Abstract
Microbubbles interact with ultrasound in various ways to enable their applications in ultrasound imaging and diagnosis. To generate high contrast and maximize therapeutic efficacy, microbubbles of high uniformity are required. Microfluidic technology, which enables precise control of small volumes of fluid at the sub-millimeter scale, has provided a versatile platform on which to produce highly uniform microbubbles for potential applications in ultrasound imaging and diagnosis. Here, we describe fundamental microfluidic principles and the most common types of microfluidic devices used to produce sub-10 µm microbubbles, appropriate for biomedical ultrasound. Bubbles can be engineered for specific applications by tailoring the bubble size, inner gas and shell composition and by functionalizing for additional imaging modalities, therapeutics or targeting ligands. To translate the laboratory-scale discoveries to widespread clinical use of these microfluidic-based microbubbles, increased bubble production is needed. We present various strategies recently developed to improve scale-up. We conclude this review by describing some outstanding problems in the field and presenting areas for future use of microfluidics in ultrasound.
Keywords: Microfluidics, Contrast agent, Microbubble
INTRODUCTION
Ultrasound imaging is a widely used, inexpensive diagnostic tool, second only in clinical imaging use to X-ray radiography (Shung 2011). Its popularity results from its tolerability, low cost, portability and ability to monitor dynamic processes in real time. However, ultrasound can suffer from a low signal-to-noise ratio when imaging small blood vessels because of the weak scattering of signal by blood cells, resulting in low contrast. To alleviate this problem, contrast agents can be used to enhance acoustic signal. Current commercially available contrast agents consist of sub-10 µm gas-filled bubbles that are stabilized with proteins or other surface active agents. Because of their high compressibility and low density, their acoustic response differs significantly from that of tissue and fluids, resulting in high contrast. Their small size ensures tolerability and also enables travel through capillaries to monitor angiogenesis (Anderson et al. 2011), atherosclerotic plaque (DeMaria et al. 2006) or carcinogenesis (Kiessling et al. 2009). In addition to imaging, microbubbles can also be used in gene therapy (Sirsi and Borden 2012), as a drug delivery vehicle (Tsutsui et al. 2004), as a drug delivery enabler through sonoporation (Liu et al. 2014; van Wamel et al. 2006) or directly as a therapeutic for cancer treatment through antivascular effects (Hunt et al. 2015; Levenback et al. 2012; Wood and Sehgal 2015). Antivascular cancer therapy is particularly advantageous in that it does not depend on the cellular features of a specific type of cancer and should be applicable to any cancerous vasculature (Wood and Sehgal 2015). How microbubble characteristics such as size uniformity and the mechanical properties of the stabilizing shell affect the efficacy of this treatment is still not well understood, and many opportunities exist for engineering bubbles with enhanced therapeutic effect.
To generate high contrast and maximize therapeutic efficacy, microbubble size and surface properties must be tightly controlled. An ideal microbubble sample will be highly stable over time and monodisperse with respect to size (Talu et al. 2007) and acoustic behavior (Segers et al. 2016a), with shell mechanical properties that strike a balance between maximizing re-radiation damping (imaging) and shell viscous damping (therapy) (Qin et al. 2009). Generating monodisperse microbubbles, however, is non-trivial, and a variety of methods have been developed to address this challenge (Lee et al. 2015; Stride and Edirisinghe 2008). The most common clinically used bubble generation methods are sonication and mechanical agitation, which, although simple and inexpensive, lead to a wide distribution of bubble sizes. For current commercially available microbubbles, 95% to 98% are <5 µm (average size: 1.1 2.4 µm), with standard deviations of 0.35 0.47 µm (Levenback et al. 2012), at a concentration of ~108 to 1010 microbubbles/mL (Wu et al. 2017). Commercially available bubbles are most commonly stabilized with phospholipids or proteins adsorbed to their gas liquid interfaces and are usually filled with a perfluorocarbon gas, such as octofluoropropane (Optison, Definity) or sulfur hexafluoride (SonoVue), to improve stability.
Various methods have been explored to enhance the uniformity of microbubbles for theranostic applications. Centrifugation of the polydisperse suspension prepared by sonication can lead to narrower size distributions (Feshitan et al. 2009); however, this approach requires extra processes and is difficult to scale up. In addition, it results in wasted materials and thus an undesirable reduction in bubble yield. Other methods exist to prepare small microbubbles such as coaxial electrohydrodynamic atomization (CEHDA) (Ahmad et al. 2008; Farook et al. 2007, 2009), inkjet printing hollow polymer particles (Böhmer et al. 2006) and membrane emulsification (Kukizaki and Baba 2008; Kukizaki and Wada 2008). CEHDA involves flowing two immiscible fluids (two liquids or a gas and a liquid) coaxially through an electric field, where individual bubbles or droplets break off. Although an improvement over the wide bubble size distributions generated by sonication, CEHDA does not reliably produce bubbles significantly more uniform than commercially available contrast agents (Yan et al. 2017). Inkjet printing, which relies on a polymer solution being pushed through piezo-driven nozzles, is another approach that improves control over bubble size. However, stiff polymer-coated particles are produced, which may not have the appropriate mechanical properties for theranostic ultrasound. Finally, membrane emulsification has been proposed to increase bubble monodispersity. In this technique, gas is forced through a porous membrane into a surfactant solution. Monodispersity is again still not ideal using this method, and the surfactant must be carefully selected to prevent undesirable interactions with the membrane that lead to bubble coalescence (Kukizaki and Baba 2008).
Among the various techniques proposed, microfluidic-based approaches have emerged as one of the most powerful methods to produce a monodisperse bubble suspension with an average bubble diameter <10 µm (Dhanaliwala et al. 2013; Lee et al. 2017; Peyman et al. 2012; Segers et al. 2016b; Talu et al. 2006). By control of fluid flow through micron-sized channels, monodisperse bubble populations can be precisely generated. Several types of microfluidic devices have been developed to produce microbubbles with diameters appropriate for clinical ultrasound applications, which we review here. First, we introduce the concepts underlying the operation of microfluidics. Then we discuss important bubble characteristics to consider and microbubble functionalization and conclude the review by discussing the outstanding challenges that must be addressed to enable translation of laboratory-scale demonstration of micro-fluidic-based microbubble generation to commercial success. For further reading on other microbubble production techniques, the reader is directed to other reviews (Lee et al. 2015; Stride and Edirisinghe 2008). Any animal studies reviewed received Institutional Animal Care and Use Committee approval.
PRINCIPLES OF GAS BUBBLE PRODUCTION IN MICROFLUIDICS
Microfluidics relies on the manipulation of fluids on the microscale, typically within channels <1000 µm (Whitesides 2006). It enables the miniaturization and integration of multiple processes that normally require many pieces of lab equipment into a single, small chip. As such, microfluidic devices generally require very low volumes of fluids, decreasing the total cost of experiments and reagents and the time for analysis. In addition to advantages related to their small size, microfluidic devices also feature advantageous fluid mechanics, with the narrow channels preventing turbulent flow and thus enabling smooth and predictable transport of materials through the device via laminar flow. These advantages have led to the use of microfluidics in a variety of applications (Kaminski and Garstecki 2017) including detection (Guo et al. 2012) and separation (Bhagat et al. 2010) (particularly related to diagnostics) (Chin et al. 2012; Sackmann et al. 2014), small-scale chemical (Liu and Jiang 2017) and micro-/nano-particle synthesis (Hou et al. 2017; Lignos et al. 2017; Marre and Jensen 2010), single-cell biology (Joensson and Andersson Svahn 2012; Yin and Marshall 2012) and drug delivery (Bhise et al. 2014; Duncanson et al. 2012; Zhao 2013). Microfluidics also offers a unique approach for engineering microbubbles by creating local gas liquid interfacial instabilities to “pinch off” small volumes of gases in microbubbles. To produce microbubbles, a microfluidic channel containing gas (gas phase) meets with a microfluidic channel containing liquid (continuous phase). Bubbles can be formed by having these channels flow perpendicular to each other or by being forced through a small orifice together. Stable production of bubbles is controlled by several dimensionless parameters, including the flow rate ratio and viscosity ratio of the gas phase and continuous phase, and the capillary number, Ca (Anna 2016):
| (1) |
where µo is the viscosity of the continuous (outer) phase; γ is the interfacial tension between the continuous phase and the gas phase; and U is a characteristic velocity, which is determined by the device geometry and often defined in terms of the bubble radius, ao, and characteristic deformation rate, G. Ca represents the relative importance of viscous and surface tension forces in the bubble breakup and is indicative of the bubble formation mechanism (Anna 2016). At low capillary numbers, Ca <0 (Eq.1), where surface tension dominates, uniform bubbles are formed either through a squeezing mechanism, where the gas liquid interface obstructs the junction until a bubble pinches off, or through a dripping mechanism, where bubble breakup is induced via an intricate balance between capillary and draft forces. In contrast, at high capillary numbers, Ca >0 (Eq.1), a long fluid thread is formed through a jetting mechanism (when inertia and capillary forces dominate), and polydisperse bubbles are formed (Anna 2016; Utada et al. 2007). By changing the composition of the continuous phase or gas phase or by changing the microfluidic device design, bubbles can be engineered for specific applications. In the next section, we summarize the principle underlying the formation of uniform gas bubbles and describe different microfluidic devices that have been used for bubble production.
There are two major categories of microbubble microfluidic devices, T-junction (Thorsen et al. 2001) and flow-focusing (Anna et al. 2003), which can be further subdivided into asymmetric and axisymmetric devices (Fig. 1). T-Junction and axisymmetric flow-focusing devices are typically built using glass or steel capillaries, whereas asymmetric flow-focusing devices are generally based on soft lithography, which takes advantage of replication of microchannels using elastomeric stamps on hard masters as illustrated in Figure 2 (Tang and White-sides 2010; Xia and Whitesides 1998). While glass or steel capillaries offer the advantage of high solvent resistance, soft lithography can be advantageous in fabricating microchannels with complex geometry, though specialized equipment and clean room access are required, increasing the fabrication cost, in addition to the expense of photoresists and chrome photomasks. Progress has been made in improving the cost-effectiveness of soft lithography techniques, however, through use of wax molds (Kaigala et al. 2007; Li et al. 2014), double-casting prototyping (Kwapiszewska et al. 2016) and micromilling replica molding (Carugo et al. 2016). Early examples of microfluidic-produced microbubbles small enough for clinical ultrasound applications were based on axisymmetric flow-focusing (Gañán-Calvo and Gordillo 2001) or T-junction (Pancholi et al. 2008b) devices. With advances in soft lithography, however, asymmetric flow-focusing devices have become much more widespread, because of the ease of fabrication and wide possibilities for customization.
Fig. 1.
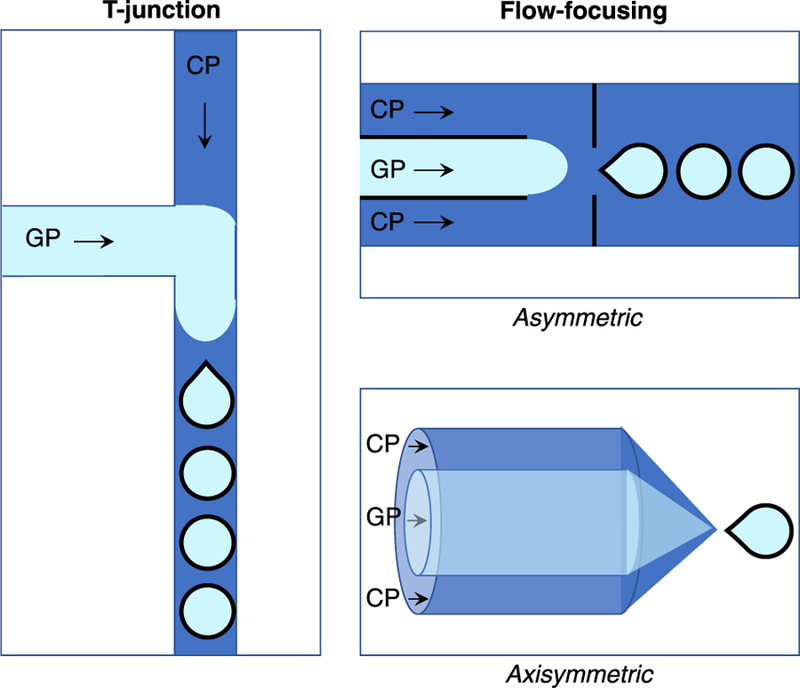
Types of microfluidic devices most commonly used to produce microbubbles small enough for ultrasound applications, all of which feature narrow channels for gas and liquid (continuous phase) to flow. The two major categories are T-junction and flow-focusing, which can be further subdivided into asymmetric and axisymmetric devices. All of the devices feature the convergence of a gas phase (GP, pale blue) and a continuous phase (CP, dark blue) to generate bubbles, though the exact mechanism of bubble formation depends on the device.
