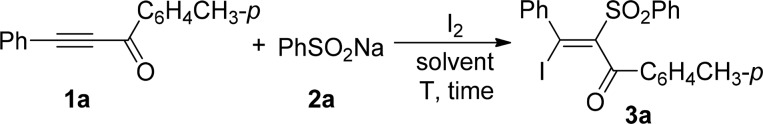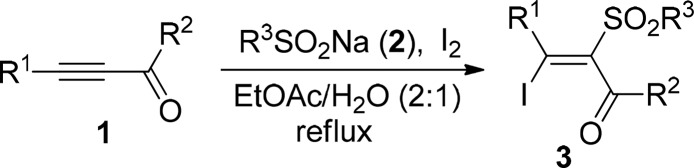Abstract
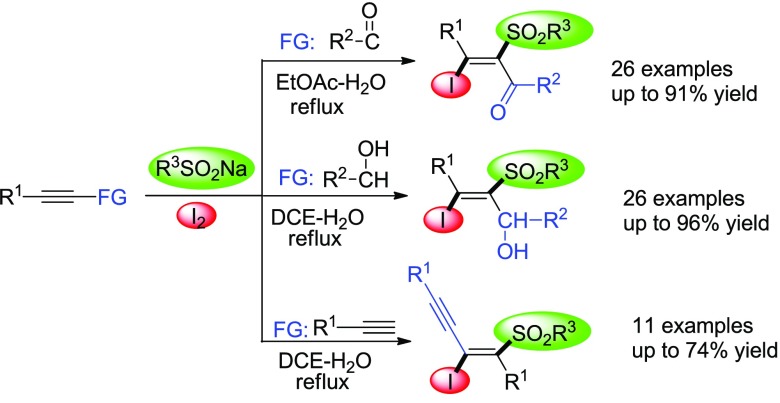
An operationally simple, efficient, and metal-/peroxide-free three-component reaction of alkynes, iodine, and sodium sulfinates has been developed for the construction of highly functionalized tetrasubstituted alkenes. Iodo- and sulfonyl-containing tetrasubstituted alkenes, such as vinyl ketones, allylic alcohols, and conjugated enynes, could be obtained regio- and stereoselectively in up to 96% yield.
Introduction
Polysubstituted alkenes are widely present in many natural products, biologically active molecules, and pharmaceuticals such as tamoxifen, doxepin, and combretatstatins A,1 as well as among the most useful structural units in organic transformations, such as the generation of quarternary centers by conjugate addition, osmylations, and epoxidations.2 Thus, it was of particular interest to find an effective and convenient protocol to synthesize functionalized polysubstituted alkenes. Despite the fact that numerous methods have been developed for the synthesis of alkenes, access to tetrasubstituted alkenes remains a challenging task for the synthetic community due to the congested nature of the double bond.2d,3 Additionally, new and general strategies that could provide tetrasubstituted alkenes with diverse functional groups for further transformations are in much demand.
Owing to the distinctive structure and electronic features of the sulfonyl group, vinylsulfones have broad utilizations as valuable building blocks in medicinal chemistry, materials chemistry, and organic synthesis.4 Among them, halo vinylsulfones are of significant interest because both the vinylsulfone and vinyl halide are versatile functional units in organic synthesis. Therefore, the simultaneous introduction of sulfonyl and halo into alkenes has drawn great attention from chemists. Transition-metal-catalyzed reaction of terminal alkynes with sulfonyl chlorides or sulfonylhydrazides has emerged as a powerful means to construct halo vinylsulfones,5 yet the use of toxic metals makes it limited for wide application. Alternatively, metal-free synthesis of halo vinylsulfones has been developed during recent years. For example, β-iodo vinylsulfones could be synthesized by metal-free iodosulfonylation of alkynes with sulfonyl hydrazides.6 Wang et al.7 reported the synthesis of β-iodovinyl sulfones by difunctionalization of alkynes with sulfinic acids and iodine without any metal catalyst. Jiang8 and Sun9 disclosed iodosulfonylation of alkynes with sodium sulfinates and iodine under metal-free conditions, respectively. Despite the synthetic efficiency of these reactions, almost all of these protocols are limited to the synthesis of trisubstituted alkenes from terminal alkynes. Very recently, Reddy and Wang, respectively, reported the stereoselective synthesis of tetrasubstituted alkenes by iodosulfonylation of electron-deficient internal alkynes, where TBHP (3 equiv) is used as oxidant or sulfonylhydrazide is used as the sulfur source.10 Given the importance of tetrasubstituted alkenes and our ongoing interest in the construction of sulfonyl compounds,11 we present herein a convenient and efficient synthesis of iodo- and sulfonyl-contained tetrasubstituted alkenes, e.g., vinyl ketones, allylic alcohols, and conjugated enynes, by three-component reaction of internal alkynes, iodine, and sodium sulfinates under metal- and peroxide-free conditions.
Results and Discussion
Initially, the reaction of 3-phenyl-1-(p-tolyl)prop-2-yn-1-one and sodium benzenesulfinate was chosen as the model reaction to optimize the reaction conditions. The experimental results were compiled in Table 1. With the treatment of 1.0 equiv of acetylenic ketone (1a), 1.0 equiv of sodium benzenesulfinate (2a), and 1.0 equiv of I2 in ethyl acetate at room temperature for 24 h, (E)-3-iodo-3-phenyl-2-(phenylsulfonyl)-1-(p-tolyl)prop-2-en-1-one (3a) could be isolated in 12% yield along with recovery of 1a (entry 1, Table 1). Considering the solubility of sodium sulfinate, ethyl acetate/water (v:v = 2:1) was used as the solvent, and 3a was isolated in 28% yield (entry 2, Table 1). Only a trace of 3a was detected in water (entry 3, Table 1). The yield of 3a was increased to 65% when excess sodium benzenesulfinate was used (entries 4 and 5, Table 1). Screening various solvents suggested ethyl acetate/water (v:v = 2:1) as the best candidate for this reaction (entries 5–10, Table 1). The yield of 3a was further improved to 83% by increasing the reaction temperature to reflux, while only a trace of 3a was detected by performing the reaction at 0 °C (entries 11–13, Table 1). An increase in the amount of I2 has no apparent effect on the yield of 3a, and a reduced amount of I2 was detrimental to the yield (entries 14 and 15, Table 1). Based on these experimental results, we defined that acetylenic ketones react with 2.0 equiv of sodium sulfinates and 1.0 equiv of I2 in ethyl acetate/water (v:v = 2:1) at reflux as the optimal reaction conditions for the scope of study.
Table 1. Optimization of the Reaction Conditionsa.
| entry | solvent | I2 (equiv) | 2a (equiv) | T (°C) | time (h) | yield (%)b |
|---|---|---|---|---|---|---|
| 1 | EtOAc | 1 | 1 | r.t. | 24 | 12 |
| 2c | EtOAc/H2O | 1 | 1 | r.t. | 24 | 28 |
| 3 | H2O | 1 | 1 | r.t. | 24 | trace |
| 4c | EtOAc/H2O | 1 | 1.5 | r.t. | 24 | 40 |
| 5c | EtOAc/H2O | 1 | 2 | r.t. | 24 | 65 |
| 6c | THF/H2O | 1 | 2 | r.t. | 24 | 40 |
| 7c | CH2Cl2/H2O | 1 | 2 | r.t. | 24 | 61 |
| 8c | Toluene/H2O | 1 | 2 | r.t. | 24 | 57 |
| 9c | DMF/H2O | 1 | 2 | r.t. | 24 | trace |
| 10c | DMSO/H2O | 1 | 2 | r.t. | 24 | trace |
| 11c | EtOAc/H2O | 1 | 2 | 0 | 24 | trace |
| 12c | EtOAc/H2O | 1 | 2 | 50 | 19 | 78 |
| 13c | EtOAc/H2O | 1 | 2 | reflux | 12 | 83 |
| 14c | EtOAc/H2O | 2 | 2 | reflux | 12 | 82 |
| 15c | EtOAc/H2O | 0.8 | 2 | reflux | 12 | 76 |
Reaction conditions: 1a (0.5 mmol), 2a, I2, solvent (3 mL).
Isolated yields based on 1a.
2 mL of organic solvent and 1 mL of H2O.
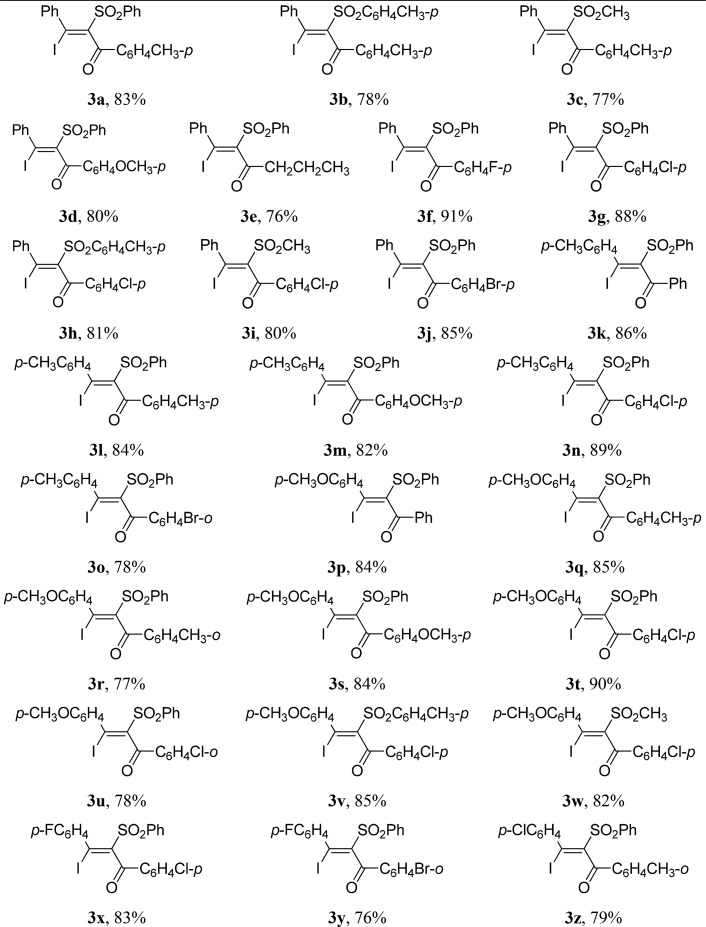
With the optimized reaction conditions in hand, a range of acetylenic ketones and sodium sulfinates were employed in the reaction to evaluate the scope and generality of this protocol, and results are summarized in Table 2. The reaction worked well with various substituted acetylenic ketones, and the corresponding β-iodo-α-sulfonyl vinyl ketones 3 were obtained in the range of 76%–91% yields. R1 in acetylenic ketones can be phenyl (3a–3j, Table 2), p-tolyl (3k–3o, Table 2), p-methoxyphenyl (3p–3w, Table 2), p-fluorophenyl (3x and 3y, Table 2), and p-chlorophenyl (3z, Table 2), respectively. The compatibility of R2 in acetylenic ketones was also investigated, by revealing that phenyl, electron-rich and electron-deficient aryl, and n-propyl could be well tolerated in the reaction. Several commercially available sodium sulfinates 2 such as sodium phenylsulfinate, sodium p-tolylsulfinate, and sodium methanesulfinate could conduct this reaction successfully to give the corresponding products. The substituents in acetylenic ketones or sodium sulfinates had no apparent effect on the reaction. Unfortunately, no desired product was obtained when R1 in acetylenic ketones is alkyl.
Table 2. Three-Component Reaction of Acetylenic Ketones, Iodine, and Sodium Sulfinatesa.
Reaction conditions: 1 (0.25 mmol), 2 (0.5 mmol), and I2 (0.25 mmol) in 3 mL of EtOAc/H2O (v:v = 2:1) at reflux.
Isolated yields based on 1.
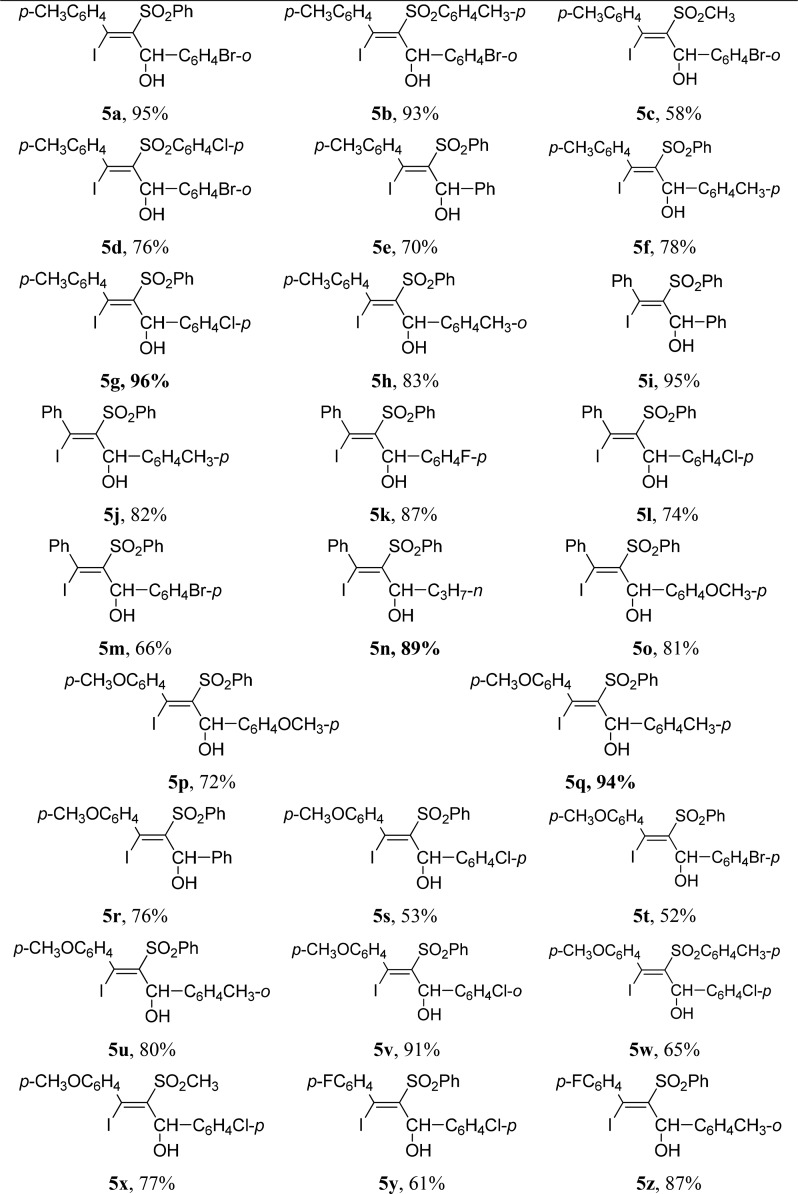
Allylic alcohols are pervasive in natural products and serve as ubiquitous intermediates in organic synthesis.12 The construction of allylic alcohols has been a focus in methodology development for decades.13 Encouraged by the experimental results of acetylenic ketones, we investigated the similar transformation of propargyl alcohols. The results show that iodosulfonylation reaction can be readily extended to propargyl alcohols. The desired functionalized tetrasubstituted allylic alcohols 5 were isolated in up to 96% yield when propargyl alcohols reacted with 2.0 equiv of sodium sulfinates and 1.5 equiv of iodine in DCE/H2O (v:v = 2:1) at reflux (Table 3). R1 in propargyl alcohols can be p-tolyl (5a–5h, Table 3), phenyl (5i–5o, Table 3), p-methoxyphenyl (5p–5x, Table 3), and p-fluorophenyl (5y and 5z, Table 3), respectively. R2 in propargyl alcohols can be phenyl, n-propyl, and electron-rich and electron-deficient aryl. Similarly, no desired product was obtained when R1 in propargyl alcohols is alkyl.
