Abstract
In this study, we merged infant hospitalization data to get the first nationally representative study of fine particulate matter (PM2.5) and bronchiolitis hospitalization charges and costs. We found that a small increase (1 microgram/cubic meter) in lifetime PM2.5 was associated with $285 in additional charges and $127 in incremental costs. In aggregate, each microgram/cubic meter of fine particulate matter represents at least an annual $15 million, nationally, in additional health care utilization and should be considered alongside costs of pollution prevention.
Introduction
Bronchiolitis – a lower respiratory tract infection - is the most common cause of hospitalization in children <1 year old (1), with in-patient care costing greater than $500 million annually. (2) Studies are increasingly documenting evidence for a role of outdoor air pollutants as a risk factor for bronchiolitis. (3–10) The air pollutants linked to pediatric respiratory disease, in general, are particulate matter, ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO). These are 5 of the 6 air pollutants regulated by the Clean Air Act. A plausible mechanism by which outdoor air pollution could worsen bronchiolitis is by contributing to ongoing inflammation in the lungs. (11)
Particulate matter is an area of particular interest. This type of air pollutant is, unlike other pollutants which are labeled by their elemental composition, defined by its size. The category of particulate matter that is the subject of the most health research is called fine particulate matter (particles with a diameter less than 2.5 microns, also called PM2.5). (12) The smaller the particle the farther into the lungs it can penetrate when inhaled thereby affecting more of the lung tissue and potentially even being absorbed from the lungs into the blood.
While acute air pollutant exposure has been associated with bronchiolitis clinic visits and hospitalizations, (6, 9, 10) other studies have associated subchronic exposure to outdoor air pollutants with bronchiolitis episodes. These include a study in the Czech Republic, which found that children (<2 years old) had an increased bronchitis risk from sub-chronic (defined as the average exposure over 30 days) PM2.5 exposure, (4) and an Italian study which identified a positive association between parental report of increased traffic and bronchiolitis occurrence in the first 2 years of life. (3)
In North America, studies limited to single geographic areas have identified chronic exposures to outdoor air pollution to be of concern. For infants this is typically represented by the average of all daily or monthly averages of various pollutant levels for the lifetime of the infant, or the ‘lifetime’ exposure. Catherine Karr and colleagues (7) examined a British Columbia population of infants and found a positive association both of lifetime and prior month exposure to NO2, SO2 and CO with bronchiolitis episodes. In a western Washington state study that looked at lifetime as well as shorter durations of exposures to PM2.5 and NO2, positive but non-significant associations – meaning that almost all odds ratios were greater than one but the 95% confidence intervals crossed one - were seen between these air pollutants and risk of hospitalization for bronchiolitis. (8) In an area of higher ambient air pollution levels in Southern California, infant bronchiolitis requiring hospitalization was associated with lifetime and month prior to hospitalization averages of PM2.5 but not CO or NO2 exposures. (5)
A major limitation of past studies is their focus on one geographic area, especially because – due to regional variation - findings from the Western US and southwestern Canada may not be generalizable across the broader US to inform regulation of outdoor air pollutant emissions. PM2.5 has been documented to have varying effects on cardiovascular admissions in adults, based on season and location within the U.S. which may represent different chemical composition of the particulate matter in different parts of the country, increasing concern about extrapolating from single-region studies. (11, 13)
We therefore decided to link two nationally representative data sets, the 1999–2007 Nationwide Inpatient Sample (NIS) and the Aerometric Information Retrieval System to assess the impact of air pollution in a national sample of bronchiolitis hospitalizations. (14, 15) Our analysis also assesses the variable impact of differing lengths of exposure (e.g. 1-month versus longer) to PM2.5 on bronchiolitis outcomes.
