Short abstract
Hyaloid vascular system (HVS) is a transient capillary network nourishing developing eye. Better study of the HVS regression correlated with eye development is essential for in-depth understanding of the nature of vision system. In this study, we demonstrate the feasibility of longitudinal optical coherence tomography (OCT) and OCT angiography (OCTA) monitoring of the HVS in C57BL/6J mice. OCT enables morphological monitoring of the HVS regression, and OCTA allows physiological assessment of the HVS involution correlated with eye development. Functional OCTA reveals early physiological dysfunction before morphological regression of the hyaloid vasculature in developing mouse eye. We anticipate that noninvasive, simultaneous OCT/OCTA observation of morphological regression and physiological degradation in normal and diseased animal models will be valuable to unravel the complex mechanisms of the HVS regression correlated with normal eye development and abnormal persistent hyaloid conditions.
Impact statement
Hyaloid vascular system (HVS) is known to have an essential role in the eye development. However, established knowledge of the HVS largely relies on end-point studies with biochemically fixed tissues, lacking a full description of the natural dynamics of the HVS correlated with eye development. An imaging methodology for noninvasive, longitudinal, and high-resolution monitoring of the HVS is important not only for better understanding of the nature of the vision system and is also valuable for better study of abnormal eye conditions. Here, we report the feasibility of in vivo optical coherence tomography (OCT) and OCT angiography (OCTA) imaging of the HVS regression in developing mouse eye. OCT enables morphological imaging of the HVS structure, and OCTA allows functional assessment of the HVS physiology correlated with eye development.
Keywords: Hyaloid vascular system, hyaloid vessel regression, vitreous, macrophages, optical coherence tomography, optical coherence tomography angiography
Introduction
Hyaloid vascular system (HVS) is a transient vascular network, nourishing immature intraocular components of the developing embryonic and fetal eye. The HVS are all arteries and the venous drain is achieved by the choroidal veins,1 and the HVS undergoes spontaneous regression by the fifth month of gestation in humans as the growing retinal vasculature starts to deliver oxygen to the retina.2 However, failure of the regression causes a congenital eye disease referred to as persistent hyperplastic primary vitreous (PHPV) or persistent fetal vasculature (PFV), which can lead to cataract, intraocular hemorrhage, and retinal detachment.3 Despite its clinical importance, the exact mechanism of the HVS regression correlated with eye development is not fully understood, since experimental study on the living fetus and infant is almost impossible. Thus, established knowledge is mainly elucidated by histological studies of postmortem human tissues.4
Among laboratory animals, the highly vascularized mouse eye offers several advantages to the HVS study. Unlike human, newborn mice have the immature retinal vasculature and persistent hyaloid vessels, which mostly regress at postnatal day 12 (P12) to P16.5,6 Moreover, the ocular vascular systems of the mouse can be experimentally or genetically manipulated, and various strains with known genomes are commercially available for lab research. Nevertheless, even the mouse study has traditionally relied on end-point analysis with biochemically fixed tissues, lacking a full description of the natural dynamics of the HVS correlated with eye development.7 This significantly limits the investigation of the relationship between hyaloid vessels and surrounding ocular tissues including the retina, the lens, and the vitreous chamber.
