Abstract
Background:
Cancer patients with chemotherapy-induced peripheral neuropathy (CIPN) have deficits in sensory and motor skills leading to inappropriate proprioceptive feedback, impaired postural control and high fall risk.
Objective:
This proof-of-concept study investigated the acceptability and effect of an interactive motor adaptation balance training program based on wearable sensors for improving balance in older cancer patients with CIPN.
Methods:
Twenty-two patients (age 70.3±8.7 years) with objectively confirmed CIPN (Vibration perception threshold, VPT > 25 Volts) were randomized to either intervention (IG) or control (CG) group. The IG received interactive game-based balance training including repetitive weight shifting and virtual obstacle crossing tasks. Wearable sensors provided real-time visual/auditory feedback from lower limb trajectory and allowed perception of motor-errors during each motor-action. The CG received no intervention, while recommended to perform regular exercise at home. Outcome measures were changes in sway of ankle, hip, and center of mass (CoM) in both medio-lateral (ML) and anterior-posterior (AP) directions during 30-second balance tests with increasing task difficulty (i.e. standing in feet-closed-position with eyes open (EO) and eyes closed (EC), and in semi-tandem-position with EO), at baseline and post-intervention. Additionally, gait performance (speed, variability) and fear of falling (Falls-Efficacy-Scale-International, FES-I) were measured.
Results:
Training was safe and well accepted despite participants’ impaired health status, high severity of CIPN (VPT=49.6±26.7 Volts) and high fear of falling (FES-I=35.10±13.78). Post intervention, sway of hip, ankle, and CoM were significantly reduced in the IG compared to CG during standing in feet-close-position with EO (p=.010–.022, except AP CoM sway) and semi-tandem-position (p=.008–.035, except ankle sway). While improvement trends were observed for balance with EC (8–39%), gait speed (8%), and FES-I (7%) in IG, they did not achieve statistical significance (p>0.05).
Conclusions:
This study demonstrates that older cancer patients with CIPN can significantly improve their postural balance with specifically tailored, sensor-based exercise training. The training approach is well accepted in the target group and has potential as a therapy for improving CIPN-related postural control deficits. However, future studies comparing the proposed technology-based training with traditional balance training are required to evaluate the benefit of the interactive joint movement feedback.
Keywords: cancer, chemotherapy, peripheral neuropathy, exercise training, rehabilitation, postural balance
INTRODUCTION
With the aging of the population and the improved survival of cancer patients, rehabilitation of older cancer survivors is an increasingly common problem [1]. Studies have shown that older adults are more likely to experience chemotherapy side effects, and chemotherapy doses often are reduced clinically, based on concern for comorbidities [2].
One major side-effect of antineoplastic agents is chemotherapy-induced peripheral neuropathy (CIPN), which, affects up to 40% of patients suffering from cancer [3]. The risk for and severity of CIPN increases with advancing age [4]. Unfortunately, in most instances, the CIPN is only partly reversible and in the worst cases damage is completely irreversible [5]. CIPN therefore represents an important sequela of cancer treatment that can have significant, often long-term impact on quality of life.
CIPN-associated sensory deficits can lead to inadequate proprioceptive feedback and increased fall risk [6]. Cancer patients during ongoing or recently completed (≤12 months) chemotherapy often show impairments in postural control [7–9]. Persistent mobility disability and falls related to neurotoxic chemotherapy and CIPN are more pronounced in older adults [7].
Mobility deficits and balance disorder in patients with peripheral neuropathy have been found to be associated with abnormal somatosensory feedback (i.e., diminished sensation in ankles and feet) [10–12]. This misinformation alters the formation of an internal representation of body position and motion in the central nervous system [13,14]. It is well established that based on error-dependent learning rules between prior motor action and desired action, an internal model is formed and tuned with practice [14–16]. This internal model enables individuals to produce motor commands (feedforward prediction) appropriate for arbitrary actions.
CIPN-associated balance impairment requires the design of novel tailored exercise programs [17], particularly in older patients who may be less able to compensate for the loss of proprioception. Several factors need to be considered when designing exercise programs for patients with peripheral neuropathy including compensation for lost joint perception, controlled intensity to avoid overtaxing, and tailoring of exercise to meet the needs of the target group [18]. With advancements in technology, interactive exergaming and virtual-reality systems have been evaluated for training of motor control in older adults and patient populations [19,20]; benefits include concordance of visual and proprioceptive information, enhanced information about joint movements in order to compensate for deteriorated joint position sense via repetitive practice, and incorporation of gaming features. CIPN patients with impaired lower-extremity proprioception may particularly benefit from interactive game-based balance training programs. However, to our knowledge, no study has evaluated such training program in this particular target population.
The current research focuses on the evaluation of a new interactive training regimen specifically developed to improve balance [18,21,22]. This exercise system integrates data from wearable sensors into a human-computer interface designed for game-based motor adaptation training. A key feature of the system is its ability to provide lower extremity real-time feedback in order to visualize motor errors during exercise. Our group has shown the effectiveness of this interactive balance training program in patients with diabetic peripheral neuropathy [18] and frail older adults [22]. This study sought to estimate the effectiveness of the new balance exercise regimen for improving balance in older cancer patients with confirmed CIPN. We hypothesized that 4-weeks of balance training (twice a week) would result in improved balance performance in our study cohort.
