Abstract
Objectives:
We sought to conduct a meta-analysis to compare N-acetylcysteine in combination with sodium bicarbonate for the prevention of contrast-induced acute kidney injury (AKI).
Background:
Contrast-induced AKI is a serious consequence of cardiac catheterizations and percutaneous coronary interventions (PCI). Despite recent supporting evidence for combination therapy, not enough has been done to prevent the occurrence of contrast-induced AKI prophylactically.
Methods:
Published randomized controlled trial data were collected from OVID/PubMed, Web of Science, and conference abstracts. The outcome of interest was contrast-induced AKI, defined as a ≥25% or ≥0.5 mg/dL increase in serum creatinine from baseline. Secondary outcome was renal failure requiring dialysis.
Results:
Ten randomized controlled trials met our criteria. Combination treatment of N-acetylcysteine with intravenous sodium bicarbonate reduced contrast-induced AKI by 35% (RR: 65; 95%CI: 0.40, 1.05). However, the combination of N-acetylcysteine plus sodium bicarbonate did not significantly reduce renal failure requiring dialysis (RR: 0.47; 95%CI: 0.16, 1.41).
Conclusions:
Combination prophylaxis with N-acetylcysteine and sodium bicarbonate significantly reduced the occurrence of contrast-induced AKI overall, but not dialysis-dependent renal failure. Combination prophylaxis should be incorporated for all high-risk patients (emergent cases or patients with chronic kidney disease) and should be strongly considered for all interventional radio-contrast procedures.
Keywords: acute kidney injury, renal pharmacology, contrast, epidemiology, meta-analysis
Introduction
Contrast-induced acute kidney injury (AKI) is a serious consequence of the more than 1.3 million cardiac catheterizations and percutaneous coronary interventions (PCI) in the United States each year. Researchers hypothesize contrast-induced AKI results from direct toxicity to the renal tubules by contrast medium or renal hemodynamic changes.(1,2) Up to fifteen percent of patients develop contrast-induced AKI following PCI with a 5-fold increased risk of in-hospital(3) and long-term mortality.(4)
Contrast-induced AKI is commonly defined as a 25% increase or 0.5 mg/dL increase in serum creatinine from baseline within 48 hours of exposure.(4–7) Rihal and Chertow, showed that contrast-induced AKI was associated with an increased risk of in-hospital mortality.(8,9) Patients with contrast-induced AKI had a 22% mortality rate compared to 1.4% for those without AKI.(9) Patients admitted to the hospital for all causes and developing contrast-induced AKI were 6.5-times more likely to die in the hospital compared to patients not developing AKI; on average these patients had 3.5 more days in the hospital and $7,500 additional hospital costs.(8)
Variation exists in prophylactic strategies and there is a lack of consensus on prevention tactics according to a recent taskforce.(10) Despite the ease of identifying patients at risk,(11,12) preventive measures to reduce contrast-induced AKI have not been consistent.(13) However, two recent randomized controlled trials (RCT) among high-risk patients using N-acetylcysteine and sodium bicarbonate(14,15) demonstrated the combination of N-acetylcysteine and sodium bicarbonate were significantly effective at preventing the contrast-induced AKI. Both N-acetylcysteine and sodium bicarbonate are oxygen-derived free radical scavengers and therefore block injury to the renal tubules.(16–21) There has been no formal synthesis of the combination prophylactic trial data. Consequently, there are no consensus protocols for prophylactic strategies or contrast dosing to prevent contrast-induced AKI. These gaps have identified opportunities to adopt effective evidence-based measures to reduce contrast-induced AKI.
Therefore, we sought to synthesize randomized controlled trial evidence for prophylactic combination strategies incorporating oral or intravenous N-acetylcysteine and intravenous sodium bicarbonate in cardiac catheterization or PCI.
Materials and Methods
Data and Sources of Searches
We conducted a meta-analysis of randomized controlled trials using combination prophylaxis of N-acetylcysteine and sodium bicarbonate among patients undergoing catheterization or percutaneous coronary intervention. MEDLINE (OVID and Pubmed, 1960 through February 2009), Web of Knowledge, Cochrane Library databases and conference abstracts (American Heart Association, American College of Cardiology, Transcatheter Cardiovascular Therapeutics, National Kidney Foundation, American Society of Nephrology Renal Week) were used to identify published randomized controlled trials from 2006 through February 2009.
