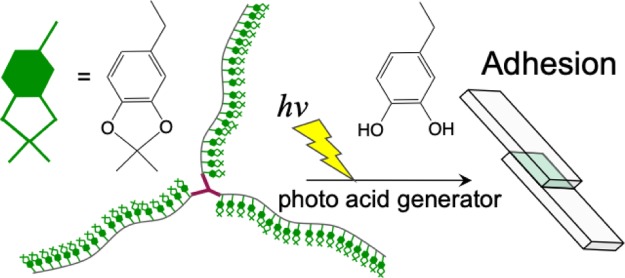
Abstract
Biomimetic material design is a useful method for producing new functional materials. In recent years, catecholic polymers inspired from the adhesion mechanism of marine organisms have attracted attention. Here, we demonstrated the preparation of catecholic polymers by reversible addition–fragmentation chain transfer (RAFT) polymerization of an acetonide-protected catecholic monomer, that is, N-(2-(2,2-dimethylbenzo-1,3-dioxol-5-yl)ethyl)-acrylamide (DDEA). By selecting the specific RAFT reagents, well-defined branched PDDEA and linear PDDEA were obtained. These PDDEA samples showed stronger adhesion strength after deprotection by acid stimulation compared with that before deprotection. In addition, we demonstrated the adhesion control of synthetic polymers by photoirradiation in the presence of photoacid generators, which decompose under light and release an acid.
Introduction
Nature produces a wide range of materials with various functions, and the creation of biomimetic materials that mimic these functions attracts attention.1−5 Adhesion is a key property in the design of biomimetic materials, and new materials are inspired from the adhesion mechanisms of various organisms.6−10 In the adhesion mechanism of mussels, the catechol group plays an important role,11,12 and various catechol-containing polymers have been reported.13 A number of catecholic polymers have been reported, including a main chain-type, a side chain-type, and an end chain-type. Polydopamine, which is an example of a main chain-type, is a mimetic polymer of the mussel’s adhesive protein,14 and many studies have progressed due to its ability to be coated on various substrates.15−18 We have also reported a design of a colorless polydopamine layer and the modification of various material surfaces.19 Using polydopamine-based coating techniques, a variety of functional materials were successfully prepared.20−22 However, adhesion control is usually difficult because polydopamine is obtained by in situ polymerization of dopamine on the surface of substrates.14 In contrast, catechol-functionalized synthetic polymers, such as side chain-types23−28 and end chain-types,29,30 can be used as adhesives after their synthesis. For example, the wet adhesion of polymers having catechol groups in their side chains was dramatically increased compared to that of noncatecholic polymers.23 It has also been reported that polyethers prepared with catechol groups at the end were useful as a surface modifier.29
The adhesion strength usually decreases when catechols are oxidized to produce quinone.31,32 Thus, catechol group-protected polymers have been of interest due to their potential use in active materials, because they can be used to control adhesive properties by appropriate deprotection. Catechol derivatives having various protective groups, including acetonide,27−29,33,34 acetyl,35,36 borate,37,38 silyl,39,40 carboxybenzyl,41 cyclic ethyl orthoformate,42 2-nitrobenzyl,43 and methyl ether groups,44 have been synthesized. Among them, acetonide-protecting groups are useful for forming functional polymers because they can be easily removed without affecting the polymer backbone under mild acidic conditions.
The shape of the polymer is also important for catecholic polymer applications.45 While many catecholic polymers have already been synthesized for use as surface modifiers or adhesives, the majority of them are linear polymers with a low density of catechol groups.45 Recently, compared to the conventional linear catecholic polymers, the dendritic,46 hyperbranched,47 and multi-armed48 catecholic polymers have been reported to provide superior properties related to surface modification, adhesion, and antifouling efficacy. It is necessary to further accelerate this work for engineering applications by controlling the shape and adhesion ability of the catecholic polymers.
Here, we prepared branched catecholic polymers by reversible addition–fragmentation chain transfer (RAFT) polymerization of an acetonide-protected monomer, that is, N-(2-(2,2-dimethylbenzo-1,3-dioxol-5-yl)ethyl)-acrylamide (DDEA). The main objective of this study is to examine the effect of well-defined branched catecholic polymers on adhesion and achieve adhesion control of the polymer by stimulation. By selecting the kind of RAFT reagents, both branched PDDEA and linear PDDEA with controlled chain lengths were obtained. The synthesized products are designated as branched PDDEA54, branched PDDEA19, and linear PDDEA25 (Figure 1). The adhesive strengths before and after deprotection with acids of the three kinds of polymers were compared. In addition to adhesion control by pH change, the use of photocontrollable materials is important for practical applications such as local adhesion.49,50 With the knowledge we gained, photoinduced adhesion control using catechol groups is attractive, but rarely studied. Takahara et al. reported the usefulness of photoirradiation for adhesion control of polymers, with the catechol protected by an ortho-nitrobenzyl group.43 It remains a challenge to develop an effective strategy to create a photocontrollable system for adhesion of catecholic polymers. Photoacid generators decompose by photoirradiation and release an acid.51 We also demonstrated adhesion control of designed polymers in the presence of photoacid generators.
Figure 1.
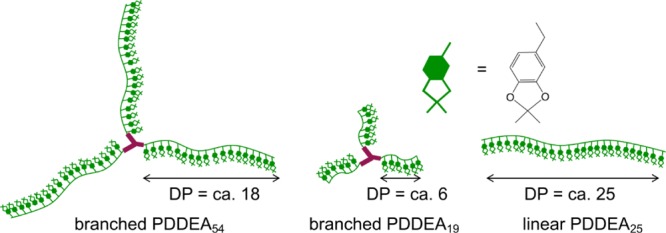
Schematic illustration of designed polymers.
