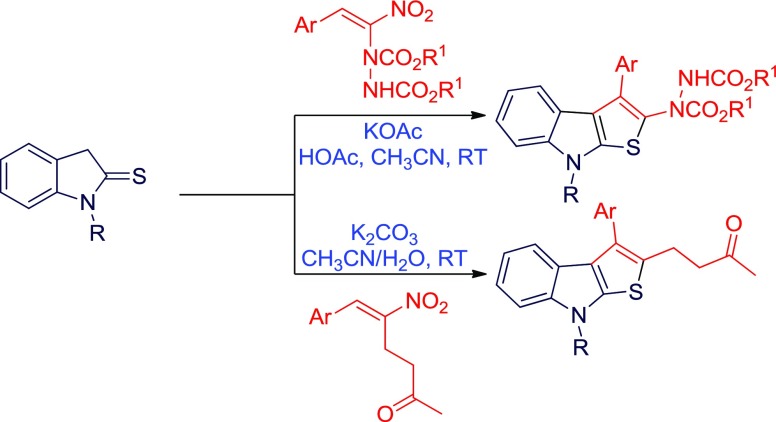
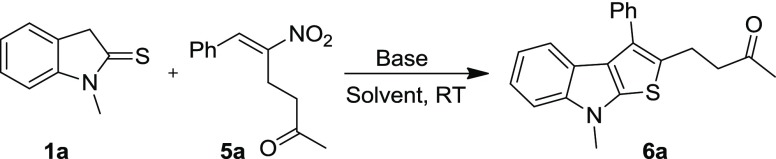
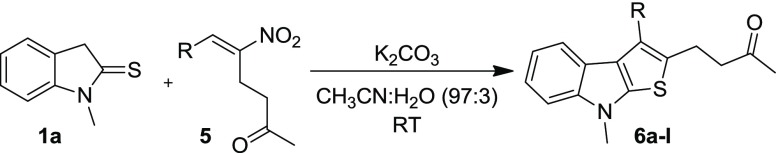
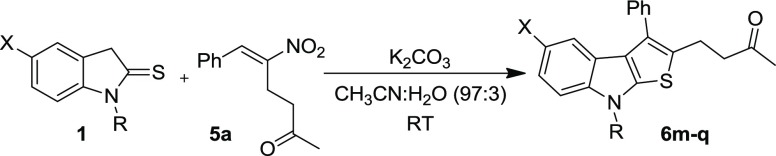
Abstract
A straightforward protocol for the synthesis of functionalized thieno[2,3-b]indoles by base-mediated [3 + 2]-annulation of indoline-2-thione with Morita–Baylis–Hillman and Rauhut–Currier adducts of nitroalkenes is described. Complete regioselectivity, broad substrate scope, and mild reaction conditions make this strategy very valuable. Moreover, the thieno[2,3-b]indoles comprising functional groups such as hydrazine and ketoalkyl moieties are amenable for further synthetic elaboration.
Introduction
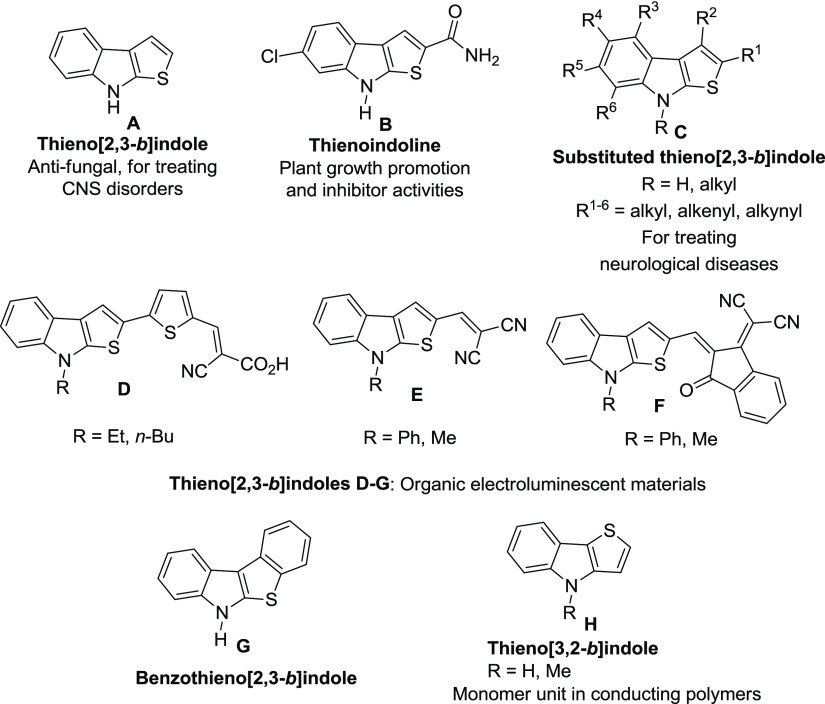
Organosulfur compounds exhibit drug-like properties and thus they have been widely exploited for medicinal chemistry applications.1 Thiophene and their fused analogues stand out among various sulfur-containing compounds because of their excellent medicinal properties.2 The indole-annulated thiophene, particularly, thieno[2,3-b]indole, constitutes a privileged structural motif and has gained considerable attention among synthetic and medicinal chemists.3 Structures of some of the bioactive indole-annulated thiophene derivatives are listed in Figure 1. The core structure, thieno[2,3-b]indole A exhibits antifungal activity4 and the substituted thieno[2,3-b]indoles have potential medicinal applications for the treatment of neurological diseases such as epilepsy, senile dementia, Parkinson’s disease, and deficiencies of mental and motoric performance observed after conditions of brain ischemia.5 Also, the natural product thienodolin B has the same structural framework which displays plant growth-promoting and -inhibiting activities in rice seedlings.3,6 In addition to their pronounced pharmacological properties, some of these scaffolds (D–G) are also employed in organic electronics as electroluminescent materials7 and in the field of conducting polymers (H, Figure 1).8
Figure 1.
Bioactive (A–C) and functional materials (D–H) containing the thieno[2,3-b]indole skeleton.
Owing to their wide range of biological and electronic material applications, various synthetic methods have been developed for the synthesis of thieno[2,3-b]indole skeletons.9 The prominent strategies include deoxygenative or palladium-catalyzed reductive cyclization of 3-(2-nitrophenyl)thiophenes,10 electrophilic cyclization of 2-alkyl-5-(2-isothiocyanoaryl)furans in the presence of AlCl3,11 oxidative cycloamination of benzothiophenes,12 radical or palladium-catalyzed cyclization of 3-(2-bromoindol-3-yl)acrylonitriles,13 Paal–Knorr cyclization of oxindoles in the presence of Lawesson’s reagent,14 and so forth. Recently, Deng et al. demonstrated efficient methods for the regioselective synthesis of thieno[2,3-b]indoles by Brønsted acid-promoted multicomponent reactions.15
Indoline-2-thione has been recognized as a suitable binucleophilic synthon for the synthesis of various indole-annulated heterocycles,16 and few reports are also available for the synthesis of thieno[2,3-b]indoles starting from indoline-2-thione.17 Although many elegant methods are documented in the literature, the development of novel and efficient diversity-oriented strategies for the construction of functionalized indole-annulated thiophenes are still desirable considering their synthetic and biological significance.
Our group has long-term interest in the Morita–Baylis–Hillman (MBH)18 and Rauhut–Currier (RC)19 reactions of nitroalkenes and their applications toward the synthesis of several carbo- and heterocycles.20 Specifically, α-hydrazinonitroalkenes prepared via MBH reaction of nitroalkenes with azodicarboxylates21 and an RC adduct of nitroalkene with MVK22 are well utilized as synthons for the preparation of several functionalized carbo- and heterocycles.23,24 These are excellent Michael acceptors which participate in cascade Michael addition–cyclization sequences.22−26 There is also the possibility of further exploitation of the reactivity of hydrazino and ketoalkyl moieties that are retained in the products. α-Hydrazinonitroalkenes were employed as substrates for the synthesis of functionalized triazoles and arenofurans,23 whereas pyrazoles, furans, decalins, cycloalkanones, spirocycles, and a bridged heterobicyclic compound epibatidine were efficiently synthesized from RC-adducts.22,24,25 Very recently, we reported an effective strategy for the synthesis of aminophenanthrenes and benzoquinolines from RC-adducts of nitroalkenes using Hauser–Kraus reaction of sulfonyl phthalide as the key step.26 As part of our ongoing program to synthesize functionalized heterocycles, herein, we report a novel approach for the construction of indole-annulated thiophenes from MBH/RC-adducts of nitroalkenes and indoline-2-thione.
Results and Discussion
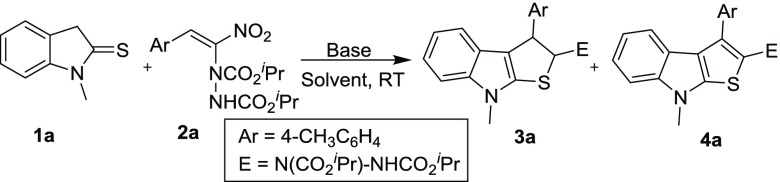
Our investigation commenced with a model reaction between N-methylated indoline-2-thione 1a and hydrazinated nitroalkene 2a in the presence of a mild base such as KOAc at room temperature (Table 1, entry 1). The desired product, fused thienoindole 4a, was formed in 75% yield after 4 days of stirring. The role of inorganic and organic bases was studied to improve the yield in short reaction time. When the reaction was carried out in the presence of inorganic bases such as K2CO3, Cs2CO3, and NaOH, instead of the expected aromatized fused thienoindole, the dihydrothienoindole 3a was formed in 58, 27 and 62% yields, respectively, in a relatively short reaction period (Table 1, entries 2–4). Subsequently, the role of an organic base was evaluated by performing the reaction in the presence of Et3N. In this case also the reaction exclusively furnished the dihydrothienoindole 3a in 62% yield (Table 1, entry 5). Among several bases screened, only KOAc delivered the desired product. After successful screening of bases, we have investigated the effect of solvents by using the optimized base KOAc. A brief evaluation of solvents was then performed by conducting the reaction in tetrahydrofuran (THF), methanol, and toluene (entries 6–8). Among the solvents screened, THF and methanol provided the desired product 4a after 8 d of reaction, but the yield was inferior as compared to that in CH3CN (entry 1). When the reaction was carried out in the presence of 1 mol % acid additive, viz, acetic acid, the yield improved to 78% and the reaction was completed in 4 days. These reaction conditions were the best for this transformation (Table 1, entry 9).
Table 1. Optimization Studiesa.
| % yieldb |
|||||
|---|---|---|---|---|---|
| entry | base | solvent | time | 3ac | 4a |
| 1 | KOAc | CH3CN | 4 d | 75 | |
| 2 | K2CO3 | CH3CN | 30 min | 58 | |
| 3 | Cs2CO3 | CH3CN | 30 min | 27 | |
| 4 | NaOH | CH3CN | 25 min | 62 | |
| 5 | Et3N | CH3CN | 25 min | 62 | |
| 6 | KOAc | THF | 8 d | 60 | |
| 7 | KOAc | MeOH | 8 d | 63 | |
| 8 | KOAc | toluene | 8 d | ||
| 9 | KOAc | CH3CN | 4 d | 78d | |
Reaction scale: 1a (0.25 mmol, 1.0 equiv), 2a (0.25 mmol, 1.0 equiv), base (0.25 mmol, 1.0 equiv), and solvent (3 mL) at RT.
After silica gel column chromatography.
Slowly gets converted to 4a during purification and upon storage; prolonging the reaction time beyond the indicated time or heating to 60–80 °C in the case of entries 2–5 led to complex mixtures.
Acetic acid as the additive (1 mol %).
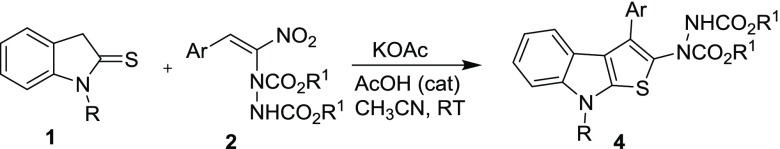
After establishing the best reaction conditions for this transformation, we have investigated the generality of the reaction (Table 2). Various N-substituted indoline-2-thiones 1 and different hydrazinonitroalkenes 2 were well tolerated, and the thienoindole derivatives 4 were formed in moderate to good yields. The electronic nature of groups present on the aryl ring of hydrazinonitroalkenes 2 did not influence the product yield. The hydrazinonitroalkenes 2a and 2c bearing an isopropyl ester moiety and weakly and strongly electron-donating para-substituted aryl groups on reaction with 1a afforded the corresponding products 4a and 4c in 78 and 66% yield (entries 1 and 3), respectively. Likewise, the hydrazinonitroalkene 2e having a weakly electron-withdrawing aryl substituent also produced the product 4e in 66% yield (entry 5). Comparable to those bearing electron-donating and electron-withdrawing aryl groups, a lower yield (58%) was obtained in the case of the electron-neutral substrate 2b (entry 2). The methodology was further generalized with the heterocycle-substituted hydrazinonitroalkene 2f, which also reacted smoothly with 1a to provide the desired fused thienoindole derivative 4f in 40% yield (entry 6). Additionally, the bulky tert-butyl dicarboxylate-containing hydrazinonitroalkene 2d is also compatible for this transformation and delivered 4d in 75% yield (entry 4).
