To the editor,
The interstitial lung diseases (ILDs) are a diverse group of diffuse parenchymal lung disorders that commonly result in pulmonary fibrosis. ILDs are broadly classified according to known and unknown etiologies. Connective tissue disease-associated ILD (CTD-ILD) and chronic hypersensitivity pneumonitis (CHP) are among the most common ILDs of known etiology, while idiopathic pulmonary fibrosis (IPF) and unclassifiable ILD (U-ILD) are among the most common of unknown etiology.(1–4)
There exists substantial heterogeneity in natural history between ILD subtypes, but clinical characteristics such as age, gender and pulmonary function have been shown to predict mortality across ILD subtypes.(5) Baseline BMI has also been linked to outcomes in IPF,(6) but the clinical significance of longitudinal changes in BMI has not been reported. We hypothesized that weight loss would be associated with worse outcome in patients with commons forms of ILD
Methods
We conducted a retrospective cohort study (IRB approved protocol #875917) of patients with ILD at the University of California at Davis. Consecutive consenting patients with a multi-disciplinary diagnosis of IPF, CTD-ILD, CHP or U-ILD and at least two pulmonary function tests (PFTs) performed >90 days apart were identified using our ILD registry. The electronic medical record was used to extract pertinent clinical data. Vital status was determined by review of medical records and telephone communication. Weight loss was assessed using BMI calculated from height and weight reported on serial PFT reports, which are collected per institutional protocol. All available PFTs performed within 48 months of ILD center evaluation were included for review.
Statistical analysis
Longitudinal change in BMI was assessed using a maximum likelihood linear mixed effects model with time aligned to 3-month intervals. Baseline BMI, ILD diagnosis, GAP score,(7) race, smoking history, anti-fibrotic exposure and immunsuppressant exposure were included as fixed effects variables to adjust for potential confounders of weight change over time, with intercept term used to account for random effects. Mortality risk was assessed using Poisson regression with a robust variance estimator(8) and survival time assessed and plotted using the log rank test and Kaplan Meier estimator, respectively. BMI was modeled as a continuous measure and binary variable stratified by greater or less than 5% mean yearly decline. Statistical significance was defined as p<0.05. All statistical analyses were performed using Stata (Release 14; StataCorp, College Station, TX, USA).
Results
Of 291 patients screened, 225 met inclusion criteria. The mean age was 70 years and a majority was male (56%), white (74%) and reported a history of smoking (56%). The mean cohort BMI was 28.2, with 5% underweight, 15% normal weight, 41% overweight and 36% obese. The mean percent predicted forced vital capacity and diffusion capacity was 72% and 54%, respectively. A majority of patients carried a diagnosis of IPF (33%), followed by CTD-ILD (31%), CHP (19%) and U-ILD (17%).
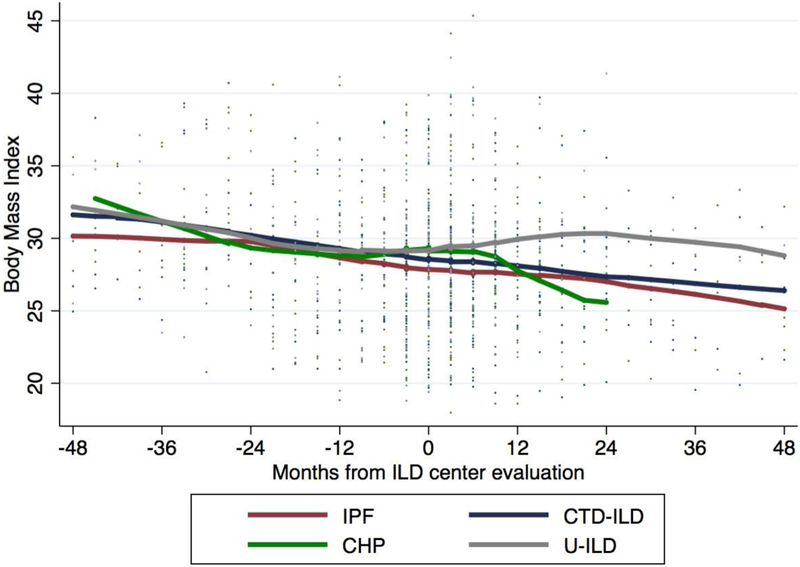
Forty-eight percent (n=108) of patients experienced ≥1% yearly BMI decline and 18% (n=40) experienced ≥5% yearly BMI decline during the study period. The estimated yearly decline in BMI was similar in across ILD subtypes (0.12 kg/m2; 95% CI 0.07–0.17; p<0.001 for IPF, 0.08 kg/m2; 95% CI 0.01–0.14; p=0.03 for CTD-ILD, 0.11 kg/m2; 95% CI −0.07–0.28; p=0.23 for CHP and 0.14 kg/m2; 95% CI 0.03–0.25; p=0.01 for U-ILD)(Figure 1a), but did not reach statistical significance in the CHP cohort. Baseline BMI was marginally associated with differential BMI decline, with obese individuals demonstrating the largest yearly decline.