METHODS
Study design
The study was designed as a single blinded, randomized, controlled trial. Investigators were not aware of group assignment. The study was approved by the University of Arizona Institutional Review Committee (project no 12-0616-01).
Study Population
Individuals were recruited from the University of Arizona Cancer Center (Tucson, AZ). Recruitment started in September 2013 and follow-up was completed in August 2014. Inclusion criteria were (1) age ≥55 years; (2) ability to provide written informed consent, (3) diagnosis of current or prior malignancy; (4) neurotoxic chemotherapy exposure; (5) ability to walk without an assistive device for a minimum of 10 meters; and (6) presence of CIPN as confirmed by (a) presence of clinical key symptoms including numbness, tingling or pain in feet [23] and (b) objective assessment of vibration perception threshold score at the hallux bilaterally using a Biothesiometer (Diabetica Solutions, Inc. San Antonio, TX, USA). A cut-off of >25 Volt was defined as an indicator of peripheral neuropathy [24]. Vibration perception threshold measured in the feet has been identified as a sensitive method for assessment of CIPN [23]. Exclusion criteria included (1) diabetes; (2) foot ulcers or infection; (3) neurological disorders including stroke, Parkinson’s disease, multiple sclerosis, and dementia; and (4) severe visual impairment.
Participants meeting the inclusion criteria were randomly assigned to the intervention group (IG), or the control group (CG), using the urn design [25] (numbered containers). The sequence was concealed until group assignment (after baseline measurement) was completed. A person unrelated to the study performed the randomization procedure. The progress through the phases of screening, enrolment, allocation, follow-up, and data analysis is illustrated in figure 1.
Figure 1.
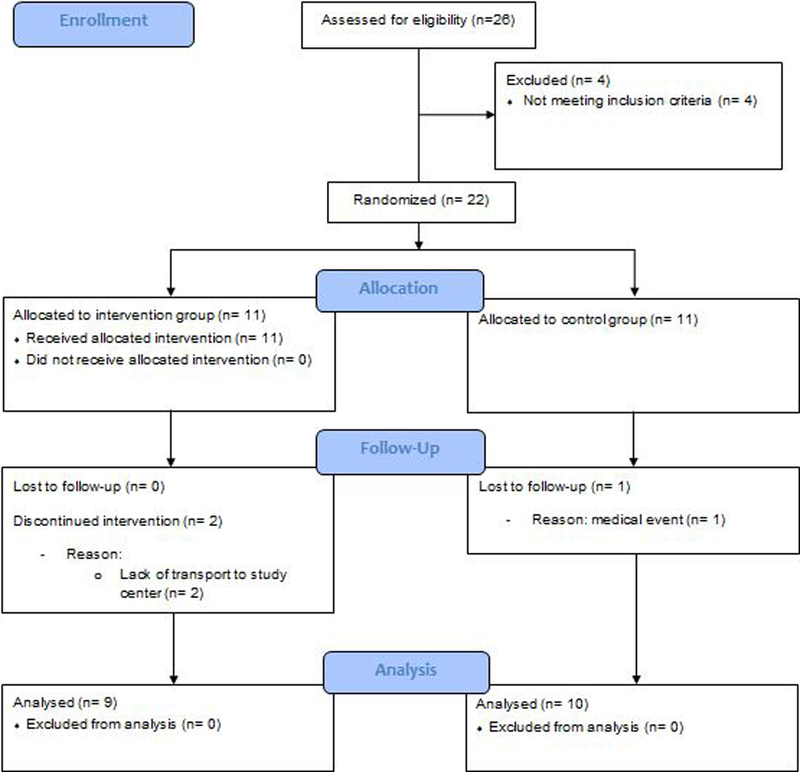
CONSORT flow diagram of progress through the phases of screening, enrolment, allocation, follow-up, and data analysis
Intervention
Balance training technology:
The technology used has been specifically developed for measuring and improving postural control, as described previously [18,22,26]. It consisted of a personal computer with 17 inch screen, a virtual user interface, and five inertial sensors (LegSysTM, BioSensics LLC, MA, USA) equipped with tri-axial accelerometer, gyroscope, and magnetometer; for estimation of joint angles and position [26]. Sensors were mounted on each shank, thigh, and on the lower back using elastic straps (Figure 2 and 3). Sensor data were acquired and transmitted in real-time at a 100 Hz sample frequency for real-time visualization of ankle and knee movement trajectory on the computer screen as well as providing audio/visual reward or notification of motor-error at the end of execution of each trial.
Figure 2. An illustration of the ankle reaching balance task.
