Abstract
The leading cause of bacterial gastroenteritis, Campylobacter jejuni, is a Gram-negative pathogen that contains a unique O-methyl phosphoramidate (MeOPN) on its capsular polysaccharide. Previously, MeOPN has been linked to the evasion of host immune responses and serum resistance. Despite the involvement of MeOPN in pathogenicity, the complete biosynthesis of this modification is unknown; however, the first four enzymatic steps have been elucidated. The second enzyme in this pathway, Cj1416, is a CTP:phosphoglutamine cytididylyltransferase that catalyzes the displacement of pyrophosphate from MgCTP by L-glutamine phosphate to form CDP-L-glutamine. Initially, Cj1416 was predicted to use phosphoramidate to form cytidine diphosphoramidate but no activity was detected with MgATP as a substrate. However, in the presence of MnCTP, Cj1416 can directly catalyze the formation of cytidine diphosphoramidate from phosphoramidate and MnCTP. Here we characterize the manganese-induced promiscuity of Cj1416. In the presence of Mn2+, Cj1416 catalyzes the formation of 12 different reaction products using L-glutamine phosphate, phosphoramidate, methyl phosphate, methyl phosphonate, phosphate, arsenate, ethanolamine phosphate, glycerol-1-phosphate, glycerol-2-phosphate, serinol phosphate, L-serine phosphate or 3-phospho-D-glycerate as the nucleophile to displace pyrophosphate from CTP.
Graphical Abstract
INTRODUCTION
Campylobacter jejuni, a Gram-negative pathogenic bacteria, is the leading cause of bacterial gastroenteritis world wide.1, 2 Like many Gram-negative bacteria, C. jejuni produces a capsular polysaccharide (CPS), which helps improve the overall fitness and pathogenicity of this organism.3 Approximately 70% of all C. jejuni strains contain an O-methyl phosphoramidate (MeOPN) modification to the capsular polysaccharide (CPS).4 This decoration is unique to the genus Campylobacter and phosphorus nitrogen bonds are relatively rare in nature.5 In the NCTC11168 strain of C. jejuni, several gene knock-out studies have been conducted and eight genes appear to be responsible for the biosynthesis of the MeOPN modification to the CPS.4 The role of MeOPN was examined using mutants that lacked this modification of its CPS and it was shown that MeOPN contributes to serum resistance and evasion of host immune responses.6
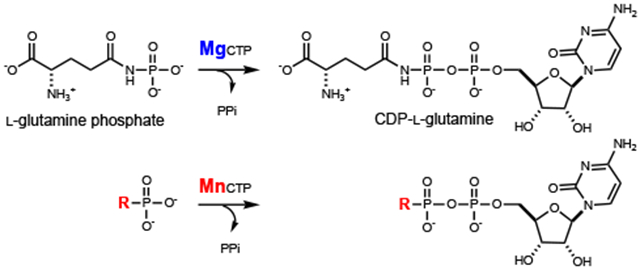
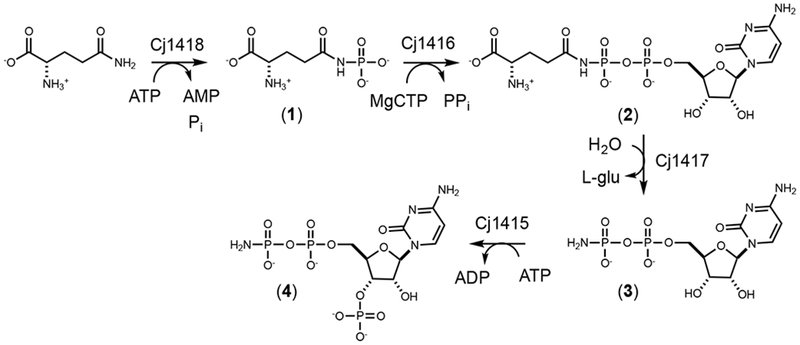
Of the eight genes directly linked to the presence of MeOPN, four have previously been characterized. The first enzyme in the pathway, Cj1418, represents a novel L-glutamine kinase that catalyzes the ATP-dependent phosphorylation of L-glutamine, resulting in L-glutamine phosphate (1).7, 8 The second enzyme, Cj1416, uses L-glutamine phosphate and MgCTP to form CDP-L-glutamine (2).9 CDP-L-glutamine is then hydrolyzed by Cj1417 to generate L-glutamate and cytidine diphosphoramidate (3).9 Finally, Cj1415 catalyzes the phosphorylation of the 3’-hydroxyl group of cytidine diphosphoramidate to make 3’-phosphocytidine 5’-diphosphoramidate (4), a cofactor that shares similarity with 3’-phosphoadenosine 5’-phosphosulfate (PAPS), which is used in the transfer of sulfate to various acceptors.10 The remaining four enzymes, Cj1419, Cj1420, Cj1421, and Cj1422 are likely responsible for the transfer of the phosphoramidate moiety from 3’-phosphocytidine 5’-diphosphoramidate to a carbohydrate substrate and subsequent methylation to generate the O-methyl phosphoramidate product.
