Abstract
Electrospun nanofibers have been widely studied for many medical applications. They can be designed with specific features, including mucoadhesive properties. This review summarizes the polymeric scaffolds obtained by the electrospinning process that has been applied for drug release in different mucosal sites such as oral, ocular, gastroenteric, vaginal, and nasal. We analyzed the electrospinning parameters that have to be optimized to create reproducible and efficient mucoadhesive nanofibers, among them are: electrical field, polymer concentration, viscosity, flow rate, needle-collector distance, solution conductivity, solvent, environmental parameters, and electrospinning setup. We also revised the mucoadhesive theories as well as the mucoadhesive properties of the polymers used. This review shows that the most studied mucosal site is the oral cavity, because it is accessible and easy to evaluate, while the rest are uncomfortable for the patient and difficult to assess in vivo. We found problems that need to be solved for mucoadhesive electrospun nanofibers, such as improving adhesion strength and mucosal permanence time, and the design of unidirectional release, multilayer systems for the treatment of several pathologies, to ensure the drug concentration in the tissue or target organ.
Keywords: mucoadhesives, electrospinning, drug delivery systems, polymers
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
In the last decades, pharmaceutical research has focused on the search for new molecules with better therapeutic effects, but also on the development of novel drug delivery systems that increase drug bioavailability, reduce fluctuations in plasma concentration, and improve patients' therapeutic compliance.1
The quantity of drug absorbed could be determined by the time of residence of the drug in the site of absorption. Therefore, there is an increasing need to search for drug delivery systems that can secure enough contact time in the absorption site. This is how the mucoadhesive delivery systems have become one of the most studied systems in recent research.2,3
While the traditional formulations must be ingested or injected, mucoadhesion delivery systems can be easily applied near the affected zone, with better patient acceptance. Most studies have been done on buccal, nasal, ocular, gastrointestinal, and vaginal mucosa.
Electrospun nanofibers have been extensively used in drug delivery systems,4–6 some of these fibers possess mucoadhesive properties,7–10 which can be strategically applied in several mucosal tissues as a controlled delivery system for specific pharmaceutical drugs to treat several pathologies.
Among many of the interesting characteristics of nanofibers are their high encapsulation efficiency and flexible encapsulation capacity. Moreover, the mucoadhesion property is used to temporarily immobilize a delivery device on a specific site, for targeted release and optimal drug delivery due to intimacy and duration of contact.11 On the other hand, fabrication of mucoadhesive nanofibers gives the opportunity to control the drug delivery rate through the degradation of the fibers or the diffusion of drugs from core-shell nanofibers, providing flexibility to position it in any part of the mucosa. The tri-dimensional scaffolds create more surface area and more contact points between the system and mucosa.12
In this review, a summary is presented of the existing literature regarding mucoadhesive systems consisting of nano-microfibers produced by the electrospinning method. There is also an overview of the electrospinning technique and the parameters that affect the process, and the mechanisms proposed for mucoadhesion.
Electrospinning
Electrospinning is a technique used to generate fibers at different scales based on different electromagnetic concepts. Since the 19th century, electrospinning has been widely studied, starting with Rayleigh in 1897 but patented by Formhals in 1934, in the textile industry, where it was used to create a series of continuous cross-linked fibers used for sewing and rope making, among other applications. These fibers were produced by using cellulose acetate, with acetone and monomethyl ether of ethylene glycol as solvents.13–15
For a few decades, electrospinning was not relevant for the research community, until 1957 when Vonnegut and Newbauer worked on a novel device to form highly electrified fibers of about 0.1 mm, using an electrical atomization device. Following them, Drozin and Simon made relevant contributions in the dispersion of liquids and production of thin and low weight fibers. In 1971 a US researcher named Peter K Baumgarten created an electrospinning machine and produced acrylic fibers with diameters between 0.5 and 1.1 µm; after these events, the electrospinning process regained attention, caused by the emergence of nanotechnology. Due to the success, in the past decade, a substantial number of patents have been issued related to the production of nano- and microfibers.13,14,16
Since the fiber producing technique was developed, the number of institutions focusing on this process has increased. A significant number of parameters of this technique have been studied, and not just in the research community, but also in the industrial setting, for example, the eSpin NanoTechnics and The Donaldson Company, which have been using electrospinning for the past two decades, producing scaffolds, nanostructures, and air filtration devices.16–18
Parameters
Electrical field
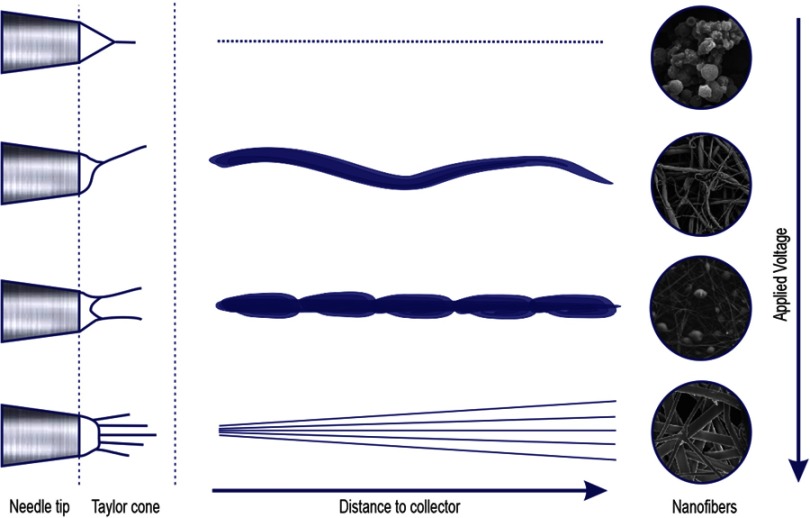
The electrospinning technique has an essential parameter for fiber formation which needs to be considered: this is the current flow generated by a high voltage power supply, forming an electric field between the needle and the collector plate, with the objective of creating a Taylor cone, the Taylor cone forms when the applied voltage brakes the tension of the drop. Without this amount of voltage, the electrospinning process cannot be initiated. According to several authors, an increase in voltage can decrease the fibers' diameters due to the stretching of the polymeric solution. Moreover, it can improve the solvent vaporization.13,18,19
Nevertheless, an excessive raising of voltage flow can lead to spherical deformation among the fibers (beads), this is due to an increase in flow rate and a decrease of Taylor cone shape, becoming asymmetrical (Figure 1). In contrast, a decrease in voltage can result in a different technique known as “Electrospraying”. The most commonly used voltage is between 10 and 20 KV, depending on the polymer properties.13,15,18,19
Figure 1.
The relation between voltage and distance with fiber production.
Polymer concentration and viscosity
In the electrospinning process, polymer solution concentration and viscosity are fundamental parameters to predict fibers' morphology and diameter; this is because the electrospinning process is based on the stretching of a charged unidirectional jet. These two parameters are closely related, and it is known that viscosity depends strongly on the polymer solution concentration. The stretching of the jet in the polymer solution is directly affected by the change of concentration.13,18
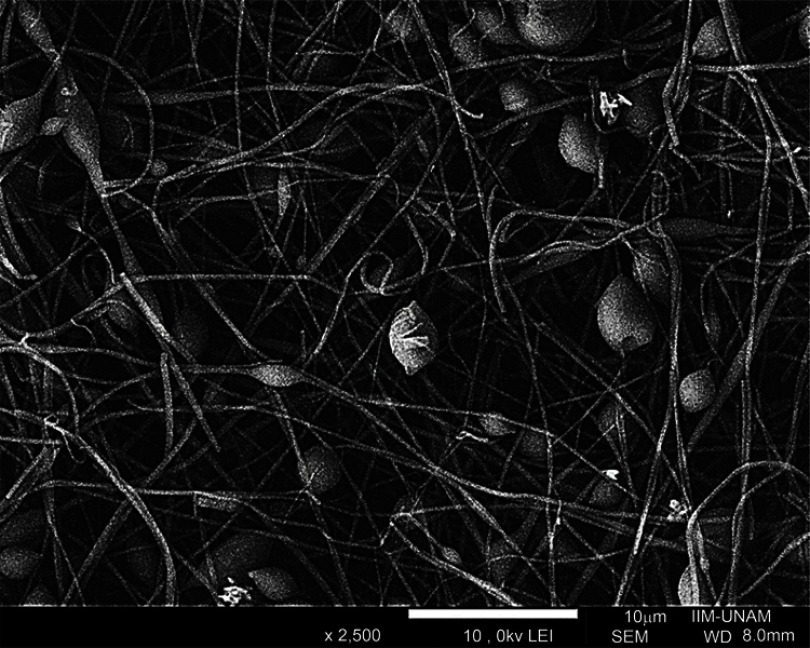
When the solution concentration is too low, the electrical field between the needle and the collector modifies the drop surface tension causing the jet´s partial fragmentation while crossing the space, producing protuberance or nodules, forming the beaded fibers effect (Figure 2). On the other hand, when the solution polymer concentration is too high the viscosity increases, the movement is difficult through the needle, leading to a needle clogging and therefore no fiber formation.13,15,18,20
Figure 2.
