Abstract
Mesenchymal stromal cells are multipotent cells that are being used to treat a variety of medical conditions. Over the past decade, there has been considerable excitement about using MSCs to treat neurodegenerative diseases, which are diseases that are typically fatal and without other robust therapies. In this review, we discuss the proposed MSC mechanisms of action in neurodegenerative diseases, which include growth factor secretion, exosome secretion, and attenuation of neuroinflammation. We then provide a summary of preclinical and early clinical work on MSC therapies in amyotrophic lateral sclerosis, multiple system atrophy, Parkinson’s disease, and Alzheimer’s disease. Continued rigorous and controlled studies of MSC therapies will be critical in order to establish efficacy and protect patients from possible untoward side effects.
Neurodegenerative diseases are a broad class of disorders characterized by progressive neuronal death that leads to debilitating neurological impairments. Examples of ultimately fatal neurodegenerative diseases include Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and multiple system atrophy (MSA). While there have been significant advances in the symptomatic management of these diseases that improve quality of life and at times survival, the available medications likely only slow the progression of neuronal death by a few months. The idea of using cell therapy to treat neurodegenerative diseases has been around for decades, most notably in PD where a variety of cell transplant investigations have been performed with varying success.1 Mesenchymal stromal cells (also sometimes referred to as mesenchymal stem cells; MSCs; see recent commentary by Sipp et al., for discussion about the nomenclature controversy)2 are multipotent cells that have become increasingly studied as a therapy for a variety of neurological diseases. As of October 2018, there were 939 clinical studies listed at www.clinicaltrials.gov that report using MSCs. 218 of these clinical studies are for diseases of the nervous system, making them the most represented system in the body (Table 1). As MSCs have entered clinical trials for devastating neurodegenerative diseases, the excitement and interest in MSCs has become at times fevered. This, in part, has led to many for-profit entities that provide MSC therapies for a range of diseases, some of which make dubious claims and have unclear product safety. Conversely, rigorous basic science and clinical research are being performed widely in this rapidly growing and exciting field. In this review, we aim to discuss MSC therapeutic modes of action and how these cells are being utilized in neurodegenerative disease preclinical models and early phase clinical trials.
Table 1:
MSC-related studies listed at http://www.clinicaltrials.gov sorted by disease systems as of October 2018.
| Disease Type | Number of Studies |
|---|---|
| Nervous System | 218 |
| Musculoskeletal | 179 |
| Immune System | 177 |
| Cardiovascular | 140 |
| Wounds/Injuries | 133 |
| Gastrointestinal | 102 |
| Genetic/Congenital | 92 |
| Endocrine | 77 |
| Urogenital | 67 |
| Respiratory Tract | 57 |
| Skin | 53 |
| Graft-versus-Host | 45 |
| Hematological | 29 |
| Infection | 22 |
MSCs secrete growth factors and modulate immune system
While MSCs are considered to be a type of stem cell, they have limited differentiation capacity. Unlike embryonic (or induced) pluripotent stem cells, which may differentiate into all cell types, MSCs are primarily limited to differentiating into cells of mesenchymal origin (fibroblast, osteocyte, adipocyte, chondrocyte). It is still controversial whether MSCs can be readily differentiated into cells of endodermal or ectodermal (including neuronal) fates. Therefore, it is not expected that MSCs would mediate any beneficial effect by incorporating into neuronal networks to replace dying neurons, which may be anticipated in other neural stem cell approaches.
MSCs reside within several tissues in vivo, including adipose, bone marrow, Wharton’s jelly, and dental pulp, and may arise from pericytes3. They are further defined by the International Society for Cellular Therapy as expressing CD90, CD73, CD105 and CD44 while not expressing CD45 and CD31. 4, 5 Within the body, it is thought that normal MSC function is to migrate to areas of injury and participate in the reparative process.6 Both allogeneic and autologous MSC therapies are in development. Unlike most other allogeneic cell therapies in clinical development, allogeneic MSC therapies may be used without concomitant immunosuppression due to their paucity of MHC Class II proteins and decreased propensity to trigger an immune response.7
The precise mechanism by which MSCs may exert beneficial effects in neurological disease is still being elucidated, but it appears that multiple different mechanisms may contribute (Figure). First, MSCs have been shown to secrete neurotrophic growth factors, including glial cell-derived neurotrophic factor (GDNF), vascular endothelial growth factor, and brain-derived neurotrophic factor (BDNF),8, 9 which can be further enhanced under specific culture conditions.10 Neurotrophic growth factors have been shown to improve neuronal survival in a number of preclinical models of neuron injury, including ALS, PD, and MSA transgenic animals 11–17 and nerve injury models. 12, 18, 19 Second, MSCs strongly modulate the immune system and can aid wound healing, and this mechanism has been exploited in disorders such as graft versus host disease20 and Crohn’s disease.21 From a neurodegenerative perspective, it has become increasingly recognized that neuroinflammation plays a significant pathomechanistic role. Neuroinflammation in this context is defined as the negative contribution of non-neuronal cells (immune cells, glial cells, etc.) to neurodegenerative disease. While all of the details are not worked out, it is clear that activated microglia, astrocytes, and T-cells are able to interact and increase neuronal death due to proinflammatory and reactive oxygen species production.22,23 Interestingly, MSCs may be either anti-inflammatory or pro-inflammatory depending on the milieu within which they exist. When entering an inflammatory milieu (interferon-gamma, tumor necrosis factor-alpha), MSCs become anti-inflammatory wherein they secrete transforming growth factor-beta1, indoleamine-2,3-dioxygenase, and prostaglandin E2 and can convert macrophage/microglia from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype.24 Furthermore, MSCs can induce upregulation of forkhead box P3+ regulatory T cells, which are thought to play a key role in ALS.25
Figure.
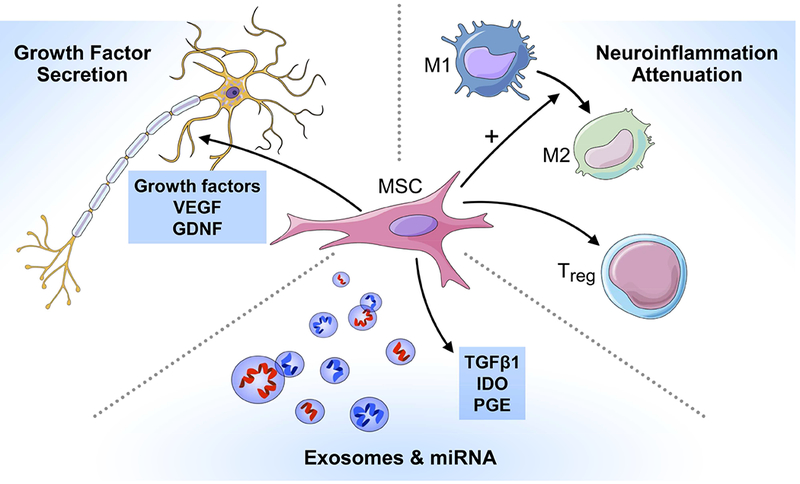
Putative MSC mechanisms of action to treat neurodegenerative diseases: 1) growth factor secretion, 2) neuroinflammation attenuation, and 3) exosome and miRNA secretion.
