Abstract
Glucosinolates, which are unique to Brassicaceae vegetables, have diverse biological activities, including antimicrobial, antioxidant, and anticancer actions. In this study, we applied hydrophilic interaction chromatography–tandem mass spectrometry (HILIC–MS/MS) to the simultaneous quantification of 22 glucosinolates in 12 Brassicaceae vegetables, including pak choi, choy sum, Chinese cabbage, cauliflower, cabbage, broccoli, Kai Lan, Brussels sprouts, rocket salad, daikon radish, red cherry radish, and watercress. Significant differences in concentration and composition of glucosinolates were observed among these vegetables. Cabbage had the highest level of total glucosinolates (μg/g dry weight: 19 551.2 ± 1317.7), whereas Kai Lan had the lowest level (7611.3 ± 868.4). Aliphatic and indole glucosinolates were the major components in the 12 vegetables ranging from 76 to 100%, except watercress (37%). On the basis of the content of glucosinolates, the 12 vegetables were well distinguishable and classified according to their morphological taxonomy. This study presents a HILIC–MS/MS approach for quantification of glucosinolates, and demonstrates the potential of glucosinolate profiles for Brassicaceae species identification.
Introduction
Glucosinolates are secondary plant metabolites that occur naturally in the family Brassicaceae,1 which are a group of sulfur-containing glucosides having a common core structure with a β-d-thioglucose group, a sulfonated aldoxime moiety, and a variable side chain (=R) derived from amino acids. Depending on the variable R group, glucosinolates can be categorized into three major classes: aliphatic, indole, and aromatic glucosinolates.2 To defend against attacks by harmful organisms, glucosinolates can be hydrolyzed by the endogenous enzyme myrosinase into various breakdown products, including isothiocyanates, nitriles, thiocyanates, epithionitriles, and oxazolidinethiones.3 Because of its characteristic aroma, glucosinolate-containing plant material is commonly used in the biofumigation for plant protection against soil-borne pathogens in agriculture and horticulture.4 Recently, glucosinolates have been of interest for their diverse biological activities, ranging from antimicrobial, antioxidant, and anticancer actions.1,5−7
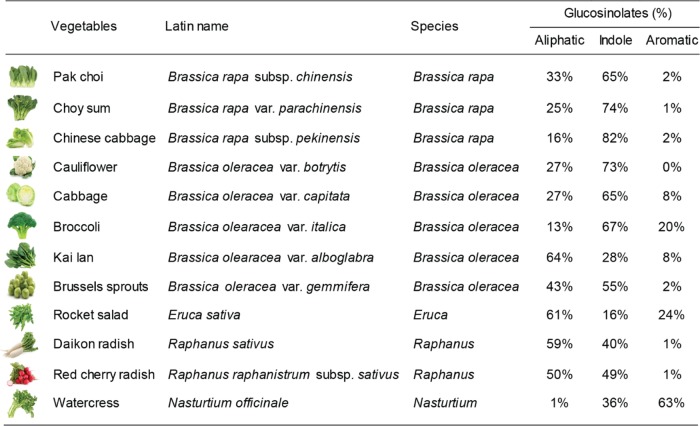
Brassicaceae vegetables are widely distributed and consumed in Asia, and include species such as Brassica oleracea (e.g., cauliflower, Brussels sprouts, cabbage, broccoli, and Kai Lan), Brassica rapa (e.g., pak choi, choy sum, and Chinese cabbage), Nasturtium officinale (e.g., watercress), Raphanus sativus (e.g., daikon radish and red cherry radish), and Eruca sativa (e.g., rocket salad).8 Evidence indicates that the concentration and composition of glucosinolates may vary among and within individual Brassicaceae species.9 Considering the enormous potential of biodiversity, screening of glucosinolate-enriched Brassicaceae species is of particular interest for nutritionists, agribusiness, and consumers at large. Moreover, recent studies indicate that species classification based on the secondary metabolites has emerged as an effective chemotaxonomic tool, providing detailed biochemical information about the differences and similarities among plant species.10 So far, there are only a handful of studies on quantification of glucosinolates in cruciferous or Brassica species; thus, it is interesting to explore the potential of use of quantitative glucosinolate profiles for the chemotaxonomic classification of Brassicaceae species.
To date, more than 130 glucosinolates have been reported in Brassicaceae vegetables;11 however, only a small portion of them (<10%) have been quantified because of the difficulties in trace-level determination of most glucosinolates using routine analytical methods. In general, reversed-phase liquid chromatography (RPLC) coupled to diode-array detector (DAD) or mass spectrometry (MS) is the most frequently used method for quantification of glucosinolates.12,13 In a recent study,14 a total of 12 glucosinolates were measured in 9 Brassica crops using RPLC–DAD, which is the largest number of glucosinolates quantified in an individual study so far. Owing to their biochemical properties, high volume of consumption, and health effects, it is thus time to develop more sensitive and selective methods for quantification of glucosinolates in vegetables. In 2007, Wade et al. for the first time applied hydrophilic interaction liquid chromatography (HILIC) for quantification of glucosinolates, but only three glucosinolates were measured in their study including glucoraphanin, glucoriberin, and sinigrin.15 HILIC utilizes a gradient of increasing aqueous content to elute analytes from a hydrophilic stationary phase, which is a more appropriate analytical method for analysis of polar components.16,17 Thus, it is a more effective technique than RPLC for separation of glucosinolates because of their highly polar nature.