Results and Discussion
Synthesis of Branched PDDEA
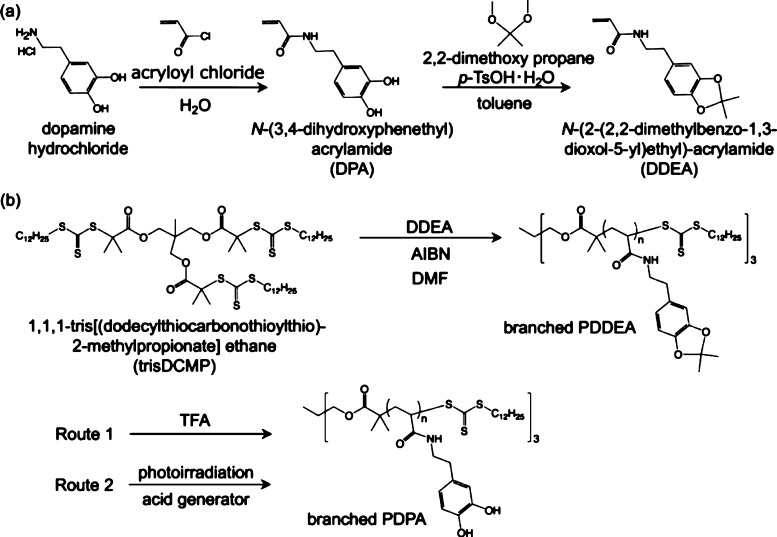
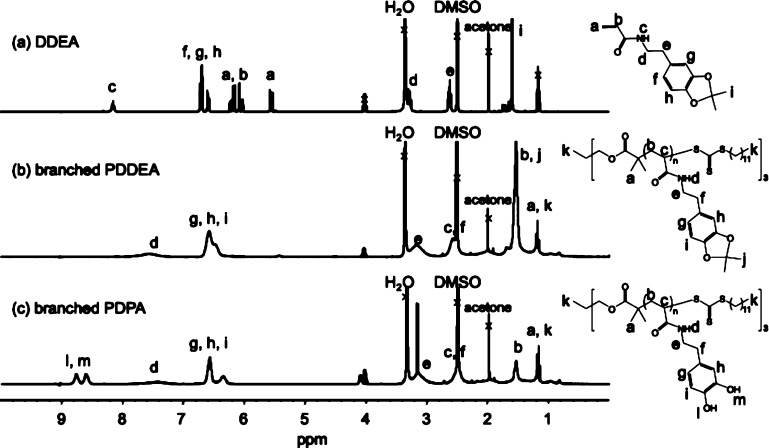
Figure 2 shows the synthetic route of a designed monomer and polymer. It is known that dopamine hydrochloride is polymerized by self-oxidative polymerization under basic conditions.14 To prevent the self-oxidative reaction, N-(3,4-dihydroxyphenethyl) acrylamide (DPA) was synthesized using dopamine hydrochloride and acryloyl chloride in the presence of boronic compounds. In this reaction, sodium tetraborate decahydrate (Na2B4O7·10H2O) was added to protect the catechol groups with the borate ion, forming a catechol–borate complex and preventing self-oxidative polymerization under basic conditions.52 Additionally, sodium carbonate (Na2CO3) dissolved in H2O was added during the reaction in order to maintain pH 9. When the synthesized DPA compound was purified by silica gel chromatography, the yield was very low. This phenomenon is probably due to the adsorption of catechol groups to silica gel. Thus, the reaction proceeded without purification to synthesize DDEA. The proton nuclear magnetic resonance (1H NMR) spectrum of DDEA purified by column chromatography is shown in Figure 3a. Protection of the catechol group by acetonide was confirmed by the acetonide methyl group peak found in the DDEA measurement.
Figure 2.
(a) Synthesis of acetal-protected catecholic monomer (DDEA). (b) Preparation of branched PDDEA by RAFT polymerization, and deprotection of acetal group by acid stimulation.
Figure 3.
1H NMR spectra of (a) DDEA, (b) branched PDDEA, and (c) branched PDPA in DMSO-d6.
RAFT polymerization of DDEA was carried out using 1,1,1-tris[(dodecylthiocarbonothioylthio)-2-methylpropionate]ethane (trisDCMP) having a three-branched structure as a RAFT reagent. Figure 3a,b shows the 1H NMR spectra of the DDEA monomer and the branched PDDEA after purification by dialysis. Progress of the polymerization was confirmed by the disappearance of the proton peak (a, b: δ = 5.6 and 6.2 ppm) of the DDEA monomer vinyl group. The conversion of RAFT polymerization, calculated from the proton peak (g–i: δ = 6.3–6.6 ppm) of the benzene ring of unpurified PDDEA and the peak derived from the DDEA monomer (a, b: δ = 6.2 ppm), was calculated to be ca. 97%. The degree of polymerization (n) and number average molecular weight (Mn,NMR) of the product, calculated by comparing the methyl group peak (a: δ = 0.8–1.4 ppm) and the benzene ring proton peak (g–i: δ = 6.3–6.6 ppm), were n = 54 and Mn,NMR = 14 500, respectively. Another branched PDDEA was similarly obtained under the condition of [DDEA]/[trisDCMP] = 25:1. The conversion, degree of polymerization, and Mn,NMR, calculated from NMR measurements, were 84%, 19, and 5800, respectively. To investigate the effect of branching of the polymer, linear PDDEA was also prepared by DPA polymerization using 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid as a RAFT reagent. Subsequent experiments were performed using three synthesized polymers, that is, branched PDDEA54, branched PDDEA19, and linear PDDEA25 (Figure 1).
