Abstract
Background: High sensitivity C-reactive protein (hsCRP) predicts myocardial dysfunction after acute coronary syndromes. We aimed to study the association of hsCRP estimation at first acute myocardial infarction (AMI) with myocardial dysfunction and heart failure.
Methods: This research was carried out at the Department of Physiology and Department of Emergency Medicine, King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia. In this prospective study, 227 patients were studied. hsCRP levels were estimated when patients came to the emergency department at AMI, 7 days post AMI, and at 12 weeks of follow up after AMI. The outcome was change in myocardial functions, especially heart failure, 12 months after the attack.
Results: Based on a cutoff mean value of hsCRP levels at admission (10.05±12.68 mg/L), patients were grouped into high and low C-reactive protein (CRP.) The ejection fraction was significantly lower at follow up in the high CRP group (37.29±12.97) compared to the low CRP group (43.85±11.77, p<0.0198). hsCRP had significant inverse correlation with left ventricular ejection fraction (r=−0.283, p<0.01). About 38.1% patients showed heart failure, with 23.6% in the high CRP group and 14.5% in the low CRP group (OR 2.4, p=0.028). Receiver operating characteristic curve analysis showed that CRP levels at AMI had a specificity of 79% and sensitivity of 83% to predict heart failure.
Conclusion: A high hsCRP level measured at first AMI predicts myocardial dysfunction and heart failure. It is suggested that hsCRP plays an important role in the development of heart failure after myocardial infarction.
Keywords: high sensitivity C-reactive protein, Lipoprotein(a), heart failure, acute myocardial infarction, ejection fraction
Introduction
The development of heart failure after acute coronary syndromes (ACS) is the most common complication associated with a high mortality rate.1,2 Circulating levels of C-reactive protein (CRP) have been shown to be linked to poor prognosis in patients with atherothrombotic problems, heart failure, arrhythmias, and myocarditis.3 Fourteen predictors of heart failure were studied by Ho et al.4,5 Important predictors identified were older age, diabetes mellitus, valvular disease, hypertension, higher heart rate, left ventricular hypertrophy, left bundle branch block, cardiovascular disease, body mass index, smoking, gender, and dyslipidemia.4,5 Few studies have reported the association between high sensitivity C-reactive protein (hsCRP) and heart failure after ACS.6,7 High levels of hsCRP at myocardial infarction also predict early mortality.6,8 Diabetic patients also have grave prognosis after acute myocardial infarction (AMI).9 A high level of hsCRP earlier in ACS, before development of myocardial necrosis, is an important indicator of poor prognosis with cardiovascular comorbidities. Its assessment during the time course of ACS may assist in risk stratification for myocardial dysfunction.10 The major function of biomarkers is in ACS, heart failure prediction, and risk stratification for recurrent cardiovascular events. They provide a way for guiding further treatment needs.11 There are few data on the prognostic role of hsCRP measured at and after first myocardial infarction. Therefore, we aimed to study the association of serial measurements of hsCRP estimation at AMI with development of heart failure in patients who had suffered myocardial infarction for the first time.
Methodology
Study design and settings
This prospective study was conducted at the Department of Physiology, Department of Emergency Medicine, and Department of Cardiology of the College of Medicine and King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia. The study protocol was approved by the Research Ethics Committee of the CMRC.
Study population andpatient enrollment
The study was approved by the College of Medicine Institutional Review Board committee. All patients signed the consent form with Arabic and English versions. The patient consent was written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
A detailed history was recorded and clinical examination was performed. All information was recorded on a predesigned proforma. Inclusion criteria included adult patients of any sex with AMI. For the diagnosis of AMI and other ACS protocols we used standard criteria as previously reported.12,13 Heart failure was diagnosed on the basis of symptoms, clinical signs, and documented left ventricular impairment (left ventricular ejection fraction≤40%) according to the current guidelines.14 At AMI, the Killip classification was used to evaluate patients for heart failure, and at follow up the New York Heart Association Functional Classification scores were recorded. Patients with associated systemic disorders like acute infections, stroke, acute diabetic status, rheumatic diseases, chronic liver diseases, renal failure, cancers, and sepsticemia, patients with an ongoing or recent infectious disorder, and patients with a surgical procedure in the past 3 months time were excluded.
