Abstract
Introduction:
Poor glycemic control, assessed by higher glycated hemoglobin (HbA1c) levels, is associated with greater risk of diabetic complications.
Aim:
The aim of this study was to assess the association of triglyceride - to - HDL cholesterol (TG/HDL-C) ratio and triglyceride glucose (TyG) index with HbA1c and to evaluate their potential role as predictors of glycemic control in patients with diabetes mellitus type 2 (DM2).
Patients and methods:
This cross-sectional study was conducted in Health Center Banovici and included a total of 113 patients with DM2 classified according to their HbA1c values in two groups: DM2 HbA1c <7% - DM2 patients with good glycemic control (n=39) and DM2 HbA1c ≥7% - DM2 patients with poor glycemic control (n=74). Anthropometric, biochemical parameters and blood pressure values were measured, while TG/HDL-C ratio and TyG index were calculated.
Results:
TG/HDL-C ratio and TyG index were significantly higher in DM2 HbA1c≥7% compared to DM2 HbA1c<7% group (p=0.003 and p<0.001; respectively). Both TG/HDL-C ratio and TyG index were positively associated with HbA1c levels (Rho=0.29; p=0.002; Rho=0.37; p<0.001; respectively). In linear regression analysis TG/HDL-C ratio and BMI, and also TyG index and BMI were significantly independently associated with HbA1c even after controlling for age, gender, diabetes duration and smoking. When we stratified patients according to BMI values, independent association between TG/HDL-C ratio and HbA1c remained significant only in normal weight subjects (OR 0.21; 95%CI: 0.05-0.37; β=0.65; p=0.017), while independent association between TyG index and HbA1c remained significant only in overweight and obese subjects (OR 0.063; 95%CI: 0.01- 0.12; β=0.24; p =0.027).
Conclusion:
TG/HDL-C ratio might be a useful predictor of glycemic control in normal weight, and TyG index in overweight and obese patients with DM2.
Keywords: TG/HDL-C ratio, TyG index, glycemic control, HbA1c, patients with diabetes mellitus type 2
1. INTRODUCTION
Diabetes mellitus is a major health and socioeconomic problem. The number of patients with diabetes mellitus type 2 (DM2) is growing worldwide. Also, the number of premature deaths caused by diabetic macro- and micro-vascular complications is rising (1).
Glycemic control is fundamental to diabetes management because a good glycemic control reduces risk of complications. Glycated hemoglobin A1c (HbA1c) is the gold standard of glycemic control that the reflects average blood glucose in patients over approximately 3 months. Achieving HbA1c target value less than 7% has been shown to reduce diabetic vascular complication (1–3). In diabetic patients, for each 1% increase in absolute HbA1c value estimated risk of cardiovascular diseases (CVD) increases by 18% (4, 5).
Another risk factor for CVD in patients with DM2 is diabetic dyslipidemia. It consists of increased triglycerides (TG), reduced high density lipoprotein cholesterol (HDL-C), and postprandial lipemia. In addition, low density lipoprotein cholesterol (LDL-C) is converted to small, dens LDL that is more atherogenic. The serum triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, known as atherogenic index of plasma, is one of the major risk factors for CVD and metabolic syndrome (6). Higher TG/HDL C ratio has been associated with the presence of endothelial dysfunction (7). Furthermore, TG/HDL-C ratio has been proposed as a marker of insulin resistance (8). Quispe et al. (9) have shown that TG/HDL-C can also be a marker of glycemic control, especially in obese patients with DM2. Additional marker associated with insulin resistance is triglyceride to glucose (TyG) index. It helps in identification of subjects at high risk of CVD in asymptomatic subjects with DM2 (10).
Majority of studies conducted to date have explored association between TG/HDL-C ratio and insulin resistance in diabetic patients (8, 11). However, studies that aimed to assess association between TG/HDL-C ratio and glycemic control, and association between TyG index and glycemic control are limited.
2. AIM
Therefore, the aim of this study was to explore the association of Tg/HDL ratio and TyG index with HbA1c and to evaluate their potential role as predictors of glycemic control in patients with DM2.