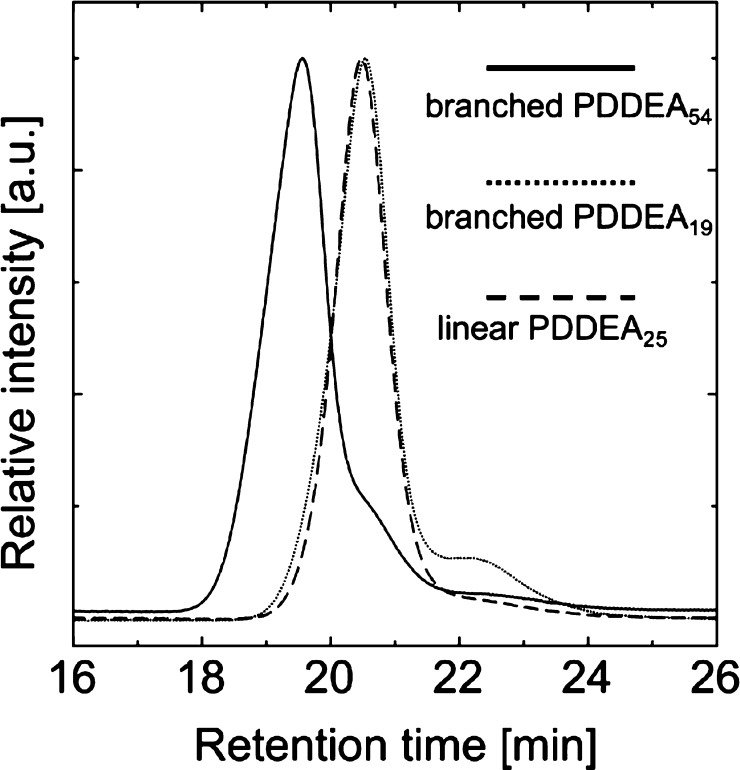
Figure 4 shows the gel permeation chromatography (GPC) charts of branched PDDEA54, branched PDDEA19, and linear PDDEA25. The GPC curve for the linear PDDEA is monomodal, whereas the curves for the branched PDDEAs exhibited small peaks at low molecular weight region besides main peaks. This phenomenon is probably due to insufficient reaction. Because each polymer was formed from three initiating groups, it was suggested that the polymer did not uniformly polymerize. The conversion, molecular weight, and molecular weight distribution are summarized in Table 1. The polydispersity Mw/Mn was calculated to be approximately 1.2–1.6. While a small amount of low molecular weight compound was present, it was shown that relatively monodisperse polymers were obtained. Two kinds of branched PDDEA having different molecular weights with relatively controlled molecular weight distributions were successfully synthesized.
Figure 4.
GPC charts of branched PDDEA54, branched PDDEA19, and linear PDDEA25.
Table 1. Conversion, Molecular Weight, and Molecular Weight Distribution of Synthetic Polymers.
| sample | Mn,theorya | conversion [%]b | Mn,NMRc | Mn,GPCd | Mw/Mnd |
|---|---|---|---|---|---|
| branched PDDEA54 | 19 000 | 97 | 14 500 | 17 000 | 1.6 |
| branched PDDEA19 | 6300 | 84 | 5800 | 5900 | 1.4 |
| linear PDDEA25 | 6400 | 98 | 6400 | 6720 | 1.2 |
Calculated by ([DDEA]/[RAFT reagent]) × molecular weight of DDEA × conversion + molecular weight of the RAFT reagent.
Determined by 1H NMR spectra before purification.
Determined by 1H NMR spectra after purification by dialysis.
Determined by GPC measurements using PMMA as a standard.
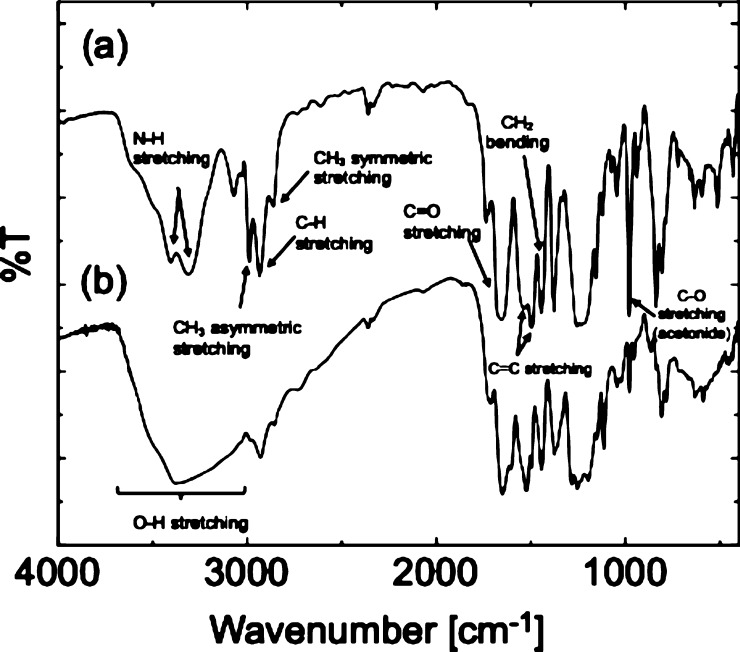
Acetal-protecting groups such as acetonide bridge-protecting groups are capable of protecting two hydroxy groups at the same time. Acetal-protecting groups are also resistant to basic conditions and high temperatures and can be deprotected by an acid. Deprotection of acetonide using trifluoroacetic acid (TFA) was carried out to investigate its influence on adhesion properties before and after deprotection of catechol groups. Figure 5 shows the infrared (IR) spectra of branched PDDEA54 before and after deprotection. The IR spectrum after deprotection showed a broad absorption band from 2500 to 3600 cm–1 as compared with that before deprotection. This is believed to be a peak due to hydroxy groups of the deprotected catechol group. Additionally, the strong absorption peak at around 1000 cm–1 is due to ether on the protected catechol moiety were decreased after deprotection of catechol moiety. From the 1H NMR spectra of branched PDDEA54 before and after deprotection, the appearance of catechol groups (l, m: δ = 8.5 and 8.8 ppm) and the disappearance of acetonide (j: δ = 1.4 ppm) were confirmed (Figure 3c). In addition, the division of the aromatic ring proton peaks (g–i: δ = 6.5 ppm) was observed, suggesting the deprotection of acetonide groups.