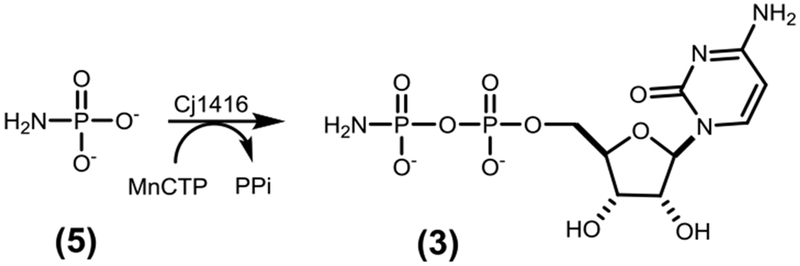
Throughout the characterization of the first four steps in the formation of 3’-phospho-5’-cytidine diphosphoramidate (4) as illustrated in Scheme 1, alternative pathways for the biosynthesis of the phosphoramidate cofactor were postulated. In these other transformations, Cj1416 was initially proposed to use phosphoramidate (5) as a substrate with a nucleotide triphosphate (NTP) to form the corresponding NDP-amidate (3). Cj1416 was initially assayed for its ability to catalyze the formation of an NDP-amidate using phosphoramidate and the Mg2+ complexes of various nucleotide triphosphates.9 However, no significant activity with phosphoramidate was detected with any of the NTPs tested in the presence of Mg2+, but catalytic activity was detected when the Mn2+ was used as the divalent cation (Scheme 2). This promiscuous catalytic activity with Cj1416 and MnCTP was unexpected, but other nucleotide utilizing enzymes have previously been shown to alter their substrate profile when different divalent cations have been substituted for Mg2+. Pyruvate kinase, for example, catalyzes the Mg2+-dependent phosphorylation of pyruvate with ATP. If Zn2+ is used in place of Mg2+, the enzyme is able to phosphorylate hydroxylamine to form hydroxylamine phosphate.11 DNA polymerase has been shown to use Mn2+ but significantly more errors are made in copying DNA, compared with the fidelity observed with Mg2+.12, 13 Error-prone PCR uses the enhanced Mn2+-dependent error rate to generate libraries of mutants for enzyme evolution investigations.14 Dihydroxyacetone kinase catalyzes the phosphorylation of dihydroxyacetone with MgATP, but in the presence of Mn2+ the enzyme acts as a cyclase, catalyzing the cyclization of flavin adenine dinucleotide (FAD) to riboflavin 4’,5’-cyclic phosphate and AMP.15
Scheme 1:
Biosynthesis of 3’-phospho-5’-cytidine diphosphoramidate.
Scheme 2:
Formation of cytidine diphosphoramidate by Cj1416 and MnCTP.
In this paper we have more fully characterized the expansion in the substrate profile of Cj1416 when the reaction is catalyzed in the presence of MnCTP. We have shown that Cj1416 can catalyze the formation of 12 different reaction products when MnCTP is used as one of the substrates. The products have been characterized by 31P-NMR spectroscopy and ESI (negative mode) mass spectrometry.
MATERIALS and METHODS
Materials.
All buffers and salts were purchased from Sigma-Aldrich, unless otherwise specified. Phosphate, methyl phosphonate, arsenate, ethanolamine phosphate, 3-phospho-D-glycerate, racemic glycerol-1-phosphate, glycerol-2-phosphate, and L-serine phosphate were purchased from Sigma-Aldrich. Phosphoramidate (5), L-glutamine phosphate (1) and (R/S)-serinol phosphate (14) were synthesized as reported previously.7, 16, 17 Methyl phosphate (7) was synthesized by adding dichloromethyl phosphate to water. The plasmid (pET-30b) for expression of Cj1416 from C. jejuni NCTC 11168 was obtained from Professor Christine Szymanski of the University of Georgia.
Purification of CTP:Phosphoglutamine Cytidylyltransferase.
The plasmid used for the expression of Cj1416 (UniProt: Q0P8J8) with a C-terminal poly-histidine purification tag was used to transform Rosetta (DE3) Escherichia coli cells by electroporation. Cultures (5 mL) of LB medium supplemented with 50 μg/mL kanamycin and 25 μg/mL chloramphenicol were inoculated with a single colony and grown overnight at 37 °C. These cultures were used to inoculate 1 L of LB medium containing 50 μg/mL kanamycin and 25 μg/mL chloramphenicol, and then incubated at 30 °C until an OD600 of ~0.6-0.8 was achieved. Gene expression was induced with 1.0 mM isopropyl β-thiogalactoside (IPTG), grown for 16 h at 16 °C and then harvested by centrifugation at 6300 g at 4 °C. The resulting cell pellet was resuspended in loading buffer (50 mM HEPES/K+, 300 mM KCl, 20 mM imidazole, pH 8.0) and lysed by sonication. Cell debris was removed by centrifugation at 26,000 g, and then passed through a 0.45 μm filter before being loaded onto a prepacked 5-mL HisTrap HP (GE Healthcare) nickel affinity column. Protein was eluted with 30 column volumes using a gradient of 20-400 mM imidazole in 50 mM HEPES/K+ pH 8.0, 300 mM KCl. Excess imidazole was removed by exchanging the buffer against 50 mM HEPES/K+, pH 8.0, and 100 mM KCl, using a 20-mL (10 kDa molecular weight cutoff) concentrator (GE Healthcare). Enzyme was divided into smaller aliquots, frozen in liquid nitrogen and stored at −80 °C until needed.
31P-NMR Experiments.
31P-NMR analysis was performed on a Bruker Ascend 400 MHz instrument with phosphoric acid as the reference at 0.0 ppm. Reactions (600 μL) contained 2.5 mM CTP, 5.0 mM substrate and 5.0 mM MnCl2 in 100 mM HEPES/K+, 100 mM KCl, pH 8.0, with 15 μM Cj1416 for 8 h at 30 °C while shaking. Control reactions were conducted under the same conditions with no added enzyme. After incubation, 50 μL of 1 M KOH was added to the reaction mixture. The samples were vortexed and the oxidized manganese was removed by centrifugation at 18,000 g in a microcentrifuge for 5 min. The reaction mixtures were centrifuged two more times to remove all of the remaining manganese. After the third centrifugation, 10 mM EDTA and 100 μL of D2O was added to the sample and the product analyzed by 31P-NMR spectroscopy.