Table 3. Three-Component Reaction of Propargyl Alcohols, Iodine, and Sodium Sulfinatesa.
Reaction conditions: 4 (0.25 mmol), 2 (0.5 mmol), and I2 (0.375 mmol) in 3 mL of ClCH2CH2Cl/H2O (v:v = 2:1) at reflux.
Isolated yields based on 4.
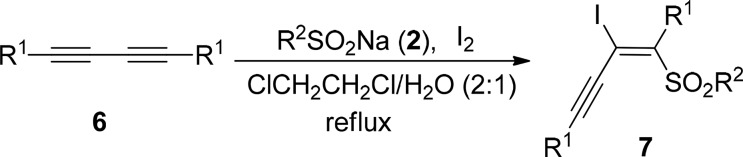
On the other hand, conjugated enynes are of great interest in organic synthesis, materials science, and biochemistry due to their ubiquitous occurrence in bioactive natural products and functional materials.14 Transition-metal-catalyzed cross coupling of vinyl halides or acetylenic halides15 and dimerization of alkynes16 are the most commonly used synthetic methods for access to conjugated enynes. However, direct stereoselective synthesis of highly substituted conjugated enynes from 1,3-diynes is scarce.17 To further demonstrate the synthetic utility of this protocol, we extended the iodosulfonylation reaction to 1,3-diynes. It was found that the reaction proceeded smoothly, and the polysubstituted conjugated enynes 7 were isolated in 48–74% yields when 1,3-diynes 6 reacted with 2 equiv of sodium sulfinates and 1.5 equiv of iodine in DCE/H2O (v:v = 2:1) at reflux (Table 4). The R1 in 1,3-diynes can be phenyl (7a–7c, Table 4), p-methylphenyl (7d–7f, Table 4), p-fluorophenyl (7g–7i, Table 4), p-methoxyphenyl (7j, Table 4), and p-chlorophenyl (7k, Table 4). R2 in sodium sulfinates can be phenyl, methyl, and p-tolyl. Unfortunately, complex mixtures were observed in the case of unsymmetrical 1,3-diynes.
Table 4. Three-Component Reaction of 1,3-Diynes, Iodine, and Sodium Sulfinatesa.
Reaction conditions: 6 (0.25 mmol), 2 (0.5 mmol), and I2 (0.375 mmol) in 3 mL of ClCH2CH2Cl/H2O (v:v = 2:1) at reflux.
Isolated yield based on 6.
The configurations of compounds 3, 5, and 7 were unambiguously confirmed by X-ray crystallographic analysis of 3a, 5c, and 7i (see Supporting Information), indicating that the iodosulfonylation of carbon–carbon triple bond proceeds in anti-fashion.
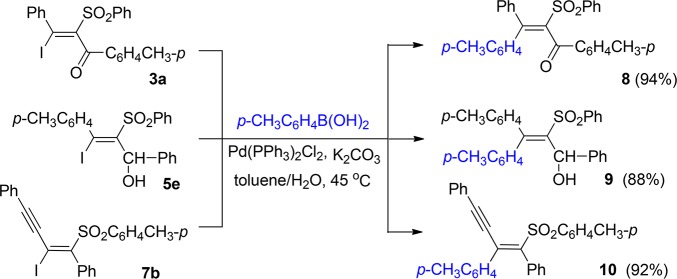
To examine the synthetic application of the obtained functionalized tetrasubstituted alkenes, the palladium-catalyzed Suzuki coupling reaction of the obtained iodo- and sulfonyl-substituted alkenes was investigated. The Suzuki coupling reactions of p-tolylboronic acid with compounds 3a, 5e, and 7b proceeded smoothly, and the desired products 8, 9, and 10 were isolated in excellent yields (Scheme 1).
Scheme 1. Pd-Catalyzed Suzuki Coupling Reactions of 3a, 5e, and 7b with p-Tolylboronic Acid.
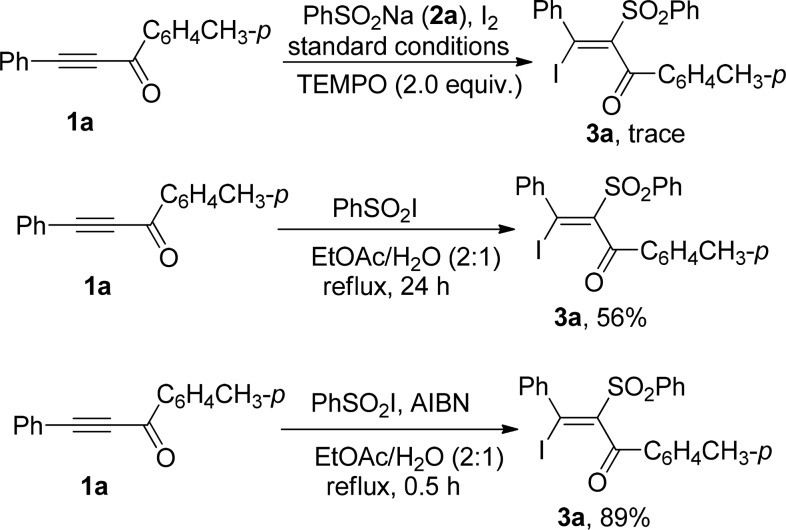
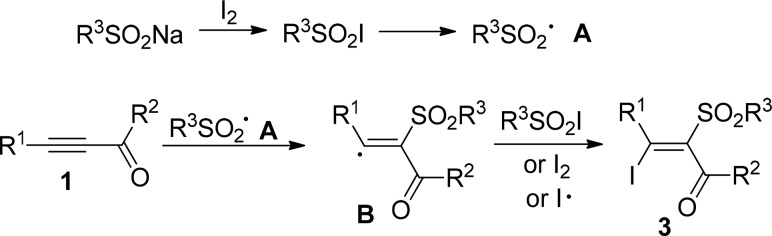
To investigate the reaction mechanism, some control experiments were conducted (Scheme 2). When the radical scavenger TEMPO (2 equiv) was added to the standard reaction conditions, inhibition of the reaction was observed. The desired product 3a was isolated in 56% yield when acetylenic ketone 1a directly reacts with benzenesulfonyl iodode under the optimized reaction conditions. The reaction proceeded much faster to provide 89% yield of 3a after 0.5 h when the reaction of acetylenic ketone 1a with benzenesulfonyl iodode was conducted in the presence of 5 mol % of AIBN. On the basis of the experimental results and related literature,7,8 a mechanism for the iodosulfonylation of alkynes is proposed as shown in Scheme 3. First, the sodium sulfinate reacts with iodine to give sulfonyl iodide intermediate, which may undergo homolytic cleavage to yield a sulfonyl radical A. Addition of A to acetylenic ketone 1a chemoselectively generates the more stable vinyl radical intermediate B. Subsequently, vinyl radical B reacts with sulfonyl iodide, I2, or iodine radical to afford the functionalized alkenes 3. The regioselectivity of the reaction may be determined by the stability of the vinyl radical intermediate B. In the case of acetylenic ketones (or propargyl alcohols), the β-carbonyl (or γ-hydroxyl) vinyl radical intermediate is more stable than the α-carbonyl (or β-hydroxyl) vinyl radical intermediate because of the electron-withdrawing effect of carbonyl (or hydroxyl), giving α-sulfonyl vinyl ketones (or β-sulfonyl allylic alcohols). In the case of 1,3-diynes, the α-alkynyl vinyl radical intermediate is more stable than the β-alkynyl vinyl radical intermediate due to the conjugation effects of the carbon–carbon triple bond, affording 2-iodo-1-sulfonyl 1,3-enynes.
Scheme 2. Control Experiment.
Scheme 3. Possible Reaction Mechanism.
Conclusion
In summary, we have developed a convenient and general method for the construction of highly functionalized tetrasubstituted alkenes, e.g., iodo- and sulfonyl-substituted vinyl ketones, allylic alcohols, and conjugated enynes, from three-component reaction of acetylenic ketones, propargyl alcohols, or 1,3-diynes with iodine and sodium sulfinates. The method is characterized by its wide substrate scope, high regio- and stereoselectivity, mild reaction conditions, and readily available starting materials. It is predictable that the resulting highly functionalized alkenes are potential precursors for differently tetrasubstituted alkenes.
Experimental Section
General Information
Unless otherwise stated, all commercial materials and solvents were used directly without further purification. 1H NMR and 13C NMR spectra were recorded on a 300 or 500 MHz spectrometer (1H at 300 MHz, 13C at 75 MHz or 1H at 500 MHz, 13C at 125 MHz), using CDCl3 as the solvent with tetramethylsilane (TMS) as an internal standard at room temperature. Chemical shifts are given in δ relative to TMS, and the coupling constants (J) are given in hertz. The multiplicities are reported as follows: singlet (s), doublet (d), multiplet (m), triplet (t), and broad resonances (br). High-resolution mass spectra were recorded on an Agilent model G6220 mass spectrometer. All reactions under a nitrogen atmosphere were conducted using standard Schlenk techniques. Melting point data are uncorrected.
Typical Experimental Procedure for the Synthesis of Propargyl Alcohols 4 and Acetylenic Ketones 1.18
Alkyne (6.00 mmol, 1.20 equiv) and anhydrous THF (15 mL) were added to a 50 mL two-neck round-bottom flask. The mixture was cooled to 0 °C, and n-BuLi (2.5 M in hexanes, 2.50 mL, 6.25 mmol, 1.25 equiv) was added slowly via syringe. The mixture was stirred at 0 °C for 1 h and then at room temperature for 1 h and then recooled to 0 °C. Aldehyde (5.00 mmol) in THF (5 mL) was added dropwise via syringe. The reaction was stirred at 0 °C for 1 h and then at room temperature for an additional 30 min. Upon the completion of the reaction, the reaction was quenched with saturated aqueous NH4Cl and extracted with ethyl acetate. The combined organic extracts were washed with brine (50 mL) and dried over anhydrous Na2SO4. After filtration and removal of the solvent under reduced pressure, the residue was purified by flash column chromatography (silica gel, petroleum ether/ethyl acetate = 10/1) to afford the desired propargyl alcohol 4.
MnO2 (15 equiv) was added to a solution of propargyl alcohol 4 (5 mmol) in CH2Cl2 (15 mL) at room temperature, and the resulting mixture was stirred overnight. Then the solid was filtered, and the solvents were removed under reduced pressure. The residue was purified by flash column chromatography (silica gel, petroleum ether/ethyl acetate = 50/1) to give acetylenic ketone 1.
Typical Experimental Procedure for the Synthesis of 1,3-Diynes 6.19
In the open air, alkyne (3.0 mmol) and Cu(OAc)2·H2O (0.3 mmol) were added to a 100 mL tube successively. Then the tube was sealed, and the resulting mixture was allowed to react at 110 °C for 7 h. After completion of the reaction, 30 mL of ethyl acetate was added. The mixture was filtered, and the filtration residue was washed with ethyl acetate. Ethyl acetate was removed under reduced pressure, and the residue was purified by column chromatography (silica gel, petroleum ether as eluent) to afford the 1,3-diyne 6.
Typical Experimental Procedure for the Synthesis of PhSO2I.4a
A saturated solution of iodine (2.0 g, 8.0 mmol) in alcohol (7 mL) was added gradually to the solution of sodium benzenesulfinate (1.6 g, 10 mmol) in 35 mL of distilled water until a slight excess of iodine was present. During this period, the yellow precipitate was formed gradually. The precipitate was filtered, washed with cold water, and dried carefully at room temperature to give benzenesulfonyl iodide as a yellow solid.
Typical Experimental Procedure for the Synthesis of Functionalized Tetrasubstituted Vinyl Ketones 3
Under argon atmosphere, a Schlenk flask equipped with a condenser was charged with 0.25 mmol (55 mg) of 3-phenyl-1-(p-tolyl)prop-2-yn-1-one (1a), 0.5 mmol (82 mg) of sodium benzenesulfinate (2a), 0.25 mmol (64 mg) of I2, and 3 mL of ethyl acetate/H2O (v/v 2/1). The reaction mixture was stirred at reflux until the complete consumption of 1a (monitored by TLC). The reaction was then quenched with saturated NH4Cl and extracted with ethyl acetate (3 × 15 mL). The organic layer was washed with saturated Na2S2O3 and dried over anhydrous Na2SO4. After filtration and removal of the solvent in vacuo, the crude product was purified by flash column chromatography on silica gel (silica gel, petroleum ether/ethyl acetate = 15/1) to give product 3a.
(E)-3-Iodo-3-phenyl-2-(phenylsulfonyl)-1-(4-tolyl)prop-2-en-1-one (3a)
White solid; mp 167–168 °C; yield 83%. 1H NMR (300 MHz, CDCl3): δ 8.15 (d, J = 8.0 Hz, 2H), 7.55–7.40 (m, 5H), 7.35–7.29 (m, 5H), 7.14 (d, J = 6.4 Hz, 2H), 2.50 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.6, 149.8, 146.4, 140.8, 140.4, 134.0, 131.8, 130.8, 130.4, 129.9, 129.1, 128.7, 128.4, 127.9, 114.4, 22.4. IR (KBr, ν, cm–1): 3055, 2920, 2360, 1666, 1600, 1446, 1303, 1145, 781, 551. HRMS m/z (ESI) calcd for C22H18IO3S (M + H)+ 489.0016, found 489.0016.
(E)-3-Iodo-3-phenyl-1-(4-tolyl)-2-tosylprop-2-en-1-one (3b)
White solid; mp 151–152 °C; yield 78%. 1H NMR (300 MHz, CDCl3): δ 8.13 (d, J = 9.0 Hz, 2H), 7.39 (d, J = 9.0 Hz, 2H), 7.34–7.28 (m, 5H), 7.18–7.10 (m, 4H), 2.49 (s, 3H), 2.38 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.7, 149.9, 146.3, 145.2, 140.5, 137.9, 131.8, 130.8, 130.4, 129.9, 129.7, 128.9, 128.3, 127.9, 113.9, 22.4, 22.1. IR (KBr, ν, cm–1): 3049, 2918, 1668, 1602, 1323, 1255, 1151, 1082, 773, 565, 545. HRMS m/z (ESI) calcd for C23H20IO3S (M + H)+ 503.0172, found 503.0167.
