Abstract
Despite persistence of leukemic stem cells, patients with chronic myeloid leukemia who achieve and maintain deep molecular responses may successfully stop the tyrosine kinase inhibitor imatinib. However, questions remain unanswered regarding the biological basis of molecular relapse after imatinib cessation. In IMMUNOSTIM, we monitored 51 patients from the French Stop IMatinib trial for peripheral blood T cells and natural killer cells. Molecular relapse-free survival at 24 months was 45.1% (95% CI: 31.44%–58.75%). At the time of imatinib discontinuation, non-relapsing patients had significantly higher numbers of natural killer cells of the cytotoxic CD56dim subset than had relapsing patients, while CD56bright natural killer cells, T cells and their subsets did not differ significantly. Furthermore, the CD56dim natural killer-cell count was an independent prognostic factor of molecular-relapse free survival in a multivariate analysis. However, expression of natural killer-cell activating receptors, BCR-ABL1+ leukemia cell line K562-specific degranulation and cytokine-induced interferon-gamma secretion were decreased in non-relapsing and relapsing patients as compared with healthy individuals. After imatinib cessation, the natural killer-cell count increased significantly and stayed higher in non-relapsing patients than in relapsing patients, while receptor expression and functional properties remained unchanged. Altogether, our results suggest that natural killer cells may play a role in controlling leukemia-initiating cells at the origin of relapse after imatinib cessation, provided that these cells are numerous enough to compensate for their functional defects. Further research will decipher mechanisms underlying functional differences between natural killer cells from patients and healthy individuals and evaluate the potential interest of immunostimulatory approaches in tyrosine kinase inhibitor discontinuation strategies. (ClinicalTrial.gov Identifier NCT00478985)
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasia caused by the fusion of the BCR and ABL1 genes, as the result of the acquired reciprocal t(9;22)(q34;q11) translocation. In the early 2000s, imatinib, the first ATP-competitive inhibitor of the BCR-ABL1 oncoprotein, revolutionized the management of CML, providing most patients a dramatic progression-free survival benefit.1 Since then, newer generations of tyrosine kinase inhibitors (TKI) have been developed in order to overcome some of the drawbacks of imatinib, but imatinib remains one of the key initial therapies for newly diagnosed patients.2
When imatinib treatment is addressed appropriately, life expectancy of adult patients diagnosed with chronic-phase CML (CP-CML) is close to that of the general population.3,4 However, the current recommendation is to administer treatment lifelong because of the inability of imatinib and other TKI to eliminate quiescent leukemic stem cells.5–8 This recommendation represents a substantial challenge with respect to long-term safety, quality of life and economic burden. Therefore in the past few years, clinical trials have investigated the feasibility of discontinuing imatinib treatment in patients with sustained deep molecular responses. In the pioneering STIM trial, patients on imatinib therapy for a minimum of 3 years in whom BCR-ABL1 transcripts were undetectable for at least 2 years had a probability of maintaining deep molecular responses without any treatment of about 40%, challenging the statement that TKI may never be stopped.9 These findings were rapidly corroborated by the independent TWISTER trial.10 However, a definitive cure remains uncertain in patients who do not relapse. Indeed, serial assessments with reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) showed that peripheral blood BCR-ABL1 transcripts could be detected in patients who successfully stopped imatinib, albeit in low amounts.9 The use of genomic DNA-based PCR as a monitoring tool revealed that patients continued to harbor the BCR-ABL1 gene after discontinuation of imatinib, even when the corresponding transcripts were undetectable.11 In patients who had been off TKI therapy for several years, BCR-ABL1 transcripts could be amplified in CD34+ cell-derived colony-forming cells and long-term culture-initiating cells despite undetectable residual disease in the peripheral blood.8 Altogether, these results indicate that a reservoir of primitive leukemic cells persists in most if not all TKI-treated patients regardless of outcome after treatment discontinuation.
There is great clinical interest in trying to identify patients who are more likely to succeed in discontinuing imatinib in order to minimize potential risks of a leukemic rebound and to avoid undesirable drug-withdrawal symptoms.12 So far, the search for clinical variables predictive of outcome has been challenging but factors such as the Sokal score, duration of therapy, depth of molecular response and duration of deep molecular response have provided some insights into the probability of successful imatinib discontinuation in several studies.9,13,14 However, biological factors directing the fate of residual leukemic cells once TKI pressure is released are unclear. Given the susceptibility of CML to adaptive and innate immune cellular attack, an efficient autologous anti-CML response might help to control the leukemic load beyond cessation of TKI treatment.15,16 We designed and conducted an ancillary biological study within the STIM trial, named IMMUNOSTIM, with the goal of analyzing peripheral blood T cells and natural killer (NK) cells and investigated whether immune parameters were associated with molecular relapse-free survival.
Methods
Patients
IMMUNOSTIM is a sub-study of the STIM trial approved by French health authorities (NCT00478985).9 Written informed consent was given in agreement with the Declaration of Helsinki. Imatinib was stopped after ≥3 years of therapy and ≥2 years of undetectable BCR-ABL1 transcripts. Stringent monitoring by RT-qPCR was performed after imatinib discontinuation to detect a molecular relapse.9 The assay sensitivity was ≥4.5 log. Consecutively detectable peripheral blood BCR-ABL1 transcripts showing a ≥1 log increase or loss of a major molecular response [BCR-ABL1/ABL1 internationally standardized (IS) ratio ≤0.1%] defined molecular relapse and triggered imatinib resumption. In IMMUNOSTIM, heparinized blood was collected at baseline, bimonthly for 6 months then every 6 months until 24 months unless imatinib was resumed. Healthy donors were recruited through the Paris Saint-Louis Blood Donation Center and gave informed consent. Experiments were performed in a centralized fashion, allowing ≤48 h from blood collection to processing.