A noninvasive imaging approach for high spatiotemporal resolution investigation of the HVS regression is essential for better understanding of the three-dimensional (3D) dynamics of the hyaloid vessels correlated with the retinal development. Micro-CT8 and ultrasonography9 demonstrated in vivo hyaloid vascular imaging. However, long acquisition duration and processing time, and low spatial resolution were limiting factors. On the other hand, optical coherence tomography angiography (OCTA) is an emerging noninvasive imaging modality that visualizes the presence of blood flow and forms detailed 3D images of ocular vasculatures with high spatiotemporal resolution.10,11 Both OCT and OCTA of retinal vasculatures have been well established, but their application for the HVS study has not been well explored. This study aims to demonstrate longitudinal OCT imaging of morphological regression as well as OCTA observation of physiological distortion of the HVS in C57BL/6J mice. Some of these data were presented in preliminary form at the 2019 SPIE Photonics West Conference.12
Materials and methods
OCT imaging system
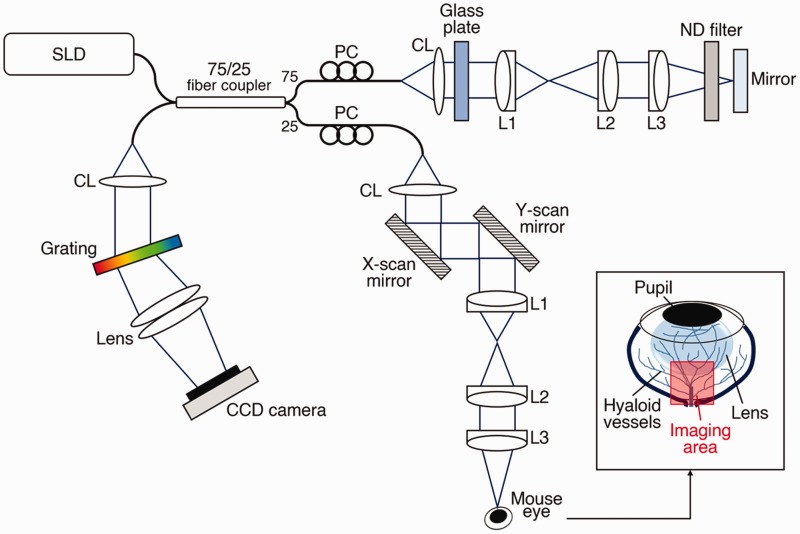
Figure 1 shows a schematic diagram of a custom-designed OCT system employed for in vivo hyaloid vascular imaging. A wide bandwidth near-infrared (NIR; Δλ = 100 nm, λ = 840 nm) super-luminescent diode (SLD; D840, Superlum, Carrigtwohill, County Cork, Ireland) was used as the OCT light source to provide high axial resolution (∼3 µm), and the lateral resolution of the system was estimated at 12 µm. A linear CCD camera (AViiVA EM4, e2v Technologies, Chelmsford, UK) used in the custom-built OCT spectrometer provided the frame speed of 30 kHz A-scan rate. The light power incident on the mouse cornea was set to 0.95 mW.
Figure 1.
Optical diagram of the OCT setup. CL: collimation lens; L1, L2, L3: lens; PC: polarization controller; SLD: super luminescent diode (λ = 840 nm). The inset figure indicates the imaging area in the mouse eye. (A color version of this figure is available in the online journal.)
Animal preparation
All animal experiments were performed by following the protocols approved by the Animal Care Committee at the University of Illinois at Chicago. Three wild-type mice (C57BL/6J, The Jackson Laboratory, Bar Harbor, ME) were used in longitudinal OCT/OCTA measurements of the HVS. The mouse was first anesthetized with a mixture of 100 mg/kg ketamine and 5 mg/kg xylazine given by intraperitoneal injection. A drop of ophthalmic mydriatic (1% atropine sulfate ophthalmic solution, Akorn, Lake Forest, IL) was then applied to the left eye of the mouse to dilate the pupil where imaging was performed. After the pupil was fully dilated, the mouse was mounted onto the animal holder equipped with a bite bar and ear bars to minimize motion artefacts. A drop of eye gel (Severe; GenTeal, Novartis, Basel, Switzerland) was further applied on both eyes, and a cover glass (12–545-80; Microscope cover glass, Fisherbrand, Waltham, MA) was placed on the imaging eye.
Image acquisition
The hyaloid vascular images were acquired in a laboratory room under ambient light condition. During the image acquisition, the optic nerve head (ONH) was placed at the middle of the imaging area as a reference imaging point. Figure 2(a) represents an OCT volumetric image of the back of the mouse eye, acquired over 1.2 mm (width) × 1.2 mm (length) × 1.4 mm (height). This imaging region contains part of the lens, the retina, and the hyaloid vessels with floaters. OCT 3D volume consisted of 500 B-scans and each B-scan was composed of 500 A-scans. In addition, the OCTA images were constructed by the speckle variance (SV) calculation using four repeated OCT B-scans at each scanning position (Figure 2(b)). Thus, OCTA 3D volume was obtained by a total of 4 × 500 × 500 A-scans.
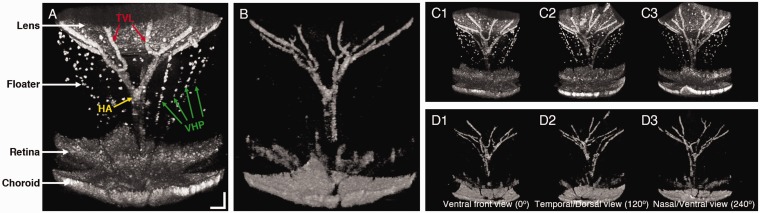
Figure 2.