3. MATERIAL AND METHODS
This cross-sectional study was conducted in Health Center Banovici and included a total of 113 patients with DM2 classified according to their values of HbA1c in two groups: DM2 HbA1c<7% - DM2 patients with good glycemic control (n=39) and DM2 HbA1c≥7% - DM2 patients with poor glycemic control (n=74).
All participants were informed about the study and signed a written consent for the participation. Also, they were asked to complete a questionnaire including general characteristics, medical history and lifestyle activities, such as smoking, alcohol intake and exercise. Subjects who had any condition known to affect lipid metabolism (e.g. chronic diseases such as heart and kidney diseases, thyroid diseases, liver diseases, serious infections, malignancy) or taking any drugs known to cause disturbance of lipid metabolism were excluded from the study.
The study was approved by the Ethics Committee of Health Center Banovici and by the Ethics Committee of Faculty of Medicine, University of Sarajevo. Research was conducted in accordance with Helsinki Declaration as revised in year 2000.
All subjects were advised to be on a low-fat diet and to abstain from intensive physical activity and alcohol intake 3 days before blood samples were taken. Blood samples were collected after an overnight fast. Fasting glucose (FG) level was determined by the glucose oxidase method. HbA1c was determined by a high-performance liquid chromatography. Lipid parameters were measured by automated auto-analyzer using standard methods: serum total cholesterol (TC) by the cholesterol oxidase method, the high-density lipoprotein cholesterol (HDL-C) levels by a direct homogeneous enzymatic method, while serum triglyceride (TG) levels were assayed after enzymatic hydrolysis, by a simultaneous enzymatic determination of glycerol. The low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald’s equation as previously described (12).
The normal ranges for measured parameters were: fasting blood glucose 3.3-6.1 mmol/L, triglycerides 0.11-1.7 mmol/L, total cholesterol 3.1-5.2 mmol/L, HDL-C 1.06-1.94 mmol/L, and LDL-C 2.0-4.3 mmol/L.
The TG/HDL-C ratio was calculated by dividing the serum concentration of TG by HDL-C measured in mg/dL (13). TyG index was calculated based on formula: Ln [TG (mg/dL) x FG (mg/dL)/2], according to the previous studies (10, 14).
Body weight was measured in light clothing without shoes, using the electronic scale. The body height was measured using a portable stadiometer. Body mass index (BMI) was calculated by dividing body weight and the square of subjects height [BMI = weight (kg) / height (m2)]. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) values were measured three times on the right arm using standard mercury sphygmomanometer. The mean value was recorded. Before the BP measurement, the participants were resting quietly in a sitting position for 5 min.
Statistical analysis
All statistical analyses were performed using the SPSS statistical package for Windows (version 16.0, SPSS Inc, Chicago, Illinois, USA). Kolmogorov–Smirnov test was used in order to test the normality of distribution of variables. The differences between groups were analyzed by the Man-Whitney test or independent t-test where appropriate. Univariate correlation coefficients were determined by Spearman analysis.
A multiple regression analysis was applied to examine independent relationship between HbA1c and a set of clinical parameters that were associated with HbA1c including age, gender, smoking, BMI, duration of DM, TG/HDL-C ratio and TyG index. Since TG/HDL-C and HbA1c were non-normally distributed in this study, we normalized than by log transformation before entering into multiple regression. The significant independent variables were defined as regression coefficient/standard error of the regression (β). Odds ratios (ORs) and 95 % confidence interval (CIs) were calculated. Statistical significance was considered for p < 0.05.