Fig. 2.
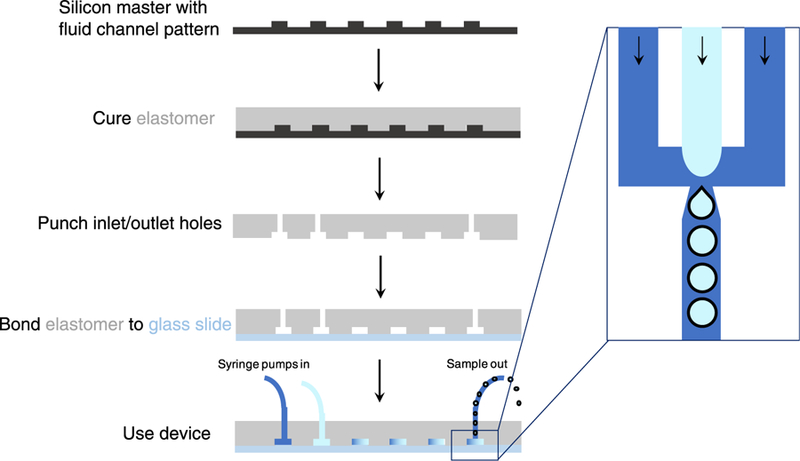
Fabrication of microfluidic devices using soft lithography. A silicon wafer with a raised surface of the fluid channels is coated with elastomer mixed with a cross-linker. After curing, the elastomer is peeled off, and holes for inserting inlet and outlet ports are punched. The punched elastomer is then bonded to a glass slide to seal off the fluid channels. The device can then be used. Zoom inset: Top-down view of bubble formation in the fluid channels, with arrows representing the direction of flow.
T-Junction
T-Junction devices feature a continuous-phase channel perpendicular to a gas-phase channel. As the gas phase moves into the continuous phase channel, a neck begins to form and narrow, until an individual bubble pinches off, as illustrated in Figure 1 (Christopher and Anna 2007). This pinch-off is caused by instability at the gas liquid interface. Faster pinch-off results in smaller bubble diameters, which is controlled by the ratio of gas to liquid flow rates.
T-Junction devices were first employed to produce microbubbles for ultrasound by using 150 µm plastic capillaries to form the perpendicular channels (Pancholi et al. 2008b). Bubbles of ~100 µm were produced, with a low polydispersity index (defined as bubble diameter standard deviation divided by average bubble diameter × 100%) of ±1%. Under insonation using a 5 MHz broadband pulse, the bubbles exhibited greater contrast compared with a sample of water or the continuous-phase phospholipid solution, but less contrast than bubbles produced by sonication. This was attributed to the low bubble concentration from the T-junction device, as well as large bubble size (significant mismatch between insonation frequency and resonance frequency of bubbles). To make small microbubbles more appropriate for clinical ultrasound settings, a hybrid electrospray/T-junction device was developed (Pancholi et al. 2008a) using 50 µm glass capillaries embedded in a plastic block, with a voltage applied at an outlet capillary of 500 µm. By optimizing gas and continuous phase flow rates, as well as applied electrospray voltage, bubbles of 5.1 ± 2 µm were produced. The bubbles were stable for up to 2 h, an improvement over the previously published 20 min. Another device was built using a polydimethylsiloxane (PDMS) continuous-phase channel partially intersected by a thin glass capillary for gas delivery (Chen et al. 2009). By varying how far the gas capillary blocked the continuous-phase channel, the liquid velocity could be increased enough that neither high flow rates nor gas pressures were needed to reliably produce bubbles, and bubble size could be reduced below 10 µm. Bubble diameter was also dependent on gas capillary diameter, with 2-, 5- and 10 µm capillaries tested. With a 2 µm capillary, bubbles of 7.8 µm were produced, whereas with 10 µm capillaries, bubbles >60 µm were generated. However, no information regarding bubble stability was discussed.
Flow-focusing
Because of their geometry, it can be difficult to produce sufficiently small bubbles for ultrasound applications using T-junction devices compared with flow-focusing. Flow-focusing is therefore much more frequently used in producing microbubbles for ultra-sound. In flow-focusing devices, inner gas-phase channels and outer continuous-phase channels are focused through a small orifice, leading to the formation of microbubbles. This can be done by radially surrounding the gas phase with continuous phase, as in axisymmetric devices (Fig. 1), or by flanking the gas-phase channel with two continuous-phase channels, as in asymmetric devices (Fig. 1). The size of gas bubbles is determined by the intricate balance between the surface tension and viscous forces, leading to the formation of highly uniform bubbles.
Similar to T-junction devices, axisymmetric devices can be made using glass or steel capillaries. The continuous-phase velocity in these devices is high enough that the Reynolds number (Re) for the liquid is between 1 and 1000, whereas the Reynolds number for the gas is significantly smaller, <14. This results in liquid inertia as the dominating force in bubble formation (Gañán-Calvo and Gordillo 2001). The bubbles formed are smaller than the device orifice. The first example of an axisymmetric device used to produce microbubbles was published by Gañán-Calvo and Gordillo (2001). A capillary tube 400 mm in diameter was used to flow gas into water ethanol or water glycerol mixtures flowing through a 100 or 210 µm focusing hole. Bubbles ranging from 5 to 200 µm in diameter were produced by tuning the flow rates of gas or liquid and by tuning the liquid viscosity. From measurements, the relationship between bubble diameter (db) and gas capillary diameter (D) was found to be dependent on the ratio of gas flow rate (Qg) and liquid flow rate (Ql) as outlined in the equation
| (2) |
Because of the ease of their fabrication using standard soft lithography techniques and nearly limitless options for customization, asymmetric flow-focusing devices are currently the devices most widely used to generate sub-10 µm microbubbles. Asymmetric devices generally have similar geometries, with two flanking continuous-phase channels joining a gas-phase channel just before an orifice. The continuous-phase channels are usually perpendicular to the gas-phase channel, but widen to form a diamond-shaped chamber before the orifice. It is not clearly understood how such a geometry is able to facilitate the production of more stable and uniform microbubbles, but this shape likely provides larger interfacial area for surfactant adsorption and lowers the breakthrough pressure for air to form gas bubbles. Often, exit channels are not a uniform width, but broaden to form a V-like structure (Dixon et al. 2013; Hettiarachchi et al. 2009; Segers et al. 2016b; Talu et al. 2008). With this channel geometry, the combination of high shear force at the orifice and a flow velocity gradient post-orifice enables controlled break-off of individual bubbles solely at the orifice (Hettiarachchi et al. 2007). Variations in the angle of the expanding exit channel (17.5° vs. 45°) or the height of the channels (14 µm vs. 28 µm) were reported not to affect bubble formation (Segers et al. 2017). By increasing the continuous-phase flow rate and decreasing the ratio of gas flow rate to continuous-phase flow rate, bubbles smaller in diameter than the orifice can be produced (Anna et al. 2003). Gas bubbles can also be produced by inducing instability in a gaseous jet that is formed by flow-focusing gas and liquid flows through a long exit channel. This mechanism is different from the squeezing mechanism typical of flow-focusing devices, where a gas jet blocks the orifice and is squeezed through with the continuous phase to form one gas bubble at a time (Castro-Hernandez et al. 2011). To produce bubbles <10 µm in diameter with this device geometry, the continuous-phase flow rate must be significantly higher than the gas flow rate.
IMPORTANT MICROBUBBLE CHARACTERISTICS
A number of microbubble characteristics are important to tailor for optimal bubble behavior and stability depending on the particular application. These include diameter and inner gas, as well as the structure and mechanical properties of the shell (which depend on its composition). The size and shell properties are particularly important for optimizing the resonance frequency of a bubble, and matching it to that of the ultrasound transducer used gives the greatest acoustic response. Resonance frequency fr for a bubble with a shell can be described by various models. The radius-dependent surface tension model (van der Meer et al. 2007)
| (3) |
describes the bubble shell as a 2-D viscoelastic medium with radius-dependent surface tension. Over a certain range of radii the bubble has elastic behavior, while below the range the bubble buckles and above the range the shell breaks. f0 is the eigenfrequency of the bubble, and d its damping coefficient. The finite-shell-thickness model (Hoff et al. 2000) considers the shell to be a 3-D continuous medium of finite thickness
| (4) |
where r is bubble radius, κ is the polytropic index of the inner gas, pe is equilibrium pressure inside the bubble, Gs is the shear modulus of the shell, ds is the shell thickness and ρ is the density of surrounding medium. Although the radius-dependent surface tension model more completely describes bubbles’ behavior, for the practical design of microbubbles the finite-thickness model is more intuitive. The use of microfluidic approaches provides unprecedented control over the variables in the preceding equations, save for ρ, which in clinical use will be the density of a patient’s blood. We next explore how different bubble sizes, inner gases and shell compositions (which determine shell shear modulus and thickness) have been used to engineer the characteristics of microfluidic-produced microbubbles.
Controlling microbubble size in microfluidic-produced microbubbles
The size of a microbubble has a significant effect on its behavior under ultrasound (Chomas et al. 2001), especially its resonance frequency, which in turn affects its contrast and therapeutic efficacy. In general, stronger echoes from larger bubbles must be balanced with a need for bubbles small enough to maneuver through narrow capillaries, as well as avoid embolism. Resonant microbubbles with high size uniformity have been predicted to generate more heat, which can be used to disrupt tumor blood vessels (Levenback et al. 2012). Calculations have been used to determine the percentage of microbubbles with resonance frequency matching that of a clinical ultrasound transducer for a given bubble size distribution (Talu et al. 2007). It was found that ~50% of the microbubble population in commercially available agents has a resonance frequency outside the range of the transducer used. Therefore, ~50% of the contrast agents do not contribute substantially to contrast enhancement and result in inefficient use of the materials. There are two main ways to tune microbubble size and dispersity: adjusting the flow rate of the continuous phase or the gas phase (changing gas pressure), or changing the geometry of the microfluidic device (changing the width or height of the orifice where bubbles are formed). Increasing the gas pressure will lead to larger bubbles as more gas is forced through the orifice over a given period. Decreasing the continuous-phase flow rate will have the same effect.
Using a microfluidic flow-focusing device, gas bubbles are hydrodynamically pinched off one at a time by flow-focusing a gas stream with a continuous phase as discussed above. In addition to the flow rates of gas and continuous phases, the viscosity of the continuous phase also affects the size of the gas microbubbles produced (Talu et al. 2007). Slow gas flow rates and high continuous-phase flow rates lead to smaller bubbles, while decreased viscosity leads to larger bubbles. Lowering both gas- and continuous-phase flow rates (<5 psi, <60 µL/min) can improve bubble uniformity (Talu et al. 2007). Using PDMS-based flow-focusing devices, Dhanaliwala et al. (2013) also observed decreasing bubble diameter with decreasing gas pressure, with a near-linear relationship. They found that the most stable bubbles produced had a diameter smaller than the height of the channel but larger than that of the orifice. Kaya et al. (2010) explored the effect of microbubble size on ultrasound imaging, also with a PDMS-based flow-focusing device. By adjusting the gas- and continuous-phase flow rates, they generated microbubbles with diameters from 1.8 to 4.8 µm. They found that the largest acoustic signal was measured when bubbles were excited at their resonance frequency. For bubbles excited off-resonance, larger bubbles gave greater signal.