Of the 227 patients recruited, 181 patients had evidence of AMI on the basis of the standard criteria. The remaining 46 patients were used as a control. Among them, 31 had unstable angina, 9 had chronic CAD, and 6 turned out to be having nonischemic problems. Only 150 patients completed the study: 5 patients died during follow up and 26 patients did not turn up and were excluded from the study. Patients were divided into two groups based on a cutoff point of the mean value of hsCRP levels on admission, which was 10.05±12.68 mg/L. Group A had CRP<10 mg/L on admission while group B had CRP≥10 mg/L on admission.
Analytical details and assays
Venous blood samples were tested for lipid profile including total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein (HDL), and hsCRP. TC, TG, LDL ,and HDL were analyzed by a Dimension autoanalyzer (USA). hsCRP assays were performed with the turbidimetric assay method (Quantex hsCRP kits) supplied by BIOKIT Spain using an Hitachi 911 autoanalyzer manufactured by ROCHE Diagnostics (Hoffmann-La Roche Ltd., Basel, Switzerland). The kit detected hsCRP in the range of 0.10–20.0 mg/L. We followed the American Heart Association criteria for measurement, evaluation, and expression of hsCRP.15 Lp(a) was analyzed by turbidimetric immunoassay with the quantex Lp(a) kits supplied by BIOKIT Spain.
Patient follow up and study endpoints
Blood samples were taken for analysis of hsCRP on admission to hospital at first AMI in the acute phase (<12 hours from onset of symptoms) from the antecubital vein. After centrifugation, the serum was separated and stored at −70 °C until assayed. 12 months after recruitment each patient was called for a complete clinical check-up and echocardiography was performed using standard procedures. The patients were followed up for recurrent events and complications that occurred after the first attack of AMI. The doctors examining the patients and those who performed the echocardiography were unaware of the hsCRP levels. In about 16 patients the echocardiographic images were technically not suitable and were discarded from the final data analysis. Any mortalities during this time, with dates and causes, were obtained from hospital records or from the patient’s family doctor.
Statistical analysis
We used the Statistical Package for Social Sciences (SPSS version 20; IBM Corporation, Armonk, NY, USA). The demographic data and lipid profiles were expressed as the mean±SD for continuous variables and as percentage values for categorical variables. Independent sample Student’s t-test was used for comparison between studied groups. Nonnormally distributed data (skewed) were analyzed by Mann–Whitney U-test. Categorical variables were compared between various groups using the chi-square test. p<0.05 was considered statistically significant.
Results
We prospectively examined the association of hsCRP with heart failure in patients with first myocardial infarction.
Clinical and demographic data details are expressed in detail for all CAD patients in Table 1. hsCRP (19.5±16.2 vs 1.8±0.90, p=0.0001) and Lp(a) (26.65±26.92 vs 16.78±13.31, p=0.0132) levels were significantly higher in CAD patients compared to control subjects. Table 1 also shows the mean peak cardiac enzymes attained. Among the peak cardiac enzymes, only AST levels were significantly high in AMI patients divided into low and high CRP groups based on a cutoff mean value of hsCRP level on admission (10.05±12.68 mg/L). The average time for the first sample was 7 h and 20 min in the low CRP group and was 6 h and 45 min in the high CRP group. Table 2 shows a comparison of clinical and demographic characteristics between AMI patients with low and high hsCRP levels. Serum AST levels were significantly higher in the high CRP group (p=0.0198), and also the diabetes mellitus frequency (p=0.0106). We observed significantly lower levels of ejection fraction at 1 year of follow up in patients of Group B (37.29±12.97) compared to Group A (43.85±11.77, p=0.0198) (Table 3).
Table 1.