Immunophenotyping
Patients’ whole blood cell counts were determined using a Sysmex XS 1000i analyzer. T cells and NK cells were quantified by dual-platform flow cytometry using monoclonal antibodies recognizing CD3, CD4, CD127, CD25, CD8, CD45RA, CCR7, CD27, CD56, CD16 and NKG2D (Online Supplementary Methods). Peripheral blood mononuclear cells (PBMC) were purified with Ficoll density-gradient centrifugation and cryopreserved in liquid nitrogen. NK-cell receptor expression was studied by flow cytometry using thawed PBMC stained with monoclonal antibodies recognizing CD56, CD16, CD3, DNAM-1, KIR2D, NKp46, NKp30, NKG2A, CD94 and CD57 (Online Supplementary Methods).
Natural killer-cell functional assays
To assess degranulation, PBMC were thawed, maintained in 10% fetal calf serum-RPMI-1640 overnight at 37°C and incubated with or without the HLA class I-deficient BCR-ABL1+ leukemia cell line K562 at a 1:1 effector to target ratio for 18 h at 37°C with a CD107a monoclonal antibody. Thereafter, PBMC were stained with monoclonal antibodies recognizing CD137, CD56, CD3 and CD16. CD137+ and CD107a+ NK cells were detected by flow cytometry (Online Supplementary Methods). To study interferon (IFN)-γ production, thawed PBMC were activated overnight in medium supplemented with interleukin (IL)-12 and IL-18 (10 ng/mL each). The following day, brefeldin A was added for 4 h. IFN-γ–producing CD3−CD56brightCD16−/low NK cells were detected by flow cytometry as previously described (Online Supplementary Methods).17
Statistical analyses
A Mann-Whitney U-test, a Kruskal-Wallis test and a Wilcoxon matched-pairs signed ranked test were used to compare, respectively, quantitative variables from two independent groups, more than two groups with a Dunn test for multiple comparisons, and quantitative variables following imatinib discontinuation. Molecular relapse-free survival was estimated with the Kaplan-Meier method.9 Clinical variables and NK cells were assessed as potential prognostic factors for molecular relapse-free survival. Quantitative factors were categorized into two groups with cutoffs set at the median. All variables were assessed by univariate analysis using the Kaplan-Meier method and the two-tailed log-rank test. A backward stepwise multivariate Cox proportional analysis was performed to determine the influence of variables potentially associated with outcome in univariate analysis (P≤0.10). In the final model, P values <0.05 were considered statistically significant. Quantitative variables were categorized into two groups with cut-offs set at the median. Hazard ratios and 95% confidence intervals (CI) were estimated from the Cox regression analysis.
Results
Patients’ characteristics
Fifty-one of the 100 patients enrolled in the STIM trial who agreed to participate to IMMUNOSTIM and from whom blood samples were received and processed within 48 h of collection were included; their baseline characteristics are detailed in Table 1. Thirty-three patients (64.7%) were females and the Sokal score at diagnosis of CP-CML was low in 28 (54.9%). Single-agent imatinib was given as first-line TKI therapy in all patients, either soon after the diagnosis of CML (n=24) or after resistance or intolerance to IFN-α (n=27). None of the patients had a history of allogeneic stem cell transplantation. The median duration of imatinib treatment was 57 months (range; 36–94). The median age at imatinib discontinuation was 63 years (range; 39–81) and the median follow-up after imatinib discontinuation was 63 months (range; 47–72). After treatment cessation, 28 patients (54.9%) experienced a protocol-defined molecular relapse and all restarted imatinib treatment. The median BCR-ABL1/ABL1 IS ratio was 0.015% (range; 0.0004–0.201) at first detection of molecular relapse, 0.056% (range; 0.006–1.54) at confirmation of molecular relapse and 0.165% (range; 0.024–4.49) at imatinib resumption (Figure 1A). The median time from imatinib discontinuation to first detection of molecular relapse was 2 months (range, 1–20) and all molecular relapses but one occurred before 6 months. The median time until imatinib resumption was 4 months (range, 3–29). At 24 months, molecular relapse-free survival was 45.1% (95% CI: 31.44%–58.75%) (Figure 1B). These findings were consistent with those of the entire STIM study population.18 The median follow-up for non-relapsing patients was 63 months (range, 48–72).
Table 1.
Baseline characteristics of the patients (n=51).
Figure 1.
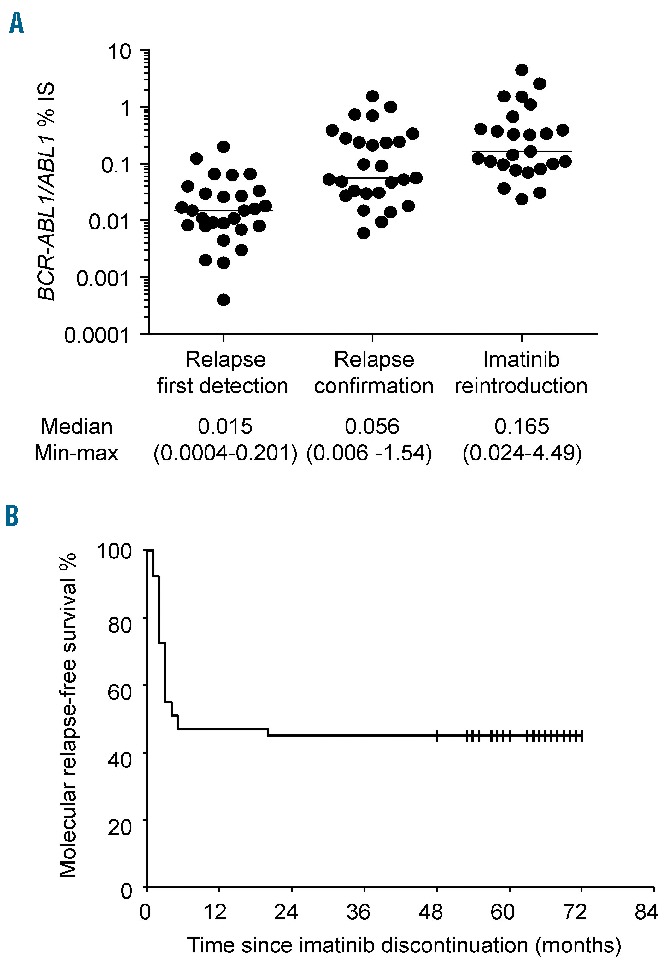
Evolution of BCR-ABL1 transcripts in relapsing patients and molecular relapse-free survival. (A) Scatter dot plots represent BCR-ABL1 IS % for each individual relapsing patient at first detection of relapse, relapse confirmation and at imatinib reintroduction; median values (horizontal bars) are also shown. (B) Kaplan-Meier estimate of molecular relapse-free survival defined as the time interval between imatinib discontinuation and first occurrence of molecular relapse or death, whichever came first. Data were censored at last molecular assessment for patients who were alive and had not relapsed.
