Short abstract
Infants born with neonatal abstinence syndrome increased 5-fold between 2000 and 2012. Prenatal exposure to opioids has been linked to altered brain development, congenital heart defects, neural tube defects, and clubfoot. In the study presented here, placental transfer rate of six opioids; morphine, heroin, fentanyl, methadone, oxycodone, and naloxone was compared in vitro using a human trophoblast cell monolayer model, BeWo b30. The rate and concentration of opioid translocation were investigated individually and in mixtures. The opioid transfer was quantified at 5, 15, 30, 60, and 120 min, using target liquid chromatography-mass spectrometry. The transfer was lowest for heroin (<1.2% of administered dose at 120 min; the permeability across the cells [P]: 0.21 × 10−5 cm/s) and highest for oxycodone (13.2% of administered dose at 120 min; P: 2.46 × 10−5 cm/s). As oxycodone is a preferred drug for both short- and long-term pain treatment, more knowledge is needed to understand the potential adverse impact on fetal development, growth, and well-being. Opioid exposure is likely to happen as mixtures, and we therefore investigated the transfer of selected mixtures. Permeability was significantly decreased for heroin when administered together with fentanyl. This study demonstrates that the transfer rate between individual opioids varies significantly and that some mixtures may impact the transfer of some individual opioids like heroin.
Impact statement
Gaining knowledge about opioid exposure and placental transfer of opioids is an important part of providing clinicians with the necessary information for therapeutic strategies during pregnancy in the rising opioid epidemic. Opioids exposure often includes a mixture rather than an exposure to an individual opioid. We investigated placental trophoblast permeability to opioids in vitro for six individual opioids and selected mixtures. The rate and concentration of opioid translocation across placental trophoblast monolayer varied considerably between the six investigated opioids. Oxycodone had the highest level of translocation. We also found that the transfer of heroin decreased when administered together with fentanyl, while no change was observed for fentanyl transfer in the mixture. This suggests that opioid mixtures may decrease translocation of some opioids while others are unaffected.
Keywords: Opioid transfer, trophoblasts, BeWo b30, opioid mixtures, transfer kinetics, in vitro
Introduction
The opioid epidemic in the U.S. is accelerating. In 2012, an estimated 21,732 infants were born with neonatal abstinence syndrome due to maternal opioid use, a 5-fold increase since 2000.1,2 In laboratory animal studies, prenatal exposure to opioids has been demonstrated to affect myelination in the developing brain,3,4 to modulate the fetal hypothalamic-pituitary-adrenal axis,5,6 and to have neurobehavioral consequences in offspring; including hyperactivity,7 elevated startle response,6 and depression-like neurobehavior.8 Clinical data have also linked prenatal opioid exposure to altered brain development,9 as well as congenital heart defects, neural tube defects, and clubfoot (reviewed in Yazdy et al. and Lind et al.10,11).
The placenta is one of the least understood human organs and perturbations in cell growth and signaling can disrupt temporally sequenced developmental processes in the developing fetus and result in long-term functional deficits and detrimental health impacts for offspring. A challenge with studying placental transport using laboratory animals is the inter-species variation which can make the translational aspects of animal studies to human health outcome complicated (as reviewed in literature12–15). The ex vivo human placenta perfusion model addresses several of these challenges; however, the perfusion model require access to human placental tissue and a sophisticated instrumental setup which makes it challenging to use for comparison studies of multiple individual and mixture of compounds. The in vitro model of monolayer forming immortalized human trophoblastic cell lines, like BeWo B30, is therefore more suited for larger comparison studies of transport rate and permeability.16–18
Opioid peptides have been suggested to cross the placental trophoblast monolayer models, BeWo b30 cells19,20 mainly via a paracellular route.19 However, transporter proteins like ABC transporters and solute carrier transporters are also involved in opioid transport (as reviewed in Yang et al.21). Data from a dually perfused human placental lobule model suggest that levo-alpha-acetylmethadol and methadone, but not buprenorphine, is extruded by the ABC transporter P-glycoprotein (PGP).22,23
In this brief communication, the placental transfer kinetics for six individual opioids and selected opioid mixtures were investigated in vitro using a BeWo b30 monolayer model to compare the permeability across the cells (P) between opioids and to understand how P changes when cells are exposed to a mixture of opioids.
Methods
Materials
Cell culture medium DMEM:F12, 50/50 Mix (Cellgro, Corning, NY), MEM nonessential amino acids (NEAA) (ATCC, Manassas, VA), and 200 mM L-glutamine, 100 U penicillin–streptomycin (P/S) and fetal bovine serum (FBS) (Gibco, Gaithersburg, MD). Transwell 12-well plates, polycarbonate, 0.4 μm pore size (Corning, NY). Human placental collagen type IV and opioids were purchased from Sigma-Aldrich (St Louis, MO). Chemicals and solvents for LC-MS analysis were purchased from Thermo Fisher Scientific (Hampton, NH) and an Acquity UPLC HSS C18 column (2.1 × 50 mm with VanGuard HSS C18 1.8 μm) was purchased from Waters, (Milford, MA).