Study Selection
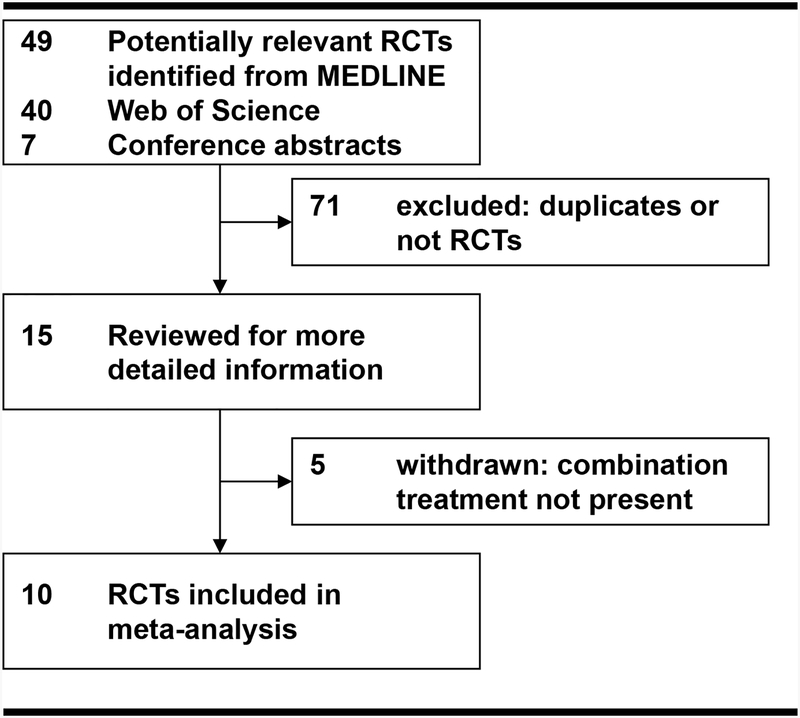
Key words used to search included: (N-acetylcysteine) and (sodium bicarbonate) and (catheterization or angiography or percutaneous coronary intervention or PCI). The search yielded ten published human randomized controlled trials (Figure 1, Table 1).(14,15,22–29) We searched ClinicalTrial.gov; we found one additional trial: CONTRAST (Singapore). However, the trial is still enrolling patients and has not reported the interim results.
Figure 1: Study Selection.
Table 1.
Study Characteristics*
| Total Patients | Treatment Group, N | Control Group, N | Jadad23 Score | |||||
|---|---|---|---|---|---|---|---|---|
| Trial (Year) | Treatment Protocol | Control Protocol | Enrollment Criteria | Blinding | ||||
| Saidin28 (2006) | 57 | 29 | Oral N-acetylcysteine plus sodium bicarbonate. Oral N-acetylcysteine plus sodium bicarbonate 2 hours before and 6 hours after procedure. | 28 | Oral N-acetylcysteine plus normal saline. Oral N-acetylcysteine plus normal saline 2 hours before and 6 hours after procedure. | Coronary angiography or angioplasty, CKD stages 2–4. | Double-Blind | 2 |
| Brigouri14 (2007) | 351 | 10 8 | Oral N-acetylcysteine plus sodium bicarbonate. Isotonic saline (0.9%) IV at 1 mL/kg/hr (0.5mL/kg/hr for EF<40) for 12 hours before and 12 hours after contrast exposure. Oral 1,200mg N-acetylcysteine twice a day for day before and after procedure with 154 mEq/L sodium bicarbonate in dextrose and H2O (Merten Protocol29): initial IV bolus 3mL/kg/h for 1 hour immediately prior to contrast; during and for 6 hours after contrast exposure with same dose at 1mL/kg/hr. | 111 | Oral N-acetylcysteine plus IV hydration. Isotonic saline (0.9%) IV at 1 mL/kg/hr (0.5mL/kg/hr for EF<40) for 12 hours before and 12 hours after contrast exposure. Oral 1,200mg N-acetylcysteine twice a day for day before and after procedure. | Coronary and/or peripheral angiography and/or angioplasty with chronic kidney disease, Creatinine ≥2mg/dL or eGFR<40 mL min−1 1.73m−2, age ≥18. | Double-Blind | 3 |
| 107 | Oral N-acetylcysteine plus IV hydration plus IV ascorbic acid. Isotonic saline (0.9%) IV at 1 mL/kg/hr (0.5mL/kg/hr for EF<40) for 12 hours before and 12 hours after contrast exposure. Oral 1,200mg N-acetylcysteine twice a day for day before and after procedure with 3g IV ascorbic acid 2 hours before contrast and 2g the night and morning after contrast. | Double-Blind | 3 | |||||
| Heguillen23 (2007) | 27 | 9 | Oral N-acetylcysteine plus sodium bicarbonate. Oral 600mg N-acetylcysteine twice a day for day before and of procedure with 154 mEq/L sodium bicarbonate at 3mL/kg/h for 1 hour immediately prior to procedure; and at 1mL/kg/h for 6 hours after procedure. | 9 | Oral N-acetylcysteine plus normal saline. Oral 600mg N- acetylcysteine twice a day for day before and of procedure with 154 mEq/L normal saline at 3mL/kg/h for 1 hour immediately prior to procedure; and at 1mL/kg/h for 6 hours after procedure. | Coronary angiography or angioplasty, serum creatinine >1.25 (mg/dL), eGFR<50 mL min-1 1.73m-2, age ≥18. | Single-Blind | 2 |
| Kim24 (2007) | 100 | 31 | Oral N-acetylcysteine plus sodium bicarbonate. Oral 600mg N-acetylcysteine twice a day for two days with 80 mEq/L sodium bicarbonate at 1mL/kg/h for 12 hour prior to procedure and for 12 hours after procedure. | 20 | Oral N-acetylcysteine plus normal saline. Oral 600mg N- acetylcysteine twice a day for two days with 80 mEq/L normal saline at 1mL/kg/h for 12 hour prior to procedure and for 12 hours after procedure. | Elective coronary angiography, serum creatinine >1.5 (mg/dL), proteinurea>5 00 mg/day. | Single-Blind | 1 |
| Lin25 (2007) | 45 | 21 | Oral N-acetylcysteine plus sodium bicarbonate. Oral 600mg N-acetylcysteine twice a day for day of and day after procedure with 154 mEq/L sodium bicarbonate at 3mL/kg/h for 1 hour prior to procedure and for 6 hours after procedure. | 24 | Oral N-acetylcysteine plus normal saline. Oral 600mg N- acetylcysteine twice a day for day of and day after procedure with 154 mEq/L normal saline at 3mL/kg/h for 1 hour prior to procedure and for 6 hours after procedure. | Coronary angiography, angioplasty, serum creatinine <2.0 (mg/dL). | Single-Blind | 1 |
| Recio- Mayoral15 (2007) | 111 | 56 | IV N-acetylcysteine plus sodium bicarbonate. Initial IV bolus 5mL/kg/hr alkaline saline with 154 mEq/L sodium bicarbonate in 5% glucose and H2O plus 2,400mg N- acetylcysteine in same solution over 1 hour. Following contrast, same fluids continued without N- acetylcysteine at 1.5 mL/kg/hr for 12 hours plus 2 oral doses of 600mg N-acetylcysteine the day after contrast. | 55 | Oral N-acetylcysteine plus hydration. Isotonic saine (0.9%) at 1mL/kg/hr for 12 hours after contrast plus 2 oral doses of 600mg N-acetylcysteine the day after contrast. | Patients with MI undergoing primary or rescue PCI or high-risk non- STEMI requiring urgent PCI. | Single-Blind | 2 |