GDNF = glial cell-derived neurotrophic factor, IDO = Indoleamine-2,3-dioxygenase, miRNA = microRNA, PGE = prostaglandin, TGFB1 = transforming growth factor beta 1, Treg = Regulatory T cell, VEGF = vascular endothelial growth factor.
MSCs mediate their immunomodulatory effects via direct cell-cell interactions, but also have strong paracrine influences via secreted cytokines and growth factors. One of the key methods that MSCs secrete biological factors is via extracellular vesicles (EVs), which are divided into either microvesicles (> 200nm diameter that are exocytosed from plasma membrane) or exosomes (50-200 nm diameter that arise from endosomal trafficking).26 EVs are packed with thousands of proteins,27 mRNA, and/or microRNA,28 many of which are enriched in EVs compared to MSCs, and have been demonstrated to enhance neuronal growth and health in model systems.29–31 Given that much of MSC paracrine actions are mediated via EVs, these subcellular packages are being developed as a cell-free biological therapeutic in their own right, which would obviate the theoretical teratogenic concerns of cell therapy.
Finally, an intriguing new hypothesis to explain the positive effects of MSCs is they may improve neuronal health by donating their mitochondria.32 This mechanism of mitochondrial transfer has been observed between astrocytes and neurons in stroke model,33 as well as between MSCs and alveoli in a lung injury model.34 Through this mechanism, MSCs conceivably could improve neuronal health by donating healthy mitochondria to neurons that harbor dysfunctional mitochondria.
MSC therapy for neurodegenerative diseases
MSCs are being investigated as a therapy for a host of neurological diseases, and clinical trials have been performed in cerebrovascular diseases35–37 and inflammatory demyelinating disorders.38, 39 In this review, we will focus on data gathered in the study of neurodegenerative diseases, which have overlapping neuroinflammatory pathomechanisms that MSC therapy may impact. Table 2 lists all clinical trials for the below conditions that are registered with ClinicalTrials.gov and are signified as “recruiting”, “enrolling by invitation”, “active not recruiting”, or “not yet recruiting”.
Table 2.
Clinical trials for the discussed neurological conditions that are registered with ClinicalTrials.gov and signified as “recruiting”, “enrolling by invitation”, “active not recruiting”, or “not yet recruiting” (as of October 2018).
| Conditions | ClinicalTrials.gov# | Phase | Allo/Auto | MSC source | Site of Injection | Locations |
|---|---|---|---|---|---|---|
| Amyotrophic Lateral Sclerosis | 1 | Autologous | Bone Marrow | Intrathecal | Poland | |
| 1 | Allogeneic | Wharton’s Jelly | Intrathecal | Poland | ||
| 1 | Autologous | Adipose | Intraspinal & Intrathecal | Poland | ||
| 1/2 | Autologous | Bone Marrow | Intrathecal | Brazil | ||
| 1/2 | Autologous | Adipose | Intravenous | Spain | ||
| 2 | Autologous | Adipose | Intrathecal | USA | ||
| 3 | Autologous | Bone Marrow | Intrathecal | USA | ||
| Alzheimer’s Disease | 1 | Allogeneic | Bone Marrow | Intravenous | USA | |
| 1/2 | Allogeneic | Umbilical Cord | Intracerebroventricular | South Korea | ||
| 1/2 | Allogeneic | Umbilical Cord | Intravenous | China | ||
| 1/2 | Autologous | Adipose | Intravenous | USA | ||
| 2 | Allogeneic | Bone Marrow | Intravenous | USA | ||
| Multiple System Atrophy | 1 | Autologous | Adipose | Intrathecal | USA | |
| 1 | Autologous | Bone Marrow | Intracarotid | South Korea | ||
| Parkinson’s Disease | 1 | Allogeneic | Umbilical Cord | Intravenous | China | |
| 1/2 | Allogeneic | Bone Marrow | Intravenous | USA | ||
| 1/2 | Allogeneic | Umbilical Cord | Intrathecal & Intravenous | Jordan |
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal paralytic neurodegenerative disorder due to death of motor neurons in the brain and spinal cord.40 The median lifespan following symptom onset is 3 years. The only medications that alter the disease course are riluzole and edaravone. Riluzole decreases glutamate excitotoxicity, but only prolongs life for ~3 months and edaravone was recently approved, but is currently without demonstrated survival benefit data (although it is presumed). Several mechanisms have been implicated in the pathogenesis of ALS and insights from hereditary forms of ALS strongly implicate RNA processing and protein aggregation as key early steps in the disease initiation. Two primary mechanisms of MSC putative benefits, neurotrophic growth factor secretion and neuroinflammation modulation, have been targets for ALS therapy development for years.
There is considerable evidence from animal studies that neurotrophic factors (Insulin-like Growth Factor-1, BDNF, GDNF, etc.) may slow neurodegeneration.11, 13, 14, 18, 41–45 Unfortunately, subsequent human studies have not confirmed a benefit for patients.46–48 The reason for this lack of concurrence between the results of animal model studies and subsequent human trials may be because the animals do not faithfully model the human disease, because therapeutic agents are handled differently in animal systems compared with humans, or because clinical trials have been too short or inadequately sensitive to detect the degree of change detectable in animal models.49 The role of the blood brain barrier may also be relevant, and notably some of the most compelling animal studies have used direct delivery of growth factors into the CNS.14 This mode of delivery has not been thoroughly investigated in human trials.50–52
The role of neuroinflammation in ALS has been hypothesized since the 1970’s, but recent data has increased the recognition of this mechanism.53 Microglia and inflammatory leukocytes are thought to be key players in this process and there are alterations of these cells in autopsies of patients with ALS.54, 55 While it is not clear whether neuroinflammation causes ALS, it is theorized that it greatly determines the rate of disease progression. For example, in transgenic Superoxide dismutase-1 (SOD-1) rodent models of ALS, it has been shown that mutant SOD-1 in motor neurons primarily determines disease onset, whereas mutant SOD-1 in astrocytes and the immune system primarily determines the rate of progression.56–59 Human studies have also demonstrated abnormalities in the peripheral immune system in ALS patients.60–68 More data supporting the role of the immune system in ALS has arisen from studies of leukocyte microRNA, where specific upregulation of distinct microRNA has been reported in people with ALS,69–71 and treatment of these leukocytes in animal models ameliorates the disease.69, 72
Animal studies of MSCs in ALS have been promising. MSCs can be safely infused into the intrathecal space of animals and survive for up to 6 months after injection.73–77 Animal models of ALS have revealed MSC therapeutic potential, with efficacy data in several preclinical studies in ALS,78–89 including MSC conditioned-media81 and exosomes.79
Early phase human clinical trials have been completed using MSC treatment in ALS and demonstrate a favorable safety profile. Our group recently studied the effect of intrathecal therapy with autologous adipose-derived MSCs in a Phase I, dose-escalation study. Overall, the safety was acceptable, with some temporary back/leg pain at the highest tested doses.90 Other groups have found similar safety profiles using naïve bone marrow-derived MSCs91–93 and bone marrow-derived MSCs cultured to enhance neurotrophic factor secretion.94 While none of these studies were designed to study efficacy, there did not appear to be any worsening of ALS progression rates, and there were some signs that suggested benefit.90–95 These studies have now progressed to ongoing Phase M/MI clinical trials.