Changing the size of the orifice is another way to tune bubble diameter. In fact, the dimension of the orifice sets the range in which the bubble size can be varied by changing the flow rates in a single device. This tendency indicates that multiple devices with different orifice dimensions must be used to control the bubble size for different applications. Angilèx et al. (2014) reported a dynamically tunable flow-focusing PDMS microfluidic device that generated bubbles with diameters ranging from 1.8 to 7.2 µm. Dynamic size control was achieved by incorporating an air-actuated valve into their microfluidic device, with air-filled arms on both sides of the bubble generation orifice (Fig. 3a). On increasing the gas pressure in the valve, the arms narrowed the orifice, leading to smaller bubbles. Decreasing the gas pressure enabled the formation of larger bubbles, up to a maximum of 7.2 µm, similar to the orifice size when no valve pressure was applied. Dynamic control of orifice size can also enable the production of gas microbubbles with prescribed size distribution, which could be extremely powerful in the production of microbubble ensembles that are tailored to meet multiple requirements.
Fig. 3.
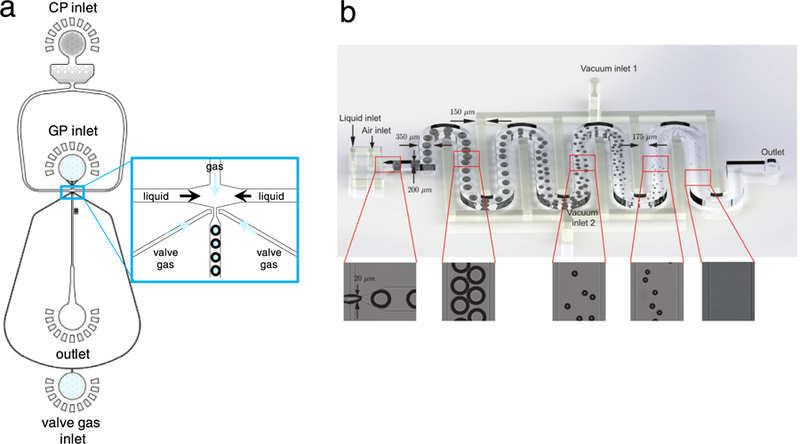
Novel devices for dynamically tuning bubble size. (a) Air-actuated valve enables more precise and dynamic control over microbubble diameter. As the valve gas pressure is increased, the two valve arms exert more pressure on the orifice, making it smaller in width. This leads to smaller bubble diameters. Reproduced, with permission, from Ref. 24. (b) Applying vacuum to interdigitated channels shrinks bubbles to appropriate size for use in ultrasound. After large bubbles are formed in a flow-focusing orifice, applied vacuum removes gas from the channels, leading to bubble shrinkage and small bubble collection at the outlet. Adapted from Gnyawali et al. (2017) with permission of The Royal Society of Chemistry.
One novel way to tune microbubble size was described by Gnyawali et al. (2017), who applied vacuum to interdigitated channels as bubbles post-formation flowed through a 350-mm-long serpentine channel in a PDMS-based flow-focusing device (Fig. 3b). By tuning the vacuum pressure between 0 and 90 kPa, they could control bubble size from 1 µm to greater than the channel dimensions (80 µm). At −70 kPa, bubbles with diameter <7 µm were generated. Because the PDMS from which the device was fabricated is permeable to air, the applied vacuum can remove air dissolved in the continuous phase and the core of the bubbles. Without vacuum applied, bubbles shrink as they travel to the device exit, a phenomenon that has been observed by other researchers (Segers et al. 2016b; Talu et al. 2008). This shrinkage phenomenon is due to gas dissolving to lower the effective interfacial tension through compression of the bubble shell (Segers et al. 2016b). Bubbles with diameters small enough for ultrasound applications, however, were observed only after vacuum was applied. On collection, bubbles without vacuum continued to shrink by 10 µm in diameter (95 – 85 µm), but the diameter of vacuum bubbles remained constant at 5 µm over 25 min.
All of the aforementioned examples are for bubbles with diameters >1 µm. However, nano-sized bubbles may be desirable in certain applications, for example, for deeper penetration into tumors (Yin et al. 2012). Their preparation has been difficult because of the resolution limits in fabrication techniques. In addition, the Laplace pressure (difference in pressure between the inside and outside of the bubble) is extremely high for nanobubbles (German et al. 2016). To circumvent these challenges, nanodroplets with liquid rather than gaseous cores (Hughes et al. 2005) have been developed. On exposure to ultrasound, the liquid core is converted to gas, and signal enhancement is observed (Sheeran and Dayton 2012). Nanobubbles have been produced by both typical sonication/mechanical agitation methods and microfluidic devices. All of these methods usually require the removal of larger micron-sized bubbles, either through centrifugation (Yin et al. 2012, 2013) or by removal of the more buoyant larger bubbles by pipetting (Peyman et al. 2016). By use of microfluidics, bubbles were produced with stable concentration and size over days (Peyman et al. 2016), but the major advantage of using microfluidics to produce nanobubbles stems from its lower energy consumption and lower liquid volume requirement. A more detailed study to understand the difference between the nanobubbles generated from conventional and microfluidic-based methods is warranted.
To generate nanobubbles, Peyman et al. (2016) designed a flow-focusing device that produced a fine spray of bubbles (stabilized with a lipid shell and filled with C4F10 gas) ranging from 4 µm to <1 µm. Larger bubbles were removed by pipetting away the top portion of the solution, while nanobubbles and nanodroplets remained dispersed below. Through the use of particle tracking, which infers the size of particles (gas bubbles and droplets in this case) by monitoring their Brownian diffusion in a medium of known viscosity, the majority of the bubbles and droplets were measured to be 100 300 nm. In vivo ultrasound imaging in mice revealed these nanobubbles to have higher contrast, compared with microbubbles prepared in the same batch. Because of their size, they also were predicted to have a higher resonance frequency than the larger microbubbles. This more closely matched the 40 MHz transducer used, which likely explains the higher contrast observed. Although 40 MHz is considerably higher than the frequencies typically used in clinical ultrasound scanners, high-frequency imaging (>20 MHz) is considered to be the next frontier in ultrasound (Shung 2010), finding use in imaging small anatomic features in dermatology, ophthalmology, neonatology and pediatric medicine. Even in routine clinical scanners that operate between 3 and 15 MHz, there is a growing trend to expand imaging to higher frequencies to improve spatial and contrast resolution.
Microbubble acoustic sorting
In addition to producing small microbubbles, microfluidic devices can also be used to sort bubbles to enhance the size uniformity of bubble suspensions, based on acoustic response. Segers and Versluis (2014) developed a device that sorted bubbles using a traveling acoustic wave immediately after production in a flow-focusing geometry (Fig. 4). The traveling acoustic wave causes bubble displacement away from the source of the wave, with resonating bubbles moving the farthest. Thus, bubbles with similar acoustic responses (dependent on their size and shell coating) can be isolated. Sorting was also successfully performed on a commercial microbubble sample. The acoustic responses of sorted microbubbles were characterized to confirm uniformity (Segers et al. 2016a). Bubble scattering and attenuation were measured under varying acoustic pressures and were representative of uniform samples, as well as in good agreement with theoretical models (Segers et al. 2016a). Microfluidic sorting is highly useful for careful bubble characterization and highlights the importance of shell composition uniformity in addition to size, although the efficacy of these sorted bubbles in enhancing the echogenicity was not tested.
Fig. 4.
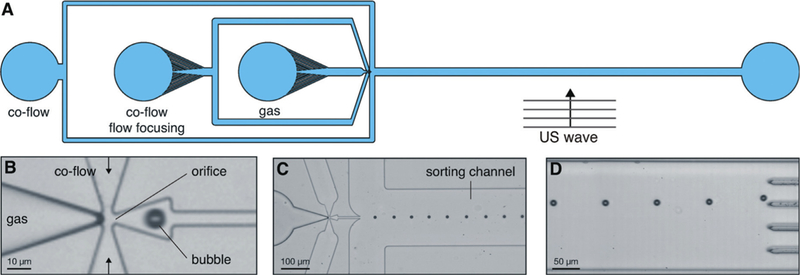
(A) Overview of microbubble acoustic sorting device, where exposure to ultrasound displaces bubbles according to their acoustic response and bubbles collected in different channels. (B) Close-up of bubble generation orifice. (C) Close-up of sorting channel. (D) Under traveling acoustic wave, bubbles are displaced toward one of five sorting channels. US = ultrasound. Reproduced, with permission, from Segers and Versluis (2014) with permission of The Royal Society of Chemistry.
Effect of gas phase in microfluidic-produced microbubbles
The inner gas phase of microbubbles can affect the performance of microbubbles in a clinical setting by changing the stability and size of microbubbles, which will affect the response of gas bubbles to ultrasound. Gas bubbles change size over time via coalescence (multiple bubbles fusing with each other), dissolution (gas diffusing out of the bubble and dissolving in the solution), growth (gas diffusing into the bubble from the solution) or Ostwald ripening (gas transferring from small to large bubbles because of differences in the Laplace pressure). Using a gas that is less soluble in water than common gases such as nitrogen (N2) and air can lead to greater bubble stability, as less gas diffuses out of the bubble, maintaining the bubble size over an extended period. Commercially available microbubbles contain octafluoropropane, perfluorobutane or sulfur hexafluoride as inner gases to enhance their stability (Fig. 5). The combination of gas phase used and the gas with which the continuous phase is saturated can also affect bubble size and stability. Talu et al. (2008) explored different combinations of N2, air and perfluoro-butane (C4F10) as inner and solution-saturated gases for bubbles prepared in a flow-focusing microfluidic device. For N2-filled bubbles in air-saturated solution, smaller bubbles (initial diameter: 5.0 ± 0.2 µm) were observed to have high stability compared with larger bubbles (initial diameter: 12.0 ± 0.2 µm) and maintained their diameters over the course of 9 h of observation. C4F10-filled bubbles in air-saturated solution, however, increased in diameter over time and became more poly-disperse. C4F10-filled bubbles in C4F10-saturated solution also underwent Ostwald ripening, but to a smaller extent than the air-saturated sample. These somewhat counterintuitive results were attributed to differences in gas exchange kinetics. C4F10 has slower mass transport because of its larger molecular weight than N2 or O2 in air, and therefore, air could diffuse into the bubbles post-generation, increasing bubble diameter. If bubbles are given enough time to equilibrate before encountering other bubbles, Ostwald ripening is reduced, as was the case for N2-filled, N2-saturated bubbles.
Fig. 5.
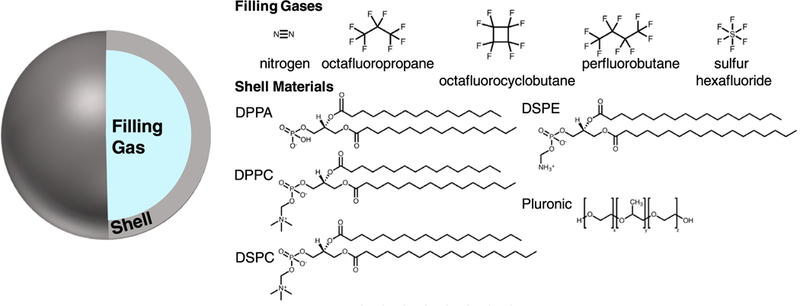
Structures of compounds used for microbubble filling gas and stabilizing shell. Perfluorocarbon gases are often used to improve bubble stability because of their lower solubility in water. Common shell stabilizers include phospholipid and polymer surfactants. DPPA = 1,2-dipalmitoyl-sn-glycero-3-phosphate; DPPC = 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPC = 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPE = 1,2-distearoyl-sn-glycero-3-phosphoethanolamine.