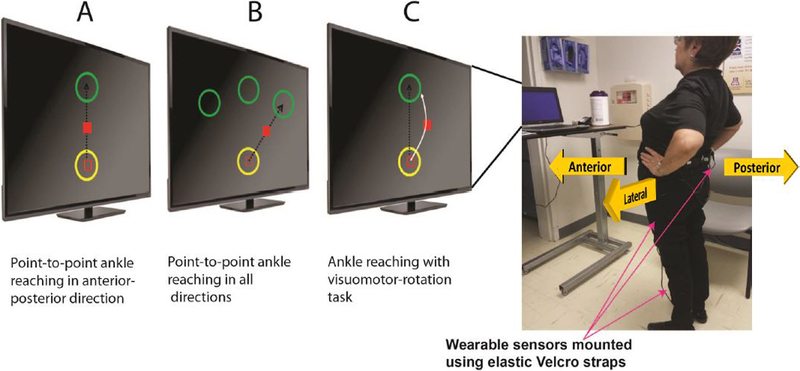
The participant performs the ankle reaching balance task in front of a monitor providing real-time ankle joint feedback as a red cursor. A: The ankle reaching task involves moving a red cursor from a start circle (yellow) to a target circle (green) in a straight line by rotating the ankle joint by leaning forward. Task repetition in the opposite direction (leaning backward) completes one cycle. B: The ankle reaching task is conducted in anterior-posterior and medial-lateral direction. C: The trajectory of the cursor is rotated by the computer by an angle of 20°. The participant needs to observe this change in trajectory and compensate by adjusting ankle/hip coordination. All exercises were administered inside the clinic without the need of supervision and through on-screen feedback. However, a supervisor was present in the room to guarantee safety.
Figure 3. An illustration of the virtual obstacle crossing task.
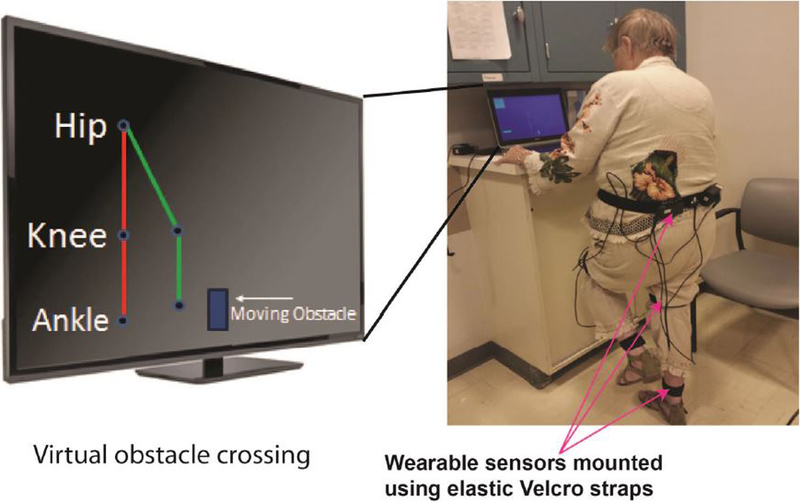
The participant is challenged to cross virtual obstacles appearing on the screen. Lower extremity real-time feedback is given using a stick figure avatar representing the participant’s hip and knee movements. The avatar replicates lower limb movements including lifting of designated leg to appropriate height to cross an obstacle. When needed, the participant can use support by holding on to a desk, as illustrated in the figure.
Training procedure:
Training was conducted in a separate room in the University of Arizona Cancer Center clinic. During the exercises the participant stood in front of the computer screen placed on an elevated desk (Figure 2 and 3). A supervisor gave instructions during the first training session. In subsequent sessions, participants conducted exercises using interactive sensor feedback only. The supervisor remained with the participant to guarantee safety.
Training protocol:
Participants attended two sessions per week (45 min. each), for four weeks. Sessions included: 1) ankle point-to-point reaching tasks; and 2) virtual obstacle crossing tasks (described below). Balance exercises included repetitive, error-dependent forward/backward/sideward/diagonal leaning tasks and cognitively challenging dynamic weight shifting tasks designed to improve postural balance [27]. The graphical interface was designed to be intuitive and easy to navigate, while avoiding complex animations, which may distract the user from observing relevant information related to motion performance and motor error.
Ankle point-to-point reaching task:
This task has been described previously in detail [22]. In summary, the exercise required forward/backward/sideward/diagonal leaning (Figure 2A–C) and partial weight transfer during standing in front of the computer screen. Data from shank mounted sensors provided real-time visual feedback about 2D ankle trajectory during exercises. The kinematic of the ankle joint rotation was translated to a linear cursor movement on a screen. The participant had to navigate the cursor from a start circle to a target circle by ankle joint rotation during standing (Figure 2A). Task repetition in the opposite direction completed one cycle. The participant had to navigate rapidly (<1 second) and accurately (in middle of circle) from one circle to another. Correct execution was awarded by visual (exploding of the target) and auditory (positive sound) feedback used as incentive to be engaged in the exercise and perceive the improvement in the course of exercise. Motor-error due to inaccuracy of navigating to and stopping at the center of the target circle in a timely manner was also notified to the subject via an audio-visual feedback at the end of execution of each trial. In order to move the cursor forward/backward, the participant had to move the hip in anterior-posterior direction to generate ankle dorsi-flexion or plantar-flexion (Figure 2A). Medial-lateral hip movement navigated the cursor sideward (Figure 2B).