Alzheimer’s Disease
Dementia is a progressive neurodegenerative disorder of the brain that alters normal cognition to such a degree that an individual is no longer able to function independently in society. Alzheimer’s disease (AD) is the most common cause of dementia. The typical clinical dementia syndrome associated with AD is that of a slowly progressive decline in memory appearing early in the clinical phase of the disease.96 As the disease progresses, other cognitive domains become involved. Atypical clinical presentations of AD occur with initial prominent symptoms in visual-spatial, motor, language, or executive functioning.97 AD is a progressive degenerative disorder with associated clinical symptoms during life, but the definitive diagnosis can only be made on the post-mortem examination of the brain. The classic neuropathological features are neuritic plaques and neurofibrillary tangles.98 Biomarkers, such as amyloid positron emission tomography, have been shown to predict AD pathology99 allowing for the characterization of these processes in vivo during life. The pathologic changes that are characteristic of AD are known to occur decades before clinical symptoms are present leading to a long preclinical prodromal disease phase100 before progressing to mild cognitive impairment101 and then dementia.102 Recent biomarker criteria for diagnosing the AD continuum during life for research purpose have recently been proposed.103 These research criteria will be important for future clinical trials, especially in preclinical phases of AD where therapeutics are hoped to be most effective before cognitive symptoms and pathologic changes become irreversible. Once MSCs trials have progressed to large clinical trials in humans, the entire AD continuum (preclinical, mild cognitive impairment, and dementia) and the heterogeneity in clinical symptoms should be carefully considered during trial design.
It has long been known that the pathologic hallmarks of AD (i.e., plaques and neurofibrillary tangles) are composed of beta-amyloid and tau protein aggregates. However, the pathogenic mechanisms that drive the association between these protein aggregates and the clinical symptoms are not known. A wide-array of global, molecular, and cellular processes have been hypothesized to play a role in AD pathogenies including network failure104, pathologic plasticity,105 mitochondrial dysfunction,106 innate immunity,107 inflammation,108 autophagy109, and toxicity of protein aggregates and oligomers like amyloid.110 The ability of MSCs to secrete neurotrophic factors may improve the cellular milieu and limit cell loss in the setting of this complex AD pathophysiology. In addition, MSCs’ known immunomodulatory effects may limit the damage effects of activated glial cell related synaptic pruning and inflammation in general. MSCs also have the potential to deliver a healthy supply of mitochondria to the CNS thereby mitigating the impact of age and AD-related mitochondrial dysfunction.
In amyloid precursor protein/presenilin 1 mouse models of AD, bone marrow-derived MSCs have been shown to reduce microglia cell counts, but not to alter the number of amyloid plaques.111 However, others have seen a decrease in amyloid deposits with bone marrow-derived MSCs112 consistent with what has been reported using human umbilical cord derived MSCs to rescue memory deficits and reduce amyloid-beta deposition in an amyloid precursor protein/presenilin 1 transgenic mouse.113 The effect of MSCs on mouse models of AD pathology and cognition may be mediated through modulatory effects on neuroinflammation.114 This same group also postulated that inhibition of apoptosis115 may also be playing a role. Others have reported enhanced neurogenesis via the Wnt signaling pathway in the hippocampus is playing an important role in the effect of MSC on mouse models of AD.116 There has not been a detailed study of the effects of MSC on mitochondrial function in mouse models of AD. Human trials in AD are under development.117
Multiple System Atrophy
MSA is a rare sporadic and fatal multi-system progressive disorder characterized by progressive autonomic failure (including orthostatic hypotension, neurogenic bladder, and erectile dysfunction), cerebellar ataxia, corticospinal dysfunction, and parkinsonism that is often poorly responsive to levodopa therapy (unlike PD)118. The disease is relentlessly progressive with a median survival from diagnosis to death of approximately 3 years.119–121 MSA represents one of the motor synucleinopathies which also include PD and diffuse Lewy body disease. Neuropathologically, these conditions are linked by positivity of inclusions to α-synuclein with reduced solubility.122 But in contrast to neuronal α-synuclein (Lewy bodies), MSA is characterized by glial cytoplasmic inclusions of abnormally aggregated α-synuclein, primarily found in the striatum, cerebellum, brainstem, cortex, and spinal cord, regions also associated with the most pronounced neuronal loss.123–125 Many lines of evidence highlight the pathological importance of α-synuclein aggregation, and disease progression is thought to be directly linked to accumulation and aggregation of conformationally changed α-synuclein.126–129 Although the precise mechanisms by which α-synuclein aggregation leads to neuronal loss are not completely understood, there are a number of downstream effects contributing to neuronal pathology. A central mechanism appears to be that of glial dysfunction with resulting deficiency of growth factors, especially BDNF and GDNF, which are critical for neuronal survival.130 Another important mechanism appears to be neuroinflammation as microglial activation can be demonstrated in certain, stages of the pathophysiologic cascade.131, 132 Lastly, although glial cytoplasmic inclusions are the primary neuropathological hallmark of MSA, neuronal cytoplasmic and nuclear inclusions of α-synuclein have also been reported.125, 133 Furthermore, recent work with transgenic models suggests that neuronal/oligodendroglial propagation of α-synuclein may partake in the pathophysiology of MSA.128
The ability of MSCs to produce and secrete neurotrophic factors known to be deficient in MSA, and MSCs’ known immunomodulatory effects therefore provide a compelling rationale for the pursuit of delivering MSCs with therapeutic intent. Such an approach is further supported by animal studies demonstrating that human MSCs have a protective effect against progressive dopaminergic and striatal neuronal loss.15, 16 Recently, the neuroprotective and immunomodulatory effects of MSCs were confirmed in a transgenic mouse model of MSA.17
When applied to humans, the blood brain barrier comprises a potential hurdle in cell delivery to the central nervous system. Although it has been shown that this barrier is less tight in MSA, it remains a major hurdle for MSC access.134 Since growth factors also do not generally cross the blood-brain barrier, the desired effects may not reach target neurons in the relevant areas of brain if delivered systemically.135, 136 In order to reach those areas of interest, strategies have to be pursued to efficiently and safely overcome that barrier.