| Shaikh29 (2007) | 320 | 80 | IV N-acetylcysteine plus sodium bicarbonate. IV N-acetylcysteine with 154 mEq/L sodium bicarbonate at 3mL/kg/h for 1 hour prior to procedure and 1mL/kg/h for 6 hours after procedure. | 81 | IV N-acetylcysteine plus normal saline. IV N-acetylcysteine with 154 mEq/L normal saline at 3mL/kg/h for 1 hour prior to procedure and 1mL/kg/h for 6 hours after procedure. | High risk catheterization. | Single-Blind | 1 |
| Brar22 (2008) | 353 | 73 | Oral N-acetylcysteine plus sodium bicarbonate. Oral 600mg N-acetylcysteine twice a day for day before and day of the procedure with sodium bicarbonate at 3mL/kg/h for 1 hour prior to procedure and 1.5mL/kg/h during and for 6 hours after procedure. | 78 | Oral N-acetylcysteine plus normal saline. Oral 600mg N- acetylcysteine twice a day for day before and day of the procedure with normal saline at 3mL/kg/h for 1 hour prior to procedure and 1.5mL/kg/h during and for 6 hours after procedure. | Coronary angiography eGFR<60 mL min−1 1.73m−2, age ≥18, 1 + of either diabetes, congestive heart failure, hypertension, or age>75 years. | Single-Blind | 3 |
| Maioli26 (2008) | 502 | 25 0 | Oral N-acetylcysteine plus sodium bicarbonate. Oral 600mg N-acetylcysteine twice a day for day before and after procedure with 154 mEq/L sodium bicarbonate in dextrose and H2O (Merten Protocol29): initial IV bolus 3mL/kg/h for 1 hour immediately prior to contrast; during and for 6 hours after contrast exposure with same dose at 1mL/kg/hr. | 252 | Oral N-acetylcysteine plus normal saline. Isotonic saline (0.9%) IV at 1 mL/kg/hr for 12 hours before and 12 hours after contrast exposure. Oral 600mg N-acetylcysteine twice a day for day before and after procedure. | Coronary angiography eGFR<60 mL min−1 1.73m−2, age ≥18, 1 + of either diabetes, congestive heart failure, hypertension, or age>75 years. | Single-Blind | 3 |
| Ruiz27 (2008) | 128 | 32 | Oral N-acetylcysteine plus sodium bicarbonate. Methods not described. | 32 | Oral N-acetylcysteine plus normal saline. Methods not described. | Coronary and/or peripheral angiography, angioplasty, creatinine >1.5 (mg/dL) or diabetics creatinine >1.2 (mg/dL). | Single-Blind | 1 |
All studies investigating combination of N-acetylcysteine plus sodium bicarbonate were included. IV: intravenous; EF: ejection fraction (%); MI: myocardial infarction; STEMI: ST-elevation MI; PCI: percutaneous coronary intervention; eGFR: estimated glomerular filtration rate using MDRD equation.
Data Abstraction and Quality Assessment
We abstracted data from the trials on contrast-induced AKI (defined as ≥25%, ≥0.5 mg/dL, ≥25% and ≥0.5 mg/dL increase in creatinine from baseline) and renal failure (new onset of dialysis). We followed the appropriate methods for conducting a meta-analysis as stipulated in the QUORUM statement.(30) Two independent reviewers (JB, CB) selected trials for information outcomes and recorded data on spreadsheets. The Jadad criteria were used to assess study quality and reported with study the characteristics (Table 1).(31)
Data Synthesis and Analysis
All outcome comparisons and treatment effects were calculated using the Cochrane Collaborative software, RevMan 4.2.8. We calculated the I2 to evaluate the percentage of heterogeneity among all the trials incorporated in the summary estimate.(32) Heterogeneity was observed in the three comparisons; therefore, we used random effects modeling. For all comparisons, a fixed effects relative risk (RR) and 95%CI was calculated for each independent study and for the summary statistic. Methods for the calculation of the above statistics have been reported previously.(33,34)
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Ten trials met our eligibility criteria for combination therapy including sodium bicarbonate plus N-acetylcysteine before and after contrast administration (Table 1). All studies reported contrast-induced AKI as ≥25% increase in serum creatinine; four reported contrast-induced AKI separately by ≥0.5 (mg/dL) increase in serum creatinine. Nine studies compared combination treatment (sodium bicarbonate and N-acetylcysteine) with N-acetylcysteine and hydration with normal saline; one study compared combination therapy with N-acetylcysteine alone; one study compared combination therapy with N-acetylcysteine with normal saline and a separate arm with N-acetylcysteine and ascorbic acid (we have included this arm in the analysis).