(E)-3-Iodo-2-(methylsulfonyl)-3-phenyl-1-(4-tolyl)prop-2-en-1-one (3c)
White solid; mp 159–160 °C; yield 77%. 1H NMR (300 MHz, CDCl3): δ 8.08 (d, J = 8.2 Hz, 2H), 7.50–7.45 (m, 4H), 7.42–7.37 (m, 3H), 2.91 (s, 3H), 2.48 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 191.0, 147.6, 146.6, 140.8, 131.4, 130.9, 130.5, 128.7, 127.6, 115.1, 44.8, 22.4. IR (KBr, ν, cm–1): 3003, 2920, 1668, 1600, 1323, 1255, 1138, 964, 783, 513, 480. HRMS m/z (ESI) calcd for C17H16IO3S (M + H)+ 426.9859, found 426.9859.
(E)-3-Iodo-1-(4-methoxyphenyl)-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-one (3d)
White solid; mp 135–136 °C; yield 80%. 1H NMR (300 MHz, CDCl3): δ 8.22 (d, J = 8.8, 2H), 7.55–7.46 (m, 3H), 7.35–7.28 (m, 5H), 7.14–7.07 (m, 4H), 3.94 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 189.5, 165.3, 149.9, 140.9, 140.5, 134.0, 133.2, 129.9, 129.1, 128.7, 128.4, 127.9, 127.3, 115.1, 114.3, 56.1. IR (KBr, ν, cm–1): 2839, 1660, 1593, 1573, 1315, 1253, 1166, 1026, 840, 748, 540. HRMS m/z (ESI) calcd for C22H18IO4S (M + H)+ 504.9965, found 504.9966.
(E)-1-Iodo-1-phenyl-2-(phenylsulfonyl)hex-1-en-3-one (3e)
White solid; mp 97–98 °C; yield 76%. 1H NMR (300 MHz, CDCl3): δ 7.51 (t, J = 4.5 Hz, 1H), 7.40–7.39 (m, 2H), 7.31 (t, J = 4.5 Hz, 2H), 7.26–7.25 (m, 1H), 7.21 (t, J = 7.4 Hz, 2H), 7.02 (d, J = 4.4 Hz, 2H), 3.10 (t, J = 4.3 Hz, 2H), 1.89–1.82 (m, 2H), 1.07 (t, J = 4.5 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ 200.3, 151.1, 140.6, 140.1, 134.1, 129.9, 129.2, 128.5, 128.3, 127.8, 112.2, 44.9, 17.1, 13.9. IR (KBr, ν, cm–1): 3021, 1677, 1523, 1157, 832, 755, 643. HRMS m/z (ESI) calcd for C18H18IO3S (M + H)+ 441.0016, found 441.0023.
(E)-1-(4-Fluorophenyl)-3-iodo-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-one (3f)
White solid; mp 146–147 °C; yield 91%. 1H NMR (300 MHz, CDCl3): δ 8.30–8.26 (m, 2H), 7.56–7.45 (m, 3H), 7.36–7.29 (m, 7H), 7.13 (d, J = 6.0 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 189.5, 167.2 (d, J = 257 Hz), 149.5, 140.7, 140.2, 134.2, 133.4 (d, J = 9.7 Hz), 130.8, 130.1, 129.2, 128.7, 128.4, 127.8, 117.0 (d, J = 22.1 Hz), 114.9. IR (KBr, ν, cm–1): 3068, 1676, 1595, 1319, 1234, 1145, 1078, 848, 686, 547. HRMS m/z (ESI) calcd for C21H15FIO3S (M + H)+ 492.9765, found 492.9760.
(E)-1-(4-Chlorophenyl)-3-iodo-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-one (3g)
White solid; mp 141–142 °C; yield 88%. 1H NMR (300 MHz, CDCl3): δ 8.18 (d, J = 8.5 Hz, 2H), 7.60–752 (m, 3H), 7.46 (d, J = 7.3 Hz, 2H), 7.36–7.25 (m, 5H), 7.13 (d, J = 7.2 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 189.8, 149.3, 141.8, 140.6, 140.1, 134.2, 132.7, 132.0, 130.1, 129.2, 128.7, 128.4, 127.8, 115.1. IR (KBr, ν, cm–1): 3091, 3057, 1668, 1585, 1442, 1330, 1152, 1083, 844, 684, 553, 540. HRMS m/z (ESI) calcd for C21H15ClIO3S (M + H)+ 508.9470, found 508.9467.
(E)-1-(4-Chlorophenyl)-3-iodo-3-phenyl-2-tosylprop-2-en-1-one (3h)
White solid; mp 159–160 °C; yield 81%. 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.6 Hz, 2H), 7.57 (d, J = 8.6 Hz, 2H), 7.33–7.29 (m, 5H), 7.17–7.12 (m, 4H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 189.9, 149.4, 145.4, 141.7, 140.3, 137.6, 132.7, 131.9, 130.0, 129.8, 128.9, 128.4, 127.8, 114.6, 22.1. IR (KBr, ν, cm–1): 3064, 2920, 1676, 1583, 1323, 1255, 1151, 1082, 844, 698, 545. HRMS m/z (ESI) calcd for C22H17IClO3S (M + H)+ 522.9626, found 522.9625.
(E)-1-(4-Chlorophenyl)-3-iodo-2-(methylsulfonyl)-3-phenylprop-2-en-1-one (3i)
White solid; mp 157–158 °C; yield 80%. 1H NMR (300 MHz, CDCl3): δ 8.11 (d, J = 8.5 Hz, 2H), 7.56 (d, J = 8.5 Hz, 2H), 7.47–7.40 (m, 5H), 2.92 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.3, 147.2, 141.9, 140.5, 132.3, 132.0, 130.6, 130.1, 128.8, 127.5, 115.8, 44.9. IR (KBr, ν, cm–1): 2997, 2914, 1670, 1583, 1323, 1143, 964, 839, 778, 511, 399. HRMS m/z (ESI) calcd for C16H13ClIO3S (M + H)+ 446.9313, found 446.9313.
(E)-1-(4-Bromophenyl)-3-iodo-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-one (3j)
White solid; mp 144–145 °C; yield 85%. 1H NMR (300 MHz, CDCl3): δ 8.10 (d, J = 8.5 Hz, 2H), 7.76 (d, J = 8.5 Hz, 2H), 7.54 (t, J = 7.3 Hz, 1H), 7.46–7.44 (m, 2H), 7.36–7.29 (m, 5H), 7.13 (d, J = 6.6 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 190.1, 149.3, 140.6, 140.1, 134.2, 133.1, 132.0, 130.7, 130.1, 129.2, 128.7, 128.4, 127.8, 115.1. IR (KBr, ν, cm–1): 3068, 1672, 1581, 1325, 1240, 1153, 1083, 835, 779, 732, 549. HRMS m/z (ESI) calcd for C21H15BrIO3S (M + H)+ 552.8964, found 552.8963.
(E)-3-Iodo-1-phenyl-2-(phenylsulfonyl)-3-(4-tolyl)prop-2-en-1-one (3k)
White solid; mp 136–137 °C; yield 86%. 1H NMR (300 MHz, CDCl3): δ 8.24 (d, J = 7.4 Hz, 2H), 7.70 (t, J = 7.2 Hz, 1H), 7.63–7.48 (m, 5H), 7.33 (t, J = 7.5 Hz, 2H), 7.07 (s, 4H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 191.0, 149.3, 140.8, 140.4, 137.6, 135.1, 134.3, 134.1, 130.7, 129.6, 129.1, 129.0, 128.8, 127.9, 115.3, 21.8. IR (KBr, ν, cm–1): 3041, 2922, 1681, 1593, 1504, 1446, 1323, 1151, 788, 684, 555, 540; HRMS m/z (ESI) calcd for C22H18IO3S (M + H)+ 489.0016, found 489.0010.
(E)-3-Iodo-2-(phenylsulfonyl)-1,3-di-4-tolylprop-2-en-1-one (3l)
White solid; mp 168–169 °C; yield 84%. 1H NMR (300 MHz, CDCl3): δ 8.13 (d, J = 8.1 Hz, 2H), 7.55–7.48 (m, 3H), 7.41–7.30 (m, 4H), 7.06 (s, 4H), 2.49 (s, 3H), 2.38 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.6, 149.4, 146.4, 140.9, 140.3, 137.7, 134.0, 131.9, 130.8, 130.4, 129.1, 129.0, 128.8, 127.9, 115.0, 22.4, 21.8. IR (KBr, ν, cm–1): 3028, 2920, 1676, 1598, 1301, 1141, 1080, 815, 727, 553, 542. HRMS m/z (ESI) calcd for C23H20IO3S (M + H)+ 503.0172, found 503.0169.
(E)-3-Iodo-1-(4-methoxyphenyl)-2-(phenylsulfonyl)-3-(4-tolyl)prop-2-en-1-one (3m)
White solid; mp 153–154 °C; yield 82%. 1H NMR (300 MHz, CDCl3): δ 8.21 (d, J = 8.7 Hz, 2H), 7.55–7.48 (m, 3H), 7.32 (t, J = 7.6 Hz, 2H), 7.08–7.06 (m, 6H), 3.94 (s, 3H), 2.38 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 189.5, 165.3, 149.5, 140.9, 140.2, 137.8, 134.0, 133.1, 129.0, 128.8, 127.8, 127.4, 115.0, 56.1, 21.8. IR (KBr, ν, cm–1): 3018, 2846, 1647, 1589, 1315, 1261, 1165, 1147, 1020, 837, 549, 470. HRMS m/z (ESI) calcd for C23H20IO4S (M + H)+ 519.0121, found 519.0115.
(E)-1-(4-Chlorophenyl)-3-iodo-2-(phenylsulfonyl)-3-(4-tolyl)prop-2-en-1-one (3n)
White solid; mp 174–175 °C; yield 89%. 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.5 Hz, 2H), 7.59–7.46 (m, 5H), 7.34 (t, J = 7.5 Hz, 2H), 7.10–7.03 (m, 4H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 189.9, 148.9, 141.7, 140.7, 140.5, 137.4, 134.2, 132.8, 132.0, 130.1, 129.1, 129.0, 128.8, 127.8, 115.7, 21.8. IR (KBr, ν, cm–1): 3080, 3030, 1681, 1581, 1301, 1141, 1080, 817, 732, 682, 543. HRMS m/z (ESI) calcd for C22H17ClIO3S (M + H)+ 522.9626, found 522.9622.
(E)-1-(2-Bromophenyl)-3-iodo-2-(phenylsulfonyl)-3-(4-tolyl)prop-2-en-1-one (3o)
Yellow solid; mp 136–137 °C; yield 78%. 1H NMR (300 MHz, CDCl3): δ 8.23 (d, J = 7.7 Hz, 1H), 7.79 (d, J = 7.8 Hz, 1H), 7.58–7.44 (m, 5H), 7.36 (t, J = 7.5 Hz, 2H), 7.10–7.00 (m, 4H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 189.6, 149.3, 141.0, 140.4, 137.6, 136.0, 134.5, 134.4, 134.1, 133.9, 129.1, 128.9, 128.8, 128.1, 127.7, 124.0, 116.3, 21.9. IR (KBr, ν, cm–1): 3066, 3030, 1683, 1583, 1444, 1319, 1228, 1151, 1020, 785, 684, 559, 540. HRMS m/z (ESI) calcd for C22H17BrIO3S (M + H)+ 566.9121, found 566.9117.
(E)-3-Iodo-3-(4-methoxyphenyl)-1-phenyl-2-(phenylsulfonyl)prop-2-en-1-one (3p)
White solid; mp 111–112 °C; yield 84%. 1H NMR (300 MHz, CDCl3): δ 8.24 (d, J = 7.4 Hz, 2H), 7.70 (t, J = 7.2 Hz, 1H), 7.62–7.48 (m, 5H), 7.34 (t, J = 7.4 Hz, 2H), 7.13 (t, J = 8.7 Hz, 2H), 6.78 (d, J = 8.7 Hz, 2H), 3.85 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 191.1, 161.0, 149.2, 140.9, 135.1, 134.3, 134.1, 132.6, 130.7, 129.8, 129.6, 129.1, 128.7, 115.4, 113.8, 55.9. IR (KBr, ν, cm–1): 3059, 2918, 1662, 1604, 1319, 1305, 1251, 1149, 1085, 823, 736, 582, 528. HRMS m/z (ESI) calcd for C22H18IO4S (M + H)+ 504.9965, found 504.9965.
(E)-3-Iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)-1-(4-tolyl)prop-2-en-1-one (3q)
White solid; mp 176–177 °C; yield 85%. 1H NMR (300 MHz, CDCl3): δ 8.13 (d, J = 8.1 Hz, 2H), 7.54–7.49 (m, 3H), 7.41–7.31 (m, 4H), 7.12 (d, J = 8.7 Hz, 2H), 6.77 (d, J = 8.8 Hz, 2H), 3.85 (s, 3H), 2.48 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.7, 160.9, 149.4, 146.3, 140.9, 134.0, 132.7, 131.9, 130.8, 130.4, 129.8, 129.1, 128.7, 115.1, 113.7, 55.9, 22.4. IR (KBr, ν, cm–1): 3063, 2833, 1668, 1504, 1307, 1250, 823, 559, 540. HRMS m/z (ESI) calcd for C23H20IO4S (M + H)+ 519.0121, found 519.0118.
(E)-3-Iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)-1-(2-tolyl)prop-2-en-1-one (3r)
Yellow solid; mp 152–153 °C; yield 77%. 1H NMR (300 MHz, CDCl3): δ 8.21 (d, J = 7.4 Hz, 1H), 7.53–7.41 (m, 5H), 7.35–7.30 (m, 3H), 7.09 (d, J = 8.2 Hz, 2H), 6.75 (d, J = 8.3 Hz, 2H), 3.83 (s, 3H), 2.72 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 192.3, 160.9, 150.4, 142.6, 141.0, 134.0, 133.2, 133.1, 132.9, 132.6, 129.9, 129.0, 128.6, 126.7, 115.0, 113.7, 55.9, 22.6. IR (KBr, ν, cm–1): 3007, 2843, 1674, 1321, 1151, 1033, 832, 555, 538. HRMS m/z (ESI) calcd for C23H20IO4S (M + H)+ 519.0121, found 519.0126.