In this study, we established an analytical method based on HILIC–MS/MS to quantify and compare the glucosinolate profiles across 12 commonly consumed Brassicaceae vegetables in Asia, including cauliflower, Brussels sprouts, cabbage, broccoli, Kai Lan, pak choi, choy sum, Chinese cabbage, watercress, daikon radish, red cherry radish, and rocket salad. Moreover, the potential use of glucosinolate profiles for Brassicaceae species classification was explored (Figure 1).
Figure 1.
Twelve commercial Brassicaceae vegetables and their glucosinolate composition.
Results and Discussion
Method Validation
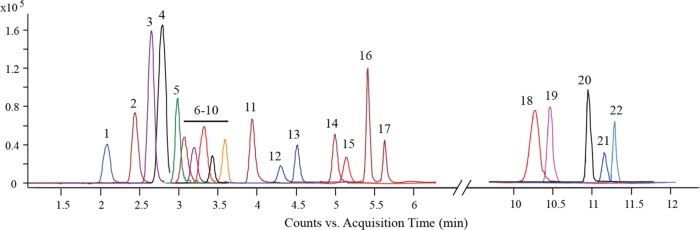
In this study, an analytical method based on HILIC–MS/MS was developed for the quantitative analysis of glucosinolates. Compared to the previously reported HILIC method for determination of limited glucosinolates,15 our present method allowed simultaneous quantification of a panel of 22 glucosinolates using the multiple reaction monitoring (MRM) mode, with a powerful separation in a relatively short analysis time (Figure 2). The optimized MRM conditions of 22 glucosinolates are shown in Table 1. In the validation process for the new method, we examined its limit of detection (LOD), limit of quantification (LOQ), linearity, accuracy, precision, and recovery (Table 2). LODs under the present chromatographic conditions, measured with a signal-to-noise ratio (S/N) of 3, ranged from 0.001 to 0.028 μg/g dry weight. LOQs, measured with a signal-to-noise ratio (S/N) of 10, ranged from 0.003 to 0.093 μg/g dry weight. The calibration curves and correlation coefficients were determined using a linear regression model. Good linear regression with r2 > 0.997 was obtained in all relevant ranges. For intrabatch and interbatch precision and accuracy, the relative standard deviation (RSD) values ranged from 2.00 to 9.24 and 3.33 to 9.95%, respectively. The mean recoveries were from 76.46 to 120.14% with RSD less than 9.07%. These results indicated that the newly developed method was satisfactory with acceptable precision, accuracy, reproducibility, and recovery.
Figure 2.
Representative HILIC–MS/MS chromatogram of 22 glucosinolates.
Table 1. Multiple Reaction Monitoring (MRM) Conditions of 22 Glucosinolates in HILIC–MS/MS Analysisa.
| glucosinolates | RT (min) | precursor ion | product ion | CE (V) | internal standard | calibration curve |
|---|---|---|---|---|---|---|
| aliphatic | ||||||
| sinigrinb | 3.94 | 358.0 | 97, 195 | 24 | glucosinalbin | sinigrin |
| glucoiberinb | 11.30 | 422.0 | 259, 358 | 26 | glucosinalbin | glucoiberin |
| glucocheirolinb | 5.19 | 438.0 | 97, 259 | 26 | glucosinalbin | glucocheirolin |
| glucoraphasatin | 2.79 | 418.0 | 97, 259 | 26 | glucosinalbin | glucoerucin |
| glucosativin | 3.45 | 406.0 | 97, 259 | 26 | glucosinalbin | glucoerucin |
| glucoerucinb | 3.00 | 420.0 | 97, 259 | 26 | glucosinalbin | glucoerucin |
| glucorapheninb | 10.30 | 434.0 | 97, 259 | 24 | glucosinalbin | glucoraphenin |
| glucoraphaninb | 11.20 | 436.0 | 97, 372 | 26 | glucosinalbin | glucoraphanin |
| gluconapinb | 3.60 | 372.0 | 97, 259 | 26 | glucosinalbin | gluconapin |
| epiprogoitrinb | 5.42 | 388.0 | 97, 259 | 24 | glucosinalbin | epiprogoitrin |
| progoitrinb | 5.72 | 388.0 | 97, 259 | 24 | glucosinalbin | progoitrin |
| glucoalyssinb | 10.46 | 450.1 | 97, 259 | 26 | glucosinalbin | glucoalyssin |
| glucobrassicanapinb | 3.10 | 386.1 | 97, 259 | 26 | glucosinalbin | glucobrassicanapin |
| gluconapoleiferin | 5.00 | 402.1 | 97, 259 | 26 | glucosinalbin | glucobrassicanapin |
| indole | ||||||
| glucobrassicinb | 3.23 | 447.1 | 97, 259 | 26 | glucosinalbin | glucobrassicin |
| neoglucobrassicin | 2.21 | 477.1 | 97, 259 | 24 | glucosinalbin | glucobrassicin |
| 4-methoxyglucobrassicin | 3.29 | 477.1 | 97, 259 | 26 | glucosinalbin | glucobrassicin |
| 4-hydroxyglucobrassicinb | 4.52 | 463.0 | 97, 259 | 26 | glucosinalbin | 4-hydroxyglucobrassicin |
| aromatic | ||||||
| glucotropaeolinb | 2.65 | 408.0 | 97, 259 | 24 | glucosinalbin | glucotropaeolin |
| gluconasturtiinb | 2.49 | 422.1 | 97, 259 | 24 | glucosinalbin | gluconasturtiin |
| glucobarbarinb | 10.96 | 438.1 | 97, 259 | 26 | glucosinalbin | glucobarbarin |
| glucosinalbinb,c | 4.30 | 424.0 | 97, 259 | 26 | glucoraphenin | glucosinalbin |
RT, retention time; CE, collision energy.