Table 2. Synthesis of Thienoindole 4 by Cascade Reaction of Indoline-2-thione 1 with Hydrazinonitroalkene 2a.
| entry | 1, R | 2, Ar | R1 | 3 or 4 | time | % yieldb |
|---|---|---|---|---|---|---|
| 1 | 1a, Me | 2a, 4-MeC6H4 | iPr | 4a | 4 d | 78 |
| 2 | 1a, Me | 2b, C6H5 | iPr | 4b | 4 d | 58 |
| 3 | 1a, Me | 2c, 4-OMeC6H4 | iPr | 4c | 2 d | 66 |
| 4 | 1a, Me | 2d, 4-OMeC6H4 | tBu | 4d | 3 d | 75 |
| 5 | 1a, Me | 2e, 4-ClC6H4 | iPr | 4e | 3 d | 66 |
| 6 | 1a, Me | 2f, 2-thienyl | iPr | 4f | 3 d | 40c |
| 7 | 1b, Et | 2a, 4-MeC6H4 | iPr | 4g | 8 d | 43c |
| 8 | 1c, nPr | 2a, 4-MeC6H4 | iPr | 4h | 7 d | 65 |
| 9 | 1d, Bn | 2a, 4-MeC6H4 | iPr | 4i | 8 d | 64 |
| 10 | 1a, Me | 2a, 4-MeC6H4 | iPr | 3a | 3 h | 46d |
| 11 | 1a, Me | 2g, Cy | iPr | 4j | 1 d | e |
Reaction scale: 1 (0.7 mmol, 1.0 equiv), 2 (0.7 mmol, 1.0 equiv), KOAc (0.7 mmol, 1.0 equiv), and acetic acid (0.4 μL, 1 mol %) in CH3CN (3 mL) at RT.
After silica gel column chromatography.
10–20% of 1 and 2 was recovered; prolonged reaction led to a complex mixture.
Short reaction time allows isolation of the product before aromatization in this case.
Complex mixture.
Next, the substrate scope with regard to N-substituted indoline-2-thione was studied. It was found that various N-protected thioindoles 1b–d, including ethyl, n-propyl, and benzyl, reacted efficiently and provided the desired products in decent to good yields (43–64%) (Table 2, entries 7–9). It is worth to stress that the synthesis of desired aromatized thienoindoles requires a prolonged reaction time (2–8 days), but it is impressive by considering the outcome of the reaction. When the reaction was allowed to proceed for short time, for instance, the reaction of N-methylated indoline-2-thione 1a with hydrazinonitroalkene 2a under the optimized reaction conditions provided dihydrothienoindole 3a in 46% yield after 3 h (Table 2, entry 10). Unfortunately, hydrazinonitroalkenes bearing an alkyl group such as cyclohexyl as in 2g was not suitable for our reaction (Table 2, entry 11).
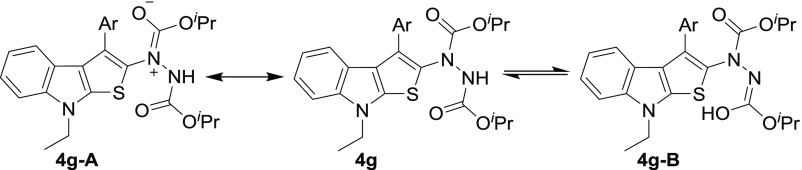
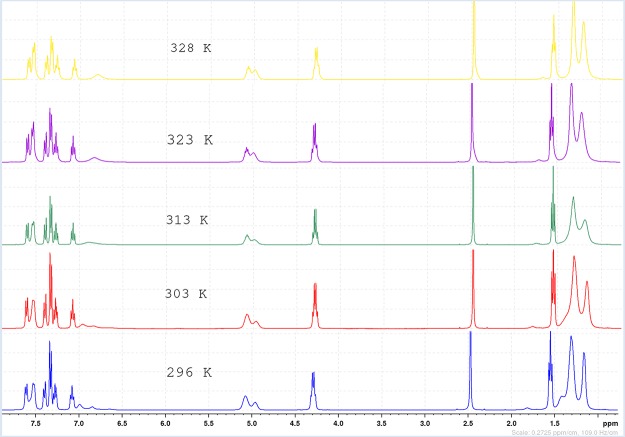
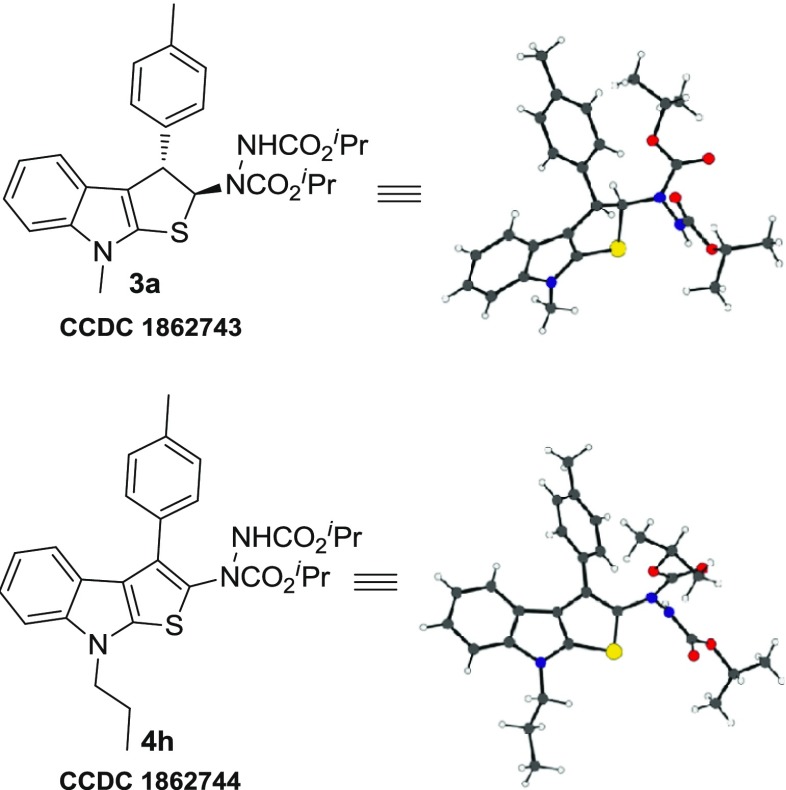
The synthesized indole-annulated thiophene derivatives 4a–i were characterized by usual spectroscopic analyses. In most cases, these compounds showed signal broadening in the NMR spectrum because of the presence of rotamers. The partial double bond character of carbamate in the hydrazine moiety is responsible for the existence of rotamers, and additionally, the quadrupolar effect of N-atoms present in the hydrazine moiety complicates the NMR spectra (Scheme 1). To study this dynamic NMR phenomenon, variable-temperature 1H NMR experiments were conducted by taking 4g as the representative compound (Figure 2). The spectra were recorded in the range of 296–328 K, but only marginal changes were observed in the spectral pattern. Although the signals for most of the aromatic and N–Et protons were reasonably well resolved and methyl protons of the isopropyl group remained unresolved at all of the temperatures studied, gradual resolution of one of the isopropyl methine protons resonating at 4.98–5.08 ppm was discernible upon increasing the temperature. A similar resolution was observed for two of the aromatic protons resonating at ∼7.50 ppm. The two broad signals appearing at ∼7.00 and 6.85 ppm in approximately 2:1 ratio, assigned for the N–H of two rotamers, in the spectrum recorded at 296 K coalesce at 313 K and become sharper at higher temperature. Overall, the sharper signals observed for the protons upon increasing the temperature are attributed to faster rotation about the C–N bond. Furthermore, the structure and regiochemistry of both indole-fused thiophenes and dihydrothienoindole were established by single-crystal X-ray analysis of compounds 4h and 3a (Figure 3).
Scheme 1. Possible Isomers of 4g.
Figure 2.
Variable-temperature 1H NMR spectra of 4g recorded at different temperatures in the range 296–328 K.
Figure 3.
ORTEP representation of compounds 3a and 4h.
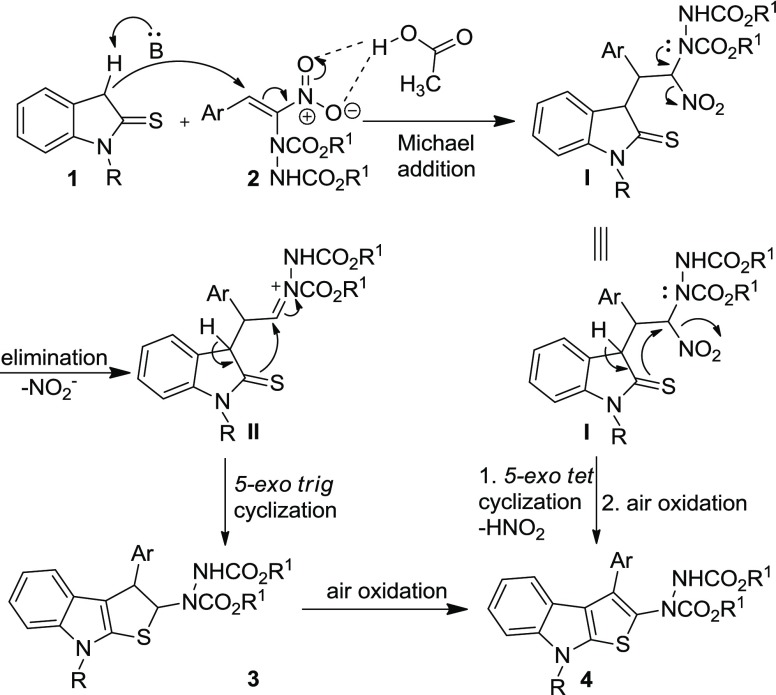
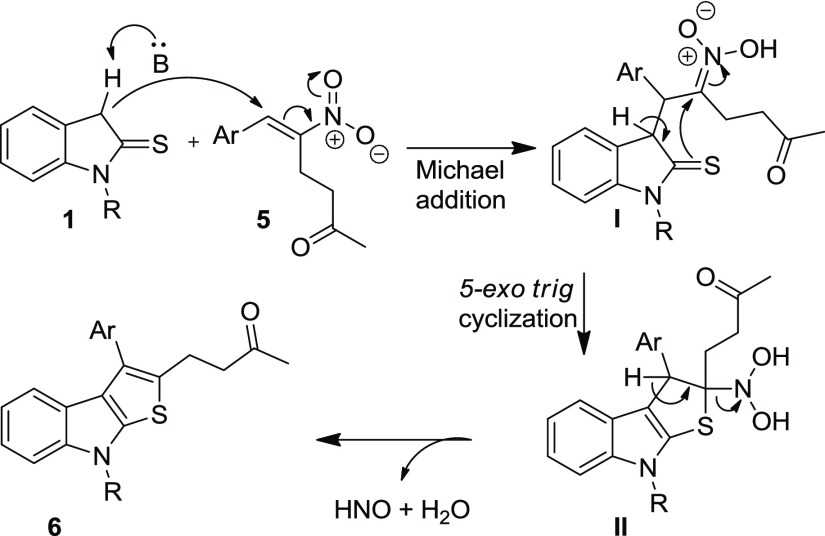
On the basis of the results obtained and the literature precedents,16,17 a logical mechanism for the formation of indole-annulated thiophene 4 is proposed in Scheme 2. The anion, generated from indoline-2-thione 1 by abstraction of a proton from C-3 position, adds in a Michael fashion to hydrazinonitroalkene 2, which is activated by AcOH via H-bonding, leading to the formation of intermediate I. The lone pair of electrons on the nitrogen atom of the hydrazine moiety participates in the elimination of the nitro group in intermediate I and generates a transient acyliminium-type intermediate II. Subsequently, the intramolecular cyclization of II occurs in a 5-exo-trig fashion, resulting in the formation of dihydrothienoindole 3 which on air oxidation gives the desired aromatized thienoindole 4. Alternatively, the reaction can proceed in another pathway starting from intermediate I. Thio-enolization of I followed by intramolecular 5-exo-tet cyclization and subsequent air oxidation results in the formation of thieno[2,3-b]indole 4 (Scheme 2).
Scheme 2. Mechanism of Formation of Thienoindole 4 from Hydrazinonitroalkene 2 and Indoline-2-thione 1.