In a first human study utilizing MSC delivery to patients with MSA, a Korean group pursued an open-label study of 29 patients with MSA, of which 11 received bone marrow-derived MSCs while 18 did not.137 A total of 4×107 MSCs were administered as infusions into both internal carotid arteries and the dominant vertebral artery. Additionally, patients received 3 subsequent intravenous MSC injections. The investigators compared the clinical course between MSC-treated and control patients and reported significantly less progression based on clinical assessments using the unified MSA rating scale in MSC-treated patients compared to control patients at all visits throughout the 12-month study period. Serial positron emission tomography scans in the MSC-treated group showed increased fluorodeoxyglucose uptake from baseline in cerebellum and frontal white matter while fluorodeoxyglucose uptake in the follow-up scan of the control group decreased significantly in the cerebellum and brainstem. No serious adverse effects related to MSC therapy occurred, although transient ischemic changes, evident on MRI, without clinical correlate, were seen with the intra-arterial infusions.137 The same group published the results of a double-blind placebo controlled single-center study on 33 subjects in 2012.138 Although the effect was less dramatic than what was observed in the open-label study, this trial confirmed a significantly smaller increase in total and unified MSA rating scale scores (part II) compared with the placebo group. Concordantly, cerebral glucose metabolism and gray matter density showed less decrease in the cerebellum and the cerebral cortical areas in the MSC compared to the placebo group. Again seen were small ischemic lesions on magnetic resonance imaging as a result of intra-arterial infusions which were asymptomatic except for one patient who developed basal ganglia infarcts with transient dystonia.138
We have since pursued a phase I/II dose-escalation trial utilizing a different approach to overcoming the blood-brain barrier and allowing for more widespread CNS delivery: the intrathecal route. 24 patients with probable MSA based on clinical consensus criteria and autonomic testing were enrolled and received escalating doses of adipose-derived MSCs ranging from one injection of 1 × 107 to two injections (one month apart) of 1 × 108 cells each. The primary aim of this study was to determine the safety and tolerability of this approach, and secondary aims relate to exploring signals of potential efficacy using clinical, autonomic, and imaging markers. This study has recently been completed and the manuscript is currently under review. Findings were sufficiently intriguing to pursue an ongoing compassionate extension study.
Parkinson’s disease
PD is the most common synucleinopathy and the second most common neurodegenerative disease after AD, with a prevalence of approximately 1% in people over 60 years of age in industrialized countries.139 PD is clinically characterized by a combination of tremor, bradykinesia, and rigidity, but a number of non-motor symptoms and findings commonly associated with PD, including cognitive dysfunction, mood disorders, sleep disturbances, autonomic dysfunction, go beyond the classification of PD as a movement disorder.140–144 The pathologic hallmark of PD are Lewy bodies - intracytoplasmic neuronal alpha-synuclein inclusions - and neuronal loss in selected areas within the brain, including substantia nigra, locus ceruleus, dorsal vagal nucleus, and cerebral cortex; however, Lewy pathology is also found in spinal cord, sympathetic ganglia, as well as the cardiac and myenteric plexus.145–149 Cardiac sympathetic noradrenergic denervation is also a common finding.150 The precise mechanisms leading to neuronal loss in PD are incompletely understood, but appear to be multifactorial and include genetic factors, oxidative stress, glial dysfunction and lack of trophic factors, excitotoxicity, inflammation, and mitochondrial dysfunction.151–154
Considering the ability of MSCs to secrete neurotrophic factors, modulate inflammation, and possibly even act as mitochondria “donor”, it comes as no surprise that there is a lot of interest in the use of MSCs in the treatment of PD, and a multitude of animal studies has shown promise. Direct striatal administration of bone marrow-derived MSCs with or without prior use of neuronal differentiation medium resulted in improvement of motor function, protection of the nigrostriatal system, and improved striatal dopamine release in several studies using toxic lesion rodent models of PD 155–162 as well as a proteasome model of PD 16. Similar effects were reported with adipose-derived and umbilical cord-derived MSCs with or without prior differentiation.163–170 For example, in a study using autologous transplantation into the substantia nigra, McCoy and colleagues reported improvement of motor function, reduced microglial activation, and decreased loss of TH immunoreactivity, associated with local production of trophic factors.165 Intrastriatal administration was furthermore shown to enhance neurogenesis in the subventricular zone and to induce neuroblast migration to the striatum.167,171
Similar findings were reported with venous administration of MSCs in some studies, but concerns have been expressed about the non-selectivity of this administration route, limited crossing of the blood-brain barrier, and lack of long-term survival. 16, 172–177 A study on intracarotid infusion of MSCs in the brain of rats bearing a 6-hydroxydopamine-induced lesion of the nigrostriatal tract showed that the infused cells did not efficiently cross the blood-brain barrier without using a permeabilizing agent.178 MSCs were detected in various brain regions, but there was no convincing modification of the progression of motor impairment. A study using intranasal administration showed neuroprotective and anti-inflammatory effects with localization of MSCs documented in the olfactory bulb, cortex, hippocampus, striatum, cerebellum, brainstem, amygdala, hippocampus and spinal cord; MSCs were still found in these regions 4.5 months after injection.179
Several rodent studies utilized engineered MSCs expressing tyrosine hydroxylase gene, vascular endothelial growth factor, or transduced to produce increased GDNF or cerebral dopamine neurotrophic factor showed mixed but overall positive results. 180–187 In a Rhesus monkey model of PD, vector-engineered umbilical cord-derived MSCs which showed neuronal differentiation were transplanted into the striatum resulting in recovery of behavior and neuroprotective effects.188 Combined adipose-derived MSC delivery along with gene therapy delivering tyrosine hydroxylase and neurturin to the striatum in that monkey model showed better neuroprotective effects than gene therapy alone.189
In a rat nigrostriatal lesion model of PD the effects of human amniotic fluid stem cells and bone marrow derived mesenchymal stromal cells injected into the lesion site were assessed with a focus on bladder dysfunction. There was a temporary improvement of cystometry assessed bladder function in both stem cells groups compared to sham-treated rats.190
Only preliminary data are available on the use of MSCs in human PD. In an open-label study in 2010 and Indian researchers administered 106 autologous bone marrow-derived MSCs per kilogram body weight unilaterally into the sublateral ventricular zone via stereotactic surgery in 7 patients with PD.191 The procedure was well tolerated. 3 out of 7 patients were reported to have lasting improvement in the unified Parkinson’s disease rating scale and other rating scales compared to baseline. The same group reported another open-label study in 2012 during which 8 PD and 8 “PD plus” patients received 2×106 allogeneic bone-marrow derived MSCs per kilogram body weight into the bilateral sublateral ventricular zone. 192 Improvement in unified Parkinson’s disease rating scale and other measures was seen on follow-up, which was persistent in those with PD and transient in those with PD plus.
Future Directions in MSC Studies
Despite promising preclinical and early clinical findings, there continue to be many unanswered questions regarding the use of MSCs as a therapeutic approach in neurodegenerative disorders. Ultimately, the most important question is: do MSCs provide benefit to patients with neurodegenerative diseases? This efficacy question must be answered with well-designed clinical trials, which is a challenging task in neurodegenerative diseases. Within these clinical trials; however, we argue that it is equally important that biomarkers are investigated that can answer questions about MSC biology and their effects in the nervous system. Biomarker investigations into the immune system, neuroinflammation, growth factors, microRNA, EVs are examples of what need to be studied in human MSC clinical trials. Once we begin to more clearly understand why MSCs are beneficial in neurodegenerative diseases, we can rationally design future trials to optimize these therapies. As an example, if it is discovered that MSC efficacy correlates primarily with a specific effect on biomarker studies of neuroinflammation, the next set of experiments can be designed to maximize that effect. Variables that may be explored to optimize therapy should include dose, frequency, route of delivery, or autologous versus allogeneic therapy (some of which may be able to be answered in preclinical models). Furthermore, next-generation MSCs manipulated by gene therapy or specific culturing condition can then be developed specifically to alter that aspect of neuroinflammation. In this way, these novel MSC therapies not only can be properly validated, but also may reveal new mechanisms of MSC therapeutics that lead to further targeted drug development, which will be essential to move the field forward.