These 17 glucosinolates have available standards.
Glucosinalbin is present only in rocket salad and does not exist in other 11 examined vegetables. In this study, it was used as an internal standard for quantitative analysis of glucosinolates in the 12 vegetables, except rocket salad, in which glucoraphenin was used as the internal standard for quantitative analysis of glucosinolates, as it is absent in rocket salad.
Table 2. Method Validation of HILIC–MS/MS for 17 Glucosinolates with Available Standardsa.
| calibration
curve |
accuracy (%) |
recovery (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| glucosinolates | formulation | R2 | concentration (ng/mL) | LOD (μg/g dry weight) | LOQ | intrabatch | interbatch | low | high |
| sinigrin | y = 0.037x – 0.106 | 0.9997 | 1–500 | 0.007 | 0.023 | 4.35 | 5.96 | 79.44 | 103.06 |
| glucoiberin | y = 0.006x + 0.012 | 0.9994 | 1–500 | 0.003 | 0.010 | 4.82 | 6.32 | 112.10 | 84.36 |
| glucocheirolin | y = 0.038x – 0.036 | 0.9998 | 1–500 | 0.004 | 0.013 | 4.17 | 3.34 | 95.74 | 106.37 |
| glucoerucin | y = 0.073x – 0.725 | 0.9991 | 1–1000 | 0.009 | 0.030 | 7.21 | 6.76 | 84.04 | 110.19 |
| glucoraphenin | y = 0.47x – 1.149 | 0.9985 | 1–500 | 0.001 | 0.003 | 2.00 | 8.85 | 90.45 | 84.25 |
| glucoraphanin | y = 0.018x – 0.020 | 0.9998 | 1–500 | 0.013 | 0.043 | 4.06 | 7.67 | 85.26 | 98.40 |
| gluconapin | y = 0.054x – 0.012 | 0.9999 | 1–250 | 0.003 | 0.010 | 8.61 | 5.96 | 78.36 | 87.43 |
| epiprogoitrin | y = 0.033x – 0.068 | 0.9994 | 1–250 | 0.007 | 0.023 | 6.10 | 5.70 | 117.01 | 96.59 |
| progoitrin | y = 0.015x – 0.061 | 0.9997 | 1–1000 | 0.028 | 0.093 | 4.23 | 5.35 | 84.69 | 95.64 |
| glucoalyssin | y = 0.017x + 0.029 | 0.9991 | 1–500 | 0.013 | 0.043 | 6.74 | 9.04 | 95.26 | 114.17 |
| glucobrassicanapin | y = 0.079x – 0.936 | 0.9987 | 1–1000 | 0.008 | 0.027 | 9.24 | 9.95 | 81.67 | 91.45 |
| glucobrassicin | y = 0.042x – 0.133 | 0.9995 | 1–500 | 0.019 | 0.063 | 5.51 | 8.75 | 92.30 | 120.31 |
| 4-hydroxyglucobrassicin | y = 0.004x – 0.016 | 0.9996 | 1–1000 | 0.021 | 0.070 | 2.43 | 9.02 | 76.46 | 107.01 |
| glucotropaeolin | y = 0.082x – 0.265 | 0.9998 | 1–500 | 0.004 | 0.013 | 5.58 | 7.29 | 120.14 | 106.33 |
| gluconasturtiin | y = 0.052x – 0.129 | 0.9990 | 1–500 | 0.006 | 0.020 | 4.29 | 7.16 | 105.76 | 102.56 |
| glucobarbarin | y = 0.002x + 0.003 | 0.9979 | 5–500 | 0.023 | 0.077 | 6.54 | 3.33 | 114.79 | 99.05 |
| glucosinalbin | y = 0.003x + 0.008 | 0.9991 | 5–500 | 0.007 | 0.023 | 2.02 | 8.99 | 95.01 | 101.22 |
LOD, limit of detection; LOQ, limit of quantification.