Cell culture
The choriocarcinoma human trophoblast cell line BeWo b30 was obtained from Dr. Erik Rytting (University of Texas Medical Branch, Galveston, TX, USA) and was cultured as described.24 Briefly, cells were cultured at 37°C with 5% CO2 in a humidified atmosphere in DMEM:F12 medium supplemented with 1% NEAA, 1% L-glutamine, 1% P/S, and 10% FBS.
Opioid transport studies
Cells between passages 25–45 were seeded onto permeable membrane inserts coated with collagen at 2.0 × 105 cells/cm2 and cultured for 10 days. Transepithelial electrical resistance (TEER) was measured with an Endohm 12 chamber and EVOM voltohmeter (World Precision Instruments Ltd, Sarasota, FL) to ensure a confluent cell monolayer prior to transport studies. TEER was measured in DMEM:F12 media and calculated by subtracting the resistance of a blank transwell without cells. Only transwells with a cell monolayer measuring between 35 and 70 ohm cm−2 were used.17,25 Six opioids were investigated: heroin, methadone, morphine, naloxone, fentanyl, and oxycodone. Cells were exposed to opioids on the apical side at a final concentration of 10 μg/mL and samples collected from the basolateral side at 5, 10, 15, 30, 60, and 120 min after administration. Vehicle, methanol (1%) was used as control. Studies were conducted in biological triplicates and experimental duplicates. The permeability across the cells (P [cm/s]) was calculated as described;19 P (cm/s)=X/(A×t×Cd), where X is the amount of substance (mol) in the receiver chamber at time t (s), A is the diffusion area (1.12 cm2), and Cd is the concentration of the substance in the donor chamber (mol/cm3). An unpaired, two-tailed t-test was used to evaluate significant differences using GraphPad Prism 7.04.
Ultra-performance liquid chromatography mass spectrometry
All liquid chromatography was performed on a waters Acquity ultra-performance liquid chromatography (UPLC). For reversed phase analysis, 2 μL of each sample were injected into an Acquity UPLC HSS C18 column at 30°C. Water with 0.1% formic acid (mobile phase A) and acetonitrile (mobile phase B) were the mobile phases. The elution gradient consisted of initial conditions at 98% mobile phase A, held for 0.75 min, then linearly increased B to 80% over 3.25 min, and to 95% over 1 min, and then returned to initial conditions. MS analysis was performed using an API 5000 (Applied Biosystems/MDS SCIEX, Foster City, CA) triple quadrupole mass spectrometer. Multiple reaction monitoring was conducted with the following transitions used for quantitation: morphine: 285.8→152.1, naloxone: 328.2→212.0, oxycodone: 316.3→241.2, heroin: 369.9→165.2, fentanyl: 336.9→188.2, and methadone: 309.7→265.2.
Results and discussion
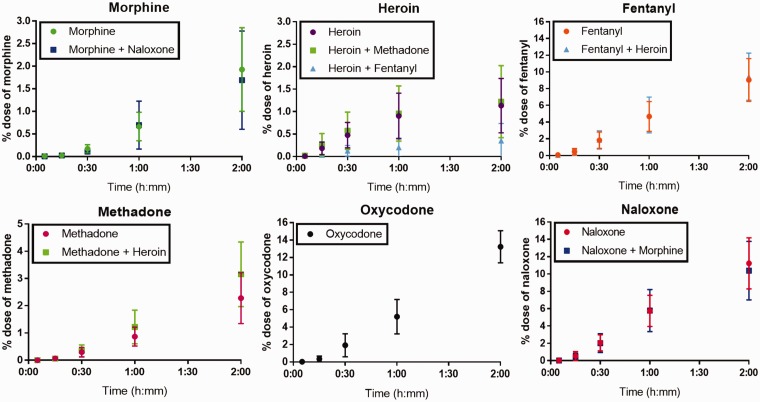
The opioid transfer kinetics across human placental trophoblast, BeWo b30, monolayer of six opioids were tested individually and in combination over a time frame of 120 min (Figure 1). The transfer rate varied between the individual opioids. Permeability for individually tested opioids ranked as following oxycodone > naloxone ∼ fentanyl >methadone ∼ morphine ∼ heroin (Table 1).
Figure 1.
Translocation of opioids across the trophoblast monolayer over time expressed as percentage of dose, showing mean ± standard deviation. The doses were either individual opioids (morphine, heroin, fentanyl, methadone, oxycodone, or naloxone) or mixtures of opioids (morphine + naloxone, heroin + methadone, or heroin + fentanyl). (A color version of this figure is available in the online journal.)