ABBREVIATIONS:
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- BDNF
Brain-derived neurotrophic factor
- EV
Extracellular vesicles
- GDNF
Glial cell-derived neurotrophic factor
- MSA
multiple system atrophy
- MSC
mesenchymal stromal cell
- PD
Parkinson’s disease
- SOD-1
superoxide dismutatse-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bjorklund A, Lindvall O. Replacing Dopamine Neurons in Parkinson’s Disease: How did it happen? J Parkinsons Dis. 2017;7:S23–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561:455–457. [DOI] [PubMed] [Google Scholar]
- 3.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 4.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–145. [DOI] [PubMed] [Google Scholar]
- 6.Seppanen E, Roy E, Ellis R, Bou-Gharios G, Fisk NM, Khosrotehrani K. Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: evidence from a murine fetal microchimerism model. PLoS One. 2013;8:e62662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira FG, Carvalho MM, Panchalingam KM, et al. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. Stem Cells Transl Med. 2017;6:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whone AL, Kemp K, Sun M, Wilkins A, Scolding NJ. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res. 2012;1431:86–96. [DOI] [PubMed] [Google Scholar]
- 10.Gothelf Y, Abramov N, Harel A, Offen D. Safety of repeated transplantations of neurotrophic factors-secreting human mesenchymal stromal stem cells. Clin Transl Med. 2014;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsumoto H, Ikeda K, Klinkosz B, Cedarbaum JM, Wong V, Lindsay RM. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994;265:1107–1110. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima H, Uchida K, Kobayashi S, et al. Rescue of rat anterior horn neurons after spinal cord injury by retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene. J Neurotrauma. 2007;24:703–712. [DOI] [PubMed] [Google Scholar]
- 13.Narai H, Nagano I, Ilieva H, et al. Prevention of spinal motor neuron death by insulin-like growth factor-1 associating with the signal transduction systems in SODG93A transgenic mice. J Neurosci Res. 2005;82:452–457. [DOI] [PubMed] [Google Scholar]
- 14.Storkebaum E, Lambrechts D, Dewerchin M, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Bang G, Lee BR, Kim HO, H. LP. Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy-parkinsonism. Cell Transplant. 2011;20:827–835. [DOI] [PubMed] [Google Scholar]
- 16.Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J Neurochem. 2008;107:141–151. [DOI] [PubMed] [Google Scholar]
- 17.Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS ONE. 2011;6:e19808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barati S, Hurtado PR, Zhang SH, Tinsley R, Ferguson IA, Rush RA. GDNF gene delivery via the p75(NTR) receptor rescues injured motor neurons. Exp Neurol. 2006;202:179–188. [DOI] [PubMed] [Google Scholar]
- 19.Watabe K, Hayashi Y, Kawazoe Y. Peripheral nerve avulsion injuries as experimental models for adult motoneuron degeneration. Neuropathology. 2005;25:371–380. [DOI] [PubMed] [Google Scholar]
- 20.Te Boome LC, Mansilla C, van der Wagen LE, et al. Biomarker profiling of steroid-resistant acute GVHD in patients after infusion of mesenchymal stromal cells. Leukemia. 2015;29:1839–1846. [DOI] [PubMed] [Google Scholar]
- 21.Panes J, Garcia-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. [DOI] [PubMed] [Google Scholar]
- 22.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. [DOI] [PubMed] [Google Scholar]
- 23.Richards RI, Robertson SA, O’Keefe LV, et al. The Enemy within: Innate Surveillance-Mediated Cell Death, the Common Mechanism of Neurodegenerative Disease. Front Neurosci. 2016;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol. 2013;8:888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851–858. [DOI] [PubMed] [Google Scholar]
- 27.Eirin A, Zhu XY, Puranik AS, et al. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016;6:36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eirin A, Riester SM, Zhu XY, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. [DOI] [PubMed] [Google Scholar]
- 30.Nakano M, Nagaishi K, Konari N, et al. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6:24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Chopp M, Liu XS, et al. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol Neurobiol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babenko VA, Silachev DN, Zorova LD, et al. Improving the Post-Stroke Therapeutic Potency of Mesenchymal Multipotent Stromal Cells by Cocultivation With Cortical Neurons: The Role of Crosstalk Between Cells. Stem Cells Transl Med. 2015;4:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayakawa K, Esposito E, Wang X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke. 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oskarsson B, Gendron TF, Staff NP. Amyotrophic lateral sclerosis: An update for 2018. Mayo Clin Proc. 2018. [DOI] [PubMed] [Google Scholar]
- 41.Gorio A, Lesma E, Madaschi L, Di Giulio AM. Co-administration of IGF-I and glycosaminoglycans greatly delays motor neurone disease and affects IGF-I expression in the wobbler mouse: a long-term study. J Neurochem. 2002;81:194–202. [DOI] [PubMed] [Google Scholar]
- 42.Kurek JB, Radford AJ, Crump DE, et al. LIF (AM424), a promising growth factor for the treatment of ALS. J Neurol Sci. 1998;160 Suppl 1:S106–113. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Brakefield D, Pan Y, Hunter D, Myckatyn TM, Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203:457–471. [DOI] [PubMed] [Google Scholar]
- 44.Mennini T, De Paola M, Bigini P, et al. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med. 2006;12:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Den Bosch L, Storkebaum E, Vleminckx V, et al. Effects of vascular endothelial growth factor (VEGF) on motor neuron degeneration. Neurobiol Dis. 2004;17:21–28. [DOI] [PubMed] [Google Scholar]
- 46.A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III). Neurology. 1999;52:1427–1433. [DOI] [PubMed] [Google Scholar]
- 47.Miller RG, Petajan JH, Bryan WW, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol. 1996;39:256–260. [DOI] [PubMed] [Google Scholar]
- 48.Sorenson EJ, Windbank AJ, Mandrekar JN, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71:1770–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benatar M Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis. 2007;26:1–13. [DOI] [PubMed] [Google Scholar]
- 50.Nagano I, Shiote M, Murakami T, et al. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. 2005;27:768–772. [DOI] [PubMed] [Google Scholar]
- 51.Ochs G, Penn RD, York M, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:201–206. [DOI] [PubMed] [Google Scholar]
- 52.Van Damme P, Robberecht W. Developments in treatments for amyotrophic lateral sclerosis via intracerebroventricular or intrathecal delivery. Expert Opin Investig Drugs. 2014;23:955–963. [DOI] [PubMed] [Google Scholar]
- 53.Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. [DOI] [PubMed] [Google Scholar]
- 54.Engelhardt JI, Appel SH. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol. 1990;47:1210–1216. [DOI] [PubMed] [Google Scholar]
- 55.Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50:30–36. [DOI] [PubMed] [Google Scholar]