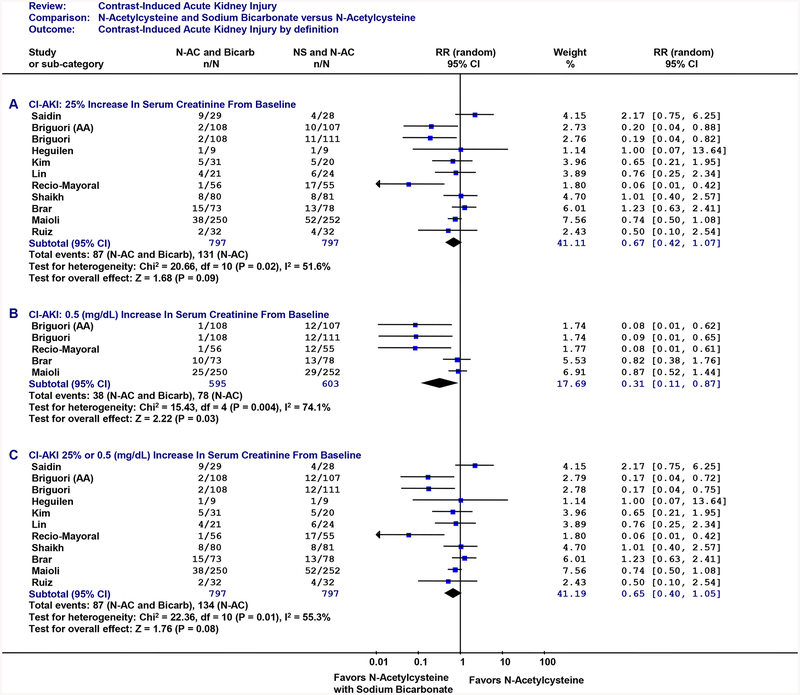
Contrast-induced AKI was defined in three ways. The first analysis with contrast-induced AKI defined as a ≥25%ΔCr (Figure 2A) demonstrated the combination of N-acetylcysteine plus sodium bicarbonate did not significantly reduce contrast-induced AKI (≥25%ΔCr) by 33% with the combined RR of 0.67 (95%CI: 0.42, 1.07), however, this effect demonstrated a strong trend towards protection against contrast-induce AKI. Alternatively, when using an alternative definition for contrast-induced AKI (≥0.5Cr: Figure 2B), a statistically significant benefit was observed for combination treatment with a significant 69% reduction (RR: 0.31; 95%CI: 0.11, 0.87). When using the greater of the two definitions (≥25%ΔCr and ≥0.5: Figure 2C), the results were similar to the ≥25%ΔCr definition with a non-significant 35% reduction in contrast-induced AKI (RR: 65; 95%CI: 0.40, 1.05).
Figure 2: Contrast-Induced AKI.
Individual randomized controlled trials are listed in order by year of publication. Outcome is contrast-induced AKI. Figure 2A: contrast-induced AKI (25% relative increase in serum creatinine from baseline). Figure 2B: contrast-induced AKI (≥0.5 mg/dL increase in serum creatinine from baseline). Figure 2C: contrast-induced AKI (≥25% or ≥0.5 mg/dL increase in serum creatinine from baseline). CI: 95 percent confidence interval. The size of each square denotes the weight of each trial’s relative risk in calculating the combined relative risk. The diamond represents the combined relative risk at the center; opposing points of the diamond represent the 95% confidence intervals. Treatment: N-acetylcysteine plus sodium bicarbondate. AA: N-acetylcysteine plus ascorbic acid.
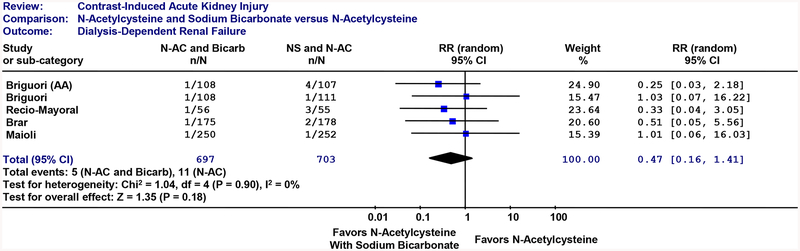
Dialysis (Figure 3): When the combination of N-acetylcysteine and sodium bicarbonate was compared to controls or head-to-head with N-acetylcysteine, the combination treatment did not significantly reduce dialysis dependent renal failure (RR: 0.47; 95%CI: 0.16, 1.41).
Figure 3: Renal Failure Requiring Dialysis.