Table 1.
Permeability [cm/s] across the cells at 120 min for opioids tested individually or in mixtures.
| Permeability (cm/s)*10-5 | |
|---|---|
| Single doses | |
| Morphine | 0.36 ± 0.16 |
| Heroin | 0.21 ± 0.10 |
| Fentanyl | 1.76 ± 0.48 |
| Methadone | 0.58 ± 0.22 |
| Oxycodone | 2.46 ± 0.031 |
| Naloxone | 2.09 ± 0.50 |
| Mixed doses | |
| Heroin + fentanyl | H: 0.066* ± 0.066; F: 1.76 ± 0.48 |
| Heroin + methadone | H: 0.23 ± 0.14; M: 0.59 ± 0.20 |
| Morphine + naloxone | M: 0.32 ± 0.19; N: 1.93 ± 0.57 |
Note: Permeability was significantly decreased (P < 0.05) for heroin when administered together with fentanyl as indicated by ‘*’.
In the study presented here, oxycodone had the highest level of transfer. Studies of oxycodone’s impact on birth weight have been inconclusive (as reviewed in Yazdy et al.10). As oxycodone is a commonly prescribed drug for both short- and long-term pain treatment, more knowledge is needed to understand the potential adverse impact on fetal development, growth, and well-being. Furthermore, it has been suggested that the transfer rate is slower in the BeWo b30 transwell model compared to the human placenta perfusion system,25 which could indicate that the transfer of oxycodone in the human placenta is even higher than reported in this study.
The permeability was significantly decreased for heroin when administered together with fentanyl. Our findings suggest that heroin transfer was decreased by fentanyl, while fentanyl transfer was not affected by heroin. It is also possible that fentanyl increases the heroin metabolism. Fentanyl is a fast-acting opioid with a potency of 100:1 to morphine, and it is therefore concerning that the permeability of fentanyl is the third highest for individual opioid exposure tested here and does not seem affected by the presence of another opioids. The drug–drug interactions between heroin and fentanyl need to be investigated further.
With the exception of heroin, which is metabolized by carboxylesterases,26 Cytochrome P450 CYP3A4 is involved in the metabolism of all the investigated opioids.27,28 While other CYPs are also involved, the overlap in CYPs makes it unlikely that the explanation for the difference in transfer rate is due to differences in metabolism. Transporter proteins involved in opioid translocation include ABC transporters (PGPs, multidrug resistance proteins (MRPs), and breast cancer resistance protein (BCRP)) and solute carrier transporters (as reviewed21). Methadone, morphine, and fentanyl21,23,29 are PGP substrates, while oxycodone, naloxone, and heroin are not,21,29,30 suggesting that PGP-mediated efflux is not the determining factor in the transfer rates observed in BeWo b30 cells. The data presented here demonstrate the need to better understand the role of PGPs, MRPs, and BCRPs in placental opioid transport, as well as how potential activation of nuclear receptors and modulated expression of ABC transporters may influence the placental transfer of opioids.
ACKNOWLEDGEMENT
The authors would like to thank Dr. Erik Rytting, University of Texas Medical Branch, for the BeWo b30 cells. The authors would like to thank RTI International for financial support.
Authors’ contributions
Mortensen designed the studies, analyzed the data and wrote manuscript. Moreno Caffaro performed the in vitro studies. Snyder and Yueh developed and validated the LCMS methods for detection of opioids. Fennell has extensive experience in small molecule research including ADME and PK studies and contributed with his experience in this field.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by RTI International Internal Research and Development grant.