Methods
This was a multi-year, cross-sectional study, including hospitalizations between 1999 and 2007 for children ages > 1 month to 1 year of age with a primary diagnosis of bronchiolitis (ICD-9 code 466). Hospitalizations with no corresponding air pollutant data were excluded. Weights accounting for sampling design were included in the NIS; these sample weights were incorporated into all analyses except descriptive air pollutant data. SAS software version 9.2 (SAS Institute, Inc, Cary, NC) was used for database management. Analyses were conducted using SAS-callable SUDAAN software version 10.0.1 (Research Triangle Institute, NC) to take into account the complex sampling design used by NIS. The Institutional Review Board/Program for the Protection of Human Subjects at our institution deemed that this project, analyzing data from a previously collected, publicly available, de-identified data set, was not considered human research according to federal regulations, and as such was exempt from review.
Databases
The NIS is the largest all-payer inpatient care database in the US and contains data from approximately 8 million hospital stays each year. (14) Details regarding sample design, data collection and weighting are described elsewhere.(14) The dataset includes diagnostic codes and basic patient demographics, as well as length of stay (LOS) and total hospital charges.
The EPA Aerometric Information Retrieval System (AIRS) contains pollutant data recorded at defined intervals, ranging from hourly to every few days, depending on regulatory requirements. (15) Publicly available text files for six air pollutants (PM2.5, PM10, O3, NO2, SO2, CO) from 1999 through 2007 were merged with the NIS hospitalization data.
Because the NIS does not contain identifying information about patient residence, air pollutant levels were determined for a defined area surrounding the hospital. To compare with the publicly available hospital latitude and longitude, we determined the latitude and longitude of all hospitals in NIS for which addresses were available. The distance between NIS hospitals and AIRS monitors was determined using the following equation, which calculates the hypotenuse of a right triangle formed by the two locations:
where D is distance in miles between a hospital and a monitor, lat1 and lat2 are latitudes of first and second locations respectively and lon1 and lon2 are longitudes of those same locations.
Outcomes: hospitalization data
Outcomes in this study included length of stay (LOS), total charges, and total costs for infant bronchiolitis hospitalizations. The LOS for each admission was provided in days. Total charges represent the amount billed for each hospitalization. Charges were controlled for inflation by adjusting to 2005 dollars using the Healthcare Consumer Price Index from the Bureau of Labor. (16) Costs represent the amount of money actually paid to the hospital, which in general, are significantly less than the charges. Because data on costs are not directly collected, cost estimates are created using specific files that accompany the NIS called group-weighted cost-to-charge ratio files, with further detail provided in the On-line Supplement. (17)
Predictor variables: air pollutants
The main predictor for our examination of infant bronchiolitis hospitalizations was average ambient PM2.5 level. To calculate the monthly average air pollutant levels, we averaged data from all monitors located within 10 miles of the hospital at which hospitalization occurred. Specifically for PM2.5, personal air monitoring has shown high correlation with ambient levels within a 20 kilometer radius of the central monitor. (18, 19)
We performed correlations among the pollutants to determine which ones to include in multivariable analyses. Any two pollutants correlated with each other very strongly (by Pearson correlation coefficient ≥ absolute value of 0.3) were not included together in multivariable models, and we prioritized the inclusion of PM2.5 and O3, the pollutants with the strongest evidence linked to adverse respiratory outcomes.
As our analyses progressed in a three pollutant model (PM2.5, O3, NO2), we identified a multivariate association with PM2.5 exposure, which we decided to explore further by increasing chronicity of PM2.5. For PM2.5 two-month averages (which included the month of admission as well as the month prior) as well as three- to eleven-month averages were also calculated and linked to the hospital data. To calculate the lifetime PM2.5, the monthly averages for as many months as the child was old were averaged and then also linked to the hospital data.