(E)-3-Iodo-1,3-bis(4-methoxyphenyl)-2-(phenylsulfonyl)prop-2-en-1-one (3s)
White solid; mp 136–137 °C; yield 84%. 1H NMR (300 MHz, CDCl3): δ 8.20 (d, J = 8.9 Hz, 2H), 7.54–7.49 (m, 3H), 7.34 (t, J = 7.1 Hz, 2H), 7.13–7.05 (m, 4H), 6.76 (d, J = 8.8 Hz, 2H), 3.93 (s, 3H), 3.84 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 189.6, 165.3, 160.9, 149.4, 140.9, 133.9, 133.2, 132.8, 129.8, 129.0, 128.7, 127.4, 115.0, 113.7, 56.1, 55.9. IR (KBr, ν, cm–1): 2931, 2837, 1660, 1593,1504, 1259, 1168, 1024, 829, 557, 543. HRMS m/z (ESI) calcd for C23H20IO5S (M + H)+ 535.0071, found 535.0066.
(E)-1-(4-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)prop-2-en-1-one (3t)
White solid; mp 163–164 °C; yield 90%. 1H NMR (300 MHz, CDCl3): δ 8.17 (d, J = 8.4 Hz, 2H), 7.59–7.48 (m, 5H), 7.35 (t, J = 7.5 Hz, 2H), 7.11 (d, J = 8.6 Hz, 2H), 6.78 (d, J = 8.6 Hz, 2H), 3.85 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.0, 161.1, 148.8, 141.7, 140.7, 134.2, 132.8, 132.4, 132.0, 130.1, 129.8, 129.1, 128.7, 115.8, 113.8, 55.9. IR (KBr, ν, cm–1): 3063, 2835, 1672, 1600, 1504, 1258, 1149, 1082, 827, 543. HRMS m/z (ESI) calcd for C22H17ClIO4S (M + H)+ 538.9575, found 538.9572.
(E)-1-(2-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)prop-2-en-1-one (3u)
Yellow solid; mp 132–133 °C; yield 78%. 1H NMR (300 MHz, CDCl3): δ 8.22 (d, J = 7.4 Hz, 1H), 7.57–7.48 (m, 6H), 7.37 (t, J = 7.2 Hz, 2H), 7.10 (d, J = 8.7 Hz, 2H), 6.78 (d, J = 8.7 Hz, 2H), 3.85 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 189.3, 161.0, 149.5, 141.1, 135.7, 134.6, 134.1, 133.7, 133.1, 132.5, 132.4, 129.7, 129.1, 128.8, 127.6, 115.7, 113.7, 55.9. IR (KBr, ν, cm–1): 3007, 1683, 1604, 1506, 1321, 1259, 1033, 831, 786, 557. HRMS m/z (ESI) calcd for C22H17ClIO4S (M + H)+ 538.9575, found 538.9578.
(E)-1-(4-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-tosylprop-2-en-1-one (3v)
Yellow solid; mp 175–176 °C; yield 85%. 1H NMR (300 MHz, CDCl3): 8.16 (d, J = 8.5 Hz, 2H), 7.56 (d, J = 8.5 Hz, 2H), 7.35 (d, J = 8.2 Hz, 2H),7.14 (d, J = 8.4 Hz, 2H), 6.80 (d, J = 8.7 Hz, 2H), 3.87 (s, 3H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.0, 161.1, 148.9, 145.4, 141.6, 137.8, 132.8, 132.5, 132.0, 130.0, 129.8, 129.7, 128.8, 115.3, 113.7, 55.9, 22.1. IR (KBr, ν, cm–1): 2980, 2839, 1672, 1583, 1325, 1083, 833, 543, 524. HRMS m/z (ESI) calcd for C23H19ClIO4S (M + H)+ 552.9732, found 552.9735.
(E)-1-(4-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-(methylsulfonyl)prop-2-en-1-one (3w)
White solid; mp 177–178 °C; yield 82%. 1H NMR (300 MHz, CDCl3): δ 8.11 (d, J = 8.5 Hz, 2H), 7.55 (d, J = 8.6 Hz, 2H), 7.46 (d, J = 8.8 Hz, 2H), 6.95 (d, J = 8.8 Hz, 2H), 3.86 (s, 3H), 2.89 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 190.3, 161.4, 146.7, 141.8, 132.4, 132.0, 130.1, 129.8, 116.4, 114.2, 55.8, 44.7. IR (KBr, ν, cm–1): 3020, 2918, 1668, 1614, 1583, 1506, 1315, 1249, 1139, 960, 835, 530, 499. HRMS m/z (ESI) calcd for C17H15ClIO4S (M + H)+ 476.9419, found 476.9419.
(E)-1-(4-Chlorophenyl)-3-(4-fluorophenyl)-3-iodo-2-(phenylsulfonyl)prop-2-en-1-one (3x)
Yellow solid; mp 163–164 °C; yield 83%. 1H NMR (300 MHz, CDCl3): δ 8.09 (d, J = 8.5 Hz, 2H), 7.53–7.48 (m, 3H), 7.44–7.41 (m, 2H), 7.34–7.29 (m, 2H), 7.09–7.05 (m, 2H), 6.90 (t, J = 8.6 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 189.7, 163.50 (d, J = 250.4 Hz), 150.0, 141.7, 140.5, 136.2, 134.4 (d, J = 6.2 Hz), 132.6, 131.9, 130.1 (d, J = 8.2 Hz), 129.3, 129.2, 128.7, 115.6 (d, J = 21.9 Hz), 113.6. IR (KBr, ν, cm–1): 3105, 3066, 1666, 1585, 1502, 1328, 1151, 1083, 833, 553. HRMS m/z (ESI) calcd for C21H14ClFIO3S (M + H)+ 526.9375, found 526.9378.
(E)-1-(2-Bromophenyl)-3-(4-fluorophenyl)-3-iodo-2-(phenylsulfonyl)prop-2-en-1-one (3y)
Yellow solid; mp 134–135 °C; yield 76%. 1H NMR (300 MHz, CDCl3): δ 8.21 (d, J = 7.7 Hz, 1H), 7.80 (d, J = 7.8 Hz, 1H), 7.60–7.54 (m, 4H), 7.50–7.45 (m, 1H), 7.42–7.37 (m, 2H), 7.14–7.09 (m, 2H), 6.97 (t, J = 8.6 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 189.3, 163.3 (d, J = 250.0 Hz), 150.2, 140.7, 136.2 (d, J = 2.5 Hz), 135.9, 134.5, 134.2, 133.7, 129.9 (d, J = 8.8 Hz), 129.1, 128.6, 128.0, 123.9, 115.3 (d, J = 22.1 Hz), 113.8. IR (KBr, ν, cm–1): 3109, 3062, 1683, 1600, 1502, 1327, 1230, 1155, 837, 688, 557. HRMS m/z (ESI) calcd for C21H14BrFIO3S (M + H)+ 570.8870, found 570.8868.
(E)-3-(4-Chlorophenyl)-3-iodo-2-(phenylsulfonyl)-1-(2-tolyl)prop-2-en-1-one (3z)
Yellow solid; mp 171–172 °C; yield 79%. 1H NMR (300 MHz, CDCl3): δ 8.18 (d, J = 7.1 Hz, 2H), 7.58–7.50 (m, 4H), 7.46–7.42 (m, 1H), 7.40–7.35 (m, 3H), 7.23 (d, J = 8.6 Hz, 2H), 7.06 (d, J = 8.4 Hz, 2H), 2.72 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 191.9, 151.5, 142.7, 140.8, 138.8, 136.1, 134.2, 134.1, 133.2, 133.0, 132.9, 129.3, 129.2, 128.7, 128.6, 126.8, 112.3, 22.5. IR (KBr, ν, cm–1): 3066, 3014, 1674, 1602, 1483, 1323, 1153, 1082, 827, 785, 555. HRMS m/z (ESI) calcd for C22H17ClIO3S (M + H)+ 522.9626, found 522.9622.
Typical Experimental Procedure for the Synthesis of Functionalized Tetrasubstituted Allylic Alcohols 5
Under argon atmosphere, a Schlenk flask equipped with a condenser was charged with 0.25 mmol (75 mg) of 1-(2-bromophenyl)-3-(4-tolyl)prop-2-yn-1-ol (4a), 0.5 mmol (82 mg) of sodium benzenesulfinate (2a), 0.375 mmol (95 mg) of I2, and 3 mL of 1,2-dichloroethane/H2O (v/v 2/1). The reaction mixture was stirred at reflux until the complete consumption of 4a (monitored by TLC). The reaction was then quenched with saturated NH4Cl and extracted with ethyl acetate (3 × 15 mL). The organic layer was washed with saturated Na2S2O3 and dried over anhydrous Na2SO4. After filtration and removal of the solvent in vacuo, the crude product was purified by flash column chromatography on silica gel (silica gel, petroleum ether/ethyl acetate = 15/1) to give product 5a.
(E)-1-(2-Bromophenyl)-3-iodo-2-(phenylsulfonyl)-3-(p-tolyl)prop-2-en-1-ol (5a)
Yellow solid; mp 105–106 °C; yield 95%. 1H NMR (500 MHz, CDCl3): δ 7.88 (d, J = 7.6 Hz, 1H), 7.50–7.44 (m, 2H), 7.42–7.39 (m, 1H), 7.23–7.17 (m, 6H), 6.98 (br, 3H), 6.19 (d, J = 11.6 Hz, 1H), 4.50 (d, J = 11.6 Hz, 1H), 2.33 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 145.5, 140.5, 140.0, 139.4, 138.9, 133.0, 132.9, 129.6, 129.5, 128.3, 127.5, 127.4, 124.7, 123.5, 83.0, 21.4. IR (KBr, ν, cm–1): 3616, 3090, 2852, 2893, 1602, 1571, 1500, 1465, 1253, 820, 743; HRMS m/z (ESI) calcd for C22H17BrIO2S (M – OH)+ 550.9177, found 550.9184.
(E)-1-(2-Bromophenyl)-3-iodo-3-(p-tolyl)-2-tosylprop-2-en-1-ol (5b)
White solid; mp 153–154 °C; yield 93%. 1H NMR (300 MHz, CDCl3): δ 7.87 (d, J = 7.7 Hz, 1H), 7.52–7.43 (m, 2H), 7.22 (t, J = 7.4 Hz, 1H), 7.06–7.05 (m, 3H), 6.99–6.96 (m, 5H), 6.18 (d, J = 11.5 Hz, 1H), 4.53 (d, J = 11.6 Hz, 1H), 2.34 (s, 6H). 13C NMR (125 MHz, CDCl3): δ 146.2, 144.3, 140.6, 139.7, 139.4, 138.0, 133.4, 130.0, 129.9, 129.3, 128.7, 128.1, 127.8, 124.7, 124.6, 124.0, 83.4, 22.0, 21.8. IR (KBr, ν, cm–1): 3622, 2918, 1662, 1595, 1577, 1501, 816, 770. HRMS m/z (ESI) calcd for C23H19BrIO2S (M – OH)+ 564.9334, found 564.9342.
(E)-1-(2-Bromophenyl)-3-iodo-2-(methylsulfonyl)-3-(p-tolyl)prop-2-en-1-ol (5c)
White solid; mp 177–178 °C; yield: 58%. 1H NMR (300 MHz, CDCl3): δ 7.76 (d, J = 7.7 Hz, 1H), 7.63 (d, J = 7.7 Hz, 1H), 7.44 (t, J = 7.4 Hz, 1H), 7.22 (t, J = 10.22 Hz 5H), 6.15 (d, J = 11.8 Hz, 1H), 4.18 (d, J = 11.5 Hz, 1H), 2.44 (s, 3H), 2.37 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 143.9, 140.8, 139.9, 138.8, 133.0, 130.0, 129.6, 128.9, 127.7, 126.4, 123.5, 82.4, 45.6, 21.5. IR (KBr, ν, cm–1): 3566, 2928, 1581, 1502, 1463, 825, 777. HRMS m/z (ESI) calcd for C17H15BrIO2S (M – OH)+ 488.9021, found 488.9025.
(E)-1-(2-Bromophenyl)-2-((4-chlorophenyl)sulfonyl)-3-iodo-3-(p-tolyl)prop-2-en-1-ol (5d)
Yellow solid; mp 134–135 °C; yield: 76%. 1H NMR (500 MHz, CDCl3): δ 7.86 (d, J = 7.6 Hz, 1H), 7.51–7.50 (m, 1H), 7.46 (t, J = 7.7 Hz, 1H), 7.25–7.21 (m, 1H), 7.15–7.09 (m, 5H), 7.01 (br, 2H), 6.79 (br, 1H), 6.19 (d, J = 11.2 Hz, 1H), 4.39 (d, J = 11.5 Hz, 1H), 2.35 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 145.3, 140.0, 139.7, 139.6, 138.8, 138.7, 133.0, 129.7, 129.4, 129.0, 128.6, 127.4, 125.1, 123.4, 82.9, 21.4. IR (KBr, ν, cm–1): 3564, 2856, 1672, 1529, 898, 816, 701. HRMS m/z (ESI) calcd for C22H16BrClIO2S (M – OH)+ 584.8788, found 584.8786.
(E)-3-Iodo-1-phenyl-2-(phenylsulfonyl)-3-(p-tolyl)prop-2-en-1-ol (5e)
White solid; mp 163–164 °C; yield 70%. 1H NMR (300 MHz, CDCl3): δ 7.66 (d, J = 7.3 Hz, 2H), 7.49–7.38 (m, 4H), 7.15 (t, J = 7.7 Hz, 2H), 7.06–7.04 (m, 2H), 6.93 (br, 4H), 6.38 (d, J = 8.9 Hz, 1H), 4.69 (d, J = 10.8 Hz, 1H), 2.33 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 149.2, 140.7, 140.0, 139.2, 138.9, 132.9, 128.7, 128.3, 128.2, 128.0, 127.9, 127.4, 125.3, 120.4, 83.3, 21.3. IR (KBr, ν, cm–1): 3628, 2914, 1639, 1537, 1462, 816. HRMS m/z (ESI) calcd for C22H18IO2S (M – OH)+ 473.0072, found 473.0074.
(E)-3-Iodo-2-(phenylsulfonyl)-1,3-(di-p-tolyl)prop-2-en-1-ol (5f)
White solid; mp 171–172 °C; yield 78%. 1H NMR (300 MHz, CDCl3): δ 7.54 (d, J = 7.8 Hz, 2H), 7.40 (t, J = 7.2 Hz, 1H), 7.28 (s, 1H), 7.18–7.08 (m, 5H), 6.92 (br, 4H), 6.34 (d, J = 11.9 Hz, 1H), 4.64 (d, J = 12.0 Hz, 1H), 2.40 (s, 3H), 2.33 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 149.2, 140.8, 139.2, 138.9, 137.7, 136.9, 132.8, 129.7, 129.4, 128.3, 127.9, 127.4, 125.2, 120.0, 83.3, 21.4, 21.2. IR (KBr, ν, cm–1): 3535, 2908, 2910, 1527, 1444, 846, 806. HRMS m/z (ESI) calcd for C23H20IO2S (M – OH)+ 487.0229, found 487.0236.