Clinical and demographic data of AMI patients compared with control subjects
| Control N=46 |
AMI N=150 |
P-value | |
|---|---|---|---|
| Male/female | 27/19 | 112/38 | |
| Age (years) | 54.62±10.60 | 55.16±9.97 | 0.7745 |
| Height (cm) | 164.13±9.00 | 162.39±9.24 | 0.3435 |
| Weight (kg) | 75.84±15.83 | 75.61±16.04 | 0.6125 |
| BMI | 28.12±6.08 | 28.59±6.57 | 0.5767 |
| SBP (mmHg) | 129.93±19.07 | 132.11±18.28 | 0.7798 |
| DBP (mmHg) | 75.83±12.26 | 76.94±11.69 | 0.8113 |
| Pulse | 81.22 13.09 | 81.91±14.23 | 0.1518 |
| Temperature (°C) | 36.46±0.47 | 36.54±0.48 | 0.1747 |
| Respiratory rate (/min) | 20.98±3.47 | 21.28±3.83 | 0.4319 |
| hsCRP (mg/L) at AMI | 1.8±0.90 | 9.01±10.75 | 0.0001 |
| Lp(a) | 16.78±13.31 | 26.65±26.92 | 0.0132 |
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index; DBP, diastolic blood pressure; hsCRP, high sensitivity C-reactive protein; SBP, systolic blood pressure.
Table 2.
Comparison of clinical and demographic data between AMI patients with low and high hsCRP levels
| Low CRP group N=99, CRP<10 mg/L |
High CRP group N=51, CRP≥10 mg/L |
P-value | |
|---|---|---|---|
| Male/female | 75/24 | 37/14 | |
| Age (years) | 54.22±12.35 | 55.33±13.42 | 0.6394 |
| Height (cm) | 163.80±10.37 | 161.88±7.54 | 0.4173 |
| Weight (kg) | 78.02±16.53 | 73.93±12.74 | 0.2443 |
| BMI | 29.29±6.01 | 27.18±6.57 | 0.1475 |
| SBP (mmHg) | 131.62±19.19 | 131.21±23.64 | 0.9210 |
| DBP (mmHg) | 77.76±14.58 | 75.12±15.37 | 0.3637 |
| Pulse | 81.93±14.30 | 85.86±16.69 | 0.1892 |
| Temperature (°C) | 36.48±0.48 | 36.63±0.67 | 0.1785 |
| Respiratory rate (/min) | 20.65±3.29 | 20.53±1.28 | 0.8300 |
| hsCRP (mg/L) | 3.2±02.5 | 26.1±12.6 | 0.0001 |
| Troponin T | 2.35±1.44 | 3.60±2.93 | 0.5962 |
| CKMB | 116.88±109.08 | 196.50±110.74 | 0.6094 |
| AST | 24.50±8.96 | 108.50±73.73 | 0.0198 |
| LDH | 190.20±49.51 | 271.90±198.49 | 0.3892 |
| History of angina | 15 (15.1%) | 9 (17.6%) | 0.8729 |
| Current smoking | 30 (30.3%) | 21 (41.2%) | 0.2502 |
| Hypertension | 49 (49.5%) | 33 (64.7%) | 0.1096 |
| Diabetes mellitus | 53 (53.5%) | 39 (76.5%) | 0.0106 |
| Thrombolysis | 32 (32.3%) | 19 (37.2%) | 0.6729 |
| Gensini score | 64.21±51.89 | 64.65±36.21 | 0.9682 |
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; hsCRP, high sensitivity C-reactive protein; SBP, systolic blood pressure.
Table 3.
Echocardiographic findings in AMI patients divided into low and high hsCRP at 1 year of follow up
| Low CRP group N=93, CRP<10 mg/L |
High CRP group N=41, CRP≥10 mg/L |
P-value | |
|---|---|---|---|
| ARD | 28.62±5.77 | 29.05±5.15 | 0.3135 |
| LAD | 37.47±3.64 | 38.73±5.98 | 0.9429 |
| LVIDd | 50.15±5.98 | 53.53±8.28 | 0.0271 |
| LVIDs | 32.88±5.74 | 37.12±10.20 | 0.0134 |
| PW | 10.12±1.45 | 10.04±1.78 | 0.7310 |
| IVSep | 10.35±1.81 | 10.68±2.13 | 0.1494 |
| Ejection fraction | 45.87±9.96 | 38.42±13.45 | 0.0048 |
Abbreviations: AMI, acute myocardial infarction; CRP, C-reactive protein; hsCRP, high sensitivity C-reactive protein; LAD, left atrial dimension; ARD, aortic root dimension; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; PW, left ventricular posterior wall thickness; IVS, interventricular septal thickness.