Statistical Analyses
For more normal distribution of the variables, we converted total charges and costs into their logarithmic equivalents (e.g. log10-total charges and log10-costs) and included air pollutants in linear regression models based on results of correlation analysis. We also included other factors associated with bronchiolitis outcomes and/or hospital charges: age; race (white, black, Hispanic, Asian/Pacific Islander, Native American or other); gender; income; insurance (Medicare, Medicaid, private, self-pay, no charge, or other); hospital region of the country (Northeast, South, Midwest, or West); hospital location (urban, rural); teaching status of the hospital; and month of admission. As a proxy for income, we used a variable included in the NIS which is the median household income quartile for patient’s zip code. Because we used log10-total charges and log10-costs in our analysis but then needed dollar values for later interpretation, we used a technique called Duan transformation to convert back to total charges and costs.(20)
Confirming the Absence of a Spurious Association with Air Pollution
To validate findings between air pollutants and bronchiolitis outcomes, we ran similar multivariable models for gastroenteritis, a frequent cause of infant hospitalization that should have no association with change in air pollutant levels. We hypothesized that there would be no significant relationships found.
There are several important limitations of this study. First is the lack of personal exposure measurement and residential address of the hospitalized individuals. However, previous studies have shown that ambient PM2.5 levels measured at central monitoring sites correlate well with average personal PM2.5 exposure in contrast to ambient O3, NO2, and SO2 concentrations correlations. (18, 19, 21) Patient address would more be a precise point from which to estimate air pollution exposure, but in this sample, air pollutant monitoring data were almost always obtained for urban zip codes for which the results obtained using patient address would not significantly deviate from that obtained using patient zip code. (22) Our metric for PM2.5 is a reasonable proxy for average exposure provided that the majority of patients live in the vicinity of the hospital. Another limitation is our ability to discern what drives the increased charges and costs because the NIS does not contain details regarding management during hospitalization, e.g. medications used and intensive care. An additional potential limitation is residual confounding by socioeconomic status. We control for income and insurance as markers of socioeconomic status. Lastly, our analysis is limited to the areas of the country with available air quality data. Air monitors are most densely located in urban areas and in the bicoastal regions of the country.
Results
Between 1999 and 2007, there were 70,052,217 hospital admissions in the NIS database; 156,889 were for infants, ages 30 days to 1 year, with a primary diagnosis of bronchiolitis. Hospital admissions with no corresponding air pollutant data were excluded for a final sample of 47,822 hospitalizations. Compared to all infant bronchiolitis hospitalizations, discharges included in the analysis had more patients who were black or Hispanic, were more likely to be in teaching hospitals, almost exclusively located in urban settings, and less likely to be located in the South and Midwest (Exhibit 1). These differences occur due to the national distribution of air monitors which are more numerous in more densely populated areas. Many states do not report race data to NIS and thus this variable was unreported for a large percentage of patients. (23, 24)
Exhibit 1 (table).
Characteristics of Patients and Hospitals among Infant Bronchiolitis Hospitalizations in the Nationwide Inpatient Sample, 1999–2007
| All infant bronchiolitis hospitalizations (N=769,875 weighted) |
Infant bronchiolitis hospitalizations with corresponding air pollutant data1 (N=248,216 weighted) |
|
|---|---|---|
|
Patient Characteristics |
weighted % | weighted % |
| Female | 39.9 | 39.4 |
| Race | ||
| White | 33.2 | 25.2 |
| Black | 10.2 | 14.9 |
| Hispanic | 16.5 | 36.1 |
| Asian / Pac. Islander | 1.6 | 3.1 |
| Unreported | 34.3 | 14.5 |
| Median household income for patient zip code | ||
| 1st quartile | 23.0 | 24.0 |
| 2nd quartile | 29.5 | 24.1 |
| 3rd quartile | 24.4 | 23.5 |
| 4th quartile | 23.1 | 28.5 |
| Primary Payer | ||
| Medicaid | 55.4 | 57.8 |
| Private | 38.6 | 35.9 |
| Admission month | ||
| March – May | 24.9 | 24.5 |
| June – August | 3.9 | 5.0 |
| Sept – Nov | 12.2 | 13.2 |
| Dec – Feb | 58.9 | 57.3 |
| Hospital characteristics | ||
| Region | ||
| Northeast | 20.0 | 31.6 |
| Midwest | 28.2 | 14.1 |
| South | 26.4 | 7.3 |
| West | 25.3 | 47.0 |
| Urban location | 79.2 | 99.6 |
| Teaching hospital | 49.7 | 68.8 |
Source: Authors’ analysis from the following sources 1) U.S. Environmental Protection Agency Aerometric Information Retrieval System and 2) Agency for Healthcare Quality and Research Nationwide Inpatient Sample.