Carbon dioxide has also been used as an inner gas, taking advantage of its ability to alter solution pH. Using a flow-focusing device to prepare bubbles filled with CO2 and its subsequent dissolution led to bubble shrinkage from an initial diameter >100 µm to <10 µm (Park et al. 2010a). Dissolution of CO2 lowered the pH of the continuous phase via formation of carbonic acid, inducing adsorption of cationic lysozyme, followed by adsorption of anionic nanoparticles (Fe3O4, Au or SiO2-capped CdSe/ZnS) and anionic alginate. Using N2 as an inner gas under these conditions did not lead to stable bubbles, highlighting the importance of choosing an appropriate filling gas depending on the desired bubble shell composition.
Depending on the nature of the surfactant that is used for bubble production, however, changing the inner gas may have less of an effect on the bubble stability. Angilè et al. (2014) found that microbubbles produced in a flow-focusing device and stabilized with a recombinant amphiphilic protein, oleosin, and Pluronic F68 surfactant were stable for weeks at a time, whether the inner gas was N2 or C4F8. The bubbles also remained echogenic after a week of storage. The stability, in this case, depends more strongly on the composition/properties of the stabilizing shell than on the inner gas.
Effect of shell composition in microfluidic-produced microbubbles
To stabilize the gas liquid interface of a microbubble, various surface active agents (surfactants) are used (Fig. 5). These stabilizing agents adsorb onto the surface of gas bubbles to provide kinetic barriers to bubble coalescence and can impart solid-like properties to the surface of the bubbles to slow down gas dissolution. These stabilizers can also imbue bubbles with additional functionality, such as targeting to a site of interest (Klibanov 2008, 2009) or additional imaging modalities (Benchimol et al. 2013; Ke et al. 2009), which we discuss in more detail later in this review. For microfluidic-produced microbubbles, lipids have been the most commonly used surfactants in the bubble shell (Campo-Cortés et al. 2016; Hettiarachchi et al. 2009; Parrales et al. 2014; Peyman et al. 2012; Segers et al. 2016b; Shih et al. 2013; Talu et al. 2007, 2008). Such amphiphilic surfactants preferentially align with the hydrophobic portion facing toward the inner gas, with the hydrophilic portion in the aqueous solution. Primary lipids are usually phosphocholines such as 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-dipalmitoyl-sn-glycero-3-phosphocho-line (DPPC). Ethanolamines, such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(cap biotinyl) (DOPE-B) and modified 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) (see Fig. 5 for structures), as well as 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA), have also been used as stabilizers for gas microbubbles. Other stabilizers used in microfluidic production of microbubbles include polymers such as triblock copolymer poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) ((PEO)x-(PPO)y-(PEO)x, also known as Pluronic) (Angilè et al. 2014; Kendall et al. 2012; Park et al. 2010b; Shih et al 2013), proteins (Angilè et al. 2014; Park et al. 2010b; Seo et al. 2010), and nanoparticles (Abou-Saleh et al. 2013; Park et al. 2009; Seo et al. 2010).
Bubbles produced via sonication versus microfluidics may have differing stability and performance, as the heat produced during sonication can affect surfactant adsorption to the interface, affecting their structure, composition and properties (Hosny et al. 2013). Hosny et al., for example, determined that DSPC-stabilized bubbles produced using sonication had higher shell viscosity and therefore greater stability compared with bubbles produced using a T-junction microfluidic device. Careful selection of shell surfactant(s) is critical in the production of stable bubbles using microfluidics. Angilè et al. (2014), found that the combination of oleosin recombinant protein and Pluronic F68 led to stable (size distribution unchanging and bubbles echo-genic for at least a week), monodisperse (coefficient of variation <5%) bubbles produced in a flow-focusing device. Using Pluronic F127 ((PEO)100 (PPO)65 (PEO)100) with oleosin at non-optimal concentrations and ratios in the continuous phase led to initially uniform bubbles that rapidly coalesced on collection. Likewise, using only Pluronic F68 or only oleo-sin led to bubble coalescence. These results highlight the importance of selecting appropriate shell stabilizers in obtaining stable, uniform bubbles in microfluidic production of microbubbles.
Often one of the surfactants is modified with poly(ethylene glycol) (PEG), which can suppress non-specific protein adsorption and immune system recognition and, thus, prolong circulation of gas bubbles and decrease undesirable interactions with off-target proteins and cells (Amoozgar and Yeo 2012). PEGylation can have further effects on microbubble size, concentration, stability and stiffness. In one study using a flow-focusing device, increasing the mole percentage of PEGylation up to 5% led to an increase in microbubble concentration (greater bubble stability on collection), with further increases in PEG concentration leading to decreased bubble concentration (Abou-Saleh et al. 2014). A similar trend was observed for bubble lifetime, with 4% to 5% PEG exhibiting maximum bubble stability, with further increases leading to slight decreases in life-time. The molecular weight of the PEG used can also affect bubble stability: increasing PEG molecular weight was observed to enhance bubble stability, as well as decrease final bubble size (compared with the initial size immediately after formation) using a flow-focusing device (Segers et al. 2017).
The choice of surfactant has a large effect on the stability and the behavior of the bubble under ultrasound. Bubbles with stiffer shells are more stable, but their lack of flexibility makes them less compressible and, therefore, less effective contrast agents (Qin et al. 2009). Because of this trade-off, it is important to understand the composition mechanical property relationship of microbubble shell, which requires an accurate method of mechanical characterization. Microbubble shell mechanical properties can be tested using atomic force microscopy (AFM) (Abou-Saleh et al. 2013), where a scanning probe measures the force between the probe and the bubble surface (Fig. 6a). The advantage of AFM is that gas bubbles that are smaller than 10 µm can be directly characterized. However, in the process of attaching the bubbles to the solid surface used for AFM, an adhesion molecule must be used, which can accumulate on the bubble surface, slightly influencing the observed stiffness measured. DPPC and DOPE (90:10) in a flow-focusing device were used to prepare microbubbles that were then functionalized with streptavidin or nanoparticles. Both coatings were found to increase shell stiffness (by calculating the elastic modulus), with nanoparticles increasing to a greater extent than streptavidin. The effect of adding PEG to the shell was also explored by substituting DOPE with DSPE-B-PEG2000, with the presence of PEG leading to small increases in stiffness compared with either coating.
Fig. 6.
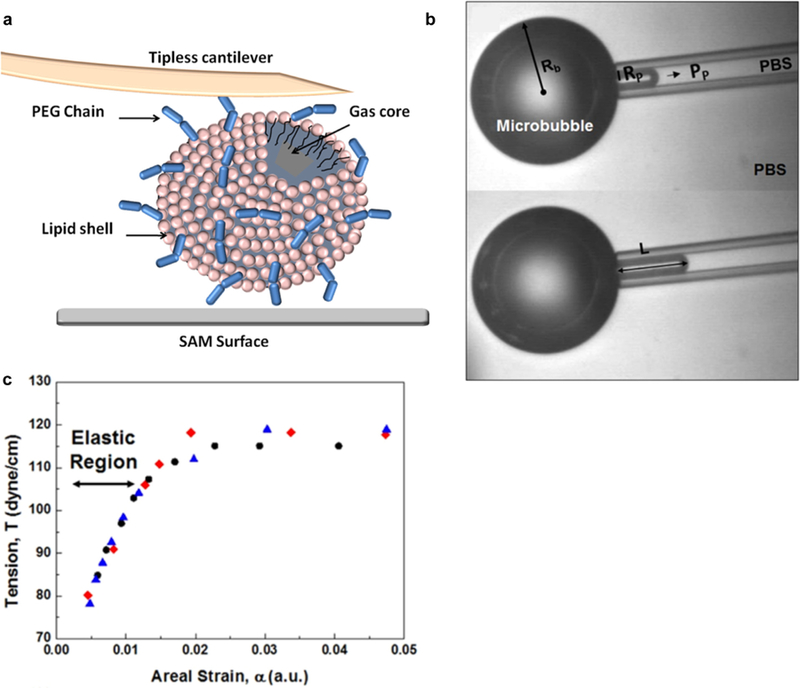
Materials characterization of microbubbles. (a) Atomic force microscopy measurement of a microbubble using a tipless cantilever on a self-assembled monolayer (SAM) surface. Adapted, with permission, from Abou-Saleh et al. (2014). Copyright 2014 American Chemical Society. (b) Micropipet aspiration of a microbubble. The relationship between the aspiration pressure and how far the bubble travels up the pipet is dependent on the stiffness of the bubble shell. (c) By generating a tension-versus-areal strain curve from aspiration measurements, the bubble shell stiffness can be approximated by the areal strain modulus, equal to the slope of the linear portion of the curve. Adapted, with permission, from Jang et al. (2016). Copyright 2016 American Chemical Society.
The mechanical properties of the microbubble shell can also be characterized using micropipet aspiration (Evans and Needham 1987; Kim et al. 2003; Rawicz et al. 2000), relying on the relationship between applied suction pressure through a pipet and the areal strain in the bubble shell (Fig. 6b). This technique was first applied to bubbles by Kim et al. (2003) for the characterization of polycrystalline phospholipid bubble shells, illustrating a dependence of shell mechanical properties on phospholipid acyl chain length, lipid domain size and lipid domain distribution. As seen in Figure 6c, the slope of the tension (Tv, eqn [4]) and areal strain (α, eqn [5]) generated through this measurement is equal to the areal strain modulus (Ka, eqn [6]), giving a measure of the stiffness of the bubble shell (Jang et al. 2015; Kamat et al. 2011):
| (4) |
| (5) |
| (6) |
where Pp is the aspiration pressure, Rb is the bubble radius, Rp is the pipet radius and L is the bubble aspiration length in the pipet. Using micropipet aspiration, the effect of Pluronic composition on bubble stiffness was explored for bubbles stabilized by Pluronic surfactant and oleosin protein (Jang et al. 2016). For increasing amounts of Pluronic F68 with a constant amount of oleosin, the bubbles had increasing areal expansion moduli, indicating increased stiffness. The effect of changing the composition of the Pluronic was also investigated. When the hydrophilicity of the Pluronic was decreased by using a Pluronic with a lower number of PEO blocks compared with PPO, bubble stiffness decreased. It is not clear by what mechanism this shell softening occurs, but there is a clear dependence of bubble shell mechanical properties on the molecular characteristics of the stabilization surfactant used. Although micropipet aspiration provides a precise method of shell characterization, because of challenges in pipet preparation and the resolution of optical microscopy, bubble diameters need to be >50 µm for accurate measurement, which is larger than bubbles used for biomedical ultrasound (Jang et al. 2016).
Two optical methods can also be used to probe the viscosity and lipid packing of the microbubble shell, namely, fluorescence lifetime imaging microscopy (FLIM) and spectral microscope imaging, respectively. For FLIM, a molecular rotor molecule is embedded in the bubble shell. Its fluorescence lifetime is directly related to the viscosity of its environment, and therefore, fluorescence lifetime measurements can be used to measure bubble shell viscosity of individual bubbles, as well as how viscosity varies across a single bubble surface (Hosny et al. 2013). In spectral microscope imaging, polarity-sensitive fluorescent probes embedded in the bubble surface exhibit shifts in emission spectra with changes in lipid packing (Aron et al. 2017). The spectral changes can be quantified using a generalized polarization parameter, varying from −1 (more disordered packing) to 1 (more ordered packing). These methods should provide greater information about bubble shell structure and resulting properties, enabling further engineering of bubbles with desired characteristics.