The difference for Lp(a) levels was nonsignificant between the two groups (23.39±20.67 vs 28.43±28.25, p=0.2532). This was also the case for TC, TG, LDL, and HDL levels (Table 4).
Table 4.
Lipid and Lp(a) profile of myocardial infarction patients divided into CRP<10 mg/L and CRP≥10 mg/L at AMI
| Lipid profile | Low CRP group N=99, CRP<10 mg/L |
High CRP group N=51, CRP≥10 mg/L |
P-value |
|---|---|---|---|
| TC (mmol/L) | 4.28±1.40 | 4.48±1.14 | 0.4923 |
| TG (mmol/L) | 1.84±1.10 | 1.72±0.94 | 0.6039 |
| LDL (mmol/L) | 2.71±1.20 | 2.93±0.84 | 0.4789 |
| HDL (mmol/L) | 0.71±0.25 | 0.81±0.15 | 0.1220 |
| Lp(a) (mg/dl) | 23.39±20.67 | 28.43±28.25 | 0.2532 |
Abbreviations: AMI, acute myocardial infarction; CRP, C-reactive protein; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglycerides; TC, serum cholesterol.
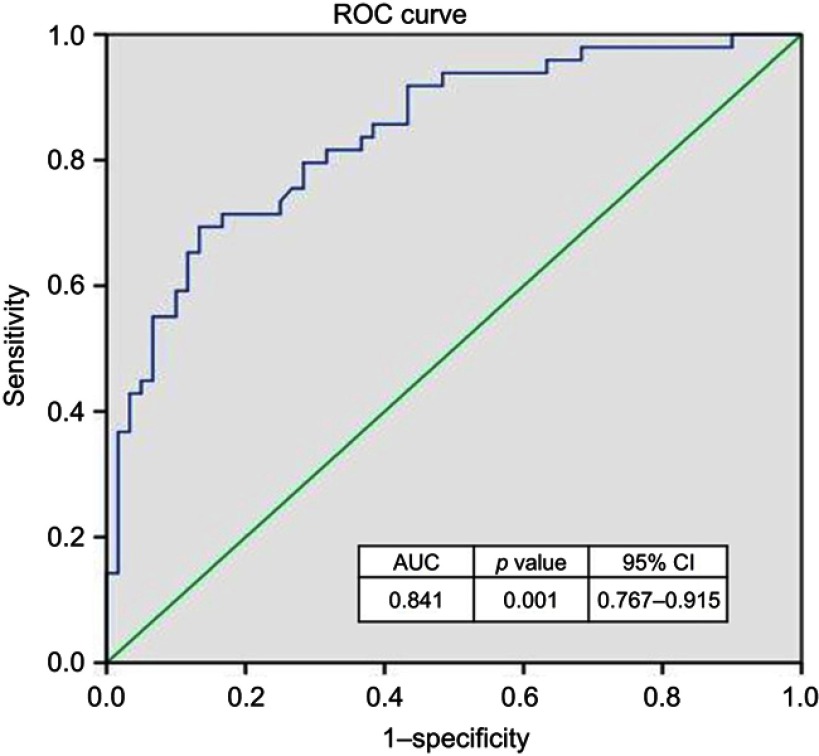
Troponin (r=−0.382, p<0.001) and hsCRP (r=−0.283, p<0.01) levels on admission correlated negatively with ejection fraction (Table 5). 38.1% of patients had heart failure, with 23.6% in the high CRP group and 14.5% in the low CRP group (OR 2.4, CI=1.084–5.225, p=0.028). CRP levels at AMI had a specificity of 79% and sensitivity of 83% to predict heart failure at a cutoff level of 10 mg/L (Figure 1). Correlation of CRP at AMI with clinical characteristics, CAD severity, and lipid profile in all myocardial infarction patients is expressed in Tables 6 and 7. CRP levels were correlated significantly with Killip scores at AMI (r=0.311, p=0.008) and clinical signs of severity scores at follow up (r=0.255, p=0.032). There was no relation of CRP with CAD severity determined by the Gensini score.