Representative (a) OCT (Video 1) and (b) OCTA (Video 2) images of a wild-type mouse eye at P14 (Video 1, AVI, 2.4 MB; Video 2, AVI, 1.1 MB). Ventral (c1 and d1), temporal/dorsal (c2 and d2), and nasal/ventral (c3 and d3) views of the OCT (c) and OCTA (d) images. Scale bars: 100 µm. (A color version of this figure is available in the online journal.)
Results
The OCT image in Figure 2(a) shows three types of the HVS branches: (1) the hyaloid artery (HA), which arises from the primitive dorsal ophthalmic artery and enters the eyecup through the embryonic fissures and extends to the posterior pole of the lens, (2) the vasa hyaloidea propria (VHP), which is capillary branches from the HA into the vitreous chamber and anastomoses anteriorly with a vessel network surrounding the lens, and (3) the tunica vasculosa lentis (TVL), which is terminal branches of the HA and cups the posterior surface of the lens.13 However, multiple VHP capillaries observed in the OCT image (green arrows, Figure 2(a)) were absent in the OCTA image (Figure 2(b)), indicating functional loss, i.e. lacking blood flow in the VHP vessels. Comparative OCT (Figure 2(c)) and OCTA (Figure 2(d)) images viewed from different orientations confirmed the observation. The VHP is known to supply oxygen to the inner retina until the formation of retinal vasculature.4 Thus, it largely regresses at P14,5,9 when three retinal vascular plexuses are formed.14
In order to further confirm the difference between the morphological regression and functional loss of the HVS, Figure 3 illustrates representative OCT and OCTA images captured at P14, P21, and P28. Comparative en face projection images of OCT (Figure 3(a4) to (a6)) and OCTA (Figure 3(b4) to (b6)) clearly revealed functionally impaired vessels from intact vascular structures (yellow arrows). Retinal vasculature was excluded from the en face OCT and OCTA of the HVS. Figure 3(a) and (b) also confirm that the regression process begins with atrophy of the VHP, followed by the capillaries of the TVL, and finally the HA. Moreover, different populations of floaters in the vitreous were observed with aging (Figure 3(a)). These floaters are mainly composed of macrophages and retrolental cell mass,15 and eventually cleaned by phagocytosis.16 Therefore, the vitreous chamber became transparent with aging.
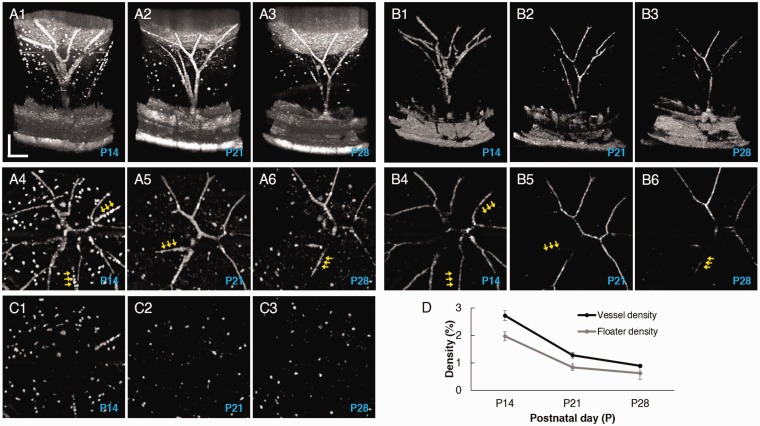
Figure 3.
Comparative (a) OCT and (b) OCTA images from a wild-type mouse eye measured at P14, P21, and P28. a1, a2, and a3 illustrate side views of OCT images and a4, a5, and a6 illustrate en face OCT projection images at the three time points. b1, b2, and b3 illustrate side views of OCTA images and b4, b5, and b6 illustrate en face OCTA projection images at the three time points. Yellow arrows indicate capillaries in functional loss. c1, c2, and c3 show floater images, which isolated from a4, a5, and a6, respectively. (d) Hyaloid vessel density and floater density measurements. Scale bars: 200 µm. (A color version of this figure is available in the online journal.)
Quantitative analysis of vessel and floater densities was performed with three wild-type mice, and OCT/OCTA images were longitudinally acquired at P14, P21, and P28. OCTA vessel images (Figure 3(b4) to (b6)) were used for the density calculation after binarization, while the isolated floaters (Figure 3(c)), derived from en face OCT images (Figure 3(a4) top (a6)) after vessel removal, were used for the floater density calculation. The result in Figure 3(d) shows that the vessel density steadily decreases with age as the vascular regression proceeds (2.72 ± 0.2% [P14] to 0.89 ± 0.1% [P28]). The floater density also gradually decreases (1.97 ± 0.2% [P14] to 0.62 ± 0.2% [P28]). The two density curves present a similar biphasic trend, i.e. a fast regression period between P14 and P21 and a slow regression period between P21 and P28.