FUNCTIONALIZATION OF MICROFLUIDIC-FORMULATED MICROBUBBLES
Microbubbles can be functionalized with various features for more specialized applications and increased capabilities. Attaching targeting ligands to the microbubble surface can increase bubble residence time at a site of interest (Klibanov 2009), while nanoparticles or organic dyes can be used to add additional imaging modalities (e.g., magnetic resonance or fluorescence). Microbubbles can also be loaded with small-molecule drugs, via an oil layer. Microfluidics can enable the controlled formation of multi-layered bubbles by slight modifications of the device types discussed above, such as adding channels containing the drug (often in oil) that combines with the gas and aqueous channels at the bubble-generating orifice in a flow-focusing device (see Hettiarachchi et al. [2009], Kandadai et al. [2016] and Shih et al [2013] for design examples). Bubbles feature multiple potential functionalization sites. The shell components can be chemically modified pre- or post-bubble fabrication. In the case of a protein shell, the protein can also be modified genetically, fused with another protein with desired activity (Angilè et al. 2014). Compounds or materials can be added to the continuous phase which in turn will adsorb to the gas water interface with the shell material to add functionality to the shell as well. Finally, hydrophobic compounds can intercalate in the hydrophobic inner layer of the shell.
Additional imaging modalities
Microbubbles produced by microfluidics have also been functionalized with additional imaging modalities including fluorescence and magnetic resonance. Fluorescent microbubbles have been produced using a number of different fluorescent tags. Talu et al. (2007) used intercalation of hydrophobic DiI dye in phospholipid bubble shells to functionalize their flow-focusing-produced microbubbles, by adding the dye to the continuous phase containing a mixture of DSPC and DSPE-PEG2000 lipids during bubble formation. Angilè et al. (2014) created a genetic fusion of shell-stabilizing oleosin protein and green fluorescent protein, and used this fusion protein to prepare microbubbles in a flow-focusing device, confirming the incorporation of the protein on the bubble surface. Fluorescent avidin was used to generate fluorescent microbubbles by using biotin-labeled lipids to form microbubbles in a flow-focusing device, followed by conjugation with high-affinity avidin (Talu et al. 2007). Quantum dots have also been used to generate fluorescent microbubbles. By first incorporating quantum dots into the liposomes used in the continuous phase to prepare the microbubbles, quantum dot-functionalized bubbles were generated in a flow-focusing device (Peyman et al. 2012). A more involved functionalization method was developed by Seo et al. (2010). Microbubbles were prepared using a flow-focusing device with lysozyme protein and DPPC and MPEG-5000-DPPE lipids in the continuous phase. The bubbles were generated at pH 11.5, where lysozyme has a negative charge, to strengthen hydrophobic interaction between the protein and perfluorocarbon gas used. The solution pH was then decreased via addition of 0.1 M HCl to pH 7.4, where lysozyme is positively charged. Quantum dots with negatively charged silica shell adsorb readily to the lysozyme-functionalized microbubbles. The quantum dots were tightly bound to the microbubble surface, with multiple washes leading to no loss of fluorescence. Addition of quantum dots had no effect on microbubble monodispersity and stability in saline compared with unfunctionalized bubbles, and both functionalized and unfunctionalized bubbles were echogenic under low-pressure ultrasound.
Magnetic resonance imaging (MRI) is another non-invasive, high-resolution imaging technique free from ionizing radiation. Microbubbles produced using a flow-focusing device and functionalized with Fe3O4 (Park et al. 2010a; Seo et al. 2010) nanoparticles were found to have dual contrast activity. The nanoparticles preferentially adsorbed to the gas water interface and, because of their magnetic properties, generated magnetic resonance contrast. Bubbles were also functionalized with Gd-loaded silica, another magnetic resonance contrast agent (Seo et al. 2010). However, data reflecting dual contrast were not presented for these bubbles. Fe3O4- and Gd-loaded silica particles were conjugated to microbubbles using the lysozyme pH-variation method described above (Park et al. 2010a).
Drug-loaded microbubbles
Microfluidics is ideal for producing drug-loaded microbubbles, as bubble uniformity will ensure greater uniformity over drug loading and bubble behavior under ultrasound, both of which will ensure greater control over drug dosage (Choi et al. 2010). In addition, microfluidic devices can be operated at low temperatures (if needed for thermally labile drugs) and avoid generating significant heat, unlike sonication methods. However, very few examples are found in the literature, and this is an area that is ripe for rapid and significant growth. One of the key challenges lies in the low loading capacity of gas bubbles compared with solid particles or emulsions. This limitation was addressed by using microfluidic channels to produce gas-in-oil-in-water compound microbubbles, which are gas bubbles with thin oil layers between the gas bubble and the continuous phase. High concentrations of hydrophobic molecules can be loaded in the oil phase. Hettiarachchi et al. (2009) successfully loaded the anti-cancer drug doxorubicin at a concentration of 15 mg/mL using a flow-focusing device and found that these bubbles localized to MDA-MB-231 breast cancer cells through avidin biotin binding. How-ever, detailed information regarding the delivery of the drug to the cells through release from the bubble oil layer was not provided. Protein-based thrombolytic drug recombinant tissue-plasminogen activator (rt-PA) was loaded in bubbles using a flow-focusing device for treatment of acute ischemic stroke (Kandadai et al. 2016). Using a chromogenic method to quantify the rt-PA present, the researchers found that 64.30 ± 3.80 µg/mL associated with the bubble shell (compared with 148.70 ± 30.80 µg/mL present if the device was operated without gas and subsequent bubble formation).
Even if the drug is not directly linked to the microbubble or encapsulated within an oil layer of compound microbubbles, the cavitation under ultrasound can also lead to improved drug delivery. At high ultrasound intensities, bubbles will burst, and the resulting forces can enhance cell membrane or blood vessel permeability, enhancing drug uptake (Lentacker et al. 2009; van Wamel et al. 2006). Microfluidic microbubbles with high uniformity and engineered properties could be ideal in such an approach as their response to ultrasound will be very uniform and can be tailored to meet delivery needs.
Targeted microbubbles
Targeted delivery of drugs or contrast agent is a major area in translational research, with the promise of enabling more efficient treatment (Sakhrani and Padh 2013), minimizing side effects (Bae and Park 2011) and leading to molecular imaging (James and Gambhir 2012). Targeting enables the specific localization of bubbles to a site of interest (cell type or specific organ) through the attachment of ligands to the bubble that bind specifically to particular cellular markers. Because targeting is often dependent on the density of ligands on a particle surface (Elias et al. 2014), microfluidics provides a powerful platform to control the bubble surface composition while ensuring uniformity (Kamaly et al. 2012). Despite such a great potential, there are a surprisingly small number of published examples of taking this approach (most targeted bubbles are produced using sonication or other techniques), and thus there is much room for growth in this area. Bubbles can be functionalized with a targeting moiety by modifying the shell material pre-microfluidics or by modifying the bubble surface after fabrication (Yeh et al. 2015). Proprietary lipids conjugated with cyclic RGD peptide enabled formation of targeted microbubbles with a flow-focusing device (Talu et al. 2007). Cyclic RGD peptide has high affinity for αvβ3 integrin, which is involved in angiogenesis and is overexpressed in many types of cancer cells (Liu et al. 2008). The targeted bubbles were shown to bind to human melanoma A375 m cells. Targeted microbubbles were also generated using anti-vascular endothelial growth factor 2 (VEGFR-2) antibodies (Peyman et al. 2012). The VEGFR family of proteins is highly important in angiogenesis and represents another broad cancer target (Shibuya 2011). The bubbles were prepared using DPPC and DSPE-biotin-PEG2000 in a flow-focusing device, and neutravidin was added to the bubbles post-formation. Biotinylated antibody was then added, resulting in microbubbles functionalized through a biotin avidin biotin linkage. The targeted bubbles were found to preferentially bind to mouse pancreatic islet endothelial cells, which overexpress VEGFR-2.
SCALE-UP OF MICROFLUIDIC BUBBLE GENERATION
One disadvantage of using microfluidic devices instead of sonication or chemical methods to produce microbubbles is the low production rate. In fact, the inability to scale up bubble production could become the main impediment to translating the exciting laboratory-scale discoveries to commercialization of microbubble agents produced based on microfluidic methods. Laboratory-scale sonication produces ~108 microbubbles per second (mB/s) (Qin et al. 2009; Shih et al. 2013). Assuming a bubble diameter of 5 µm, this translates to a production rate of 23 mL/h. Industrial-scale processes can generate emulsions at a very high rate of hundreds to thousands of liters per hour, albeit with broad size distributions (coefficient of variation >30%) (Joscelyne and Trägårdh 2000). Microfluidics offers a significant advantage over the sonication-based method in producing highly uniform microbubbles, with the disadvantage of lower productivity. Microfluidic throughput is on average much lower than sonication, if the strategies described below are not taken, with a production rate of <104 mB/s (~2 µL/h). Devices running at higher production rates need to be more robust, which can be achieved through the use of hard materials, such as glass and plastic (polymethylmethacrylate, polycarbonate) rather than PDMS (Jeong et al. 2016). Although more expensive, they can operate for longer periods and can be cleaned using harsh chemicals.
To overcome limitations in production rate, several strategies for increasing microbubble production in microfluidic flow-focusing devices have been explored. It is important to be able to continuously deliver fluids without significant fluctuations as well. A well-designed parallel device can produce highly uniform microbubbles reproducibly, as long as the channel dimensions are uniform throughout the microfluidic device (Jeong et al. 2017). Campo-Cortés et al. (2016) developed a PDMS-on-PDMS device with a narrow groove in the exit channel. Illustrated in Figure 7, these two features help to stabilize a thin gas stream, resulting in bubble sizes significantly smaller than the orifice, while also increasing bubble production up to 105 mB/s (23 µL/h).
Fig. 7.
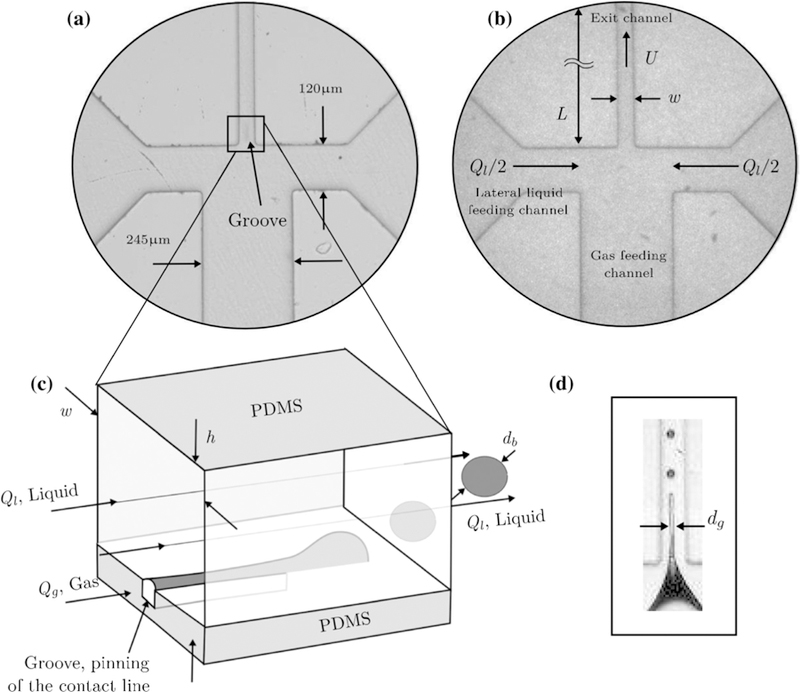
(a) Grooved exit channel helps to stabilize thin gas ligament, increasing bubble productivity and narrowing diameter. (b) Device shown without the exit channel groove. (c) Schematic illustrating bubble generation. (d) Image of gas ligament and bubble formation. PDMS = polydimethylsiloxane. Reprinted by permission from: Springer, Microfluidics and Nanofluidics, The effect of contact line pinning favors the mass production of monodisperse microbubbles, Campo-Cortés F, Riboux G, Gordillo JM, 2016.