Reaction Rates.
The rates of the reactions catalyzed by Cj1416 were determined by measuring the change in substrate and product concentrations using anion exchange chromatography and following the reaction on an FPLC by monitoring the wavelength of 255 nm. The column used was a prepackaged 1-mL HiTrap Q HP column from GE Healthcare. A sample of 600 μL was prepared, which contained 1.0 mM CTP, 4.0 mM MnCl2, 10 mM of the substrate to be tested, and 5.0 μM Cj1416 in 100 mM HEPES/K+, 100 mM KCl, pH 8.0. Samples of 100 μL were injected onto the column every 12.5 min. A total of 17 column volumes were used to elute the sample using a gradient of 0-17% 10 mM triethanolamine, 2 M KCl, pH 8.0 with a flow rate of 2.0 mL min−1. CMP, CDP and CTP standards were used to determine their relative elution times as 5.5, 7.1 and 8.3 min, respectively.
Negative ESI Mass Spectrometry.
Electrospray ionization mass spectrometry (ESI-MS) experiments were performed using a Thermo Scientific Q Exactive Focus. The sample was directly infused at a flow rate of 10 μL/min. The Q Exactive Focus HESI source was operated in full MS in negative mode. The mass resolution was tuned to 17500 FWHM at m/z 200. The spray voltage was set to 3.30 kV, and the sheath gas and auxiliary gas flow rates were set to 7 and 0 arbitrary units, respectively. The transfer capillary temperature was held at 250 °C and the S-Lens RF level was set at 50 v. Exactive Series 2.8 SP1/Xcalibur 4.0 software was used for data acquisition and processing.
Samples for negative mode ESI mass spectrometry were prepared as follows, unless otherwise stated. Reaction of 1.0 mL containing 5.0 mM CTP, 9.0 mM MnCl2, 10 mM substrate, and 30 μM Cj1416 were incubated together in 100 mM HEPES/K+, 100 mM KCl, pH 8.0 for 16 h, while shaking. Samples were then centrifuged for 5 min at 15,500 g in a microcentrifuge. The supernatant solution was loaded onto an anion exchange column and the product eluted with a total of 35 column volumes using a gradient of 0-35% of 1.0 M ammonium bicarbonate, pH 8.0. At high pH, the initial products formed using either 3-phospho-D-glycerate (11), glycerol-1-phosphate (12) or glycerol-2-phosphate (13) degrade to a cyclic phosphate species and CMP. Unfractionated reactions using these three substrates were submitted for ESI analysis. All conditions were the same as before, except ammonium acetate (pH 8.0) was used in place of the HEPES buffer.
RESULTS and DISCUSSION
Substrate Specificity of Cj1416.
Cj1416 was previously characterized as a CTP:phosphoglutamine cytididylyltransferase, which catalyzes the formation of CDP-L-glutamine (2) from L-glutamine phosphate (1) and MgCTP (Scheme 1).9 When Cj1416 was incubated with MgCTP and phosphoramidate (5) and the reaction followed by anion exchange chromatography, no significant activity could be detected (kcat <1 h−1). Since Cj1416 appeared inactive with phosphoramidate using Mg2+ as a cofactor, Mn2+ was substituted as a potential divalent cation for the enzyme. Incubation of Cj1416 with MnCTP and phosphoramidate resulted in the consumption of CTP and formation of PPi and cytidine diphosphoramidate (3), as demonstrated by changes in the FPLC chromatogram (Figure 1) and 31P-NMR spectroscopy. The 31P-NMR spectrum of the reaction products revealed the appearance of two new resonances at −0.40 and −10.15 ppm, consistent with the formation of cytidine diphosphoramidate and an additional singlet (−5.01 ppm) from the PPi reaction product (Figure 2).
Figure 1:
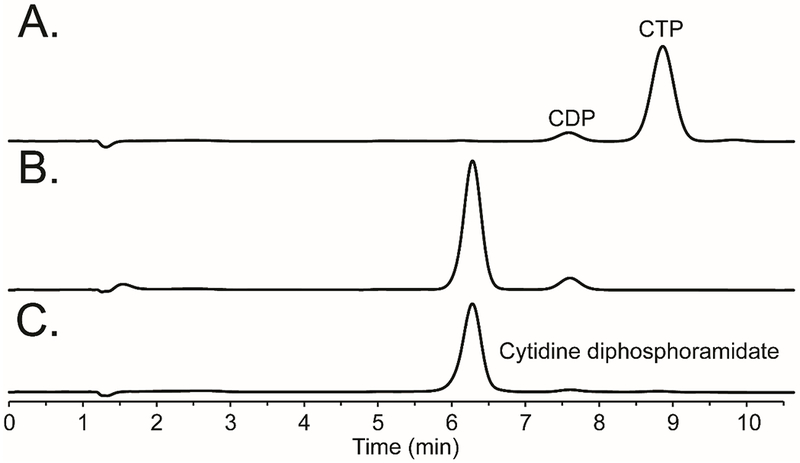
Anion exchange chromatograms for the reaction of MnCTP and phosphoramidate (5). (A) Control sample containing 1.0 mM CTP, 4.0 mM MnCl2, and 10 mM phosphoramidate in 100 mM HEPES/K+, pH 8.0, and 100 mM KCl. (B) Same reaction mixture as in (A), plus the addition of 5.0 μM Cj1416. (C) Authentic cytidine diphosphoramidate standard.