Figure 5.
FT-IR spectra of (a) branched PDDEA and (b) branched PDPA.
Adhesive Strength Measurement by Tensile Testing
The adhesion properties of unprotected PDDEA (PDPA) prepared by acid treatment just before use were investigated. The PDDEA or PDPA sample (5 mg) was dissolved in 1 mL of methanol and then dropped on a glass substrate at 60 °C to evaporate solvents. Another glass substrate was placed on top of them. The sample was fixed by clamps and allowed to stand for 24 h at room temperature to obtain a measurement sample. The sample prepared was set on a tensile tester, and the shear adhesion strength was measured (Figure 6a).
Figure 6.
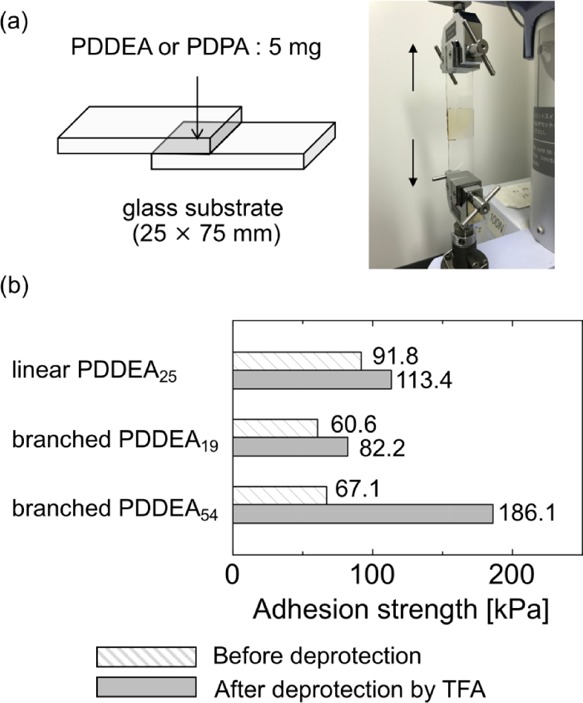
(a) Schematic illustration of the setup for measuring adhesion strength. (b) Maximum value of adhesion strength of glass plates adhered by PDDEA samples before and after deprotection of acetonide groups. The polymer samples were sandwiched between two glass substrates, and then the adhesive strength was measured. Because adhesive area (m2) was slightly different for each sample, the adhesive strengths were converted Pa (N m–2).
First, an adhesion test using linear PDDEA25 was conducted. As shown in Figure 6b, the adhesive strength increased from 91.8 to 113.4 kPa after deprotection. Next, the adhesion properties of branched PDDEA19, which has a similar molecular weight as linear PDDEA25, were examined. While the glass plate adhered by the branched PDDEA19 before deprotection was broken at 60.6 kPa, the maximum tensile stress after deprotection increased to 82.2 kPa. We believe that the adhesion of branched PDDEA19 was lower than that of linear PDDEA25 because of the effect of the length of the branch chain. Assuming that the lengths of branched polymers are equal, the degree of polymerization of the side chain of branched PDDEA19 is about 6. This caused lower adhesive ability. On the other hand, the maximum tensile stress after deprotection of branched PDDEA54 with a large molecular weight reached 186.1 kPa, showing stronger adhesion compared with branched PDDEA19. Additionally, the difference between the maximum tensile stress values before and after deprotection was also large. In general, the molecular weight of the polymer influences its properties, such as the glass transition temperature, melting temperature, viscoelasticity, and viscosity.53 The adhesion strength increased with increasing molecular weight of PDDEA due to the entanglement of polymer chains and the amount of catechol groups introduced at adhesion sites. While the branched chain length of branched PDDEA54 was approximately 18, it showed higher adhesive strength than linear PDDEA25. While the increase in adhesion strength will be mainly due to the total increase in molecular weight, it seems to be the multiple binding occurred due to the branched structure. Compared with the adhesive strength before deprotection, all samples showed stronger adhesion strength after deprotection, suggesting the usability of deprotection of catechol groups. Although the adhesion strength was effectively controlled by acid stimulation, it remains unclear whether the effect of branched structures on adhesion. Detailed study of branched polymers might be necessary. Further experiments are in progress to control the branched structures and molecular weights by changing the polymerization conditions. After adhesion measurements, broken polymers remained on the failed area of each glass plate surface, indicating that cohesive failure took place within the polymers.
Finally, control of the adhesive strength by the photoacid generator was investigated. Diphenyl-2,4,6-trimethylphenylsulfonium p-toluenesulfonate, which produces p-toluenesulfonic acid by photoirradiation, was used as an acid generator (Figure 7a). Branched PDDEA62 (100 mg) was dissolved in 1 mL acetone in the presence of an acid generator (4.7 mg), sandwiched between two glass plates. After irradiated with light (14 mW cm−2, 254 nm) for 30 min, samples were left to stand for 24 h. Figure 7b shows the adhesion strength of the samples. The glass plates adhered by branched PDDEA62 without photoirradiation broke at an adhesion strength of 141.0 kPa. In contrast, the adhesion strength of branched PDDEA62 with photoirradiation increased to 194.4 kPa, indicating that adhesion was improved by exposure of catechol groups. Results show that the photo-activated adhesive process was achieved with combined use of the acetonide-protecting group and photoacid generators.