Parallelization of bubble-generating devices can also increase microbubble production, although simple parallelization in doubling the number of bubble generation orifices does not necessarily lead to a doubling in production. The key in parallelization is to deliver uniform fluid flow rates to multiple gas generators operating in parallel from a single set of inlet ports for the dispersed gas and continuous phases. One device was built by Dhanaliwala et al. (2013), using a pressurized liquid chamber (Fig. 8). A single-orifice device could be optimized for bubble production by increasing the gas pressure up until the jetting regime, resulting in a production rate of 6.6 × 105 mB/s (155 µL/h). A slight increase in production rate was observed using a parallelized device featuring two orifices with only one gas inlet and two liquid inlets. Bubbles were produced at the rate of 6.7 × 105 mB/s (158 µL/h). It was concluded that the design of the device led to each orifice receiving half the liquid flow rate and thus operating at approximately half the rate of the single-orifice device. Doubling the flow rate to compensate prevented microbubble formation, highlighting the need for careful device engineering.
Fig. 8.
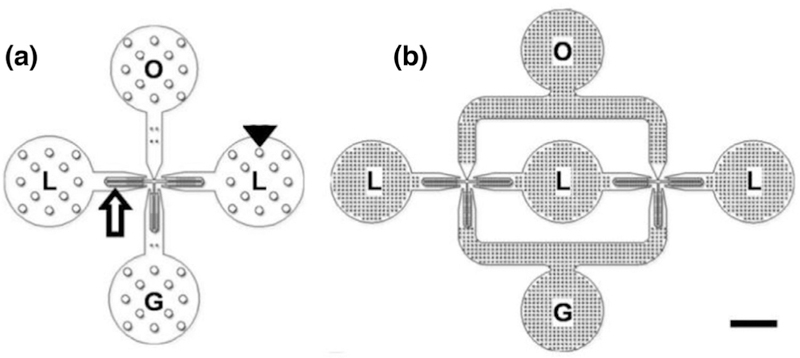
(a) Single-orifice device with gas inlet (G), two liquid inlets (L) and a bubble outlet (O). (b) Dual-orifice device featuring three liquid inlets, one gas inlet and one bubble outlet. G = gas, L = lipid, O = oil. Bar = 1 mm. Adapted by permission from: Springer, Microfluidics and Nanofluidics, The effect of contact line pinning favors the mass production of monodisperse microbubbles, Dhanaliwala AH, Chen JL, Wang S, Hossack JA, 2013.
One modified flow-focusing device developed by Peyman et al. (2012) featured two bubble formation regimes: the typical single bubble stream and a bubble spray (Fig. 9). This was accomplished by introducing a sudden pressure drop after the flow-focusing orifice, by widening the outlet channel and abruptly doubling the channel depth. In the bubble spray regime, 106 mB/s (235 µL/h) was produced, leading to a bubble concentration of 108 to 109 mB/mL. The average bubble size was highly reproducible at 1.7 ± 0.07 µm, based on three devices built and tested by four different users in three different laboratories (this rigor is notable and should be encouraged). To increase bubble production even further, multiplexed devices were built to produce 109 – 1010 mB/mL in 10 min, approaching the production rate of sonication with the advantage of greater monodispersity.
Fig. 9.
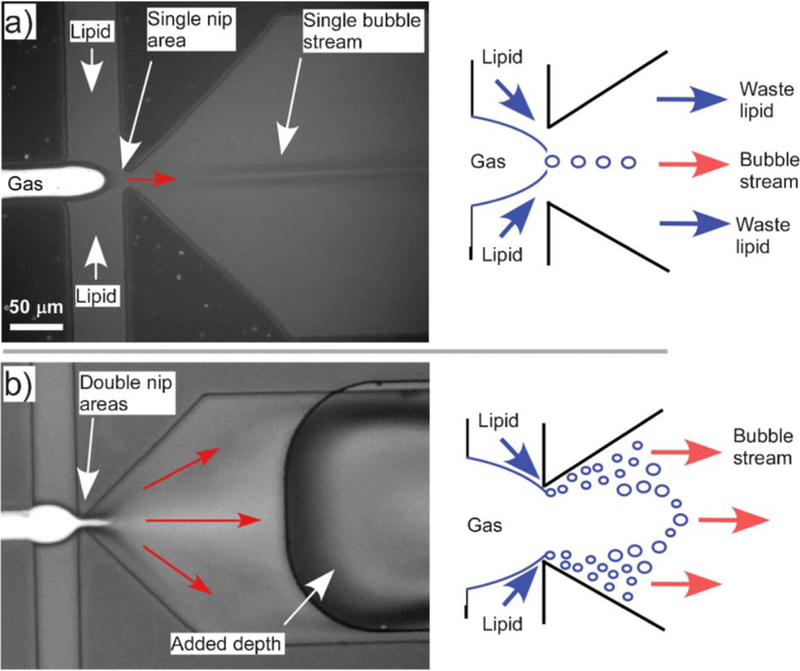
Modified flow-focusing device for increased microbubble production. (a) Typical bubble formation with single bubbles pinching off at the orifice. (b) By widening the outlet channel and introducing an abrupt increase in channel depth, a spray of bubbles was formed, significantly increasing bubble production. Adapted from Peyman et al. (2012) with permission of The Royal Society of Chemistry.
Kendall et al. (2012) developed a circular, parallelized flow-focusing device featuring eight orifices that could produce oil-layered bubbles at production rates of up to 5 × 104 mB/s (11 µL/h) (Fig. 10). The device was built using three PDMS layers, with single gas, oil and liquid inlets. On entering the top PDMS layer, the gas was split among the eight orifices and passed through serpentine channels that acted as resistors to better separate individual orifices and prevent disruptive cross-talk between bubble generators. The oil and liquid entered at the bottom PDMS layer were split at the bottom and middle layers, respectively. Vertical passages acting as reservoirs to minimize errors in distribution led the oil and liquid to the orifices on the top PDMS layer. The PDI of the resulting bubbles collected from all eight parallelized channels was higher than that of a single channel (≤9% vs. <3%, respectively). This was attributed to small variations and imperfections in the individual devices: the orifice size had a coefficient of variation of 9% because of resolution limits in soft lithography. As is often a challenge, at the highest volumetric production rates, bubble size was larger than desired for ultrasound applications at >18 µm.
Fig. 10.
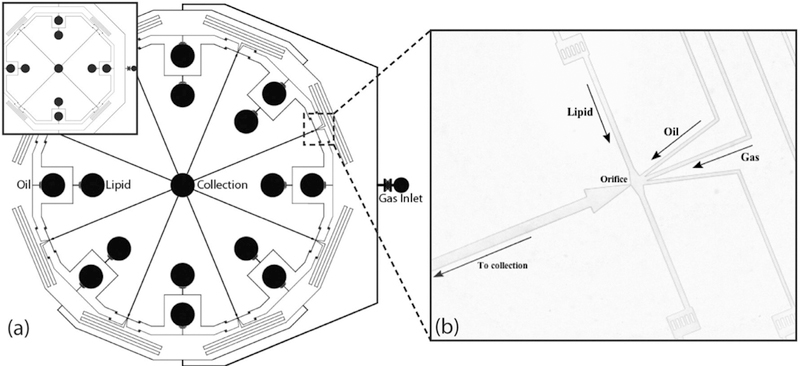
(a) Parallelized bubble generation device featuring eight orifices (four in inset). (b) Close-up revealing gas, oil and lipid channels focused at single orifice “Scaled-up production of monodisperse, dual layer microbubbles using multi-array microfluidic module for medical imaging and drug delivery,” (Kendall et al. 2012) Bubble Science Engineering and Technology, 2012, reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com)
CONCLUSIONS AND OUTLOOK
As highlighted in this review, efforts to combine microfluidic-based approaches and recent advances in surfactant chemistry and physics have led to many exciting possibilities in engineering microbubbles for ultrasound theranostic applications. Though much work has been focused on the production of stable, functionalized bubbles with precise control over size and uniformity, there is significant room for additional advances.
Despite the enormous potential and success of microfluidics, there are a very limited number of studies on the in vivo investigation of microfluidic microbubbles. One example is the direct injection of microbubbles produced by a flow-focusing device into mice (Dhanaliwala et al. 2015). The bubbles were stabilized by bovine serum albumin and dextrose. The mice exhibited no respiratory distress, despite injection of bubbles up to 19 µm in diameter. A follow-up study revealed the therapeutic application of direct injection of microfluidic-produced microbubbles for sonothrombolysis using an in vitro blood clot model (Dixon et al. 2018). Further work is needed to understand the behavior of microfluidic-produced microbubbles in vivo, including immunogenicity studies, determining circulation time and actual targeting capabilities of targeted bubbles, stability under ultrasound and improving understanding of how specific microbubble properties affect their imaging and therapeutic efficacy.
Toward this goal, using microfluidics to test microbubbles in a simulated environment could potentially be transformative. This possibility is even more attractive with recent interest in producing organ-on-a-chip devices that can be used as a platform for drug screening and testing (Benam et al. 2015; Vladisavljevi et al. 2013). Progress along these lines of investigation is underway. Park et al. (2016) used a microfluidic chip to explore the effect of microbubbles under ultrasound on drug delivery to a solid tumor microvasculature model. The greatest cell toxicity was observed when targeted doxorubicin liposomes were used, in the presence of microbubbles, after exposure to ultrasound under stable cavitation conditions. The increased delivery and subsequent toxicity were attributed to an increase in receptor-mediated endocytosis, rather than disruption of blood vessel integrity, as a combination of drug-free liposomes, microbubbles and ultrasound did not lead to cell mortality. The experiments were not performed under flow conditions, which would more completely model a real microvasculature system. However, adapting the device presented to incorporate flow conditions was noted as feasible. Exploring the effect of microbubbles under ultrasound in the presence of flow should be well suited to microfluidic devices in general and will be a welcome area of future research in the field.
Although work has been reported to increase bubble production, as described above, continuing to develop scale-up strategies will be paramount to the clinical use of microfluidic-produced microbubbles. Recent work by Jeong et al. (2017) illustrated the production of microbubbles at liter-per-hour rates, using a 5 × 5 cm2 chip with 400 parallelized flow-focusing devices. Although the bubbles produced were >10 µm, further engineering could lead to the production of bubbles appropriately sized for biomedical ultrasound applications, at commercially relevant scale.
In summary, microfluidics has been used to produce the most monodisperse microbubble contrast agents reported and enables careful control over bubble size, shell composition and inner gas. Although work remains to increase production rates before microfluidic-produced bubbles can be practical for use in the clinic, their uniform acoustic behavior warrants continued research in the field, especially toward targeted, drug-loaded, multi-functional bubbles. While it is generally believed that highly uniform bubbles will lead to uniform acoustic behavior and thus higher efficacy in enhancing imaging contrast and therapeutic treatments, few demonstrations have been reported. With the advent of uniform bubbles with engineered properties, it is also becoming increasingly important to be able to predict and model the behavior of such microbubbles under conditions that are relevant to clinical use (Qin and Ferrara 2006; Qin et al. 2009; Wiedemair et al. 2012). In particular, a detailed understanding of the energy dissipation mechanisms of microbubbles under ultrasound could lead to production of engineered bubbles that will have transformative impacts on the clinical use of microbubbles for diagnostic and therapeutic applications.
Acknowledgments
This work was supported by the National Institutes of Health (Grant R01 EB022612).