Individual randomized controlled trials are listed in order by year of publication. Outcome is dialysis. CI: 95 percent confidence interval. The size of each square denotes the weight of each trial’s relative risk in calculating the combined relative risk. The diamond represents the combined relative risk at the center; opposing points of the diamond represent the 95% confidence intervals. Treatment: N-acetylcysteine plus sodium bicarbondate. AA: N-acetylcysteine plus ascorbic acid.
Discussion
We conducted a meta-analysis on the clinical effectiveness of N-acetylcysteine in combination with intravenous sodium bicarbonate compared to N-acetylcysteine. We found ten randomized controlled trials that met our criteria. Collectively, combination treatment of N-acetylcysteine with intravenous sodium bicarbonate reduced contrast-induced AKI by 35% (RR: 65; 95%CI: 0.40, 1.05). Therefore, combination treatment with N-acetylcysteine plus sodium bicarbonate prevented contrast-induced AKI in 4 out of 10 patients over N-acetylcysteine alone. However, the combination of N-acetylcysteine plus sodium bicarbonate did not significantly reduce renal failure requiring dialysis (RR: 0.47; 95%CI: 0.16, 1.41), although only five patients (0.7%) receiving the combination therapy went on dialysis compared with eleven (1.6%) receiving N-acetylcysteine.
Multiple strategies have been used independently to reduce contrast-induced AKI: hydration alone, sodium bicarbonate alone, N-acetylcysteine alone, and others. However, there has been a lack of consensus about the implementation of these strategies in practice, likely due to much confusion about their clinical efficacy.
Hydration:
In a small prospective randomized control trial (RCT), hydration with 0.45% normal saline for 12 hours before and after angiography has been shown to be effective in reducing contrast-induced AKI by 65%.(35) In a large prospective RCT, hydration with half isotonic (0.45%) or isotonic (0.9%) saline for the morning before elective PCI and immediately prior to emergency PCI reduced contrast-induced AKI by 0.7% and 2.0%, respectively.(36)
Sodium Bicarbonate (NaHCO3):
Isotonic sodium bicarbonate through the alkalinization of renal tubular fluid and subsequent reduction in free oxygen radicals has shown beneficial results (although mixed) in reducing contrast-induced AKI. Merten reported patients receiving isotonic (154 mEq/L) infusion of sodium bicarbonate before and after contrast administration (370 mg iodine/mL) had an 89% reduction in contrast-induced AKI compared with patients that received hydration with isotonic sodium chloride.(37) Four recent meta-analyses evaluating the protective effects of hydration with sodium bicarbonate compared with hydration with normal saline have shown sodium bicarbonate to be more effective in preventing contrast-induced AKI by 54–63%: RR: 37 (95%CI: 0.18, 0.74);(38) RR: 0.45 (95%CI: 0.26, 0.79);(39) RR: 0.46 (95%CI: 0.26, 0.82);(40) and RR: 0.52 (95%CI: 0.34, 0.80).(41)
N-acetylcysteine:
N-acetylcysteine is postulated to act as a free radical scavenger. Tepel was the first to report a protective effect of N-acetylcysteine (600 mg twice daily on the day before and day of intervention with half isotonic saline) in reducing contrast-induced AKI by 91%.(42) Seven meta-analyses of N-acetylcysteine have shown beneficial treatment effects in reducing contrast-induced AKI.(43–49) However, five meta-analyses were inconclusive. (50–54) Marenzi et al., demonstrated a dose-dependent effect of N-acetylcycsteine (600 mg IV before and 600 mg orally twice daily for 48hrs post), whereby both single and double doses of N-acetylcysteine reduced contrast-induced AKI and in-hospital mortality with the more beneficial treatment being the double dose of N-acetylcysteine in patients undergoing primary PCI.(55) Unfortunately, N-acetylcysteine might cause an artificial transient decline in serum creatinine without changing renal function and therefore additional markers of renal function should be incorporated to confirm these effects, such as Cystatin C.(45,56) Recently, Kelly et al., performed a meta-analysis of N-acetylcyteine compared to hydration alone. They found oral or intravenous N-acetylcysteine significantly reduced contrast-induced AKI by 38% when compared with hydration controls (RR: 0.62; 95%CI: 0.44–0.88).(57) Current systematic reviews and meta-analyses have identified a statistically significant benefit for either hydration with sodium bicarbonate and prophylaxis with N-acetylcysteine. Our meta-analysis focus on the question of combined hydration and prophylaxis with both sodium bicarbonate and N-acetylcysteine, demonstrating a significant benefit for combination prophylaxis over N-acetylcysteine with or without hydration alone.