To further demonstrate the scope of the developed method for the synthesis of diversely functionalized fused thienoindoles, we employed the RC adduct of nitroalkene 5 with MVK 5 as the reaction partner with indoline-2-thione 1. The initial reaction was performed by treating 1a with the RC adduct 5a under the established reaction conditions (Table 3, entry 1). As expected, the indole-annulated thiophene 6a was formed, but the yield was quite low (20%) after 19 h stirring at room temperature. To improve the yield of the reaction, optimization studies were conducted by varying the bases, solvents, and additives by choosing 1a and 5a as the model substrates. Initially, we screened several organic and inorganic bases in acetonitrile medium. When the reaction was carried out in the presence of an organic base, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), the yield slightly improved to 44% in 1 h (Table 3, entry 2). Subsequent screening of milder inorganic bases such as K2CO3 and Cs2CO3 and stronger inorganic bases such as NaOH and KOtBu revealed that the milder base K2CO3 promoted the reaction efficiently and delivered the product in 71% yield in 15 h (entries 3–6). Different solvents were then screened by selecting K2CO3 as the optimal base (entries 7–9). A considerable decline in yield was observed when the reaction was performed in other solvents such as THF, CHCl3, and toluene, and the initially employed medium CH3CN was found to be suitable for this transformation. Further, the loading of the base was varied which resulted in the formation of the product in reduced yield upon decreasing or increasing the amount of K2CO3 from 1 equiv (entries 10 and 11). Few additional experiments were performed by employing the additives such as LiCl and H2O (entries 12 and 13). The additive, LiCl was found to be inefficient for improving the yield (entry 12). The reaction was complete in 8 h and the yield improved (76%) when H2O was used as an additive (entry 13). When the reaction was performed under microwave irradiation at 40 °C, the reaction was complete in 15 min, but a substantial drop in yield (43%) was observed (entry 14). Finally, in terms of chemical yield and reaction time, entry 14 has been considered as optimal for this transformation. It may be noted that unlike in the reaction of thienoindole 1 with hydrazinonitroalkene 2, the aromatized fused thienoindole 6a was isolated in all of the cases when RC-adducts of nitroalkene 5a was employed.
Table 3. Optimization Studiesa.
| entry | base (equiv) | time (h) | solvent | % yieldb |
|---|---|---|---|---|
| 1 | KOAc (1.0) | 19 | CH3CN | 20 |
| 2 | DBU (1.0) | 1 | CH3CN | 44 |
| 3 | K2CO3 (1.0) | 15 | CH3CN | 71 |
| 4 | Cs2CO3 (1.0) | 15 | CH3CN | 57 |
| 5 | NaOH (1.0) | 4 | CH3CN | 50 |
| 6 | KOtBu (1.0) | 4 | CH3CN | 52 |
| 7 | K2CO3 (1.0) | 17 | THF | 45 |
| 8 | K2CO3 (1.0) | 18 | CHCl3 | 35 |
| 9 | K2CO3 (1.0) | 26 | toluene | 30 |
| 10 | K2CO3 (0.5) | 23 | CH3CN | 47 |
| 11 | K2CO3 (2.0) | 12 | CH3CN | 55 |
| 12 | K2CO3 (1.0) (LiCl)c | 15 | CH3CN | 44 |
| 13 | K2CO3(1.0) (H2O)d | 8 | CH3CN | 76e |
| 14 | K2CO3 (1.0) | 15 min | CH3CN | 43f |
Reaction scale: 1a (0.23 mmol, 1.0 equiv), 5a (0.23 mmol, 1.0 equiv), and solvent (3 mL) at RT until complete consumption of at least one of the starting materials.
After column chromatography.
LiCl (0.23 mmol, 1.0 equiv).
CH3CN/H2O (97:3 v/v).
59% yield at 60 °C for 6 h.
Under microwave at 40 °C.
With the optimal reaction conditions in hand, the scope of the reaction was investigated with different substituted RC-adducts of nitroalkenes 5 and indoline-2-thiones 1. Initially, we focused on studying the reactivity of various substituted RC-adducts of nitroalkenes 5b–l, taking thienoindole 1a as the representative reaction partner, and the results are summarized in Table 4. In general, the electron-donating substituents on the aryl group of RC-adducts of nitroalkenes 5 afforded the thieno[2,3-b]indole derivatives 6 in low to moderate yields (28–51%, entries 2, 3, 5, and 6). In some cases, fairly good yields (59 and 70%) were obtained when multiple electron-donating groups were placed at different positions (entries 4 and 7). RC-adducts of nitroalkenes bearing weakly electron-withdrawing groups (5h–j) were also subjected to the reaction. While high yield (63%) was observed in the case of 4-chloro-substituted RC-adduct 5i, modest yields were obtained from RC-adducts 5h and 5j containing bromo- and fluoro substituents (entries 8–10). The RC-adduct of nitroalkene 5k containing bulky 1-naphthyl group also participated in the reaction and furnished the corresponding fused thienoindole derivative 6k in comparable yield. Notably, the heteroaryl-derived RC-adduct of nitroalkene 5l also reacted with 1a and delivered the product, though in low yield (31%).
Table 4. Synthesis of Thienoindoles (6a–l) from Indoline-2-thione 1a and Various Aryl-Substituted RC Adducts 5a.
| entry | 5, R | 6 | time (h) | % yieldb |
|---|---|---|---|---|
| 1 | 5a, C6H5 | 6a | 8 | 76 |
| 2 | 5b, 4-MeC6H4 | 6b | 9 | 37c |
| 3 | 5c, 4-OMeC6H4 | 6c | 5 | 35c |
| 4 | 5d, 3,4-(OMe)2C6H3 | 6d | 9 | 59 |
| 5 | 5e, 3-OMeC6H4 | 6e | 7 | 28c |
| 6 | 5f, 3-(PhCH2O)C6H4 | 6f | 7 | 51 |
| 7 | 5g, 2,5-(OMe)2C6H3 | 6g | 9 | 70 |
| 8 | 5h, 4-BrC6H4 | 6h | 7 | 46c |
| 9 | 5i, 4-ClC6H4 | 6i | 12 | 63 |
| 10 | 5j, 4-FC6H4 | 6j | 7 | 48c |
| 11 | 5k, 1-naphthyl | 6k | 7 | 56 |
| 12 | 5l, 2-thienyl | 6l | 8 | 31c |
Reaction scale: 1a (0.75 mmol, 1.0 equiv), 5 (0.75 mmol, 1.0 equiv), K2CO3 (0.75 mmol, 1.0 equiv) in CH3CN and H2O (97:3 v/v, 3 mL) at RT.
After silica gel column chromatography.
10–20% 1a and 5 was recovered; prolonged reaction led to a complex mixture.
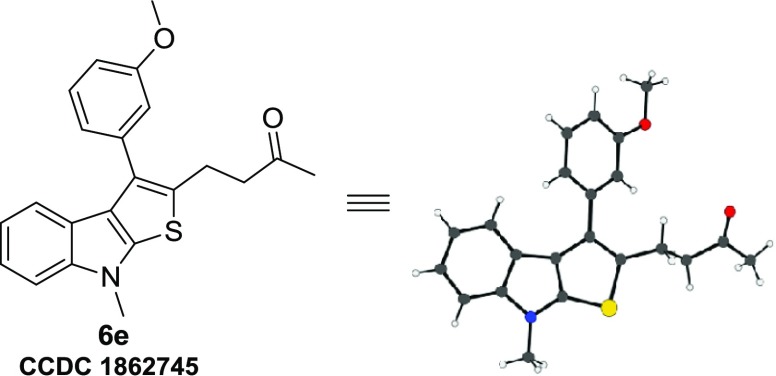
Subsequently, the scope of indoline-2-thione 1 was explored by reacting a representative RC-adduct 5a with different N-substituted and aryl-substituted thienoindoles 1 under the optimized conditions (Table 5). Various N-protecting groups such as ethyl, n-propyl, and benzyl were tested. Among these, N-ethyl- and N-benzyl-protected indoline-2-thiones 1b and 1d afforded the product in excellent yields (87 and 94%, respectively, entries 1 and 3), whereas the N-propyl derivative 1c provided the corresponding product in much lower yield (entry 2). Indoline-2-thione 1e having chlorine on the aryl group (located at C-5 position) was subjected to reaction with 1a, and the expected product was isolated in moderate yield (44%, entry 4). It is noteworthy that the unprotected indoline-2-thione 1f is also compatible for this transformation and furnished the desired product in 44% yield (entry 5). Finally, the structures of thienoindoles 6a–q synthesized from RC-adducts 5a–l were characterized by analysis of their spectral data and further unambiguously confirmed by single-crystal X-ray analysis of a representative compound 6e (Figure 4). (Table 5
Table 5. Synthesis of Thienoindoles (6m–q) from RC Adduct 5a and Differently Substituted Indoline-2-thiones 1a.
| entry | 1, R | X | 6 | time (h) | % yieldb |
|---|---|---|---|---|---|
| 1 | 1b, Et | H | 6m | 12 | 87 |
| 2 | 1c, nPr | H | 6n | 12 | 55 |
| 3 | 1d, Bn | H | 6o | 13 | 94 |
| 4 | 1e, Me | Cl | 6p | 5 | 44 |
| 5 | 1f, H | H | 6q | 7 | 44 |
Reaction scale: 1a (0.75 mmol, 1.0 equiv), 5 (0.75 mmol, 1.0 equiv), K2CO3 (0.75 mmol, 1.0 equiv) in CH3CN, and H2O (97:3 v/v, 3 mL) at RT.
After silica-gel column chromatography.
Figure 4.
ORTEP representation of compound 6e.
A plausible mechanism for the one-pot synthesis of thiophene-annulated indole derivatives 6 from RC-adducts is outlined in Scheme 3. The base-mediated Michael addition of indoline-2-thione 1 to the RC-adduct 5 generates intermediate I. Subsequently, an intramolecular thio-Mannich-type reaction occurs in a 5-exo-trig manner resulting in the intermediate II which undergoes elimination of HNO and H2O to afford the aromatized product 6.
Scheme 3. Mechanism of Formation of Thienoindole 6 from RC-Adduct 5 and Indoline-2-thione 1.
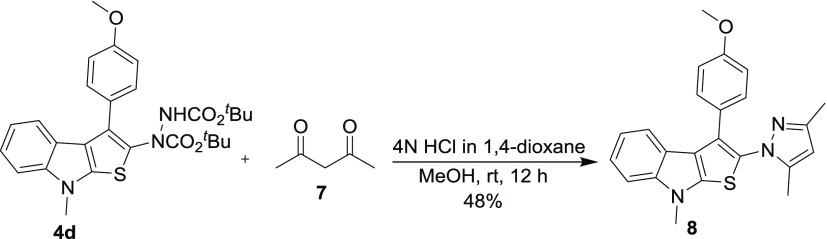
Because the thieno[2,3-b]indole frameworks prepared from hydrazinonitroalkene 2 and RC-adducts 5 consist of functional moieties such as hydrazine and ketone, there exist enormous possibilities for further synthetic manipulation. The synthetic potential of these compounds was then highlighted by exemplifying a specific one-pot transformation of thieno[2,3-b]indole 4d containing a hydrazine ester moiety to pyrazole-tethered thieno[2,3-b]indole 8 (Scheme 4). Acid hydrolysis of 4d followed by decarboxylation generated the corresponding hydrazine derivative which on reaction in situ with acetyl acetone (Knorr-pyrazole synthesis) provided 8, though in moderate yield (48%).
Scheme 4. Synthesis of Pyrazole-Tethered Thieno[2,3-b]indole.
Conclusions
In summary, we have developed a convenient one-pot strategy for the synthesis of diverse functionalized thieno[2,3-b]indoles from easily accessible starting materials. The base-mediated [3 + 2] annulation of indoline-2-thione and α-hydrazinonitroalkenes derived from the MBH-reaction of nitroalkenes and azodicarboxylates provided the functionalized indole-annulated thiophene motifs in moderate to excellent yields. In a similar fashion, the RC-adducts of nitroalkenes underwent cascade cyclization when reacted with indoline-2-thione and afforded corresponding indole-fused thiophenes in moderate to good yields. Although a prolonged reaction time was required for the construction of thieno[2,3-b]indoles bearing a hydrazine moiety (2–8 d), the mild and metal-free reaction conditions make this protocol quite attractive. Moreover, the hydrazine moiety on the thieno[2,3-b]indole skeleton could be transformed to pyrazole, which further broadens the synthetic applicability of the developed strategy. Studies to further extend the scope of the developed method for synthesizing novel heterocycle-fused indoles by employing various indolines and functionalized nitroalkenes are currently underway in our laboratory.
Experimental Section
General Information
The melting points recorded are uncorrected. NMR spectra (1H, 1H decoupled 13C, 19F, APT, 1H–1H COSY) were recorded with tetramethylsilane as the internal standard. The coupling constants (J values) are given in Hz. IR spectra were recorded on a Fourier transform infrared spectrometer, and the values are expressed in cm–1. High-resolution mass spectra were recorded under ESI Q-TOF conditions. X-ray data were collected on a diffractometer equipped with graphite monochromated Mo Kα radiation. The structure was solved by direct methods shelxs97 and refined by full matrix least squares against F2 using shelxl97 software. The starting materials indoline-2-thiones,27 MBH-adducts,21 and RC-adducts22 were prepared by literature methods.