REFERENCES
- Abou-Saleh RH, Peyman SA, Critchley K, Evans SD, Thomson NH. Nanomechanics of lipid encapsulated microbubbles with functional coatings. Langmuir 2013;29:4096–4103. [DOI] [PubMed] [Google Scholar]
- Abou-Saleh RH, Swain M, Evans SD, Thomson NH. Poly(ethylene glycol) lipid-shelled microbubbles: Abundance, stability, and mechanical properties. Langmuir 2014;30:5557–5563. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Zhang H, Farook U, Edirisinghe M, Stride E, Colombo P. Generation of multilayered structures for biomedical applications using a novel tri-needle coaxial device and electrohydrodynamic flow. J R Soc Interface 2008;5:1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobio-technol 2012;4:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CR, Hu X, Tlaxca J, Houghtaling R, Sharma K, Lawrence M, Ferrara K, Rychak JJ, Diego S. Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Invest Radiol 2011;46:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilè FE, Vargo KB, Sehgal CM, Hammer DA, Lee D. Recombinant protein-stabilized monodisperse microbubbles with tunable size using a valve-based microfluidic device. Langmuir 2014;30: 12610–12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anna SL. Droplets and bubbles in microfluidic devices. Annu Rev Fluid Mech 2016;48:285–309. [Google Scholar]
- Anna SL, Bontoux N, Stone HA. Formation of dispersions using “flow focusing” in microchannels. Appl Phys Lett 2003;82:364–366. [Google Scholar]
- Aron M, Browning R, Carugo D, Sezgin E, Bernardino de la Serna J, Eggeling C, Stride E Spectral imaging toolbox: Segmentation, hyperstack reconstruction, and batch processing of spectral images for the determination of cell and model membrane lipid order. BMC Bioinformatics 2017;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. J Control Release 2011;153:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang K, Karalis K, Kim HJ, Macqueen L, Mahmoodian R, Musah S, Torisawa Y, Van Der Meer AD, Villenave R, Yadid M, Parker KK, Ingber DE. Engineered in vitro disease models. Annu Rev Pathol Mech Dis 2015;10:195–262. [DOI] [PubMed] [Google Scholar]
- Benchimol MJ, Hsu MJ, Schutt CE, Hall DJ, Mattrey RF, Esener SC. Phospholipid/carbocyanine dye-shelled microbubbles as ultrasound-modulated fluorescent contrast agents. Soft Matter 2013;9:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat AAS, Bow H, Hou HW, Tan SJ, Han J, Lim CT. Microfluidics for cell separation. Med Biol Eng Comput 2010;48:999–1014. [DOI] [PubMed] [Google Scholar]
- Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, Dokmeci MR, Khademhosseini A. Organ-on-a-chip platforms for studying drug delivery systems. J Control Release 2014;190:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmer MR, Schroeders R, Steenbakkers JAM, de Winter SHPM, Dui-neveld PA, Lub J, Nijssen WPM, Pikkemaat JA, Stapert HR Preparation of monodisperse polymer particles and capsules by ink-jet printing. Colloids Surf A 2006;289:96–104. [Google Scholar]
- Campo-Cortés F, Riboux G, Gordillo JM. The effect of contact line pinning favors the mass production of monodisperse microbubbles. Microfluid Nanofluidics 2016;20:21. [Google Scholar]
- Carugo D, Lee JY, Pora A, Browning RJ, Capretto L, Nastruzzi C, Stride E. Facile and cost-effective production of microscale PDMS architectures using a combined micromilling-replica moulding (µMi-REM) technique. Biomed Microdevices 2016;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Hernández E, van Hoeve W, Lohse D, Gordillo JM. Microbubble generation in a co-flow device operated in a new regime. Lab Chip 2011;11:2023–2029. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhu Y, Leech PW, Manasseh R. Production of monodispersed micronsized bubbles at high rates in a microfluidic device. Appl Phys Lett 2009;95 144101. [Google Scholar]
- Chin CD, Linder V, Sia SK. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012;12:2118. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Feshitan JA, Baseri B, Wang S, Tung Y, Borden MA, Konofagou EE. Microbubble-size dependence of focused ultrasound-induced blood brain barrier opening in mice in vivo. IEEE Trans Biomed Eng 2010;57:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomas JE, Dayton P, May D, Ferrara K. Threshold of fragmentation for ultrasonic contrast agents. J Biomed Opt 2001;6:141–150. [DOI] [PubMed] [Google Scholar]
- Christopher GF, Anna SL. Microfluidic methods for generating continuous droplet streams. J Phys D Appl Phys 2007;40:R319–R336. [Google Scholar]
- DeMaria AN, Narula J, Mahmud E, Tsimikas S. Imaging vulnerable plaque by ultrasound. J Am Coll Cardiol 2006;47:32–39. [DOI] [PubMed] [Google Scholar]
- Dhanaliwala AH, Chen JL, Wang S, Hossack JA. Liquid flooded flow-focusing microfluidic device for in situ generation of monodisperse microbubbles. Microfluid Nanofluidics 2013;14: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanaliwala AH, Dixon AJ, Lin D, Chen JL, Klibanov AL, Hossack JA. In vivo imaging of microfluidic-produced microbubbles. Biomed Microdevices 2015;17:23. [DOI] [PubMed] [Google Scholar]
- Dixon AJ, Dhanaliwala AH, Chen JL, Hossack JA. Enhanced intracellular delivery of a model drug using microbubbles produced by a microfluidic device. Ultrasound Med Biol 2013;39:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AJ, Rickel JMR, Shin BD, Klibanov AL, Hossack JA. In vitro sonothrombolysis enhancement by transiently stable microbubbles produced by a flow-focusing microfluidic device. Ann Biomed Eng 2018;46:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncanson WJ, Lin T, Abate AR, Seiffert S, Shah RK, Weitz DA. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab Chip 2012;12:2135. [DOI] [PubMed] [Google Scholar]
- Elias DR, Poloukhtine A, Popik V, Tsourkas A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nano-medicine 2014;9:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Needham D. Physical properties of surfactant bilayer membranes: Thermal transitions, elasticity, rigidity, cohesion, and colloidal interactions. J Phys Chem 1987;91:4219–4228. [Google Scholar]
- Farook U, Stride E, Edirisinghe MJ, Moaleji R. Microbubbling by coaxial electrohydrodynamic atomization. Med Biol Eng Comput 2007;45:781–789. [DOI] [PubMed] [Google Scholar]
- Farook U, Stride E, Edirisinghe MJ. Preparation of suspensions of phospholipid-coated microbubbles by coaxial electrohydrodynamic atomization. J R Soc Interface 2009;6:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feshitan JA, Chen CC, Kwan JJ, Borden MA. Microbubble size isolation by differential centrifugation. J Colloid Interface Sci 2009;329:316–324. [DOI] [PubMed] [Google Scholar]
- Gañán-Calvo AM, Gordillo JM. Perfectly monodisperse microbubbling by capillary flow focusing. Phys Rev Lett 2001;87 274501. [DOI] [PubMed] [Google Scholar]
- German SR, Edwards MA, Chen Q, White HS. Laplace pressure of individual H2 nanobubbles from pressure—Addition electrochemistry. Nano Lett 2016;16:6691–6694. [DOI] [PubMed] [Google Scholar]
- Gnyawali V, Moon B-U, Kieda J, Karshafian R, Kolios MC, Tsai SSH. Honey, I shrunk the bubbles: Microfluidic vacuum shrinkage of lipid-stabilized microbubbles. Soft Matter 2017;13:4011–4016. [DOI] [PubMed] [Google Scholar]
- Guo MT, Rotem A, Heyman JA, Weitz DA. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012;12:2146. [DOI] [PubMed] [Google Scholar]
- Hettiarachchi K, Talu E, Longo ML, Dayton PA, Lee AP. On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging. Lab Chip 2007;7:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettiarachchi K, Lee AP, Zhang S, Feingold S, Dayton PA. Controllable microfluidic synthesis of multiphase drug-carrying lipospheres for site-targeted therapy. Biotchnol Prog 2009;25:938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff L, Sontum PC, Hovem JM. Oscillations of polymeric microbubbles: Effect of the encapsulating shell. J Acoust Soc Am 2000;107:2272–2280. [DOI] [PubMed] [Google Scholar]
- Hosny NA, Mohamedi G, Rademeyer P, Owen J, Wu Y, Tang MX, Eckersley RJ, Stride E, Kuimova MK. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proc Natl Acad Sci 2013;110:9225–9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Zhang YS, Santiago GTDe, Alvarez MM, Ribas J, Jonas SJ, Weiss PS, Andrews AM, Aizenberg J, Khademhosseini A Inter-play between materials and microfluidics. Nat Rev Mater 2017;2:17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MS, Marsh JN, Hall CS, Fuhrhop RW, Lacy EK, Lanza GM, Wickline SA. Acoustic characterization in whole blood and plasma of site-targeted nanoparticle ultrasound contrast agent for molecular imaging. J Acoust Soc Am 2005;117:964–972. [DOI] [PubMed] [Google Scholar]
- Hunt SJ, Gade T, Soulen MC, Pickup S, Sehgal CM. Antivascular ultrasound therapy. J Ultrasound Med 2015;34:275–287. [DOI] [PubMed] [Google Scholar]
- James ML, Gambhir SS. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol Rev 2012;92:897–965. [DOI] [PubMed] [Google Scholar]
- Jang WS, Park SC, Kim M, Doh J, Lee D, Hammer DA. The effect of stabilizer on the mechanical response of double-emulsion-templated polymersomes. Macromol Rapid Commun 2015;36:378–384. [DOI] [PubMed] [Google Scholar]
- Jang Y, Jang WS, Gao C, Shim TS, Crocker JC, Hammer DA, Lee D. Tuning the mechanical properties of recombinant protein-stabilized gas bubbles using triblock copolymers. ACS Macro Lett 2016;5:371–376. [DOI] [PubMed] [Google Scholar]
- Jeong HH, Issadore D, Lee D. Recent developments in scale-up of microfluidic emulsion generation via parallelization. Korean J Chem Eng 2016;33:1757–1766. [Google Scholar]
- Jeong HH, Yadavali S, Issadore D, Lee D. Liter-scale production of uniform gas bubbles via parallelization of flow-focusing generators. Lab Chip 2017;17:2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensson HN, Andersson Svahn H. Droplet microfluidics—A tool for single-cell analysis. Angew Chem Int Ed 2012;51:12176–12192. [DOI] [PubMed] [Google Scholar]
- Joscelyne SM, Trägårdh G. Membrane emulsification—A literature review. J Memb Sci 2000;169:107–117. [Google Scholar]
- Kaigala GV, Ho S, Penterman R, Backhouse CJ. Rapid prototyping of microfluidic devices with a wax printer. Lab Chip 2007;7:384–387. [DOI] [PubMed] [Google Scholar]
- Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev 2012;41:2971–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat NP, Lee MH, Lee D, Hammer DA. Micropipette aspiration of double emulsion-templated polymersomes. Soft Matter 2011;7:9863. [Google Scholar]
- Kaminski TS, Garstecki P. Controlled droplet microfluidic systems for multistep chemical and biological assays. Chem Soc Rev 2017;46:6210–6226. [DOI] [PubMed] [Google Scholar]
- Kandadai MA, Mukherjee P, Shekhar H, Shaw GJ, Papautsky I, Holland CK. Microfluidic manufacture of rt-PA-loaded echogenic liposomes. Biomed Microdevices 2016;18:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M, Feingold S, Hettiarachchi K, Lee AP, Dayton PA. Acoustic responses of monodisperse lipid-encapsulated microbubble contrast agents produced by flow focusing. Bubble Sci Eng Technol 2010;2:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H, Xing Z, Zhao B, Wang J, Liu J, Guo C, Yue X, Liu S, Tang Z, Dai Z. Quantum-dot-modified microbubbles with bi-mode imaging capabilities. Nanotechnology 2009;20 425105. [DOI] [PubMed] [Google Scholar]
- Kendall MR, Bardin D, Shih R, Dayton PA, Lee AP. Scaled-up production of monodisperse, dual layer microbubbles using multiarray microfluidic module for medical imaging and drug delivery. Bubble Sci Eng Technol 2012;4:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling F, Huppert J, Palmowski M. Functional and molecular ultrasound imaging: concepts and contrast agents. Curr Med Chem 2009;16:627–642. [DOI] [PubMed] [Google Scholar]