Electrospun beaded fibers.
The effect of viscosity and concentration has been studied widely in the past two decades, and it has been concluded that the optimum viscosity value for uniform and appropriate shape fibers' formation is between 100 and 21,500 cp. However, most studies report good fiber production using 100–2,000 cp.13,15,18,20
Flow rate and distance needle collector
The flow rate is a crucial parameter in the electrospinning process because it establishes the polymer solution quantity available in the needle tip to be electrospun, so the Taylor cone can be formed. Modification of these parameters can lead to the production of variations in fiber morphologies.13,15
It is better to use a controlled minimum flow rate from the syringe, which can be adjusted from one polymer to another. Raising of flow rate over a critical value can lead to the formation of undesired structures such as nodules (beaded fibers) or ribbon-like fibers. Nevertheless, increasing flow rate is used to produce porous fiber (Figure 3), or when there is desire to increase the fiber diameter, this is because of the lack of solvent vaporization time during the transition between the needle and the collector. On the other hand, if the flow rate is below the critical value, a plug can be formed inside the needle.18,21
Figure 3.
Electrospun porous fibers.
Previous studies have demonstrated that flow rate and electric field are tightly related to the desired formation of fibers.18,19
The distance between the metallic needle and the collector plate plays a crucial role in the creation of homogeneous fiber, as the viscosity and flow rate. The distance is specific for each polymer solution, and is also related to the correct solvent vaporization before reaching the collector; otherwise, morphological abnormalities can be found, except for some polymers where no difference has been recorded.13,21
If the needle-collector distance is too small, beaded fibers and flat ribbon-like fibers will be formed, owing to the excess humidity coming from the non-vaporized solvent. While the distance between the tip and collector plate is increasing, the fiber diameter is descending; however, when the distance is excessive, the fibers tend to break due to its weight, especially whenever the diameter is too small.13,15,18,22
Solution conductivity and solvent
Most of the polymer solutions are conductive, which is imperative for fiber production. Solution conductivity is determined by the polymer chemicals' characteristics, type of solvent, and the presence of ions. This parameter affects the formation of the Taylor cone and contributes to the fiber diameter since the jet ratio is inversely proportional to the cubic root of the solution's electrical conductivity.13,18,22
Ions raise the electrical charge that flows through the jet applied by the power supply. It has been observed that the use of some salts, such as potassium phosphate monobasic (KH2PO4), sodium phosphate monobasic monohydrate (NaH2PO4), and sodium chloride (NaCl), increases the fiber homogeneity and avoids the formation of beaded fibers.22
For polymer solutions with low conductivity, the drop surface does not possess enough charge to form a Taylor cone. Hence, the electrospinning process will never initiate, while an increase of the solution conductivity helps to start the electrospinning process. However, raising conductivity over a critical value might prevent the formation of Taylor cone and the complete electrospinning process.19,22
The correct selection of solvent is an essential parameter to determine fiber morphology, and a critical key to this selection is the polymer solubility, to get an ideal electrically charged jet and get the polymer molecules to the collector plate. The solvent volatility and solubility are key factors for the electrospinning process due to the contribution in the solvent vaporization time, which reduces bead formation and fiber diameter.15,17,18,22
Enviromental parameters
Besides the solution and electrospinning process parameters, there are other considerations to take into account given the fact that they can affect the process of fiber formation, like humidity and temperature. The effect of these parameters on the electrospinning process and fiber morphology has been studied by Mit-uppatham et al (2004), and they concluded that they have an essential effect on the entire process.22,23
When the temperature is raised, there is a high production of low diameter fibers due to the decline in the polymer solution's viscosity, hence, temperature is inversely proportional to viscosity and at a lower temperature the viscosity rises leading to a slow flow rate and needle obstruction.23
Humidity modifies the fiber diameter by varying the solidification process, its increment stimulates the production of beaded fibers and can generate continuous pores in the fiber surface; while with very low humidity, it has been observed that the solvent volatility increases, leading to faster solvent vaporization, entails obstruction on the needle tip.23–25
Different setup for electrospinning
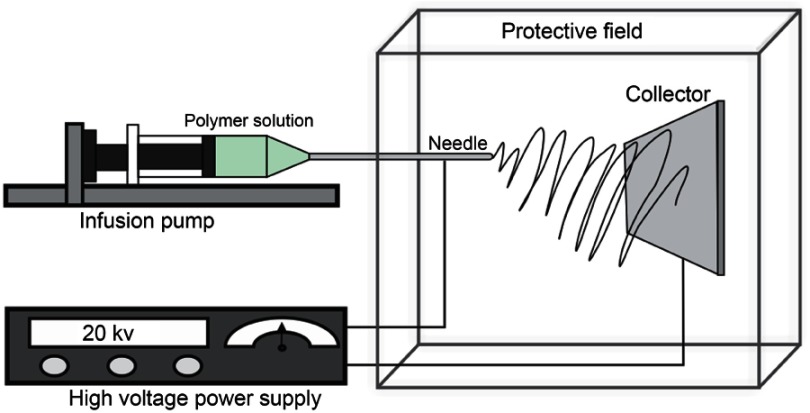
This technique can be performed in two ways: vertical and horizontal position. In the horizontal electrospinning setup, the syringe isplacedparallel to the base and the collector must be placed in avertical position; in this setup, aflow pump must be used to impulse the polymer solution (Figure 4).13,22
Figure 4.
Diagram of the horizontal electrospinning device.
In vertical electrospinning device the syringe is placed in a vertical position over the collector while the collector must be placed on a base horizontally; in this setup, the flow is stimulated by the polymer solution's viscosity and the gravitational forces (Figure 5).22,26
Figure 5.
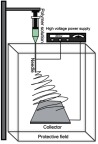
Diagram of the vertical electrospinning device.
Mucoadhesion
Since 1986 the bioadhesion term has been widely explored, being defined as the bonding between a biological or synthetic molecule and epithelial tissue or mucus; this concept has remained intact over the last years.27,28
In molecular terms, it can be organized as follows:
type 1: union between two biological stratums without the intervention of any synthetic material.
Type 2: union between a biological layer and a synthetic substrate.
Type 3: union between a synthetic material and a biological substrate.2,27
Mucoadhesion theories
The union between a mucosal surface is named mucoadhesion. The mucosal layer is covered entirely by mucus, where the most abundant components are mucins.29 Mucins are highly glycosylated glycoproteins with a large peptide core and 8–10 monosaccharides side chains with sialic acid or sulfonic acid ends. Due to this, mucus is charged negatively at human pH. Mucins are known for the formation of extensive mass distribution, for this reason, they are the main responsible for bioadhesion.27,28,30
With the objective of understanding the interaction requirements present between the two layers of mucoadhesion, several theories have been suggested. Unfortunately, these theories can only explain a few interactions of the entire bioadhesion process. These theories are explained as follows:
Wetting theory: in this theory, bioadhesion is expressed as an incrustation process where the bioadhesive polymers penetrate between the mucosal surface irregularities. Here, mucoadhesion is described as total superficial tension from the two phases, less the apparent interfacial tension between these two, according to this, the dispersion coefficient is determined by the difference between the surface energies (Yb + Yt) and the interfacial energy Ybt:
| (1) |
When the contact angle is greater than 0, the polymer bioadhesive does not spread over the mucosal surface, the closer it gets to 0, the mucin humects the polymer inducing the spreading.12,31
Diffusion theory: this theory proposes the penetration of bioadhesive polymeric chains on the mucin chains when a deepness between 0.2 and 0.5 µm a semi-permanent union is produced leading to a cross-linking between these two layers.2,31
The properties involved in this process are molecular weight, cross-linking density, chain flexibility, and expansion capacities of both polymer networks.32
Therefore, the maximum diffusion can be reached when the solubility parameter on both polymer networks is similar, and this can be measured in time units with an FTIR technique.33
This diffusional depth of the polymeric bioadhesive can be represented as:
| (2) |
Where “t” is the contact angle and “D” is the diffusion coefficient.3,31
Electrostatic or electronic theory: this theory describes adhesion through electron transference between the mucosal layer and the polymer mucoadhesive producing a charged double layer due to the formation of attraction forces between them.27
- Absorption theory: the adhesion is defined as a result of several interactions between two surfaces, and it can be divided in:
- Primary: ionic chemicals, covalent and metallic unions, which are not desired because they are permanent.