Notes:
Hospitalizations with data available for three pollutants PM2.5, NO2, O3.
The mean total charge for infant bronchiolitis hospitalizations was $14,027, with a maximum charge of $967,799. The median charges and interquartile range were $7,909 and $9,268 respectively. The mean total cost was $5,493, with a maximum cost of $446,077. The median costs and interquartile range were $3,173 and $3,513 respectively.
Regarding the air monitoring around the hospitals, 1309 out of 2426 hospitals in the NIS sample from the years 1999–2007 had at least one PM2.5 monitor for at least one year. Of those hospitals, the median number of air monitors within 10 miles was 2, with a mean of 2.9, a maximum of 18, and a standard deviation of 2.8. The average distance of air monitors to the hospitals was 5.5 miles. While difficult to make direct comparisons to national air quality standards because of different averaging times, the mean PM2.5 and NO2 levels (monthly averages) are slightly above the annual standard, and the maximum monthly O3 is below the 8 hour maximum standard, as shown in the On-line Supplement.
Among the different air pollutants, PM2.5 and PM10 were most highly correlated (rho=0.5). SO2 and CO were negatively correlated with O3 (rho= −0.3), and PM2.5 and CO were positively correlated (rho=0.3), as shown in the On-line Supplement. As mentioned above, we prioritized PM2.5 and O3 for inclusion in final models because of a priori hypotheses about bronchiolitis morbidity. Therefore, PM2.5, O3, and NO2 were further examined in three-pollutant multivariable analyses.
In multivariable analyses, the 1-month averages of the three pollutants and the lifetime PM2.5 were not significant predictors (defined as p-value < 0.05) of LOS (data not shown). Monthly PM2.5 was nearly significant as a predictor of charges and was weakly significant for costs. Neither O3 nor NO2 were significantly associated with charges or costs. To examine the effect of chronicity, we explored the association of increasing months of PM2.5 exposure which showed increasing incremental association with charges (Exhibit 3), with limited power to assess 6–12 month exposures due to the smaller number of 6–12 month olds in the sample (See On-line Supplement).
Exhibit 3 (figure).
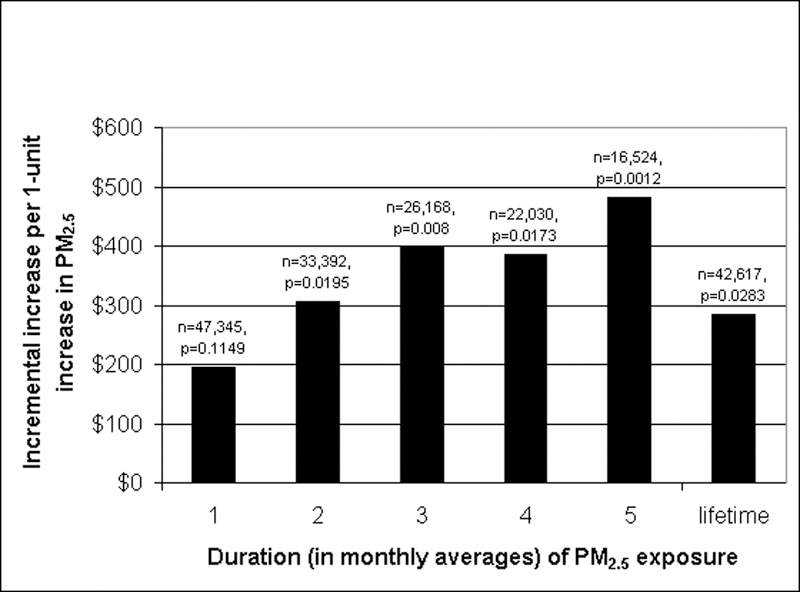
The Association of Increasing Chronicity of Outdoor PM2.5 Exposure and Bronchiolitis Hospital Charges for Infants. Source: Authors’ analysis from the following sources 1) U.S. Environmental Protection Agency Aerometric Information Retrieval System and 2) Agency for Healthcare Quality and Research Nationwide Inpatient Sample.
