incubation was similar as reported,3 be it with a lower concentration of the compound per RBC. We argued that the published conditions would require the presence of approximately 20 mM VCR in whole blood, a level that would be orders of magnitude higher than can be expected in the clinic. Our in vitro incubation, in the presence of 100 µM VCR was also many folds higher than that seen in vivo. However, even under these relatively extreme conditions, the osmotic deformability pattern, a sensitive assessment of membrane skeletal alterations, was not affected (not shown).
Figure 3.
Osmotic deformability profile of blood samples collected on two different treatment cycles, three months separated. (a) Blood collected immediately before VCN injection (λ) and 1 h after VCN treatment (ν). (b) Blood collected immediately before VCN injection (λ), 1 h after VCN treatment (ν) and four days after VCN treatment (υ). (A color version of this figure is available in the online journal.)
As indicated in Figure 1, Hb and RBC count dropped dramatically in the four days after treatment. Assessment with the Advia 120 analyzer confirmed the drop in Hb, RBC, and reticulocyte count as obtained from the routine clinical lab data. Although Hb and the number of RBCs decreased significantly, RBC characteristics (MCV, MCHC) on day 4 after treatment were not significantly different from day 1, as shown in Figure 1. Markers of hemolysis (total bilirubin, LDH) were not significantly higher at the RBC nadir following VCR treatment. Blood analyzed by ektacytometry at day 4 after treatment, and before transfusion, did not show a shift (Figure 3(b)).
Together, these data indicate that rather than damage of peripheral blood RBCs, a dysregulation of erythropoiesis likely led to the rapidly developing anemia after treatment. The rapid drop in marrow output seems only temporary. After transfusion at day 4, the levels stabilize and reticulocytes reversed to baseline levels after a month, before the next VCR treatment. Figure 3(a) and (b) shows the ektacytometric deformability profile two months apart and indicate that at different times of treatment the deformability profile was virtually identical. Several months after treatment, the hematologic parameters returned to those typical for HS.
Discussion
HS is a common inherited hemolytic anemia characterized by spheroidal, osmotically fragile, RBCs with a shortened life span.7,8 The severity of the disease varies from clinically mild to severe and is based on altered interactions between the RBC membrane skeleton and the membrane bilayer. Most patients are moderately affected, with incompletely compensated hemolysis and, as seen in this patient, a mild-to-moderate anemia. Hemolytic crises, usually triggered by viral infections, may lead to the need for RBC transfusions, and patients may benefit from splenectomy.9 In addition to transfusion, steroid treatment10,11 or erythropoietin stimulating agents12 have been used in some cases to modulate the hemolytic events in these patients.
The alkaloid anticancer drugs vinblastine and VCR exert their effects by binding to tubulin,1 thus inhibiting microtubule formation in the mitotic spindle and resulting in an arrest of dividing cells at the metaphase stage. Vinblastine has been shown to induce alterations in the RBC that lead to increased cell rigidity and a spherocytic morphology.2,3 Hence, it was logical to assume that the hemolytic crisis induced in this HS patient might have been related to an additional RBC membrane insult in addition to the alteration that led to spherocytosis. This in turn would lead to an increased filtration of the more rigid cells in the spleen. Our data do not support this hypothesis. We cannot conclude that no additional cells are removed from the circulation during the four days after treatment, but there was no evidence of hypersplenism or increased hemolysis. The RBC counts and characteristics were not affected. Moreover, although the total RBC mass decreased after a few days, the remaining RBCs were virtually identical to the cells on the day of treatment. When VCR was added to blood from the patient in vitro, at concentrations approximately 100-fold greater than those experienced by RBC in vivo, no change in deformability was observed and other RBC characteristics remained the same. It cannot be excluded that vinblastine acts differently from VCR, and it should be taken into account that the conditions used in the published reports are more extreme.2,3 RBC rigidity was measured by the resistance of RBC to filtrate through artificial filters2,3 rather than the measure of deformability with ektacytometry.
Our data show that a negative effect of VCR on erythropoiesis is likely the major cause of the acute anemia. It is likely that VCR, in addition to affecting its primary treatment target, caused a temporary arrest of the erythroid marrow precursors. VCR has been observed to decrease erythropoiesis13 and lower reticulocyte count in chemotherapy protocols.14,15 In vivo murine and human studies demonstrate VCR can induce a marked metaphase arrest in early erythroblasts without concomitant changes in the myeloid and megakaryocytic precursors. The laboratory observations in this patient mimic the erythropoietic changes observed in the murine model. In animal studies, VCR-induced a rapid metaphase arrest reaching a nadir in two to five days.13,16,17
This patient received a common treatment protocol in which the maintenance phase of therapy does not typically cause severe anemia in pediatric patients. This is likely explained by the fact that a temporary reduction in erythropoiesis in patients with a normal RBC survival and a normal low reticulocyte count is unlikely to be noticed. However, in a patient with a drastically decreased RBC survival and highly increased erythropoiesis, a disruption in RBC generation will be apparent. The drop in reticulocytes will disrupt the compensation for the reduced RBC lifespan, and the already low Hb levels will drop in a few days to levels that require transfusion. This may not be limited to a membrane disorder such as described here. Patients with hemoglobin disorders such as thalassemia and sickle cell anemia have a high rate of erythropoiesis, increased reticulocytosis, and reduced RBC lifespan. We speculate that VCR therapy may also have an unwanted impact in these cases.