General Procedure for the Synthesis of Thienoindole (4)
To a stirred solution of indoline-2-thione 1 (0.7 mmol, 1 equiv), KOAc (104 mg, 0.7 mmol, 1 equiv), and AcOH (0.4 μL, 1 mol %) in CH3CN (3 mL) at room temperature was added the MBH adduct 2 (0.7 mmol, 1 equiv). After the completion of the reaction [monitored by thin-layer chromatography (TLC)], the reaction mixture was concentrated in vacuo and the crude product was purified by silica gel (60–120 mesh) column chromatography by gradient elution with ethyl acetate/pet ether (10–25%).
Diisopropyl 1-(8-Methyl-3-(p-tolyl)-3,8-dihydro-2H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (3a)
White solid; yield 155 mg, 46%; mp 158–160 °C; IR (KBr, cm–1): 3292 (br m), 2980 (m), 1720 (vs), 1384 (m), 1107 (s); 1H NMR (CDCl3, 500 MHz): δ 1.10–1.40 (br poorly resolved, 12H), 2.32 (s, 3H), 3.68 (s, 3H), 4.90–5.10 (br unresolved, 3H), 6.60 (br s, 1H), 6.72 (br unresolved d, 1H), 6.95 (br d, J = 7.3 Hz, 2H), 7.06 (t, J = 8.1 Hz, 1H), 7.11 (d, J = 7.3 Hz, 2H), 7.21 (d, J = 8.1 Hz, 1H), 7.24 (br unresolved, 2H); 13C NMR (CDCl3, 125 MHz): δ 21.2, 22.0, 22.1, 32.1, 50.8, 70.3, 71.6, 83.9, 109.0, 117.3, 117.5, 119.7, 119.8, 125.1, 127.5, 127.8, 129.5, 136.9, 138.0, 141.0, 154.5, 156.0; MS (ES+, Ar) m/z (rel intensity): 520 (MK+, 30), 504 (100), 482 (MH+, 40); HRMS (ES+): calcd for C26H31N3O4SNa (MNa+, 100), 504.1927; found, 504.1925.
Diisopropyl 1-(8-Methyl-3-(p-tolyl)-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4a)
White solid; yield 261 mg, 78%; mp 192–194 °C; IR (KBr, cm–1): 3289 (br m), 2980 (m), 1725 (vs), 1483 (m), 1469 (m), 1372 (m), 1306 (m), 1241 (m), 1180 (m), 1106 (s), 1038 (m), 742 (s); 1H NMR (CDCl3, 500 MHz): δ 1.14 (br unresolved d, 6H), 1.25–1.40 (br unresolved, 6H), 2.45 (s, 3H), 3.84 (s, 3H), 4.90–5.00 (br unresolved m, 1H), 5.00–5.10 (br unresolved m, 1H), 6.90 (br s, 1H), 7.07 (t, J = 8.0 Hz, 1H), 7.27 (t, J = 8.0 Hz, 1H), 7.31 (d, J = 7.7 Hz, 2H), 7.37 (d, J = 8.0 Hz, 1H), 7.49 (d, J = 7.7 Hz, 2H), 7.58 (d, J = 8.0 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 21.5, 21.8, 22.1, 32.2, 70.4, 71.6, 109.1, 117.8, 119.2, 119.4, 122.2, 122.9, 128.7, 129.5, 131.5, 133.2, 133.8, 137.8, 141.5, 142.0, 155.7, 156.0; MS (ES+, Ar) m/z (rel intensity): 518 (MK+, 85), 502 (MNa+, 100), 480 (MH+, 6), 377 (18); HRMS (ES+): calcd for C26H29N3O4SNa (MNa+, 100), 502.1771; found, 502.1778.
Diisopropyl 1-(8-Methyl-3-phenyl-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4b)
White solid; yield 189 mg, 58%; mp 179–172 °C; IR (KBr, cm–1): 3291 (br w), 2980 (m), 1724 (s), 1482 (s), 1466 (s), 1374 (m), 1304 (m), 1244 (s), 1106 (s), 740 (m); 1H NMR (CDCl3, 500 MHz): δ 1.11 (br unresolved d, 6H), 1.25–1.40 (br unresolved, 6H), 3.84 (s, 3H), 4.90–5.00 (br unresolved m, 1H), 5.00–5.10 (br unresolved m, 1H), 7.01 (br s), 7.08 (t, J = 8.0 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.52 (t, J = 7.6 Hz, 2H), 7.57 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 7.6 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 21.8, 22.1, 32.2, 70.4, 71.6, 109.1, 117.7, 119.1, 119.4, 122.2, 122.8, 128.0, 128.8, 128.9, 129.2, 133.1, 134.5, 141.5, 142.0, 155.6, 156.1; MS (ES+, Ar) m/z (rel intensity): 504 (MK+, 100); HRMS (ES+): calcd for C25H27N3O4SK (MK+, 100), 504.1354; found, 504.1355.
Diisopropyl 1-(3-(4-Methoxyphenyl)-8-methyl-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4c)
White solid; yield 229 mg, 66%; mp 96–98 °C; IR (KBr, cm–1): 3304 (br m), 2980 (m), 1726 (s), 1726 (vs), 1486 (m), 1246 (s), 1107 (m); 1H NMR (CDCl3, 500 MHz): δ 1.13 (br unresolved, 6H), 1.27 (d, J = 6.2 Hz, 6H), 3.84 (s, 3H), 3.89 (s, 3H), 4.90–5.08 (unresolved m, 2H), 6.85 (br s, 1H), 7.02 (d, J = 8.6 Hz, 2H), 7.06 (t, J = 7.5 Hz, 1H), 7.26 (t, J = 7.5 Hz, 1H), 7.37 (d, J = 8.6 Hz, 2H), 7.51 (d, J = 7.5 Hz, 1H), 7.56 (d, J = 7.5 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 21.9, 22.1, 32.2, 55.5, 70.1, 71.6, 109.1, 114.3, 117.9, 119.1, 119.4, 122.2, 122.9, 126.8, 128.6, 130.1, 132.9, 141.5, 142.0, 155.8, 156.0, 159.4; MS (ES+, Ar) m/z (rel intensity): 534 (MK+, 32), 518 (MNa+, 30) 496 (100); HRMS (ES+): calcd for C26H30N3O5S (MH+, 100), 496.1901; found, 496.1911.
Diisobutyl 1-(3-(4-Methoxyphenyl)-8-methyl-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4d)
White solid; yield 275 mg, 75%; mp 201–203 °C; IR (KBr, cm–1): 3302 (br w), 2979 (m), 1725 (s), 1482 (s), 1247 (s), 1159 (vs); 1H NMR (CDCl3, 500 MHz): δ 1.30, 1.53 (s, 18H), 3.84 (s, 3H), 3.89 (s, 3H), 6.90 (br s, 1H), 7.03–7.12 (br unresolved, 3H), 7.25–7.31 (br unresolved, 1H), 7.35–7.40 (br unresolved, 1H), 7.54–7.66 (br unresolved, 3H); 13C NMR (CDCl3, 125 MHz): δ 28.0, 28.3, 32.2, 55.4, 81.9, 82.5, 109.1, 114.2, 117.7, 119.1, 119.3, 122.0, 122.9, 127.0, 129.5, 130.0, 132.2, 141.4, 141.9, 154.6, 155.5, 159.3; MS (ES+, Ar) m/z (rel intensity): 562 (MK+, 100); HRMS (ES+): calcd for C28H33N3O5SK (MK+, 100), 562.1773; found, 562.1769.
Diisopropyl 1-(3-(4-Chlorophenyl)-8-methyl-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4e)
White solid; yield 231 mg, 66%; mp 164–166 °C; IR (KBr, cm–1): 3284 (br w), 2980 (w), 1725 (vs), 1481 (m), 1466 (m), 1374 (m), 1306 (m), 1245 (m), 1105 (s), 741 (m); 1H NMR (CDCl3, 500 MHz): δ 1.12 (br unresolved d, 6H), 1.20–1.40 (br unresolved, 6H), 3.85 (s, 3H), 4.90–5.00 (br unresolved m, 1H), 5.00–5.10 (br unresolved m, 1H), 6.91 (br s, 1H), 7.07 (t, J = 7.9 Hz, 1H), 7.28 (t, J = 7.9 Hz, 1H), 7.37 (d, J = 7.9 Hz, 1H), 7.48 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 8.2 Hz, 2H), 7.53–7.57 (br unresolved, 1H); 13C NMR (CDCl3, 125 MHz): δ 21.9, 22.2, 32.3, 70.6, 71.8, 109.3, 117.6, 119.0, 119.6, 122.4, 122.7, 129.1, 129.7, 130.3, 132.0, 133.0, 134.0, 141.6, 142.2, 155.6, 156.2; MS (ES+, Ar) m/z (rel intensity): 524 ([MNa+2]+, 11), 526 (MNa+, 40), 502 ([MH+2]+, 35), 500 (MH+, 100); HRMS (ES+): calcd for C25H27N3O4SCl (MH+, 100), 500.1405; found, 500.1404.
Diisopropyl 1-(8-Methyl-3-(thiophen-2-yl)-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4f)
White solid; yield 132 mg, 40%; mp 145–147 °C; IR (KBr, cm–1): 3299 (br w), 2980 (w), 1728 (vs), 1599 (w), 1480 (m), 1237 (m), 1105 (m); 1H NMR (CDCl3, 500 MHz): δ 1.13 (br d, J = 5.3 Hz, 6H), 1.35–1.45 (br unresolved, 6H), 3.83 (s, 3H), 4.95–5.00 (br m, 1H), 5.02–5.12 (br unresolved m, 1H), 7.06 (br s, 1H), 7.15 (t, J = 7.9 Hz, 1H), 7.21 (dd, J = 4.7, 4.2 Hz, 1H), 7.31 (t, J = 7.9 Hz, 1H), 7.38 (d, J = 7.9 Hz, 1H), 7.42 (d, J = 4.7 Hz, 1H), 7.50 (br unresolved, 1H), 7.92 (d, J = 7.9 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 21.8, 22.1, 32.2, 70.5, 71.8, 109.2, 117.1, 119.4, 119.5, 122.4, 122.6, 126.0, 126.1, 127.2, 127.4, 129.3, 134.8, 141.5, 142.0, 155.5, 156.0; MS (ES+, Ar) m/z (rel intensity): 510 (MK+, 100); HRMS (ES+): calcd for C23H25N3O4S2K (MK+, 100), 510.0918; found, 510.0919.
Diisopropyl 1-(8-Ethyl-3-(p-tolyl)-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4g)
White solid; yield 148 mg, 43%; mp 184–186 °C; IR (KBr, cm–1): 3289 (br m), 2980 (s), 1726 (vs), 1479 (s), 1466 (s), 1374 (m), 1302 (m), 1242 (s), 1181 (m), 1106 (s), 1038 (m), 740 (m); 1H NMR (CDCl3, 500 MHz): δ 1.11 (br unresolved d, 6H), 1.20–1.40 (br resolved, 6H), 1.51 (t, J = 7.2 Hz, 3H), 2.43 (s, 3H), 4.26 (q, J = 7.2 Hz, 2H), 4.88–4.95 (br unresolved m, 1H), 4.98–5.08 (br unresolved, 1H), 6.84 (br s, 1H), 7.03 (t, J = 8.1 Hz, 1H), 7.24 (t, J = 8.1 Hz, 1H), 7.28 (d, J = 7.2 Hz, 2H), 7.37 (d, J = 8.1 Hz, 1H), 7.46 (br d, J = 7.2 Hz, 2H), 7.55 (d, J = 8.1 Hz, 1H), no appreciable change in the pattern even at 328 K, see VT NMR spectra; 13C NMR (CDCl3, 125 MHz): δ 13.9, 21.4, 21.8, 22.0, 40.8, 70.3, 71.5, 109.1, 118.1, 119.2, 122.0, 122.9, 128.7, 129.4, 131.4, 133.1, 133.6, 137.7, 140.4, 155.6, 156.0; MS (ES+, Ar) m/z (rel intensity): 532 (MK+, 100), 516 (MNa+, 65), 494 (35); HRMS (ES+): calcd for C27H31N3O4SK (MK+, 100), 532.1667; found, 532.1665.
Diisopropyl 1-(8-Propyl-3-(p-tolyl)-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4h)
White solid; yield 230 mg, 65%; mp 169–171 °C; IR (KBr, cm–1): 3290 (br w), 2979 (m), 1726 (vs), 1478 (m), 1244 (m), 1107 (s); 1H NMR (CDCl3, 500 MHz): δ 1.03 (t, J = 7.2 Hz, 3H), 1.14 (br unresolved d, 6H), 1.25–1.40 (br unresolved, 6H), 1.99 (sextet, J = 7.2 Hz, 2H), 2.46 (s, 3H), 4.18 (t, J = 7.2 Hz, 2H), 4.92–4.98 (br unresolved m, 1H), 5.02–5.10 (br unresolved m, 1H), 6.95 (br s, 1H), 7.06 (t, J = 8.0 Hz, 1H), 7.26 (t, J = 8.0 Hz, 1H), 7.32 (d, J = 7.1 Hz, 2H), 7.40 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 7.1 Hz, 2H), 7.60 (d, J = 8.0 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 11.8, 21.5, 21.8, 22.1, 22.3, 47.9, 70.4, 71.5, 109.3, 118.0, 118.1, 119.2, 122.0, 122.9, 128.7, 129.5, 131.5, 133.1, 133.6, 137.7, 141.0, 141.2, 155.7, 156.1; MS (ES+, Ar) m/z (rel intensity): (MK+, 100); HRMS (ES+): calcd for C28H33N3O4SK (MK+, 100), 546.1823; found, 546.1821.