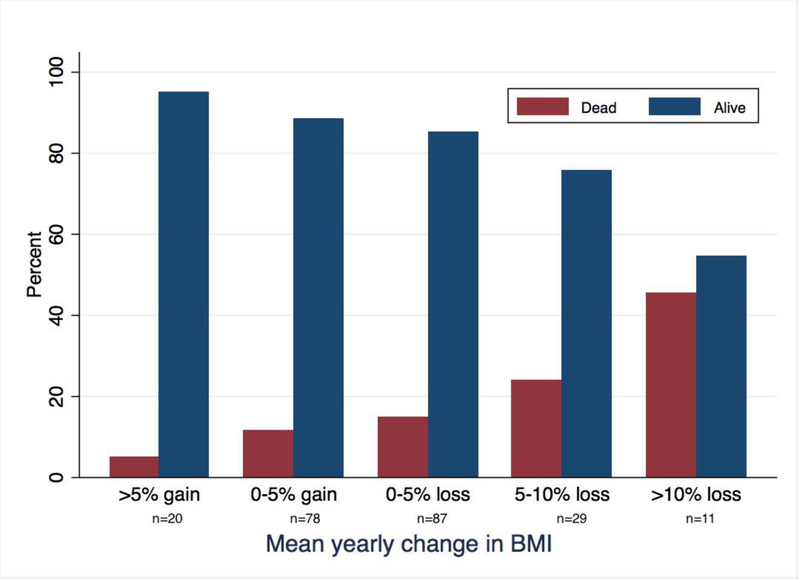
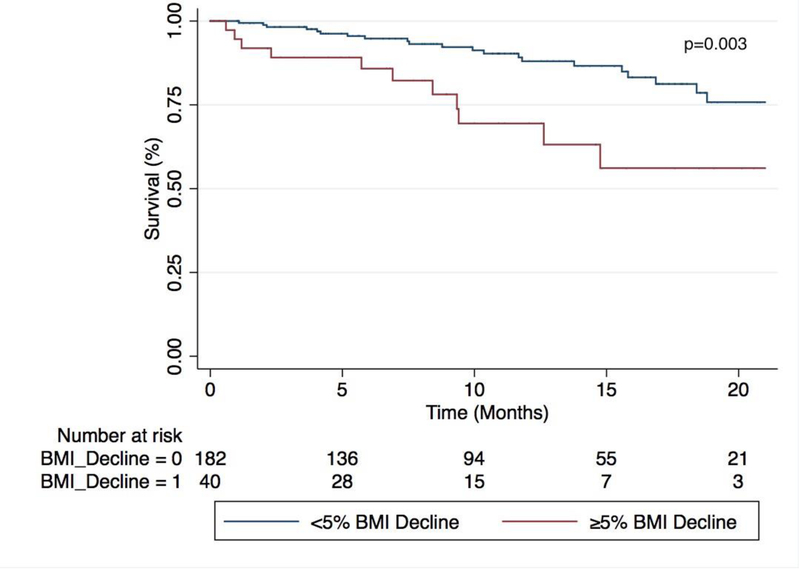
Figure 1.
Mean BMI change over time among ILD subtypes (a). Proportion of patients surviving with each 5% yearly change in BMI (b). Survival time stratified by BMI decline, with those experiencing ≥5% yearly BMI decline displaying significantly worse survival compared to those with <5% yearly BMI decline (p=0.003)(c).
In outcome analysis, there was no association between baseline BMI and mortality, but longitudinal BMI decline did correlate with increasing mortality (Figure 1b). Each 1% yearly decline in BMI was associated with a 5% increase in mortality risk in unadjusted analysis (RR 1.05; 95% CI 1.03–1.08, p<0.001), which persisted after multivariable adjustment (RR 1.04; 95% CI 1.01–1.06, p=0.004). These findings were driven primarily by patients with IPF (RR 1.06; 95% CI 1.01–1.10; p=0.008) and U-ILD (RR 1.09; 95% CI 1.0–1.2; p=0.05), as no association was observed in the CTD-ILD and CHP cohorts.
Survival time was significantly better in those with <5% yearly BMI decline when compared to those with ≥5% yearly BMI decline (Figure 1c)(p=0.003). Those with ≥5% yearly BMI decline had 2-fold higher risk of death compared to those with <5% yearly BMI decline (RR 2.41, 95% CI 1.31–4.44; p=0.005) in unadjusted analysis, which persisted after multivariable adjustment (RR 2.0, 95% CI 1.08–3.71; p=0.03). These findings were again driven primarily by patients with IPF (RR 3.1; 95% CI 1.08–8.88; p=0.03) and U-ILD (RR 9.21; 95% CI 1.18–72.0; p=0.03), as no association was observed in the CTD-ILD and CHP cohorts.
Discussion
In this study we found that weight loss is common among patients with ILD and is associated with an increased risk of death in those with IPF and U-ILD. Just as pulmonary function decline is a hallmark of progressive ILD,(9–11) our findings suggest that weight loss may also serve as a longitudinal marker of ILD progression. To our knowledge, this study if the first to explore the clinical implications of weight loss among patients with ILD.