Fracture theory: the adhesive bonding between the two surfaces is related to the required force to pull them apart, this bonding is stronger when the polymeric network is longer or if the cross-linking grade is reduced. This concept is named “fracture energy” and it is represented as:
| (3) |
Where “E” represents Young´s modulus of elasticity, “ε” is the fracture energy, and “L” the critical crack length if two surfaces are separated.3,27,28,34
Mucoadhesives forces
The interaction between the biological surface and the mucoadhesive polymer solution is the base of the unions produced among them. This interaction determines the time of residence and adhesive force, and they can be classified into two groups.32
Physical and mechanical interactions: these interactions appear when the irregular polymer surface and the mucoadhesive polymer get in contact producing interpenetration between the polymer’s molecules and the cross-linked network of mucins forming semi-permanent bonding. Other factors that are included are the mechanical tension, fluidity, and molecular flexibility of the polymers, as well as bioadhesive viscosity and substrate.
- Chemical interactions: the presence of a primary chemical bond, such as covalent and ionic bond between the biological surface and the polymeric layer, produces very stable attachments. This interaction is of great interest in odontology and orthopedic fields. While the secondary bonds such as hydrogen bonds and Van der Waals forces, according to bioadhesion theories, have more relevance to the mucoadhesion purpose because these bonds have less energy and possess ideal transitory characteristics, which are very important to the bioadhesion process. These molecular interactions are a result of attraction and repulsion forces. For the mucoadhesion phenomenon to take place, the attractive interactions have to be greater than the repulsive attractions.27,32,35
- Van der Waals forces: always present between molecules, even in the neutral ones; these forces play an important part in several kinds of phenomena such as adhesion, superficial tension, adsorption, particles' aggregation, and more. The interactions created by Van der Waals forces decrease rapidly when the distance gets larger between the surfaces.36
- Hydrogen bonds: the multiple formations of this kind of interactions increase the intermolecular forces so that it could lead to precipitation in the polymer solution. In the mucoadhesive polymer, the carboxylic groups in a no-ionizable form are responsible for the formation of these attractions, because of this, the polymer pka and the environment pH are imperative factors for the establishment of correct bioadhesion.27,37
- Disulfide bridging: a disulfide bond is a strong covalent attraction with thiol groups containing cysteines where one sulfhydryl (-SH) group present on the mucins in the mucus layer react with the polymer sulfhydryl group producing an oxidation reaction producing a sulfur-sulfur bridge. Thiomers have the strongest mucoadhesion properties owing to thiol-disulfide and its oxidation reaction.37–39
Absorption pathways for drugs in mucoadhesive systems
The absorption pathway on a mucoadhesive polymer according to literature suggests that the administrated substance can permeate through the mucosal membrane by diverse ways, depending on the chemical nature of the molecule, anatomical and physic-chemical properties of the mucosal layers. Another crucial factor is the mucosal epithelium thickness, lipid percentage, and keratinization grade.30
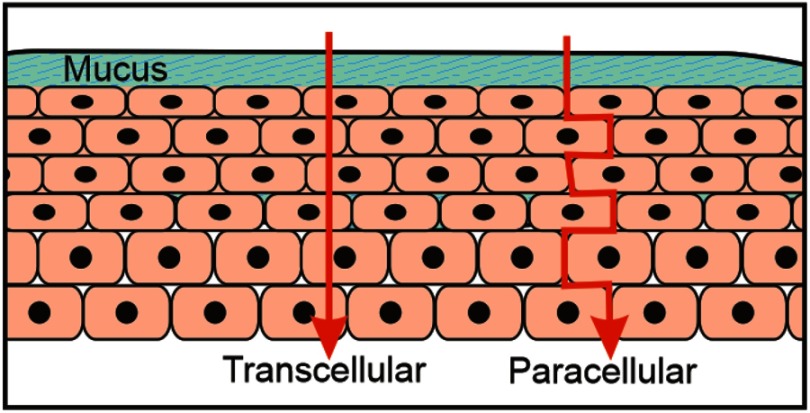
Passive diffusion is the principal route used by molecules to cross through the mucosal membrane, owing to the meager resistance to penetration, this process can happen in two ways: transcellular or paracellular. Lipophilic molecules usually use the transcellular way, while the hydrophilic molecules use the paracellular way which is distinguished by a low quantity of polar intercellular lipids. Although, if a molecule has amphoteric properties it can use both ways at the same time. (Figure 6).40–43
Figure 6.
Schematic representation of transcellular and paracellular pathways.
Mucoadhesive polymers
All mucoadhesive systems' bioadhesive characteristics are due to the physical and chemical properties of the polymer. During the appropriate conditions, interactions with a mucosal surface are established, allowing longer residence time for drug delivery. For this purpose, natural and synthetic polymers have been used, the latter have been designed to achieve optimal results in adhesiveness.31,35,44
According to literature, there is an extensive list of bioadhesive polymers which express different adhesive force, some of them are enlisted in Table 1.
Table 1.
Polymers' adhesive forces
| Polymer | Adhesive force (%) |
|---|---|
| Poly (acrylic acid) | 185.0 |
| Poly (methyl vinyl ether) | 147.7 |
| Methylcellulose | 128.0 |
| Hydroxypropyl methylcellulose | 125.2 |
| Methyl ethyl cellulose | 117.4 |
| Gelatin | 115.8 |
| Pectin | 100.0 |
| Poly (vinyl pyrrolidone) | 97.6 |
| Poly (ethylene glycol) | 96.0 |
| Poly (vinylic alcohol) | 94.8 |
| Poly (hydroxyethyl methacrylate) | 88.4 |
Note: Reproduced with permission from Roy SK, Prabhaka B. Bioadhesive polymeric platforms for transmucosal drug delivery systems - a review. Trop J Pharm Res 2010; 9(1):91–104.45
Several studies have indicated that the molecular weight of bioadhesive polymers has a powerful influence over the adhesive force, since interpenetration and cross-linking of the polymers is favored in low molecular weight molecules.46–49 Similarly, the linear polymers have better interpenetration than branched ones.27,34
The functional groups in the polymers have a substantial effect on the mucoadhesion. The mucins present in the mucus surface establish a stable bonding with the polycationic polymers. In contrast, in an acidic environment these polymers will have less effect, in this case the polyanionic polymers will be the best choice for a mucoadhesive system. The hydrophilic groups capable of establishing more hydrogen bonds are better mucoadhesives. The order for such bonding is amine, hydroxyl, carboxyl, sulfate.27,34,45
The chain flexibility on a mucoadhesive polymer is an imperative parameter for interpenetration and cross-linking. With a higher density, the effective chain length for interpenetration decreases, reducing the adhesive force.45,50
Polyacrylates
These polymers are derivates of acrylic acid. The most commonly used in the past years is poly (acrylic acid), which has demonstrated its mucoadhesive properties, owing to the presence of a significant number of carboxylic acid groups which form hydrogen bonds. However, they are not the only interaction responsible for the adhesiveness, hydrophobic interactions and Van der Waals forces are also involved.51
Nowadays, the most studied of the polyacrylates are polycarbophil (Noveon) and carbomer (Carbopol [CP]) for its mucoadhesion features, since they generate high cross-linking formation and excellent bonding attraction due to its carboxylic acid groups.34,44,51
Cellulose derivatives
These polymers are classified into three groups depending on the applied chemical treatment, they are acetate, esters, and ether celluloses. The majority of applications of these polymers are in the textile, paper, and food industry for acetate cellulose. However, ethyl, methyl, and hydroxypropyl have been widely used as a carrier for drug loading materials. Specifically, hydroxypropyl derivates have shown the best mucoadhesive features for buccal adhesion due to their hydrophilic character which allows them to form an extensive network of hydrogen bonding. Also, sodium carboxymethylcellulose (NaCMC) has been shown to have adequate mucoadhesive features.21,34,44
Chitosan
A cationic polymer, the second-most abundant on earth. This is a linear polysaccharide and has been widely studied in drug delivery for its properties of mucoadhesion owing to the presence of hydroxyl groups (-OH) and amine groups (-NH2) that favor interaction with mucins to form hydrogen bonds that allow a greater time of residence as a mucoadhesive.34,44,52
Another characteristic of this polymer that improves mucoadhesion is the molecular mass and flexibility. When the polymer is interlaced with another polymer or molecule that generates a reaction with its amino groups, it decreases the level of interaction and hence reduces the mucoadhesion features.34,44