4. RESULTS
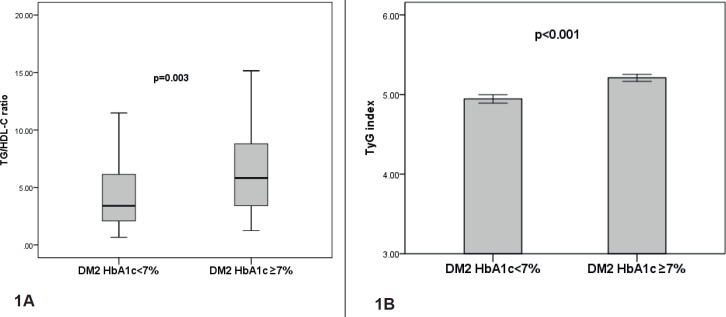
Baseline characteristics of both groups of patients with DM2 are shown in Table 1. Median duration of DM2 in HbA1c<7% group was not significantly different compared to HbA1c≥7% group [4 (2-10.5) vs. 6 (2.0-10.0) years; p=0.39]. Patients in the DM2 HbA1c≥7% group had significantly higher values of DBP, FG, HbA1c and TG levels compared to DM2 patients in DM2 HbA1c<7% group. There was no significant difference in age, gender, smoking, BMI, SBP, TC, HDL-C and LDL-C level between the study groups (Table 1). TG/HDL-C ratio was significantly higher in DM2 patients with HbA1c ≥7% compared to DM2 patients with HbA1c<7% [5.82 (3.41-8.98) vs. 3.40 (2.08-6.38); p=0.003] (Figure 1A).
Table 1. Baseline characteristics of patients with DM2 according to HbA1c value. Data are presented as median and interquartile ranges and mean ± SEM or absolute values and corresponding percentages for each group DM2 HbA1c<7% - DM2 patients with good glycemic control; DM2 HbA1c≥7% - DM2 patients with poor glycemic control; BMI: Body Mass Index; SBP: systolic blood pressure; DBP: diastolic blood pressure; FG: fasting glucose; HbA1c: glycated hemoglobin; TC: total cholesterol; TG: triglycerides; HDL-C: HDL-cholesterol; LDL-C: LDL-cholesterol; p–probability.
| Variables | DM2 HbA1c<7% (n=39) | DM2 HbA1c≥7% (n=74) | p-value |
|---|---|---|---|
| Age (years) | 64.18±2.06 | 59.92±1.4 | NS |
| Gender (n; %) M | 14 (35.9%) | 41 (55.4%) | NS |
| Smoking (n; %) Yes | 13 (33.3%) | 31 (41.9%) | NS |
| BMI (kg/m2) | 29.45 ± 0.74 | 29.81 ± 0.54 | NS |
| SBP (mmHg) | 130 (120-150) | 140 (130 -150) | NS |
| DBP (mmHg) | 80 (80-90) | 90 (80-100) | 0.004 |
| FG (mmol/L) | 6.8 (6.0-8.10) | 8.2 (7.0-10.52) | <0.001 |
| HbA1c (%) | 6.4 (6.10-6.70) | 9.0 (7.80-10.40) | <0.001 |
| TC (mmol/L) | 5.6 (5.00 -6.02) | 5.6 (4.67 – 6.32) | NS |
| TG (mmol/L) | 1.72 (1.30-2.36) | 2.32 (1.50-3.44) | 0.011 |
| HDL-C (mmol/L) | 1.11 (0.88-1.40) | 1.00 (0.86-1.15) | NS |
| LDL-C (mmol/L) | 3.60±0.17 | 3.37±0.16 | NS |
Figure 1. TG/HDL-C ratio and TyG index in DM2 patients according to HbA1c value. Data are presented as median and interquartile range (25-75 percentiles) and as mean ± SEM DM2 HbA1c<7% - DM2 patients with good glycemic control; DM2 HbA1c≥7% - DM2 patients with poor glycemic control; p– probability.
Also, TyG index was significantly higher in DM2 HbA1c≥7% group compared to DM2 HbA1c<7% group (5.21±0.04 vs. 4.94±0.05; p<0.001) (Figure 1B).
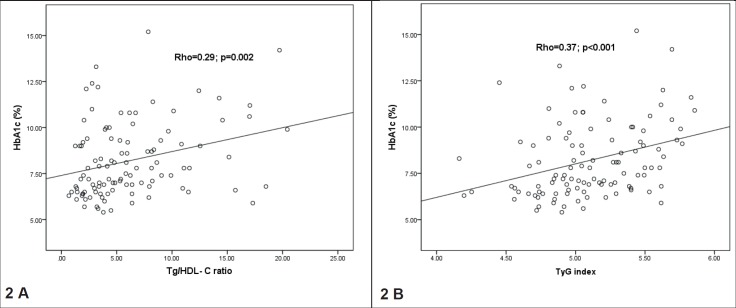
In DM2 patients, TG/HDL-C ratio was significantly negatively associated with age, DM2 duration and HDL-C levels and positively associated with BMI, DBP and TG, while TyG index was positively associated with BMI, DBP, FG, TG, TC and LDL-C levels and negatively associated only with HDL-C (Table 2). Both TG/HDL-C ratio and TyG index were positively associated with HbA1c levels in patients with DM2 (Figure 2).