Leukocyte, T-cell and natural kill-cell counts at imatinib discontinuation
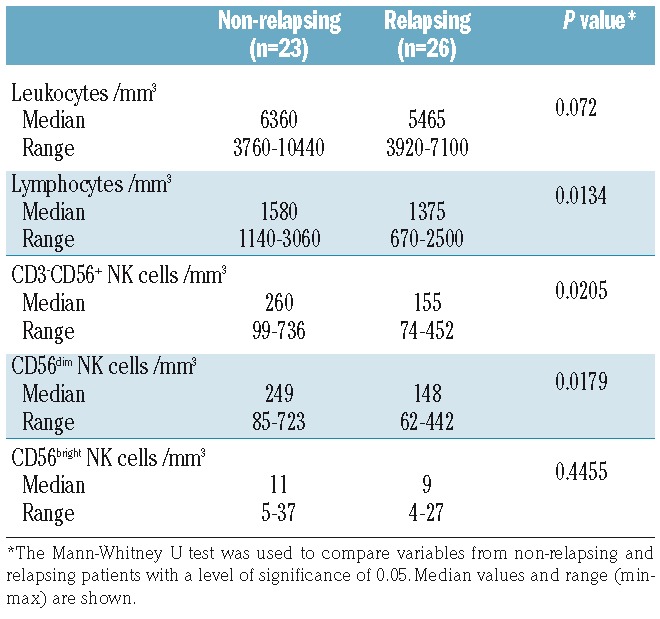
Leukocyte and lymphocyte counts at the time of imatinib discontinuation did not differ significantly between non-relapsing and relapsing patients (Online Supplementary Table S1). When CD3+ T cells, CD4+ and CD8+ T cells, naïve, central memory, effector memory CD4+ and CD8+ subsets and CD4+CD25+CD127low/− regulatory T cells were analyzed, no significant differences were observed between non-relapsing and relapsing patients (Online Supplementary Table S1). In contrast, although CD3−CD56+ NK cells were within the range established in our laboratory using samples from healthy donors (data not shown), non-relapsing patients had significantly higher frequencies and counts of CD3−CD56+ NK cells than had relapsing patients, with a median count of 233/mm3 (range, 70–727) in the former and 145/mm3 (range, 67–450) in the latter (P=0.014) (Figure 2A,B). This finding was not explained by differences in age or imatinib doses between the two groups (data not shown). In addition, we did not find any association between CD3−CD56+ NK-cell counts and duration of imatinib therapy (data not shown). CD3−CD56+ NK cells were thus subtyped based upon cell-surface density of the adhesion molecule CD56 and the FcRγIII receptor CD16. Counts of the cytotoxic CD3-CD56dimCD16+ population (hereafter named CD56dim) were significantly greater in non-relapsing patients [median: 216/mm3 (range, 55–723)] than in relapsing patients [median: 139/mm3 (range, 58–438)] (P=0.011) and accounted for higher total NK-cell numbers in the former (Figure 2C). Indeed, counts of the cytokine-secreting CD3-CD56brightCD16−/low fraction (hereafter named CD56bright) did not differ between the two groups of patients (Figure 2D).
Figure 2.
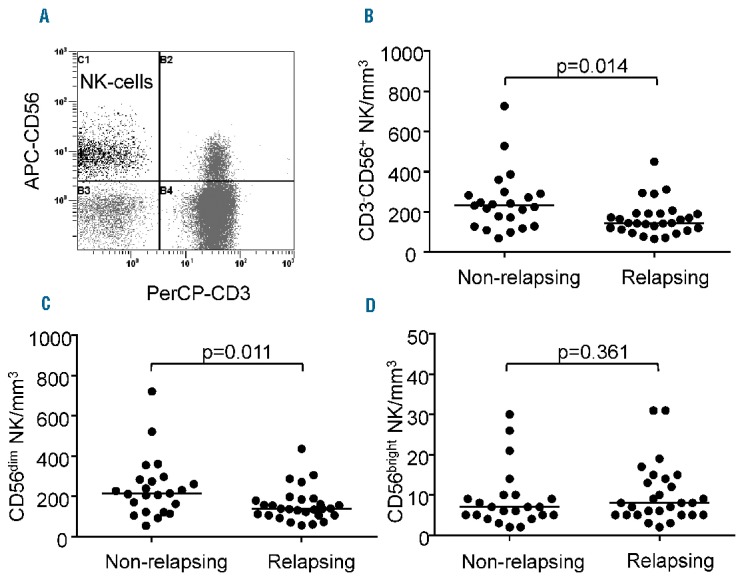
Natural killer cells at imatinib discontinuation in non-relapsing and relapsing patients. (A) Flow cytometry dot plot from a representative patient showing CD3−CD56+ NK cells in the upper left quadrant after lymphocyte gating using the side and forward scatter display. (B) Scatter dot plots represent CD3−CD56+ NK-cell counts for each individual non-relapsing and relapsing patient; median values (horizontal bars) are also shown. (C) Scatter dot plots represent CD3−CD56dim NK-cell counts for each individual non-relapsing and relapsing patient; median values (horizontal bars) are also shown. (D) Scatter dot plots represent CD3−CD56bright NK-cell counts for each individual non-relapsing and relapsing patient; median values (horizontal bars) are also shown. P values (by the Mann-Whitney U-test) are indicated for each panel.