Alginates
Anionic polymers. Its performance has been tested as a mucoadhesive for the creation of hydrogen bonds with the interaction between mucin proteins and carboxylic groups. The pharmaceutical industry has widely explored them for their hydrophilic polysaccharide features.34
Pectins
An anionic polysaccharide, which is a common part of the human diet. These polymers are hydrophilic and establish direct contact with mucins using electrostatic repulsion forces. These polymers uncoil, allowing interpenetration, which increases the polymer and mucin entanglement and the formation of hydrogen bonds. In recent research, it has been established that these polymers are an excellent mucoadhesion molecule for gastrointestinal drug delivery.34,44
The new generation polymers (thiomers)
Thiomers are the most studied polymers for mucoadhesion. They are hydrophilic molecules attached with lateral chains containing thiol groups (-SH). The thiolated groups mimic the natural binding mechanism of glycoproteins present on the mucosal layer. Given this fact, groups that form disulfuric bonds with cysteine present on mucins in the mucosal surface demonstrate excellent mucoadhesive properties.31,53
Drug delivery
Mucoadhesive drug delivery systems
Mucoadhesive drug delivery systems have shown some advanced features such as bypassing hepatic first-pass metabolism, enhancing barrier permeability, better accessibility, unidirectional drug release, raising drug biocompatibility, and better patient acceptance.2,54
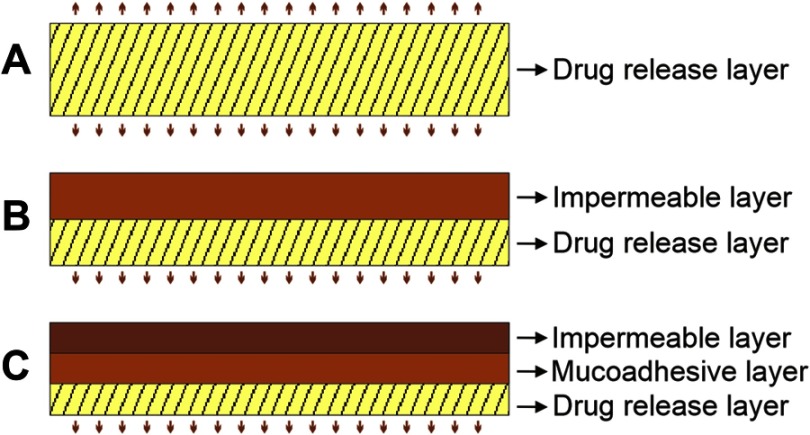
There are several pharmaceutical forms that have been developed, such as matrix tablets, patches, ointments, nanoparticles, and films.2,14,55–72 The electrospun nanofiber scaffold has become one of the most promising among them due to the concurrent delivery of different drugs, elevated loading capacity, user-friendly operation, and low-cost technique.73–79 There are various possible designs for delivery systems that can be formulated depending on the kind of drug to be administered, such as a fast dissolving polymer for a bidirectional drug release system, two-layer drug delivery system, one water resistant polymer and one fast dissolving polymer for a unidirectional drug release, and finally a three-layer mucoadhesive with a bioadhesive polymer core, a fast dissolving drug release layer, and a water-resistant layer (Figure 7).6,54
Figure 7.
Possible designs for mucosal drug delivery systems. (A) Bidirectional delivery system, (B) unidirectional delivery system, (C) mucoadhesive delivery system.
Applications of mucoadhesives nanofibers
Vaginal mucosa
In the case of the vaginal cavity, Huang (2012), reported electrospun cellulose acetate phthalate (CAP) microfibers loaded with anti-HIV drug which presented stability in the vaginal fluid, which has a pH under 4.5, however, CAP microfibers dissolve between a pH of 7.4–8.4, caused by the presence of semen. Hence, the idea of these researchers was to use this electrospun mucoadhesive system to protect women from getting infected with HIV, due to having coitus with infected men. Once the system releasing the anti-HIV drug is applied in the vagina, it is degraded by the presence of semen.20 After that, Blakney et al (2013), proposed that the vaginal mucoadhesive system loaded with an anti-HIV drug can deliver a wide range of agents, incorporating multiple agents via composites, and facilitating controlled release over relevant time frames for pericoital and sustained coitally-independent use. It is also technologically feasible to scale up production of fiber-based microbicides, as these scaffolds showed a residence time ranging from 1 hour in rapid release formulations to 70 days in sustained release ones.80
Also, Hua et al (2016), reported more specialized fibers, which are pH-responsive. These poly (urethane) (core)/CAP (shell) fibers were designed to improve mechanical properties as the tensile strength. Moreover, these fibers are sensitive to the presence of semen, and release rhodamine B for the treatment of HIV.81
Besides, the anti-HIV vaginal mucoadhesive systems have been prepared with poly (vinyl alcohol) (PVA) loaded with fluconazole, and it was demonstrated that these fibers release the drug in a sustained manner over a period of 6 hours. These mucoadhesives were tested against Candida albicans and showed a superior antimicrobial activity compared with the pure drug.82
Vaginal mucoadhesives can also be used against cervical cancer, electrospun poly (ethylene oxide)/poly (lactide) composite nanofibers loaded with cisplatin83 showed a residence time of 72 hours longer than gel.83 The studies by Aggarwal et al, demonstrated an improvement of mucoadhesion on cisplatin loaded poly(caprolactone) (PCL)/CS scaffold, for local treatment of cervical cancer84 (500 N/m2 mucoadhesive strength) compared to blank matrices (200 N/m2).84
Buccal mucosa
Several studies have used mucoadhesive electrospun fibers for administration in the oral cavity.64,85–89 These mucoadhesives have been proposed for the delivery of drugs with poor absorption due to its limited solubility. In vitro and in vivo studies have demonstrated electrospun nanofiber superiority in release rate, compared to standard administration.90
Morales and McConville91 reported that mucoadhesives can be fabricated with a retaining dosage characteristic and can deliver the drug directly into a biological substrate. These specific mucoadhesives were prepared to obtain small size and reduced thickness, compared to standard tablets. Mucoadhesives involve the casting of aqueous solutions and organic solvents, they can also be prepared by hot-melt extrusion and by the electrospinning method.91
Grewal et al (2012)92 developed a transmucosal mucoadhesive composed of PCL nanofibers loaded with diclofenac sodium for analgesic and anti-inflammatory purposes. These fibers were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and in vitro release using a Franz diffusion cell. It was proven that these fibers improved therapeutic efficacy compared to a standard method of administration.92
A nanofibrous matrix system composed of PVA in different concentrations was prepared for rapid oral mucosal drug release; separately, a hydroxypropyl methylcellulose (HPMC) and PVA with a variety of glycerol amounts backing film were synthesized which presented high surface area to volume ratio, with improved dissolution rate for fast delivery in the oral mucosa. This system exhibited an adequate detachment force and good work of adhesion (WA). The nanofibrous matrix was observed to be more mucoadhesive than a film.10 Tyagi et al prepared mucoadhesive PVA nanofibers associated with PVA/HPMC backing layer, in this research the mucoadhesive fibers were loaded with diphenhydramine, which showed an average disintegration time of 7–60 seconds, the system presented 42%–82% drug permeation in the oral mucosal cavity. In addition, it demonstrated an increase in WA with an increase in glycerol concentration in formulations containing 0.5% (w/v) HPMC.93
In another case, an in situ biodegradable implant for the local release of metronidazole in the periodontal pockets was formulated. These mucoadhesives included poly (lactic acid-co-glycolic acid) (PLGA) nanofibers loaded with the drug. These systems were applied to the in itu implants to alter the properties of the delivery complex toward a longer dwelling time in the oral cavity. The polymer also improvedthe adhesiveness and increased viscosity, achieving 10 days' sustained release.94
A fast releasing, oral electrospun poly (vinyl pyrrolidone) (PVP) and cyclodextrin (CD) nanofibers with taste-masked meloxicam have been reported. In this case, CD was used to enhance the stability of the fibers. This mucoadhesive was characterized using SEM, physical, and mechanical properties. In this study, nanofibers were tested in healthy human volunteers. These mats were demonstrated to have adequate tensile strength, fibers presented a homogeneous shape without any beads, and fibers were physically stable without any hygroscopic issue for approximately 6 months. Mats disintegrated fast in the mouth.95