Figure 2:
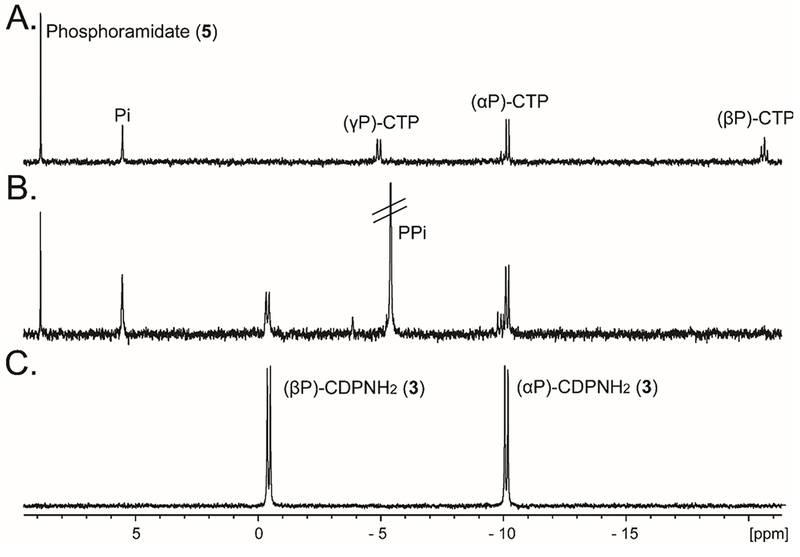
31P-NMR spectra for the reaction of MnCTP and phosphoramidate (5). (A) 31P-NMR spectrum for the reaction mixture containing 2.5 mM CTP, 5.0 mM phosphoramidate and 5.0 mM MnCl2 in 100 mM HEPES/K+, 100 mM KCl , at pH 8.0 for 8 h at 30 °C. The pH was adjusted to 12 to oxidize the manganese, centrifuged and then 10 mM EDTA was added. (B) Same reaction conditions as in (A), except 15 μM Cj1416 was added. (C) Control sample of authentic cytidine diphosphoramidate (3).
Since Cj1416 is able to catalyze the formation of cytidine diphosphoramidate (3) using MnCTP, the breadth of this promiscuous activity with other substrates was explored. Methyl phosphate (7) and methyl phosphonate (8) were both assayed as possible substrates, since they are each similar in size and shape to phosphoramidate (5). Cj1416 catalyzed the formation of the O-methyl ester of CDP (17) and CMP methyl phosphonate (18). In total, 35 different compounds were assayed for catalytic activity with Cj1416 and MnCTP (Scheme S1). Of these 35 compounds, 12 showed product formation when analyzed by 31P-NMR spectroscopy after an incubation period of 8 h with 15 μM Cj1416 and 2.5 mM MnCTP (Scheme 3). The apparent rate constants for the reaction of Cj1416 with 1.0 mM MnCTP and 10 mM substrate are presented in Table 1.
Scheme 3:
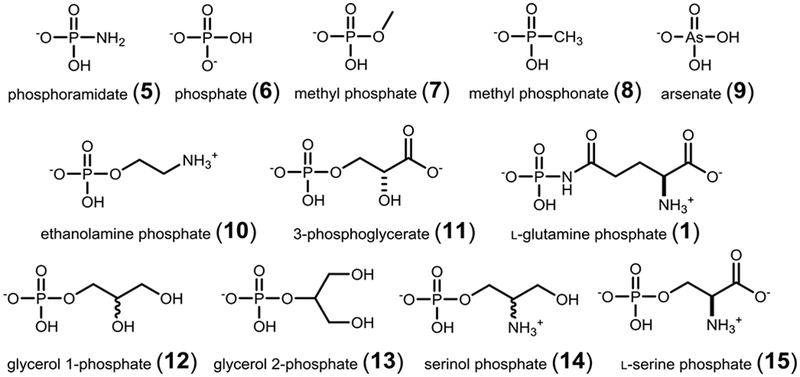
Compounds identified as substrates with MnCTP and Cj1416.
Table 1:
Characterization of Reaction Products Using Cj1416 with MnCTP and Various Substrates.
| Substrate | Expected [M-H]− | Observed [M-H]− | Rate Constant | α−31P (ppm) | β−31P (ppm) |
|---|---|---|---|---|---|
| phosphoramidate (5) | 401.02 | 401.02 | 36 ± 2 h−1 | −10.15 | −0.40 |
| phosphate (6) | 402.01 | 402.01 | 11 ± 1 h−1 | −9.85 | −5.33 |
| methyl phosphate (7) | 416.02 | 416.02 | 4 ± 0.3 h−1 | −10.53 | −8.84 |
| methyl phosphonate (8) | 400.03 | 400.03 | 9 ± 1 h−1 | −10.59 | 18.18 |
| arsenate (9) | 446.96 | 322.04 | 11 ± 1 h−1 | 4.51a | NA |
| ethanolamine phosphate (10) | 445.05 | 445.05 | 1 ± 0.2 h−1 | −10.58 | −9.91 |
| 3-phospho-D-glycerate (11) | 490.02 | 490.02 | 220 ± 20 h−1 | 4.51a | 18.88b |
| L-glutamine phosphate (1) | 530.06 | 530.06 | 21 ± 2 h−1 | −10.79 | −15.64 |
| (R/S)-glycerol-1-phosphate (12) | 476.04 | 476.04 | 4 ± 0.7 h−1 | 4.51a | 19.16b |
| glycerol-2-phosphate (13) | 476.04 | 476.04 | 5 ± 0.5 h−1 | 4.51a | 19.16b |
| (R/S)-serinol phosphate (14) | 475.06 | 475.06 | 1 ± 0.2 h−1 | −10.56 | −9.96 |
| L-serine phosphate (15) | 489.04 | 489.04 | 11 ± 2 h−1 | −10.47 | −10.11 |
The α-phosphoryl group is for CMP
Cyclic degradation products
NA: Not applicable for arsenate.