(E)-1-(4-Chlorophenyl)-3-iodo-2-(phenylsulfonyl)-3-(p-tolyl)prop-2-en-1-ol (5g)
White solid; mp 145–146 °C; yield 96%. 1H NMR (300 MHz, CDCl3): δ 7.59 (d, J = 8.4 Hz, 2H), 7.43 (d, J = 8.2 Hz, 3H), 7.21–7.08 (m, 4H), 6.92 (br, 4H), 6.32 (d, J = 11.6 Hz, 1H), 4.69 (d, J = 11.9 Hz, 1H), 2.33 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 148.9, 140.7, 139.5, 138.8, 138.7, 134.0, 133.2, 129.0, 128.5, 128.0, 127.5, 126.9, 120.9, 83.0, 21.5. IR (KBr, ν, cm–1): 3568, 3028, 3030, 1517, 1446, 1083, 848. HRMS m/z (ESI) calcd for C22H17ClIO2S (M – OH)+ 506.9682, found 506.9680.
(E)-3-Iodo-2-(phenylsulfonyl)-1-(o-tolyl)-3-(p-tolyl)prop-2-en-1-ol (5h)
Yellow solid; mp 143–144 °C; yield 83%. 1H NMR (500 MHz, CDCl3): δ 7.76 (d, J = 7.6 Hz, 1H), 7.45 (t, J = 7.4 Hz, 1H), 7.34–7.29 (m, 4H), 7.26–7.23 (m, 3H), 7.18–7.16 (m, 1H), 7.02 (br, 3H), 6.20 (d, J = 11.6 Hz, 1H), 4.43 (d, J = 11.8 Hz, 1H), 2.47 (s, 3H), 2.34 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 147.2, 141.0, 139.6, 139.2, 137.9, 137.0, 133.0, 130.7, 128.4, 128.3, 127.6, 127.4, 126.6, 126.1, 121.7, 82.1, 21.4, 20.2. IR (KBr, ν, cm–1): 3547, 2922, 1635, 1622, 1550, 1485, 815, 746. HRMS m/z (ESI) calcd for C23H20IO2S (M – OH)+ 487.0229, found 487.0235.
(E)-3-Iodo-1,3-diphenyl-2-(phenylsulfonyl)prop-2-en-1-ol (5i)
White solid; mp 126–127 °C; yield 95%. 1H NMR (500 MHz, CDCl3): δ 7.67 (d, J = 8.1 Hz, 2H), 7.48 (t, J = 7.4 Hz, 2H), 7.40 (t, J = 7.3 Hz, 2H), 7.19–7.14 (m, 4H), 7.10 (br, 1H), 7.04–7.02 (m, 3H), 6.84 (br, 1H), 6.40 (d, J = 12.0 Hz, 1H), 4.71 (d, J = 12.0 Hz 1H). 13C NMR (75 MHz, CDCl3): δ 149.4, 141.6, 140.6, 139.9, 133.0, 128.9, 128.7, 128.4, 128.0, 127.7, 127.4, 125.3, 119.8, 83.3. IR (KBr, ν, cm–1): 3500, 3057, 1707, 1583, 1487, 748, 684. HRMS m/z (ESI) calcd for C21H16IO2S (M – OH)+ 458.9916, found 458.9921.
(E)-3-Iodo-3-phenyl-2-(phenylsulfonyl)-1-(p-tolyl)prop-2-en-1-ol (5j)
White solid; mp 169–170 °C; yield 82%. 1H NMR (300 MHz, CDCl3): δ 7.55 (d, J = 6.7 Hz, 2H), 7.39 (d, J = 6.9 Hz, 1H), 7.29–7.25 (m, 2H), 7.19–7.14 (m, 4H), 7.13–7.04 (m, 4H), 6.79 (br, 1H), 6.35 (d, J = 11.1 Hz, 1H), 4.65 (d, J = 11.5 Hz, 1H), 2.41 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 149.8, 142.0, 141.1, 138.1, 137.3, 133.3, 129.8, 129.3, 128.8, 128.1, 127.8, 125.6, 119.8, 83.7, 21.6. IR (KBr, ν, cm–1): 3495, 2945, 1598, 1577, 1512, 1444, 833, 734, 688. HRMS m/z (ESI) calcd for C22H20IO3S (M + H)+ 491.0172, found 491.0179.
(E)-1-(4-Fluorophenyl)-3-iodo-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-ol (5k)
White solid; mp 173–174 °C; yield 87%. 1H NMR (300 MHz, CDCl3): δ 7.70–7.61 (m, 2H), 7.42 (t, J = 7.3 Hz, 1H), 7.21–7.14 (m, 7H), 7.09–7.07 (m, 3H), 6.78 (br, 1H), 6.34 (d, J = 10.0 Hz, 1H), 4.72 (d, J = 11.5 Hz, 1H). 13C NMR (75 MHz, CDCl3): δ 162.5 (d, J = 244.8 Hz), 149.1, 141.5, 140.5, 135.7 (d, J = 2.9 Hz), 133.1, 129.0, 128.5, 127.8, 127.3, 127.2, 127.1 (d, J = 8.2 Hz), 120.0, 115.6 (d, J = 21.5 Hz), 82.9. IR (KBr, ν, cm–1): 3473, 3047, 1602, 1581, 1448, 839, 781, 732, 680. HRMS m/z (ESI) calcd for C21H15FIO2S (M – OH)+ 476.9821, found 476.9828.
(E)-1-(4-Chlorophenyl)-3-iodo-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-ol (5l)
White solid; mp 155–156 °C; yield 74%. 1H NMR (300 MHz, CDCl3): δ 7.60 (d, J = 8.3 Hz, 2H), 7.42 (t, J = 8.7 Hz, 3H), 7.18 (t, J = 7.7 Hz, 5H), 7.07 (d, J = 7.9 Hz, 3H), 6.77 (br, 1H), 6.33 (d, J = 9.9 Hz, 1H), 4.71 (d, J = 12.0 Hz, 1H). 13C NMR (75 MHz, CDCl3): δ 149.0, 141.4, 140.5, 138.5, 133.9, 133.1, 129.0, 128.9, 128.5, 127.8, 127.3, 126.8, 120.2, 82.9. IR (KBr, ν, cm–1): 3645, 3057, 1575, 1489, 1442, 841, 734, 696. HRMS m/z (ESI) calcd for C21H15ClIO2S (M – OH)+ 492.9526, found 492.9524.
(E)-1-(4-Bromophenyl)-3-iodo-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-ol (5m)
White solid; mp 174–175 °C; yield 66%. 1H NMR (300 MHz, CDCl3): δ 7.56 (q, J = 9.6 Hz, 3H), 7.42 (t, J = 7.4 Hz, 1H), 7.25–7.10 (m, 5H), 7.07 (d, J = 7.7 Hz, 3H), 6.77 (br, 2H), 6.31 (d, J = 12.0 Hz, 1H), 4.70 (d, J = 12.0 Hz, 1H). 13C NMR (75 MHz, CDCl3): δ 148.9, 141.4, 140.4, 139.1, 133.1, 131.8, 129.1, 128.5, 127.8, 127.3, 127.1, 122.1, 120.3, 82.9. IR (KBr, ν, cm–1): 3543, 3055, 1685, 1610, 1575, 1512, 1463, 840, 734, 688. HRMS m/z (ESI) calcd for C21H15BrIO2S (M – OH)+ 536.9021, found 536.9025.
(E)-1-Iodo-1-phenyl-2-(phenylsulfonyl)hex-1-en-3-ol (5n)
White solid; mp 106–107 °C; yield 89%. 1H NMR (300 MHz, CDCl3): δ 7.41 (t, J = 7.0 Hz, 1H), 7.28–7.17 (m, 4H), 7.14–7.06 (m, 2H), 7.02–6.91 (m, 2H), 6.59 (br, 1H), 5.10–5.01 (m, 1H), 3.93 (d, J = 11.9 Hz, 1H), 2.27–2.06 (m, 2H), 1.77–1.61 (m, 2H), 1.09 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 149.6, 141.7, 141.0, 132.8, 128.7, 128.5, 127.6, 127.1, 116.8, 82.9, 38.7, 19.5, 14.0. IR (KBr, ν, cm–1): 3614, 3064, 2867, 1602, 1570, 1481, 734, 684. HRMS m/z (ESI) calcd for C18H18IO2S (M – OH)+ 425.0072, found 425.0080.
(E)-3-Iodo-1-(4-methoxyphenyl)-3-phenyl-2-(phenylsulfonyl)prop-2-en-1-ol (5o)
Yellow solid; mp 148–149 °C; yield 81%. 1H NMR (300 MHz, CDCl3): δ 7.59 (d, J = 8.3 Hz, 2H), 7.41 (t, J = 6.9 Hz, 1H), 7.24–7.14 (m, 5H), 7.13–7.06 (m, 3H), 7.01 (d, J = 8.4 Hz, 2H), 6.79 (br, 1H), 6.33 (d, J = 11.9 Hz, 1H), 4.67, (d, J = 12.0 Hz, 1H), 3.86 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 159.4, 149.3, 141.6, 140.7, 133.0, 131.9, 128.9, 128.5, 127.7, 127.4, 126.6, 119.4, 114.1, 83.1, 55.4. IR (KBr, ν, cm–1): 3651, 3105, 1601, 1577, 1506, 1440, 737, 691. HRMS m/z (ESI) calcd for C22H18IO3S (M – OH)+ 489.0021, found 489.0024.
(E)-3-Iodo-1,3-bis(4-methoxyphenyl)-2-(phenylsulfonyl)prop-2-en-1-ol (5p)
White solid; mp 100–101 °C; yield 72%. 1H NMR (300 MHz, CDCl3): δ 7.58 (d, J = 7.8 Hz, 2H), 7.40 (t, J = 6.7 Hz, 1H), 7.19–7.11 (m, 4H), 7.01–6.80 (m, 4H), 6.62 (br, 2H), 6.30 (d, J = 11.5 Hz, 1H), 4.66 (d, J = 11.9 Hz, 1H), 3.87 (s, 3H), 3.80 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 160.0, 159.3, 149.1, 140.9, 134.1, 132.9, 132.0, 129.7, 128.4, 127.3, 126.6, 119.9, 114.1, 113.1, 83.2, 55.4, 55.3. IR (KBr, ν, cm–1): 3622, 3003, 2977, 2847, 1674, 1587, 837, 761. HRMS m/z (ESI) calcd for C23H20IO4S (M – OH)+ 519.0127, found 519.0135.
(E)-3-Iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)-1-(p-tolyl)prop-2-en-1-ol (5q)
White solid; mp 131–132 °C; yield 94%. 1H NMR (300 MHz, CDCl3): δ 7.55 (d, J = 7.9 Hz, 2H), 7.40 (t, J = 7.3 Hz, 1H), 7.28 (d, J = 7.9 Hz, 2H), 7.19 (t, J = 7.7 Hz, 2H), 7.12 (d, J = 7.9 Hz, 2H), 6.95 (br, 2H), 6.63 (s, 2H), 6.33 (d, J = 12.1 Hz, 1H), 4.65 (d, J = 12.1 Hz, 1H), 3.81 (s, 3H), 2.41 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 160.4, 149.7, 141.2, 138.0, 137.4, 134.5, 133.3, 130.2, 129.8, 128.7, 127.7, 125.6, 120.4, 113.5, 83.8, 55.8, 21.6. IR (KBr, ν, cm–1): 3601, 2836, 2900, 1641, 1500, 1481, 833, 733, 685. HRMS m/z (ESI) calcd for C23H20IO3S (M – OH)+ 503.0178, found 503.0170.
(E)-3-Iodo-3-(4-methoxyphenyl)-1-phenyl-2-(phenylsulfonyl)prop-2-en-1-ol (5r)
White solid; mp 127–128 °C; yield 76%. 1H NMR (500 MHz, CDCl3): δ 7.67 (d, J = 7.9 Hz, 2H), 7.48 (t, J = 7.6 Hz, 2H), 7.40 (q, J = 7.1 Hz, 2H), 7.18 (t, J = 7.9 Hz, 2H), 7.08 (d, J = 7.6 Hz, 2H), 6.92 (br, 2H), 6.64 (s, 2H), 6.38 (d, J = 12.1 Hz, 1H), 4.70 (d, J = 12.1 Hz, 1H), 3.81 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 160.4, 149.7, 141.2, 140.4, 134.4, 133.3, 130.2, 129.1, 128.7, 128.3, 127.7, 125.6, 120.7, 113.5, 83.8, 55.8. IR (KBr, ν, cm–1): 3626, 2895, 2897, 1602, 1578, 1474, 837, 746, 685. HRMS m/z (ESI) calcd for C22H18IO3S (M – OH)+ 489.0021, found 489.0023.
(E)-1-(4-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)prop-2-en-1-ol (5s)
White solid; mp 170–171 °C; yield 53%. 1H NMR (300 MHz, CDCl3): δ 7.59 (d, J = 7.7 Hz, 2H), 7.43 (d, J = 7.9 Hz, 3H), 7.20 (t, J = 7.2 Hz, 2H), 7.13–7.10 (m, 2H), 6.90 (br, 2H), 6.63 (s, 2H), 6.31 (d, J = 10.8 Hz, 1H), 4.69 (d, J = 11.3 Hz, 1H) 3.81 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 160.1, 148.8, 140.6, 138.6, 133.8, 133.0, 129.7, 128.8, 128.4, 127.2, 126.8, 120.8, 113.1, 83.0, 55.4. IR (KBr, ν, cm–1): 3566, 3050, 1668, 1585, 1475, 829, 762, 684. HRMS m/z (ESI) calcd for C22H17IClO3S (M – OH)+ 522.9632, found 522.9640.
(E)-1-(4-Bromophenyl)-3-iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)prop-2-en-1-ol (5t)
White solid; mp 176–177 °C; yield 52%. 1H NMR (300 MHz, CDCl3): δ 7.56 (q, J = 8.5 Hz, 4H), 7.42 (t, J = 7.3 Hz, 1H), 7.21 (t, J = 7.5 Hz, 2H), 7.15–7.08 (m, 2H), 6.91 (br, 2H), 6.63 (d, J = 6.4 Hz, 2H), 6.29 (d, J = 9.7 Hz, 1H), 4.69 (d, J = 11.2 Hz, 1H), 3.81, (s, 3H). 13C NMR (75 MHz, CDCl3): δ 160.1, 148.8, 140.6, 139.2, 133.0, 131.8, 129.7, 128.4, 127.2, 127.1, 122.0, 120.8, 113.2, 83.0, 55.4. IR (KBr, ν, cm–1): 3487, 3051, 2960, 2827, 1589,1502, 1527, 1467, 829, 731, 681. HRMS m/z (ESI) calcd for C22H17IBrO3S (M – OH)+ 566.9126, found 566.9128.