Table 5.
Pearson correlation analysis of Troponin & CRP levels at AMI with Echocardiography in all AMI patients
| Troponin | CRP | ARD | LAD | LVIDD | LVIDS | PW | IVS | Ejection fraction | |
|---|---|---|---|---|---|---|---|---|---|
| Troponin | 1.000 | .057 | .026 | .078 | .205 | .318** | −.126 | −.090 | −.382** |
| CRP | 1.000 | .039 | .028 | .130 | .171 | .141 | .259* | −.283** | |
| ARD | 1.000 | .176 | .318** | .365** | .150 | .118 | −.016 | ||
| LAD | 1.000 | .400*** | .380*** | .147 | −.003 | −.270** | |||
| LVIDd | 1.000 | .798*** | −.015 | −.027 | −.545 | ||||
| LVIDs | 1.000 | −.010 | −.129 | −.610*** | |||||
| PW | 1.000 | .731*** | .160 | ||||||
| IVS | 1.000 | .197 | |||||||
| Ejection fraction | 1.000 |
Note: *p< 0.05, **p< 0.01, ***p< 0.001.
Abbreviations: AMI, acute myocardial infarction; Trop, troponin; CRP, reactive protein; LAD, left atrial dimension; ARD, aortic root dimension; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; PW, left ventricular posterior wall thickness; IVS, interventricular septal thickness; EF, ejection fraction; LAD, left atrial dimension; ARD, aortic root dimension; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; PW, left ventricular posterior wall thickness; IVS, interventricular septal thickness.
Figure 1.
ROC curve analysis to determine the cutoff points and AUC for hsCRP levels on admission with acute myocardial infarction for heart failure prediction. Abbreviations: AUC, area under the curve; hsCRP, high sensitivity C-reactive protein; ROC, receiver operating characteristic.
Table 6.
Pearson correlation of CRP levels at AMI with clinical characteristics in all AMI patients
| CRP | Age | BMI | Pulse | SBP | DBP | Killip | CLIN | |
|---|---|---|---|---|---|---|---|---|
| CRP | 1.000 | 0.002 | 0.201 | 0.233** | −0.028 | −0.046 | 0.311** | 0.255* |
| Age | 1.000 | −0.234* | −0.018 | 0.140 | −0.064 | 0.101 | 0.116 | |
| BMI | 1.000 | 0.078 | −0.105 | −0.042 | −0.002 | 0.011 | ||
| Pulse | 1.000 | 0.147 | 0.291** | 0.176* | 0.071 | |||
| SBP | 1.000 | 0.552 | −0.178 | −0.154 | ||||
| DBP | 1.000 | −0.071 | −0.049 | |||||
| Killip | 1.000 | 0.761** | ||||||
| CLIN | 1.000 |
Note: *p< 0.05, **p< 0.01, ***p< 0.001.
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; hsCRP, high sensitivity C-reactive protein; SBP, systolic blood.
Table 7.
Pearson correlation of CRP at AMI with CAD severity and lipid profile in all AMI patients
| CAD severity | Sex | CRP | TC | TG | LDL | HDL | |
|---|---|---|---|---|---|---|---|
| CAD severity | 1.000 | 0.155 | 0.105 | 0.010 | 0.053 | 0.065 | 0.037 |
| Gender | 1.000 | 0.022 | 0.047 | −0.002 | 0.010 | −0.044 | |
| CRP | 1.000 | −0.089 | 0.032 | −0.180 | −0.010 | ||
| TC | 1.000 | 0.422*** | 0.903*** | −0.469** | |||
| TG | 1.000 | 0.192 | 0.080 | ||||
| LDL | 1.000 | −0.386** | |||||
| HDL | 1.000 |
Note: *p< 0.05, **p< 0.01, ***p< 0.001.
Abbreviations: AMI, acute myocardial infarction; CRP, C-reactive protein; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglycerides; TC, serum cholesterol.