Baseline prognostic factors for molecular relapse
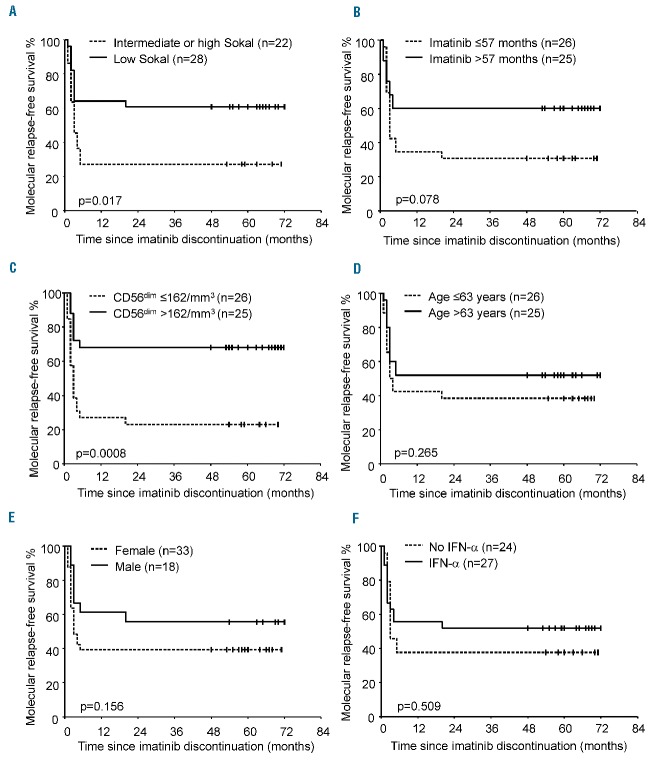
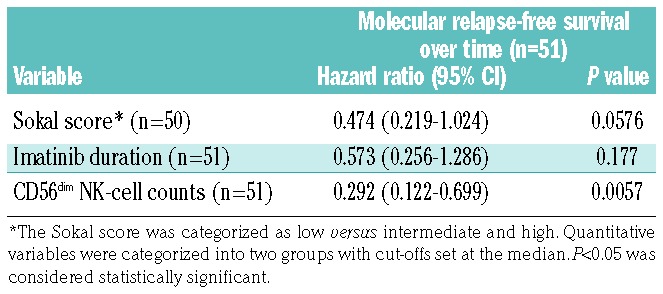
In the STIM trial, the Sokal risk group and duration of imatinib treatment were associated with outcome following imatinib discontinuation.9,18 Here, clinical and biological variables and NK-cell counts at baseline were analyzed as potential prognostic factors for molecular relapse-free survival using univariate analysis. Patients with a low Sokal score had a significantly higher molecular relapse-free survival rate than patients with an intermediate or high Sokal score (P=0.017) (Figure 3A). There was a trend toward a longer duration of imatinib treatment and higher estimated molecular relapse-free survival rate (P=0.078) (Figure 3B). Notably, patients with CD56dim NK-cell counts higher than the median (>162/mm3) at baseline had a significantly higher molecular relapse-free survival rate than those with a lower CD56dim NK-cell count (P=0.0008) (Figure 3C). No significant association with age, sex, or prior IFN-α therapy was found (Figure 3D–F). After multi-variable analysis, CD56dim NK-cell count was identified as an independent prognostic factor for molecular-relapse-free survival (Table 2). These findings led us to analyze NK cells further in the setting of immunoprofiling and functional experiments.
Figure 3.
Molecular relapse-free survival after discontinuation of imatinib according to clinico-biological factors. (A) Sokal risk group, (B) imatinib treatment duration, (C) CD56dim NK-cell counts at baseline, (D) age, (E) sex, and (F) prior exposure to IFN-α. For each survival plot, a corresponding log-rank P value is shown.
Table 2.
Potential prognostic factors of molecular relapse-free survival: multivariable Cox proportional analysis.
Natural killer-cell receptors and maturation marker at imatinib discontinuation
NK-cell receptors play a key role in recognizing targets and transducing activating or inhibitory signals upon binding to their ligands, thereby controlling cell function.19 We thus examined the expression of a large panel of NK-cell receptors and that of the carbohydrate antigen CD57, a marker linked to NK-cell terminal differentiation.20,21 No statistically significant differences were found on NK cells between non-relapsing and relapsing patients with respect to expression of the activating receptors CD16, NKG2D, NKp46, NKp30 and DNAM-1, the maturation marker CD57, the activating and inhibitory KIR2D isoforms and the C-type lectin inhibitory receptor NKG2A/CD94 (data not shown). However, when we compared NK-cell receptor expression profile of patients with that of healthy individuals, significant alterations were found. The proportions of NK cells expressing the activating receptors NKp46 and DNAM-1 were significantly lower in the CD56dim and CD56bright subsets of non-relapsing and relapsing patients (Figure 4). In addition, the CD56bright subset of non-relapsing and relapsing patients showed significantly increased KIR2D and CD57 and decreased NKG2A expression as compared to the expression in healthy donors (Figure 4).
Figure 4.
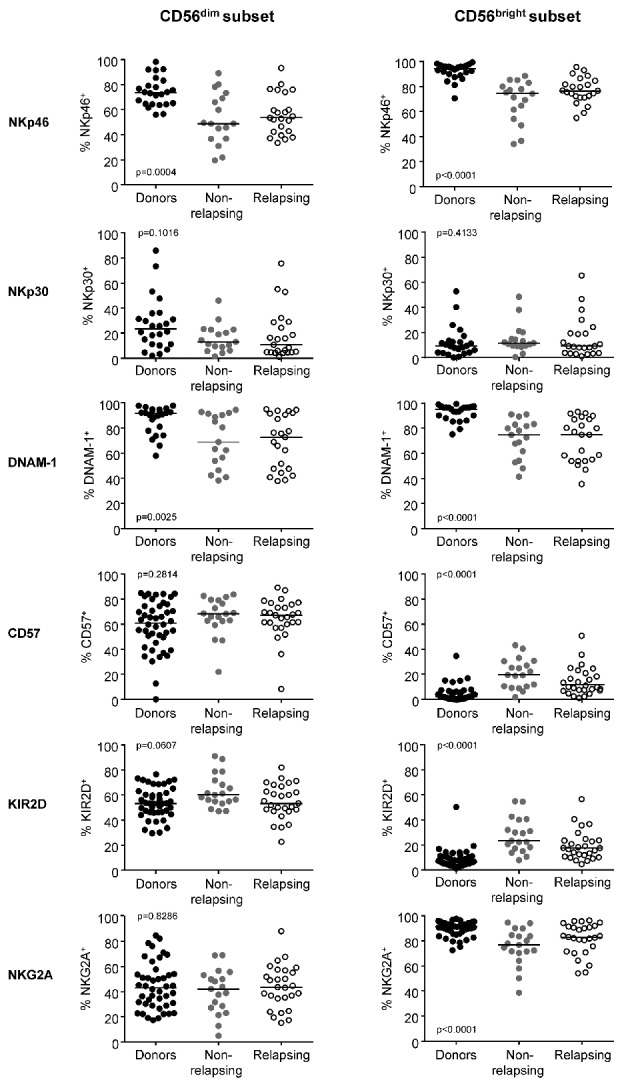
Expression of natural killer cell receptors in CD56dim and CD56bright natural killer cell subsets. Scatter dot plots for healthy donors (black dots, n=44), non-relapsing patients (filled gray dots, n=19) and relapsing patients (empty black dots, n=27) with median values (horizontal bars) are shown. Overall P value (by the Kruskall-Wallis test) is indicated for each panel.