Also, Illangakoon et al loaded paracetamol and caffeine in mucoadhesive scaffolds by electrospinning. These fibers were proposed for oral administration, with a thickness between 120–130 nm folding the membranes around 20 times. This study claimed that a flavoring agent can be easily incorporated into the formulation, and the membrane dissolved completely within 0.5 seconds in an artificial saliva solution. Because of that, the research group proposed that these mucoadhesives can be used particularly for children and patients with swallowing difficulties.96 Moreover, docetaxel was incorporated into PVA nanofibers for local transmucosal delivery with promising results.97
Finally, Tonglairoum et al (2015), fabricated a scaffold with clotrimazole microemulsion-containing nanofibers made by the electrospinning technique for the treatment of candidiasis, this microemulsion is composed of oleic acid, Tween 80, and the surfactant benzyl alcohol, ethyl alcohol, and isopropyl alcohol. The mucoadhesives were prepared with PVA and CS. It demonstrated an extended drug release of approximately 4 hours, delivering about 64.81%–74.15% of the drug.88
Gastroenteric mucosa
Few studies have reported the capability of mucoadhesive nanofibers as a drug delivery system in the gastroenteric tract. In some cases, conventional oral strategies present low bioavailability due to the incomplete release of drug and short retaining time at the absorption zone. It is claimed that nanofibers increase the bioavailability of the drug in the gastroenteric site. Hence, nanofibers provide a stomach-specific drug release for a longer time and increase the local action due to prolonged contact time with the gastric mucosa.63,89
For example, Brako et al (2018)98 prepared a mucoadhesive of carboxymethyl cellulose (CMC) fibers in various concentrations of the polymer, loaded with progesterone. These mats showed about 10 times better adhesion with an artificial cellulose acetate membrane compared to that of lamb esophageal mucosa, demonstrating that CMC affects the roughness of the fibers and enhances interpenetration, improving its mucoadhesion.98
Malik et al (2016)63 also proposed a mucoadhesive prepared with poly (L-lactic acid) nanofibers loaded with diacerein. The objective of this research was to describe the ability of nanofibers as a gastro-retentive dosage form and the capacity to improve the solubility of diacerein. These nanofibers were smooth, discrete, and non-woven and demonstrated a 61.3% drug release in about 30 hours.63
Additionally, Moreno et al (2011) showed a sustained release of lactate dehydrogenase via electrospun PVA nanofibers prepared by a coaxial electrospinning technique. The encapsulated enzyme was detected by FTIR and X-ray photoelectron spectroscopy. The study showed that most of the encapsulated protein was released in a sustained manner in a period of a month.99
Ocular mucosa
Mucosa samples are difficult to access, so not many reports are available in the literature reporting mucoadhesives as drug delivery systems in the ocular mucosa. However, Garg et al (2014) presented polymeric nanofiber patches for the treatment of glaucoma. The drugs used for this approach were dorzolamide hydrochloride and timolol maleate. Final formulations used in glaucoma induced in rabbits obtained satisfactory results showing an important reduction in the intraocular pressure compared to commercial eye drops.100
Finally, mucoadhesives can be used in tissue engineering of the retina by releasing a functional retinal pigment epithelium from nanofibers. This study showed that these kinds of membranes lead to better cell proliferation and were proposed to be a marketable ocular implant.101
Nasal mucosa
The nasal site is easily accessed but uncomfortable for the patient. Due to this, there are few studies on the subject. Lee et al (2017) developed a sinonasal mucoadhesive delivery system with electrospun nanostructured carrier microparticles loaded with resveratrol. It was proven that the electrospun nanostructure had an improved in vivo residence time on site of action, as well as improved local bioavailability.102
Future perspective and challenges
Studies of mucoadhesive electrospun nanofibers should focus on areas with limited studies, such as drug release at the nasal and ocular mucosa. These areas have a wide variety of conditions that could be treated using these administration routes. For example, macular edema treated with steroids,103 and nasal vestibulitis treated with a topical antibiotic (mupirocin) for several days,104 to mention some.
Nasal mucoadhesive electrospun fibers could be useful for the delivery of biological drugs such as proteins and peptides, as well as for DNA and RNA therapies, considering the high vasculature of the nasal cavities.
Another option is to apply these fibers in the vaginal mucosa. Bacterial vaginosis is the most common vaginal infection in women, associated with the imbalance of the vaginal flora treated with antibiotics for 5–7 days,105 where a mucoadhesive prolonged release nanofiber would be convenient.
The controlled delivery of antifungal, antibiotic, and anti-inflammatory drugs to treat infections in the buccal cavity, such as periodontitis and candidiasis, could highly benefit from bioadhesive drug loaded electrospun fibers.
Challenges in the field of mucoadhesive electrospun nanofibers include the development of innovative drug release systems improving adhesion and residence time, as well as the discovery and utilization of one-way release multilayer systems in order to ensure the drug concentration “in situ”.
Conclusion
This work discusses several studies that show evidence of the potential role of mucoadhesive nanofiber scaffolds as drug delivery systems in mucosal tissue, to improve the bioavailability of some drugs “in situ”. There is still much research to be done in order to advance in this innovative field.
Hence, despite all the clear advantages of nanofiber mucoadhesives, such as prolongation of residence time at the absorption site and controlled drug release,2,3 these strategies still present some challenges for researchers committed to this area. Some of these disadvantages come from the electrospinning technique. For example, a commercial electrospinning device is expensive for a university to purchase, thus, researchers working in these institutions regularly build an in-house-made device.106 Although these devices can produce nanofibers of excellent quality, a specific quantity of drug cannot be loaded into a specific area. Drug loading in fibers is not easily reproducible, because of the non-controllable environmental parameters such as altitude, pressure, humidity, and temperature of the place where nanofibers are fabricated.5
Another disadvantage is the high cost of polymers and solvents used in the method, even if the technique is easy, fast, and versatile, US Food and Drug Administration approved polymers have become expensive. Nevertheless PVA, PCL, and PVP are available at affordable prices. In this manner, a limited set of polymers have been reported for nanofiber mucoadhesive scaffolds, Hu et al (2014) reported some electrospun polymeric nanofibers for drug delivery systems, among them were: PLA, PLGA, PEVA, PCL, PVP, PVA, poly (ethylene oxide) (PEO), and poly (ethylene glycol) (PEG)107. All these polymers must present good spreadability, wetting, swelling, solubility, biocompatibility, biodegradability, adequate pH, viscoelasticity, sufficient mechanical strength, bioactive surface, tensile strength, shear strength, and bioadhesiveness.27
Several polymers have been proposed for mucoadhesive technology, for the oral administration of different drugs, but they are not prepared using the electrospinning technique. For example, we can enlist: CMC, CP, ethylcellulose, hyaluronic acid, hydroxyethylcellulose, hydroxypropylcellulose, HPMC, hydroxypropyl pea starch polymer, poly (methacrylic acid), methylcellulose, maltotriose polysaccharide, NaCMC, poly (methacrylic acid-co- methylmethacrylate) sodium salt, poloxamer 407, PEG, poly (ethylene glycol-dimethacrylate), PEO, PLGA, PVA, PVP, and trimethyl-chitosan, among others.44
Finally, many studies have prepared mucoadhesive nanofibers for drug administration in buccal cavity, but a limited number of reports are available in the literature for mucoadhesive nanofibers as a drug delivery system in the gastroenteric tract and nasal and ocular mucosa, due to lack of in vitro models to predict in vivo performance.