Table 2. Spearman correlation coefficients between TG/HDL-C ratio, TyG index and clinical/laboratory parameters in the total sample of DM2 patients. BMI: Body Mass Index; SBP: systolic blood pressure; DBP: diastolic blood pressure; FG: fasting glucose; TG: triglycerides; TC: total cholesterol; HDL-C: HDL-cholesterol; LDL-C: LDL-cholesterol. *p<0.05; **p<0.01.
| Variables | TG/HDL-C ratio | TyG index |
|---|---|---|
| Age | -0.21* | -0.16 |
| DM2 duration | -0.23* | -0.12 |
| BMI | 0.2* | 0.23* |
| SBP | 0.1 | 0.16 |
| DBP | 0.26* | 0.23* |
| FG | 0.16 | 0.58** |
| TG | 0.95** | 0.9** |
| TC | 0.12 | 0.39** |
| HDL-C | -0.6** | -0.24* |
| LDL-C | 0.06 | 0.28** |
Figure 2. Relationship between TG/HDL-C ratio, TyG index and HbA1c in the total sample of DM2 patients . Data are presented as rho Spearman coefficients.
Furthermore, we analysed whether the observed association between TG/HDL-C and HbA1c and between TyG index and HbA1c remained significant after controlling for variables that were associated with HbA1c levels in univariate analysis. In linear regression analysis TG/HDL C ratio and BMI were significantly independently associated with HbA1c even after controlling for age, gender, diabetes duration and smoking (β=0.28; 95%CI:0.024-0.14; p=0.007 for TG/HDL-C ratio and β=-0.24; 95%CI:-0.6- -0.06; p=0.017 for BMI). When we stratified patients according to BMI values, independent association between TG/HDL-C ratio and HbA1c remained significant only in normal weight subjects (OR 0.21; 95%CI: 0.05-0.37; β=0.65; p=0.017) (Table 3), while in overweight and obese patients no significant independent association between TG/HDL-C ratio and HbA1c was observed. In linear regression analysis TyG index and BMI were significantly independently associated with HbA1c even after controlling for age, gender, diabetes duration and smoking (β=0.47; 95%CI:0.04-0.2; p=0.05 for TyG index and β=-0.27; 95%CI:-0.66:- -0.1; p=0.007 for BMI). When we stratified patients according to BMI values independent association between TyG index and HbA1c remained significant only in overweight and obese subjects (OR 0.063; 95%CI: 0.01- 0.12; β=0.24; p =0.027) (Table 3), while no significant independent association was found between TyG index and HbA1c in normal weight subjects.
Table 3. Linear regression analysis with independent predictors of HbA1c in normal weight and overweight/obese DM2 patients. OR: Odds Ration; CI: Confidence Interval.