Diisopropyl 1-(8-Benzyl-3-(p-tolyl)-8H-thieno[2,3-b]indol-2-yl)hydrazine-1,2-dicarboxylate (4i)
White solid; yield 249 mg, 64%; mp 166–168 °C; IR (KBr, cm–1): 3278 (br m), 2980 (m), 1720 (vs), 1454 (m), 1373 (m), 1303 (s), 1244 (s), 1106 (s), 1037 (m), 738 (m); 1H NMR (CDCl3, 500 MHz): δ 1.13 (br unresolved d, 6H), 1.22–1.35 (br unresolved, 6H), 2.46 (s, 3H), 4.90–5.00 (br unresolved m, 1H), 5.00–5.08 (br unresolved m, 1H), 5.38 (s, 2H), 6.92 (br s, 1H), 7.08 (t, J = 7.5 Hz, 1H), 7.23–7.43 (m, 7H), 7.32 (d, J = 7.8 Hz, 2H), 7.51 (br d, J = 7.8 Hz, 2H), 7.62 (d, J = 7.5 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 21.5, 21.9, 22.1, 50.0, 70.4, 71.5, 109.6, 118.5, 119.3, 119.6, 122.3, 123.1, 127.5, 127.8, 128.1, 128.8, 129.0, 129.4, 129.5, 131.4, 133.0, 135.9, 137.8, 141.2, 155.7, 155.9; MS (ES+, Ar) m/z (rel intensity): 612 ([MK + H2O]+, 25), 594 (MK+, 100), 578 (MNa+, 45); HRMS (ES+): calcd for C32H33N3O4SK (MK+, 100), 594.1823; found, 594.1825.
General Procedure for the Synthesis of Thienoindoles (6)
To a stirred solution of indoline-2-thione 1 (0.75 mmol, 1 equiv) and K2CO3 (104 mg, 0.75 mmol, 1 equiv) in CH3CN/H2O (97:3, 3 mL) at room temperature was added the RC adduct 5 (0.75 mmol, 1 equiv). After the completion of the reaction (monitored by TLC), the reaction mixture was concentrated in vacuo and the crude residue was purified by silica gel column chromatography by gradient elution with ethyl acetate/pet ether (10–25%).
4-(8-Methyl-2-phenyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6a)
White solid; yield 189 mg, 76%; mp 129–131 °C; IR (KBr, cm–1): 3052 (w), 2922 (m), 1715 (vs), 1590 (m), 1360 (m), 1330 (s), 1163 (m), 743 (s); 1H NMR (CDCl3, 500 MHz): δ 2.10 (s, 3H), 2.76 (t, J = 7.4 Hz, 2H), 3.15 (t, J = 7.4 Hz, 2H), 3.80 (s, 3H), 6.99 (t, J = 8.2 Hz, 1H), 7.21 (t, J = 8.2 Hz 1H), 7.30 (d, J = 8.2 Hz, 1H), 7.41–7.45 (m, 2H), 7.50 (t, J = 7.4 Hz, 2H), 7.55 (t, J = 7.4 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 23.1, 30.1, 32.2, 45.9, 109.0, 118.9, 119.1, 121.7, 122.1, 122.4, 127.5, 128.7, 129.6, 130.2, 131.7, 136.1, 141.4, 142.0, 207.6; MS (ES+, Ar) m/z (rel intensity): 379 (M2Na+, 100), 372 (MK+, 70), 356 (MNa+, 80); HRMS (ES+): calcd for C21H19NOSK (MK+), 372.0819; found, 372.0825.
4-(8-Methyl-2-(p-tolyl)-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6b)
White solid; yield 95 mg, 37%; mp 122–124 °C; IR (KBr, cm–1): 3050 (w), 2925 (m), 1715 (s), 1484 (m), 1465 (m), 1266 (m), 1162 (m), 822 (m), 740 (vs), 705 (m); 1H NMR (CDCl3, 500 MHz): δ 2.12 (s, 3H), 2.47 (s, 3H), 2.78 (t, J = 4.0 Hz, 2H), 3.15 (t, J = 4.0 Hz, 2H), 3.83 (s, 3H), 7.02 (t, J = 7.5 Hz, 1H), 7.23 (t, J = 7.5 Hz, 1H), 7.31–7.34 (m, 3H), 7.43–7.47 (m, 3H); 13C NMR (CDCl3, 125 MHz): δ 21.5, 23.2, 30.2, 32.2, 46.0, 109.0, 119.0, 119.1, 121.7, 122.3 (×2), 129.4, 129.5, 129.9, 131.7, 133.0, 137.2, 141.4, 142.0, 207.7; MS (ES+, Ar) m/z (rel intensity): 386 (MK+, 30), 370 (MNa+, 100); HRMS (ES+): calcd for C22H21NOSNa (MNa+), 370.1236; found, 370.1240.
4-(2-(4-Methoxyphenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6c)
White solid; yield 95 mg, 35%; mp 132–134 °C; IR (KBr, cm–1): 3053 (w), 2930 (m), 1715 (s), 1610 (m), 1502 (s), 1490 (s), 1465 (s), 1245 (vs), 1174 (s), 1030 (s), 742 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.13 (s, 3H), 2.78 (t, J = 7.4 Hz, 2H), 3.16 (t, J = 7.4 Hz, 2H), 3.83 (s, 3H), 3.91 (s, 3H), 7.03 (t, J = 8.1 Hz, 1H), 7.06 (d, J = 8.6 Hz, 2H), 7.24 (t, J = 8.1 Hz, 1H), 7.33 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 8.1 Hz, 1H), 7.49 (d, J = 8.6 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 23.2, 30.2, 32.2, 46.0, 55.5, 109.0, 114.1, 118.9, 119.1, 121.7, 122.2, 122.4, 128.3, 129.7, 130.7, 131.4, 141.3, 142.0, 159.1, 207.7; MS (ES+, Ar) m/z (rel intensity): 402 (MK+, 25), 386 (100), 364 (MH+, 20); HRMS (ES+): calcd for C22H21NO2SNa (MNa+), 386.1185; found, 386.1178.
4-(2-(3,4-Dimethoxyphenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6d)
Brown sticky solid; yield 174 mg, 59%; IR (KBr, cm–1): 3054 (w), 2931 (m), 1714 (s), 1507 (s), 1487 (s), 1465 (s), 1260 (s), 1245 (s), 1026 (s), 742 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.13 (s, 3H), 2.78 (t, J = 7.3 Hz, 2H), 3.19 (t, J = 7.3 Hz, 2H), 3.81 (s, 3H), 3.91 (s, 3H), 3.98 (s, 3H), 7.01 (t, J = 8.2 Hz, 1H), 7.04 (d, J = 7.3 Hz, 1H), 7.09 (dd, J = 7.3, 1.3 Hz, 1H), 7.14 (d, J = 1.3 Hz, 1H), 7.24 (t, J = 8.2 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.40 (d, J = 8.2 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 23.1, 30.2, 32.2, 45.8, 56.0 (×2), 109.0, 111.2, 112.7, 118.8, 119.0, 121.7, 121.8, 122.1, 122.2, 128.5, 129.7, 131.3, 141.2, 141.9, 148.4, 148.9, 207.6; MS (ES+, Ar) m/z (rel intensity): 432 (MK+, 25), 416 (MNa+, 100); HRMS (ES+): calcd for C23H23NO3SNa (MNa+), 416.1291; found, 416.1298.
4-(2-(3-Methoxyphenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6e)
White solid; yield 76 mg, 28%; mp 97–99 °C; IR (KBr, cm–1): 3052 (w), 2933 (m), 1714 (s), 1599 (m), 1578 (m), 1491 (s), 1465 (s), 1223 (m), 740 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.14 (s, 3H), 3.80 (t, J = 7.3 Hz, 2H), 3.20 (t, J = 7.3 Hz, 2H), 3.83 (s, 3H), 3.88 (s, 3H), 7.00 (d, J = 7.8 Hz, 1H), 7.05 (t, J = 8.2 Hz, 1H), 7.15 (d, J = 7.8 Hz, 1H), 7.17 (s, 1H), 7.25 (t, J = 8.2 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.44 (t, J = 7.8 Hz, 1H), 7.49 (d, J = 8.2 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 23.2, 30.2, 32.3, 46.0, 55.5, 109.1, 113.4, 115.0, 119.1, 119.2, 121.8, 122.1, 122.2, 129.7, 130.3, 131.6, 137.4, 141.4, 142.1, 159.9, 207.6; MS (ES+, Ar) m/z (rel intensity): 402 (MK+, 50), 386 (MNa+, 100), 364 (MH+, 5), 306 (5); HRMS (ES+): calcd for C22H21NO2SNa (MNa+), 386.1185; found, 386.1182.
4-(2-(3-(Benzyloxy)phenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6f)
Brown sticky solid; yield 167 mg, 51%; IR (KBr, cm–1): 2928 (w), 1715 (s), 1713 (s), 1491 (m), 1465 (m), 1264 (m), 738 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.14 (s, 3H), 2.77 (t, J = 7.3 Hz, 2H), 3.18 (t, J = 7.3 Hz, 2H), 3.83 (s, 3H), 5.16 (s, 2H), 7.06 (t, J = 7.0 Hz, 1H), 7.09 (dd, J = 7.5 Hz, J = 1.8 Hz, 1H), 7.21 (d, J = 7.5 Hz, 1H), 7.23 (t, J = 7.0 Hz, 1H), 7.26 (t, J = 7.5 Hz, 1H), 7.34–7.38 (m, 2H), 7.41 (t, J = 8.2 Hz, 2H), 7.46 (d, J = 7.0 Hz, 1H), 7.47–7.51 (m, 3H); 13C NMR (CDCl3, 125 MHz): δ 23.1, 30.1, 32.2, 45.9, 70.1, 109.0, 114.4, 115.8, 119.0, 119.1, 121.7, 122.0, 122.1, 122.3, 127.6, 128.1, 128.7, 129.7, 130.3, 131.4, 137.1, 137.4, 141.4, 142.0, 159.0, 207.6; MS (ES+, Ar) m/z (rel intensity): 478 (MK+, 50), 462 (MNa+, 100); HRMS (ES+): calcd for C28H25NO2SNa (MNa+), 462.1498; found, 462.1504.
4-(2-(2,5-Dimethoxyphenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6g)
White solid; yield 205 mg, 70%; mp 131–133 °C; IR (KBr, cm–1): 2935 (m), 1714 (s), 1493 (m), 1465 (m), 1268 (m), 1218 (s), 1047 (s), 1024 (m), 738 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.14 (s, 3H), 2.80 (t, J = 7.3 Hz, 2H), 3.08 (t, J = 7.3 Hz, 2H), 3.71 (s, 3H), 3.81 (s, 3H), 3.82 (s, 3H), 6.96–7.01 (m, 2H), 7.01–7.04 (m, 2H), 7.22 (t, J = 7.8 Hz, 1H), 7.30 (ABq collapsed to t, J = 9.0 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 23.7, 30.1, 32.2, 45.6, 55.9, 56.3, 108.9, 112.7, 114.2, 117.1, 118.9, 119.0, 121.5, 122.4, 122.6, 125.6, 127.2, 131.2, 141.2, 141.9, 151.6, 153.7, 207.9; MS (ES+, Ar) m/z (rel intensity): 432 (MK+, 20), 416 (MNa+, 80), 394 (MH+, 35), 390 (100); HRMS (ES+): calcd for C23H23NO3SNa (MNa+), 416.1291; found, 416.1291.
4-(2-(4-Bromophenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6h)
White solid; yield 142 mg, 46%; mp 122–124 °C; IR (KBr, cm–1): 3049 (w), 2925 (m), 1715 (vs), 1479 (s), 1465 (s), 1332 (m), 1162 (m), 827 (m), 741 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.15 (s, 3H), 2.79 (t, J = 7.3 Hz, 2H), 3.15 (t, J = 7.3 Hz, 2H), 3.84 (s, 3H), 7.05 (t, J = 8.0 Hz, 1H), 7.26 (t, J = 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.41 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 8.2 Hz, 2H), 7.66 (d, J = 8.2 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 23.0, 30.2, 32.3, 45.8, 109.2, 118.8, 119.3, 121.6, 121.8, 121.9, 130.4, 130.6, 131.3, 131.9, 135.0, 141.5, 142.0, 207.4; MS (ES+, Ar) m/z (rel intensity): 452 ([MK+2]+, 15), 450 (MK+, 14), 436 [MNa+2]+, 100), 434 (MNa+, 98); (HRMS (ES+): calcd for C21H18NOSBrNa (MNa+), 434.0185; found, 434.0183.