Discussion
This study reports that higher levels of CRP at admission in AMI patients predicts heart failure at follow up with an OR of about 2.4 (p<0.01). Inflammatory markers are important indicators for discrimination of different clinical forms of ACS. In one study, neutrophil-to-lymphocyte ratios (NLRs) were significantly higher in patients with AMI in relation to patients with UA. There was a positive correlation between the NLR and markers of myocardial necrosis, and between the NLR and CRP, indicating the importance of the NLR in the assessment of the extent of myocardial necrosis and inflammation severity.15 We have reported previously that even in chronic stable CAD patients, higher levels of CRP are present.16
Long-term risks of high CRP levels in ACS patients have been reported in a number of studies.17,18 After AMI in patients presenting with persistent ST elevation, high CRP levels predict worse outcomes.19 The time course of hsCRP in STEMI differs from NSTEMI, which may be predictive of worse outcomes.10 Suleiman et al18 have reported similar results to our previous study that in AMI, the CRP levels obtained up to 24 h after symptom onset act as an independent marker of mortality and heart failure after 30 days.20 hsCRP levels after AMI predicted emergence of heart failure in a study by Stumpf et al.7 Peak CRP is also a strong predictor of global and cardiovascular mortality during the following year after STEMI.7 Bursi et al21 reported in a recent study that CRP at admission to hospital is useful for predicting the time course of heart failure in patients with AMI. Obtaining peak hsCRP values is reported to be a strong independent predictor of global mortality and heart failure-related mortality in the following year of follow up.21 In line with our study is the report by Berton et al that measuring hsCRP on first day of AMI was independently associated with myocardial dysfunction, with heart failure indications in both models' age of patient, CK, past heart attacks of myocardial infarction, anterior site of AMI, and aldosterone levels all being other independent predictors for development of heart failure. When statin treatment was included in the models, the results remained virtually unchanged. Therefore, hsCRP measurement on admission to hospital significantly predicts the time course of heart failure in patients with AMI and is a strong independent predictor of global and heart failure mortality.22 Acute phase levels of hsCRP prior to marked elevations of cardiac troponin I (cTnI) may make the body prime to respond to any necrotic or injured tissue. This evidence is supported by De Servi et al23 depicting that in ACS patients there is a large variability in hsCRP levels, with those presenting with high hsCRP titers at baseline probably more hyperresponsive to circulating cTnI. Thus, myocardial necrosis, indicated by troponin increase, is the strongest stimulus for CRP increase in AMI, causing a disproportionate increase of troponin. Thus, the highest CRP values during ACS are likely to be observed in patients with already elevated CRP values at baseline (increasing the probability of mortality risk and subsequent attacks) and the highest troponin values (increasing the probability of mortality risk but not of subsequent heart attacks).
The study by Kavsak et al24 indicated that high CRP titers, independent of the subjects’ age, gender, and cTnI concentrations, predict long-term heart failure and mortality. Not only did CRP≥15 mg/L identify patients with heart failure at entry, it also predicted worsening of left ventricular function in patients presenting without clinical signs of heart failure at entry. Furthermore, in agreement with their results, CRP levels were a significant predictor of heart failure in our study in patients who did not have had any signs of heart failure at their first myocardial infarction. Although the exact role of CRP requires further exploration, our study focusing on hsCRP measurements within the first 3 days after AMI may prove useful for identification and prediction in patients who are at greater risk of heart failure and mortality. Scirica et al have reported that both hsCRP and BNP measured 30 days after ACS are independently associated with the risk of heart failure and cardiovascular mortality, with the greatest risk occurring when both markers are simultaneously high.6 CRP has been a marker of interest as a predictor of heart failure and pharmacological treatment benefits. In a metaanalysis by Zhou et al,25 trimetazidine administration in heart failure patients significantly improved symptoms and echocardiographic functions and improved CRP levels. cTnI and cardiac troponin C along with CRP are related to heart failure and left ventricular hypertrophy in chronic renal failure, which may prove useful in risk stratification of these subjects.26 Levocarnitine treatment has been shown to have a beneficial effect on hsCRP, cardiac troponin, and left ventricular diastolic dysfunction.27 Low-grade chronic inflammation indicated by CRP is reported to be a significant contributor to left atrial stiffening regardless of left ventricular size.28
Conclusions
CRP levels on admission to hospital after first myocardial infarction are associated with an increased risk of heart failure. Our study shows that inflammatory processes play an independent role in the development of heart failure after myocardial infarction. CRP is a useful marker for predicting the time course of heart failure in patients with AMI. Thus, hsCRP measurements may assist in risk stratification after myocardial infarction. Measurement of hsCRP levels in patients earlier in AMI could help clinicians to discriminate those patients who are at increased risk of heart failure in the future.