Lifetime PM2.5 was a significant predictor of both charges and costs. A 1-unit (μg/m3) increase – approximately 3% above the 24-hour PM2.5 standard (35 μg/m3) and 7% above the annual PM2.5 standard (15 μg/m3)- in lifetime PM2.5 led to a $285 increase in charges (95% CI: +$25 to +$734, p=.028) and a $127 increase in costs (95% CI: +$28 to +$302, p=.0041) (Exhibit 2, and On-line Supplement for comparison with National Air Quality Standards). Monthly O3 and NO2 were not significant predictors of charges or costs. NO2 was weakly significant predictor of charges when controlling for LOS. Other significant predictors of charges included Hispanic, Asian, or Pacific Islander race and hospital location in the south (p<0.01 for all associations).
Exhibit 2 (table).
Multivariable results for Infant Bronchiolitis Hospitalization Charges and Costs as predicted by Microgram per Cubic meter Increase in the Level of Fine Particulate Matter
| PM2.5 level1 | Increment Change (95% CI) in Total Charges2 |
Increment Change (95% CI) in Total Costs2 |
|---|---|---|
| 1 month average | $195 (−39 to +563) | $114 (8 to +298)* |
| Lifetime3 | $285 (25 to +734)* | $127 (28 to + 302)** |
Source: Authors’ analysis from the following sources 1) U.S. Environmental Protection Agency Aerometric Information Retrieval System and 2) Agency for Healthcare Quality and Research Nationwide Inpatient Sample.
Notes:
Average level within a 10-mile radius of the hospital
Increment change in dollars calculated using Duan transformation for linear regression
Monthly averages from birth month to month of hospitalization
p-value ≤ 0.05
p-value ≤ 0.01
Controlled for age, race (white, black, Hispanic, Asian/Pacific Islander, Native American, or other), gender, income, insurance (Medicare, Medicaid, private, self-pay, no charge, or other), hospital region of the country (Northeast, South, Midwest, or West), teaching status of the hospital, and month of admission.
Gastroenteritis Outcomes
Linear regression models with the outcomes of total charges for gastroenteritis hospitalizations including the same predictors as in the other models did not show a significant association with PM2.5. Although some variables were significant predictors, demonstrating the potential variability in gastroenteritis charges, PM2.5 was not significantly associated with the outcome, contrary to the findings with bronchiolitis hospitalizations.
Discussion
Drawing from a national sample of hospital discharge data, we found a significant association between lifetime PM2.5 levels around hospitals and infant bronchiolitis hospitalization total charges and costs. We interpret these findings to reflect greater healthcare resource consumption due to more severe cases of bronchiolitis. As mentioned above, fine particulate matter could contribute to a kind of smoldering inflammation in the lungs of exposed infants and result in a more severe illness when these infants contract bronchiolitis.
We found no association between monthly PM2.5 averages and gastroenteritis charges thereby supporting the hypothesis that the association seen between air pollution and bronchioltis is not spurious. Also of interest is the lack of statistically significant association seen between monthly PM2.5 averages and LOS. A possible explanation for this last observation might be that the units of LOS are too coarse (i.e. measured in days not hours) to be associated with small changes monthly PM2.5. While LOS contributes to overall charges, other factors such as procedures and intensity of care likely contribute more to the differences in charges between hospitalizations. Unfortunately, variables for assessing types of procedures and intensity of care are limited or not available in the NIS.
Our sample differed, as stated, from the entire sample of infant bronchiolitis hospitalizations because it was primarily bi-coastal urban population which should be considered when generalizing these findings. The race (more Black or Hispanic) and type of hospital (more teaching hospitals) differences of our sample compared to all infant bronchiolitis hospitalizations are thought to be primarily a result of demographics and hospital characteristics within dense urban settings.