(E)-3-Iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)-1-(o-tolyl)prop-2-en-1-ol (5u)
Yellow solid; mp 116–117 °C; yield 80%. 1H NMR (300 MHz, CDCl3): δ 7.77 (d, J = 7.3 Hz, 1H), 7.33–7.30 (m, 3H), 7.29–7.24 (m, 3H), 7.20–7.16 (m, 2H), 6.95 (br, 2H), 6.66 (s, 2H), 6.20 (d, J = 11.5 Hz, 1H), 4.46 (d, J = 11.8 Hz, 1H), 3.81, (s, 3H), 2,48 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 160.0, 147.4, 141.1, 137.9, 137.0, 134.7, 133.0, 130.8, 129.4, 128.5, 128.3, 127.5, 126.8, 126.1, 121.6, 113.1, 82.3, 55.4, 20.2. IR (KBr, ν, cm–1): 3626, 2910, 2837, 1676, 1537, 1475, 1423, 840, 773, 750, 686. HRMS m/z (ESI) calcd for C23H20IO3S (M – OH)+ 503.0178, found 503.0173.
(E)-1-(2-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-(phenylsulfonyl)prop-2-en-1-ol (5v)
Yellow solid; mp 138–139 °C; yield 91%. 1H NMR (300 MHz, CDCl3): δ 7.88 (d, J = 7.6 Hz, 1H), 7.44–7.31 (m, 5H), 7.21–7.12 (m, 5H), 6.62 (br, 2H), 6.28 (d, J = 11.6 Hz, 1H), 4.60 (d, J = 11.7 Hz, 1H), 3.79, (s, 3H). 13C NMR (75 MHz, CDCl3): δ 160.2, 145.5, 140.5, 137.3, 134.9, 133.2, 132.9, 129.7, 129.4, 128.9, 128.4, 127.8, 127.3, 126.8, 123.6, 113.8, 81.7, 55.4. IR (KBr, ν, cm–1): 3500, 3065, 2962, 2914, 1600, 1577, 1500, 1460, 839, 758, 685, 650. HRMS m/z (ESI) calcd for C22H17ClIO3S (M – OH)+ 522.9632, found 522.9639.
(E)-1-(4-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-tosylprop-2-en-1-ol (5w)
White solid; mp 160–161 °C; yield 65%. 1H NMR (300 MHz, CDCl3): δ 7.58 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 8.3 Hz, 2H), 7.08–6.92 (m, 6H), 6.65 (d, J = 7.6 Hz, 2H), 6.30 (d, J = 12.0 Hz, 1H), 4.71 (d, J = 11.9 Hz, 1H), 3.81 (s, 3H), 2.34 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 160.1, 149.0, 144.1, 138.7, 137.7, 134.0, 133.8, 129.7, 129.0, 128.8, 127.4, 126.8, 120.5, 113.1, 83.0, 55.4, 21.6. IR (KBr, ν, cm–1): 3630, 3058, 3048, 3009, 1604, 1504, 831, 760, 694. HRMS m/z (ESI) calcd for C23H19ClIO3S (M – OH)+ 536.9788, found 536.5783.
(E)-1-(4-Chlorophenyl)-3-iodo-3-(4-methoxyphenyl)-2-(methylsulfonyl)prop-2-en-1-ol (5x)
Yellow solid; mp 191–192 °C; yield 77%. 1H NMR (300 MHz, CDCl3): δ 7.52 (d, J = 8.2 Hz, 2H), 7.39 (t, J = 9.0 Hz, 4H), 6.93 (d, J = 8.6 Hz, 2H), 6.22 (d, J = 8.4 Hz, 1H), 4.36 (d, J = 10.4 Hz, 1H), 3.84 (s, 3H), 2.53 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 160.6, 147.2, 138.3, 134.2, 134.0, 129.4, 128.9, 126.8, 113.7, 82.8, 55.4, 45.8. IR (KBr, ν, cm–1): 3585, 2891, 2839, 1651, 1607, 1508, 837, 775, 669. HRMS m/z (ESI) calcd for C17H15ClIO3S (M – OH)+ 460.9475, found 460.9469.
(E)-1-(4-Chlorophenyl)-3-(4-fluorphenyl)-3-iodo-2-(phenylsulfonyl)prop-2-en-1-ol (5y)
White solid; mp 178–179 °C; yield 61%. 1H NMR (300 MHz, CDCl3): δ 7.59 (d, J = 8.5 Hz, 2H), 7.46 (t, J = 8.7 Hz, 3H), 7.25 (t, J = 3.8 Hz, 3H), 7.16–7.12 (m, 2H), 6.80 (br, 3H), 6.32 (d, J = 10.1 Hz, 1H), 4.67 (d, J = 11.3 Hz, 1 H). 13C NMR (125 MHz, CDCl3): δ 163.0 (d, J = 250.0 Hz), 150.2, 140.9, 138.7, 137.9, 134.4, 133.7, 130.5 (d, J = 5.3 Hz), 129.3, 129.0, 127.6, 127.1, 118.9, 115.3 (d, J = 21.6 Hz), 83.18. IR (KBr, ν, cm–1): 2643, 3060, 2804, 1602, 1501, 1306, 806, 733, 687. HRMS m/z (ESI) calcd for C21H14ClFIO2S (M – OH)+ 510.9432, found 510.9426.
(E)-3-(4-Fluorophenyl)-3-iodo-2-(phenylsulfonyl)-1-(o-tolyl)prop-2-en-1-ol (5z)
Yellow solid; mp 129–130 °C; yield 87%. 1H NMR (500 MHz, CDCl3): δ 7.75 (d, J = 7.5 Hz, 1H), 7.49 (t, J = 7.3 Hz, 1H), 7.36–7.33 (m, 3H), 7.32–7.28 (m, 4H), 7.19 (d, J = 7.3 Hz, 1H), 6.85 (br, 3H), 6.20 (d, J = 11.8 Hz, 1H), 4.41 (d, J = 11.8 Hz, 1H), 2.49 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 162.60 (d, J = 249.38), 148.39, 140.91, 138.30, 137.37 (d, J = 39.0 Hz), 133.28, 130.83, 129.72, 128.67, 128.52, 127.50, 126.47 (d, J = 47.78 Hz), 119.57, 114.88 (d, J = 22.1 Hz), 20.15. IR (KBr, ν, cm–1): 3626, 3014, 1649, 1589, 1527, 1477, 836, 781, 681. HRMS m/z (ESI) calcd for C22H17FIO2S (M – OH)+ 490.9978, found 490.9985.
Typical Experimental Procedure for the Synthesis of Funtionalized Conjugated Enynes 7
Under argon atmosphere, a Schlenk flask equipped with a condenser was charged with 0.25 mmol (51 mg) of 1,4-diphenylbuta-1,3-diyne (6a), 0.5 mmol (82 mg) of sodium benzenesulfinate (2a), 0.375 mmol (95 mg) of I2, and 3 mL of 1,2-dichloroethane/H2O (v/v 2/1). The reaction mixture was stirred at reflux until the complete consumption of 6a (monitored by TLC). The reaction was then quenched with saturated NH4Cl and extracted with ethyl acetate (3 × 15 mL). The organic layer was washed with saturated Na2S2O3 and dried over anhydrous Na2SO4. After filtration and removal of the solvent in vacuo, the crude product was purified by flash column chromatography on silica gel (silica gel, petroleum ether/ethyl acetate = 20/1) to give product 7a.
(E)-2-Iodo-1,4-diphenyl-1-phenylsulfonylbut-1-en-3-yne (7a)
Yellow solid; mp 95–96 °C; yield 62%. 1H NMR (300 MHz, CDCl3): δ 7.76 (d, J = 7.6 Hz, 2H), 7.62–7.56 (m, 3H), 7.45–7.41 (m, 5H), 7.35 (d, J = 7.6 Hz, 3H), 7.05 (d, J = 6.1 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 152.6, 140.0, 138.4, 134.1, 132.4, 130.5, 130.3, 130.0, 129.5, 129.3, 129.0, 128.7, 122.0, 107.8, 89.9, 89.7. IR (KBr, ν, cm–1): 3076, 2181, 1573, 1496, 1313, 1153, 835, 576. HRMS m/z (ESI) calcd for C22H16IO2S (M + H)+ 470.9910, found 470.9917.
(E)-2-Iodo-1,4-diphenyl-1-tosylbut-1-en-3-yne (7b)
Yellow solid; mp 121–122 °C; yield 71%. 1H NMR (300 MHz, CDCl3): δ 7.65–7.60 (m, 4H), 7.43–7.32 (m, 6H), 7.22 (d, J = 7.0 Hz, 2H), 7.06 (d, J = 7.5 Hz, 2H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 153.0, 145.2, 138.5, 137.1, 132.4, 130.5, 130.3, 129.9, 129.6, 129.0, 128.9, 128.7, 122.1, 107.6, 89.7, 89.5, 22.1. IR (KBr, ν, cm–1): 3059, 2194, 1597, 1332, 1149, 569. HRMS m/z (ESI) calcd for C23H18IO2S (M + H)+ 485.0067, found 485.0074.
(E)-2-Iodo-1,4-diphenyl-1-(methylsulfonyl)but-1-en-3-yne (7c)
White solid; mp 102–103 °C; yield 54%. 1H NMR (300 MHz, CDCl3): δ 7.59–7.56 (m, 2H), 7.48–7.46 (m, 3H), 7.41–7.37 (m, 3H), 7.36–7.33 (m, 2H), 3.03(s, 3H). 13C NMR (125 MHz, CDCl3): δ 152.6, 138.0, 132.4, 130.7, 130.4, 130.0, 129.2, 129.0, 121.6, 108.0, 89.2, 89.1, 42.7. IR (KBr, ν, cm–1): 3012, 2175, 1438, 1313, 1132, 767, 538. HRMS m/z (ESI) calcd for C17H14IO2S (M + H)+ 408.9754, found 408.9758.
(E)-2-Iodo-1,4-di(p-tolyl)-1-phenylsulfonylbut-1-en-3-yne (7d)
Yellow solid; mp 115–116 °C; yield 69%. 1H NMR (300 MHz, CDCl3): δ 7.78 (d, J = 7.4 Hz, 2H), 7.58 (t, J = 7.4 Hz, 1H), 7.50 (d, J = 8.0 Hz, 2H), 7.42 (t, J = 7.6 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 7.8 Hz, 2H), 6.95 (d, J = 8.0 Hz, 2H), 2.41 (s, 3H), 2.37 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 152.2, 141.1, 140.3, 140.1, 135.5, 133.9, 132.3, 130.2, 129.7, 129.4, 129.2, 128.9, 119.0, 108.3, 90.3, 89.5, 22.1, 21.9. IR (KBr, ν, cm–1): 3064, 2194, 1327, 1149, 837, 569. HRMS m/z (ESI) calcd for C24H20IO2S (M + H)+ 499.0223, found 499.0229.
(E)-2-Iodo-1,4-di(p-tolyl)-1-tosylbut-1-en-3-yne (7e)
Yellow solid; mp 134–135 °C; yield 74%. 1H NMR (300 MHz, CDCl3): δ 7.66 (d, J = 8.0 Hz, 2H), 7.49 (d, J = 7.8 Hz, 2H), 7.23–7.21 (m, 4H), 7.16 (d, J = 7.7 Hz, 2H), 6.96 (d, J = 7.7 Hz, 2H), 2.41 (s, 3H), 2.39 (s, 3H), 2.38 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 152.6, 145.0, 141.0, 140.0, 137.3, 135.7, 132.3, 130.2, 129.9, 129.7, 129.4, 129.0, 119.1, 108.0, 89.9, 89.6, 22.1, 22.0, 21.9. IR (KBr, ν, cm–1): 3030, 2189, 1570, 1321, 1145, 817, 582. HRMS m/z (ESI) calcd for C25H22IO2S (M + H)+ 513.0380, found 513.0386.
(E)-2-Iodo-1,4-di(p-tolyl)-1-methylsulfonylbut-1-en-3-yne (7f)
Yellow solid; mp 152–153 °C; yield 67%. 1H NMR (300 MHz, CDCl3): δ 7.46 (d, J = 8.0 Hz, 2H), 7.29–7.18 (m, 6H), 3.03 (s, 3H), 2.41 (s, 3H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 151.7, 140.9, 140.2, 134.6, 131.9, 129.5, 129.4, 129.3, 118.2, 108.0, 89.0, 88.6, 42.3, 21.8, 21.6. IR (KBr, ν, cm–1): 3023, 2188, 1575, 1311, 1132, 825, 540. HRMS m/z (ESI) calcd for C19H18IO2S (M + H)+ 437.0067, found 437.0073.
(E)-2-Iodo-1,4-di(p-fluorophenyl)-1-methylsulfonylbut-1-en-3-yne (7g)
Yellow solid; mp 98–99 °C; yield 48%. 1H NMR (300 MHz, CDCl3): δ 7.59–7.55 (m, 2H), 7.34–7.32 (m, 2H), 7.19–7.07 (m, 4H), 3.02 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 164.2 (d, J = 251.7 Hz), 163.9 (d, J = 249.5 Hz), 151.5, 134.7 (d, J = 8.75 Hz), 133.8 (d, J = 3.4 Hz), 132.2 (d, J = 8.5 Hz), 117.7 (d, J = 3.1 Hz), 116.5 (d, J = 22.1 Hz), 107.3, 89.8, 89.0, 42.6. IR (KBr, ν, cm–1): 3063, 2198, 1597, 1489, 1325, 1149, 840, 572. HRMS m/z (ESI) calcd for C17H12F2IO2S (M + H)+ 444.9565, found 444.9572.
(E)-2-Iodo-1,4-di(p-fluorophenyl)-1-(phenylsulfonyl)but-1-en-3-yne (7h)
Yellow solid; mp 117–118 °C; yield 57%. 1H NMR (300 MHz, CDCl3): δ 7.73 (d, J = 7.4 Hz, 2H), 7.64–7.58 (m, 3H), 7.44 (t, J = 7.8 Hz, 2H), 7.12 (t, J = 8.7 Hz, 2H), 7.02 (d, J = 6.9 Hz, 4H). 13C NMR (125 MHz, CDCl3): δ 164.9 (d, J = 251.5 Hz), 162.9 (d, J = 248.9 Hz), 151.5, 139.7, 134.6 (d, J = 8.7 Hz), 134.2, 134.1 (d, J = 3.4 Hz), 132.5 (d, J = 8.5 Hz), 129.4, 128.8, 118.1 (d, J = 7.5 Hz), 116.5 (d, J = 22 Hz), 116.0 (d, J = 22.1 Hz), 107.0, 90.2, 89.4. IR (KBr, ν, cm–1): 3070, 2189, 1575, 1313, 1153, 842, 582. HRMS m/z (ESI) calcd for C22H14F2IO2S (M + H)+ 506.9722, found 506.9725.