In conclusion, our report demonstrates that VCR administration to patients with an RBC disorder may require additional clinical interventions to compensate for the VCR-induced anemia.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; JM and EV provided patient care and treatment, SL and FAK processed the laboratory measurements, FK and JM analyzed the data and wrote the manuscript.
Consent for publication
The study was undertaken with the patient’s consent.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4:253–65 [DOI] [PubMed] [Google Scholar]
- 2.Jacob H, Amsden T, White J. Membrane microfilaments of erythrocytes: alteration in intact cells reproduces the hereditary spherocytosis syndrome (vinblastine-colchicine-strychnine-electron microscopy-cell rigidity). Proc Natl Acad Sci UsaU S A 1972; 69:471–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob HS. Effect of drugs on red cell membranes: insights into normal red cell shape. J Clin Pathol Suppl (R Coll Pathol) 1975; 9:40–5 [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Climent JA, Lopez-Andreu JA, Ferris-Tortajada J, Perez-Sirvent ML, Castel-Sanchez V. Acute lymphoblastic leukaemia in a child with hereditary spherocytosis. Eur J Pediatr 1995; 154:7534 [DOI] [PubMed] [Google Scholar]
- 5.Clark MR, Mohandas N, Shohet SB. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood 1983; 61:899–910 [PubMed] [Google Scholar]
- 6.Kuypers FA, Scott MD, Schott MA, Lubin B, Chiu DT. Use of ektacytometry to determine red cell susceptibility to oxidative stress. J Lab Clin Med 1990; 116:535–45 [PubMed] [Google Scholar]
- 7.Christensen RD, Henry E. Hereditary spherocytosis in neonates with hyperbilirubinemia. Pediatrics 2010; 125:120–5 [DOI] [PubMed] [Google Scholar]
- 8.Grace RF, Lux SE. Disorders of the red cell membrane In: Nathan DG, Orkin SH, Look T, Ginsburg MD. (eds) Nathan and Oski’s hematology of infancy and childhood. 7th ed Philadelphia: WB Saunders, 2009. p. 659 [Google Scholar]
- 9.Schilling RF. Risks and benefits of splenectomy versus no splenectomy for hereditary spherocytosis–a personal view. Br J Haematol 2009; 145:728–32 [DOI] [PubMed] [Google Scholar]
- 10.Ballin A, Waisbourd-Zinman O, Saab H, Yacobovich J, Zoldan M, Barzilai-Birenbaum S, Yaniv I, Tamary H. Steroid therapy may be effective in augmenting hemoglobin levels during hemolytic crises in children with hereditary spherocytosis. Pediatr Blood Cancer 2011; 57:303–5 [DOI] [PubMed] [Google Scholar]
- 11.Coleman DH, Finch CA. Effect of adrenal steroids in hereditary spherocytic anemia. J Lab Clin Med 1956; 47:602–10 [PubMed] [Google Scholar]
- 12.Morrison JF, Neufeld EJ, Grace RF. The use of erythropoietin-stimulating agents versus supportive care in newborns with hereditary spherocytosis: a single centre’s experience. Eur J Haematol 2014; 93:161–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse BS, Stohlman F., Jr. Regulation of erythropoiesis. 18. The effect of vincristine and erythropoietin on bone marrow. J Clin Invest 1966; 45:1241–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiktor-Jedrzejczak W, Szczylik C, Siekierzynski M, Rychowiecka E. Critical evaluation of the usefulness of different reticulocyte parameters in monitoring the erythropoiesis reaction to cancer chemotherapy. Arch Geschwulstforsch 1982; 52:303–6 [PubMed] [Google Scholar]
- 15.Haioun C, Salar A, Pettengell R, Johnsen HE, Duehrsen U, Rossi FG, Verhoef G, Schwenkglenks M, Jaeger U, Hamilton L, Pujol B, Lugtenburg PJ. Anemia and erythropoiesis-stimulating agent administration in patients with non-Hodgkin lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone +/– rituximab chemotherapy: results from an observational study. Leuk Lymphoma 2011; 52:796–803 [DOI] [PubMed] [Google Scholar]
- 16.Marmont AM. Selective metaphasic arrest of erythroblasts by vincristine in patients receiving high doses of recombinant human erythropoietin for myelosuppressive anemia. Leukemia 1992; 6:167–70 [PubMed] [Google Scholar]
- 17.Frei E, 3rd, Whang J, Scoggins RB, Vanscott EJ, Rall DP, Ben M. The stathmokinetic effect of vincristine. Cancer Res 1964; 24:1918–25 [PubMed] [Google Scholar]