Each session included 6 blocks each with 20 cycles of ankle reaching. Blocks 1+2 were performed in anterior-posterior direction (Figure 2A). Block 3+4 combined anterior-posterior and medial-lateral direction for a diagonal movement (Figure 2B). Block 5+6 were conducted with a visuomotor rotation task [28], in order to increase motor and cognitive challenge. The trajectory of the cursor was rotated by a 20° angle (Figure 2C). The participant had to observe this change in trajectory during the exercise and adjust ankle coordination to navigate the cursor towards the target circle. Visuomotor rotation aimed to improve postural adaptation and postural calibration, as described earlier [29]. Participants could rest between blocks to avoid fatigue.
Virtual obstacle crossing task:
The participant crossed virtual obstacles (boulders) moving on the computer screen from the left to the right side (Figure 3). Real-time feedback was given using a stick figure avatar representing the participant’s hip and knee movements. The avatar replicated lower limb movements including lifting of designated leg to appropriate height to cross an obstacle. Each session included three series of obstacle crossing with ten repetitions each, with progressive increase in obstacle height (10%, 15%, 20% of leg length). To cognitively challenge participants, the program required that they crossed obstacle alternatively with the left or the right leg. If the sequence of leg lifting was mistaken, the subject was notified via audio-visual feedback. The next obstacle was released only after the participant had either crossed or hit the previous obstacle and was now in double-stance support for at least 2 sec (for safety). Participants received audio feedback at the end of each obstacle-crossing trial which indicated whether they successfully crossed the obstacle or not. They could hold on to a sturdy desk for support, if required (see Figure 3). However, they were encouraged to perform the exercises without support. The CG continued their normal activity but did not receive any formal exercise, while were verbally encouraged to do home exercise and be active.
Measurements
Clinical characteristics including type and duration of cancer, severity of CIPN (VPT score), neuropathy-related pain (Numeric rating scale, NRS, score 0–10 [30]), neuropathy-related numbness in feet (NRS, score 0–10), health-related quality of life (Short-Form Health Survey, SF-12 [31]), fear of falling (Falls-Efficacy-Scale International, FES-I [32]), body mass index (BMI), and history of falls (past year) were documented by standardized interviewer-administered assessment.
Outcome measures:
Measurements were performed at baseline and after 4 weeks in the Arizona Cancer Center clinic. Balance was measured using three wearable sensors (BalanSens™, BioSensics, MA, USA) attached to both shanks and lower back. Participants were instructed to stand for 30-seconds under 3 conditions: 1) feet close together (but not touching) with eyes open (EO), 2) feet close together and eyes closed (EC), 3) semi-tandem position with EO. CoM was quantified as anterior-posterior (AP, cm) sway and medial-lateral (ML, cm) sway using validated algorithms [12]. Reduction in ML CoM sway with EO was defined as the primary study endpoint. This sway component is an established predictor for future falls [33,34]. Additionally, hip sway (deg2) and ankle sway (deg2) were calculated [12].
Gait performance was measured using wearable sensors attached to each shank and thigh (LegSys™, BioSensics, MA, USA). Participants walked ten meters at usual pace. Gait speed and variability (coefficient of variation of stride velocity) were calculated [35].
Fear of falling was evaluated with the FES-I [32], by direct interview. The FES-I assesses the participants’ confidence (1= concerned, 4= very concerned, total range: 16–64) in performing different physical and social activities of daily living without falling. Higher FES-I scores indicate a lower fall related self-efficacy.
Statistical Analysis
Unpaired t-tests and Chi-square-tests were used for baseline comparisons according to the scale of the investigated variable. Analysis of covariance was used to compare the effect of the intervention on outcome parameters at follow-up adjusting for baseline values [36]. Effect sizes were calculated as partial eta squared (ηp2). Values ranging from 0.01 to 0.06 indicate small; from 0.06 to 0.25 moderate, and above 0.25 large effects [37]. Univariate linear regression analyses were performed to delineate predictive factors of training response for the primary study endpoint (pre- to post-changes in ML CoM sway with EO). Variables included age, severity of CIPN (VPT score), numbness, pain, fear of falling (FES-I), health-related quality of life (SF-12), and baseline balance. Results are given as regression coefficients β and fit of the model is reported by coefficient of determination R2. SPSS statistics 22.0 (IBM, Armonk, NY, USA) was used for analysis.
RESULTS
Twenty-two participants were recruited into the study (Figure 1). Three participants (13.6%) dropped out during the study period (IG n=2: reasons: lack of transport to the study center; CG n=1: reason: medical event unrelated to the study). The remaining IG participants completed all sessions. All participants felt comfortable in using the sensor-based technology and enjoyed the interactive balance training. Training was safe despite the participant’s impaired health status, high severity of CIPN (average VPT=49.6±26.7 Volts), high concerns about falling (FES-I=35.10 ± 13.78), and functional impairment and no adverse events occurred.