Figure 7.
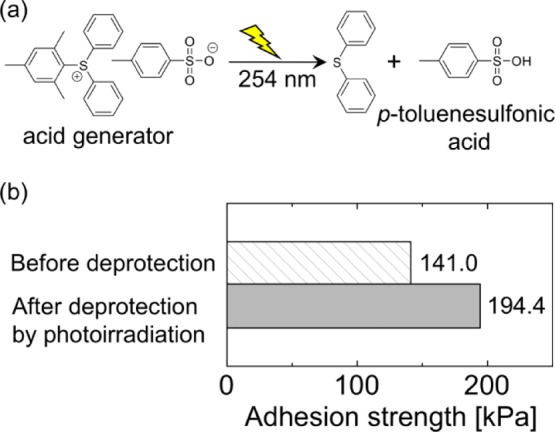
(a) Photochemical generation of acid by a photoacid generator. (b) Adhesion strength of glass plates adhered by branched PDDEA62 with or without photoirradiation.
Conclusions
In conclusion, we demonstrated the preparation of well-defined branched PDDEA and linear-PDDEA by RAFT polymerization of DDEA, which is an acetonide-protected catecholic monomer. Both branched PDDEA and linear PDDEA showed stronger adhesion after deprotection of acetonide groups by acid stimulation compared with that before deprotection. The adhesion strength increased with increasing PDDEA molecular weight, and branched polymers showed higher adhesive strength than linear polymers due to binding at multiple points of the branched structure. Additionally, the adhesion of acetonide-protected catecholic polymers was controlled by photoirradiation in the presence of photoacid generators. Because adhesion control by light irradiation enables local adhesion, our strategy, which includes control of the shape and adhesion ability of the catecholic polymers, will be useful for practical applications.
Experimental Section
Materials
Dopamine hydrochloride, sodium tetraborate decahydrate (Na2B4O7·10H2O), sodium carbonate (Na2CO3), trisDCMP, and 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid were purchased from Sigma-Aldrich Co. LLC. Hydrochloric acid (HCl), 2,2-dimethoxypropane, ethyl acetate, sodium sulfate (Na2SO4), hexane, TFA, and ethyl acetate were purchased from Kanto Chemical Co. Inc. Acryloyl chloride, 2,2′-azobis(isobutyronitrile) (AIBN), N,N-dimethylformamide (DMF), tetrahydrofuran (THF), and p-toluenesulfonic acid monohydrate (p-TsOH·H2O) were obtained from Tokyo Chemical Industry Co., Ltd. Diphenyl-2,4,6-trimethylphenylsulfonium p-toluenesulfonate was obtained from Wako Pure Chemical Co. All of the chemicals and solvents were of reagent grade and were used as received.
Measurements
1H NMR spectra were recorded on a Bruker DPX-300 MHz Fourier transform NMR spectrometer. The Fourier transform IR (FT-IR) spectra were recorded using a JASCO FT-IR-420. GPC was conducted (Tosoh TSKgel α3000) with THF as the mobile phase at a flow rate of 0.3 mL/min at 40 °C. Polymethyl methacrylate was used as a standard. Glass substrates were treated with oxygen plasma (SEDE-GE, Meiwafosis). The adhesion strength was determined by measuring the shear adhesion force with a tensile tester (Shimadzu EZ-S) at 298 K in an ambient atmosphere. The crosshead speed was set to 1 mm min–1 in tensile mode. The shear strength was defined as the stress corresponding to the failure force divided by an adhesion area.
Synthesis of DPA
Dopamine hydrochloride (3.00 g, 15.8 mmol) was added to a stirred solution of Na2B4O7·10H2O (12.1 g, 31.6 mmol) and Na2CO3 (5.0 g, 47 mmol) in H2O (450 mL) under an argon atmosphere. Acryloyl chloride was added to the resulting mixture (5.1 mL, 63.2 mmol) in a dropwise manner at 0 °C; then, the mixture was stirred at room temperature under an argon atmosphere. After 12 h, Na2CO3 (5.0 g, 47 mmol) in H2O (50 mL) was added. The pH of the sample was adjusted to approximately 1 with 4 M HCl aq and stirred under an open system. After stirring for 1 h at room temperature, the reaction mixture was washed with ethyl acetate and 0.1 M HCl aq. The solvent was removed with an evaporator to obtain a brown viscous substance (2.78 g, yield: 86%). 1H NMR (300 MHz, DMSO-d6, δ(ppm)): 7.9 (t, 1H, −NHC=O), 6.4–6.6 (m, 3H, Ph), 5.6 (s, 1H, CH2=C–CH3), 5.3 (s, 1H, CH2=C–CH3), 3.2 (q, 2H, −CH2–CH2–NH−), 2.5 (t, 2H, −CH2–CH2–NH−), 1.8 (s, 3H, CH2=C–CH3).
Synthesis of DDEA
A solution of DPA (2.78 g, 13.4 mmol) and p-TsOH·H2O (0.12 g, 0.65 mmol) in toluene (150 mL) was refluxed for 2 h under argon atmosphere. 2,2-Dimethoxypropane was added to the mixture (15.2 mL, 124 mmol) and stirred at 40 °C for 2 h and then refluxed for 2 h. After removing the solvent by evaporation, ethyl acetate was added and the mixture was washed with H2O. The organic layer was dried with Na2SO4 and filtered, and the filtrate was concentrated with an evaporator. Column chromatography was carried out using hexane/ethyl acetate = 1:1 as a developing solvent. The solvent was removed to obtain a white solid (1.75 g, yield: 54%). 1H NMR (300 MHz, DMSO-d6, δ(ppm)): 8.2 (t, 1H, −NH–C=O), 6.5–6.7 (m, 3H, Ph), 6.2 (dd, 1H, CH2=CH−), 6.0 (dd, 1H, CH2=CH−), 5.6 (dd, 1H, CH2=CH−), 3.3 (q, 2H, −CH2–CH2–NH−), 2.6 (t, 2H, −CH2–CH2–NH−), 1.6 (s, 6H, (CH3)2–C−).