Characterization of Cj1416 Reaction Products:
The predicted initially formed products for Cj1416 with MnCTP and the substrates in Scheme 3 are shown in Scheme 4. The Cj1416 catalyzed reaction products, cytidine diphosphoramidate (3), CDP-L-glutamine (2), CDP (16), and CDP-ethanolamine (20) using phosphoramidate (5), L-glutamine-P (1), phosphate (6), and ethanolamine-P (10) are consistent with their previously published 31P-NMR spectra (Figures 2 and 3).9, 18 The chemical shifts for four other reaction products, O-methyl CDP (17), CMP methyl phosphonate (18), CDP-L-serine (25) and CDP-serinol (24) are also consistent with their predicted structures (Figure S1). The remaining four substrates, 3-phospho-D-glycerate (11), (R/S)-glycerol-1-phosphate (12), glycerol 2-phosphate (13) and arsenate (9) all exhibited 31P-NMR spectra that were inconsistent with the predicted products.
Scheme 4:
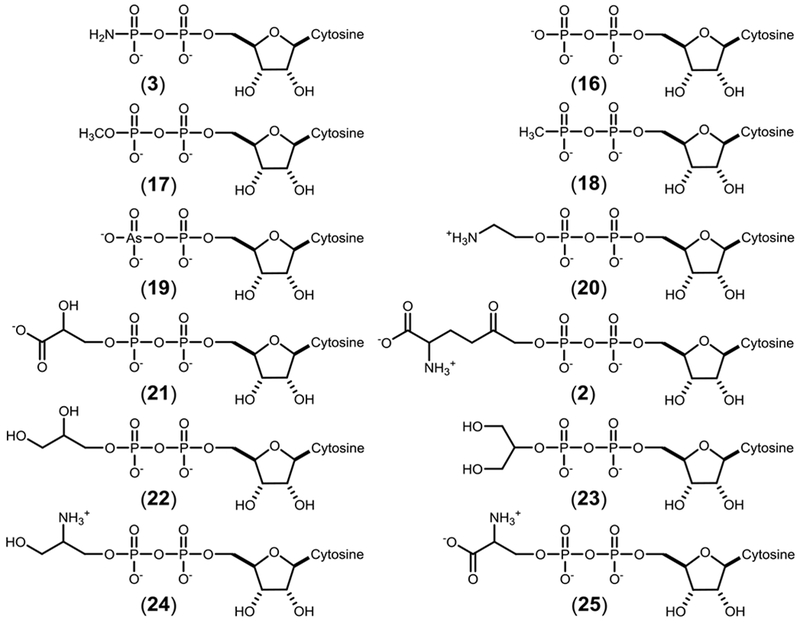
Initial reaction products formed by Cj1416 using MnCTP and the substrates shown in Scheme 3.
Figure 3:
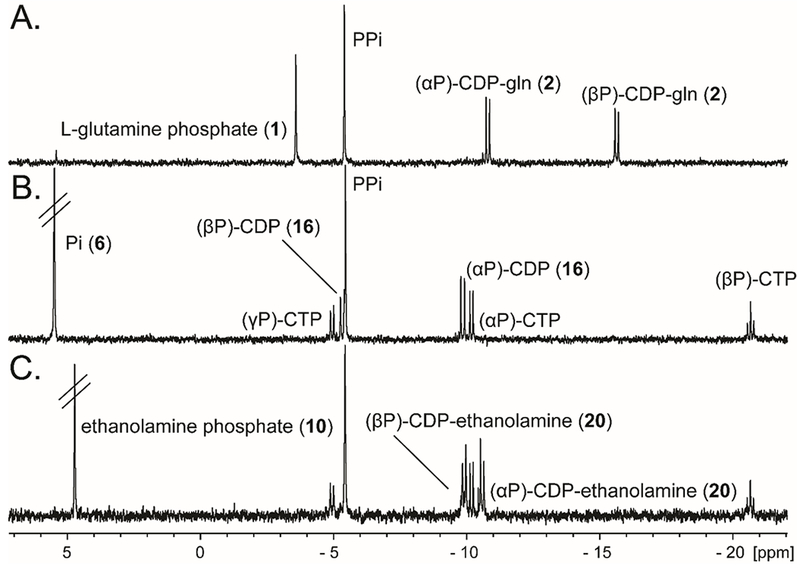
31P-NMR spectra of the Cj1416 catalyzed reaction products. Samples initially contained 2.5 mM CTP, 5.0 mM substrate and 5.0 mM MnCl2 in 100 mM HEPES/K+, 100 mM KCl, pH 8.0, and were incubated for 8 h at 30 °C while shaking with 15 μM Cj1416. The pH was adjusted to 12 to oxidize the manganese. After removal of manganese by centrifugation, 10 mM EDTA was added to the sample to sequester any remaining Mn2+. (A) L-glutamine phosphate and formation of L-glutamine CDP (2). (B) Phosphate and formation of CDP (16). (C) Ethanolamine phosphate and formation of ethanolamine CDP (20).