The participant’s average age was 70.3±8.7 (range 55–86) years. Cancer diagnosis included lung (n=11, 50%), multiple myeloma (n=2, 9.1%), breast (n=2, 9.1%), colorectal (n=1, 4.5%), melanoma (n=1, 4.5%), bladder (n=1, 4.5%), prostate (n=1, 4.5%), pancreas (n=1, 4.5%), ovarian (n=1, 4.5%), and chronic lymphoid leukemia (n=1, 4.5%). Gait speed averaged 0.94 ± 0.22 meters per second, which is comparable to the speed found in pre-frail older adults (mean age 83 years) [38], indicating substantial functional impairment in our study participants. Eight participants (36.4%) reported 1 or more falls in the last year. No differences between IG and CG were found at baseline (Table 1).
Table 1.
Baseline characteristics of study participants
| Characteristic | Intervention (n = 11) | Control (n = 11) | P-value |
|---|---|---|---|
| Age, years | 68.73 ± 8.72 | 71.82 ± 8.85 | .419 |
| Women, number | 7 (63.6) | 6 (54.4) | .665 |
| BMI, kg/m2 | 27.91 ± 8.47 | 23.00 ± 3.58 | .092 |
| Duration Cancer, month | 49.91 ± 44.11 | 44.63 ± 56.78 | .810 |
| Vibration perception threshold, Volts | 47.47 ± 25.20 | 51.7 ± 29.24 | .720 |
| Numbness NRS, score (0–10) | 5.90 ± 3.25 | 4.91 ± 3.08 | .482 |
| Pain NRS, score (0–10) | 3.10 ± 3.31 | 2.44 ± 2.83 | .651 |
| Falls Efficacy Scale – International, score | 35.10 ± 13.78 | 27.64 ± 8.09 | .130 |
| SF-12, Physical Component, score | 31.48 ± 10.04 | 32.95 ± 6.77 | .693 |
| SF-12, Mental Component, score | 55.75 ± 8.52 | 52.13 ± 5.87 | .260 |
| Habitual gait speed, meter/sec | 0.86 ± 0.25 | 1.02 ± 0.17 | .098 |
Data are mean ± standard deviation or number (%); P- values are given for difference between the intervention and control group; NRS, Numeric Rating Scale; SF-12, Short Form Health Survey
Effect of the intervention on outcome parameters
Outcome measures are shown in table 2. Post-intervention, ML CoM sway, hip sway, and ankle sway were reduced in the IG compared to CG during balance assessment with feet close and EO (p= .010– .022). Significant reductions in postural sway parameters were also found during the more challenging semi-tandem position (p= .008– .035), except for ankle sway (p= .294). Greatest effects were found for hip sway during semi-tandem stance (ηp2= .388; −74.8%).
Table 2.
Effect of the interactive balance training on outcome parameters
| Parameters | Control Group |
Intervention Group |
P valueb | Effect sizec | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline n=10 | Follow Up n=10 | % changea | Baseline n=9 | Follow Up n=9 | % changea | |||
| Balance | ||||||||
| Feet close – eyes open | ||||||||
| CoM sway, ML, cm | 2.26 ± 1.27 | 1.78 ± 0.91 | 21.2 | 2.72 ±1.42 | 1.21 ± 0.45 | 55.5 | .022 | .286 |
| CoM sway, AP, cm | 1.48 ± 1.00 | 1.53 ± 0.87 | − 3.4 | 1.61 ± 0.95 | 1.33 ± 0.86 | 17.4 | .251 | .081 |
| Hip sway, deg2 | 2.34 ± 2.11 | 1.35 ± 0.97 | 42.3 | 2.00 ± 1.91 | 0.65 ± 0.37 | 67.5 | .010 | .344 |
| Ankle sway, deg2 | 2.10 ± 1.89 | 1.65 ± 1.25 | 21.4 | 2.92 ± 4.01 | 0.93 ± 1.03 | 68.2 | .011 | .338 |
| Semi tandem – eyes open | ||||||||
| CoM sway, ML, cm | 2.13 ± 0.81 | 2.47 ± 1.37 | −15.9 | 3.15 ± 1.54 | 1.65 ± 0.65 | 47.6 | .035 | .264 |
| CoM sway, AP, cm | 1.99 ± 1.57 | 1.94 ± 1.17 | 2.5 | 2.23 ± 1.06 | 1.25 ± 0.48 | 43.9 | .012 | .355 |
| Hip sway, deg2 | 1.94 ± 1.84 | 2.15 ± 1.71 | −10.8 | 3.22 ± 3.58 | 0.81 ± 0.67 | 74.8 | .008 | .388 |
| Ankle sway, deg2 | 1.84 ± 1.70 | 1.97 ± 1.43 | −7.1 | 3.77 ± 3.41 | 1.01 ± 0.91 | 73.2 | .294 | .069 |
| Feet close – eyes closed | ||||||||
| CoM sway, ML, cm | 2.34 ± 1.27 | 2.53 ± 1.14 | −8.1 | 3.78 ± 1.64 | 2.46 ± 1.36 | 34.9 | .304 | .070 |
| CoM sway, AP, cm | 1.74 ± 1.13 | 1.99 ± 1.45 | −14.4 | 2.60 ±1.15 | 2.39 ± 1.91 | 8.07 | .550 | .024 |
| Hip sway, deg2 | 2.68 ± 3.45 | 3.06 ± 2.82 | −14.2 | 4.22 ± 3.47 | 2.57 ± 2.24 | 39.1 | .305 | .070 |
| Ankle sway, deg2 | 2.42 ± 2.14 | 3.10 ± 2.99 | −28.1 | 5.96 ± 5.14 | 4.49 ± 6.65 | 24.7 | .277 | .078 |
| Gait | ||||||||
| Speed, m/sec | 1.03 ± 0.17 | 1.07 ± 0.20 | 3.9 | 0.85 ± 0.27 | 0.92 ± 0.18 | 8.2 | .445 | .037 |
| Step time variability, CV | 3.64 ± 1.03 | 2.95 ± 1.97 | 19.0 | 4.38 ± 2.81 | 3.42 ± 2.02 | 21.9 | .734 | .007 |
| Fear of falling | ||||||||
| Falls Efficacy scale, score | 28.20 ± 8.30 | 31.10 ± 9.46 | −10.3 | 35.10 ± 13.18 | 32.60 ± 14.83 | 7.1 | .308 | .061 |
Data presented as mean ± standard deviation
positive scores indicate improvement