Acknowledgments
The authors are thankful to Antar Al Omani, Mujeeb ul Haq, and Miss Ester for their cooperation and technical support. Work on this study was supported by the Deanship of Scientific Research and International Research Group (IRG14-26) from King Saud University, Saudi Arabia.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gho JMIH, Postema PG, Conijn M, et al. Heart failure following STEMI: a contemporary cohort study of incidence and prognostic factors. Open Heart. 2017;4(2):e000551. doi: 10.1136/openhrt-2016-000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannsverk J, Steigen T, Wang H, et al. Trends in clinical outcomes and survival following prehospital thrombolytic therapy given by ambulance clinicians for ST-elevation myocardial infarction in rural sub-arctic Norway. Eur Heart J Acute Cardiovasc Care. 2017;2048872617748550. doi: 10.1177/2048872617748550 [DOI] [PubMed] [Google Scholar]
- 3.Osman R, L’Allier PL, Elgharib N, Tardif JC. Critical appraisal of C-reactive protein throughout the spectrum of cardiovascular disease. Vasc Health Risk Manag. 2006;2(3):221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2012;6(2):279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho JE, Gona P, Pencina MJ, et al. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J. 2012;33(14):1734–1741. doi: 10.1093/eurheartj/ehs070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu D, Qi X, Li Q, et al. Increased complements and high-sensitivity C-reactive protein predict heart failure in acutemyocardial infarction. Biomed Rep. 2016;5(6):761–765. doi: 10.3892/br.2016.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stumpf C, Sheriff A, Zimmermann S, et al. C-reactive protein levels predict systolic heart failure and outcome in patients with first ST-elevation myocardial infarction treated with coronary angioplasty. Arch Med Sci. 2017;13(5):1086–1093. doi: 10.5114/aoms.2017.69327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshinaga R, Doi Y, Ayukawa K, Ishikawa S. High-sensitivity C reactive protein as a predictor of inhospital mortality in patients with cardiovascular disease at an emergency department: a retrospective cohort study. BMJ Open. 2017;7(10):e015112. doi: 10.1136/bmjopen-2016-015112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juhaeri J, Gao S, Dai WS. Incidence rates of heart failure, stroke, and acute myocardial infarction among Type 2 diabetic patients using insulin glargine and other insulin. Pharmacoepidemiol Drug Saf. 2009;18(6):497–503. doi: 10.1002/pds.1741 [DOI] [PubMed] [Google Scholar]
- 10.Habib SS, Kurdi MI, Al Aseri Z, Suriya MO. CRP levels are higher in patients with ST elevation than non-ST elevation acute coronary syndrome. Arq Bras Cardiol. 2011;96(1):13–17. [DOI] [PubMed] [Google Scholar]
- 11.Vavuranakis M, Kariori MG, Kalogeras KI, et al. Biomarkers as a guide of medical treatment in cardiovascular diseases. Curr Med Chem. 2012;19(16):2485–2496. [DOI] [PubMed] [Google Scholar]
- 12.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/S0735-1097(00)00804-4 [DOI] [PubMed] [Google Scholar]
- 13.Braunwald E, Morrow DA. Unstable angina: is it time for a requiem? Circulation. 2013;127(24):2452–2457. doi: 10.1161/CIRCULATIONAHA.113.001258 [DOI] [PubMed] [Google Scholar]
- 14.Swedberg K, Cleland J, Dargie H, et al., Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the task force for the diagnosis and treatment of chronic heart failure of the european society of cardiology. Eur Heart J. 2005;26(11):1115–1140. doi: 10.1093/eurheartj/ehi204 [DOI] [PubMed] [Google Scholar]
- 15.Tahto E, Jadric R, Pojskic L, Kicic E. Neutrophil-to-lymphocyte ratio and its relation with markers of inflammation and myocardial necrosis in patients with acute coronary syndrome. Med Arch. 2017;71(5):312–315. doi: 10.5455/medarh.2017.71.312-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habib SS. Level of high-sensitivity C-reactive protein in Saudi patients with chronic stable coronary artery disease. J Ayub Med Coll Abbottabad. 2008;20(2):3–6. [PubMed] [Google Scholar]
