Abstract
In this work, nanoporous carbon (NPC) was synthesized by direct carbonization of MOF-5 (a famous metal–organic framework). The structure and morphology of the prepared MOF-derived nanoporous carbon (MOF-NPC) were investigated by X-ray diffraction, N2 adsorption/desorption isotherm, Raman spectroscopy, thermogravimetric analysis, and scanning electron microscopy methods. The MOF-NPC was then used to adsorb copper ions from aqueous solutions. To evaluate the performance of the prepared MOF-NPC to remove copper ions, both adsorption kinetics and adsorption equilibrium experiments were carried out and then the obtained data were modeled with various models. Also, the efficacy of temperature and the pH of the solution on the removal efficiency were checked. The results show that the prepared MOF-NPC is a superadsorbent for the removal of copper ions from aqueous solutions. Finally, the removal percentage of copper ions by the prepared MOF-NPC was compared with other activated carbon adsorbents to show its incredible efficiency.
1. Introduction
In recent years, the soil and water pollution by different contaminants is a serious threat to the environment. Among these pollutants, there are heavy metal ions, such as copper ions, which are released from various industries including mining, electroplating, extracting, and pigments.1,2 Heavy-metals ions are not biodegradable. Copper ions is an indispensable element for the health of the human body; however, it is toxic at high uptakes and may cause serious problems to humans, such as memory loss, kidney and liver damage, schizophrenia, and hypertension.3
Up to now, different methods have been allocated to remove copper ions from aqueous solutions, including membrane processes, electrodeposition, reverse osmosis, ion exchange, nanofiltration, and adsorption.4−6 Among the above-mentioned methods, adsorption is preferred due to its high efficiency, cost-effectiveness, and convenience.7 So far, a great number of adsorbents have been reported to adsorb copper ions, such as Ag-doped ZnO,8 microstructured zinc oxides,9 γ-alumina,10 graphene oxide–chitosan aerogel,11 natural manganese dioxide,12 metal–organic framework (MOF)-derived nanoporous carbon (NPC),13 and nanoporous MOF-5.14
New materials, such as nanoporous carbon, with features like high pore volume and specific surface area, good mechanical/thermal stability, and fast kinetics, exhibit a very high potential for use as catalyst supports, electrode, and gas storage and adsorbent materials.15−19 Recently, metal–organic frameworks (MOFs), which are ordered crystalline structure, have been used as a precursor to fabrication of nanoporous carbon.13,20−29 In our recent work,14 we have synthesized MOF-5, which shows excellent efficiency, and used it as an absorbent for copper ions.
In this research, we selected MOF-5 as a precursor. Then, it was directly carbonized to fabricate a nanoporous carbon (MOF-NPC) and employed to adsorb the copper ions from aqueous solutions. The adsorption of Cu ions was investigated by MOF-NPC under different conditions.
2. Results and Discussion
2.1. Characterization
Morphology of the obtained MOF-5 and MOF-NPC were illuminated by scanning electron microscopy (SEM) images at different magnifications (Figure 1a–d). These images indicate that the morphology of MOF-NPC with cubic texture is similar to the parent MOF-5 structure.30 Thus, the carbonization process does not change the morphology of the precursor at 530 °C.
Figure 1.
Scanning electron microscopy (SEM) images of (a, b) MOF-5 and (c, d) MOF-NPC with different magnifications.