- 56.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105:15558–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beers DR, Zhao W, Liao B, et al. Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain Behav Immun. 2011;25:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boillee S, Cleveland DW. Gene therapy for ALS delivers. Trends Neurosci. 2004;27:235–238. [DOI] [PubMed] [Google Scholar]
- 59.Boillee S, Yamanaka K, Lobsiger CS, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. [DOI] [PubMed] [Google Scholar]
- 60.Nardo G, Pozzi S, Pignataro M, et al. Amyotrophic lateral sclerosis multiprotein biomarkers in peripheral blood mononuclear cells. PLoS One. 2011;6:e25545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ragheb S, Lisak R, Lewis R, Van Stavern G, Gonzales F, Simon K. A potential role for B-cell activating factor in the pathogenesis of autoimmune myasthenia gravis. Arch Neurol. 2008;65:1358–1362. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R, Miller RG, Gascon R, et al. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2009;206:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saleh IA, Zesiewicz T, Xie Y, et al. Evaluation of humoral immune response in adaptive immunity in ALS patients during disease progression. J Neuroimmunol. 2009;215:96–101. [DOI] [PubMed] [Google Scholar]
- 65.Zhang R, Gascon R, Miller RG, et al. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2005;159:215–224. [DOI] [PubMed] [Google Scholar]
- 66.Mantovani S, Garbelli S, Pasini A, et al. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J Neuroimmunol. 2009;210:73–79. [DOI] [PubMed] [Google Scholar]
- 67.Gustafson MP, Staff NP, Bornschlegl S, et al. Comprehensive immune profiling reveals substantial immune system alterations in a subset of patients with amyotrophic lateral sclerosis. PLoS One. 2017;12:e0182002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murdock BJ, Zhou T, Kashlan SR, Little RJ, Goutman SA, Feldman EL. Correlation of Peripheral Immunity With Rapid Amyotrophic Lateral Sclerosis Progression. JAMA Neurol. 2017;74:1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butovsky O, Siddiqui S, Gabriely G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Felice B, Annunziata A, Fiorentino G, et al. miR-338–3p is over-expressed in blood, CFS, serum and spinal cord from sporadic amyotrophic lateral sclerosis patients. Neurogenetics. 2014;15:243–253. [DOI] [PubMed] [Google Scholar]
- 71.De Felice B, Guida M, Coppola C, De Mieri G, Cotrufo R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene. 2012;508:35–40. [DOI] [PubMed] [Google Scholar]
- 72.Butovsky O, Jedrychowski MP, Cialic R, et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol. 2015;77:75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin K, Sun Y, Xie L, et al. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol Dis. 2005;18:366–374. [DOI] [PubMed] [Google Scholar]
- 74.Nishida K, Tanaka N, Nakanishi K, et al. Magnetic targeting of bone marrow stromal cells into spinal cord: through cerebrospinal fluid. Neuroreport. 2006;17:1269–1272. [DOI] [PubMed] [Google Scholar]
- 75.Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266–278. [DOI] [PubMed] [Google Scholar]
- 76.Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976). 2004;29:1971–1979. [DOI] [PubMed] [Google Scholar]
- 77.Chen BK, Staff NP, Knight AM, et al. A safety study on intrathecal delivery of autologous mesenchymal stromal cells in rabbits directly supporting Phase I human trials. Transfusion. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boido M, Piras A, Valsecchi V, et al. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2014;16:1059–1072. [DOI] [PubMed] [Google Scholar]
- 79.Bonafede R, Scambi I, Peroni D, et al. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 2016;340:150–158. [DOI] [PubMed] [Google Scholar]
- 80.Boucherie C, Schafer S, Lavand’homme P, Maloteaux JM, Hermans E. Chimerization of astroglial population in the lumbar spinal cord after mesenchymal stem cell transplantation prolongs survival in a rat model of amyotrophic lateral sclerosis. J Neurosci Res. 2009;87:2034–2046. [DOI] [PubMed] [Google Scholar]
- 81.Fontanilla CV, Gu H, Liu Q, et al. Adipose-derived Stem Cell Conditioned Media Extends Survival time of a mouse model of Amyotrophic Lateral Sclerosis. Sci Rep. 2015;5:16953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forostyak S, Homola A, Turnovcova K, Svitil P, Jendelova P, Sykova E. Intrathecal Delivery of Mesenchymal Stromal Cells Protects the Structure of Altered Perineuronal Nets in SOD1 Rats and Amends the Course of ALS. Stem cells. 2014;32:3163–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forostyak S, Jendelova P, Kapcalova M, Arboleda D, Sykova E. Mesenchymal stromal cells prolong the lifespan in a rat model of amyotrophic lateral sclerosis. Cytotherapy. 2011;13:1036–1046. [DOI] [PubMed] [Google Scholar]
- 84.Frakes AE, Braun L, Ferraiuolo L, Guttridge DC, Kaspar BK. Additive amelioration of ALS by co-targeting independent pathogenic mechanisms. Ann Clin Transl Neurol. 2017;4:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim H, Kim HY, Choi MR, et al. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett. 2010;468:190–194. [DOI] [PubMed] [Google Scholar]
- 86.Marconi S, Bonaconsa M, Scambi I, et al. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience. 2013;248:333–343. [DOI] [PubMed] [Google Scholar]
- 87.Uccelli A, Milanese M, Principato MC, et al. Intravenous mesenchymal stem cells improve survival and motor function in experimental amyotrophic lateral sclerosis. Mol Med. 2012;18:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vercelli A, Mereuta OM, Garbossa D, et al. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. [DOI] [PubMed] [Google Scholar]
- 89.Zhao CP, Zhang C, Zhou SN, et al. Human mesenchymal stromal cells ameliorate the phenotype of SOD1-G93A ALS mice. Cytotherapy. 2007;9:414–426. [DOI] [PubMed] [Google Scholar]
- 90.Staff NP, Madigan NN, Morris J, et al. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology. 2016;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazzini L, Mareschi K, Ferrero I, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14:56–60. [DOI] [PubMed] [Google Scholar]
- 93.Oh KW, Moon C, Kim HY, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrou P, Gothelf Y, Argov Z, et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients With Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA neurology. 2016:1–8. [DOI] [PubMed] [Google Scholar]
- 95.Petrou P, Argov A, Lennon VA, et al. Rare combination of myasthenia and motor neuronopathy, responsive to Msc-Ntf stem cell therapy. Muscle Nerve. 2014;49:455–457. [DOI] [PubMed] [Google Scholar]
- 96.Fleming KC, Adams AC, Petersen RC. Dementia: diagnosis and evaluation. Mayo Clin Proc. 1995;70:1093–1107. [DOI] [PubMed] [Google Scholar]
- 97.Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012;8:451–464. [DOI] [PubMed] [Google Scholar]
- 98.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murray ME, Lowe VJ, Graff-Radford NR, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain. 2015;138:1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of internal medicine. 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 102.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jones DT, Knopman DS, Gunter JL, et al. Cascading network failure across the Alzheimer’s disease spectrum. Brain. 2016;139:547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. [DOI] [PubMed] [Google Scholar]
- 106.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta. 2010;1802:2–10. [DOI] [PubMed] [Google Scholar]