Natural killer-cell function at imatinib discontinuation
The capacity of NK cells to degranulate cytolytic vesicles was measured through the delocalization of the lysosomal-associated membrane protein-1 CD107a onto the cell surface after stimulation with the NK-sensitive BCR-ABL1+ cell line K562. This assay is considered a general indicator of NK activity and particularly of tumor cell lysis.22 Raw quantification of CD107a in the presence of K562 indicated a comparable degranulation in non-relapsing and relapsing patients and in healthy donors (Figure 5A). However, the propensity of NK cells for surface CD107a expression in the absence of K562 revealed significantly higher levels of spontaneous degranulation in both groups of patients (Figure 5A). Consequently, K562-specific degranulation of NK cells from non-relapsing and relapsing patients was significantly weaker than that of NK cells from healthy donors (Figure 5B). This finding was corroborated by a lower induction of the tumor necrosis factor receptor CD137 (also known as 4-1BB) upon K562 encounter in patients, a marker upregulated following NK-cell activation (Figure 5C). We also investigated the ability of the CD56bright NK-cell subset to secrete IFN-γ after stimulation by IL-12 and IL-18. We found that IFN-γ production was comparable in non-relapsing and relapsing patients and significantly reduced compared to that in healthy donors (Figure 5D).
Figure 5.
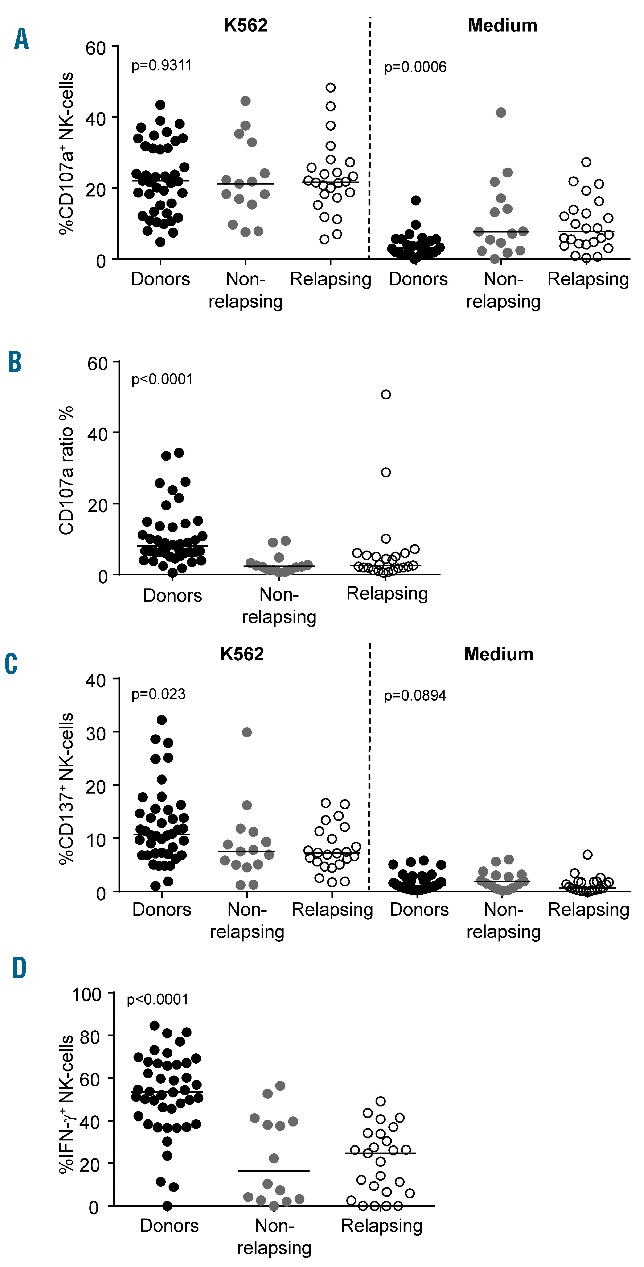
Natural killer-cell function. (A) NK-cell degranulation assay against K562 or medium control. (B) Target-specific degranulation estimated by the CD107a ratio. (C) NK-cell activation estimated by CD137 expression in the presence of K562 or medium control. (D) Production of IFN-γ upon stimulation with IL-12 and IL-18. Scatter dot plots for healthy donors (black dots, n=43), non-relapsing patients (filled gray dots, n=15) and relapsing patients (empty black dots, n=25) at baseline with median values (horizontal bars) are shown. Overall P value (by the Kruskall-Wallis test) is indicated for each panel.