- Kim DH, Costello MJ, Duncan PB, Needham D. Mechanical properties and microstructure of polycrystalline phospholipid monolayer shells: Novel solid microparticles. Langmuir 2003;19:8455–8466. [Google Scholar]
- Klibanov AL. Targeted microbubbles: Ultrasound contrast agents for molecular imaging. In: Bulte JWM, Modo MMJ, (eds). Nanoparticles in biomedical imaging New York: Springer New York; 2008:327–341. [Google Scholar]
- Klibanov AL. Preparation of targeted microbubbles: Ultrasound contrast agents for molecular imaging. Med Biol Eng Comput 2009;47:875–882. [DOI] [PubMed] [Google Scholar]
- Kukizaki M, Baba Y. Effect of surfactant type on microbubble formation behavior using Shirasu porous glass (SPG) membranes. Colloids Surf A 2008;326:129–137. [Google Scholar]
- Kukizaki M, Wada T. Effect of the membrane wettability on the size and size distribution of microbubbles formed from Shirasu-porousglass (SPG) membranes. Colloids Surf A 2008;317:146–154. [Google Scholar]
- Kwapiszewska K, Żukowski K, Kwapiszewski R, Brzózka Z. Double casting prototyping with a thermal aging step for fabrication of 3-D microstructures in poly(dimethylsiloxane). AIMS Biophys 2016;3: 553–562. [Google Scholar]
- Lee M, Lee EY, Lee D, Park BJ. Stabilization and fabrication of microbubbles: Applications for medical purposes and functional materials. Soft Matter 2015;11:2067–2079. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim H, Han H, Lee M, Lee S, Yoo H, Chang JH, Kim H. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed Eng Lett 2017;7: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentacker I, De Smedt SC, Sanders NN. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter 2009;5:2161. [Google Scholar]
- Levenback BJ, Sehgal CM, Wood AKW. Modeling of thermal effects in antivascular ultrasound therapy. J Acoust Soc Am 2012;131:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hou L, Zhang W, Zhu L. Preparation of PDMS microfluidic devices based on drop-on-demand generation of wax molds. Anal Methods 2014;6:4716–4722. [Google Scholar]
- Lignos I, Maceiczyk R, DeMello AJ. Microfluidic technology: Uncovering the mechanisms of nanocrystal nucleation and growth. Acc Chem Res 2017;50:1248–1257. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017;17:3960–3978. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang F, Chen X. Integrin αvβ3-targeted cancer therapy. Drug Dev Res 2008;69:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HL, Fan CH, Ting CY, Yeh CK. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2014;4:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre S, Jensen KF. Synthesis of micro and nanostructures in micro-fluidic systems. Chem Soc Rev 2010;39:1183. [DOI] [PubMed] [Google Scholar]
- Pancholi K, Stride E, Edirisinghe M. Generation of microbubbles for diagnostic and therapeutic applications using a novel device. J Drug Target 2008;16:494–501. [DOI] [PubMed] [Google Scholar]
- Pancholi KP, Farook U, Moaleji R, Stride E, Edirisinghe MJ. Novel methods for preparing phospholipid coated microbubbles. Eur Biophys J 2008;37:515–520. [DOI] [PubMed] [Google Scholar]
- Park JI, Nie Z, Kumachev A, Abdelrahman AI, Binks BP, Stone HA, Kumacheva E. A microfluidic approach to chemically driven assembly of colloidal particles at gas liquid interfaces. Angew Chem Int Ed 2009;48:5300–5304. [DOI] [PubMed] [Google Scholar]
- Park JI, Jagadeesan D, Williams R, Oakden W, Chung S, Stanisz GJ, Kumacheva E. Microbubbles loaded with nanoparticles: A route to multiple imaging modalities. ACS Nano 2010;4:6579–6586. [DOI] [PubMed] [Google Scholar]
- Park JI, Tumarkin E, Kumacheva E. Small, stable, and monodispersed bubbles encapsulated with biopolymers. Macromol Rapid Commun 2010;31:222–227. [DOI] [PubMed] [Google Scholar]
- Park YC, Zhang C, Kim S, Mohamedi G, Beigie C, Nagy JO, Holt RG, Cleveland RO, Jeon NL, Wong JY. Microvessels-on-a-chip to assess targeted ultrasound-assisted drug delivery. ACS Appl Mater Interfaces 2016;8:31541–31549. [DOI] [PubMed] [Google Scholar]
- Parrales MA, Fernandez JM, Perez-saborid M, Kopechek JA, Porter TM. Acoustic characterization of monodisperse lipid-coated microbubbles: Relationship between size and shell viscoelastic properties. J Acoust Soc Am 2014;136:1077–1084. [DOI] [PubMed] [Google Scholar]
- Peyman SA, Abou-Saleh RH, Mclaughlan JR, Ingram N, Johnson BRG, Critchley K, Freear S, Evans JA, Markham AF, Louise P, Evans SD. Expanding 3-D geometry for enhanced on-chip microbubble production and single step formation of liposome modified microbubbles. Lab Chip 2012;12:4544–4552. [DOI] [PubMed] [Google Scholar]
- Peyman SA, McLaughlan JR, Abou-Saleh RH, Marston G, Johnson BRG, Freear S, Coletta PL, Markham AF, Evans SD. On-chip preparation of nanoscale contrast agents towards high-resolution ultrasound imaging. Lab Chip 2016;16:679–687. [DOI] [PubMed] [Google Scholar]
- Qin S, Ferrara KW. Acoustic response of compliable microvessels containing ultrasound contrast agents. Phys Med Biol 2006;51:5065–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Caskey C, Ferrara K. Ultrasound contrast microbubbles in imaging and therapy: Physical principles and engineering. Phys Med Biol 2009;54:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans EA. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J 2000;79:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature 2014;507:181–189. [DOI] [PubMed] [Google Scholar]
- Sakhrani NM, Padh H. Organelle targeting: Third level of drug targeting. Drug Des Devel Ther 2013;7:585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers T, Versluis M. Acoustic bubble sorting for ultrasound contrast agent enrichment. Lab Chip 2014;14:1705–1714. [DOI] [PubMed] [Google Scholar]
- Segers T, de Jong N, Versluis M. Uniform scattering and attenuation of acoustically sorted ultrasound contrast agents: Modeling and experiments. J Acoust Soc Am 2016;140:2506–2517. [DOI] [PubMed] [Google Scholar]
- Segers T, De Rond L, De Jong N, Borden M, Versluis M. Stability of monodisperse phospholipid-coated microbubbles formed by flow-focusing at high production rates. Langmuir 2016;32: 3937–3944. [DOI] [PubMed] [Google Scholar]
- Segers T, Lohse D, Versluis M, Frinking P. Universal equations for the coalescence probability and long-term size stability of phospholipid-coated monodisperse microbubbles formed by flow-focusing. Langmuir 2017;33:10329–10339. [DOI] [PubMed] [Google Scholar]
- Seo M, Gorelikov I, Williams R, Matsuura N. Microfluidic assembly of monodisperse, nanoparticle-incorporated perfluorocarbon microbubbles for medical imaging and therapy. Langmuir 2010;26: 13855–13860. [DOI] [PubMed] [Google Scholar]
- Sheeran PS, Dayton PA. Phase-change contrast agents for imaging and therapy. Curr Pharm Des 2012;18:2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011;2: 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih R, Bardin D, Martz TD, Sheeran PS, Dayton PA, Lee AP. Flow-focusing regimes for accelerated production of monodisperse drug-loadable microbubbles toward clinical-scale applications. Lab Chip 2013;13:4816–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shung KK. High frequency ultrasonic imaging. J Med Ultrasound 2010;17:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shung KK. Diagnostic ultrasound: Past, present, and future. J Med Biol Eng 2011;31:371–374. [Google Scholar]
- Sirsi SR, Borden MA. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics 2012;2:1208–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride E, Edirisinghe M. Novel microbubble preparation technologies. Soft Matter 2008;4:2350. [Google Scholar]
- Talu E, Lozano MM, Powell RL, Dayton PA, Longo ML. Long-term stability by lipid coating monodisperse microbubbles formed by a flow-focusing device. Langmuir 2006;22:9487–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talu E, Hettiarachchi K, Zhao S, Powell RL, Lee AP, Longo ML, Dayton PA. Tailoring the size distribution of ultrasound contrast agents: Possible method for improving sensitivity in molecular imaging. Mol Imaging 2007;6:384–392. [PMC free article] [PubMed] [Google Scholar]
- Talu E, Hettiarachchi K, Powell RL, Lee AP, Dayton PA, Longo ML. Maintaining monodispersity in a microbubble population formed by flow-focusing. Langmuir 2008;24:1745–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Whitesides G. Basic microfluidic and soft lithographic techniques. In: Fainman Y, Lee LP, Psaltis D, Yang C, (eds). Optofluidics: Fundamentals, devices and applications New York: McGraw-Hill; 2010:7–32. [Google Scholar]
- Thorsen T, Roberts RW, Arnold FH, Quake SR. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys Rev Lett 2001;86:4163–4166. [DOI] [PubMed] [Google Scholar]
- Tsutsui JM, Xie F, Porter RT. The use of microbubbles to target drug delivery. Cardiovasc Ultrasound 2004;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utada AS, Fernandez-Nieves A, Stone HA, Weitz DA. Dripping to jetting transitions in coflowing liquid streams. Phys Rev Lett 2007;99:1–4. [DOI] [PubMed] [Google Scholar]
- van der Meer SM, Dollet B, Voormolen MM, Chin CT, Bouakaz A, de Jong N, Versluis M, Lohse D. Microbubble spectroscopy of ultrasound contrast agents. J Acoust Soc Am 2007;121:648–656. [DOI] [PubMed] [Google Scholar]
- van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, de Jong N. Vibrating microbubbles poking individual cells: Drug transfer into cells via sonoporation. J Control Release 2006;112:149–155. [DOI] [PubMed] [Google Scholar]
- Vladisavljevi GT, Khalid N, Neves MA, Kuroiwa T, Nakajima M, Uemura K, Ichikawa S, Kobayashi I, Anderssonsvahn H. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv Drug Deliv Rev 2013;65:1626–1663. [DOI] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature 2006;442:368–373. [DOI] [PubMed] [Google Scholar]
- Wiedemair W, Tukovic Z, Jasak H, Poulikakos D, Kurtcuoglu V. On ultrasound-induced microbubble oscillation in a capillary blood vessel and its implications for the blood brain barrier. Phys Med Biol 2012;57:1019–1045. [DOI] [PubMed] [Google Scholar]
- Wood AKW, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol 2015;41:905–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SK, Chu PC, Chai WY, Kang ST, Tsai CH, Fan CH, Yeh CK, Liu HL. Characterization of different microbubbles in assisting focused ultrasound-induced blood brain barrier opening. Sci Rep 2017;7: 46689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YN, Whitesides GM. Soft lithography. Annu Rev Mater Sci 1998;37:551–575. [Google Scholar]
- Yan WC, Chua QW, Ong XJ, Sharma VK, Tong YW, Wang CH. Fabrication of ultrasound-responsive microbubbles via coaxial electro-hydrodynamic atomization for triggered release of tPA. J Colloid Interface Sci 2017;501:282–293. [DOI] [PubMed] [Google Scholar]
- Yeh JSM, Sennoga CA, McConnell E, Eckersley R, Tang MX, Nourshargh S, Seddon JM, Haskard DO, Nihoyannopoulos P. A targeting microbubble for ultrasound molecular imaging. PLoS One 2015;10:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Marshall D. Microfluidics for single cell analysis. Curr Opin Biotechnol 2012;23:110–119. [DOI] [PubMed] [Google Scholar]
- Yin T, Wang P, Zheng R, Zheng B, Cheng D, Zhang X, Shuai X. Nano-bubbles for enhanced ultrasound imaging of tumors. Int J Nanomed 2012;7:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Wang P, Li J, Zheng R, Zheng B, Cheng D, Li R, Lai J, Shuai X. Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials 2013;34:4532–4543. [DOI] [PubMed] [Google Scholar]
- Zhao CX. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv Drug Deliv Rev 2013;65:1420–1446. [DOI] [PubMed] [Google Scholar]