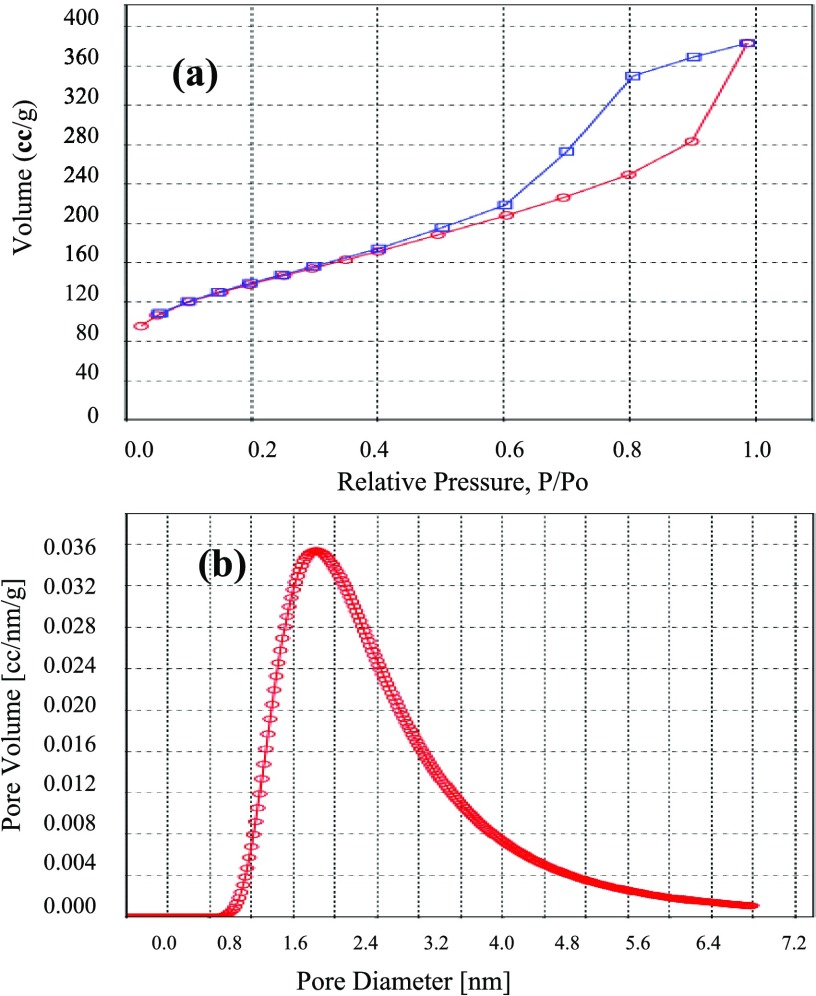
To determine the structural properties of MOF-NPC, N2 adsorption–desorption experiment was carried out, and the results are shown in Figure 2a,b. The MOF-NPC displays a type IV isotherm with a hysteresis loop, which demonstrates the presence of mesopores. The obtained hysteresis loop for MOF-NPC is similar to the simulated hysteresis loop (isotherm 7 in ref (31)), which corresponds to a porous solid with the following structural parameters: very low ratio of macropore surface area to the total surface area and also medium ratio of micropore volume to total pore volume. The structural properties of the MOF-NPC are listed in Table 1.
Figure 2.
(a) Isotherm of N2 adsorption/desorption for the MOF-NPC. (b) Distribution graph of the MOF-NPC pore size.
Table 1. Porosity Characteristics of the Prepared MOF-NPC.
| sample | BET surface area (m2/g) | pore volume (cm3/g) | average pore size (nm) |
|---|---|---|---|
| MOF-NPC | 473 | 0.593 | 1.82 |
The X-ray diffraction (XRD) schema of the MOF-NPC is shown in Figure S1. A board peak centered at 2θ = 21° approves the formation of graphitic carbon structures.
The structure of nanoporous carbon derived from MOF-5 was investigated using Raman spectroscopy. In Figure S2, the spectra shows two main peaks related to carbon; the D band at 1375 cm–1 and G band at 1581 cm–1 correspond to the structural imperfections and tangential vibrations, respectively. The relative ratio of the “D” to “G” peak intensity (ID/IG) is commensurate to the number of imperfections in nanoporous carbon; because this ratio is <1, it is clear that the graphitic structure is dominant in the prepared adsorbent.
Thermogravimetric analysis (TGA) for the investigation of decomposition behavior of MOF-5 was performed under argon atmosphere with a heating rate of 10 °C/min from ambient temperature to 600 °C. The observed TGA curve of MOF-5 (Figure S3) is similar to the previously reported curves.32 As the figure shows, the main mass loss due to carbonization occurs between 420 and 510 °C.