Other pharmacological strategies have been used over the years. Theophylline causes arrythmias and therefore is not useful for cardiac patients. In a recent meta-analysis, Kelly et al., showed theophylline with a non-significant, but impressive, 51% reduction in contrast-induced AKI (RR: 0.49; 95%CI: 0.23–1.06); this report suggests a promising protective effect for theoplylline.(57) Prostaglandins can cause severe hypotension. Other agents include antioxidant ascorbic acid and trimetazidine; but, limited evidence has been reported on these agents for preventing contrast-induced AKI.(58) Hypoperfusion of the kidney through vasoconstriction might play a role in contrast-induced AKI; however, vasodilators have not been shown to be successful reducing contrast-induced AKI. All four RCTs for dopamine showed no benefit in reducing contrast-induced AKI.(59–62) Two trials have reported on fenoldopam, neither showing a protective effect against contrast-induced AKI.(57,63,64) A meta-analysis demonstrated renal replacement therapy does not reduce the risk of contrast-induced AKI (0.97; 95%CI: 0.44, 2.14).(65) Continuous veno-venus hemofiltration (CVVH) following PCI in one study was not shown to protect renal function.(66) However, a recent trial by Lee et al., reported that prophylactic hemodialysis resulted in a 95% reduction in post-catheterization dialysis for chronic kidney disease patient undergoing coronary angiography.(67)
There have been several hypotheses generated around the pharmacodynamics of N-acetylcysteine and sodium bicarbonate. Merten et al., postulated that alkalizing the renal tubule fluid with sodium bicarbonate might reduce acute tubule necrosis brought on by nephrotoxic contrast media.(37) In a recent study examining renal cell apoptosis by contrast agents, Romano et al., proposed sodium bicarbonate scavanges free-radicals and the presence of bicarbonate in the proximal convoluted tubules might work to either buffer the production of H+ from cellular hypoxia or to drive Na+ reabsorption.(68) However, they reported sodium bicarbonate did not raise the pH of the media in vitro compared to contrast alone and postulated the protective action of sodium bicarbonate works through a different mechanism than N-acetylcysteine and ascorbic acid and therefore provides an additive effect.(68) Romano et al., were able to demonstrate that N-acetylcysteine and ascorbic acid works in vitro on the proximal renal tubule and prevents renal cell apoptosis, but not sodium bicarbonate.(68) This finding was supported by an earlier report by Briguori et al., showing N-acetylcysteine works in a dose-dependent manner.(69) Our meta-analysis compares the additive effect of sodium bicarbonate compared to the use of N-acetylcysteine or ascorbic acid and demonstrates a distinct advantage in reducing CI-AKI; while the mechanism of the prophylactic effect of sodium bicarbonate in the renal tubules is not confirmed, based on the summary of evidence, there does appear to be an additive effect either through more regimented hydration or through free radical scavenging in the renal tubules.
The barriers to reducing contrast-induced AKI following PCI have been due to inconsistencies in the randomized controlled trial evidence and meta-analysis reporting either N-acetylcysteine or sodium bicarbonate. Cardiology and Nephrology historically have compartmentalized patient care within each discipline. These barriers create a chasm between current practice and the best evidence-based care for patients.
Additional trials are needed to comment on the clinical effectiveness of combination protocols for the prevention of contrast-induced AKI. Recommendations differ surrounding the prevention of contrast-induced AKI and not enough has been done to establish a working protocol to prevent contrast-induced AKI among all patients. Until a large-scale RCT can be conducted to evaluate the clinical effectiveness of these prophylactic strategies, clinical action must be taken on the evidence that exists. We recommend a comprehensive prophylactic protocol needs to be incorporated into practice to prevent contrast-induced AKI incorporating both sodium bicarbonate and N-acetylcysteine. We encourage institutions to form a multi-disciplinary team of nephrologists, cardiologists, and epidemiologists to work together to develop evidence-based benchmarks for high quality care and standardize their prophylactic strategies in preventing contrast-induced AKI.
Funding source
Funding for this research was provided by a NRSA postdoctoral training grant from the Agency for Healthcare Research and Quality (AHRQ), T32HS000070.
Footnotes
Disclosures
There are no conflicts of interest to disclose.
References
- 1.Bui KL, Horner JD, Herts BR, Einstein DM. Intravenous iodinated contrast agents: risks and problematic situations. Cleve Clin J Med 2007;74:361–4, 367. [DOI] [PubMed] [Google Scholar]
- 2.Tumlin J, Stacul F, Adam A, et al. Pathophysiology of contrast-induced nephropathy. Am J Cardiol 2006;98:14K–20K. [DOI] [PubMed] [Google Scholar]
- 3.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996;275:1489–94. [PubMed] [Google Scholar]
- 4.Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 2000;36:1542–8. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol 2006;98:5K–13K. [DOI] [PubMed] [Google Scholar]
- 6.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 1997;103:368–75. [DOI] [PubMed] [Google Scholar]