- 107.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16:229–236. [DOI] [PubMed] [Google Scholar]
- 108.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Q, Liu Y, Sun M. Autophagy and Alzheimer’s Disease. Cell Mol Neurobiol. 2017;37:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naaldijk Y, Jager C, Fabian C, et al. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2017;43:299–314. [DOI] [PubMed] [Google Scholar]
- 112.Bae JS, Jin HK, Lee JK, Richardson JC, Carter JE. Bone marrow-derived mesenchymal stem cells contribute to the reduction of amyloid-beta deposits and the improvement of synaptic transmission in a mouse model of pre-dementia Alzheimer’s disease. Curr Alzheimer Res. 2013;10:524–531. [PubMed] [Google Scholar]
- 113.Yang H, Xie Z, Wei L, et al. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AbetaPP/PS1 transgenic mouse model. Stem Cell Res Ther. 2013;4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee HJ, Lee JK, Lee H, et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588–602. [DOI] [PubMed] [Google Scholar]
- 115.Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells attenuate amyloid beta-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res. 2010;7:540–548. [DOI] [PubMed] [Google Scholar]
- 116.Oh SH, Kim HN, Park HJ, Shin JY, Lee PH. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer’s Disease Model. Cell Transplant. 2015;24:1097–1109. [DOI] [PubMed] [Google Scholar]
- 117.Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilman S, Wenning GK, Low PA, et al. Second consensus conference on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coon EA, Sletten DM, Suarez MD, et al. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain. 2015;138:3623–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geser F, Wenning GK, Seppi K, et al. Progression of multiple system atrophy (MSA): a prospective natural history study by the European MSA Study Group (EMSA SG). Movement Disorders. 2006;21:179–186. [DOI] [PubMed] [Google Scholar]
- 121.Low PA, Reich SG, Jankovic J, et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 2015;14:710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dickson DW, Liu W, Hardy J, et al. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol. 1999;155:1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benarroch EE, Schmeichel AM, Parisi JE. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology. 2002;59:944–946. [DOI] [PubMed] [Google Scholar]
- 124.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Differential involvement of hypothalamic vasopressin neurons in multiple system atrophy. Brain. 2006;129:2688–2696. [DOI] [PubMed] [Google Scholar]
- 125.Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26:338–345. [DOI] [PubMed] [Google Scholar]
- 126.Batelli S, Albani D, Rametta R, et al. DJ-1 modulates alpha-synuclein aggregation state in a cellular model of oxidative stress: relevance for Parkinson’s disease and involvement of HSP70. PLoS ONE. 2008;3:e1884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.El-Agnaf OM, Jakes R, Curran MD, et al. Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Letters. 1998;440:71–75. [DOI] [PubMed] [Google Scholar]
- 128.Rockenstein E, Ubhi K, Inglis C, et al. Neuronal to oligodendroglial alpha-synuclein redistribution in a double transgenic model of multiple system atrophy. Neuroreport. 2012;23:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Windisch M, Wolf HJ, Hutter-Paier B, Wronski R. Is alpha-synuclein pathology a target for treatment of neurodegenerative disorders? Curr Alzheimer Res. 2007;4:556–561. [DOI] [PubMed] [Google Scholar]
- 130.Ubhi K, Rockenstein E, Mante M, et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurocsci. 2010;30:6236–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol. 2004;63:43–52. [DOI] [PubMed] [Google Scholar]
- 132.Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22:2196–2203. [DOI] [PubMed] [Google Scholar]
- 133.Cykowski MD, Coon EA, Powell SZ, et al. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138:2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song SK, Lee SK, Lee JJ, et al. Blood-brain barrier impairment is functionally correlated with clinical severity in patients of multiple system atrophy. NeurobiolAging. 2011;32:2183–2189. [DOI] [PubMed] [Google Scholar]
- 135.Ochs G, Penn RD, York M, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2000;1:201–206. [DOI] [PubMed] [Google Scholar]
- 136.Winkler J, Ramirez GA, Kuhn HG, et al. Reversible Schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Annals of Neurology. 1997;41:82–93. [DOI] [PubMed] [Google Scholar]
- 137.Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723–730. [DOI] [PubMed] [Google Scholar]
- 138.Lee PH, Lee JE, Kim H-S, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. [DOI] [PubMed] [Google Scholar]
- 139.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348:1356–1364. [DOI] [PubMed] [Google Scholar]
- 140.Asahina M, Vichayanrat E, Low DA, Iodice V, Mathias CJ. Autonomic dysfunction in parkinsonian disorders: assessment and pathophysiology. J Neurol Neurosurg Psychiatry. 2013;84:674–680. [DOI] [PubMed] [Google Scholar]
- 141.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 142.Hughes AJ, Daniel SE, Lees AJ. The clinical features of Parkinson’s disease in 100 histologically proven cases. Adv Neurol. 1993;60:595–599. [PubMed] [Google Scholar]
- 143.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56:173–181. [DOI] [PubMed] [Google Scholar]
- 144.Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol. 1996;53:175–179. [DOI] [PubMed] [Google Scholar]
- 145.Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Del Tredici K, Braak H. Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord. 2012;27:597–607. [DOI] [PubMed] [Google Scholar]
- 147.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. [DOI] [PubMed] [Google Scholar]
- 148.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9:13–24. [DOI] [PubMed] [Google Scholar]
- 149.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol. 1993;50:140–148. [DOI] [PubMed] [Google Scholar]
- 150.Goldstein DS. Cardiac denervation in patients with Parkinson disease. Cleve Clin J Med. 2007;74 Suppl 1:S91–94. [DOI] [PubMed] [Google Scholar]
- 151.Greenamyre JT, Hastings TG. Biomedicine. Parkinson’s--divergent causes, convergent mechanisms. Science. 2004;304:1120–1122. [DOI] [PubMed] [Google Scholar]
- 152.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. [DOI] [PubMed] [Google Scholar]
- 153.Machado V, Zoller T, Attaai A, Spittau B. Microglia-Mediated Neuroinflammation and Neurotrophic Factor-Induced Protection in the MPTP Mouse Model of Parkinson’s Disease-Lessons from Transgenic Mice. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson’s disease: insights from animal models. Nat Rev Neurosci. 2003;4:727–738. [DOI] [PubMed] [Google Scholar]
- 155.Bouchez G, Sensebe L, Vourc’h P, et al. Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson’s disease. Neurochem Int. 2008;52:1332–1342. [DOI] [PubMed] [Google Scholar]
- 156.Chen D, Fu W, Zhuang W, Lv C, Li F, Wang X. Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson’s disease. J Neurosci Res. 2017;95:907–917. [DOI] [PubMed] [Google Scholar]
- 157.Levy YS, Bahat-Stroomza M, Barzilay R, et al. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson’s disease. Cytotherapy. 2008;10:340–352. [DOI] [PubMed] [Google Scholar]