2.2. Adsorption Kinetics
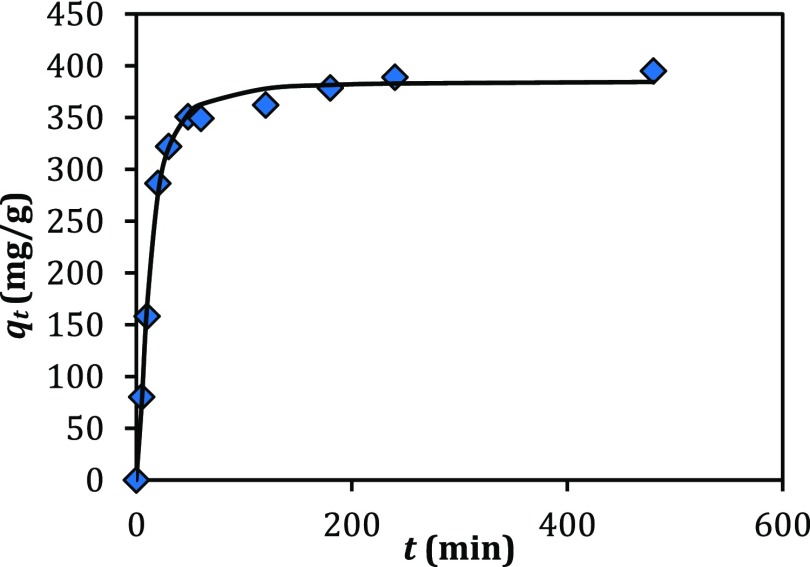
Generally, the kinetics investigations can reveal important information about the adsorption process. So, the performance of the adsorbent for the elimination of copper ions was determined by the kinetics studies of adsorption. Figure 3 shows that the adsorption of copper ions is a function of time. In Figure 3, it is clear that the uptake of copper ions into the MOF-NPC is sharp in the early moments and reaches an equilibrium after few minutes. The reason for these observations is that the number of available sites for absorption in the initial stages is high.
Figure 3.
Adsorption of copper ions (300 ppm) by MOF-NPC as a function of contact time.
To model the adsorption kinetics data, different kinetic models (Table 2) have been used. Table 3 demonstrates the results of modeling with various models. According to the values obtained for r2 (correlation coefficient) and root-mean-square (RMS) error, it is obvious that the pseudo-n-order (PnO) model represents the best fitting to the experimental kinetics data. The fitting of this model to the adsorption kinetic data reveals that the surface of the MOF-NPC as an adsorbent is heterogeneous for the adsorption of copper ions.
Table 2. Kinetics Models of Adsorption.
| kinetics model | abbreviation | nonlinear equation | refs |
|---|---|---|---|
| mixed-order | MOE |  |
(33) |
| pseudo-first-order | PFO | 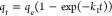 |
(34) |
| pseudo-second-order | PSO |  |
(34) |
| pseudo-n-order | PnO |  |
(35) |
| fractal-like pseudo-first order | FL-PFO |  |
(36, 37) |
| fractal-like pseudo-second order | FL-PSO | 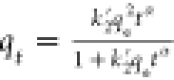 |
(36, 37) |
| mixed surface reaction and diffusion controlled | M-SR-DC |  |
(38) |
Table 3. Obtained Constants of Different Kinetics Models for the Adsorption of Copper Ions by the Prepared MOF-NPC.
| kinetics model | a (min–1) | b (min–1/2) | α | k1′ | k2′ | F2 | k1 (s–1) | k2 (g/mg) | kn | n | qe (mg/g) | r2 | RMS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOE | 9.74 | 0.061 | 381.1 | 0.9916 | 14.69 | ||||||||
| PFO | 0.059 | 381.6 | 0.9909 | 14.29 | |||||||||
| PSO | 0.0002 | 414.1 | 0.97 | 24.58 | |||||||||
| PnO | 0.071 | 0.96 | 381.2 | 0.9912 | 14.11 | ||||||||
| FL-PFO | 1.15 | 0.04 | 374.4 | 0.9906 | 14.76 | ||||||||
| FL-PSO | 1.70 | 0.0004 | 385.1 | 0.9963 | 9.76 | ||||||||
| M-SR-DC | –0.046 | –0.000025 | 383.1 | 0.9881 | 16.36 |
2.3. Adsorption Isotherm
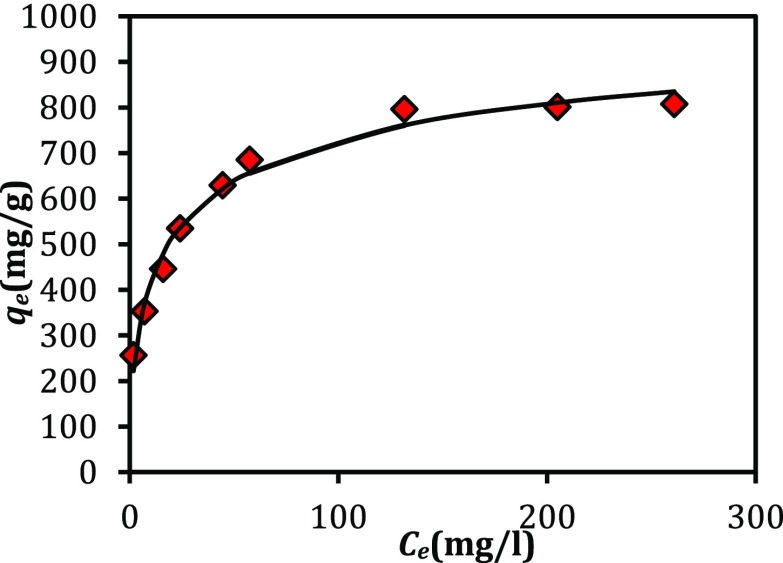
How to interact adsorbent with adsorbate when the process achieves an equilibrium condition is indicated by the adsorption isotherm. In Figure 4, the data related to the equilibrium adsorption of copper ions by the prepared MOF-NPC is shown. By increasing the initial concentration of copper ions, the qe value increases up to the saturation value.