Natural killer cells after imatinib discontinuation
Several studies support the notion that TKI exert off-target effects on NK cells and other cells of the immune system.23–26 We thus wondered whether cell counts, receptor expression and functional features assessed at baseline could have been influenced at least in part by imatinib. To address this point, experiments were repeated after cessation of imatinib. Because most relapsing patients resumed imatinib within a short time frame (64.3% within 3 months and 85.7% within 6 months), follow-up samples obtained within 6 months after imatinib discontinuation were used, prior to imatinib reintroduction. After imatinib was stopped, median leukocyte and lymphocyte counts increased in non-relapsing and relapsing patients from 4810/mm3 (range, 2250–7860) to 5950/mm3 (range, 3760–10440) (P<0.0001) and 1310/mm3 (range, 720–2610) to 1520/mm3 (range, 670–3060), respectively (P<0.0001). A rise in NK cells also occurred in both groups of patients, from a median value of 179/mm3 (range, 67–727) at baseline to 205/mm3 (range, 74–736) after imatinib discontinuation (P=0.0011). This rise was observed within both the CD56dim subset [median count 164/mm3 (range, 55–723) at baseline and 195/mm3 (range, 62–723) after imatinib discontinuation (P=0.0032)] and the CD56bright fraction [median count 7/mm3 (range, 2–31) at baseline and 10/mm3 (range, 4–37) after imatinib discontinuation (P=0.0008)]. Of note, CD3+ T cells also increased from a median value of 889/mm3 (range, 315–1949) at baseline to 990/mm3 (range, 265–1817) after imatinib discontinuation (P=0.0445). Importantly, the comparison between groups after imatinib discontinuation showed that NK cells of the CD56dim subset remained significantly higher in non-relapsing patients, with a median value of 249/mm3 (range, 85–723), than in relapsing patients, who had a median value of 148/mm3 (range, 62–442) (P=0.0179) (Table 3). Immunophenotypic analyses revealed no significant modification in the expression of NKp46, NKp30, DNAM-1 and KIR2D after imatinib discontinuation in either group of patients (Online Supplementary Figure S1AD). A significant reduction in NKG2A+ NK cells was observed in non-relapsing patients but not in relapsing patients (Online Supplementary Figure S1E). The proportion of CD57+ NK cells was significantly decreased in relapsing patients (Online Supplementary Figure S1F). NK-cell degranulation and activation and IFN-γ production did not show any significant enhancement (Online Supplementary Figure S1G–I).
Table 3.
Leukocytes, lymphocytes and natural killer-cell subsets after imatinib discontinuation.
Discussion
The current principles of CML management rely on the use of life-saving TKI through the induction of an optimal response to prevent progression to blast crisis, followed by maintenance of the optimal response by means of lifelong TKI treatment.7 However, recent demonstrations that imatinib can be successfully stopped in a substantial proportion of patients with deep and durable molecular responses is currently modifying this view and avoiding lifelong dependency on TKI treatment may become the ultimate goal.27–29 While serial molecular monitoring is essential to detect a relapse rapidly after an attempt to discontinue TKI, in order to trigger medical intervention and avoid any negative impact on the patient’s outcome, our ability to predict relapses accurately remains limited and determinants of relapse are unknown.30
Here, we demonstrated that patients free from molecular relapse after imatinib discontinuation within the STIM trial had higher numbers of circulating NK cells than had patients who relapsed. In addition to CML-related variables, such as the Sokal score and imatinib treatment duration, a greater load of CD56dim NK cells at the time of imatinib discontinuation was associated with a greater chance of treatment cessation being successful in univariate analysis. Although IFN-α has the ability to activate NK cells, no association was found between past exposure to IFN-α and molecular relapse-free survival, in agreement with what was previously found in the single-agent imatinib discontinuation studies STIM and TWISTER.9,10 Multivariable analysis enabled us to show that the amount of CD56dim NK cells was the only independent prognostic factor for molecular relapse-free survival in this population of patients. Two recent and independent studies are in line with our results. Indeed in the DADI dasatinib discontinuation trial and in the EUROSKI imatinib cessation immunological sub-study, greatest peripheral NK-cell burden at the time TKI therapy was stopped was associated with a higher probability of treatment-free remission.31,32 Given the importance of NK cells in immune surveillance against malignancies, these results and ours led us to hypothesize that NK cells and especially the differentiated and cytotoxic CD56dim subset20 might contribute to prevent overt CML relapse originating from residual leukemic cells, in a direct or indirect fashion, after imatinib discontinuation. The involvement of NK cells in immune surveillance against CML is also supported by other lines of evidence. BCR-ABL1+ CD34+ cells from CML patients express NK activating receptor ligands.33–37 In addition, BCR-ABL1+ CD34+ cells from CML patients are sensitive to NK-cell-mediated lysis in vitro. Indeed, Sconocchia and colleagues34 found that IL-2-stimulated NK cells from allogeneic HLA-matched siblings inhibited BCR-ABL1+ CD34+-derived colony-forming unit – granulocyte-macrophage growth in colony formation assays. Yong and colleagues38 reported that primitive quiescent BCR-ABL1+ CD34+ stem cells were sensitive to lysis by NK cells from HLA-identical siblings, although to a lesser extent than cycling CD34+ leukemic cells. Cervantes and colleagues39 observed that IL-2-activated NK cells were capable of suppressing autologous BCR-ABL1+ CD34+ cell-derived colony-forming cells and long-term culture-initiating cells. A further indication of a protective role of NK cells against CML is that early recovery of NK cells was able to predict a positive clinical outcome for patients undergoing T-cell-depleted allogeneic transplantation from HLA-identical siblings.40 Moreover during treatment with the NK-cell-stimulating cytokine, IFN-α, an association between the achievement of a complete cytogenetic response and NK-cell cytolytic activity was described.41,42 Finally, cross-talk between NK cells and other immune cells, such as dendritic cells, may potentiate anti-CML adaptive immune responses.43,44