(E)-2-Iodo-1,4-di(p-fluorophenyl)-1-tosylbut-1-en-3-yne (7i)
Yellow solid; mp 124–125 °C; yield 65%. 1H NMR (300 MHz, CDCl3): δ 7.63–7.59 (m, 4H), 7.23 (d, J = 8.1 Hz, 2H), 7.12 (t, J = 8.7 Hz, 2H), 7.03 (d, J = 6.9 Hz, 4H), 2.40 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 164.0 (d, J = 250.0 Hz), 163.7 (d, J = 248.9 Hz), 151.9, 145.4, 136.8, 134.6 (d, J = 8.4 Hz), 132.5 (d, J = 6.0 Hz), 130.0, 128.9, 118.1 (d, J = 3.1 Hz), 116.5 (d, J = 25 Hz), 116.0 (d, J = 19.4 Hz), 106.7, 89.8, 89.4, 22.1. IR (KBr, ν, cm–1): 2918, 2174, 1575, 1149, 817, 584. HRMS m/z (ESI) calcd for C23H16F2IO2S (M + H)+ 520.9878, found 520.9881.
(E)-2-Iodo-1,4-di(p-methoxyphenyl)-1-tosylbut-1-en-3-yne (7j)
Yellow solid; mp 143–144 °C; yield 65%. 1H NMR (300 MHz, CDCl3): δ 7.64 (d, J = 8.2 Hz, 2H), 7.55 (d, J = 8.7 Hz, 2H), 7.22 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 6.93 (d, J = 8.1 Hz, 2H), 6.86 (d, J = 8.8 Hz, 2H), 3.85 (s, 3H), 3.83 (s, 3H), 2.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 161.5, 160.8, 151.5, 144.9, 137.4, 134.2, 132.0, 130.8, 129.9, 128.9, 114.7, 114.2, 114.0, 108.2, 90.4, 89.4, 55.8, 55.7, 22.1. IR (KBr, ν, cm–1): 2964, 2175, 1570, 1496, 1249, 1149, 831, 569. HRMS m/z (ESI) calcd for C25H22IO4S (M + H)+ 545.0278, found 545.0281.
(E)-2-Iodo-1,4-di(p-chlorophenyl)-1-tosylbut-1-en-3-yne (7k)
Yellow solid; mp 145–146 °C; yield 61%. 1H NMR (300 MHz, CDCl3): δ 7.60 (d, J = 8.2 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 6.98 (d, J = 8.4 Hz, 2H), 2.40 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 152.2, 145.5, 136.9, 136.7, 136.6, 136.3, 133.6, 131.8, 130.1, 129.4, 129.1, 128.9, 120.4, 106.6, 90.3, 89.4, 22.1. IR (KBr, ν, cm–1): 3057, 2194, 1568, 1494, 1228, 1153, 840, 584. HRMS m/z (ESI) calcd for C23H16Cl2IO2S (M + H)+ 552.9287, found 552.9295.
Typical Experimental Procedure for Suzuki Coupling Reaction of Vinyl Iodides (3a, 5e, and 7b) with p-Tolylboronic Acid
Under an argon atmosphere, 0.5 mmol (244 mg) of (E)-3-iodo-3-phenyl-2-(phenylsulfonyl)-1-(4-tolyl)- prop-2-en-1-one (3a) was added to the mixture of 0.6 mmol (82 mg) of p-tolylboronic acid, 0.05 mmol (35 mg) of Pd(PPh3)2Cl2, and 0.6 mmol (83 mg) of K2CO3 in 3 mL of toluene/H2O (v/v 2:1). After stirring at 45 °C for 2 h (monitored by TLC), the reaction mixture was quenched with saturated NaCl, extracted with ethyl acetate (3 × 10 mL), and dried over anhydrous Na2SO4. After filtration and concentrated in vacuo, the crude product was purified with flash chromatography on silica gel (petroleum ether/ethyl acetate = 20/1) to give product 8.
(E)-3-Phenyl-2-(phenylsulfonyl)-1,3-di-p-tolylprop-2-en-1-one (8)
White solid; mp 170–171 °C; yield 94%. 1H NMR (300 MHz, CDCl3): δ 7.85 (d, J = 8.0 Hz, 2H), 7.49 (d, J = 7.5 Hz, 2H), 7.42 (t, J = 7.3 Hz, 1H), 7.28–7.16 (m, 5H), 7.13–7.05 (m, 4H), 6.90 (d, J = 8.0 Hz, 2H), 6.80 (d, J = 8.0 Hz, 2H), 2.28 (s, 3H), 2.08 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 191.7, 154.9, 145.3, 141.9, 141.2, 134.0, 137.4, 136.2, 134.8, 133.4, 130.3, 129.9, 129.8, 129.5, 129.3, 129.0, 128.8, 128.6, 128.1, 22.2, 21.6. IR (KBr, ν, cm–1): 3057, 2918, 1662, 1604, 1319, 1305, 1251, 1149, 1085, 823, 738, 582, 528. HRMS m/z (ESI) calcd for C29H25O3S (M + H)+ 453.1519, found 453.1509.
1-Phenyl-2-(phenylsulfonyl)-3,3-di-p-tolylprop-2-en-1-ol (9)
White solid; mp 186–187 °C; yield 92%. 1H NMR (300 MHz, CDCl3): δ 7.56 (d, J = 6.6 Hz, 2H), 7.40 (t, J = 7.23 Hz, 2H), 7.33–7.20 (m, 6H), 7.17–7.07 (m, 4H), 6.87–6.77 (m, 4H), 5.95 (d, J = 11.8 Hz, 1H), 4.86 (d, J = 11.8 Hz, 1H), 2.29 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 156.4, 142.7, 141.7, 141.5, 139.4, 138.5, 137.3, 135.6, 132.2, 129.9, 129.3, 128.9, 128.3, 127.9, 127.3, 127.2, 125.5, 73.6, 21.3, 21.2. IR (KBr, ν, cm–1): 3526, 3057, 3018, 2920, 1638, 1605, 1491, 1442, 816, 748, 698. HRMS m/z (ESI) calcd for C29H25O2S (M – OH) + 437.1570, found 437.1571.
(Z)-(2-(p-Tolyl)-1-tosylbut-1-en-3-yne-1,4-diyl)dibenzene (10)
White solid; mp 131–132 °C; yield 92%. 1H NMR (300 MHz, CDCl3): δ 7.74 (d, J = 7.7 Hz, 2H), 7.64–7.58 (m, 2H), 7.47–7.34 (m, 3H), 7.21–7.19 (m, 5H), 7.11–7.08 (m, 4H), 6.90 (d, J = 7.6 Hz, 2H), 2.38 (s, 3H), 2.21 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 146.9, 144.5, 139.0, 138.4, 135.1, 133.8, 133.7, 132.4, 132.2, 129.8, 129.7, 129.0, 128.9, 128.8, 128.4, 123.1, 104.8, 88.6, 22.1, 21.6. IR (KBr, ν, cm–1): 3041, 2198, 1600, 1298, 1143, 694, 576. HRMS m/z (ESI) calcd for C30H25O2S (M + H)+ 449.1570, found 449.1574.
Acknowledgments
We gratefully acknowledge the National Natural Science Foundation of China (Nos. 21272004 and 21702237) for financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02966.
The authors declare no competing financial interest.
Supplementary Material
References
- a Yin W. H.; Liang W. H.; Guo L. P.; Lei J. H.; Qiu F. Y. G. Stereoselective Synthesis of Homofarnesenes: Establishment of an Efficient Method for the Stereoselective Preparation of Acyclic Tetrasubstituted Olefins. ACS Omega 2018, 3, 4551–4556. 10.1021/acsomega.8b00439. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ganapathy D.; Sekar G. Efficient Synthesis of Polysubstituted Olefins Using Stable Palladium Nanocatalyst: Applications in Synthesis of Tamoxifen and Isocombretastatin A4. Org. Lett. 2014, 16, 3856–3859. 10.1021/ol5017367. [DOI] [PubMed] [Google Scholar]; c McKinley N. F.; O’Shea D. F. Carbolithiation of Diphenylacetylene as a Stereoselective Route to (Z)-Tamoxifen and Related Tetrasubstituted Olefins. J. Org. Chem. 2006, 71, 9552–9555. 10.1021/jo061949s. [DOI] [PubMed] [Google Scholar]; d Trost B. M.; Papillon J. P. N.; Nussbaumer T. Ru-Catalyzed Alkene-Alkyne Coupling. Total Synthesis of Amphidinolide P. J. Am. Chem. Soc. 2005, 127, 17921–17937. 10.1021/ja055967n. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Williams R. B.; Norris A.; Slebodnick C.; Merola J.; Miller J. S.; Andriantsiferana R.; Rasamison V. E.; Kingston D. G. I. Cytotoxic Sesquiterpene Lactones from Vernonia pachyclada from the Madagascar Rainforest. J. Nat. Prod. 2005, 68, 1371–1374. 10.1021/np050202e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews on polysubstituted alkene, see:; a Dong Z.; Ren Z.; Thompson S. J.; Xu Y.; Dong G. B. Transition-Metal-Catalyzed C-H Alkylation Using Alkenes. Chem. Rev. 2017, 117, 9333–9403. 10.1021/acs.chemrev.6b00574. [DOI] [PubMed] [Google Scholar]; b Kraft S.; Ryan K.; Kargbo R. B. Recent Advances in Asymmetric Hydrogenation of Tetrasubstituted Olefins. J. Am. Chem. Soc. 2017, 139, 11630–11641. 10.1021/jacs.7b07188. [DOI] [PubMed] [Google Scholar]; c Crossley S. W. M.; Obradors C.; Martinez R. M.; Shenvi R. A. Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev. 2016, 116, 8912–9000. 10.1021/acs.chemrev.6b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Flynn A. B.; Ogilvie W. W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745. 10.1021/cr050051k. [DOI] [PubMed] [Google Scholar]
- a Cheng L. J.; Mankad N. P. Copper-Catalyzed Borocarbonylative Coupling of Internal Alkynes with Unactivated Alkyl Halides: Modular Synthesis of Tetrasubstituted β-Borylenones. Angew. Chem., Int. Ed. 2018, 57, 10328–10332. 10.1002/anie.201804883. [DOI] [PubMed] [Google Scholar]; b Lim N. K.; Weiss P.; Li B. X.; McCulley C. H.; Hare S. R.; Bensema B. L.; Palazzo T. A.; Tantillo D. J.; Zhang H. M.; Gosselin F. Synthesis of Highly Stereodefined Tetrasubstituted Acyclic All-Carbon Olefins via a Syn-Elimination Approach. Org. Lett. 2017, 19, 6212–6215. 10.1021/acs.orglett.7b03141. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Lu N.; Zhang Z. G.; Ma N. N.; Wu C. H.; Zhang G. S.; Liu Q. F.; Liu T. X. Copper-Catalyzed Difunctionalization of Allenes with Sulfonyl Iodides Leading to (E)-α-Iodomethyl Vinylsulfones. Org. Lett. 2018, 20, 4318–4322. 10.1021/acs.orglett.8b01765. [DOI] [PubMed] [Google Scholar]; b Fu H.; Shang J. Q.; Yang T.; Shen Y. H.; Gao C. Z.; Li Y. M. Copper-Catalyzed Decarboxylative Disulfonylation of Alkynyl Carboxylic Acids with Sulfinic Acids. Org. Lett. 2018, 20, 489–492. 10.1021/acs.orglett.7b03922. [DOI] [PubMed] [Google Scholar]; c Suzol S. H.; Howlader A. H.; Wen Z. W.; Ren Y. U.; Laverde E. E.; Garcia C.; Liu Y.; Wnuk S. F. Pyrimidine Nucleosides with a Reactive (β-Chlorovinyl)sulfone or (β-Keto)sulfone Group at the C5 Position, Their Reactions with Nucleophiles and Electrophiles, and Their Polymerase-Catalyzed Incorporation into DNA. ACS Omega 2018, 3, 4276–4288. 10.1021/acsomega.8b00584. [DOI] [PMC free article] [PubMed] [Google Scholar]; d García-Domínguez A.; Müller S.; Nevado C. Nickel-Catalyzed Intermolecular Carbosulfonylation of Alkynes via Sulfonyl Radicals. Angew. Chem., Int. Ed. 2017, 56, 9949–9952. 10.1002/anie.201704862. [DOI] [PubMed] [Google Scholar]; e Xiang Y. C.; Kuang Y. Y.; Wu J. Generation of β-Halo Vinylsulfones through a Multicomponent Reaction with Insertion of Sulfur Dioxide. Chem. - Eur. J. 2017, 23, 6996–6999. 10.1002/chem.201701465. [DOI] [PubMed] [Google Scholar]; f Fang Y. Y.; Luo Z. G.; Xu X. M. Recent Advances in the Synthesis of Vinyl Sulfones. RSC Adv. 2016, 6, 59661–59676. 10.1039/C6RA10731A. [DOI] [Google Scholar]; g Singh R.; Allam B. K.; Singh N.; Kumari K.; Singh S. K.; Singh K. N. A Direct Metal-Free Decarboxylative Sulfono Functionalization (DSF) of Cinnamic Acids to α,β-Unsaturated Phenyl Sulfones. Org. Lett. 2015, 17, 2656–2659. 10.1021/acs.orglett.5b01037. [DOI] [PubMed] [Google Scholar]; h Newton A. S.; Glória P. M. C.; Gonçalves L. M.; dos Santos D. J. V. A.; Moreira R.; Guedes R. C.; Santos M. M. M. Synthesis and Evaluation of Vinyl Sulfones as Caspase-3 Inhibitors. A Structure-Activity Study. Eur. J. Med. Chem. 2010, 45, 3858–3863. 10.1016/j.ejmech.2010.05.039. [DOI] [PubMed] [Google Scholar]; i Meadows D. C.; Sanchez T.; Neamati N.; North T. W.; Gervay-Hague J. Ring Substituent Effects on Biological Activity of Vinyl Sulfones as Inhibitors of HIV-1. Bioorg. Med. Chem. 2007, 15, 1127–1137. 10.1016/j.bmc.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Frankel B. A.; Bentley M.; Kruger R. G.; McCafferty D. G. Vinyl Sulfones: Inhibitors of SrtA, a Transpeptidase Required for Cell Wall Protein Anchoring and Virulence in Staphylococcus aureus. J. Am. Chem. Soc. 2004, 126, 3404–3405. 10.1021/ja0390294. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Wan J. P.; Hu D. Q.; Bai F. C.; Wei L.; Liu Y. Y. Stereoselective Z-Halosulfonylation of Terminal Alkynes using Sulfonohydrazides and CuX (X = Cl, Br, I). RSC Adv. 2016, 6, 73132–73135. 10.1039/C6RA13737G. [DOI] [Google Scholar]; b Li X. Q.; Shi X. H.; Fang M. W.; Xu X. S. Iron Halide-Mediated Regio- and Stereoselective Halosulfonylation of Terminal Alkynes with Sulfonylhydrazides: Synthesis of (E)-β-Chloro and Bromo Vinylsulfones. J. Org. Chem. 2013, 78, 9499–9504. 10.1021/jo401581n. [DOI] [PubMed] [Google Scholar]; c Zeng X. M.; Ilies L.; Nakamura E. Iron-Catalyzed Regio- and Stereoselective Chlorosulfonylation of Terminal Alkynes with Aromatic Sulfonyl Chlorides. Org. Lett. 2012, 14, 954–956. 10.1021/ol203446t. [DOI] [PubMed] [Google Scholar]; d Liu X. Y.; Duan X. H.; Pan Z. L.; Han Y.; Liang Y. M. Stereoselective Synthesis of (Z)-β-Chlorovinyl Sulfones by Addition of Sulfonyl Chlorides to Acetylenes. Synlett 2005, 11, 1752–1754. 10.1055/s-2005-869876. [DOI] [Google Scholar]