- 158.Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci Lett. 2001;316:67–70. [DOI] [PubMed] [Google Scholar]
- 159.Offen D, Barhum Y, Levy YS, et al. Intrastriatal transplantation of mouse bone marrow-derived stem cells improves motor behavior in a mouse model of Parkinson’s disease. J Neural Transm Suppl. 2007:133–143. [DOI] [PubMed] [Google Scholar]
- 160.Sadan O, Bahat-Stromza M, Barhum Y, et al. Protective effects of neurotrophic factor-secreting cells in a 6-OHDA rat model of Parkinson disease. Stem Cells Dev. 2009;18:1179–1190. [DOI] [PubMed] [Google Scholar]
- 161.Shetty P, Ravindran G, Sarang S, Thakur AM, Rao HS, Viswanathan C. Clinical grade mesenchymal stem cells transdifferentiated under xenofree conditions alleviates motor deficiencies in a rat model of Parkinson’s disease. Cell Biol Int. 2009;33:830–838. [DOI] [PubMed] [Google Scholar]
- 162.Ye M, Wang XJ, Zhang YH, et al. Therapeutic effects of differentiated bone marrow stromal cell transplantation on rat models of Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:44–49. [DOI] [PubMed] [Google Scholar]
- 163.Kang EJ, Lee YH, Kim MJ, et al. Transplantation of porcine umbilical cord matrix mesenchymal stem cells in a mouse model of Parkinson’s disease. J Tissue Eng Regen Med. 2013;7:169–182. [DOI] [PubMed] [Google Scholar]
- 164.Mathieu P, Roca V, Gamba C, Del Pozo A, Pitossi F. Neuroprotective effects of human umbilical cord mesenchymal stromal cells in an immunocompetent animal model of Parkinson’s disease. J Neuroimmunol. 2012;246:43–50. [DOI] [PubMed] [Google Scholar]
- 165.McCoy MK, Martinez TN, Ruhn KA, et al. Autologous transplants of Adipose-Derived Adult Stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp Neurol. 2008;210:14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schwerk A, Altschuler J, Roch M, et al. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen Med. 2015;10:431–446. [DOI] [PubMed] [Google Scholar]
- 167.Schwerk A, Altschuler J, Roch M, et al. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy. 2015;17:199–214. [DOI] [PubMed] [Google Scholar]
- 168.Shetty P, Thakur AM, Viswanathan C. Dopaminergic cells, derived from a high efficiency differentiation protocol from umbilical cord derived mesenchymal stem cells, alleviate symptoms in a Parkinson’s disease rodent model. Cell Biol Int. 2013;37:167–180. [DOI] [PubMed] [Google Scholar]
- 169.Weiss ML, Medicetty S, Bledsoe AR, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. [DOI] [PubMed] [Google Scholar]
- 170.Xiong N, Cao X, Zhang Z, et al. Long-term efficacy and safety of human umbilical cord mesenchymal stromal cells in rotenone-induced hemiparkinsonian rats. Biol Blood Marrow Transplant. 2010;16:1519–1529. [DOI] [PubMed] [Google Scholar]
- 171.Cova L, Armentero MT, Zennaro E, et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Res. 2010;1311:12–27. [DOI] [PubMed] [Google Scholar]
- 172.Capitelli CS, Lopes CS, Alves AC, et al. Opposite effects of bone marrow-derived cells transplantation in MPTP-rat model of Parkinson’s disease: a comparison study of mononuclear and mesenchymal stem cells. Int J Med Sci. 2014;11:1049–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choi HS, Kim HJ, Oh JH, et al. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson’s disease. Neurobiol Aging. 2015;36:2885–2892. [DOI] [PubMed] [Google Scholar]
- 174.Kim YJ, Park HJ, Lee G, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. [DOI] [PubMed] [Google Scholar]
- 175.Park BN, Kim JH, Lee K, Park SH, An YS. Improved dopamine transporter binding activity after bone marrow mesenchymal stem cell transplantation in a rat model of Parkinson’s disease: small animal positron emission tomography study with F-18 FP-CIT. Eur Radiol. 2015;25:1487–1496. [DOI] [PubMed] [Google Scholar]
- 176.Park HJ, Shin JY, Lee BR, Kim HO, Lee PH. Mesenchymal stem cells augment neurogenesis in the subventricular zone and enhance differentiation of neural precursor cells into dopaminergic neurons in the substantia nigra of a parkinsonian model. Cell Transplant. 2012;21:1629–1640. [DOI] [PubMed] [Google Scholar]
- 177.Wang F, Yasuhara T, Shingo T, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cerri S, Greco R, Levandis G, et al. Intracarotid Infusion of Mesenchymal Stem Cells in an Animal Model of Parkinson’s Disease, Focusing on Cell Distribution and Neuroprotective and Behavioral Effects. Stem Cells Transl Med. 2015;4:1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23 Suppl 1:S123–139. [DOI] [PubMed] [Google Scholar]
- 180.Aliaghaei A, Gardaneh M, Maghsoudi N, Salehinejad P, Gharib E. Dopaminergic Induction of Umbilical Cord Mesenchymal Stem Cells by Conditioned Medium of Choroid Plexus Epithelial Cells Reduces Apomorphine-Induced Rotation in Parkinsonian Rats. Arch Iran Med. 2016;19:561–570. [PubMed] [Google Scholar]
- 181.Glavaski-Joksimovic A, Virag T, Mangatu TA, McGrogan M, Wang XS, Bohn MC. Glial cell line-derived neurotrophic factor-secreting genetically modified human bone marrow-derived mesenchymal stem cells promote recovery in a rat model of Parkinson’s disease. J Neurosci Res. 2010;88:2669–2681. [DOI] [PubMed] [Google Scholar]
- 182.Hoban DB, Howard L, Dowd E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neuroscience. 2015;303:402–411. [DOI] [PubMed] [Google Scholar]
- 183.Lu L, Zhao C, Liu Y, et al. Therapeutic benefit of TH-engineered mesenchymal stem cells for Parkinson’s disease. Brain Res Brain Res Protoc. 2005;15:46–51. [DOI] [PubMed] [Google Scholar]
- 184.Mei J, Niu C. Effects of engineered conserved dopamine neurotrophic factor-expressing bone marrow stromal cells on dopaminergic neurons following 6-OHDA administrations. Mol Med Rep. 2015;11:1207–1213. [DOI] [PubMed] [Google Scholar]
- 185.Moloney TC, Rooney GE, Barry FP, Howard L, Dowd E. Potential of rat bone marrow-derived mesenchymal stem cells as vehicles for delivery of neurotrophins to the Parkinsonian rat brain. Brain Res. 2010;1359:33–43. [DOI] [PubMed] [Google Scholar]
- 186.Xiong N, Zhang Z, Huang J, et al. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson’s disease. Gene Ther. 2011;18:394–402. [DOI] [PubMed] [Google Scholar]
- 187.Yin X, Xu H, Jiang Y, et al. The effect of lentivirus-mediated PSPN genetic engineering bone marrow mesenchymal stem cells on Parkinson’s disease rat model. PLoS One. 2014;9:e105118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yan M, Sun M, Zhou Y, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopamine neurons mediated by the Lmx1a and neurturin in vitro: potential therapeutic application for Parkinson’s disease in a rhesus monkey model. PLoS One. 2013;8:e64000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 189.Zhou Y, Sun M, Li H, et al. Recovery of behavioral symptoms in hemi-parkinsonian rhesus monkeys through combined gene and stem cell therapy. Cytotherapy. 2013;15:467–480. [DOI] [PubMed] [Google Scholar]
- 190.Soler R, Fullhase C, Hanson A, Campeau L, Santos C, Andersson KE. Stem cell therapy ameliorates bladder dysfunction in an animal model of Parkinson disease. J Urol. 2012;187:1491–1497. [DOI] [PubMed] [Google Scholar]
- 191.Venkataramana NK, Kumar SK, Balaraju S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res. 2010;155:62–70. [DOI] [PubMed] [Google Scholar]
- 192.Venkataramana NK, Pal R, Rao SA, et al. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: a pilot clinical study. Stem Cells Int. 2012;2012:931902. [DOI] [PMC free article] [PubMed] [Google Scholar]