P-values from ANCOVA comparing the effect of the intervention on outcome parameters at follow-up adjusting for baseline values
Effect size eta squared from ANCOVA; CoM, center of mass; ML, medial-lateral; AP, anterior-posterior; CV= coefficient of variation.
While noticeable trends were observed for other outcome parameters of interest, they did not achieve statistical significant level in the IG compared to the CG (Table 2). Specifically, ML CoM sway, hip sway, and ankle sway during standing with EC were improved in the IG by 34.9%, 39.1%, and 24.7% respectively. On the same note, gait speed was improved in the IG by 8.2%. Although, the observed trends were not significant compared to CG, effect sizes for these outcomes suggest presence of a moderate effect (ηp2= .037– .078), which may be verified in a larger sample.
Predictors of training response
Patients with lower baseline balance performance (i.e. higher ML CoM sway with EO) showed significantly greater improvements in balance (i.e. more reduction in ML CoM sway with EO stance: β= −.962, R2= 0.916, p< 0.001). Also, patients with higher CIPN associated numbness of feet (β= −.729, R2= 0.465, p= 0.026) or higher pain (β= −.698, R2= 0.413, p= 0.037) at baseline showed significantly greater improvements in balance. A similar trend was observed for the FES-I indicating that those with higher fear of falling a baseline had greater improvements in balance (β= −.729, R2= 0.465), although this result did not achieve statistical significance in our sample (p= 0.059). Other parameters did not significantly predict training response (p= .071– .779).
DISCUSSION
The findings of the current study indicate that interactive, error-dependent, award-based balance training is safe and effective for improving postural balance in CIPN patients. We observed significant reductions in postural sway in the IG after 4 weeks of training during balance assessments with large effect sizes during EO condition. We believe that postural coordination during ankle-reaching and dynamic weight shifting during obstacle crossing led to these improvements. We hypothesize that the training helped to restore sensory mapping by performing several repetitive and identical motor tasks and assisted patients in perceiving motor errors (i.e. difference between desired action and actual motor action), which is not well accomplished by conventional balance training programs without real-time feedback about body motion.
Previous studies have shown that ML CoM sway is more associated with falls than AP CoM sway [33,34]. In healthy individuals body sway is larger in AP compared to ML direction, related to the inherent structural mechanism of ankle and hip joints [39,40]. In contrast, CIPN patients in our study had higher baseline sway in ML direction compared to AP, increasing their risk of falling. Notably, baseline ML CoM sway in our participants (2.47 ± 1.32 cm, standing with feet close and EO) was similar that of our previously reported cohort of frail fall-prone adults (1.97 ± 0.83 cm, p= .107), who were, on average, 15 years older (84.6 ± 6.8 years) [22]. Importantly, after the training, ML CoM sway was reduced by 48–56% depending on the stance. We hypothesize that the weight shifting during the virtual obstacle crossing task, intended to improve control of ML body movements, may be an important training element that led to these improvements [41].
Our positive results in CIPN patients are in line with earlier studies reporting improvements in balance in older adults without CIPN after exergame interventions with comparable frequency and length [20,42,43]. However, previous studies used consumer exergaming devices based on force platforms (Nintendo Wii) or video system (Microsoft Kinect) which might be appropriate in more healthy populations but have limitations in functionally impaired subjects such as older patients with CIPN. Force platforms restrict the base of support during exercising which may result in falls during training [44,45]. In contrast, our sensor-based system allowed participants to exercise on the ground in a natural stance position and did not use real obstacles which could have caused tripping. Unlike camera-based exergame systems (i.e., Microsoft Kinect), our sensor-based system did not require a continuous unobstructed sightline, allowing the placement of a desk or chair in front of the participant to add to the safety of our system.