Synthesis of Branched PDDEA by RAFT Polymerization
A typical RAFT polymerization of DDEA, with a molar ratio of 75:1:1.5 ([DDEA]/[trisDCMP]/[AIBN]), was performed as follows: DDEA (1.00 g, 4.04 mmol), trisDCMP (0.063 g, 0.054 mmol), AIBN (0.013 g, 0.081 mmol), and DMF (2.0 mL) were placed in a round-bottom flask. The mixture was deoxygenated by purging with argon for 15 min, with subsequent placement in a water bath at 70 °C. After 6 h, the polymerization was stopped by purging with oxygen at 0 °C. The reaction solution was purified by dialysis using methanol/ethyl acetate at a 1:1 ratio. The solvent was removed with an evaporator to obtain a pale yellow solid.
Deprotection of Acetonide Groups by Addition of Acid
After adding 100 mg of PDDEA samples to a 1 mL sample bottle, 0.5 mL of TFA was added and stirred overnight.
Deprotection of Acetonide Groups by Photoirradiation in the Presence of Photoacid Generators
A solution of the branched PDDEA (100 mg) and acetone (1 mL) in the presence of photoacid generator, diphenyl-2,4,6-trimethylphenylsulfonium p-toluenesulfonate (4.7 mg), was irradiated at 254 nm (14 mW cm−2) for 30 min.
Measurement of Adhesive Strength by Tensile Shear Strength Test
The PDDEA samples (5 mg: before or after deprotection of acetonide groups) was dissolved in 1 mL of methanol, and the solution was dropped on an oxygen plasma-treated glass substrate and heated on a hot plate at 60 °C. Another glass substrate was sandwiched between superimposed clips from above and then allowed to stand and dry on a hot plate at 60 °C. The adhesive strength was measured by the tensile test with a speed of 1 mm min–1.
Acknowledgments
M.K. acknowledges the support of JSPS KAKENHI (grant number: 17H03110) and the Toyo Gosei Memorial Foundation.
The authors declare no competing financial interest.
References
- Tadepalli S.; Slocik J. M.; Gupta M. K.; Naik R. R.; Singamaneni S. Bio-Optics and Bio-Inspired Optical Materials. Chem. Rev. 2017, 117, 12705–12763. 10.1021/acs.chemrev.7b00153. [DOI] [PubMed] [Google Scholar]
- Huang G.; Li F.; Zhao X.; Ma Y.; Li Y.; Lin M.; Jin G.; Lu T. J.; Genin G. M.; Xu F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.; Liang W.; Guo Z.; Liu W. Biomimetic Super-Lyophobic and Super-Lyophilic Materials Applied for Oil/Water Separation: A New Strategy beyond Nature. Chem. Soc. Rev. 2015, 44, 336–361. 10.1039/c4cs00220b. [DOI] [PubMed] [Google Scholar]
- Kohri M.; Nannichi Y.; Taniguchi T.; Kishikawa K. Biomimetic Non-Iridescent Structural Color Materials from Polydopamine Black Particles that Mimic Melanin Granules. J. Mater. Chem. C 2015, 3, 720–724. 10.1039/c4tc02383h. [DOI] [Google Scholar]
- Su B.; Tian Y.; Jiang L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. 10.1021/jacs.5b12728. [DOI] [PubMed] [Google Scholar]
- Brubaker C. E.; Messersmith P. B. The Present and Future of Biologically Inspired Adhesive Interfaces and Materials. Langmuir 2012, 28, 2200–2205. 10.1021/la300044v. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Pesika N.; Zeng H.; Rosenberg K.; Zhao B.; McGuiggan P.; Autumn K.; Israelachvili J. Adhesion and Friction in Gecko Toe Attachment and Detachment. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 19320–19325. 10.1073/pnas.0608841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.; Jeong Y.; Park J. M.; Lee K. H.; Hong J. W.; Choi J. Biomimetics: Forecasting the Future of Science, Engineering, and Medicine. Int. J. Nanomed. 2015, 10, 5701–5713. 10.2147/IJN.S83642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Celiz A. D.; Yang J.; Yang Q.; Wamala I.; Whyte W.; Seo B. R.; Vasilyev N. V.; Vlassak J. J.; Suo Z.; Mooney D. J. Tough Adhesives for Diverse Wet Surfaces. Science 2017, 357, 378–381. 10.1126/science.aah6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S.; Sawada S.; Nakayama S.; Kappl M.; Ueno K.; Shitajima K.; Butt H.-J.; Nakamura Y. Pressure-Sensitive Adhesive Powder. Mater. Horiz. 2016, 3, 47–52. 10.1039/c5mh00203f. [DOI] [Google Scholar]
- Waite J. H.; Andersen N. H.; Jewhurst S.; Sun C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. 10.1080/00218460590944602. [DOI] [Google Scholar]
- Silverman H. G.; Roberto F. F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. 10.1007/s10126-007-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. P.; Messersmith P. B.; Israelachvili J. N.; Waite J. H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Dellatore S. M.; Miller W. M.; Messersmith P. B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynge M. E.; van der Westen R.; Postma A.; Städler B. Polydopamine - A Nature-Inspired Polymer Coating for Biomedical Science. Nanoscale 2011, 3, 4916–4928. 10.1039/c1nr10969c. [DOI] [PubMed] [Google Scholar]