Glucosinolate Profiles
A total of 22 glucosinolates were quantified in this study, including 14 aliphatic glucosinolates (sinigrin, glucoiberin, glucocheirolin, glucoraphasatin, glucosativin, glucoerucin, glucoraphenin, glucoraphanin, gluconapin, progoitrin, epiprogoitrin, glucoalyssin, glucobrassicanapin, and gluconapoleiferin), 4 indolic glucosinolates (glucobrassicin, neoglucobrassicin, 4-methoxyglucobrassicin, and 4-hydroxyglucobrassicin), and 4 aromatic glucosinolates (glucotropaeolin, gluconasturtiin, glucobarbarin, and glucosinalbin) (Table 1). Of these, 17 glucosinolates with available standards were absolutely quantified in the 12 vegetables examined and 5 glucosinolates without available standards were semiquantified. In this study, the five glucosinolates without available standards were tentatively identified on the basis of the specific MSn patterns of glucosinolates (Supporting Information Table S1). Briefly, glucosinolates can produce specific MS2 fragmentations at m/z 195, 241, 259, and 275, and furthermore, the most abundant MS2 fragmentation of m/z 259 gives rise to MS3 fragmentations at m/z 139 and 97.18 Two isomers, neoglucobrassicin and 4-methoxyglucobrassicin, which exhibit identical molecular masses, were differentiated by comparison with reported MS2 fragmentation ions ratio and elution sequence during reversed-phase high-performance liquid chromatography.19 The concentrations of glucosinolates were determined from the experimental peak area by analytical extrapolation in a standard calibration curve, and expressed as μg/g of dry weight. In the semiquantified analysis, glucoraphasatin and glucosativin were quantified as glucoerucin equivalents, gluconapoleiferin was quantified as glucobrassicanapin equivalent, and neoglucobrassicin and 4-methoxyglucobrassicin were quantified as glucobrassicin equivalents (Table 1).
Different Glucosinolates among 12 Brassicaceae Vegetables
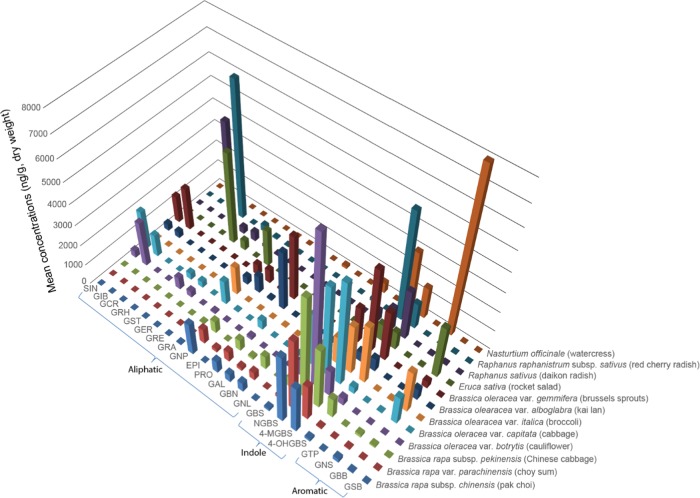
The expression of 22 glucosinolates in the 12 Brassicaceae vegetables is shown in Figure 3 and Supporting Information Table S2. As shown in Figure 3, we found that the concentration and composition of glucosinolates varied significantly among 12 vegetables. Of the 12 vegetables examined, cabbage had the highest level of total glucosinolates (in μg/g dry weight: 19 551.2 ± 1317.7), while Kai Lan had the lowest level of total glucosinolates (in μg/g dry weight: 7611.3 ± 868.4) (Supporting Information Table S2). This agrees with the concentrations of total glucosinolates in previous reports.14,20,21 Moreover, we noted that aliphatic and indolic glucosinolates were the major components in the 12 vegetables representing 76–100% of total glucosinolates, except watercress (37%) (Figure 1). Specifically, aliphatic glucosinolates constituted 61% of total glucosinolates in rocket salad. The indolic glucosinolates accounted for the major components in Brassica vegetables, including pak choi, choy sum, Chinese cabbage, cauliflower, Brussels sprouts, cabbage, and broccoli, ranging from 54 to 82%. For daikon radish and red cherry radish, subspecies of R. sativus, the contents of aliphatic and indole glucosinolates were almost equal (40–59%). These results are in accordance with a recent finding that aliphatic and indolic glucosinolates are the major components in Brassicaceae vegetables,1 although our study investigated a more comprehensive range of Brassicaceae vegetables and a wider range of glucosinolates.
Figure 3.
Mean concentrations of 22 glucosinolates in 12 Brassicaceae vegetables. SIN, sinigrin; GIB, glucoiberin; GCR, glucocheirolin; GRH, glucoraphasatin; GST, glucosativin; GER, glucoerucin; GRE, glucoraphenin; GRA, glucoraphanin; GNP, gluconapin; EPI, epiprogoitrin; PRO, progoitrin; GAL, glucoalyssin; GBN, glucobrassicanapin; GNL, gluconapoleiferin; GBS, glucobrassicin; NGBS, neoglucobrassicin; 4-MGBS, 4-methoxyglucobrassicin; 4-OHGBS, 4-hydroxyglucobrassicin; GTP, glucotropaeolin; GNS, gluconasturtiin; GBB, glucobarbarin; and GSB, glucosinalbin.