- 7.Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl 2006:S11–5. [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 9.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105:2259–64. [DOI] [PubMed] [Google Scholar]
- 10.Stacul F, Adam A, Becker CR, et al. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol 2006;98:59K–77K. [DOI] [PubMed] [Google Scholar]
- 11.Brown JR, DeVries JT, Robb JF, et al. Serious Renal Dysfunction After Percutaneous Coronary Intervention Can Be Predicted. Am Heart J 2008;155:260–6. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393–9. [DOI] [PubMed] [Google Scholar]
- 13.Elicker BM, Cypel YS, Weinreb JC. IV contrast administration for CT: a survey of practices for the screening and prevention of contrast nephropathy. AJR Am J Roentgenol 2006;186:1651–8. [DOI] [PubMed] [Google Scholar]
- 14.Briguori C, Airoldi F, D’Andrea D, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation 2007;115:1211–7. [DOI] [PubMed] [Google Scholar]
- 15.Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J Am Coll Cardiol 2007;49:1283–8. [DOI] [PubMed] [Google Scholar]
- 16.Arstall MA, Yang J, Stafford I, Betts WH, Horowitz JD. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects. Circulation 1995;92:2855–62. [DOI] [PubMed] [Google Scholar]
- 17.Atkins JL. Effect of sodium bicarbonate preloading on ischemic renal failure. Nephron 1986;44:70–4. [DOI] [PubMed] [Google Scholar]
- 18.Baroni EA, Costa RS, Volpini R, Coimbra TM. Sodium bicarbonate treatment reduces renal injury, renal production of transforming growth factor-beta, and urinary transforming growth factor-beta excretion in rats with doxorubicin-induced nephropathy. Am J Kidney Dis 1999;34:328–37. [DOI] [PubMed] [Google Scholar]
- 19.Brunet J, Boily MJ, Cordeau S, Des Rosiers C. Effects of N-acetylcysteine in the rat heart reperfused after low-flow ischemia: evidence for a direct scavenging of hydroxyl radicals and a nitric oxide-dependent increase in coronary flow. Free Radic Biol Med 1995;19:627–38. [DOI] [PubMed] [Google Scholar]
- 20.Lindinger MI, Franklin TW, Lands LC, Pedersen PK, Welsh DG, Heigenhauser GJ. NaHCO(3) and KHCO(3) ingestion rapidly increases renal electrolyte excretion in humans. J Appl Physiol 2000;88:540–50. [DOI] [PubMed] [Google Scholar]
- 21.Sporer H, Lang F, Oberleithner H, Greger R, Deetjen P. Inefficacy of bicarbonate infusions on the course of postischaemic acute renal failure in the rat. Eur J Clin Invest 1981;11:311–5. [DOI] [PubMed] [Google Scholar]
- 22.Brar SS, Shen AY, Jorgensen MB, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. Jama 2008;300:1038–46. [DOI] [PubMed] [Google Scholar]
- 23.Heguilen R, Liste A, Gabriela R, et al. Prevention Of Contrast-Induced Nephropathy: Volume Expansion, N-Acethylcysteine Or Both? Results From A Pilot Study. Nephrol Dial Transplant 2007;22:54–55. [Google Scholar]
- 24.Kim G, Kim K, Shin J, Lee CH, Kang CM. Hydration With Sodum Bicarbonate For The Prevention Of Radiocontrast-Induced Nephropathy. Nephrol Dial Transplant 2007;22:49. [Google Scholar]
- 25.Lin M, Sabeti M, Iskander E, Malhotra N, Phan PTT, Phan PCT. Prevention Of Contrast Nephropathy With Sodium Bicarbonate. J Am Soc Nephrol 2007;18:959A–960A. [Google Scholar]
- 26.Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol 2008;52:599–604. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz AS, Marchetti G, Lagioia A, et al. Randomized study of evaluation of N-acetylcysteine and sodium bicarbonate in the prevention of contrast-induced nephropathy. Circulation 2008;118:E295–E295. [Google Scholar]
- 28.Saidin R, Zainudin S, Kong NCT, Oteh K, Saaidin NF, Shah SA. Intravenous Sodium Bicarbonate Versus Normal Saline Infusion as Prophylaxis Against Contrast Nephropathy In Patients With Chronic Kidney Disease Undergoing Coroanry Artery Angiography Or Angioplasty. J Am Soc Nephrol 2006;17:766A. [Google Scholar]
- 29.Shaikh F, Maddikunta R, Museitif R, et al. A prospective randomized trial comparing normal saline and sodium bicarbonate with or without N-acetyleysteine for prevention of contrast-induced nephropathy. American Journal of Cardiology 2007;100:122L–123L.17599453 [Google Scholar]
- 30.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896–900. [DOI] [PubMed] [Google Scholar]
- 31.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Cont Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Brit Med J 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JR, Birkmeyer NJ, O’Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation 2007;115:2801–13. [DOI] [PubMed] [Google Scholar]
- 34.Henry DA, Moxey AJ, Carless PA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochr Data Syst Rev 2001. :CD001886. [DOI] [PubMed] [Google Scholar]
- 35.Solomon R, Werner C, Mann D, D’Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med 1994;331:1416–20. [DOI] [PubMed] [Google Scholar]
- 36.Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med 2002;162:329–36. [DOI] [PubMed] [Google Scholar]
- 37.Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 2004;291:2328–34. [DOI] [PubMed] [Google Scholar]
- 38.Hogan SE, L’Allier P, Chetcuti S, et al. Current role of sodium bicarbonate-based preprocedural hydration for the prevention of contrast-induced acute kidney injury: a meta-analysis. Am Heart J 2008;156:414–21. [DOI] [PubMed] [Google Scholar]
- 39.Joannidis M, Schmid M, Wiedermann CJ. Prevention of contrast media-induced nephropathy by isotonic sodium bicarbonate: a mete-analysis. Wiener Klinische Wochenschrift 2008;120:742–748. [DOI] [PubMed] [Google Scholar]