| DM2 patients with BMI<25 kg/m2 | |||||
|---|---|---|---|---|---|
| Variables | OR | 95%CI | β | t | p |
| logTG/HDL-C | 0,21 | 0,05 - 0,37 | 0,65 | 2,8 | 0,017 |
| Age | -0,001 | -0,01 - 0,05 | -0,11 | -0,45 | 0,66 |
| DM duration | 0,065 | -0,11 - 0,24 | 0,2 | 0,81 | 0,43 |
| Gender | -0,032 | -0,14 - 0,08 | -0,14 | -0,64 | 0,54 |
| Smoking | -0,008 | -0,13 - 0,12 | -0,03 | -0,14 | 0,89 |
| Dependent variable: logHbA1c | R2=0.47 | ||||
| DM2 patients with BMI>25 kg/m2 | |||||
| Variables | OR | 95%CI | β | t | p |
| TyG index | 0,063 | 0,01 - 0,12 | 0,24 | -2,3 | 0,027 |
| Age | 0,00 | -0,00 - 0,001 | -0,06 | -0,5 | 0,62 |
| DM duration | 0,025 | -0,02 - 0,07 | 0,13 | 1,3 | 0,43 |
| Gender | -0,02 | -0,06 - 0,02 | -0,1 | -1,0 | 0,31 |
| Smoking | -0,05 | -0,03 - 0,04 | 0.17 | 1,3 | 0,24 |
| Dependent variable: logHbA1c | R2=0.25 | ||||
5. DISCUSSION
The main results of our study have shown that TG/HDL-C ratio and TyG index are positively associated with HbA1c levels in DM2 patients and both were higher in patients with poor glycemic control (HbA1c≥7%) compared to patients with good glycemic control (HbA1c<7%). However, these associations were weak, hence obtained results should be interpreted with caution. Common feature of insulin resistance and DM2 is dyslipidemia, characterized with hypertriglyceridemia associated with a reduction in HDL-C levels (15). While low HDL-C is considered a consequence of insulin resistance, recent evidence suggests that low HDL-C concentrations may exacerbate abnormal glucose homeostasis (16). As triglycerides and HDL C levels are thought to be a consequence of hyperinsulinemia, their concentrations might be influenced by glycemic control in patients with diabetes (9). Furthermore, the results of our study showed that TG/HDL-C ratio and BMI were independently associated with HbA1c even after controlling for age, gender, diabetes duration and smoking. When DM2 patients were stratified according to BMI values independent association between TG/HDL-C ratio and HbA1c remained significant only in normal weight subjects, while no significant independent association between TG/HDL-C and glycemic control was found in overweight and obese subjects.
Our results are in concordance with the results of the study that included 143 patients with DM2 not taking lipid-lowering medications, which found that patients with HbA1c more than 6.5% had higher triglyceride and lower HDL-C that served as markers of poor glycemic control in mentioned study (17).
In the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial, the TG/HDL-C ratio was found to be a useful marker in individuals who achieved better glycemic control (18). Two clinical trials in patients with DM2 found that intensive glycemic control led to decreased levels of TC, LDL-C and particularly triglyceride, which could be explained by an insulin-induced enhancement of triglyceride clearance, whereas HDL-C remained unchanged (19, 20). Additionally, one study found that TG/HDL-C ratio is an effective screening tool to predict success with dose reductions of anti-diabetic medications in obese patients who successfully lose weight (21).
Previous studies have shown that TG/HDL-C ratio is independently related to insulin resistance, obesity and metabolic syndrome (22, 23). McLaughlin et al. (24) investigated the use of TG/HDL-C ratio in detecting insulin resistance in overweight individuals and found that a TG/HDL-C value above 3.0 mg/dL was a better marker of insulin resistance than triglycerides or insulin levels. In present study, average value of TG/HDL-C ratio was 3.40 mg/dL in HbA1c<7% group and 5.82 mg/dL in HbA1c ≥7% group suggesting that patients in both DM2 groups were also insulin resistant. Study that included apparently healthy individuals also showed that the fasting TG/HDL-C ratio was a better predictor of insulin resistance than triglycerides (25). Moreover, in our study TG/HDL-C ratio was significantly associated with cardiometabolic markers such as HDL-C levels, triglycerides, DBP and BMI. TyG index is a marker of insulin resistance (26) and has been correlated with the hyperinsulinaemic-euglycaemic clamp test (27) and with the homeostatic model assessment of insulin resistance (HOMA-IR) (28). Results of the present study have shown that TyG index was positively associated with BMI, DBP, fasting glucose, triglycerides, total cholesterol and LDL-C levels and negatively associated with HDL-C in patients with DM2.
In our study, TyG index and BMI were significantly independently associated with HbA1c even after controlling for age, gender, diabetes duration and smoking. When we stratified patients according to BMI values independent association between TyG index and HbA1c remained significant only in overweight and obese DM2 patients. Our results are in agreement with the results from a recent study that evaluated whether TyG index and TyG derived indices (TyG–WC and TyG-BMI) were associated with long the term glycemic control. The study found that TyG indices were significantly correlated with HbA1c and were significantly increased in the diabetics with poor glycemic control. In addition, the ROC curve analysis showed that TyG index had the largest AUC for identifying patients with poor glycemic control (29).