As shown in the more detailed data in Supporting Information Table S2, glucoraphanin, gluconapin, 4-methoxyglucobrassicin, 4-hydroxyglucobrassicin, and gluconasturtiin were the 5 most common glucosinolates found in all 12 vegetables. Sinigrin and glucoiberin were the most predominant glucosinolates in Kai Lan, Brussels sprouts, cabbage, and cauliflower, whereas glucobrassicanapin and glucoalyssin were the most predominant glucosinolates in choy sum, pak choi, and Chinese cabbage. It was also noted that glucocheirolin was detected only in cabbage and Kai Lan. Glucoerucin was a major glucosinolate in rocket salad but was also detected in white radish, red cherry radish, cabbage, and cauliflower. The highest concentration of glucoraphenin and glucoraphasatin was observed in white radish and red cherry radish, whereas notably glucoraphenin was found only in white radish and red cherry radish. The concentrations of glucosativin, glucoerucin, glucoraphanin, glucobarbarin, and glucosinalbin were detected in relatively higher quantities in rocket salad as compared to those in other vegetables. Furthermore, glucosinalbin was present only in rocket salad. In contrast to other Brassicaceae vegetables, gluconasturtiin was the predominant glucosinolate in watercress. In brief, the major profiles of the 22 glucosinolates quantified in the 12 vegetables were consistent with previous studies that focused on these vegetables.14,20,22
In addition to dry weight, we also determined fresh weight concentrations of glucosinolates in μg/g (Supporting Information Table S3). As noted, dry and fresh weights of glucosinolates showed similar glucosinolate patterns in the 12 vegetables.
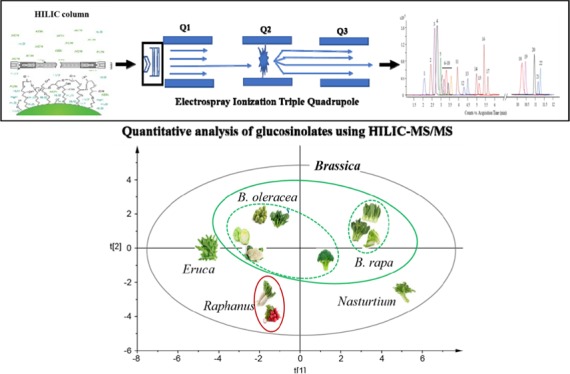
Chemotaxonomic Classification
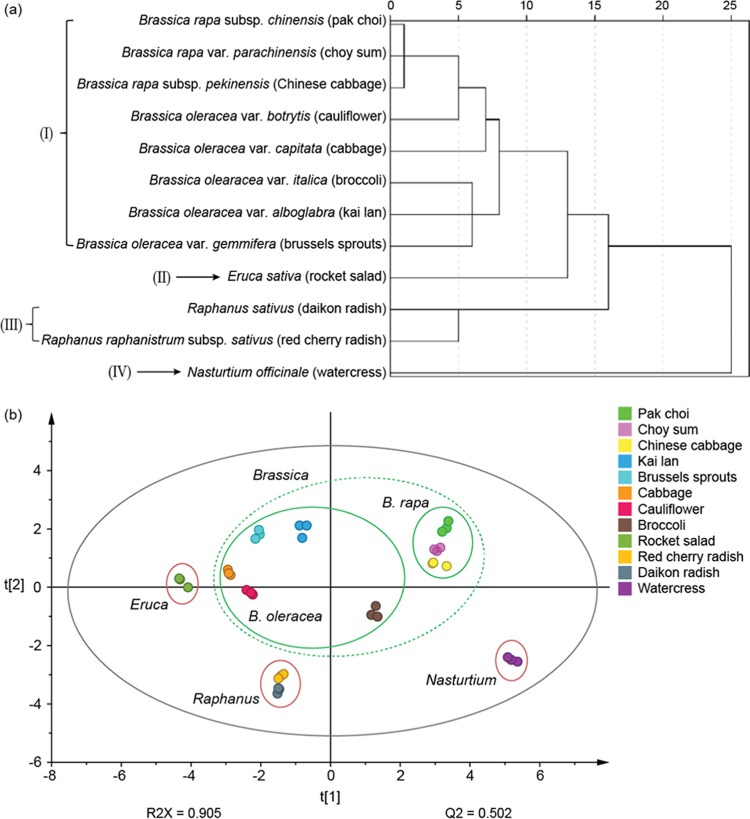
The botanical taxonomic status of the family Brassicaceae is quite complex as different concepts for the classification and status of the Brassicaceae species and subspecies exist.23 With the development of DNA sequencing methods, biological systematic analysis has increasingly been based on DNA sequence analysis.24 In addition to genetic taxonomy, phytochemical taxonomy has been shown to be able to provide supplementary information in species identification in the last decade.25 Following a previously reported protocol,26 we investigated the potential use of glucosinolate profiles as reference markers for chemotaxonomic classification of the 12 Brassicaceae vegetables using both hierarchical cluster analysis (HCA) and principal component analysis (PCA). HCA dendrogram showed the division of the 12 vegetables into 4 major branches on the basis of the average concentrations of glucosinolates in each individual vegetable (Figure 4a). Vegetables in branch I belong to the Brassica genus and were further divided into two species, B. rapa and B. oleracea. As shown here, the results of glucosinolate-based chemotaxonomic classification were closely aligned with the morphology-based classification. To further explore the relationship among the 12 vegetables across 3 different batches, PCA, an unsupervised pattern recognition method, was performed using the same data. A two-dimensional PCA score plot was utilized to depict the general variation of glucosinolate profiles among the 36 samples (12 vegetables × 3 batches). As shown in Figure 4b, the samples were primarily divided into four clusters according to their species. The clustering pattern is consistent with the classification from the HCA tree. These findings demonstrate that glucosinolate-based chemotaxonomic classification can be used to distinguish different Brassicaceae species.