Acknowledgments
The authors thank Dr Ricardo Vera Graziano, Instituto de Investigaciones en Materiales, Universidad Autónoma de Baja California, Ciudad de México, for help in acquiring SEM micrographies (Figures 2 and 3). This work was supported by “Consejo Nacional de Ciencia y Tecnología (CONACYT)” grant known as “Fondo de Cooperación Internacional en Ciencia y Tecnología del Conacyt (FONCICYT)” and grant named “Convocatoria Conjunta de Movilidad 2015 CONACYT-DST México-India” with CONACYT project number 266380 and SICASPI-UABC number 351/375/E. The work was also supported by the 20th Internal Call for Research Projects UABC and Grant for the Strengthening of Academic Bodies SEP-PRODES. The authors thank Mrs Yadira Sepulveda for proofreading the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torres-Martinez EJ, Cornejo-Bravo JM, Serrano-Medina A, Perez-Gonzalez GL, Villarreal-Gómez LJ. A summary of electrospun nanofibers as drug delivery system: drugs loaded and biopolymers used as matrices. Curr Drug Deliv. 2018;15(10):1360–1374. doi: 10.2174/1567201815666180723114326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villarreal-Gómez LJ, Cornejo-Bravo JM, Vera-Graziano R, Grande D. Electrospinning as a powerful technique for biomedical applications: a critically selected survey. J Biomater Sci Polym Ed. 2016;27(2):157–176. doi: 10.1080/09205063.2015.1116885 [DOI] [PubMed] [Google Scholar]
- 3.Son YJ, Kim WJ, Yoo HS. Therapeutic applications of electrospun nanofibers for drug delivery systems. Arch Pharm Res. 2014;37(1):69–78. doi: 10.1007/s12272-013-0284-2 [DOI] [PubMed] [Google Scholar]
- 4.Mendes AC, Sevilla Moreno J, Hanif M, Douglas T EL, Chen M, Chronakis IS. Morphological, mechanical and mucoadhesive properties of electrospun chitosan/phospholipid hybrid nanofibers. Int J Mol Sci. 2018;19(8):2266. doi: 10.3390/ijms19082266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behbood L, Karimi S, Mirzaei E, Mohammadi G, Azami M, Arkan E. Mucoadhesive chitosan electrospun nanofibers containing tetracycline and triamcinolone as a drug delivery system. Fibers Polym. 2018;19(7):1454–1462. doi: 10.1007/s12221-018-8087-1 [DOI] [Google Scholar]
- 6.Brako F, Raimi-Abraham B, Mahalingam S, Craig DQM, Edirisinghe M. Making nanofibres of mucoadhesive polymer blends for vaginal therapies. Eur Polym J. 2015;70:186–196. doi: 10.1016/j.eurpolymj.2015.07.006 [DOI] [Google Scholar]
- 7.Dott C, Tyagi C, Tomar LK, et al. A mucoadhesive electrospun nanofibrous matrix for rapid oramucosal drug delivery. J Nanomater. 2013:2013. doi: 10.1155/2013/924947. [DOI] [Google Scholar]
- 8.Mackie AR, Goycoolea FM, Menchicchi B, et al. Innovative methods and applications in mucoadhesion research. Macromol Biosci. 2017;17(8):1–32. doi: 10.1002/mabi.201600534 [DOI] [PubMed] [Google Scholar]
- 9.Weng L, Xie J. Smart electrospun nanofibers for controlled drug release: recent advances and new perspectives. Curr Pharm Des. 2015;21(15):1944–1959. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25732665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieth M, Siegel MG, Higgs RE, et al. Characteristic physical properties and structural fragments of marketed oral drugs. J Med Chem. 2004;47(1):224–232. doi: 10.1021/jm030267j [DOI] [PubMed] [Google Scholar]
- 11.Shaikh R, Raj Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3(1):89–100. doi: 10.4103/0975-7406.76478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boddupalli B, Mohammed Z, Nath R, Banji D. Mucoadhesive drug delivery system: an overview. J Adv Pharm Technol Res. 2010;1(4):381. doi: 10.4103/0110-5558.76436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–347. doi: 10.1016/j.biotechadv.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Strandman S, hu JXX, Barralet J, Cerruti M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials. 2015;37:395–404. doi: 10.1016/j.biomaterials.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 15.Valizadeh A, Mussa Farkhani S. Electrospinning and electrospun nanofibres. IET Nanobiotechnol. 2014;8(2):83–92. doi: 10.1049/iet-nbt.2012.0040 [DOI] [PubMed] [Google Scholar]
- 16.Khalf A, Madihally SV. Recent advances in multiaxial electrospinning for drug delivery. Eur J Pharm Biopharm. 2017;112:1–17. doi: 10.1016/j.ejpb.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Ahmed FE, Lalia BS, Hashaikeh R. A review on electrospinning for membrane fabrication: challenges and applications. Desalination. 2015;356:15–30. doi: 10.1016/j.desal.2014.09.033 [DOI] [Google Scholar]
- 18.Haider A, Haider S, Kang IK. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem. 2015. doi: 10.1016/j.arabjc.2015.11.015 [DOI] [Google Scholar]
- 19.Garg K, Bowlin GL. Electrospinning jets and nanofibrous structures. Biomicrofluidics. 2011;5(1):1–19. doi: 10.1063/1.3567097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–2253. doi: 10.1016/S0266-3538(03)00178-7 [DOI] [Google Scholar]
- 21.Thakkar S, Misra M. Electrospun polymeric nanofibers: new horizons in drug delivery. Eur J Pharm Sci. 2017;107(July):148–167. doi: 10.1016/j.ejps.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Duque Sánchez LM, Rodriguez L, López M. Electrospinning: the nanofibers age. Rev Iberoam Polímeros Vol Iber Polímeros. 2014;14(141):10–27. Available from: http://www.ehu.eus/reviberpol/pdf/ENE13/duque.pdf [Google Scholar]
- 23.Mit-Uppatham C, Nithitanakul M, Supaphol P. Ultratine electrospun polyamide-6 fibers: effect of solution conditions on morphology and average fiber diameter RID C-4353-2008. Macromol Chem Phys. 2004;205(17):2327–2338. doi: 10.1002/macp.200400225 [DOI] [Google Scholar]
- 24.Casper CL, Stephens JS, Tassi NG, Chase DB, Rabolt JF. Controlling surface morphology of electrospun polystyrene fibers: effect of humidity and molecular weight in the electrospinning process. Macromolecules. 2004;37(2):573–578. doi: 10.1021/ma0351975 [DOI] [Google Scholar]
- 25.Shahabadi SMS, Kheradmand A, Montazeri V, Ziaee H. Effects of process and ambient parameters on diameter and morphology of electrospun polyacrylonitrile nanofibers. Polym Sci Ser A. 2015;57(2):155–167. doi: 10.1134/S0965545X15020157 [DOI] [Google Scholar]
- 26.Rodoplu D, Mutlu M. Effects of electrospinning setup and process parameters on nanofiber morphology intended for the modification of quartz crystal microbalance surfaces. J Eng Fiber Fabr. 2012;7(2):118–123. [Google Scholar]
- 27.Shinkar DM, Dhake AS, Setty CM. Drug delivery from the oral cavity: a focus on mucoadhesive buccal drug delivery systems. PDA J Pharm Sci Technol. 2012;66(5):466–500. doi: 10.5731/pdajpst.2012.00877 [DOI] [PubMed] [Google Scholar]
- 28.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57(11):1666–1691. doi: 10.1016/j.addr.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Rubin BK, Voynow JA. Mucins, mucus, and goblet cells. Chest. 2017. doi: 10.1016/j.chest.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 30.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery - A promising option for orally less efficient drugs. J Control Release. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 31.Carvalho F, Bruschi ML, Evangelista RC, Gremiao MPD. Mucoadhesive drug delivery systems. Drug Dev Ind Pharm. 1997;23(5):489–515. doi: 10.4103/0975-7406.76478 [DOI] [Google Scholar]
- 32.Singh R, Sharma D, Garg R. Review on mucoadhesive drug delivery system with special emphasis on buccal route: an important tool in designing of novel controlled drug delivery system for the effective delivery of pharmaceuticals. J Dev Drugs. 2017;06(01):1–12. doi: 10.4172/2329-6631.1000169 [DOI] [Google Scholar]
- 33.Fieldson G, Barbari TA. The Use of FTIR-ATR Spectroscopy to Characterize Diffusion in Polymers. Vol. 34 1993. Oxford, UK: Butterworth-Heinemann Ltd. doi: 10.1016/0032-3861(93)90765-3 [DOI] [Google Scholar]
- 34.Mansuri S, Kesharwani P, Jain K, Tekade RK, Jain NK. Mucoadhesion: A promising approach in drug delivery system. React Funct Polym. 2016;100:151–172. doi: 10.1016/j.reactfunctpolym.2016.01.011 [DOI] [Google Scholar]
- 35.Rodriguez I, Cerezo A, Salem II. Sistemas de liberación bioadhesivos bioadhesive delivery systems. Ars Pharm. 2000;1(41):115–128. Available from: http://150.214.24.132/ars/pdf/186.pdf [Google Scholar]