4-(2-(4-Chlorophenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6i)
White solid; yield 175 mg, 63%; mp 95–97 °C; IR (KBr, cm–1): 3051 (w), 2927 (m), 1715 (vs), 1481 (s), 1465 (s), 1332 (m), 1162 (m), 1088 (m), 829 (m), 740 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.16 (s, 3H), 2.80 (t, J = 7.3 Hz, 2H), 3.17 (t, J = 7.3 Hz, 2H), 3.83 (s, 3H), 7.07 (t, J = 8.0 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.52–7.54 (unresolved m, 4H); 13C NMR (CDCl3, 125 MHz): δ 23.0, 30.1, 32.2, 45.7, 109.1, 118.7, 119.2, 121.8, 121.9, 128.9, 130.3, 130.6, 130.9, 133.4, 134.5, 141.4, 142.0, 207.3; MS (ES+, Ar) m/z (rel intensity): 408 ([MK+2]+, 20), 406 (MK+, 60), 392 ([MNa+2]+, 33), 390 (MNa+, 100); HRMS (ES+): calcd for C21H18ClNOSNa (MNa+), 390.0690; found, 390.0692.
4-(2-(4-Fluorophenyl)-8-methyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6j)
White solid; yield 125 mg, 48%; mp 79–81 °C; IR (KBr, cm–1): 2928 (w), 1715 (vs), 1500 (s), 1465 (m), 1221 (s), 1158 (m), 742 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.16 (s, 3H), 2.81 (t, J = 7.3 Hz, 2H), 3.17 (t, J = 7.3 Hz, 2H), 3.84 (s, 3H), 7.07 (t, J = 8.2 Hz, 1H), 7.24 (t, J = 8.2 Hz, 1H), 7.27 (dd, J = 7.7 Hz, J = 3.5 Hz, 2H), 7.36 (d, J = 8.2 Hz, 1H), 7.43 (d, J = 8.2 Hz, 1H), 7.56 (dd, J = 7.7, 5.6 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 23.0, 30.1, 32.2, 45.8, 109.1, 115.7 (d, J = 21.3 Hz), 118.7, 119.2, 121.8, 122.0, 122.1, 130.3, 130.5, 131.2 (d, J = 8.8 Hz), 132.0 (d, J = 3.8 Hz), 141.3, 142.0, 162.3 (d, J = 245.0 Hz), 207.5; 19F NMR (CDCl3, 470 MHz): δ 114.7; MS (ES+, Ar) m/z (rel intensity): 390 (MK+, 55), 374 (MNa+, 100), 352 (MH+, 8), 294 (17); HRMS (ES+): calcd for C21H18FNOSNa (MNa+), 374.0985; found, 374.0989.
4-(8-Methyl-2-(naphthalen-1-yl)-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6k)
White solid; yield 160 mg, 56%; mp 99–100 °C; IR (KBr, cm–1): 3054 (m), 2928 (m), 1715 (s), 1489 (m), 1464 (m), 1331 (m), 1265 (m), 1163 (m), 803 (m), 782 (s), 740 (vs), 703 (m); 1H NMR (CDCl3, 500 MHz): δ 2.0 (s, 3H), 2.68, 2.72 (ABqdd, J = 17.7, 7.1, 6.6 Hz, 2H), 3.00 (ddd, J = 15.1, 7.1, 6.6 Hz, 1H), 3.09 (ddd, J = 15.1, 7.1, 6.6 Hz, 1H), 3.88 (s, 3H), 6.68 (d, J = 7.9 Hz, 1H), 6.83 (t, J = 7.9 Hz, 1H), 7.18 (t, J = 7.9 Hz, 1H), 7.33 (d, J = 7.9 Hz, 1H), 7.36 (t, J = 7.2 Hz, 1H), 7.53 (t, J = 7.2 Hz, 1H), 7.59 (d, J = 7.1 Hz, 1H), 7.63 (t, J = 7.1 Hz, 1H), 7.78 (d, J = 7.2 Hz, 1H), 7.98 (d, J = 7.2 Hz, 1H), 7.99 (d, J = 7.1 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 23.4, 30.0, 32.3, 45.8, 108.9, 119.0, 119.1, 121.6, 122.1, 123.5, 125.7, 126.1, 126.3, 126.4, 127.7, 128.3, 128.4, 129.6, 131.4, 132.4, 133.8, 133.9, 141.2, 141.9, 207.6; MS (ES+, Ar) m/z (rel intensity): 422 (MK+, 100), 406 (MNa+, 50); HRMS (ES+): calcd for C25H21NOSK (MK+), 422.0975; found, 422.0995. Confirmed by 1H–1H COSY experiments.
4-(8-Methyl-2-(thiophen-2-yl)-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6l)
Brown sticky solid; yield 80 mg, 31%; IR (KBr, cm–1): 3050 (vw), 2924 (w), 1714 (vs), 1491 (m), 1464 (m), 1332 (m), 1162 (m), 742 (vs), 702 (s); 1H NMR (CDCl3, 500 MHz): δ 2.16 (s, 3H), 2.83 (t, J = 7.4 Hz, 2H), 3.25 (t, J = 7.4 Hz, 2H), 3.83 (s, 3H), 7.08 (td, J = 8.2, 0.8 Hz, 1H), 7.21 (dd, J = 3.5, 1.6 Hz, 1H), 7.26 (td, J = 8.2, 0.8 Hz, 1H), 7.27 (d, J = 1.6 Hz, 1H), 7.34 (br d, J = 8.2 Hz, 1H), 7.45 (dd, J = 5.2, 3.5 Hz, 1H), 7.67 (br d, J = 8.2 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 23.4, 30.2, 32.2, 46.0, 109.1, 119.1, 119.2, 121.9, 122.0, 122.2, 124.0, 125.7, 127.3, 127.4, 132.0, 136.5, 141.2, 142.0, 207.7; MS (ES+, Ar) m/z (rel intensity): 362 (MNa+, 95), 340 (MH+, 100); HRMS (ES+): calcd for C19H18NOS2 (MH+), 340.0824; found, 340.0825.
4-(8-Ethyl-2-phenyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6m)
White solid; yield 225 mg, 87%; mp 79–87 °C; IR (KBr, cm–1): 3052 (w), 2976 (m), 2931 (w), 1715 (vs), 1485 (s), 1467 (m), 1452 (m), 1336 (m), 1163 (m), 742 (vs); 1H NMR (CDCl3, 500 MHz): δ 1.52 (t, J = 7.2 Hz, 3H), 2.13 (s, 3H), 2.79 (t, J = 7.4 Hz, 2H), 3.16 (t, J = 7.4 Hz, 2H), 4.26 (q, J = 7.2 Hz, 2H), 7.00 (t, J = 8.2 Hz, 1H), 7.22 (t, J = 8.2 Hz, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.41 (d, J = 8.2 Hz, 1H), 7.43 (overlapped t, J = 7.4 Hz, 1H), 7.51 (t, J = 7.4 Hz, 2H), 7.56 (d, J = 7.4 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 14.1, 23.1, 30.2, 40.8, 46.0, 109.1, 118.9, 119.0, 121.7, 122.2, 122.5, 127.5, 128.7, 129.6, 130.2, 131.6, 136.0, 139.9, 141.0, 207.7; MS (ES+, Ar) m/z (rel intensity): 386 (MK+, 20), 370 (MNa+, 55), 348 (MH+, 100); HRMS (ES+): calcd for C22H22NOS (MH+), 348.1417; found, 348.1411.
4-(2-Phenyl-8-propyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6n)
Brown sticky solid; yield 150 mg, 55%; IR (KBr, cm–1): 2964 (w), 1714 (s), 1646 (s), 1484 (m), 1460 (m), 740 (vs), 703 (m); 1H NMR (CDCl3, 500 MHz): δ 1.03 (t, J = 7.2 Hz, 3H), 1.99 (sextet, J = 7.2 Hz, 2H), 2.14 (s, 3H), 2.81 (t, J = 7.4 Hz, 2H), 3.19 (t, J = 7.4 Hz, 2H), 4.18 (t, J = 7.2 Hz, 2H), 7.03 (t, J = 7.5 Hz, 1H), 7.24 (t, J = 7.5 Hz, 1H), 7.37 (d, J = 7.5 Hz), 7.45 (d, J = 7.5 Hz, 1H), 7.46 (overlapped t, J = 7.6 Hz, 1H), 7.54 (t, J = 7.6 Hz, 2H), (d, J = 7.6 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 11.9, 22.5, 23.0, 30.1, 45.9, 47.8, 109.2, 118.9, 119.0, 121.6, 122.1, 122.3, 127.5, 128.7, 129.6, 130.1, 131.5, 136.0, 140.5, 141.5, 207.7; MS (ES+, Ar) m/z (rel intensity): 400 (MK+, 20), 384 (MNa+, 50), 378 (55), 360 ([M – 1]+, 100); HRMS (ES+): calcd for C23H23NOSNa (MNa+), 384.1393; found, 384.1394.
4-(8-Benzyl-2-phenyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6o)
White solid; yield 287 mg, 94%; mp 100–102 °C; IR (KBr, cm–1): 3055 (m), 2919 (w), 1714 (vs), 1481 (s), 1456 (s), 1359 (m), 1336 (m), 1162 (m), 781 (m), 736 (vs), 703 (vs); 1H NMR (CDCl3, 500 MHz): δ 2.11 (s, 3H), 2.76 (t, J = 7.5 Hz, 2H), 3.15 (t, J = 7.5 Hz, 2H), 5.37 (s, 2H), 7.06 (t, J = 7.6 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 7.29–7.32 (m, 2H), 7.33–7.41 (m, 4H), 7.44–7.51 (m, 2H), 7.55 (t, J = 7.9 Hz, 2H), 7.60 (d, J = 7.9 Hz, 2H); 13C NMR (CDCl3, 125 MHz): δ 23.0, 30.1, 45.9, 49.8, 109.5, 119.0, 119.3, 121.9, 122.4, 122.9, 127.5, 127.6, 128.1, 128.7, 129.0, 129.6, 130.7, 131.4, 135.9, 136.0, 140.5, 141.7, 207.6; MS (ES+, Ar) m/z (rel intensity): 448 (MK+, 45), 432 (MNa+, 100); HRMS (ES+): calcd for C27H23NOSNa (MNa+), 432.1393; found, 432.1396.
4-(5-Chloro-8-methyl-2-phenyl-8H-thieno[2,3-b]indol-3-yl)butan-2-one (6p)
Brown sticky solid; yield 122 mg, 44%; IR (KBr, cm–1): 3055 (w), 2924 (m), 1715 (vs), 1488 (s), 1474 (s), 1362 (m), 1309 (m), 1163 (m), 924 (m), 793 (s), 739 (m); 1H NMR (CDCl3, 500 MHz): δ 2.12 (s, 3H), 2.77 (t, J = 7.4 Hz, 2H), 3.15 (t, J = 7.4 Hz, 2H), 3.79 (s, 3H), 7.16, 7.21 (ABq, J = 7.5 Hz, 2H), 7.35 (s, 1H), 7.43–7.46 (unresolved m, 1H), 7.51–7.54 (m, 4H); 13C NMR (CDCl3, 125 MHz): δ 23.1, 30.2, 32.4, 45.9, 109.9, 118.5, 121.5, 121.8, 122.9, 124.8, 127.8, 128.9, 129.4, 130.8, 131.5, 135.6, 140.4, 142.6, 207.5; MS (ES+, Ar) m/z (rel intensity): 408 ([MK+2]+, 5), 406 (MK+, 15), 392 (MNa+2]+, 33), 390 (MNa+, 100); HRMS (ES+): calcd for C21H18NOSClNa (MNa+), 390.0690; found, 390.0690.
4-(3-Phenyl-8H-thieno[2,3-b]indol-2-yl)butan-2-one (6q)
White solid; yield 105 mg, 44%; mp 144–146 °C; IR (KBr, cm–1): 3390 (br vs), 2962 (s), 2931 (m), 1728 (vs), 1619 (vs), 1506 (m), 1440 (m), 1286 (m), 1246 (vs), 1027 (m), 820 (m), 765 (m); 1H NMR (CDCl3, 400 MHz): δ 2.13 (s, 3H), 2.79 (t, J = 7.4 Hz, 2H), 3.19 (t, J = 7.4 Hz, 2H), 7.05 (t, J = 8.1 Hz, 1H), 7.20 (t, J = 8.1 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 7.45 (t, J = 7.3 Hz, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.54 (t, J = 7.3 Hz, 2H), 7.59 (d, J = 7.3 Hz, 2H), 8.56 (br s, 1H); 13C NMR (CDCl3, 100 MHz): δ 23.0, 30.1, 45.9, 111.4, 118.8, 119.5, 122.1, 122.3, 124.4, 127.5, 128.7, 129.6, 130.7, 131.1, 135.9, 138.1, 141.8, 208.3; MS (ES+, Ar) m/z (rel intensity): 342 (MNa+, 100); HRMS (ES+): calcd for C20H17NOSNa (MNa+), 342.0923; found, 342.0928.