Figure 4.
Cluster analysis of 12 Brassicaceae vegetables based on the content of 22 glucosinolates. (a) Hierarchical cluster analysis. (b) Principal component analysis.
In summary, in this study, we established a new, reliable, and precise analytical method for the quantitative analysis of glucosinolates using HILIC–MS/MS, and described the accumulation patterns of 22 glucosinolates in 12 Brassicaceae vegetables. Significant variations in the concentrations and compositions of glucosinolates were observed among different Brassicaceae vegetables. On the basis of the content of 22 glucosinolates, the 12 vegetables can be divided into 4 clusters, which is consistent with morphological taxonomy. The findings demonstrate that phytochemical taxonomy has the potential to be used for distinguishing among Brassicaceae species by identifying variants of chemotaxonomic markers like glucosinolates.
Materials and Methods
Chemicals and Reagents
Acetonitrile, methanol, formic acid, and ammonium formate were purchased from Sigma-Aldrich (St. Louis, MO). The 10 glucosinolate standards including glucocheirolin, progoitrin, glucoraphenin, epiprogoitrin, glucobrassicanapin, glucoalyssin, glucobrassicin, gluconasturtiin, 4-hydroxyglucobrassicin, and glucobarbarin were purchased from Cfm Oskar Tropitzsch GmbH (Marktredwitz, Germany). Another seven glucosinolate standards including sinigrin, glucoiberin, glucotropaeolin, glucoraphanin, gluconapin, glucoerucin, and glucosinalbin were purchased from ChromaDex (Santa Ana, CA). Distilled water was purified “in-house” using a Milli-Q purification system (Bedford, MA).
Sample Collection and Preparation
On the basis of earlier studies in Singapore27,28 and vegetable-consumption patterns in the Asian region, 12 commonly consumed Brassicaceae vegetables, including B. rapa subsp. chinensis (pak choi), B. rapa var. parachinensis (choy sum), B. rapa subsp. pekinensis (Chinese cabbage), B. oleracea var. botrytis (cauliflower), B. oleracea var. capitata (cabbage), Brassica olearacea var. italica (broccoli), Brassica olearacea var. alboglabra (Kai Lan), B. oleracea var. gemmifera (Brussels sprouts), E. sativa (rocket salad), R. sativus (daikon radish), Raphanus raphanistrum subsp. sativus (red cherry radish), and N. officinale (watercress), were included in this study (Figure 1). Three batches of these 12 vegetables were purchased from three local supermarkets in Singapore. The fresh vegetables (whole tissue) were washed using tap water and then snap-frozen in liquid nitrogen and freeze-dried. The freeze-dried vegetables were ground into a fine powder and stored at −80 °C before analysis. For glucosinolates extraction, 100 mg of freeze-dried samples was treated with 1 mL of 70% methanol (v/v) containing 150 ng/mL of internal standard (glucosinalbin or glucoraphenin) and incubated at 70 °C for 10 min. After being cooled in an ice bath, the extracts were centrifuged at 15 000g for 15 min, and the supernatants were collected. The extraction procedure was repeated twice with 70% methanol (v/v) to give a final extract containing 50 ng/mL of internal standard. The supernatants were then combined and evaporated until dry at 40 °C. The dried samples were reconstituted in 3 mL of 70% acetonitrile (v/v) and filtered through a 0.22 μm nylon filter for analysis. Samples were extracted and analyzed in triplicates, and data were represented as mean ± SD (n = 3).
Glucosinolate Profiling and Identification
Agilent 1290 ultra-high-performance liquid chromatography system (Waldbronn, Germany) coupled to a 6540 quadrupole time-of-flight (Q-ToF) mass detector (Agilent, Santa Clara, CA) equipped with an electrospray ionization source was used for glucosinolate profiling and identification. The separation of glucosinolates was achieved on a Waters ACQUITY UPLC BEH HILIC column (2.1 × 100 mm2, 1.7 μm). The mobile phase consisted of A (30% acetonitrile containing 10 mM ammonium formate, 0.1% formic acid) and B (95% acetonitrile containing 10 mM ammonium formate, 0.1% formic acid). The linear gradient was as follows: 0–1 min, 100–100% B; 1–5 min, 100–95% B; 5–8 min, 95–80% B; 8–12 min, 80–15% B at a column temperature of 35 °C with a flow rate of 0.4 mL/min. The autosampler was cooled at 4 °C, and 5 μL of the extract was injected. Electrospray ionization was performed in negative ion mode with the following source parameters: drying gas (N2) temperature 200 °C with a flow rate of 14 L/min, nebulizer gas pressure 30 psi, sheath gas temperature 400 °C with a flow rate of 11 L/min, capillary voltage 3000 V, and nozzle voltage 800 V. The MS/MS analysis was carried out to study the structures of glucosinolates. Moreover, the commercial standards were analyzed to support the identification of glucosinolates.
Glucosinolate Quantification
Quantitative analysis of glucosinolates was performed on an Agilent 1290 ultra-high-performance liquid chromatography system (Waldbronn, Germany) coupled to a 6490 Triple Quadrupole (QQQ) mass detector (Agilent, Santa Clara, CA) equipped with iFunnel Technology and an electrospray ionization source. Separation of glucosinolates was achieved using the same method as in Q-ToF analysis. Mass spectra were recorded in the multiple reaction monitoring (MRM) mode. Data acquisition and processing were performed using MassHunter software version B.05.00 (Agilent Technologies, CA).