- 17.Bode E, Wuppinger T, Bode T, et al. Risk stratification in stable coronary artery disease: superiority of N-terminal pro B-type natriuretic peptide over high-sensitivity C-reactive protein, gamma-glutamyl transferase, and traditional risk factors. Coron Artery Dis. 2012;23(2):91–97. doi: 10.1097/MCA.0b013e32834f1165 [DOI] [PubMed] [Google Scholar]
- 18.Suleiman M, Khatib R, Agmon Y, et al. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction. J Am Coll Cardiol. 2006;47:962–968. doi: 10.1016/j.jacc.2005.10.055 [DOI] [PubMed] [Google Scholar]
- 19.Canale ML, Stroppa S, Caravelli P, et al. Admission C-reactive protein serum levels and survival in patients with acute myocardial infarction with persistent ST elevation. Coron Artery Dis. 2006;17(8):693–698. doi: 10.1097/01.mca.0000236286.48812.8c [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro DR, Ramos AM, Vieira PL, et al. High-sensitivity C-reactive protein as a predictor of cardiovascular events after ST-elevation myocardial infarction. Arq Bras Cardiol. 2014;103(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bursi F, Weston SA, Killian JM, Gabriel SE, Jacobsen SJ, Roger VL. C-reactive protein and heart failure after myocardial infarction in the community. Am J Med. 2007;120(7):616–622. doi: 10.1016/j.amjmed.2006.07.039 [DOI] [PubMed] [Google Scholar]
- 22.Shacham Y, Topilsky Y, Leshem-Rubinow E, et al. Association between C-reactive protein level and echocardiography assessed left ventricular function in first ST-segment elevation myocardial infarction patients who underwent primary coronary intervention. J Cardiol. 2014;63(6):402–408. doi: 10.1016/j.jjcc.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 23.De Servi S, Mariani M, Mariani G, Mazzone A. C-reactive protein increase in unstable coronary disease cause or effect? J Am Coll Cardiol. 2005;46:1496–1502. doi: 10.1016/j.jacc.2005.05.083 [DOI] [PubMed] [Google Scholar]
- 24.Kavsak PA, MacRae AR, Newman AM, et al. Elevated C-reactive protein in acute coronary syndrome presentation is an independent predictor of long-term mortality and heart failure. Clin Biochem. 2007;40(5–6):326–329. doi: 10.1016/j.clinbiochem.2006.10.025 [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, Chen J. TMZ treatment in CHF patients may improve clinical symptoms and cardiac function, reduce hospitalization for cardiac causes, and decrease serum levels of BNP and CRP. Is treatment with trimetazidine beneficial in patients with chronic heart failure? PLoS One. 2014;9(5):e94660 eCollection 2014. doi: 10.1371/journal.pone.0094660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forghani MS, Jadidoleslami MS, Naleini SN, Rajabnia M. Measurement of the serum levels of serum troponins I and T, albumin and C-Reactive protein in chronic hemodialysis patients and their relationship with left ventricular hypertrophy and heart failure. Diabetes Metab Syndr. 2019;13(1):522–525. doi: 10.1016/j.dsx.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 27.Zhao G, Zhang H, Wang Y, Gao X, Liu H, Liu W. Effects of levocarnitine on cardiac function, urinary albumin, hs-CRP, BNP, and troponin in patients with coronary heart disease and heart failure. Hellenic J Cardiol. 2018;S1109-9666(18)30005–8. doi: 10.1016/j.hjc.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 28.Sani CM, Pogue EPL, Hrabia JB, et al. Association between low-grade chronic inflammation and depressed left atrial compliance in heart failure with preserved ejection fraction: a retrospective analysis. Folia Med Cracov. 2018;58(2):45–55. doi: 10.24425/fmc.2018.124657 [DOI] [PubMed] [Google Scholar]