- 36.Mahajan S, Kaur A, Aggarwal G, Harikumar SL. Mucoadhesive drug delivery system: a review. Int J Drug Dev Res. 2013;5(1):11–20. Available from: http://www.ijddr.in/drug-development/mucoadhesive-drug-delivery-system-a-review.php?aid=5005 [Google Scholar]
- 37.Schattling P, Taipaleenmäki E, Zhang Y, Städler B. A polymer chemistry point of view on mucoadhesion and mucopenetration. Macromol Biosci. 2017;1700060:1–20. doi: 10.1002/mabi.201700060 [DOI] [PubMed] [Google Scholar]
- 38.Harding SE. Mucoadhesive interactions. Biochem Soc Trans. 2003;31(5):1036–1041. doi: 10.1042/BST0311036 [DOI] [PubMed] [Google Scholar]
- 39.Quintanar-Guerrero D, Villalobos-García R, Alvarez-Colín E, Cornejo-Bravo JM. In vitro evaluation of the bioadhesive properties of hydrophobic polybasic gels containing N,N-dimethylaminoethyl methacrylate-co-methyl methacrylate. Biomaterials. 2001;22(9):957–961. doi: 10.1016/S0142-9612(00)00260-X [DOI] [PubMed] [Google Scholar]
- 40.Campisi G, Paderni C, Saccone R, Di Fede O, Wolff A, Giannola LI. Human buccal mucosa as an innovative site of drug delivery. Curr Pharm Des. 2010;16(6):641–652. doi: 10.2174/138161210790883778 [DOI] [PubMed] [Google Scholar]
- 41.Escobar-Chavez JJ, Merino-Sanjuan V, Lopez-Cervantes M, et al. The tape-stripping technique as a method for drug quantification in skin. J Pharm Pharm Sci. 2008;11(1):104–130. doi: 10.1016/j.ijpharm.2004.05.016 [DOI] [PubMed] [Google Scholar]
- 42.Hao J, Heng PWS. Buccal delivery systems. Drug Dev Ind Pharm. 2003;29(8):821–832. doi: 10.1081/DDC-120024178 [DOI] [PubMed] [Google Scholar]
- 43.Smart JD. Buccal drug delivery. Expert Opin Drug Deliv. 2005;2(3):507–517. doi: 10.1517/17425247.2.3.507 [DOI] [PubMed] [Google Scholar]
- 44.Russo E, Selmin F, Baldassari S, et al. A focus on mucoadhesive polymers and their application in buccal dosage forms. J Drug Deliv Sci Technol. 2016;32:113–125. doi: 10.1016/j.jddst.2015.06.016 [DOI] [Google Scholar]
- 45.Roy SK, Prabhakar B. Bioadhesive polymeric platforms for transmucosal drug delivery systems - A review. Trop J Pharm Res. 2010;9(1):91–104. doi: 10.4314/tjpr.v9i1.52043 [DOI] [Google Scholar]
- 46.Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57(11):1556–1568. doi: 10.1016/j.addr.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 47.Koh LD, Cheng Y, Teng CP, et al. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci. 2015;46:86–110. doi: 10.1016/j.progpolymsci.2015.02.001 [DOI] [Google Scholar]
- 48.Teodorescu M, Bercea M, Morariu S. Biomaterials of poly(vinyl alcohol) and natural polymers. Polym Rev. 2018;58(2):247–287. doi: 10.1080/15583724.2017.1403928 [DOI] [Google Scholar]
- 49.Ye M, Jiang R, Zhao J, Zhang J, Yuan X, Yuan X. In situ formation of adhesive hydrogels based on PL with laterally grafted catechol groups and their bonding efficacy to wet organic substrates. J Mater Sci Mater Med. 2015;26(12):1–13. doi: 10.1007/s10856-015-5608-y [DOI] [PubMed] [Google Scholar]
- 50.Peppas NA, Huang Y. Nanoscale technology of mucoadhesive interactions. Adv Drug Deliv Rev. 2004;56(11):1675–1687. doi: 10.1016/j.addr.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 51.Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res. 2011;2(4):215. doi: 10.4103/2231-4040.90876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garg T, Goyal AK. Biomaterial-based scaffolds–current status and future directions. Expert Opin Drug Deliv. 2014;11(5):767–789. doi: 10.1517/17425247.2014.891014 [DOI] [PubMed] [Google Scholar]
- 53.Duggan S, Cummins W, O’ Donovan O, Hughes H, Owens E. Thiolated polymers as mucoadhesive drug delivery systems. Eur J Pharm Sci. 2017;100:64–78. doi: 10.1016/j.ejps.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 54.Rossi S, Sandri G, Caramella CM. Buccal drug delivery: a challenge already won? Drug Discov Today Technol. 2005;2(1):59–65. doi: 10.1016/j.ddtec.2005.05.018 [DOI] [PubMed] [Google Scholar]
- 55.Khoshnevisan K, Daneshpour M, Barkhi M, Gholami M, Samadian H, Maleki H. The promising potentials of capped gold nanoparticles for drug delivery systems. J Drug Target. 2018;26(7):525–532. doi: 10.1080/1061186X.2017.1387790 [DOI] [PubMed] [Google Scholar]
- 56.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2012;64(SUPPL):163–174. doi: 10.1016/j.addr.2012.09.028 [DOI] [PubMed] [Google Scholar]
- 57.Pundir S, Badola A, Sharma D. Sustained release matrix technology and recent advance in matrix drug delivery system: a review. Int J Drug Res Tech. 2013;3(1):12–20. ISSN 2277-1506. [Google Scholar]
- 58.Montenegro-Nicolini M, Morales JO. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS Pharm Sci Tech. 2017;18(1):3–14. doi: 10.1208/s12249-016-0525-z [DOI] [PubMed] [Google Scholar]
- 59.Dolci LS, Liguori A, Panzavolta S, et al. Non-equilibrium atmospheric pressure plasma as innovative method to crosslink and enhance mucoadhesion of econazole-loaded gelatin films for buccal drug delivery. Colloids Surf B Biointerfaces. 2018;163:73–82. doi: 10.1016/j.colsurfb.2017.12.030 [DOI] [PubMed] [Google Scholar]
- 60.Sheikhpour M, Barani L, Kasaeian A. Biomimetics in drug delivery systems: a critical review. J Control Release. 2017;253:97–109. doi: 10.1016/j.jconrel.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 61.Schneider C, Langer R, Loveday D, Hair D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J Control Release. 2017;262(July):284–295. doi: 10.1016/j.jconrel.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 62.Badshah M, Ullah H, Khan SA, Park JK, Khan T. Preparation, characterization and in-vitro evaluation of bacterial cellulose matrices for oral drug delivery. Cellulose. 2017;24(11):5041–5052. doi: 10.1007/s10570-017-1474-8 [DOI] [Google Scholar]
- 63.Malik R, Garg T, Goyal AK, Rath G. Diacerein-Loaded novel gastroretentive nanofiber system using PLLA: development and in vitro characterization. Artif Cells Nanomed Biotechnol. 2016;44(3):928–936. doi: 10.3109/21691401.2014.1000492 [DOI] [PubMed] [Google Scholar]
- 64.Nguyen S, Hiorth M. Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv. 2015;6(5):197–210. doi: 10.4155/tde.15.5 [DOI] [PubMed] [Google Scholar]
- 65.Silva NHCS, Rodrigues AF, Almeida IF, et al. Bacterial cellulose membranes as transdermal delivery systems for diclofenac: in vitro dissolution and permeation studies. Carbohydr Polym. 2014;106(1):264–269. doi: 10.1016/j.carbpol.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 66.Mura P, Cirri M, Mennini N, Casella G, Maestrelli F. Polymeric mucoadhesive tablets for topical or systemic buccal delivery of clonazepam: effect of cyclodextrin complexation. Carbohydr Polym. 2016;152:755–763. doi: 10.1016/j.carbpol.2016.07.075 [DOI] [PubMed] [Google Scholar]
- 67.Rudzinski WE, Palacios A, Ahmed A, Lane MA, Aminabhavi TM. Targeted delivery of small interfering RNA to colon cancer cells using chitosan and PEGylated chitosan nanoparticles. Carbohydr Polym. 2016;147:323–332. doi: 10.1016/j.carbpol.2016.04.041 [DOI] [PubMed] [Google Scholar]
- 68.Geetha P, Sivaram AJ, Jayakumar R, Mohan CG. Integration of in silico modeling, prediction by binding energy and experimental approach to study the amorphous chitin nanocarriers for cancer drug delivery. Carbohydr Polym. 2016;142:240–249. doi: 10.1016/j.bcmd.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 69.Pan Q, Lv Y, Williams GR, et al. Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohydr Polym. 2016;151:812–820. doi: 10.1016/j.carbpol.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 70.Ghorbani M, Bigdeli B, Jalili-Baleh L, et al. Curcumin-lipoic acid conjugate as a promising anticancer agent on the surface of gold‑iron oxide nanocomposites: a pH-sensitive targeted drug delivery system for brain cancer theranostics. Eur J Pharm Sci. 2018;114:175–188. doi: 10.1016/j.ejps.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 71.Modaresi SMS, Mehr SE, Faramarzi MA, et al. Preparation and characterization of self-assembled chitosan nanoparticles for the sustained delivery of streptokinase: an in vivo study. Pharm Dev Technol. 2014;19(5):593–597. doi: 10.3109/10837450.2013.813542 [DOI] [PubMed] [Google Scholar]
- 72.Baharifar H, Amani A. Cytotoxicity of Chitosan/Streptokinase Nanoparticles as a Function of Size: An Artificial Neural Networks Study. Vol. 12 Elsevier Inc.;2016. Amsterdam, Netherlands. doi: 10.1016/j.nano.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 73.Unnithan AR, Gnanasekaran G, Sathishkumar Y, Lee YS, Kim CS. Electrospun antibacterial polyurethane-cellulose acetate-zein composite mats for wound dressing. Carbohydr Polym. 2014;102(1):884–892. doi: 10.1016/j.carbpol.2013.10.070 [DOI] [PubMed] [Google Scholar]