- Kang S. M.; Hwang N. S.; Yeom J.; Park S. Y.; Messersmith P. B.; Choi I. S.; Langer R.; Anderson D. G.; Lee H. One-Step Multipurpose Surface Functionalization by Adhesive Catecholamine. Adv. Funct. Mater. 2012, 22, 2949–2955. 10.1002/adfm.201200177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L.; Liu Q.; Li G.; Shi J.; Liu J.; Wang T.; Jiang G. A Mussel-Inspired Polydopamine Coating as a Versatile Platform for the in situ Synthesis of Graphene-Based Nanocomposites. Nanoscale 2012, 4, 5864–5867. 10.1039/c2nr31547e. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ai K.; Lu L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- Kohri M.; Kohma H.; Shinoda Y.; Yamauchi M.; Yagai S.; Kojima T.; Taniguchi T.; Kishikawa K. A Colorless Functional Polydopamine Thin Layer as a Basis for Polymer Capsules. Polym. Chem. 2013, 4, 2696–2702. 10.1039/c3py00181d. [DOI] [Google Scholar]
- Kohri M.; Shinoda Y.; Kohma H.; Nannichi Y.; Yamauchi M.; Yagai S.; Kojima T.; Taniguchi T.; Kishikawa K. Facile Synthesis of Free-Standing Polymer Brush Films Based on a Colorless Polydopamine Thin Layer. Macromol. Rapid Commun. 2013, 34, 1220–1224. 10.1002/marc.201300395. [DOI] [PubMed] [Google Scholar]
- Kawamura A.; Kohri M.; Morimoto G.; Nannichi Y.; Taniguchi T.; Kishikawa K. Full-Color Biomimetic Photonic Materials with Iridescent and Non-Iridescent Structural Colors. Sci. Rep. 2016, 6, 33984. 10.1038/srep33984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura A.; Kohri M.; Yoshioka S.; Taniguchi T.; Kishikawa K. Structural Color Tuning: Mixing Melanin-Like Particles with Different Diameters to Create Neutral Colors. Langmuir 2017, 33, 3824–3830. 10.1021/acs.langmuir.7b00707. [DOI] [PubMed] [Google Scholar]
- Clancy S. K.; Sodano A.; Cunningham D. J.; Huang S. S.; Zalicki P. J.; Shin S.; Ahn B. K. Marine Bioinspired Underwater Contact Adhesion. Biomacromolecules 2016, 17, 1869–1874. 10.1021/acs.biomac.6b00300. [DOI] [PubMed] [Google Scholar]
- Yamamoto S.; Uchiyama S.; Miyashita T.; Mitsuishi M. Multimodal Underwater Adsorption of Oxide Nanoparticles on Catechol-Based Polymer Nanosheets. Nanoscale 2016, 8, 5912–5919. 10.1039/c5nr08739b. [DOI] [PubMed] [Google Scholar]
- Narkar A. R.; Barker B.; Clisch M.; Jiang J.; Lee B. P. pH Responsive and Oxidation Resistant Wet Adhesive based on Reversible Catechol-Boronate Complexation. Chem. Mater. 2016, 28, 5432–5439. 10.1021/acs.chemmater.6b01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.; Glass P.; Pothen J. M.; Sitti M.; Washburn N. R. Enhanced Adhesion of Dopamine Methacrylamide Elastomers via Viscoelasticity Tuning. Biomacromolecules 2011, 12, 342–347. 10.1021/bm101076e. [DOI] [PubMed] [Google Scholar]
- Niederer K.; Schüll C.; Leibig D.; Johann T.; Frey H. Catechol Acetonide Glycidyl Ether (CAGE): A Functional Epoxide Monomer for Linear and Hyperbranched Multi-Catechol Functional Polyether Architectures. Macromolecules 2016, 49, 1655–1665. 10.1021/acs.macromol.5b02441. [DOI] [Google Scholar]
- Leibig D.; Müller A. H. E.; Frey H. Anionic Polymerization of Vinylcatechol Derivatives: Reversal of the Monomer Gradient Directed by the Position of the Catechol Moiety in the Copolymerization with Styrene. Macromolecules 2016, 49, 4792–4801. 10.1021/acs.macromol.6b00831. [DOI] [Google Scholar]
- Wilms V. S.; Bauer H.; Tonhauser C.; Schilmann A.-M.; Müller M.-C.; Tremel W.; Frey H. Catechol-Initiated Polyethers: Multifunctional Hydrophilic Ligands for PEGylation and Functionalization of Metal Oxide Nanoparticles. Biomacromolecules 2012, 14, 193–199. 10.1021/bm3015889. [DOI] [PubMed] [Google Scholar]
- Holten-Andersen N.; Harrington M. J.; Birkedal H.; Lee B. P.; Messersmith P. B.; Lee K. Y. C.; Waite J. H. pH-Induced Metal-Ligand Cross-Links Inspired by Mussel Yield Self-Healing Polymer Networks with Near-Covalent Elastic Moduli. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 2651–2655. 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Scherer N. F.; Messersmith P. B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 12999–13003. 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. K.; Lee D. W.; Israelachvili J. N.; Waite J. H. Surface-Initiated Self-Healing of Polymers in Aqueous Media. Nat. Mater. 2014, 13, 867–872. 10.1038/nmat4037. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Hu B.-H.; Messersmith P. B. Convenient Synthesis of Acetonide Protected 3,4-Dihydroxyphenylalanine (DOPA) for Fmoc Solid-Phase Peptide Synthesis. Tetrahedron Lett. 2008, 49, 5519–5521. 10.1016/j.tetlet.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black K. C. L.; Liu Z.; Messersmith P. B. Catechol Redox Induced Formation of Metal Core-Polymer Shell Nanoparticles. Chem. Mater. 2011, 23, 1130–1135. 10.1021/cm1024487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller W. D.; Verlander M. S.; Goodman M. DOPA-Containing Polypeptides. I. Improved Synthesis of High-Molecular-Weight Poly(L-DOPA) and Water-Soluble Copolypeptides. Biopolymers 1978, 17, 2939–2943. 10.1002/bip.1978.360171214. [DOI] [Google Scholar]