Method Validation
The proposed quantitative method was validated for its limit of detection (LOD), limit of quantification (LOQ), linearity, accuracy, precision, and recovery, according to Food and Drug Administration guidelines for biological methods as in our previously published report.29 Briefly, accurately weighed standards were dissolved in 70% acetonitrile separately and diluted to provide a series of standard solutions with gradient concentration to make the calibration curves. All of the solutions were stored at −20 °C. Method precision was studied by injecting the same mixed sample solution six times consecutively, both on the same day for intraday variation and on three consecutive days for interday variation. To check the reproducibility, six independently prepared samples from the mixed vegetable powder were analyzed. The recoveries were evaluated by spiking three defined amounts of glucosinolate standards (approximately equivalent to 0.8, 1.0, and 1.2 times the concentration of the matrix) into the mixed vegetable sample in triplicates and were extracted and quantified as described earlier.
Statistical Analysis
Independent measurements in triplicates were used for each sample in all statistical analyses. Values below the LOD were assigned as a proxy value of an LOD/2 as usual.30 The data were diagnosed in histogram plots to be skewed and were therefore transformed into log 2-scale to meet the assumption of normality before statistical analysis. The differences in glucosinolate concentrations among the 12 vegetables were examined using one-way analysis of variance. Both hierarchical cluster analysis (HCA) and principal component analysis (PCA) were applied to identify similarities of glucosinolate profiles in 12 examined vegetables. In HCA, the between-groups linkage method as the amalgamation rule and the squared Euclidean distance as the metric were applied to establish clusters. Statistical analyses were performed using IBM SPSS Statistics 24 and SIMCA 14. A two-sided p < 0.05 was considered statistically significant.
Glossary
Abbreviations
- RPLC
reversed-phase liquid chromatography
- DAD
diode-array detector
- MS
mass spectrometry
- HILIC
hydrophilic interaction liquid chromatography
- QQQ
triple quadrupole
- MRM
multiple reaction monitoring
- LOD
limit of detection
- HCA
hierarchical cluster analysis
- PCA
principal component analysis
- RSD
relative standard deviation
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01668.
MS and MSn information of five glucosinolates (Table S1); mean levels (μg/g dry weight, mean ± SD) of glucosinolates in 12 vegetables (Table S2); mean levels (μg/g fresh weight) of glucosinolates in 12 vegetables (Table S3) (PDF)
This work was supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Competitive Research Programme (NRF-CRP 16-2015-04) and the NUS Secondment Funds to CNO (706-000-005-133).
The authors declare no competing financial interest.
Supplementary Material
References
- Ishida M.; Hara M.; Fukino N.; Kakizaki T.; Morimitsu Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. 10.1270/jsbbs.64.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borpatragohain P.; Rose T. J.; King G. J. Fire and Brimstone: Molecular Interactions between Sulfur and Glucosinolate Biosynthesis in Model and Crop Brassicaceae. Front. Plant Sci. 2016, 7, 1735. 10.3389/fpls.2016.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask L.; Andreasson E.; Ekbom B.; Eriksson S.; Pontoppidan B.; Meijer J. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol. Biol. 2000, 42, 93–113. 10.1023/A:1006380021658. [DOI] [PubMed] [Google Scholar]
- Klopsch R.; Witzel K.; Borner A.; Schreiner M.; Hanschen F. S. Metabolic profiling of glucosinolates and their hydrolysis products in a germplasm collection of Brassica rapa turnips. Food Res. Int. 2017, 100, 392–403. 10.1016/j.foodres.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Higdon J. V.; Delage B.; Williams D. E.; Dashwood R. H. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T.; Kostov R. V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Fahey J. W.; Wehage S. L.; Holtzclaw W. D.; Kensler T. W.; Egner P. A.; Shapiro T. A.; Talalay P. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. 10.1158/1940-6207.CAPR-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L.; Paonessa J. D.; Zhang Y.; Ambrosone C. B.; McCann S. E. Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. J. Funct. Foods 2013, 5, 1996–2001. 10.1016/j.jff.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B.; Yang J.; Zhu Z. J. Variation in glucosinolates in pak choi cultivars and various organs at different stages of vegetative growth during the harvest period. J. Zhejiang Univ., Sci., B 2013, 14, 309–317. 10.1631/jzus.B1200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K. E.; Bjergegaard C.; Moller P.; Sorensen J. C.; Sorensen H. Compositional variations for alpha-galactosides in different species of leguminosae, brassicaceae, and barley: a chemotaxonomic study based on chemometrics and high-performance capillary electrophoresis. J. Agric. Food Chem. 2005, 53, 5809–5817. 10.1021/jf040471v. [DOI] [PubMed] [Google Scholar]