Despite striking quantitative differences, we found that the immunophenotypic and functional features of NK cells from non-relapsing and relapsing patients at the time of imatinib discontinuation were comparable. In addition, NK cells from both groups of patients differed phenotypically and functionally from those of healthy individuals. Expression of the activating receptors NKp46 and DNAM-1 was decreased. Overall degranulation upon encounter of K562 cells was preserved, as found in the immunological EUROSKI sub-study but contrary to EUROSKI, we were able to show lower activation marker induction and higher basal CD107a mobilization in patients, suggesting poorer K562-specific degranulation. These differences between the immunological EUROSKI sub-study and our study remain unexplained. A high level of spontaneous degranulation was described in other pathological settings, such as in human immunodeficiency virus infection, and was linked to chronic inflammation but its significance in well-controlled CML remains unclear.45 Unfortunately, it was not possible to assess degranulation toward autologous leukemic cells because it is not routine practice to collect and store CD34+ BCR-ABL1+ cells at CML diagnosis, it is challenging to find and isolate very rare residual leukemic stem cells at imatinib discontinuation and this was not planned in our study. Finally, we found that IFN-γ production in response to cytokine stimulation in the CD56bright subset was lower in patients irrespective of their relapse status. Although NK cells are not derived from the leukemic clone in CP-CML, deficient NK-cell function has been described by several groups.25,46,47 In newly diagnosed CP-CML prior to any treatment, NK-cell proliferation in response to the K562 leukemic cell line and IL-2 is reduced, as is degranulation triggered by K562 alone.25 These alterations are partially restored by imatinib, thereby suggesting that the disease itself has a deleterious impact on NK cells which persists beyond the imatinib-induced remission.25
In our study, imatinib discontinuation was accompanied by a rapid and significant increase in leukocytes and lymphocytes, as also observed by Park and colleagues.48 In addition, a significant increase in NK cells occurred. This finding is concordant with the inhibitory impact of imatinib on NK-cell expansion in vitro, although such an impact was described at higher concentrations than the trough plasma concentrations usually expected in patients treated with imatinib at a dose of 400 mg daily.25 It is also consistent with the fact that patients in deep molecular response who have successfully stopped imatinib have higher NK-cell counts than those with a comparable level of response but who do not stop the drug.49 However, it is important to note that in our study, non-relapsing patients maintained higher counts of NK cells than relapsing patients after imatinib cessation and that the imatinib dose prior to discontinuation was similar in non-relapsing and relapsing patients. Although imatinib plasma levels were not measured prior to discontinuation, this argues against a different drug exposure as the sole explanation for a lower NK-cell burden in relapsing patients. Other factors involved in NK-cell peripheral expansion, such as stromal cell factors or the immunoregulatory cytokine IL-15, need to be explored in future research studies.50
In our experimental setting, discontinuation of imatinib did not result in the disappearance of NK-cell differences with healthy individuals, ruling out a major deleterious effect of the drug on NK-cell function. Expression of the activating receptors NKp46 and DNAM-1 remained unchanged. A significant decrease in NKG2A+ NK cells in non-relapsing patients was observed but there was no gain in cytolytic and CD56bright-IFN-γ secretion capacities. Our study was not designed to decipher the underlying mechanisms of NK-cell defects in CML but several hypotheses can be made, including a negative impact of residual leukemic stem cells or their microenvironment on immune cell function or a weaker activation of the NK-cell repertoire due to HLA class I/KIR variation as compared to that in healthy individuals.36 In the immunological EUROSKI sub-study, individual KIR genes and haplotypes were not associated with imatinib discontinuation outcome but, unfortunately, comparisons with the general population were not performed.32
To conclude, we provide evidence that NK cells are associated with outcome after imatinib discontinuation in CP-CML patients in deep molecular response. Beyond functional aspects, larger amounts of cytotoxic CD56dim NK cells delivered to the leukemic reservoir in non-relapsing patients may play an important role in controlling residual CML-initiating cells and their progeny soon after cessation of imatinib treatment while a reduced CD56dim compartment, and thus a lower effector to target cell ratio in relapsing patients, may leave less chance to counterattack in an efficient manner. Of course, our results do not preclude the role of additional biological aspects in dictating patients’ outcome, such as the involvement of other immunological factors or functional heterogeneity of the residual BCR-ABL1+ hematopoietic stem-cell reservoir. Thus integration of NK-cell counts into the TKI discontinuation decision-making process is not reasonable at this stage. In addition, NK-cell alterations in patients suggest that the use of agents that stimulate NK-cell expansion or function, such as the IL-15 cytokine, or antibodies modulating NK-cell receptors may increase the chance of successfully TKI discontinuation, an aspect that may be addressed in future studies.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and the personnel from clinical centers involved in this study and Ms. Nicole Biscay, Valérie Hubert, Eliane Melgire and Véronique Delasse from the Cell Therapy Unit, Hôpital Saint-Louis, Paris, France for expert logistic and technical assistance.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/8/1368
Funding
This work was supported by research grants from the Association pour la Recherche sur le Cancer (ZK, #DOC20100600956), Association LMC France (#R13102HH), Assistance Publique-Hôpitaux de Paris (Translational Research Grant in Biology 2010 #RTB10002) and the French Ministry of Health/Institut National du Cancer (Programme Hospitalier de Recherche 2006).