- 40.Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehgal AR. Sodium Bicarbonate Therapy for Prevention of Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Am J Kidney Dis 2008. [DOI] [PubMed] [Google Scholar]
- 41.Meier P, Ko DT, Tamura A, Tamhane U, Gurm HS. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med 2009;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000;343:180–4. [DOI] [PubMed] [Google Scholar]
- 43.Alonso A, Lau J, Jaber BL, Weintraub A, Sarnak MJ. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis 2004;43:1–9. [DOI] [PubMed] [Google Scholar]
- 44.Birck R, Krzossok S, Markowetz F, Schnulle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet 2003;362:598–603. [DOI] [PubMed] [Google Scholar]
- 45.Duong MH, MacKenzie TA, Malenka DJ. N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: comprehensive meta-analysis. Catheter Cardiovasc Interv 2005;64:471–9. [DOI] [PubMed] [Google Scholar]
- 46.Guru V, Fremes SE. The role of N-acetylcysteine in preventing radiographic contrast-induced nephropathy. Clin Nephrol 2004;62:77–83. [DOI] [PubMed] [Google Scholar]
- 47.Isenbarger DW, Kent SM, O’Malley PG. Meta-analysis of randomized clinical trials on the usefulness of acetylcysteine for prevention of contrast nephropathy. Am J Cardiol 2003;92:1454–8. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Nair D, Ix J, Moore DH, Bent S. N-acetylcysteine for the prevention of contrast-induced nephropathy. A systematic review and meta-analysis. J Gen Intern Med 2005;20:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misra D, Leibowitz K, Gowda RM, Shapiro M, Khan IA. Role of N-acetylcysteine in prevention of contrast-induced nephropathy after cardiovascular procedures: a meta-analysis. Clin Cardiol 2004;27:607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagshaw SM, Ghali WA. Acetylcysteine for prevention of contrast-induced nephropathy after intravascular angiography: a systematic review and meta-analysis. BMC Med 2004;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kshirsagar AV, Poole C, Mottl A, et al. N-acetylcysteine for the prevention of radiocontrast induced nephropathy: a meta-analysis of prospective controlled trials. J Am Soc Nephrol 2004;15:761–9. [DOI] [PubMed] [Google Scholar]
- 52.Nallamothu BK, Shojania KG, Saint S, et al. Is acetylcysteine effective in preventing contrast-related nephropathy? A meta-analysis. Am J Med 2004;117:938–47. [DOI] [PubMed] [Google Scholar]
- 53.Pannu N, Manns B, Lee H, Tonelli M. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int 2004;65:1366–74. [DOI] [PubMed] [Google Scholar]
- 54.Zagler A, Azadpour M, Mercado C, Hennekens CH. N-acetylcysteine and contrast-induced nephropathy: a meta-analysis of 13 randomized trials. Am Heart J 2006;151:140–5. [DOI] [PubMed] [Google Scholar]
- 55.Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006;354:2773–82. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann U, Fischereder M, Kruger B, Drobnik W, Kramer BK. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol 2004;15:407–10. [DOI] [PubMed] [Google Scholar]
- 57.Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med 2008;148:284–94. [DOI] [PubMed] [Google Scholar]
- 58.Van Praet JT, De Vriese AS. Prevention of contrast-induced nephropathy: a critical review. Curr Opin Nephrol Hypertens 2007;16:336–47. [DOI] [PubMed] [Google Scholar]
- 59.Abizaid AS, Clark CE, Mintz GS, et al. Effects of dopamine and aminophylline on contrast-induced acute renal failure after coronary angioplasty in patients with preexisting renal insufficiency. Am J Cardiol 1999;83:260–3, A5. [DOI] [PubMed] [Google Scholar]
- 60.Gare M, Haviv YS, Ben-Yehuda A, et al. The renal effect of low-dose dopamine in high-risk patients undergoing coronary angiography. J Am Coll Cardiol 1999;34:1682–8. [DOI] [PubMed] [Google Scholar]
- 61.Hans SS, Hans BA, Dhillon R, Dmuchowski C, Glover J. Effect of dopamine on renal function after arteriography in patients with pre-existing renal insufficiency. Am Surg 1998;64:432–6. [PubMed] [Google Scholar]
- 62.Weisberg LS, Kurnik PB, Kurnik BR. Risk of radiocontrast nephropathy in patients with and without diabetes mellitus. Kidney Int 1994;45:259–65. [DOI] [PubMed] [Google Scholar]
- 63.Allaqaband S, Tumuluri R, Malik AM, et al. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc Interv 2002;57:279–83. [DOI] [PubMed] [Google Scholar]
- 64.Stone GW, McCullough PA, T umlin JA, et al. Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. Jama 2003;290:2284–91. [DOI] [PubMed] [Google Scholar]
- 65.Cruz DN, Perazella MA, Bellomo R, et al. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis 2006;48:361–71. [DOI] [PubMed] [Google Scholar]
- 66.Marenzi G, Bartorelli AL, Lauri G, et al. Continuous veno-venous hemofiltration for the treatment of contrast-induced acute renal failure after percutaneous coronary interventions. Catheter Cardiovasc Interv 2003;58:59–64. [DOI] [PubMed] [Google Scholar]
- 67.Lee PT, Chou KJ, Liu CP, et al. Renal protection for coronary angiography in advanced renal failure patients by prophylactic hemodialysis. A randomized controlled trial. J Am Coll Cardiol 2007;50:1015–20. [DOI] [PubMed] [Google Scholar]
- 68.Romano G, Briguori C, Quintavalle C, et al. Contrast agents and renal cell apoptosis. Eur Heart J 2008;29:2569–76. [DOI] [PubMed] [Google Scholar]
- 69.Briguori C, Colombo A, Violante A, et al. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J 2004;25:206–11. [DOI] [PubMed] [Google Scholar]