Figure 4.
Adsorption isotherm of copper ions by MOF-NPC.
To calculate the maximum adsorbent absorption capacity, the equilibrium adsorption data were fitted to the modified Langmuir (eq 1) and the modified Langmuir–Freundlich (eq 2) models, which have been recently presented for the adsorption from the liquid phase.39
| 1 |
| 2 |
where qe is the amount of adsorbate per unit mass of the adsorbent at equilibrium time and qm is the monolayer adsorption capacity. KML and KMLF are the equilibrium constants of modified Langmuir and modified Langmuir–Freundlich, respectively. Ce is the equilibrium concentration and Cs is the saturation concentration of the solute (for the present system, the Cs value for CuSO4·5H2O is 184 g/L40) and n is the surface heterogeneity constant. The results of the fitting are listed in Table 4.
Table 4. Obtained Constants of Various Isotherms for the Adsorption of Cu Ion onto MOF-NPC.
| isotherm | qm (mg/g) | KML | KMLF | n | r2 | RMS |
|---|---|---|---|---|---|---|
| modified Langmuir | 824.58 | 17 215 | 0.9608 | 56.42 | ||
| modified Langmuir–Freundlich | 1071.67 | 7781.83 | 0.52 | 0.9915 | 28.03 |
Due to the obtained r2 (correlation coefficient) and the RMS error values, the modified Langmuir–Freundlich model demonstrates better the adsorption of copper ions by adsorbent. The modeling of adsorption equilibrium data with modified Langmuir–Freundlich model demonstrates that the adsorbent surface is heterogeneous and provides various adsorption sites for adsorption. This result is consistent with the conclusion gained from kinetics modeling.
The maximum adsorption capacity (qm) of the prepared MOF-NPC for copper ions based on modified Langmuir–Freundlich model is almost equal to 1071 mg/g. This amount is much higher than the maximum adsorption capacity of the other activated carbons that have been used for adsorption of copper ions. Very high adsorption capacity of the prepared MOF-NPC indicated that this is a superadsorbent for copper ions.
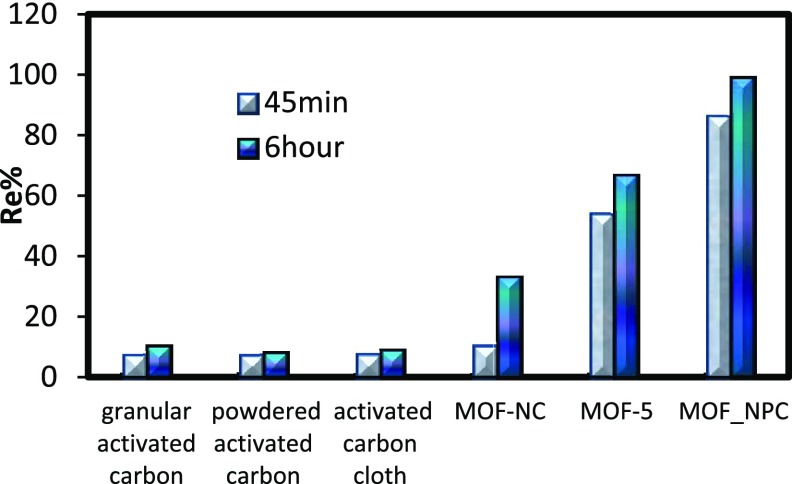
The percentage removal (Re% = 100 × (C0 – Ce)/C0) of copper ions by the prepared MOF-NPC was compared with several commercial activated carbons such as granular activated carbon, activated carbon cloth, powdered activated carbon, MOF-NC13, and MOF-514 as adsorbents. Figure 5 shows the results of the removal percentage after 45 min and also 6 h. As can be seen, the efficiency of MOF-NPC to remove copper ions is much more than that of other absorbents used (about 10 times higher than commercial activated carbons). And, this suggests that the prepared adsorbent (MOF-NPC) is an efficient superadsorbent.