- Agerbirk N.; Olsen C. E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. 10.1016/j.phytochem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Mohn T.; Cutting B.; Ernst B.; Hamburger M. Extraction and analysis of intact glucosinolates--a validated pressurized liquid extraction/liquid chromatography-mass spectrometry protocol for Isatis tinctoria, and qualitative analysis of other cruciferous plants. J. Chromatogr. A 2007, 1166, 142–151. 10.1016/j.chroma.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Peng A. J.; Tang G. F.; Lan Z. H.; Dong W. B.; Wu M. C. Determination of glucosinolates in rapeseed by reversed-phase ion-pair liquid chromatography. Se Pu 2000, 18, 85–87. [PubMed] [Google Scholar]
- Bhandari S. R.; Jo J. S.; Lee J. G. Comparison of Glucosinolate Profiles in Different Tissues of Nine Brassica Crops. Molecules 2015, 20, 15827–15841. 10.3390/molecules200915827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade K. L.; Garrard I. J.; Fahey J. W. Improved hydrophilic interaction chromatography method for the identification and quantification of glucosinolates. J. Chromatogr. A 2007, 1154, 469–472. 10.1016/j.chroma.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszewski B.; Noga S. Hydrophilic interaction liquid chromatography (HILIC)--a powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. 10.1007/s00216-011-5308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D. Q.; Zou L.; Yin X. X.; Ong C. N. HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass Spectrom. Rev. 2016, 35, 574–600. 10.1002/mas.21445. [DOI] [PubMed] [Google Scholar]
- Bialecki J. B.; Ruzicka J.; Weisbecker C. S.; Haribal M.; Attygalle A. B. Collision-induced dissociation mass spectra of glucosinolate anions. J. Mass Spectrom. 2010, 45, 272–283. 10.1002/jms.1711. [DOI] [PubMed] [Google Scholar]
- Velasco P.; Francisco M.; Moreno D. A.; Ferreres F.; Garcia-Viguera C.; Cartea M. E. Phytochemical fingerprinting of vegetable Brassica oleracea and Brassica napus by simultaneous identification of glucosinolates and phenolics. Phytochem. Anal. 2011, 22, 144–152. 10.1002/pca.1259. [DOI] [PubMed] [Google Scholar]
- Yi G.; Lim S.; Chae W. B.; Park J. E.; Park H. R.; Lee E. J.; Huh J. H. Root Glucosinolate Profiles for Screening of Radish (Raphanus sativus L.) Genetic Resources. J. Agric. Food Chem. 2016, 64, 61–70. 10.1021/acs.jafc.5b04575. [DOI] [PubMed] [Google Scholar]
- Francisco M.; Moreno D. A.; Cartea M. E.; Ferreres F.; Garcia-Viguera C.; Velasco P. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A 2009, 1216, 6611–6619. 10.1016/j.chroma.2009.07.055. [DOI] [PubMed] [Google Scholar]
- Jeon J.; Bong S. J.; Park J. S.; Park Y. K.; Arasu M. V.; Al-Dhabi N. A.; Park S. U. De novo transcriptome analysis and glucosinolate profiling in watercress (Nasturtium officinale R. Br.). BMC Genomics 2017, 18, 401. 10.1186/s12864-017-3792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daun J. K.; Eskin N.; Hickling D.. Canola: Chemistry, Production, Processing, and Utilization; AOCS Press, 2011. [Google Scholar]
- Johnston J. S.; Pepper A. E.; Hall A. E.; Chen Z. J.; Hodnett G.; Drabek J.; Lopez R.; Price H. J. Evolution of genome size in Brassicaceae. Ann. Bot. 2005, 95, 229–235. 10.1093/aob/mci016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins A. E. Natural product chemistry meets genetics: when is a genotype a chemotype?. J. Agric. Food Chem. 2008, 56, 7587–7592. 10.1021/jf801239j. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Lam H. M.; Pi E. X.; Zhan Q. L.; Tsai S. N.; Wang C. M.; Kwan Y. W.; Ngai S. M. Comparative Metabolomics in Glycine max and Glycine soja under Salt Stress To Reveal the Phenotypes of Their Offspring. J. Agric. Food Chem. 2013, 61, 8711–8721. 10.1021/jf402043m. [DOI] [PubMed] [Google Scholar]
- Jiao D.; Yu M. C.; Hankin J. H.; Low S.-H.; Chung F.-L. Total isothiocyanate contents in cooked vegetables frequently consumed in Singapore. J. Agric. Food Chem. 1998, 46, 1055–1058. 10.1021/jf9706989. [DOI] [Google Scholar]
- Isabelle M.; Lee B. L.; Lim M. T.; Koh W.-P.; Huang D.; Ong C. N. Antioxidant activity and profiles of common vegetables in Singapore. Food Chem. 2010, 120, 993–1003. 10.1016/j.foodchem.2009.11.038. [DOI] [Google Scholar]
- Lu Y.; Fang J.; Zou L.; Cui L.; Liang X.; Lim S. G.; Dan Y.-Y.; Ong C. N. Omega-6-derived oxylipin changes in serum of patients with hepatitis B virus-related liver diseases. Metabolomics 2018, 14, 26. 10.1007/s11306-018-1326-z. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Su J.; van Dam R. M.; Prem K.; Hoong J. Y. S.; Zou L.; Lu Y. H.; Ong C. N. Dietary predictors and plasma concentrations of perfluorinated alkyl acids in a Singapore population. Chemosphere 2017, 171, 617–624. 10.1016/j.chemosphere.2016.12.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.