- 74.Rogina A. Electrospinning process: versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl Surf Sci. 2014;296:221–230. doi: 10.1016/j.apsusc.2014.01.098 [DOI] [Google Scholar]
- 75.Sedghi R, Shaabani A, Mohammadi Z, Samadi FY, Isaei E. Biocompatible electrospinning chitosan nanofibers: a novel delivery system with superior local cancer therapy. Carbohydr Polym. 2017;159:1–10. doi: 10.1016/j.carbpol.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 76.Ghorbani FM, Kaffashi B, Shokrollahi P, Seyedjafari E, Ardeshirylajimi A. PCL/chitosan/Zn-doped nHA electrospun nanocomposite scaffold promotes adipose derived stem cells adhesion and proliferation. Carbohydr Polym. 2015;118:133–142. doi: 10.1016/j.carbpol.2014.10.071 [DOI] [PubMed] [Google Scholar]
- 77.Ghorani B, Tucker N. Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll. 2015;51:227–240. doi: 10.1016/j.foodhyd.2015.05.024 [DOI] [Google Scholar]
- 78.Antunes BP, Moreira AF, Gaspar VM, Correia IJ. Chitosan/arginine-chitosan polymer blends for assembly of nanofibrous membranes for wound regeneration. Carbohydr Polym. 2015;130:104–112. doi: 10.1016/j.carbpol.2015.04.072 [DOI] [PubMed] [Google Scholar]
- 79.Khoshnevisan K, Maleki H, Samadian H, et al. Cellulose acetate electrospun nanofibers for drug delivery systems: applications and recent advances. Carbohydr Polym. 2018;198:131–141. doi: 10.1016/j.carbpol.2018.06.072 [DOI] [PubMed] [Google Scholar]
- 80.Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 2013;100(SUPPL):S9–S16. doi: 10.1016/j.antiviral.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 81.Hua D, Liu Z, Wang F, et al. pH responsive polyurethane (core) and cellulose acetate phthalate (shell) electrospun fibers for intravaginal drug delivery. Carbohydr Polym. 2016;151:1240–1244. doi: 10.1016/j.carbpol.2016.06.066 [DOI] [PubMed] [Google Scholar]
- 82.Sharma R, Garg T, Goyal AK, Rath G. Development, optimization and evaluation of polymeric electrospun nanofiber: a tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol. 2016;44(2):524–531. doi: 10.3109/21691401.2014.966194 [DOI] [PubMed] [Google Scholar]
- 83.Zong S, Wang X, Yang Y, et al. The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur J Pharm Biopharm. 2015;93:127–135. doi: 10.1016/j.ejpb.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 84.Aggarwal U, Goyal AK, Rath G. Development and characterization of the cisplatin loaded nanofibers for the treatment of cervical cancer. Mater Sci Eng C. 2017;75:125–132. doi: 10.1016/j.msec.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 85.Colley HE, Said Z, Santocildes-Romero ME, et al. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials. 2018;178:134–146. doi: 10.1016/j.biomaterials.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 86.Muzzarelli RAA, El Mehtedi M, Bottegoni C, Aquili A, Gigante A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar Drugs. 2015;13(12):7314–7338. doi: 10.3390/md13127068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mašek J, Lubasová D, Lukáč R, et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles - important step towards effective mucosal vaccines. J Control Release. 2017;249:183–195. doi: 10.1016/j.jconrel.2016.07.036 [DOI] [PubMed] [Google Scholar]
- 88.Tonglairoum P, Ngawhirunpat T, Rojanarata T, Panomsuk S, Kaomongkolgit R, Opanasopit P. Fabrication of mucoadhesive chitosan coated polyvinylpyrrolidone/cyclodextrin/clotrimazole sandwich patches for oral candidiasis. Carbohydr Polym. 2015;132:173–179. doi: 10.1016/j.carbpol.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 89.Malik R, Garg T, Goyal AK, Rath G. Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target. 2015;23(2):109–124. doi: 10.3109/1061186X.2014.965715 [DOI] [PubMed] [Google Scholar]
- 90.Ignatious F, Sun L, Lee C-P, Baldoni J. electrospun nanofibers in oral drug delivery. Pharm Res. 2010;27(4):576–588. doi: 10.1007/s11095-010-0061-6 [DOI] [PubMed] [Google Scholar]
- 91.Morales JO, McConville JT. Manufacture and characterization of mucoadhesive buccal films. Eur J Pharm Biopharm. 2011;77(2):187–199. doi: 10.1016/j.ejpb.2010.11.023 [DOI] [PubMed] [Google Scholar]
- 92.Grewal H, Dhakate SR, Goyal AK, Markandeywar TS, Malik B, Rath G. Development of transmucosal patch using nanofibers. Artif Cells Blood Substit Biotechnol. 2012;40(1–2):146–150. doi: 10.3109/10731199.2011.637924 [DOI] [PubMed] [Google Scholar]
- 93.Tyagi C, Tomar L, Choonara YE, Du Toit LC, Kumar P, Pillay V. Electrospun nanofiber matrix with a mucoadhesive backing film for oramucosal drug delivery. Int J Mater Mech Manuf. 2014;2(1):81–85. doi: 10.7763/IJMMM.2014.V2.105 [DOI] [Google Scholar]
- 94.Kilicarslan M, Koerber M, Bodmeier R. In situ forming implants for the delivery of metronidazole to periodontal pockets: formulation and drug release studies. Drug Dev Ind Pharm. 2014;40(5):619–624. doi: 10.3109/03639045.2013.873449 [DOI] [PubMed] [Google Scholar]
- 95.Samprasit W, Akkaramongkolporn P, Ngawhirunpat T, Rojanarata T, Kaomongkolgit R, Opanasopit P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int J Pharm. 2015;487(1–2):213–222. doi: 10.1016/j.ijpharm.2015.04.044 [DOI] [PubMed] [Google Scholar]
- 96.Illangakoon UE, Gill H, Shearman GC, et al. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int J Pharm. 2014;477(1–2):369–379. doi: 10.1016/j.ijpharm.2014.10.036 [DOI] [PubMed] [Google Scholar]
- 97.Singh H, Sharma R, Joshi M, Garg T, Goyal AK, Rath G. Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofi bers. Artif Cells Nanomed Biotechnol. 2015;43(4):263–269. doi: 10.3109/21691401.2014.885442 [DOI] [PubMed] [Google Scholar]
- 98.Brako F, Thorogate R, Mahalingam S, Raimi-Abraham B, Craig DQM, Edirisinghe M. Mucoadhesion of progesterone-loaded drug delivery nanofiber constructs. ACS Appl Mater Interfaces. 2018;acsami.8b03329. doi: 10.1021/acsami.8b03329 [DOI] [PubMed] [Google Scholar]
- 99.Moreno I, González-González V, Romero-García J. Control release of lactate dehydrogenase encapsulated in poly (vinyl alcohol) nanofibers via electrospinning. Eur Polym J. 2011;47(6):1264–1272. doi: 10.1016/j.eurpolymj.2011.03.005 [DOI] [Google Scholar]
- 100.Garg T, Rath G, Goyal AK. Biomaterials-based nanofiber scaffold: targeted and controlled carrier for cell and drug delivery. J Drug Target. 2015;23(3):202–221. doi: 10.3109/1061186X.2014.992899 [DOI] [PubMed] [Google Scholar]
- 101.Hotaling NA, Khristov V, Wan Q, et al. Nanofiber scaffold-based tissue-engineered retinal pigment epithelium to treat degenerative eye diseases. J Ocul Pharmacol Ther. 2016;32(5):272–285. doi: 10.1089/jop.2015.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee M, Park CG, Huh BK, et al. Sinonasal delivery of resveratrol via mucoadhesive nanostructured microparticles in a nasal polyp mouse model. Sci Rep. 2017;7:40249. doi: 10.1038/srep40249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hattenbach L-O, Springer-Wanner C, Hoerauf H, et al. Intravitreal sustained-release steroid implants for the treatment of macular edema following surgical removal of epiretinal membranes. Ophthalmologica. 2017;237(4):232–237. doi: 10.1159/000464259 [DOI] [PubMed] [Google Scholar]
- 104.Rea W, Doherty VR, Perkins W, Aitchison TC, Mackie RM. Staphylococcus aureus and intra-nasal mupirocin in patients receiving isotretinoin for acne. Br J Dermatol. 1992;126(4):362–366. doi: 10.1111/j.1365-2133.1992.tb00679.x [DOI] [PubMed] [Google Scholar]
- 105.Bagnall P, Rizzolo D. Bacterial vaginosis: a practical review. J Am Acad Physician Assist. 2017;30(12):15–21. doi: 10.1097/01.JAA.0000526770.60197.fa [DOI] [PubMed] [Google Scholar]
- 106.Velasco Barraza RD, Álvarez Suarez AS, Gómez LV, Paz González JA, Iglesias AL, Vera Graziano R. Designing a low cost electrospinning device for practical learning in a bioengineering biomaterials course. Rev Mex Ing Biomédica. 2016;37(1):7–16. Available from: https://www.redalyc.org/articulo.oa?id=61943766002 [Google Scholar]
- 107.Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185(1):12–21. doi: 10.1016/j.jconrel.2014.04.018 [DOI] [PubMed] [Google Scholar]