Figure 5.
Evaluation of the removal percentages of copper ions (100 ppm) by MOF-NPC and other adsorbents.
2.4. Effect of Temperature
Because temperature is the main factor in the adsorption process, the effect of temperature (25, 35, 45, and 55 °C) on the removal percentage of Cu ion by MOF-NPC was investigated. Figure S4 represents the percentage removal of copper ions by the prepared adsorbent at several temperatures. It is obvious that as the temperature rises, the elimination efficiency also increases. This result indicates that the adsorption of copper ions from the aqueous solution by MOF-NPC is endothermic (ΔHad > 0).
2.5. Effect of Solution pH
To evaluate the effect of solution pH onto the performance of adsorption, first, the point of zero charge pH (pHpzc) of adsorbent was specified. As shown in Figure S5, the pHpzc of MOF-NPC is about 7.8; this means that, at pH < 7.8, the surface charge is positive, whereas at pH > 7.8, the surface charge is negative.
Due to the precipitation of copper ions at pH > 6, the removal efficiency of the MOF-NPC for copper ions was evaluated within the range of 3.0 < pH < 5.2. These results are shown in Figure 6. It is obvious that with increasing pH, the removal efficiency increases. In this pH range, the absorbent surface charge is positive. So, a decrease in hydrogen ion concentration (increasing of pH) leads to a reduction in the positive charge of surface, and thereby, the repulsion between the surface and Cu2+ reduces. Also, in solutions with low pH, the competition between hydrogen and copper ions leads to a decrease in the efficiency of MOF-NPC to adsorb Cu ions from the aqueous solution.
Figure 6.
Effect of initial pH of the solution on the percentage removal of copper ions by MOF-NPC.
2.6. Recycling Capability
The recycling capability of the used adsorbent was investigated by utilization of deionized water, H2SO4 (0.1 M), and HNO3 (0.1 M). Figure S6 illustrates the results of this study. As this result shows, about 40% of copper ions adsorption capacity of MOF-NPC can be recovered after exposure to sulfuric acid.
3. Conclusions
In this work, heterogenic nanoporous carbons were prepared by carbonization of MOF-5, and the morphology of the prepared adsorbent did not change during carbonization. The obtained nanoporous carbon (MOF-NPC) indicated great adsorption efficiency for the removal of copper ions compared with commercial activated carbons. Both kinetic and equilibrium experimental data indicated that MOF-NPC provides heterogenic surface for the adsorption of copper ions with different adsorption active sites.
The adsorption investigation at different temperatures shows that with increasing temperature, the removal efficiency of MOF-NPC for copper ions increases. The maximum percentage of copper ions removed by MOF-NPC was obtained at pH 5.2.
4. Experimental Methods
4.1. Preparation of MOF-5 and Nanoporous Carbon
MOF-5 was prepared according to our previous report.14 Briefly, 0.82 g of terephthalic acid (BDC) and 3.86 g of zinc nitrate tetrahydrate were added to 112.8 mL of dimethylformamide and then the resultant mixture was mildly stirred until whole of solids were dissolved at ambient conditions. The reaction mixture was heated under a reflux for 4 h at 130 °C. After about an hour, a white powder (MOF-5) appeared. The resultant sample was cooled to ambient temperature. The precipitate was detached and carefully washed. The obtained white powders were dried for 3 h at 60 °C in a vacuum oven.
For the fabrication of porous carbon, about 500 mg of MOF-5 was loaded in a ceramic crucible and then, to remove the solvent molecules, the sample was placed into the vacuum oven and degassed at 200 °C for 3 h. Afterward, the sample was heated at a constant heating rate of 5 °C min–1 in a tube furnace to 530 °C for 8 h under the protection of nitrogen flow. Finally, the sample was cooled to ambient temperature.
4.2. Adsorption Experiments
For the determination of the residual concentration of copper ions, the complexometric method was applied. In this method, Zincon41 (in a buffer solution, at pH = 7) and UV/visible spectrophotometer (at 600 nm) have been used. The performances of the MOF-NPC as an adsorbent to eliminate copper ions were evaluated from both equilibrium and kinetics viewpoint.
The kinetics experiments were performed using various samples of 2 mg of adsorbent and 10 mL of copper ions solution with a constant initial concentration (300 ppm). The samples were stirred in water bath shaker with 150 rpm at room temperature (25 °C). After centrifugation, they were sampled to measure the residual concentration of copper ions at different time intervals.