Procedure for the Synthesis of Pyrazole-Containing Thieno[2,3-b]indole (8)
To a stirred solution of hydrazinothienoindole 4d (54 mg, 0.103 mmol, 1 equiv) in MeOH (1.5 mL) was added 4N HCl in dioxane (0.77 mL, 3.093 mmol, 30 equiv) at room temperature. The reaction mixture was stirred for 10 min, and then, acetyl acetone 7 (21 mg, 0.206 mmol, 2 equiv) was added and the mixture was stirred at room temperature for 12 h. After completion of the reaction (monitored by TLC), the solvent was removed in vacuo and the crude product was partitioned between EtOAc (10 mL) and sat aq NaHCO3 (10 mL). The organic phase was separated, washed with brine (10 mL), dried over anhydrous sodium sulphate, and concentrated in vacuo. The crude product was purified by silica gel column chromatography by gradient elution with ethyl acetate-pet ether (15–20%).
2-(3,5-Dimethyl-1H-pyrazol-1-yl)-3-(4-methoxyphenyl)-8-methyl-8H-thieno[2,3-b]indole (8)
Brown solid; yield 19 mg, 48%; mp 175–177 °C; IR (KBr, cm–1): 1647 (s), 1612 (s), 1248 (m), 1029 (m), 739 (vs); 1H NMR (CDCl3, 500 MHz): 1.77 (s, 3H), 2.33 (s, 3H), 3.84 (s, 3H), 3.87 (s, 3H), 5.82 (s, 1H), 6.92 (d, J = 8.5 Hz, 2H), 7.11 (t, J = 7.5 Hz, 1H), 7.31 (t, J = 7.5 Hz, 1H), 7.40 (d, J = 8.5 Hz, 2H and d, J = 7.5 Hz, 1H overlapped), 7.75 (d, J = 7.5 Hz, 1H); 13C NMR (CDCl3, 125 MHz): δ 11.4, 13.9, 32.4, 55.4, 106.0, 109.3, 114.2, 118.3, 119.5, 119.6, 122.5, 122.9, 124.7, 126.3, 132.9, 141.1, 141.7, 143.5 (×2), 149.8, 159.4; MS (ES+, Ar) m/z (rel intensity): 410 (MNa+, 5), 388 (MH+, 100), HRMS (ES+): calcd for C23H22N3OS (MH+), 388.1478; found, 388.1475.
Acknowledgments
I.N.N.N. thanks SERB, DST India for financial assistance. V.M. thanks CSIR India for a senior Research Fellowship and T.V.B. thanks IIT Bombay for an Institute Postdoctoral Fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02147.
Crystal data and structure refinement for compounds 3a, 4h, and 6e and NMR spectra of compounds 3a, 4a–i, 6a–q, and 8 (PDF)
Single-crystal X-ray data of compound 3a: CCDC 1862743 (CIF)
Single-crystal X-ray data of compound 4h: CCDC 1862744 (CIF)
Single crystal X-ray data of compound 6e: CCDC 1862745 (CIF)
Author Present Address
† Service de Chimie Bioorganique et de Marquage (SCBM), CEA, Université Paris-Saclay, 91191 Gif-sur-Yvette, France.
The authors declare no competing financial interest.
Supplementary Material
References
- Books:; a Ingall A. H.Thiopyrans and their benzo derivatives. In Comprehensive Heterocyclic Chemistry II; Katritzky A. R., Rees C. W., Scriven E. F. V., Eds.; Pergamon: Oxford, 1996; pp 501–617. [Google Scholar]; b Schneller S. W.Thiochromanones and related compounds. In Advances in Heterocyclic Chemistry; Katritzky A. R., Boulton A. J., Eds.; Academic Press, 1975; Vol. 18, pp 59–97. [Google Scholar]; Reviews:; c Beno B. R.; Yeung K.-S.; Bartberger M. D.; Pennington L. D.; Meanwell N. A. A survey of the role of noncovalent sulfur interactions in drug design. J. Med. Chem. 2015, 58, 4383–4438. 10.1021/jm501853m. [DOI] [PubMed] [Google Scholar]; d Chauhan P.; Mahajan S.; Enders D. Organocatalytic carbon-sulfur bond-forming reactions. Chem. Rev. 2014, 114, 8807–8864. 10.1021/cr500235v. [DOI] [PubMed] [Google Scholar]
- Book:; a Biehl E. R.Five-membered ring systems: Thiophenes and Se/Te derivatives. In Progress in Heterocyclic Chemistry; Gribble G. W., Joule J. A., Eds.; Elsevier, 2015; Chapter 5.1, Vol. 27, pp 117–157. [Google Scholar]; Reviews:; b Gramec D.; Mašič L. P.; Dolenc M. S. Bioactivation potential of thiophene-containing drugs. Chem. Res. Toxicol. 2014, 27, 1344–1358. 10.1021/tx500134g. [DOI] [PubMed] [Google Scholar]; c Jha K. K.; Kumar S.; Tomer I.; Mishra R. Thiophene: The molecule of diverse medicinal importance. J. Pharm. Res. 2012, 5, 560–566. [Google Scholar]; d Mishra R.; Jha K. K.; Kumar S.; Tomer I. Synthesis, properties and biological activity of thiophene: A review. Der Pharma Chem. 2011, 3, 38–54. [Google Scholar]
- a Kanbe K.; Okamura M.; Hattori S.; Naganawa H.; Hamada M.; Okami Y.; Takeuchi T. Thienodolin, a new plant growth-regulating substance produced by a streptomycete strain: I. Taxonomy and fermentation of the producing strain, and the isolation and characterization of thienodolin. Biosci., Biotechnol., Biochem. 1993, 57, 632–635. 10.1271/bbb.57.632. [DOI] [Google Scholar]; b Engqvist R.; Javaid A.; Bergman J. Synthesis of thienodolin. Eur. J. Org. Chem. 2004, 2589–2592. 10.1002/ejoc.200400073. [DOI] [Google Scholar]
- Pedras M. S. C.; Suchy M. Design, synthesis, and antifungal activity of inhibitors of brassilexin detoxification in the plant pathogenic fungus Leptosphaeria maculans. Bioorg. Med. Chem. 2006, 14, 714–723. 10.1016/j.bmc.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Jakobsen P.; Kanstrup A.; Faarup P.; Olesen P. H.; Lundbech J. M.. Preparation of thieno[2,3-b]indoles and pyrido[2,3-b]indoles as antagonists at the metabotropic glutamate receptor. U. S. Patent 5,783,575 A, 1998; Chem. Abstr. 1998, 129, 148909f.
- Kanbe K.; Naganawa H.; Nakamura K. T.; Okami Y.; Takeuchi T. Thienodolin, a new plant growth-regulating substance produced by a streptomycete strain: II. Structure of thienodolin. Biosci., Biotechnol., Biochem. 1993, 57, 636–637. 10.1271/bbb.57.636. [DOI] [Google Scholar]
- a Baert F.; Cabanetos C.; Allain M.; Silvestre V.; Leriche P.; Blanchard P. Thieno[2,3-b]indole-based small push-pull chromophores: Synthesis, structure, and electronic properties. Org. Lett. 2016, 18, 1582–1585. 10.1021/acs.orglett.6b00438. [DOI] [PubMed] [Google Scholar]; b Qi T.; Qiu W.; Liu Y.; Zhang H.; Gao X.; Liu Y.; Lu K.; Du C.; Yu G.; Zhu D. Synthesis, structures, and properties of disubstituted heteroacenes on one side containing both pyrrole and thiophene rings. J. Org. Chem. 2008, 73, 4638–4643. 10.1021/jo800622y. [DOI] [PubMed] [Google Scholar]; c Irgashev R. A.; Karmatsky A. A.; Kozyukhin S. A.; Ivanov V. K.; Sadovnikov A.; Kozik V. V.; Grinberg V. A.; Emets V. V.; Rusinov G. L.; Charushin V. N. A facile and convenient synthesis and photovoltaic characterization of novel thieno[2,3-b]indole dyes for dye-sensitized solar cells. Synth. Met. 2015, 199, 152–158. 10.1016/j.synthmet.2014.11.024. [DOI] [Google Scholar]
- Mézlová M.; Aaron J. J.; Svoboda J.; Adenier A.; Maurel F.; Chane-Ching K. Novel conducting polymers based on thieno[3,2-b]indoles: Electrochemical properties and molecular structure. J. Electroanal. Chem. 2005, 581, 93–103. 10.1016/j.jelechem.2005.04.017. [DOI] [Google Scholar]
- a Olesen P. H.; Hansen J. B.; Engelstoft M. Thieno[2,3-b]indole. The unsubstituted ring system. J. Heterocycl. Chem. 1995, 32, 1641–1642. 10.1002/jhet.5570320540. [DOI] [Google Scholar]; b Gao H.; Xu Q.-L.; Yousufuddin M.; Ess D. H.; Kürti L. Rapid synthesis of fused N-heterocycles by transition-metal-free electrophilic amination of arene C-H bonds. Angew. Chem., Int. Ed. 2014, 53, 2701–2705. 10.1002/anie.201309973. [DOI] [PubMed] [Google Scholar]; c Appukkuttan P.; Van der Eycken E.; Dehaen W. Microwave-enhanced Cadogan cyclization: An easy access to the 2-substituted carbazoles and other fused heterocyclic systems. Synlett 2005, 127–133. 10.1055/s-2004-836030. [DOI] [Google Scholar]
- a Cadogan J. I. G. Phosphite-reduction of aromatic nitro-compounds as a route to heterocycles. Synthesis 1969, 11–17. 10.1055/s-1969-34189. [DOI] [Google Scholar]; b Smitrovich J. H.; Davies I. W. Catalytic C–H functionalization driven by CO as a stoichiometric reductant: Application to carbazole synthesis. Org. Lett. 2004, 6, 533–535. 10.1021/ol036294l. [DOI] [PubMed] [Google Scholar]
- Butin A.; Tsiunchik F.; Abaev V.; Zavodnik V. A new simple route to the thieno[2,3-b]indole ring system. Synlett 2008, 1145–1148. 10.1055/s-2008-1072720. [DOI] [Google Scholar]
- Kienle M.; Wagner A. J.; Dunst C.; Knochel P. Preparation of heterocyclic amines by an oxidative amination of zinc organometallics mediated by CuI: A new oxidative cycloamination for the preparation of annulated indole derivatives. Chem.—Asian J. 2011, 6, 517–523. 10.1002/asia.201000367. [DOI] [PubMed] [Google Scholar]
- a Singh P. P.; Yadav A. K.; Ila H.; Junjappa H. A novel radical cyclization approach to thieno-fused heterocycles. Eur. J. Org. Chem. 2011, 4001–4007. 10.1002/ejoc.201100516. [DOI] [Google Scholar]; b Yadav A. K.; Ila H.; Junjappa H. Synthesis of novel substituted phenanthrenes and polycyclic heteroarenes by Pd-catalyzed, direct, intramolecular arylation/heteroarylation. Eur. J. Org. Chem. 2010, 338–344. 10.1002/ejoc.200901036. [DOI] [Google Scholar]
- Irgashev R. A.; Karmatsky A. A.; Rusinov G. L.; Charushin V. N. A new and convenient synthetic way to 2-substituted thieno[2,3-b]indoles. Beilstein J. Org. Chem. 2015, 11, 1000–1007. 10.3762/bjoc.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Li B.; Ni P.; Huang H.; Xiao F.; Deng G.-J. Three-component thieno[2,3-b ]indole synthesis from indoles, alkenes or alkynes and sulfur powder under metal-free conditions. Adv. Synth. Catal. 2017, 359, 4300–4304. 10.1002/adsc.201701106. [DOI] [Google Scholar]; b Ni P.; Li B.; Huang H.; Xiao F.; Deng G.-J. Solvent-controlled highly regio-selective thieno[2,3-b]indole formation under metal-free conditions. Green Chem. 2017, 19, 5553–5558. 10.1039/c7gc02818k. [DOI] [Google Scholar]