In the reaction with arsenate, a new 31P-NMR resonance is consistent with the formation of PPi, indicating that there was turnover of MnCTP. However, CMP-arsenate (19) was not detected but CMP was observed. The formation of pyrophosphate is consistent with arsenate being a substrate for the enzyme, but the CMP-arsenate product is anticipated to be unstable and degrades to CMP and arsenate (Figure S1). The FPLC chromatograph of the reaction mixture is also consistent with rapid formation of the CMP degradation product. With (R/S)-glycerol-1-phosphate (12), glycerol-2-phosphate (13), and 3-phospho-D-glycerate (11) all of the initially anticipated products were predicted to have a pair of doublets in their 31P-NMR spectra due to 31P-31P spin coupling. However, for these three reaction products, new singlets are observed at 4.51 ppm (for CMP) and at ~19 ppm (Figure 4). The proton coupled 31P-NMR spectrum of the 3-phospho-D-glycerate reaction product that resonates at 4.51 is a triplet, consistent with the formation of CMP and another resonance at 18.88 ppm, consistent with the formation a cyclic phosphate product as illustrated in Scheme 5. The observed cyclic degradation products are believed to be formed during the procedure to remove the manganese from the reaction mixture. The initial formation of CDP-3-D-glycerate is observed in the FPLC chromatogram when MnCTP is incubated with 3-phospho-D-glycerate in the presence of Cj1814 (Figure S2). Other examples of five-membered cyclic phosphate species have previously been made chemically using high pH.19, 20
Figure 4:
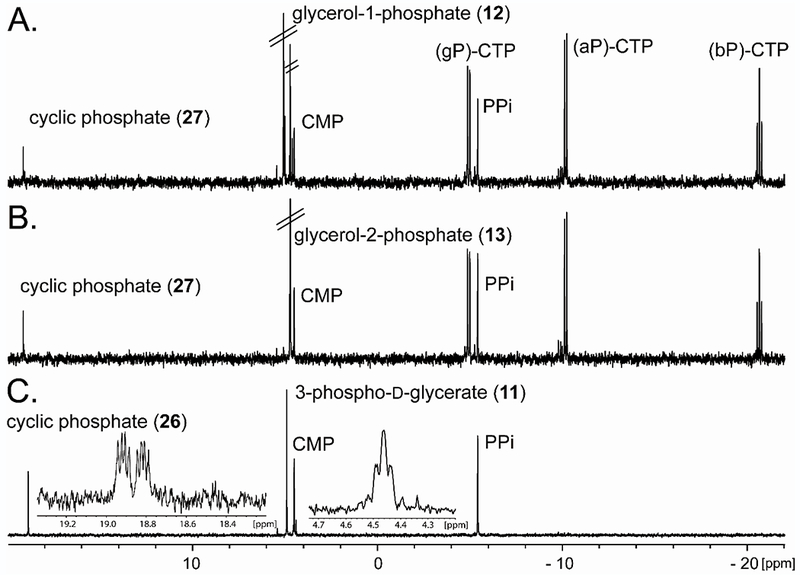
31P NMR spectra. 31P NMR of a reaction containing 2.5 mM CTP, 5.0 mM substrate and 5.0 mM MnCl2 in 100 mM HEPES/KOH, 100 mM KCl at pH 8.0 with for 8 h at 30 °C while shaking with 15 μM Cj1416. The pH was adjusted to 12 to oxidize manganese. After removal of manganese by centrifugation 10 mM EDTA was added to the sample. (A) Glycerol-1-phosphate and formation of CMP and cyclic glycerol phosphate (27). (B) Glycerol-2-phosphate and the formation of CMP and cyclic glycerol phosphate (27). (C) 3-Phospho-D-glycerate and the formation of cyclic 3-phospho-D-glycerate (26). Insets show the 31P-1H coupled spectra.
Scheme 5:
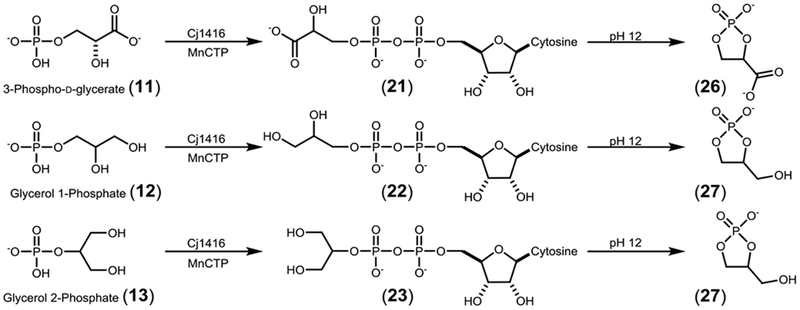
Cyclic phosphate degradation products.
ESI-negative mode mass spectrometry was used to analyze all of the Cj1416 catalyzed reaction products using MnCTP as the nucleotide substrate (Table 1). Table 1 shows the predicted and observed masses for each of the 12 products. The spectra for CDP-glycerol (22), and CDP-2-glycerol (23) both show a mass of 476.04 consistent with the formation of their respective CDP-glycerol products. With CDP-2-glycerol (23) masses of 152.99 and 322.04 are consistent with the cyclic phosphate (27) degradation product and CMP respectively. The 3-phosphoglycerate reaction indicates a mass of 490.02 consistent with the formation of CDP-3-D-glycerate (21). The mass spectrum is also consistent with formation of CMP (322.04) and the cyclic phosphate species (166.97) (26). This confirms our prediction that CDP-glycerol, CDP-2-glycerol, and CDP-3-D-glycerate are the enzymatic products, and that the degradation products occur due to the high pH in which the samples were exposed.