A major strength of this study is that our results are based on a large amount of data nationally representative of urban area hospitals. Although these results cannot be translated to non-urban areas, 80% of bronchiolitis hospitalizations do occur in urban areas. (25) Substantial health care dollars are spent each year on bronchiolitis, (2) and better control of PM2.5 levels may result in considerable savings. We found that an increase of one microgram/cubic meter in PM2.5 increased infant bronchiolitis hospitalization costs on average by $127. There are approximately 150,000 infant bronchiolitis hospitalizations in the US annually. Extrapolating our findings to urban infant bronchiolitis hospitalizations (80% of 150,000 = 120,000), reducing the average level of PM2.5 by 1 microgram/cubic meter could save about $15 million annually.
Our study findings are consistent with some studies that point to an association between bronchiolitis severity and particulate matter. The lack of association observed in some of the other studies between PM2.5 and bronchiolitis could reflect a lack of regional variability – all previous studies examined only a single airshed or region - or overall lower particulate matter levels or also variations in the actual toxicity of the specific type of particulate matter in that region, as discussed in Karr et al. (7)
Also in contrast to some other studies discussed in the introduction, we found a lack of association between NO2 and bronchiolitis charges and costs. The lack of association that we found with O3 and bronchiolitis was consistent with the two prior studies where this pollutant was included.(5, 7) These differing results could reflect a true lack of association or they could reflect limitations of the exposure metric. One explanation is that, as shown in previous studies, ambient O3 levels measured at central monitoring sites may not be representative of personal exposure, in contrast to PM2.5 for which ambient levels are highly correlated with personal exposure. (18, 19, 21)
These estimates represent only a small fraction of potential healthcare savings resulting from reduced PM2.5 levels, given that there are a number of other illnesses associated with this pollutant, such as cardiac mortality. (26–28) This study underscores the economic impacts of particulate matter on healthcare resource consumption. Furthermore, the findings indicate that fine particulate matter could have far-reaching children’s environmental health effects but the extent is still unknown based on this preliminary study. Studies such as this one should stimulate further research exploring the role of air pollution in infectious disease severity – to confirm and better understand the mechanism underlying the association of PM2.5 and increased bronchiolitis costs – and incite regulatory agencies such as the EPA to consider these chronic pollutant levels impacts when re-evaluating air pollutant standards. Our results provide economic data to reinforce the need for ongoing efforts to reduce levels of air pollutants in this country.
Supplementary Material
References
- 1.Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics 2010. February;125(2):342–9. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier AJ, Mansbach JM, Camargo CA Jr. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics 2006. December;118(6):2418–23. [DOI] [PubMed] [Google Scholar]
- 3.Ciccone G, Forastiere F, Agabiti N, Biggeri A, Bisanti L, Chellini E, et al. Road traffic and adverse respiratory effects in children. SIDRIA Collaborative Group. Occup Environ Med 1998. November;55(11):771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertz-Picciotto I, Baker RJ, Yap PS, Dostal M, Joad JP, Lipsett M, et al. Early childhood lower respiratory illness and air pollution. Environ Health Perspect 2007. October;115(10):1510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karr C, Lumley T, Schreuder A, Davis R, Larson T, Ritz B, et al. Effects of subchronic and chronic exposure to ambient air pollutants on infant bronchiolitis. Am J Epidemiol 2007. March 1;165(5):553–60. [DOI] [PubMed] [Google Scholar]