References
- 1.Druker BJ, Guilhot F, O’Brien SG, et al. ; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- 2.Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015;29(5): 1123–1132. [DOI] [PubMed] [Google Scholar]
- 3.Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553–561. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2(5):e186–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121(1):396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton A, Helgason GV, Schemionek M, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119(6):1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomel JC, Bonnet ML, Sorel N, et al. Leukemic stem cell persistence in chronic myeloid leukemia patients in deep molecular response induced by tyrosine kinase inhibitors and the impact of therapy discontinuation. Oncotarget. 2016;7(23):35293–35301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon FX, Réa D, Guilhot J, et al. ; Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11): 1029–1035. [DOI] [PubMed] [Google Scholar]
- 10.Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. [DOI] [PubMed] [Google Scholar]
- 11.Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719–1724. [DOI] [PubMed] [Google Scholar]
- 12.Richter J, Söderlund S, Lübking A, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome. J Clin Oncol. 2014;32(25):2821–2823. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Vagge E, le Coutre P, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90(10):910–914. [DOI] [PubMed] [Google Scholar]
- 14.Lee SE, Choi SY, Song HY, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101(6):717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohon P. Biological therapy and the immune system in patients with chronic myeloid leukemia. Int J Hematol. 2012;96(1):1–9. [DOI] [PubMed] [Google Scholar]
- 16.Barrett AJ, Ito S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood. 2015;125(21):3230–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khaznadar Z, Henry G, Setterblad N, et al. Acute myeloid leukemia impairs natural killer cells through the formation of a deficient cytotoxic immunological synapse. Eur J Immunol. 2014;44(10):3068–3080. [DOI] [PubMed] [Google Scholar]
- 18.Etienne G, Guilhot J, Rea D, et al. , on behalf of the Intergroupe Français des Leucémies Myéloïdes Chroniques (Fi-LMC). Long-term follow-up of the French Stop Imatinib Study (STIM1) in chronic myeloid leukemia patients. J Clin Oncol. 2017;35(3):298–305. [DOI] [PubMed] [Google Scholar]
- 19.Carrillo-Bustamante P, Ke mir C, de Boer RJ. The evolution of natural killer cell receptors. Immunogenetics. 2016;68(1):3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Björkström NK, Riese P, Heuts F, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116(19):3853–3864. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional significance of CD57 expression on human NK cells and relevance to disease. Front Immunol. 2013;4(422):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. [DOI] [PubMed] [Google Scholar]
- 23.Cebo C, Da Rocha S, Wittnebel S, et al. The decreased susceptibility of Bcr/Abl targets to NK cell-mediated lysis in response to imatinib mesylate involves modulation of NKG2D ligands, GM1 expression, and synapse formation. J Immunol. 2006;176(2):864–872. [DOI] [PubMed] [Google Scholar]
- 24.Salih J, Hilpert J, Placke T, et al. The BCR/ABL-inhibitors imatinib, nilotinib and dasatinib differentially affect NK cell reactivity. Int J Cancer. 2010;127(9):2119–2128. [DOI] [PubMed] [Google Scholar]
- 25.Chen CI, Koschmieder S, Kerstiens L, et al. NK cells are dysfunctional in human chronic myelogenous leukemia before and on imatinib treatment and in BCR-ABL-positive mice. Leukemia. 2012;26(3):465–474. [DOI] [PubMed] [Google Scholar]
- 26.Zitvogel L, Rusakiewicz S, Routy B, Ayyoub M, Kroemer G. Immunological off-target effects of imatinib. Nat Rev Clin Oncol. 2016;13(7):431–446. [DOI] [PubMed] [Google Scholar]
- 27.Rea D, Rousselot P, Guilhot J, Guilhot F, Mahon FX. Curing chronic myeloid leukemia. Curr Hematol Malig Rep. 2012;7(2):103–108. [DOI] [PubMed] [Google Scholar]
- 28.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17–23. [DOI] [PubMed] [Google Scholar]
- 29.Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8): 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deininger MW. Molecular monitoring in CML and the prospects for treatment-free remissions. Hematology Am Soc Hematol Educ Program. 2015;(2015):257–263. [DOI] [PubMed] [Google Scholar]
- 31.Imagawa J, Tanaka H, Okada M, et al. ; DADI Trial Group. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2(12):e528–e535. [DOI] [PubMed] [Google Scholar]
- 32.Ilander M, Olsson-Strömberg U, Schlums H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31(5):1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salih HR, Antropius H, Gieseke F, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102(4):1389–1396. [DOI] [PubMed] [Google Scholar]
- 34.Sconocchia G, Lau M, Provenzano M, et al. The antileukemia effect of HLA-matched NK and NK-T cells in chronic myelogenous leukemia involves NKG2D-target-cell interactions. Blood. 2005;106(10):3666–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boissel N, Rea D, Tieng V, et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J Immunol. 2006;176(8):5108–5116. [DOI] [PubMed] [Google Scholar]
- 36.Verheyden S, Demanet C. NK cell receptors and their ligands in leukemia. Leukemia. 2008;22(2):249–257. [DOI] [PubMed] [Google Scholar]
- 37.Hilpert J, Grosse-Hovest L, Grünebach F, et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189(3):1360–1371. [DOI] [PubMed] [Google Scholar]
- 38.Yong AS, Keyvanfar K, Hensel N, et al. Primitive quiescent CD34+ cells in chronic myeloid leukemia are targeted by in vitro expanded natural killer cells, which are functionally enhanced by bortezomib. Blood. 2009;113(4):875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cervantes F, Pierson BA, McGlave PB, Verfaillie CM, Miller JS. Autologous activated natural killer cells suppress primitive chronic myelogenous leukemia progenitors in long-term culture. Blood. 1996;87(6): 2476–2485. [PubMed] [Google Scholar]
- 40.Savani BN, Rezvani K, Mielke S, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107(4):1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pawelec G, Da Silva P, Max H, et al. Relative roles of natural killer- and T cell-mediated anti-leukemia effects in chronic myelogenous leukemia patients treated with interferon-alpha. Leuk Lymphoma. 1995;18(5–6):471–478. [DOI] [PubMed] [Google Scholar]
- 42.Meseri A, Delwail V, Brizard A, et al. Endogenous lymphokine activated killer cell activity and cytogenetic response in chronic myelogenous leukaemia treated with alpha-interferon. Br J Haematol. 1993;83(2):218–222. [DOI] [PubMed] [Google Scholar]
- 43.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5(2):112–124. [DOI] [PubMed] [Google Scholar]
- 44.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106(7):2252–2258. [DOI] [PubMed] [Google Scholar]
- 45.Lichtfuss GF, Cheng WJ, Farsakoglu Y, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189(3):1491–1499. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi N, Miura I, Saitoh K, Miura AB. Lineage involvement of stem cells bearing the philadelphia chromosome in chronic myeloid leukemia in the chronic phase as shown by a combination of fluorescence-activated cell sorting and fluorescence in situ hybridization. Blood. 1998;92(12):4758–4763. [PubMed] [Google Scholar]
- 47.Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;96(5): 1961–1968. [PubMed] [Google Scholar]
- 48.Park JS, Lee SE, Jeong SH, et al. Change of health-related profiles after Imatinib cessation in chronic phase chronic myeloid leukemia patients. Leuk Lymphoma. 2016;57(2):341–347. [DOI] [PubMed] [Google Scholar]
- 49.Ohyashiki K, Katagiri S, Tauchi T, et al. Increased natural killer cells and decreased CD3(+)CD8(+)CD62L(+) T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br J Haematol. 2012;157(2):254–256. [DOI] [PubMed] [Google Scholar]
- 50.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.