Cj1416 Relative Rates.
The rates observed with MnCTP and Cj1416 are relatively slow. In the presence of MnCTP, L-glutamine phosphate is utilized with an apparent kcat of 21 ± 2 h−1, compared to a kcat of 3400 ± 400 h−1 with MgCTP, resulting in a >100-fold reduction in rate.9 With phosphoramidate and MnCTP, the apparent kcat is 36 ± 2 h−1 but no significant activity (kcat <1 h−1) was detected with MgCTP as the cosubstrate. The most surprising substrate was 3-phospho-D-glycerate (11). An apparent kcat of 220 ± 20 h−1 was observed with this substrate and MnCTP, and thus the promiscuous activity of 3-phospho-D-glycerate with MnCTP is an order of magnitude faster than the activity of the physiological substrate L-glutamine phosphate when MnCTP is used as the nucleotide. When 3-phospho-D-glycerate was examined with MgCTP as the cofactor, no significant activity was observed (kcat <1 h−1), thus demonstrating a >200-fold increase in rate with Mn2+ as a divalent cation, compared to Mg2+.
Manganese-Induced Promiscuity of Cj1416.
The effects of various divalent cations on enzyme catalysis have previously been studied. In many cases the effects of divalent cations have been addressed in the terms of reaction rates, but promiscuity has rarely been examined. DNA polymerase is well known to exhibit promiscuity when Mg2+ is replaced by Mn2+. The promiscuous activity has been exploited in error-prone PCR procedures to generate random mutations in genes of interest. A physical explanation for how Mn2+ induces this effect on DNA polymerase is unknown. DNA polymerases generally require two metals for catalysis; “metal A” helps to lower the pKa of the 3’-hydroxyl group of the primer and coordinates the α-phosphoryl group of the incoming nucleotide while “metal B” is coordinated to the incoming nucleotide and helps negate the negative charge of the triphosphate moiety of the substrate.12 It is unclear which one of these two metals in DNA polymerase is responsible for the Mn2+ promiscuity. Magnesium and manganese are similar in several ways; both metals utilize an octahedral coordination geometry, have a similar ionic radius (Mg2+ 0.86 Å, Mn2+ 0.81 Å), and have a similar effect on the pKa of coordinated water (Mg2+ 11.4, Mn2+ 11.5).21 However, if the average covalent bond length is examined in all high resolution Mg2+ and Mn2+ protein crystal structures, Mg2+ has an average bond length of 2.09 Å, and Mn2+ 2.22 Å.22 The higher average bond length may help Mn2+ form distorted octahedral geometries, which can broaden or relax ligand preferences.21
The substitution of divalent cations is now known to have an effect on the substrate profile of Cj1416. Cj1416 can form a minimum of 12 unique reaction products from MnCTP. CDP-ethanolamine is a known natural product and is used in the formation of phosphatidylethanolamine, a common lipid and CDP-1-glycerol is used in teichoic acid biosynthesis.23 CDP-2-glycerol is another known natural product from Streptococcus pneumonia.24 Cj1416 is capable of forming all three of these natural products, two of which are unavailable commercially. Additionally CDP-serine, CDP-serinol and CDP-3-D-glycerate are all structurally similar to CDP-glycerol and may function as potential inhibitors or as mechanistic probes for enzymes that use CDP-glycerol. Another reaction catalyzed by Cj1416 and MnCTP is the formation of CDP from CTP and phosphate. If 18O-labeled phosphate is used as an alternate substrate, Cj1416 can be used to form β-[18O4]-CDP which can subsequently be used to synthesize β-[18O4]-CTP. Typically, β-[18O4]-CDP is made using morpholidate chemistry in dry DMSO with 18O-labeled phosphate. Labeled nucleotides of this type can be used to probe the details of enzyme-catalyzed reactions using the methodologies of positional isotope exchange (PIX) and molecular isotope exchange (MIX).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (GM 122825).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: https://doi.org/10.1021/acs.biochem.9b00189
The authors declare no competing financial interest.