- 6.Karr C, Lumley T, Shepherd K, Davis R, Larson T, Ritz B, et al. A case-crossover study of wintertime ambient air pollution and infant bronchiolitis. Environ Health Perspect 2006. February;114(2):277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karr CJ, Demers PA, Koehoorn MW, Lencar CC, Tamburic L, Brauer M. Influence of ambient air pollutant sources on clinical encounters for infant bronchiolitis. Am J Respir Crit Care Med 2009. November 15;180(10):995–1001. [DOI] [PubMed] [Google Scholar]
- 8.Karr CJ, Rudra CB, Miller KA, Gould TR, Larson T, Sathyanarayana S, et al. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ Res 2009. April;109(3):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pino P, Walter T, Oyarzun M, Villegas R, Romieu I. Fine particulate matter and wheezing illnesses in the first year of life. Epidemiology 2004. November;15(6):702–8. [DOI] [PubMed] [Google Scholar]
- 10.Segala C, Fauroux B, Just J, Pascual L, Grimfeld A, Neukirch F. Short-term effect of winter air pollution on respiratory health of asthmatic children in Paris. Eur Respir J 1998. March;11(3):677–85. [PubMed] [Google Scholar]
- 11.Bell ML, Ebisu K, Peng RD, Walker J, Samet JM, Zeger SL, et al. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am J Epidemiol 2008. December 1;168(11):1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US EPA (Environmental Protection Agency). Particulate Matter. 2010. [cited 2011 February 9]; Available from: http://www.epa.gov/pm/
- 13.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006. March 8;295(10):1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AHRQ (Agency for Healthcare Research and Quality). Overview of the Nationwide Inpatient Sample (NIS). 2009. [cited 2010 11 December]; Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 15.US EPA. Aerometric Information Retrieval System (AIRS). 2009. [cited 2010 11 December]; Available from: http://www.epa.gov/enviro/html/airs/. [Google Scholar]
- 16.United States Department of Labor. Bureau of Labor Statistics. Consumer Price Index. [cited 2010 11 December]; Available from: http://data.bls.gov/cgi-bin/surveymost?cu. [Google Scholar]
- 17.AHRQ (Agency for Healthcare Research and Quality). Cost-to-Charge Ratio Files. Healthcare Cost and Utilization Project (HCUP); 2009. [cited 2010 11 December]; Available from: http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. [Google Scholar]
- 18.Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology 2005. May;16(3):385–95. [DOI] [PubMed] [Google Scholar]
- 19.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore, MD. J Air Waste Manag Assoc 2000. July;50(7):1184–98. [DOI] [PubMed] [Google Scholar]
- 20.Duan N Smearing estimate: a nonparametric retransformation method. Journal of the American Statistical Association 1983;78(383):605–10. [Google Scholar]
- 21.Liu LJ, Box M, Kalman D, Kaufman J, Koenig J, Larson T, et al. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ Health Perspect 2003. June;111(7):909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin S, Bell EM, Liu W, Walker RJ, Kim NK, Hwang SA. Ambient ozone concentration and hospital admissions due to childhood respiratory diseases in New York State, 1991–2001. Environ Res 2008. September;108(1):42–7. [DOI] [PubMed] [Google Scholar]
- 23.Trasande L, Lee M, Liu Y, Weitzman M, Savitz D. Incremental charges, costs, and length of stay associated with obesity as a secondary diagnosis among pregnant women. Med Care 2009. October;47(10):1046–52. [DOI] [PubMed] [Google Scholar]
- 24.Trasande L, Liu Y, Fryer G, Weitzman M. Effects of childhood obesity on hospital care and costs, 1999–2005. Health Aff (Millwood) 2009. Jul-August;28(4):w751–60. [DOI] [PubMed] [Google Scholar]
- 25.AHRQ (Agency for Healthcare Research and Quality). HCUP Net. [cited 2010 20 December]; Available from: http://hcupnet.ahrq.gov/HCUPnet.jsp.
- 26.Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, et al. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 2010. May;21(3):414–23. [DOI] [PubMed] [Google Scholar]
- 27.Roy A, Sheffield PE, Wong K, Trasande L. The Effects of Outdoor Air Pollutants on the Costs of Pediatric Asthma Hospitalizations in the United States, 1999–2007. Medical Care 2010(accepted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simkhovich BZ, Kleinman MT, Kloner RA. Particulate air pollution and coronary heart disease. Curr Opin Cardiol 2009. November;24(6):604–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