- Lee B. P.; Chao C.-Y.; Nunalee F. N.; Motan E.; Shull K. R.; Messersmith P. B. Rapid Gel Formation and Adhesion in Photocurable and Biodegradable Block Copolymers with High DOPA Content. Macromolecules 2006, 39, 1740–1748. 10.1021/ma0518959. [DOI] [Google Scholar]
- Lee H.; Lee B. P.; Messersmith P. B. A Reversible Wet/Dry Adhesive Inspired by Mussels and Geckos. Nature 2007, 448, 338–341. 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- Fan X.; Lin L.; Dalsin J. L.; Messersmith P. B. Biomimetic Anchor for Surface-Initiated Polymerization from Metal Substrates. J. Am. Chem. Soc. 2005, 127, 15843–15847. 10.1021/ja0532638. [DOI] [PubMed] [Google Scholar]
- Lee B. P.; Huang K.; Nunalee F. N.; Shull K. R.; Messersmith P. B. Synthesis of 3,4-Dihydroxyphenylalanine (DOPA) Containing Monomers and Their Co-Polymerization with PEG-Diacrylate to Form Hydrogels. J. Biomater. Sci., Polym. Ed. 2004, 15, 449–464. 10.1163/156856204323005307. [DOI] [PubMed] [Google Scholar]
- Heo J.; Kang T.; Jang S. G.; Hwang D. S.; Spruell J. M.; Killops K. L.; Waite J. H.; Hawker C. J. Improved Performance of Protected Catecholic Polysiloxanes for Bioinspired Wet Adhesion to Surface Oxides. J. Am. Chem. Soc. 2012, 134, 20139–20145. 10.1021/ja309044z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Deming T. J. Synthetic Polypeptide Mimics of Marine Adhesives. Macromolecules 1998, 31, 4739–4745. 10.1021/ma980268z. [DOI] [PubMed] [Google Scholar]
- Hu B.-H.; Messersmith P. B. Protection of 3,4-Dihydroxyphenylalanine (DOPA) for Fmoc Solid-Phase Peptide Synthesis. Tetrahedron Lett. 2000, 41, 5795–5798. 10.1016/s0040-4039(00)00957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida J.; Kobayashi M.; Takahara A. Light-Triggered Adhesion of Water-Soluble Polymers with a Caged Catechol Group. ACS Macro Lett. 2013, 2, 112–115. 10.1021/mz300524q. [DOI] [PubMed] [Google Scholar]
- Westwood G.; Horton T. N.; Wilker J. J. Simplified Polymer Mimics of Cross-Linking Adhesive Proteins. Macromolecules 2007, 40, 3960–3964. 10.1021/ma0703002. [DOI] [Google Scholar]
- Faure E.; Falentin-Daudré C.; Jérôme C.; Lyskawa J.; Fournier D.; Woisel P.; Detrembleur C. Catechols as Versatile Platforms in Polymer Chemistry. Prog. Polym. Sci. 2013, 38, 236–270. 10.1016/j.progpolymsci.2012.06.004. [DOI] [Google Scholar]
- Wei Q.; Achazi K.; Liebe H.; Schulz A.; Noeske P.-L. M.; Grunwald I.; Haag R. Mussel-Inspired Dendritic Polymers as Universal Multifunctional Coatings. Angew. Chem., Int. Ed. 2014, 53, 11650–11655. 10.1002/anie.201407113. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zhao T.; Newland B.; Liu W.; Wang W.; Wang W. Catechol Functionalized Hyperbranched Polymers as Biomedical Materials. Prog. Polym. Sci. 2018, 78, 47–55. 10.1016/j.progpolymsci.2017.09.002. [DOI] [Google Scholar]
- Mizrahi B.; Khoo X.; Chiang H. H.; Sher K. J.; Feldman R. G.; Lee J.-J.; Irusta S.; Kohane D. S. Long-Lasting Antifouling Coating from Multi-Armed Polymer. Langmuir 2013, 29, 10087–10094. 10.1021/la4014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal B. T.; Spencer E. J.; Rowan S. J. Stimuli-Responsive Reversible Two-Level Adhesion from a Structurally Dynamic Shape-Memory Polymer. ACS Appl. Mater. Interfaces 2016, 8, 11041–11049. 10.1021/acsami.6b01251. [DOI] [PubMed] [Google Scholar]
- Kizilkan E.; Strueben J.; Staubitz A.; Gorb S. N. Bioinspired Photocontrollable Microstructured Transport Device. Sci. Robot. 2017, 2, eaak9454. 10.1126/scirobotics.aak9454. [DOI] [PubMed] [Google Scholar]
- Martin C. J.; Rapenne G.; Nakashima T.; Kawai T. Recent Progress in Development of Photoacid Generators. J. Photochem. Photobiol., C 2018, 34, 41–51. 10.1016/j.jphotochemrev.2018.01.003. [DOI] [Google Scholar]
- Patil N.; Falentin-Daudré C.; Jérôme C.; Detrembleur C. Mussel-Inspired Protein-Repelling Ambivalent Block Copolymers: Controlled Synthesis and Characterization. Polym. Chem. 2015, 6, 2919–2933. 10.1039/c5py00127g. [DOI] [Google Scholar]
- Tadlaoui K.; Pietrasanta Y.; Michel A.; Verney V. Influence of Molecular Weight on the Glass Transition Temperature and the Melt Rheological Behavior of Methyl Methacrylate Telomers. Polymer 1991, 32, 2234–2237. 10.1016/0032-3861(91)90052-k. [DOI] [Google Scholar]