Discussion
In summary, we demonstrated the feasibility of noninvasive, longitudinal in vivo OCT and OCTA monitoring of the HVS in developing mouse eye. OCT enables morphological monitoring of HVS structural regression, and OCTA allows physiological assessment of the HVS involution correlated with eye development. Functional OCTA reveals early physiological dysfunction, i.e. lacking blood flow, before morphological regression of hyaloid vasculature in developing mouse eye.
The exact mechanisms responsible for the involution of the hyaloid vessels during eye development are not well understood. However, several contributing factors are believed to be directly involved in the regression process of the HVS. Cessation of blood flow in hyaloid vessels is a potential triggering factor of the vascular regression.17 Reduced shear stress on the endothelial surface of the blood vessel due to reduced blood flow is known to stimulate expression of vasoconstrictors,18 and vasoconstriction of the HA preceded the involution of the HVS.19 A recent histochemical study also found a reduced vessel diameter during the regression of the VHP. They further confirmed severe vascular congestions due to neutrophilic leukocytes aggregated with numerous erythrocytes in the lumen of hyaloid vessels, followed by the vascular regression.15 In this study, we consistently observed fragmented threadlike vascular structures in OCT similar to the aggregated cellular mass in the lumen (Figure 3(a4) to (a6)), and they revealed a dysfunction, i.e. a lack of blood flow, in OCTA (Figure 3(b4) to (b6)). The early functional loss and fragmented vasculatures observed in this study can support the notion of the reduced blood flow as a major triggering factor of the vascular regression. Longitudinal observations of OCT/OCTA within a shorter time interval would provide more detailed information on the hemodynamic changes correlated with morphological deformations of the HVS.
On the other hand, under some conditions, apoptosis of endothelial cells and phagocytosis of their atrophic vessels by macrophages may be the primary mechanism triggering the HVS regression. Numerous macrophages are found around the capillaries of regressing hyaloid vessels in developing eyes,5,20 and it is reported that macrophages alone can induce the programmed hyaloid vessel regression.15,21 In this study, OCT images revealed numerous hyperreflective round-shaped floaters as well as smaller and irregular shaped floaters in the vitreous, which were absent in OCTA (Figures 2 and 3). We speculate that the hyperreflective round-shaped floaters may reflect active macrophages, directly involved in the regression process. Further OCT/OCTA study with mutant mice,21 which have persistent hyaloid vessels and lack resident macrophages in the vitreous, can verify the different signal sources from different cell types among the floaters.
Moreover, the HVS regression can also be controlled by tissue oxygenation like sprouting angiogenesis. Hyperoxia leads to vascular endothelial growth factor (VEGF) downregulation, causing substantial obliteration of retinal capillaries.22 Another intriguing contributing factor is an unknown common signaling pathway between the HVS and the retinal vasculature, considering the development of retinal vasculature occurs contemporaneously with the involution of hyaloid vessels.1 It is interesting to note that the disturbed deeper plexus formation in various mutant mouse retinas directly affects hyaloid vessel regression.23 We have recently demonstrated OCT and OCTA of retinal vascular morphology and physiology.24,25 We anticipate that the concurrent OCT and OCTA study of the retinal vasculature and the HVS in normal and diseased animal models will be valuable to unravel the complex mechanisms of the HVS regression correlated with normal eye development and abnormal persistent hyaloid conditions.
ACKNOWLEDGMENTS
The authors thank Dr. Devrim Toslak for valuable discussion on clinical significance of this study.
Authors’ contributions
TK conducted the experiments, and contributed to data analysis and manuscript preparation; TS contributed to data analysis and reviewed the manuscript; XY supervised the study, contributed to data analysis and manuscript preparation.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This research was supported in part by NIH grants R01 EY023522, R01 EY024628, P30 EY001792; by unrestricted grant from Research to Prevent Blindness; by Richard and Loan Hill endowment.