- a Yi L.; Chen K.-Q.; Liang Z.-Q.; Sun D.-Q.; Ye S. N-Heterocyclic carbene-catalyzed [3+3] annulation of indoline-2-thiones with bromoenals: Synthesis of indolo[2,3-b]dihydrothiopyranones. Adv. Synth. Catal. 2017, 359, 44–48. 10.1002/adsc.201600726. [DOI] [Google Scholar]; b Chen X.; Qi Z.-H.; Zhang S.-Y.; Kong L.-P.; Wang Y.; Wang X.-W. Enantioselective construction of functionalized thiopyrano-indole annulated heterocycles via a formal thio [3 + 3]-cyclization. Org. Lett. 2015, 17, 42–45. 10.1021/ol503210q. [DOI] [PubMed] [Google Scholar]; c Jha M.; Shelke G. M.; Cameron T. S.; Kumar A. Access to substituted dihydrothiopyrano[2,3-b]indoles via sequential rearrangements during S-alkylation and Au-catalyzed hydroarylation on indoline-2-thiones. J. Org. Chem. 2015, 80, 5272–5278. 10.1021/jo5025943. [DOI] [PubMed] [Google Scholar]; d Chen S.; Pan J.; Wang Y.; Zhou Z. Stereocontrolled construction of the 3,4-dihydrothiacarbazol-2(9H)-one skeleton by using bifunctional squaramide-catalyzed cascade reactions. Eur. J. Org. Chem. 2014, 7940–7947. 10.1002/ejoc.201403078. [DOI] [Google Scholar]; e Shelke G. M.; Jha M.; Kumar A. Synthesis of indole-annulated sulfur heterocycles using copper-catalysed C-N coupling and palladium-catalysed direct arylation. Org. Biomol. Chem. 2016, 14, 3450–3458. 10.1039/c6ob00117c. [DOI] [PubMed] [Google Scholar]; f Ni C.; Zhang Y.; Hou Y.; Tong X. Access to thiopyrano[2,3-b]indole via tertiary amine-catalyzed formal (3+3) annulations of β′-acetoxy allenoates with indoline-2-thiones. Chem. Commun. 2017, 53, 2567–2570. 10.1039/c6cc09788j. [DOI] [PubMed] [Google Scholar]; g Wu L.-L.; Zheng Y.; Wang Y.-M.; Zhou Z.-H. Organocatalyzed enantioselective [3 + 3] annulation for the direct synthesis of conformationally constrained cyclic tryptophan derivatives. RSC Adv. 2016, 6, 11602–11608. 10.1039/c5ra24288f. [DOI] [Google Scholar]; h Jha M.; Dhiman S.; Cameron T. S.; Kumar D.; Kumar A. Au-catalyzed synthesis of thiopyrano[2,3-b]indoles featuring tandem rearrangement and hydroarylation. Org. Lett. 2017, 19, 2038–2041. 10.1021/acs.orglett.7b00617. [DOI] [PubMed] [Google Scholar]; i Chen X.; Zhang J.-Q.; Yin S.-J.; Li H.-Y.; Zhou W.-Q.; Wang X.-W. Asymmetric construction of spiro[thiopyranoindole-benzoisothiazole] scaffold via a formal [3 + 3] spiroannulation. Org. Lett. 2015, 17, 4188–4191. 10.1021/acs.orglett.5b01951. [DOI] [PubMed] [Google Scholar]; j Zhou S.; Xiao G.; Liang Y. Copper-catalyzed synthesis of 2-sulfenylindoles from indoline-2-thiones and aryl iodides. Tetrahedron Lett. 2017, 58, 338–341. 10.1016/j.tetlet.2016.12.028. [DOI] [Google Scholar]; k Berg R.; Bergman J. Synthesis of thieno[2,3- b]indole-2,3-diones and their ring expansions induced by diazomethane. Tetrahedron 2017, 73, 5654–5658. 10.1016/j.tet.2017.07.059. [DOI] [Google Scholar]; l Taheri S.; Mohammadi A. A.; Ahdenov R.; Azarlak R. Ultrasound-mediated efficient synthesis of dihydrothiopyrano[2,3-b]indole-3-carbonitrile derivatives. J. Iran. Chem. Soc. 2016, 13, 1301–1306. 10.1007/s13738-016-0844-8. [DOI] [Google Scholar]
- a Boeini H. Z. Highly efficient synthesis of thieno[2,3-b]indole derivatives. Helv. Chim. Acta 2009, 92, 1268–1272. 10.1002/hlca.200800423. [DOI] [Google Scholar]; b Savych I.; Gläsel T.; Villinger A.; Sosnovskikh V. Y.; Iaroshenko V. O.; Langer P. Synthesis of functionalized 2-salicyloylfurans, furo[3,2-b]chromen-9-ones and 2-benzoyl-8H-thieno[2,3-b]indoles by one-pot cyclizations of 3-halochromones with β-ketoamides and 1,3-dihydroindole-2-thiones. Org. Biomol. Chem. 2015, 13, 729–750. 10.1039/c4ob01730g. [DOI] [PubMed] [Google Scholar]; c Moghaddam F.; Saeidian H.; Mirjafary Z.; Taheri S.; Kheirjou S. A new and facile synthesis of thieno[2,3-b]indole derivatives via condensation of isocyanide and indolin-2-thiones. Synlett 2009, 1047–1050. 10.1055/s-0028-1088107. [DOI] [Google Scholar]
- Selected book:; a Zhao M.-X.; Wei Y.; Shi M. In The Chemistry of the Morita–Baylis–Hillman Reaction; Shi M., Wang F.-J., Zhao M.-X., Wei Y., Eds.; RSC Catalysis Series; Royal Society of Chemistry: Cambridge, 2011; Vol. 8, pp 1–561. Reviews: [Google Scholar]; b Ciganek E. The catalyzed α-hydroxyalkylation and α-aminoalkylation of activated olefins (The Morita-Baylis-Hillman reaction). Org. React. 1997, 51, 201–350. 10.1002/0471264180.or051.02. [DOI] [Google Scholar]; c Declerck V.; Martinez J.; Lamaty F. aza-Baylis–Hillman Reaction. Chem. Rev. 2009, 109, 1–48. 10.1021/cr068057c. [DOI] [PubMed] [Google Scholar]; d Masson G.; Housseman C.; Zhu J. The enantioselective Morita-Baylis-Hillman reaction and its aza counterpart. Angew. Chem., Int. Ed. 2007, 46, 4614–4628. 10.1002/anie.200604366. [DOI] [PubMed] [Google Scholar]; e Basavaiah D.; Naganaboina R. T. The Baylis-Hillman reaction: A new continent in organic chemistry - our philosophy, vision and over three decades of research. New J. Chem. 2018, 42, 14036–14066. 10.1039/c8nj02483a. [DOI] [Google Scholar]; f Singh V.; Batra S. Advances in the Baylis-Hillman reaction-assisted synthesis of cyclic frameworks. Tetrahedron 2008, 64, 4511–4574. 10.1016/j.tet.2008.02.087. [DOI] [Google Scholar]
- Reviews:; a Xie P.; Huang Y. Domino cyclization initiated by cross-Rauhut-Currier reactions. Eur. J. Org. Chem. 2013, 6213–6226. 10.1002/ejoc.201300469. [DOI] [Google Scholar]; b Aroyan C. E.; Dermenci A.; Miller S. J. The Rauhut-Currier reaction: a history and its synthetic application. Tetrahedron 2009, 65, 4069–4084. 10.1016/j.tet.2009.02.066. [DOI] [Google Scholar]; c Methot J. L.; Roush W. R. Nucleophilic phosphine organocatalysis. Adv. Synth. Catal. 2004, 346, 1035–1050. 10.1002/adsc.200404087. [DOI] [Google Scholar]
- a Nair D. K.; Kumar T.; Namboothiri I. N. N. α-Functionalization of nitroalkenes and its applications in organic synthesis. Synlett 2016, 27, 2425–2442. 10.1055/s-0036-1588587. [DOI] [Google Scholar]; b Halimehjani A. Z.; Namboothiri I. N. N.; Hooshmand S. E. Part I: Nitroalkenes in the synthesis of heterocyclic compounds. RSC Adv. 2014, 4, 48022–48084. 10.1039/c4ra08828j. [DOI] [Google Scholar]; c Halimehjani A. Z.; Namboothiri I. N. N.; Hooshmand S. E. Part II: Nitroalkenes in the synthesis of heterocyclic compounds. RSC Adv. 2014, 4, 51794–51829. 10.1039/c4ra08830a. [DOI] [Google Scholar]; d Kaur K.; Namboothiri I. N. N. Morita-Baylis-Hillman and Rauhut-Currier reactions of conjugated nitroalkenes. Chimia 2012, 66, 913–920. 10.2533/chimia.2012.913. [DOI] [PubMed] [Google Scholar]; e Baiju T. V.; Namboothiri I. N. N. Synthesis of functionalized pyrazoles via 1,3-dipolar cycloaddition of α -diazo-β -ketophosphonates, sufones and esters with electron-deficient alkenes. Chem. Rec. 2017, 17, 939–955. 10.1002/tcr.201600141. [DOI] [PubMed] [Google Scholar]; See also; f Huang W.-Y.; Anwar S.; Chen K. Morita-Baylis-Hillman (MBH) reaction derived nitroallylic alcohols, acetates and amines as synthons in organocatalysis and heterocycle synthesis. Chem. Rec. 2017, 17, 363–381. 10.1002/tcr.201600075. [DOI] [PubMed] [Google Scholar]
- Dadwal M.; Mobin S. M.; Namboothiri I. N. N. Highly efficient hydrazination of conjugated nitroalkenes via imidazole or DMAP mediated Morita-Baylis-Hillman reaction. Org. Biomol. Chem. 2006, 4, 2525–2528. 10.1039/b604899d. [DOI] [PubMed] [Google Scholar]
- a Shanbhag P.; Nareddy P. R.; Dadwal M.; Mobin S. M.; Namboothiri I. N. N. Rauhut-Currier type homo- and heterocouplings involving nitroalkenes and nitrodienes. Org. Biomol. Chem. 2010, 8, 4867–4873. 10.1039/c0ob00062k. [DOI] [PubMed] [Google Scholar]; b Dadwal M.; Mohan R.; Panda D.; Mobin S. M.; Namboothiri I. N. N. The Morita-Baylis-Hillman adducts of β-aryl nitroethylenes with other activated alkenes: synthesis and anticancer activity studies. Chem. Commun. 2006, 338–340. 10.1039/b512267h. [DOI] [PubMed] [Google Scholar]
- Mane V.; Pandey J.; Ayyagari N.; Dey C.; Kale R.; Namboothiri I. N. N. Synthesis of hydrazinoheterocycles from Morita-Baylis-Hillman adducts of nitroalkenes with azodicarboxylates. Org. Biomol. Chem. 2016, 14, 2427–2438. 10.1039/c5ob02656c. [DOI] [PubMed] [Google Scholar]
- For furan synthesis:; a Mane V.; Kumar T.; Pradhan S.; Katiyar S.; Namboothiri I. N. N. One-pot regioselective synthesis of functionalized and fused furans from Morita-Baylis-Hillman and Rauhut-Currier adducts of nitroalkenes. RSC Adv. 2015, 5, 69990–69999. 10.1039/c5ra11471c. [DOI] [Google Scholar]; For pyrazole synthesis:; b Muruganantham R.; Namboothiri I. N. N. Phosphonylpyrazoles from Bestmann–Ohira reagent and nitroalkenes: Synthesis and dynamic NMR studies. J. Org. Chem. 2010, 75, 2197–2205. 10.1021/jo902595e. [DOI] [PubMed] [Google Scholar]; c Kumar R.; Namboothiri I. N. N. Regioselective synthesis of sulfonylpyrazoles via base mediated reaction of diazosulfones with nitroalkenes and a facile entry into withasomnine. Org. Lett. 2011, 13, 4016–4019. 10.1021/ol201534f. [DOI] [PubMed] [Google Scholar]; d Verma D.; Kumar R.; Namboothiri I. N. N. Synthesis of withasomnines and their non-natural analogues from aldehydes and 4-nitro-1-butanol in three Steps. J. Org. Chem. 2013, 78, 3482–3486. 10.1021/jo400207u. [DOI] [PubMed] [Google Scholar]; For decaline synthesis:; e Shanbhag P.; Mane V.; Hazra C.; Namboothiri I. N. N. Diastereoselective synthesis of substituted decalins from Rauhut-Currier adducts of conjugated nitroalkenes. J. Indian Chem. Soc. 2013, 90, 1713–1719. [Google Scholar]; For cycloalkanone synthesis: ref (20).
- For spirocycle synthesis:; a Roy S.; Amireddy M.; Chen K. Organocatalytic formal [5+1] annulation: Diastereoselective cascade synthesis of functionalized six-membered spirocyclic indane-1,3-diones/oxindoles via Michael-aldol reaction. Tetrahedron 2013, 69, 8751–8757. 10.1016/j.tet.2013.07.084. [DOI] [Google Scholar]; For epibatidine synthesis:; b Huang X.; Shi H.; Ren J.; Liu G.; Tang Y.; Zeng B. An improved practical route to (±)-epibatidine through L-proline catalyzed intramolecular Michael addition. Chin. J. Chem. 2012, 30, 1305–1309. 10.1002/cjoc.201200298. [DOI] [Google Scholar]
- Kumar T.; Mane V.; Namboothiri I. N. N. Synthesis of aminophenanthrenes and benzoquinolines via Hauser-Kraus annulation of sulfonyl phthalide with Rauhut-Currier adducts of nitroalkenes. Org. Lett. 2017, 19, 4283–4286. 10.1021/acs.orglett.7b01924. [DOI] [PubMed] [Google Scholar]
- Pedras M. S. C.; Jha M. Concise syntheses of the cruciferous phytoalexins brassilexin, sinalexin, wasalexins, and analogues: Expanding the scope of the Vilsmeier formylation. J. Org. Chem. 2005, 70, 1828–1834. 10.1021/jo0479866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.