While it is of importance to try to minimize the development of CIPN in cancer patients, we observed, interestingly, that participants with more severe CIPN signs and symptoms (foot numbness, pain, and balance deficits) and increased fear of falling had a better training response, suggesting that the most impaired participants reaped the most benefit from the training. Our findings are in accordance with earlier studies demonstrating that participants with the lowest performance benefit most from physical activity interventions [22,46].
While noticeable improvements were observed in the IG, effects obtained for balance assessed with EC condition, gait, and fear of falling, were not significant compared to the CG. The sample size of this proof-of-concept study may have been too small to show a significant effect for these outcomes. Further, it may be that our intervention primed the visual input system for balance control which may explain larger effects on balance with EO. Future studies may incorporate auditory or vibro-tactile feedback in order to allow specific balance training with EC [47]. In addition, the distance for gait assessment (10 meters) may have been too short to detect a training effect on gait fatigue, which is a frequent symptom in cancer patients.
Limitations and future research
The major limitation of this trial is the low number of participants. Further, IG participants visited the clinic twice per week during the study period, which may have slightly biased the findings. However, participants in both study groups (IG and CG) visited the cancer clinic frequently for routine care and IG participants often combined training sessions with routine care visits.
Participants had relatively few weeks of training. Despite this limitation, however, balance improvements found here are congruent with previous studies in older adults without CIPN which have used a comparable frequency of training sessions and length of intervention period [42,43].
The aim of this proof-of-concept study was to examine whether a novel technology based on wearable sensors combined with targeted balance exercises in a virtual environment can enhance balance in patients with CIPN and its applicability in a routine clinical environment. We did not aim to compare the advantage of the proposed sensor-based training to traditional supervised or unsupervised balance training. This will be investigated in a future study with a larger sample size, longer intervention period, active control group (i.e. conventional balance training, commercial exergames), and follow-up at 3–6 months to assess durability of observed improvement in balance and effect on fall frequency, fear of falling and quality of life measures.
We are currently developing a technology for autonomous usage by older adults at home utilizing Bluetooth sensors, user-friendly computer interface, and automated adjustment of task difficulty for the purpose of unsupervised home training which should improve its application broadly in the community and for more home-bound cancer patients.
CONCLUSIONS
The presented study is an initial step towards evaluating a new and minimally supervised balance training paradigm practical for implementation into clinic and possibly home environment and specifically designed for rehabilitation of CIPN-related balance deficits, which place patients at fall risk, increase fear for falling, and negatively impact on quality of life. Our study provides preliminary evidence that the intervention is beneficial for improving balance in CIPN and provides a basis for a larger trial with a more rigorous design. The ability to tailor our training approach to CIPN patients’ specific balance deficits, minimum requirement for supervision of exercise, award features to engage participants, along with the system’s safety in high risk patients, supports further study of this novel method of balance training.
ACKNOWLEDGEMENTS
The project described was supported in part by awards from Flinn Foundation (award number 1907) and National Institute on Aging (award number 1R43AG044882–01A1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions.
Abbreviations:
- AP
anterior-posterior
- BMI
Body mass index
- CIPN
chemotherapy-induced peripheral neuropathy
- CoM
center of mass
- EC
eyes closed
- EO
eyes open
- IG
intervention group
- ML
medial-lateral
- FES-I
Falls-Efficacy-Scale International
- SF-12
Short Form Health Survey
- VPT
vibration perception threshold test
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Balducci L, Fossa SD: Rehabilitation of older cancer patients. Acta Oncol 2013;52:233–238. [DOI] [PubMed] [Google Scholar]
- 2.Giovanazzi-Bannon S, Rademaker A, Lai G, Benson AB, 3rd: Treatment tolerance of elderly cancer patients entered onto phase ii clinical trials: An illinois cancer center study. J Clin Oncol 1994;12:2447–2452. [DOI] [PubMed] [Google Scholar]
- 3.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C: Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer 2008;44:1507–1515. [DOI] [PubMed] [Google Scholar]
- 4.du Bois A, Schlaich M, Luck HJ, Mollenkopf A, Wechsel U, Rauchholz M, Bauknecht T, Meerpohl HG: Evaluation of neurotoxicity induced by paclitaxel second-line chemotherapy. Support Care Cancer 1999;7:354–361. [DOI] [PubMed] [Google Scholar]
- 5.Quasthoff S, Hartung HP: Chemotherapy-induced peripheral neuropathy. J Neurol 2002;249:9–17. [DOI] [PubMed] [Google Scholar]