The equilibrium experiments were accomplished by adding 4 mg of MOF-NPC into 10 mL of several solutions with different concentrations of copper ions (10–1000 ppm). The samples were placed in a shaker at 150 rpm and 25 °C. After 24 h, the adsorbent was separated by centrifugation and the equilibrium concentration of copper ions measured.
The adsorption capacity (amount of copper ions adsorbed per unit mass of adsorbent) was obtained by using eq 3
| 3 |
where qt and qe are the amount of adsorbate per unit mass of the adsorbent at time t and equilibrium, respectively. C0, Ct, and Ce are the concentrations of copper ions in the solution at initial, any time, and equilibrium, respectively. V is the volume of the solution and M is the mass of the adsorbent.
Using a method conducted by Faria et al.,42 pHpzc (pH of point of zero charge) of MOF-NPC has been obtained.
Acknowledgments
This work was supported by the research deputy of Bu-Ali Sina University.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02278.
XRD and Raman spectra of MOF-NPC, TGA curve of MOF-5, effect of temperature on the removal percentage of Cu2+ by MOF-NPC, plot of point of zero charge pH, removal efficiency of recycled MOF-NPC (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Sudha Rani K.; Srinivas B.; Gouru Naidu K.; Ramesh K.-V. Removal of copper by adsorption on treated laterite. Mater. Today: Proc. 2018, 5, 463–469. 10.1016/j.matpr.2017.11.106. [DOI] [Google Scholar]
- Ghaee A.; Niassar M. S.; Barzin J.; Zarghan A. Adsorption of copper and nickel ions on macroporous chitosan membrane: Equilibrium study. Appl. Surf. Sci. 2012, 258, 7732–7743. 10.1016/j.apsusc.2012.04.131. [DOI] [Google Scholar]
- Cheng Z.; Liu X.; Han M.; Ma W. Adsorption kinetic character of copper ions onto a modified chitosan transparent thin membrane from aqueous solution. J. Hazard. Mater. 2010, 182, 408–415. 10.1016/j.jhazmat.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Yan N.; Feng J.; Ma J.; Wen Q.; Li N.; Dong Q. Adsorption mechanism of copper and lead ions onto graphene nanosheet/δ-MnO2. Mater. Chem. Phys. 2012, 136, 538–544. 10.1016/j.matchemphys.2012.07.023. [DOI] [Google Scholar]
- Tian Y.; Wu M.; Liu R.; Li Y.; Wang D.; Tan J.; Wu R.; Huanga R. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr. Polym. 2011, 83, 743–748. 10.1016/j.carbpol.2010.08.054. [DOI] [Google Scholar]
- Lai Y.-L.; Thirumavalavan M.; Lee J.-F. Effective adsorption of heavy metal ions (Cu2+, Pb2+, Zn2+) from aqueous solution by immobilization of adsorbents on Ca-alginate beads. Toxicol. Environ. Chem. 2010, 92, 697–705. 10.1080/02772240903057382. [DOI] [Google Scholar]
- Chen J. J.; Ahmad A. L.; Ooi B. S. Poly (N-isopropylacrylamide-co-acrylic acid) hydrogels for copper ion adsorption: Equilibrium isotherms, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2013, 1, 339–348. 10.1016/j.jece.2013.05.012. [DOI] [Google Scholar]
- Azizian S.; Bagheri M. Enhanced adsorption of Cu2+ from aqueous solution by Ag doped nano-structured ZnO. J. Mol. Liq. 2014, 196, 198–203. 10.1016/j.molliq.2014.03.043. [DOI] [Google Scholar]
- Mosayebi E.; Azizian S. Study of copper ion adsorption from aqueous solution with different nanostructured and microstructured zinc oxides and zinc hydroxide loaded on activated carbon cloth. J. Mol. Liq. 2016, 214, 384–389. 10.1016/j.molliq.2015.11.036. [DOI] [Google Scholar]
- Papas B. N.; Whitten J. L. Adsorption of copper on a γ-alumina support. Surf. Sci. 2016, 651, 22–27. 10.1016/j.susc.2016.03.019. [DOI] [Google Scholar]