Although previous studies have showed that both TG/HDL-C and TyG index could be useful markers of insulin resistance and glycemic control, previous study by Er et al. (30) showed that TyG was more efficient than TG/HDL-C for identifying insulin resistance.
The contribution of our study suggests that the utility of these two markers in predicting glycemic control might be dependent on the overweight and obesity status of DM2 patients. TG/HDL-C in our study emerged as independent predictor of glycemic control in normal weight DM2 patients. Possible mechanism that could explain obtained findings is that hypertriglyceridemia might cause fatty acid accumulation in non-adipose tissues such as the liver, muscle, and heart, which results in ectopic lipid deposition with lipotoxicity implicated as a mechanism for insulin resistance. However, in overweight and obese patients with DM2, TyG index was independent predictor of poor glycemic control. In obese patients, adipose tissue becomes a major source of proinflamatory adipokines leading to beta cells dysfunction, insulin resistance, hyperglycemia and subsequent glucotoxicity.
Another contribution of our study is that both TG/HDL-C ratio and TyG index could be good surrogate markers of glycemic control, besides HbA1c. The rationale for use of these two parameters in the clinical setting is that both are routinely measured in the primary health care setting, are cost effective, and reflect different cardiometabolic abnormalities. HbA1c is relatively expensive and not available in most primary health care centers in undeveloped countries. Thus, an alternative test that is inexpensive and routinely available could have potential use for primary care physician in follow up of patients with confirmed DM2 and for screening of prediabetic patients with poor glycemic control.
The main limitation of the present study is its cross-sectional nature that does not allow us to draw any cause-effect relations between our results. Further, our study sample was relatively small. Finally, we did not measure HOMA-IR and this may represent a potential source of bias for the obtained findings.
6. CONCLUSION
TG/HDL-C ratio might be a useful predictor of glycemic control in normal weight, and TyG index in overweight and obese patients with DM2.
Author’s contribution:
N.B. and A.V. gave substantial contribution to the conception, design, drafting the work analysis and interpretation of data for the work. A.Z. gave a substantial contribution to analysis and interpretation of data for the work. S.Z. gave a substantial contribution in the acquisition of data. N.A. and S.H. gave a substantial contribution in revising manuscript critically for important intellectual content.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent forms.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
This cross-sectional study was conducted as a part of Project financed by Federal Ministry of Education and Science, Federation of B&H (05-39-3087-16-1/16).
REFERENCES
- 1.International Diabetes Federation. IDF Diabetes Atlas Seventh Edition. 2015. https://www.oedg.at/pdf/1606_IDF_Atlas_2015_UK.pdf.
- 2.American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes - 2018. Diabetes Care. 2018;41(1):55–64. doi: 10.2337/dc18-S006. [DOI] [PubMed] [Google Scholar]
- 3.Badedi M, Solan Y, Darraj H, Sabai A, Mahfouz M, Alamodi S, Alsabaani A. Factors Associated with Long-Term Control of Type 2 Diabetes Mellitus. J Diabetes Res. 2016;2016:2109542. doi: 10.1155/2016/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-Analysis: Glycosylated Hemoglobin and Cardiovascular Disease in Diabetes Mellitus. Ann Intern Med. 2004;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-000072109542. [DOI] [PubMed] [Google Scholar]
- 5.Patel MB, Sachora WM, Pandya AR, Kothari AD, Patel JK. Can HbA1c Act as a Surrogate Marker for Cardiovascular Risk? Natl J Community Med. 2014;5(1):29–32. [Google Scholar]
- 6.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clinical biochemistry. 2001;34(7):583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 7.Keles N, Aksu F, Aciksari G, Yilmaz Y, Demircioglu K, Kostek O, et al. Is triglyceride/HDL ratio a reliable screening test for assessment of atherosclerotic risk in patients with chronic inflammatory disease? North Clin Istanb. 2016;3(1):39–45. doi: 10.14744/nci.2016.52824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chávez AG, Mendía LES, Argueta SE. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cir Cir. 2011;79(2):126–131. [PubMed] [Google Scholar]