References
- 1.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 2015; 35:650–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McQueen K, Murphy-Oikonen J. Neonatal abstinence syndrome. N Engl J Med 2016; 375:2468–79 [DOI] [PubMed] [Google Scholar]
- 3.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia 2008; 56:1017–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 2014; 36:409–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor CC, Wu D, Soong Y, Yee JS, Szeto HH. Opioid modulation of the fetal hypothalamic-pituitary-adrenal axis: the role of receptor subtypes and route of administration. J Pharmacol Exp Ther 1997; 281:129–35 [PubMed] [Google Scholar]
- 6.Hamilton KL, Harris AC, Gewirtz JC, Sparber SB, Schrott LM. HPA axis dysregulation following prenatal opiate exposure and postnatal withdrawal. Neurotoxicol Teratol 2005; 27:95–103 [DOI] [PubMed] [Google Scholar]
- 7.Sithisarn T, Legan SJ, Westgate PM, Wilson M, Wellmann K, Bada HS, Barron S. The effects of perinatal oxycodone exposure on behavioral outcome in a rodent model. Front Pediatr 2017; 5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung CJ, Wu CC, Chen WY, Chang CY, Kuan YH, Pan HC, Liao SL, Chen CJ. Depression-like effect of prenatal buprenorphine exposure in rats. PLoS One 2013; 8:e82262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, Semple SI, Bastin ME, Boardman JP. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin 2018; 18:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdy MM, Desai RJ, Brogly SB. Prescription opioids in pregnancy and birth outcomes: a review of the literature. J Pediatr Genet 2015; 4:56–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lind JN, Interrante JD, Ailes EC, Gilboa SM, Khan S, Frey MT, AlL D, Honein MA, Dowling NF, Razzaghi H, Creanga AA, Broussard CS. Maternal use of opioids during pregnancy and congenital malformations: a systematic review. Pediatrics 2018; 139:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta RC. Toxicology of the placenta. General, applied and systems toxicology. Vol. 4. 3rd ed Hoboken, NJ: John Wiley & Sons, 2009, p.1–37. [Google Scholar]
- 13.Carter AM. Animal models of human placentation – a review. Placenta 2007; 28(Suppl A):S41–7 [DOI] [PubMed] [Google Scholar]
- 14.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 2002; 23:3–19 [DOI] [PubMed] [Google Scholar]
- 15.Chavatte-Palmer P, Tarrade A. Placentation in different mammalian species. Ann Endocrinol 2016; 77:67–74 [DOI] [PubMed] [Google Scholar]
- 16.Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL. In vitro models for studying trophoblast transcellular transport. Methods Mol Med 2006; 122:225–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol 1997; 273:C1596–604 [DOI] [PubMed] [Google Scholar]
- 18.Mitra P, Audus KL. MRP isoforms and BCRP mediate sulfate conjugate efflux out of BeWo cells. Int J Pharm 2010; 384:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ampasavate C, Chandorkar GA, Vande Velde DG, Stobaugh JF, Audus KL. Transport and metabolism of opioid peptides across BeWo cells, an in vitro model of the placental barrier. Int J Pharm 2002; 233:85–98 [DOI] [PubMed] [Google Scholar]
- 20.Chandorkar GA, Ampasavate C, Stobaugh JF, Audus KL. Peptide transport and metabolism across the placenta. Adv Drug Deliv Rev 1999; 38:59–67 [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Reilly BG, Davis TP, Ronaldson PT. Modulation of opioid transport at the blood-brain barrier by altered ATP-binding cassette (ABC) transporter expression and activity. Pharmaceutics 2018; 10:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nekhayeva IA, Nanovskaya TN, Hankins GD, Ahmed MS. Role of human placental efflux transporter P-glycoprotein in the transfer of buprenorphine, levo-alpha-acetylmethadol, and paclitaxel. Amer J Perinatol 2006; 23:423–30 [DOI] [PubMed] [Google Scholar]
- 23.Nanovskaya T, Nekhayeva I, Karunaratne N, Audus K, Hankins GD, Ahmed MS. Role of P-glycoprotein in transplacental transfer of methadone. Biochem Pharmacol 2005; 69:1869–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cartwright L, Poulsen MS, Nielsen HM, Pojana G, Knudsen LE, Saunders M, Rytting E. In vitro placental model optimization for nanoparticle transport studies. Int J Nanomed 2012; 7:497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulsen MS, Rytting E, Mose T, Knudsen LE. Modeling placental transport: correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol In Vitro 2009; 23:1380–6 [DOI] [PubMed] [Google Scholar]
- 26.Kamendulis LM, Brzezinski MR, Pindel EV, Bosron WF, Dean RA. Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. J Pharmacol Exp Ther 1996; 279:713–7 [PubMed] [Google Scholar]
- 27.Smith HS. Opioid metabolism. Mayo Clin Proc 2009; 84:613–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preissner S, Kroll K, Dunkel M, Senger C, Goldsobel G, Kuzman D, Guenther S, Winnenburg R, Schroeder M, Preissner R. SuperCYP: a comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res 2010; 38:D237–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seleman M, Chapy H, Cisternino S, Courtin C, Smirnova M, Schlatter J, Chiadmi F, Scherrmann JM, Noble F, Marie-Claire C. Impact of P-glycoprotein at the blood-brain barrier on the uptake of heroin and its main metabolites: behavioral effects and consequences on the transcriptional responses and reinforcing properties. Psychopharmacology 2014; 231:3139–49 [DOI] [PubMed] [Google Scholar]
- 30.Kanaan M, Daali Y, Dayer P, Desmeules J. P-glycoprotein is not involved in the differential oral potency of naloxone and naltrexone. Fundam Clin Pharmacol 2009; 23:543–8 [DOI] [PubMed] [Google Scholar]