- Yu B.; Xu J.; Liu J. H.; Yang S. T.; Luo J.; Zhou Q.; Wan J.; Liao R.; Wang H.; Liu Y. Adsorption behavior of copper ions on graphene oxide–chitosan aerogel. J. Environ. Chem. Eng. 2013, 1, 1044–1050. 10.1016/j.jece.2013.08.017. [DOI] [Google Scholar]
- Demirkiran N. Copper adsorption by natural manganese dioxide. Trans. Nonferrous Met. Soc. China 2015, 25, 647–653. 10.1016/S1003-6326(15)63648-2. [DOI] [Google Scholar]
- Bakhtiari N.; Azizian S.; Alshehri S. M.; Torad N. L.; Malgras V.; Yamauchi Y. Study on adsorption of copper ion from aqueous solution by MOF-derived nanoporous carbon. Microporous Mesoporous Mater. 2015, 217, 173–177. 10.1016/j.micromeso.2015.06.022. [DOI] [Google Scholar]
- Bakhtiari N.; Azizian S. Adsorption of copper ion from aqueous solution by nanoporous MOF-5: A kinetic and equilibrium study. J. Mol. Liq. 2015, 206, 114–118. 10.1016/j.molliq.2015.02.009. [DOI] [Google Scholar]
- Saha D.; Deng S. Ammonia adsorption and its effects on framework stability of MOF-5 and MOF-177. J. Colloid Interface Sci. 2010, 348, 615–620. 10.1016/j.jcis.2010.04.078. [DOI] [PubMed] [Google Scholar]
- Shim W. G.; Hwang K. J.; Chung J. T.; Baek Y. S.; Yoo S. J.; Kim S. C.; Moon H.; Lee J. W. Adsorption and thermodesorption characteristics of benzene in nanoporous metal organic framework MOF-5. Adv. Powder Technol. 2012, 23, 615–619. 10.1016/j.apt.2011.07.002. [DOI] [Google Scholar]
- Szczęśniak B.; Choma J.; Jaroniec M. Gas adsorption properties of hybrid graphene-MOF materials. J. Colloid Interface Sci. 2018, 514, 801–813. 10.1016/j.jcis.2017.11.049. [DOI] [PubMed] [Google Scholar]
- Salunkhe R.; Young C.; Tang J.; Takei T.; Ide Y.; Kobayashi N.; Yamauchi Y. A high-performance supercapacitor cell based on ZIF-8-derived nanoporous carbon using an organic electrolyte. Chem. Commun. 2016, 52, 4764–4767. 10.1039/C6CC00413J. [DOI] [PubMed] [Google Scholar]
- Kaneti Y. V.; Tang J.; Salunkhe R. R.; Jiang X.; Yu A.; Wu K. C.; Yamauchi Y. Nanoarchitectured Design of Porous Materials and Nanocomposites from Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1604898 10.1002/adma.201604898. [DOI] [PubMed] [Google Scholar]
- Hu M.; Reboul J.; Furukawa S.; Radhakrishnan L.; Zhang Y.; Srinivasu P.; Iwai H.; Wang H.; Nemoto Y.; Suzuki N.; Kitagawa S.; Yamauchi Y. Direct synthesis of nanoporous carbon nitride fibers using Al-based porous coordination polymers (Al-PCPs). Chem. Commun. 2011, 47, 8124–8126. 10.1039/c1cc12378e. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan L.; Reboul J.; Furukawa S.; Srinivasu P.; Kitagawa S.; Yamauchi Y. Preparation of Microporous Carbon Fibers through Carbonization of Al-Based Porous Coordination Polymer (Al-PCP) with Furfuryl Alcohol. Chem. Mater. 2011, 23, 1225–1231. 10.1021/cm102921y. [DOI] [Google Scholar]
- Chaikittisilp W.; Torad N.; Li C.; Imura M.; Suzuki N.; Ishihara S.; Ariga K.; Yamauchi Y. Synthesis of Nanoporous Carbon–Cobalt-Oxide Hybrid Electrocatalysts by Thermal Conversion of Metal–Organic Frameworks. Chem. - Eur. J. 2014, 20, 4217–4221. 10.1002/chem.201304404. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Jiang X.; Zhao Y.; Carné-Sánchez A.; Malgras V.; Kim J.; Kim J.; Wang S.; Liu J.; Jiang J.; Yamauchi Y.; Hu M. Hollow carbon nanobubbles: monocrystalline MOF nanobubbles and their pyrolysis. Chem. Sci. 2017, 8, 3538–3546. 10.1039/C6SC04903F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C.; Wang J.; Kim J.; Sugahara Y.; Henzie J.; Yamauchi Y. Controlled Chemical Vapor Deposition for Synthesis of Nanowire Arrays of Metal–Organic Frameworks and Their Thermal Conversion to Carbon/Metal Oxide Hybrid Materials. Chem. Mater. 2018, 30, 3379–3386. 10.1021/acs.chemmater.8b00836. [DOI] [Google Scholar]