References
- 1.Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Develop Biol 2004; 48:1045–58 [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa Y, Yamada T, Tai-Nagara I, Okabe K, Kitagawa Y, Ema M, Kubota Y. Developmental regression of hyaloid vasculature is triggered by neurons. J Exp Med 2016; 213:1175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg MF. Persistent fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol 1997; 124:587–626 [DOI] [PubMed] [Google Scholar]
- 4.Lutty GA, McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog Retin Eye Res 2018; 62:58–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol 1999; 200:403–411 [DOI] [PubMed] [Google Scholar]
- 6.Ritter MR, Aguilar E, Banin E, Scheppke L, Uusitalo-Jarvinen H, Friedlander M. Three-dimensional in vivo imaging of the mouse intraocular vasculature during development and disease. Invest Ophthalmol Visual Sci 2005; 46:3021–6 [DOI] [PubMed] [Google Scholar]
- 7.McLeod DS, Hasegawa T, Baba T, Grebe R, Galtier d'Auriac I, Merges C, Edwards M, Lutty GA. From blood islands to blood vessels: morphologic observations and expression of key molecules during hyaloid vascular system development. Invest Ophthalmol Visual Sci 2012; 53:7912–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atwood RC, Lee PD, Konerding MA, Rockett P, Mitchell CA. Quantitation of microcomputed tomography-imaged ocular microvasculature. Microcirculation 2010; 17:59–68 [DOI] [PubMed] [Google Scholar]
- 9.Brown AS, Leamen L, Cucevic V, Foster FS. Quantitation of hemodynamic function during developmental vascular regression in the mouse eye. Invest Ophthalmol Visual Sci 2005; 46:2231–7 [DOI] [PubMed] [Google Scholar]
- 10.Kim T-H, Son T, Lu Y, Alam M, Yao X. Comparative optical coherence tomography angiography of wild-type and rd10 mouse retinas. Trans Vis Sci Tech 2018; 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao X, Son T, Kim TH, Lu Y. Functional optical coherence tomography of retinal photoreceptors. Exp Biol Med 2018;243:1256–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim T-H, Son T, Yao X. Longitudinal optical coherence tomography angiography of hyaloid vessels in the developing mouse eye. Proc SPIE Int Soc Opt Eng 2019; 10858 [Google Scholar]
- 13.Zhang H, Tse J, Hu X, Witte M, Bernas M, Kang J, Tilahun F, Hong YK, Qiu M, Chen L. Novel discovery of LYVE-1 expression in the hyaloid vascular system. Invest Ophthalmol Vis Sci 2010; 51:6157–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 2010; 51:2813–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto A, Kimura S, Nio-Kobayashi J, Takahashi-Iwanaga H, Park AM, Iwanaga T. Histochemical characteristics of regressing vessels in the hyaloid vascular system of neonatal mice: novel implication for vascular atrophy. Exp Eye Res 2018; 172:1–9 [DOI] [PubMed] [Google Scholar]
- 16.Vrolyk V, Haruna J, Benoit-Biancamano MO. Neonatal and juvenile ocular development in Sprague-Dawley rats: a histomorphological and immunohistochemical study. Veter Pathol 2018; 55:310–30 [DOI] [PubMed] [Google Scholar]
- 17.Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development 1996; 122:3929–38 [DOI] [PubMed] [Google Scholar]
- 18.Lobov IB, Cheung E, Wudali R, Cao J, Halasz G, Wei Y, Economides A, Lin HC, Papadopoulos N, Yancopoulos GD, Wiegand SJ. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood 2011; 117:6728–37 [DOI] [PubMed] [Google Scholar]
- 19.Browning J, Reichelt ME, Gole GA, Massa H. Proximal arterial vasoconstriction precedes regression of the hyaloid vasculature. Curr Eye Res 2001; 22:405–11 [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Asnaghi L, Gongora C, Patek B, Hose S, Ma B, Fard MA, Brako L, Singh K, Goldberg MF, Handa JT, Lo WK, Eberhart CG, Zigler JS, Jr, Sinha D. A developmental defect in astrocytes inhibits programmed regression of the hyaloid vasculature in the mammalian eye. Eur J Cell Biol 2011; 90:440–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 2005; 437:417–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claxton S, Fruttiger M. Role of arteries in oxygen induced vaso-obliteration. Exp Eye Res 2003; 77:305–11 [DOI] [PubMed] [Google Scholar]
- 23.Fruttiger M. Development of the retinal vasculature. Angiogenesis 2007; 10:77–88 [DOI] [PubMed] [Google Scholar]
- 24.Son T, Alam M, Toslak D, Wang B, Lu Y, Yao X. Functional optical coherence tomography of neurovascular coupling interactions in the retina. J Biophotonics 2018;11:e201800089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son T, Wang B, Lu Y, Chen Y, Cao D, Yao X. Concurrent OCT imaging of stimulus evoked retinal neural activation and hemodynamic responses. Proc SPIE Int Soc Opt Eng 2017; 10045 [DOI] [PMC free article] [PubMed] [Google Scholar]