- a Hou Y. L.; Zhu L. Y.; Hu H.; Chen S. W.; Li Z. F.; Liu Y. J.; Gong P. Iodine Promoted Iodosulfonylation of Alkynes with Sulfonyl Hydrazides in an Aqueous Medium: Highly Stereoselective Synthesis of (E)-β-Iodo Vinylsulfones. New J. Chem. 2018, 42, 8752–8755. 10.1039/C8NJ01145A. [DOI] [Google Scholar]; and references cited therein.; b Yang L.; Hu D. Q.; Wei L.; Liu Y. Y. KI as Iodine Source for the Synthesis of E-Iodovinyl Sulfones via Metal-Free Iodosulfonylation of Terminal Alkynes. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 1301–1304. 10.1080/10426507.2017.1359596. [DOI] [Google Scholar]
- Wei W.; Wen J. W.; Yang D. S.; Jing H. J.; You J. M.; Wang H. Direct Difunctionalization of Alkynes with Sulfinicacids and Molecular Iodine: a Simple and Convenient Approach to (E)-β-Iodovinyl Sulfones. RSC Adv. 2015, 5, 4416–4419. 10.1039/C4RA13998D. [DOI] [Google Scholar]
- Gao Y.; Wu W. Q.; Huang Y. B.; Huang K. F.; Jiang H. F. NBS-Promoted Halosulfonylation of Terminal Alkynes: Highly Regio- and Stereoselective Synthesis of (E)-β-Halo Vinylsulfones. Org. Chem. Front. 2014, 1, 361–364. 10.1039/C3QO00075C. [DOI] [Google Scholar]
- Sun Y. D.; Abdukader A.; Lu D.; Zhang H. Y.; Liu C. J. Synthesis of (E)-β-Iodo Vinylsulfones via Iodine Promoted Iodosulfonylation of Alkynes with Sodium Sulfinates in an Aqueous Medium at Room Temperature. Green Chem. 2017, 19, 1255–1258. 10.1039/C6GC03387C. [DOI] [Google Scholar]
- a Cui H. H.; He C. L.; Yang D. S.; Yue H. L.; Wei W.; Wang H. Direct Iodosulfonylation of Alkylynones with Sulfonylhydrazides and Iodine Pentoxide Leading to Multisubstituted αβ-Enones. Synlett 2018, 29, 830–834. 10.1055/s-0036-1589160. [DOI] [Google Scholar]; b Kumar R.; Dwivedi V.; Reddy M. S. Metal-Free Iodosulfonylation of Internal Alkynes: Stereodefined Access to Tetrasubstituted Olefins. Adv. Synth. Catal. 2017, 359, 2847–2856. 10.1002/adsc.201700576. [DOI] [Google Scholar]
- a Xie M. H.; Wang S. K.; Wang J.; Fang K.; Liu C. Q.; Zha C.; Jia J. Construction of Substituted Benzenes via Pd-Catalyzed Cross-Coupling/Cyclization Reaction of Vinyl Halides and Terminal Alkynes. J. Org. Chem. 2016, 81, 3329–3334. 10.1021/acs.joc.6b00308. [DOI] [PubMed] [Google Scholar]; b Xie M. H.; Wang J.; Fang K.; Wang S. K.; Yan L. Q. Regio- and Stereoselective Synthesis of (E)-β-halo Alkenyl Ketones/Sulfones via Haloallylation of Alkynyl Ketones/Sulfones. Tetrahedron Lett. 2015, 56, 4388–4391. 10.1016/j.tetlet.2015.05.098. [DOI] [Google Scholar]
- For selected examples, see:; a Kumar G. S.; Kapur M. Ruthenium-Catalyzed, Site-Selective C-H Allylation of Indoles with Allyl Alcohols as Coupling Partners. Org. Lett. 2016, 18, 1112–1115. 10.1021/acs.orglett.6b00217. [DOI] [PubMed] [Google Scholar]; b Mondal K.; Mondal B.; Pan S. C. Organocatalytic Redox Isomerization of Electron-Deficient Allylic Alcohols: Synthesis of 1,4-Ketoaldehydes. J. Org. Chem. 2016, 81, 4835–4840. 10.1021/acs.joc.6b00243. [DOI] [PubMed] [Google Scholar]; c Ricard S.; Sanapo G. F.; Rahem N.; Daoust B. Synthesis of γ,δ-Unsaturated α-Aminoaldehydes Using a Copper Catalyzed Vinylation Reaction Followed by a Claisen Rearrangement. J. Org. Chem. 2016, 81, 5066–5073. 10.1021/acs.joc.6b00512. [DOI] [PubMed] [Google Scholar]; d Aursnes M.; Tungen J. E.; Colas R. A.; Vlasakov I.; Dalli J.; Serhan C. N.; Hansen T. V. Synthesis of the 16S,17S-Epoxyprotectin Intermediate in the Biosynthesis of Protectins by Human Macrophages. J. Nat. Prod. 2015, 78, 2924–2931. 10.1021/acs.jnatprod.5b00574. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Chan J.; Jamison T. F. Enantioselective Synthesis of (−)-Terpestacin and Structural Revision of Siccanol Using Catalytic Stereoselective Fragment Couplings and Macrocyclizations. J. Am. Chem. Soc. 2004, 126, 10682–10691. 10.1021/ja0470968. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Shimkin K. W.; Montgomery J. Synthesis of Tetrasubstituted Alkenes by Tandem Metallacycle Formation/Cross-Electrophile Coupling. J. Am. Chem. Soc. 2018, 140, 7074–7078. 10.1021/jacs.8b04637. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huang Y.; Huang R. Z.; Zhao Y. Cobalt-Catalyzed Enantioselective Vinylation of Activated Ketones and Imines. J. Am. Chem. Soc. 2016, 138, 6571–6576. 10.1021/jacs.6b02372. [DOI] [PubMed] [Google Scholar]; c Nie M.; Fu W. Z.; Cao Z. P.; Tang W. J. Enantioselective Nickel-catalyzed Alkylative Alkyne–Aldehyde Cross-Couplings. Org. Chem. Front. 2015, 2, 1322–1325. 10.1039/C5QO00148J. [DOI] [Google Scholar]; d Bauer T. Enantioselective dialkylzinc-mediated alkynylation, arylation and alkenylation of carbonyl groups. Coord. Chem. Rev. 2015, 299, 83–150. 10.1016/j.ccr.2015.03.025. [DOI] [Google Scholar]; e Haynes M. T.; Liu P.; Baxter R. D.; Nett A. J.; Houk K. N.; Montgomery J. Dimer Involvement and Origin of Crossover in Nickel-Catalyzed Aldehyde-Alkyne Reductive Couplings. J. Am. Chem. Soc. 2014, 136, 17495–17504. 10.1021/ja508909u. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Montgomery J. Nickel-Catalyzed Cyclizations, Couplings, and Cycloadditions Involving Three Reactive Components. Acc. Chem. Res. 2000, 33, 467–473. 10.1021/ar990095d. [DOI] [PubMed] [Google Scholar]
- a Han J. T.; Yun J. Copper-Catalyzed Synthesis of Tetrasubstituted Enynylboronates via Chemo-, Regio-, and Stereoselective Borylalkynylation. Org. Lett. 2018, 20, 2104–2107. 10.1021/acs.orglett.8b00665. [DOI] [PubMed] [Google Scholar]; b Ye C. Q.; Qian B.; Li Y. J.; Su M.; Li D. L.; Bao H. L. Iron-Catalyzed Dehydrative Alkylation of Propargyl Alcohol with Alkyl Peroxides To Form Substituted 1,3-Enynes. Org. Lett. 2018, 20, 3202–3205. 10.1021/acs.orglett.8b01043. [DOI] [PubMed] [Google Scholar]; c Zhou Y. J.; Zhang Y.; Wang J. B. Recent Advances in Transition-metal-catalyzed Synthesis of Conjugated enynes. Org. Biomol. Chem. 2016, 14, 6638–6650. 10.1039/C6OB00944A. [DOI] [PubMed] [Google Scholar]; d Bharathiraja G.; Sengoden M.; Kannan M.; Punniyamurthy T. Expedient Synthesis of Tetrasubstituted Pyrroles via a Copper-Catalyzed Cascade Inter-/Intramolecular Cyclization of 1,3-Enynes Carry a Nitro Group with Amines. Org. Biomol. Chem. 2015, 13, 2786–2792. 10.1039/C4OB02508C. [DOI] [PubMed] [Google Scholar]; e Saito S.; Yamamoto Y. Recent Advances in the Transition-Metal-Catalyzed Regioselective Approaches to Polysubstituted Benzene Derivatives. Chem. Rev. 2000, 100, 2901–2915. 10.1021/cr990281x. [DOI] [PubMed] [Google Scholar]; f Nicolaou K. C.; Dai W. M.; Tsay S. C.; Estevez V. A.; Wrasidlo W. Designed Enediynes: A New Class of DNA-Cleaving Molecules with Potent and Selective Anticancer Activity. Science 1992, 256, 1172–1178. 10.1126/science.256.5060.1172. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Cheung C. W.; Hu X. L. Stereoselective Synthesis of Trisubstituted Alkenes through Sequential Iron-Catalyzed Reductive anti-Carbozincation of Terminal Alkynes and Base-Metal-Catalyzed Negishi Cross-Coupling. Chem. - Eur. J. 2015, 21, 18439–18444. 10.1002/chem.201504049. [DOI] [PubMed] [Google Scholar]; b Cornelissen L.; Lefrancq M.; Riant O. Copper-Catalyzed Cross-Coupling of Vinylsiloxanes with Bromoalkynes: Synthesis of Enynes. Org. Lett. 2014, 16, 3024–3027. 10.1021/ol501140p. [DOI] [PubMed] [Google Scholar]; c Saha D.; Chatterjee T.; Mukherjee M.; Ranu B. C. Copper (I) Hydroxyapatite Catalyzed Sonogashira Reaction of Alkynes with Styrenyl Bromides. Reaction of cis-Styrenyl Bromides Forming Unsymmetric Diynes. J. Org. Chem. 2012, 77, 9379–9383. 10.1021/jo3015819. [DOI] [PubMed] [Google Scholar]; d Zhou L.; Ye F.; Ma J. C.; Zhang Y.; Wang J. B. Palladium-Catalyzed Oxidative Cross-Coupling of N-Tosylhydrazones or Diazoesters with Terminal Alkynes: A Route to Conjugated Enynes. Angew. Chem. 2011, 123, 3572–3576. 10.1002/ange.201007224. [DOI] [PubMed] [Google Scholar]; e Zhu Y.; Li T. Y.; Qu X. M.; Sun P.; Yang H. L.; Mao J. C. Copper(I)-Catalyzed Synthesis of 1,3-Enynes via Coupling between Vinyl Halides and Alkynes or Domino Coupling of Vinyl Halides. Org. Biomol. Chem. 2011, 9, 7309–7312. 10.1039/c1ob06210g. [DOI] [PubMed] [Google Scholar]; f Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]
- For selected examples, see:; a Li X.; Chen X. L.; Zhang Q.; Qu L. B.; Bi W. Z.; Sun K.; Chen J. Y.; Chen X.; Zhao Y. F. CuSO4-H-phosphonate Catalyzed Highly Stereo- and regioselective dimerization of terminal alkynes. RSC Adv. 2015, 5, 5004–5009. 10.1039/C4RA10617B. [DOI] [Google Scholar]; b Midya G. C.; Parasar B.; Dhara K.; Dash J. Ligand Mediated Iron Catalyzed Dimerization of Terminal Aryl Alkynes: Scope and Limitations. Org. Biomol. Chem. 2014, 12, 1812–1822. 10.1039/c3ob42365d. [DOI] [PubMed] [Google Scholar]; c Sakurada T.; Sugiyama Y.; Okamoto S. Cobalt-Catalyzed Cross Addition of Silylacetylenes to Internal Alkynes. J. Org. Chem. 2013, 78, 3583–3591. 10.1021/jo400064b. [DOI] [PubMed] [Google Scholar]; d Tsukada N.; Ninomiya S.; Aoyama Y.; Inoue Y. Palladium-Catalyzed Selective Cross-Addition of Triisopropylsilylacetylene to Internal and Terminal Unactivated Alkynes. Org. Lett. 2007, 9, 2919–2921. 10.1021/ol071326g. [DOI] [PubMed] [Google Scholar]; e Ghosh R.; Zhang X. W.; Achord P.; Emge T. J.; Krogh-Jespersen K.; Goldman A. S. Dimerization of Alkynes Promoted by a Pincer-Ligated Iridium Complex. C-C Reductive Elimination Inhibited by Steric Crowding. J. Am. Chem. Soc. 2007, 129, 853–866. 10.1021/ja0647194. [DOI] [PubMed] [Google Scholar]
- a Kong K.; Moussa Z.; Lee C.; Romo D. Total Synthesis of the Spirocyclic Imine Marine Toxin (−)-Gymnodimine and an Unnatural C4-Epimer. J. Am. Chem. Soc. 2011, 133, 19844–19856. 10.1021/ja207385y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Takahashi T.; Aoyagi K.; Denisov V.; Suzuki N.; Choueiry D.; Negishi E. I. Zirconium Catalyzed C-C Bond Formation Reaction of Conjugated Diynes with EtMgBr. Tetrahedron Lett. 1993, 34, 8301–8304. 10.1016/S0040-4039(00)61416-8. [DOI] [Google Scholar]; c Dabdoub M. J.; Dabdoub V. B.; Comasseto J. V. Synthesis of (E)-l,4-Bis(organyl)but-1-en-3-ynes by Lithlium-Tellurium Exchange Reaction on (Z)-l-Butyltelluro-1,4-bis(organyl) But-1-en-3-ynes. Tetrahedron Lett. 1992, 33, 2261–2264. 10.1016/S0040-4039(00)74184-0. [DOI] [Google Scholar]
- Teng Q.; Xu Y.; Liang Y.; Wang H.; Wang Y.; Pan Y. Transition Metal-Free Synthesis of 3-Alkynylpyrrole-2-carboxylates via Michael Addition/Intramolecular Cyclodehydration. Adv. Synth. Catal. 2016, 358, 1897–1902. 10.1002/adsc.201501073. [DOI] [Google Scholar]
- Niu X. J.; Li C. J.; Li J.; Jia X. S. Importance of Bases on the Copper-Catalyzed Oxidative Homocoupling of Terminal Alkynes to 1,4-Disubstituted 1,3-Diynes. Tetrahedron Lett. 2012, 53, 5559–5561. 10.1016/j.tetlet.2012.08.050. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.