While obesity is a well-recognized risk factor for cardiovascular and metabolic conditions, unintentional weight loss is a well-described phenomenon in chronic illness, such as emphysema and congestive heart failure.(12, 13) It has been proposed that low BMI may be a determinant of poor survival in those with emphysema due to respiratory muscle weakness, impaired gas exchange, and impaired immune response.(14) Similar phenomena may be at play in patients with ILD.
Our observed association between BMI decline and mortality risk in IPF and U-ILD, but not CTD-ILD and CHP, suggests that progressive disease may manifest differently in fibrotic-predominant disease compared to mixed inflammatory/fibrotic disease. However, the small sample size within each ILD subtype may have limited our ability to detect this association in those with CTD-ILD and CHP.
We did not find an association between baseline BMI and survival in our analysis. A previous investigation by Alakhras and colleagues showed that a higher baseline BMI was associated with improved outcomes,(6) while Kondoh and colleagues found that higher BMI was associated with increased acute exacerbation risk.(15) The conflicting results of these investigations, along with our findings, leave it unclear the extent to which baseline BMI influences outcomes in this patient population.
Our study has several limitations. First the retrospective nature of our study allows for assessment of association, not causation. Second, the generalizability of our results may be limited given that our study was single center and performed in patients somewhat older than other reported ILD populations. Third, few patients had complete PFT data, resulting in a large number of missing time points for each patient. Finally, it was not possible to systematically ascertain whether the observed weight loss was unintentional, but the overwhelming majority of patients did not endorse intentional weight loss on chart review.
Conclusions
Weight loss is a common finding among patients with ILD and appears to be a marker of disease progression in those with IPF and U-ILD. Further research is needed elucidate the biology underpinning this observation and to determine whether this finding is generalizable to other ILD cohorts. Finally, an assessment of whether nutritional augmentation in those with ILD experiencing ongoing weight loss modulates disease course is warranted.
Funding
This study was funded by a grant from the National Heart Lung and Blood Institute (K23HL138190)
Footnotes
Publisher's Disclaimer: This manuscript has recently been accepted for publication in the European Respiratory Journal. It is published here in its accepted form prior to copyediting and typesetting by our production team. After these production processes are complete and the authors have approved the resulting proofs, the article will move to the latest issue of the ERJ online.
References
- 1.Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet 2012; 380: 689–698. [DOI] [PubMed] [Google Scholar]
- 2.Selman M, Pardo A, King TE Jr., Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. American journal of respiratory and critical care medicine 2012; 186: 314–324. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr. Kondoh Y Myers J Muller NL Nicholson AG Richeldi L Selman M Dudden RF Griss BS Protzko SL Schunemann HJ An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryerson CJ, Urbania TH, Richeldi L, Mooney JJ, Lee JS, Jones KD, Elicker BM, Koth LL, King TE Jr. Wolters PJ , Collard HR . Prevalence and prognosis of unclassifiable interstitial lung disease. The European respiratory journal 2013; 42: 750–757. [DOI] [PubMed] [Google Scholar]
- 5.Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, Elicker BM, Wolters PJ, Koth LL, King TE Jr., Collard HR. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014; 145: 723–728. [DOI] [PubMed] [Google Scholar]
- 6.Alakhras M, Decker PA, Nadrous HF, Collazo-Clavell M, Ryu JH. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 2007; 131: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 7.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD, King TE Jr., Collard HR A multidimensional index and staging system for idiopathic pulmonary fibrosis. Annals of internal medicine 2012; 156: 684–691. [DOI] [PubMed] [Google Scholar]
- 8.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt SL, Tayob N, Han MK, Zappala C, Kervitsky D, Murray S, Wells AU, Brown KK, Martinez FJ, Flaherty KR. Predicting Pulmonary Fibrosis Disease Course from Past Trends in Pulmonary Function. Chest 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zappala CJ, Latsi PI, Nicholson AG, Colby TV, Cramer D, Renzoni EA, Hansell DM, du Bois RM, Wells AU. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. The European respiratory journal 2010; 35: 830–836. [DOI] [PubMed] [Google Scholar]
- 11.Gimenez A, Storrer K, Kuranishi L, Soares MR, Ferreira RG, Pereira CAC. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax 2018; 73: 391–392. [DOI] [PubMed] [Google Scholar]
- 12.Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. The European respiratory journal 2002; 20: 539–544. [DOI] [PubMed] [Google Scholar]
- 13.Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 2003; 361: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 14.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 1999; 160: 1856–1861. [DOI] [PubMed] [Google Scholar]
- 15.Kondoh Y, Taniguchi H, Katsuta T, Kataoka K, Kimura T, Nishiyama O, Sakamoto K, Johkoh T, Nishimura M, Ono K, Kitaichi M. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG/World Association of Sarcoidosis and Other Granulomatous Disorders 2010; 27: 103–110. [PubMed] [Google Scholar]