- Salunkhe R. R.; Kaneti Y.; Kim J.; Kim J.; Yamauchi Y. Nanoarchitectures for Metal–Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. 10.1021/acs.accounts.6b00460. [DOI] [PubMed] [Google Scholar]
- Wang C.; Kaneti Y. V.; Bando Y.; Lin J.; Liu C.; Li J.; Yamauchi Y. Metal–organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horiz. 2018, 5, 394–407. 10.1039/C8MH00133B. [DOI] [Google Scholar]
- Tang J.; Yamauchi Y. Carbon materials: MOF morphologies in control. Nat. Chem. 2016, 8, 638–639. 10.1038/nchem.2548. [DOI] [PubMed] [Google Scholar]
- Chaikittisilp W.; Hu M.; Wang H.; Huang H. S.; Fujita T.; Wu K. C. W.; Chen L. C.; Yamauchi Y.; Ariga K. Nanoporous Carbon through Direct Carbonization of Zeolitic Imidazolate Framework for Supercapacitor Electrodes. Chem. Commun. 2012, 48, 7259–7261. 10.1039/c2cc33433j. [DOI] [PubMed] [Google Scholar]
- Li X.; Yuan H.; Quan X.; Chen S.; You S. Effective adsorption of sulfamethoxazole, bisphenol A and methyl orange on nanoporous carbon derived from metal-organic frameworks. J. Environ. Sci. 2018, 63, 250–259. 10.1016/j.jes.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Mueller U.; Schubert M.; Teich F.; Puetter H.; Schierle-Arndt K.; Pastre J. Metal–organic frameworks—prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. 10.1039/B511962F. [DOI] [Google Scholar]
- Efremov D. K.; Fenelonov V. B. Adsorption-desorption hysteresis in porous networks. Stud. Surf. Sci. Catal. 1991, 62, 115–122. 10.1016/S0167-2991(08)61315-4. [DOI] [Google Scholar]
- Peng M.; Jeon U.; Ganesh M.; Aziz A.; Vinodh R.; Palanichamy M.; Jang H. Oxidation of Ethylbenzene Using Nickel Oxide Supported Metal Organic Framework Catalyst. Bull. Korean Chem. Soc. 2014, 35, 3213–3218. 10.5012/bkcs.2014.35.11.3213. [DOI] [Google Scholar]
- Marczewski A. W. Application of mixed order rate equations to adsorption of methylene blue on mesoporous carbons. Appl. Surf. Sci. 2010, 256, 5145–5152. 10.1016/j.apsusc.2009.12.078. [DOI] [Google Scholar]
- Azizian S. Kinetic models of sorption: a theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. 10.1016/j.jcis.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Ozer A. Removal of Pb(II) ions from aqueous solutions by sulphuric acid-treated wheat bran. J. Hazard. Mater. 2007, 141, 753–761. 10.1016/j.jhazmat.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Haerifar M.; Azizian S. Fractal-Like Adsorption Kinetics at the Solid/Solution Interface. J. Phys. Chem. C 2012, 116, 13111–13119. 10.1021/jp301261h. [DOI] [Google Scholar]
- Haerifar M.; Azizian S. Fractal-Like Kinetics for Adsorption on Heterogeneous Solid Surfaces. J. Phys. Chem. C 2014, 118, 1129–1134. 10.1021/jp4110882. [DOI] [Google Scholar]
- Haerifar M.; Azizian S. Mixed Surface Reaction and Diffusion-Controlled Kinetic Model for Adsorption at the Solid/Solution Interface. J. Phys. Chem. C 2013, 117, 8310–8317. 10.1021/jp401571m. [DOI] [Google Scholar]
- Azizian S.; Eris S.; Wilson L. D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. 10.1016/j.chemphys.2018.06.022. [DOI] [Google Scholar]
- Perry D. L.Handbook of Inorganic Compounds, 2nd ed.; CRC Press, 2011; p 151. [Google Scholar]
- Ghasemi J.; Ahmadi S.; Torkestani K. Simultaneous determination of copper, nickel, cobalt and zinc using zincon as a metallochromic indicator with partial least squares. Anal. Chim. Acta 2003, 487, 181–188. 10.1016/S0003-2670(03)00556-7. [DOI] [Google Scholar]
- Faria P. C. C.; Orfao J. J. M.; Pereira M. F. R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. 10.1016/j.watres.2004.01.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.