- 9.Quispe R, Martin SS, Jones SR. Triglycerides to high-density lipoprotein-cholesterol ratio, glycemic control and cardiovascular risk in obese patients with type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):150–156. doi: 10.1097/MED.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 10.Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, Ko SH, Ahn YB, Cha BY, Yoon KH, Cho JH. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren X, Chen Z, Zheng S, Han T, Li Y, Liu W, et al. Association between Triglyceride to HDL-C Ratio (TG/HDL-C) and Insulin Resistance in Chinese Patients with Newly Diagnosed Type 2 Diabetes Mellitus. PLoS One. 2016;11(4):e0154345. doi: 10.1371/journal.pone.0154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasouli M, Mokhtari H. Calculation of LDL-Cholesterol vs. Direct Homogenous Assay. J Clin Lab Anal. 2017;31(3) doi: 10.1002/jcla.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masson W, Siniawski D, Lobo M, Molinero G, Huerín M. Association between triglyceride/HDL cholesterol ratio and carotid atherosclerosis in postmenopausal middle-aged women. Endocrinología y Nutrición. 2016;63(7):327–332. doi: 10.1016/j.endonu.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Simental-Mendía LE, Rodriguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 15.Quispe R, Manalac RJ, Faridi KF, Blaha MJ, Toth PP, Kulkarni KR, et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis. 2015;242(1):243–250. doi: 10.1016/j.atherosclerosis.2015.06.057. [DOI] [PubMed] [Google Scholar]
- 16.Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8(4):237–245. doi: 10.1038/nrendo.2011.235. [DOI] [PubMed] [Google Scholar]
- 17.Laverdy OG, Hueb WA, Sprandel MC, Kalil-Filho R, Maranhão RC. Effects of glycemic control upon serum lipids and lipid transfers to HDL in patients with type 2 diabetes mellitus: novel findings in unesterified cholesterol status. Exp Clin Endocrinol Diabetes. 2015;123(4):232–239. doi: 10.1055/s-0034-1396863. [DOI] [PubMed] [Google Scholar]
- 18.Zonszein J, Lombardero M, Ismail-Beigi F, Palumbo P, Foucher S, Groenewoud Y, et al. Triglyceride high-density lipoprotein ratios predict glycemia-lowering in response to insulin sensitizing drugs in type 2 diabetes: a post hoc analysis of the BARI 2D. J Diabetes Res. 2015;2015(129891) doi: 10.1155/2015/129891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chait A, Bierman EL, Albers JJ. Low-density lipoprotein receptor activity in cultured human skin fibroblasts. Mechanism of insulin-induced stimulation. J Clin Invest. 1979;64(5):1309–1319. doi: 10.1172/JCI109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emanuele N, Azad N, Abraira C, Henderson W, Colwell J, Levin S, et al. Effect of intensive glycemic control on fibrinogen, lipids, and lipoproteins: Veterans Affairs Cooperative Study in Type II Diabetes Mellitus. Arch Intern Med. 1998;158(22):2485–2490. doi: 10.1001/archinte.158.22.2485. [DOI] [PubMed] [Google Scholar]
- 21.Palamaner Subash Shantha G, Kumar AA, Kahan S, Irukulla PK, Cheskin LJ. Triglyceride/HDL ratio as a screening tool for predicting success at reducing anti-diabetic medications following weight loss. PLoS One. 2013;8(7):e69285. doi: 10.1371/journal.pone.0069285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meigs JB, Williams K, Sullivan LM, Hunt KJ, Haffner SM, Stern MP, et al. Using metabolic syndrome traits for efficient detection of impaired glucose tolerance. Diabetes Care. 2004;27(6):1417–1426. doi: 10.2337/diacare.27.6.1417. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96(3):399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 26.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13(146) doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 28.Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60:1673–1677. doi: 10.1016/j.metabol.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Hameed EK. TyG index a promising biomarker for glycemic control in type 2 Diabetes Mellitus. Diabetes Metab Syndr. 2019;13(1):560–563. doi: 10.1016/j.dsx.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS One. 2016 Mar 1;11(3):e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]