Cj1416 (UniProt: Q0P8J8)
REFERENCES
- [1].Young KT, Davis LM, and Dirita VJ (2007) Campylobacter jejuni: molecular biology and pathogenesis, Nat. Rev. Microbiol 5, 665–679. [DOI] [PubMed] [Google Scholar]
- [2].García-Sánchez L, Melero B, and Rovira J (2018) Chapter Eight - Campylobacter in the Food Chain, In Adv. Food Nutr. Res. (Rodríguez-Lázaro D, Ed.), pp 215–252, Academic Press. [DOI] [PubMed] [Google Scholar]
- [3].Roberts IS (1996) The Biochemistry and Genetics of Capsularpolysaccharide Production in Bacteria, Annu. Rev. Microbiol 50, 285–315. [DOI] [PubMed] [Google Scholar]
- [4].McNally DJ, Lamoureux MP, Karlyshev AV, Fiori LM, Li J, Thacker G, Coleman RA, Khieu NH, Wren BW, Brisson J-R, Jarrell HC, and Szymanski CM (2007) Commonality and Biosynthesis of the O-Methyl Phosphoramidate Capsule Modification in Campylobacter jejuni, J. Biol. Chem 282, 28566–28576. [DOI] [PubMed] [Google Scholar]
- [5].Petkowski JJ, Bains W, and Seager S (2019) Natural Products Containing ‘Rare’ Organophosphorus Functional Groups, Molecules 24, 866–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van Alphen LB, Wenzel CQ, Richards MR, Fodor C, Ashmus RA, Stahl M, Karlyshev AV, Wren BW, Stintzi A, Miller WG, Lowary TL, and Szymanski CM (2014) Biological Roles of the O-Methyl Phosphoramidate Capsule Modification in Campylobacter jejuni, PLoS One 9, e87051, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Taylor ZW, Brown HA, Narindoshvili T, Wenzel CQ, Szymanski CM, Holden HM, and Raushel FM (2017) Discovery of a Glutamine Kinase Required for the Biosynthesis of the O-Methyl Phosphoramidate Modifications Found in the Capsular Polysaccharides of Campylobacter jejuni, J. Am. Chem. Soc 139, 9463–9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Taylor ZW, Chamberlain AR, and Raushel FM (2018) Substrate Specificity and Chemical Mechanism for the Reaction Catalyzed by Glutamine Kinase, Biochemistry 57, 5447–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taylor ZW, Brown HA, Holden HM, and Raushel FM (2017) Biosynthesis of Nucleoside Diphosphoramidates in Campylobacter jejuni, Biochemistry 56, 6079–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taylor ZW, and Raushel FM (2018) Cytidine Diphosphoramidate Kinase: An Enzyme Required for the Biosynthesis of the O-Methyl Phosphoramidate Modification in the Capsular Polysaccharides of Campylobacter jejuni, Biochemistry 57, 2238–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cottam GL, Kupiecki FP, and Coon MJ (1968) A Study of the Mechanism of O-Phosphorylhydroxylamine Synthesis Catalyzed by Pyruvate Kinase, J. Biol. Chem 243, 1630–1637. [PubMed] [Google Scholar]
- [12].Johnson KA (2010) The kinetic and chemical mechanism of high-fidelity DNA polymerases, Biochim. Biophys. Acta 1804, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tabor S, and Richardson CC (1989) Effect of manganese ions on the incorporation of dideoxynucleotides by bacteriophage T7 DNA polymerase and Escherichia coli DNA polymerase I, Proceedings of the National Academy of Sciences 86, 4076–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fromant M, Blanquet S, and Plateau P (1995) Direct Random Mutagenesis of Gene-Sized DNA Fragments Using Polymerase Chain Reaction, Anal. Biochem 224, 347–353. [DOI] [PubMed] [Google Scholar]
- [15].Sánchez-Moreno I, Iturrate L, Martín-Hoyos R, Jimeno ML, Mena M, Bastida A, and García-Junceda E (2009) From Kinase to Cyclase: An Unusual Example of Catalytic Promiscuity Modulated by Metal Switching, ChemBioChem 10, 225–229. [DOI] [PubMed] [Google Scholar]
- [16].Wehrli W, Verheyden D, and Moffatt J (1965) Dismutation Reactions of Nucleoside Polyphosphates. II. Specific Chemical Syntheses of α-, β-, and γ-P32-Nucleoside 5’-Triphosphates1, J. Am. Chem. Soc 87, 2265–2277. [DOI] [PubMed] [Google Scholar]
- [17].Watanabe M, Sato S, and Wakasugi K (1990) The Hydrolytic Property of Imidodiphosphate in Solid and in an Aqueous Medium, Bull. Chem. Soc. Jpn 63, 1243–1245. [Google Scholar]
- [18].Ghezal S, Thomasson MS, Lefebvre-Tournier I, Périgaud C, Macnaughtan MA, and Roy B (2014) CDP-Ethanolamine and CDP-Choline: one-pot synthesis and 31P NMR study, Tetrahedron Lett. 55, 5306–5310. [Google Scholar]
- [19].Ghodge SV, Cummings JA, Williams HJ, and Raushel FM (2013) Discovery of a Cyclic Phosphodiesterase That Catalyzes the Sequential Hydrolysis of Both Ester Bonds to Phosphorus, J. Am. Chem. Soc 135, 16360–16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hove-Jensen B, McSorley FR, and Zechel DL (2011) Physiological Role of phnP-specified Phosphoribosyl Cyclic Phosphodiesterase in Catabolism of Organophosphonic Acids by the Carbon−Phosphorus Lyase Pathway, J. Am. Chem. Soc 133, 3617–3624. [DOI] [PubMed] [Google Scholar]
- [21].Vashishtha AK, Wang J, and Konigsberg WH (2016) Different Divalent Cations Alter the Kinetics and Fidelity of DNA Polymerases, J. Biol. Chem 291, 20869–20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xia S, Wang M, Blaha G, Konigsberg WH, and Wang J (2011) Structural Insights into Complete Metal Ion Coordination from Ternary Complexes of B Family RB69 DNA Polymerase, Biochemistry 50, 9114–9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schertzer JW, and Brown ED (2008) Use of CDP-Glycerol as an Alternate Acceptor for the Teichoic Acid Polymerase Reveals that Membrane Association Regulates Polymer Length, J. Bacteriol 190, 6940–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Q, Xu Y, Perepelov AV, Xiong W, Wei D, Shashkov AS, Knirel YA, Feng L, and Wang L (2010) Characterization of the CDP-2-Glycerol Biosynthetic Pathway in Streptococcus pneumoniae, J. Bacteriol 192, 5506–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