- 6.Tofthagen C, Overcash J, Kip K: Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer 2012;20:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hile ES, Fitzgerald GK, Studenski SA: Persistent mobility disability after neurotoxic chemotherapy. Phys Ther 2010;90:1649–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederer D, Schmidt K, Vogt L, Egen J, Klingler J, Hubscher M, Thiel C, Bernhorster M, Banzer W: Functional capacity and fear of falling in cancer patients undergoing chemotherapy. Gait Posture 2014;39:865–869. [DOI] [PubMed] [Google Scholar]
- 9.Wampler MA, Topp KS, Miaskowski C, Byl NN, Rugo HS, Hamel K: Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy. Arch Phys Med Rehabil 2007;88:1002–1008. [DOI] [PubMed] [Google Scholar]
- 10.Horak FB, Dickstein R, Peterka RJ: Diabetic neuropathy and surface sway-referencing disrupt somatosensory information for postural stability in stance. Somatosens Mot Res 2002;19:316–326. [DOI] [PubMed] [Google Scholar]
- 11.Lord SR, Caplan GA, Colagiuri R, Colagiuri S, Ward JA: Sensori-motor function in older persons with diabetes. Diabet Med 1993;10:614–618. [DOI] [PubMed] [Google Scholar]
- 12.Najafi B, Horn D, Marcley S, Crews RT, Wu S, Wrobel JS: Assessing postural control and postural control strategy in diabetic patients using innovative and wearable technology. J Sci Technol 2010;4:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K: Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil 1996;77:1152–1156. [DOI] [PubMed] [Google Scholar]
- 14.Smith Maurice A, Ghazizadeh Ali, Shadmehr Reza: Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biology 2006;4:e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krakauer JW, Ghez C, Ghilardi MF: Adaptation to visuomotor transformations: Consolidation, interference, and forgetting. J Neurosci 2005;25:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krakauer JW, Ghilardi M-F, Ghez C: Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 1999;2:1026. [DOI] [PubMed] [Google Scholar]
- 17.Visovsky C, Collins M, Abbott L, Aschenbrenner J, Hart C: Putting evidence into practice®: Evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clinical J Oncol Nurs 2007;11:901–913. [DOI] [PubMed] [Google Scholar]
- 18.Grewal GS, Schwenk M, Lee-Eng J, Parvaneh S, Bharara M, Menzies R, Talal TK, Armstrong DG, Najafi B: Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: A randomized controlled trial. Gerontology 2015 [DOI] [PubMed]
- 19.de Bruin ED, Schoene D, Pichierri G, Smith ST: Use of virtual reality technique for the training of motor control in the elderly. Some theoretical considerations. Z Gerontol Geriatr 2010;43:229–234. [DOI] [PubMed] [Google Scholar]
- 20.van Diest M, Lamoth CJ, Stegenga J, Verkerke GJ, Postema K: Exergaming for balance training of elderly: State of the art and future developments. J Neuroeng Rehabil 2013;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grewal G, Sayeed R, Schwenk M, Bharara M, Menzies R, Talal T, Armstrong D, Najafi B: Balance rehabilitation: Promoting the role of virtual reality in patients with diabetic peripheral neuropathy. J Am Podiatr Med Assoc 2013;103:498. [DOI] [PubMed] [Google Scholar]
- 22.Schwenk M, Grewal GS, Honarvar B, Schwenk S, Mohler J, Khalsa DS, Najafi B: Interactive balance training integrating sensor-based visual feedback of movement performance: A pilot study in older adults. J Neuroeng Rehabil 2014;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SB, Goldstein D, Krishnan AV, Lin CSY, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC: Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA: A Cancer Journal For Clinicians 2013;63:419–437. [DOI] [PubMed] [Google Scholar]
- 24.Garrow AP, Boulton AJ: Vibration perception threshold--a valuable assessment of neural dysfunction in people with diabetes. Diabetes Metab Res Rev 2006;22:411–419. [DOI] [PubMed] [Google Scholar]
- 25.Wei LJ: A class of designs for sequential clinical trials. J Am Stat Assoc 1977;72:382–386. [Google Scholar]
- 26.Grewal G, Sayeed R, Yeschek S, Menzies RA, Talal TK, Lavery LA, Armstrong DG, Najafi B: Virtualizing the assessment: A novel pragmatic paradigm to evaluate lower extremity joint perception in diabetes. Gerontology 2012;58:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe T, Rochester L, Neil F, Skelton D, Ballinger C: Exercise for improving balance in older people. Cochrane Database Syst Rev 2011:CD004963. [DOI] [PMC free article] [PubMed]
- 28.Shabbott BA, Sainburg RL: Learning a visuomotor rotation: Simultaneous visual and proprioceptive information is crucial for visuomotor remapping. Exp Brain Res 2010;203:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastian AJ: Understanding sensorimotor adaptation and learning for rehabilitation. Current Opin Neurol 2008;21:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM: Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 31.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L: Cross-validation of item selection and scoring for the sf-12 health survey in nine countries: Results from the iqola project. J Clin Epidemiol 1998;51:1171–1178. [DOI] [PubMed] [Google Scholar]
- 32.Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C: Development and initial validation of the falls efficacy scale-international (fes-i). Age Ageing 2005;34:614–619. [DOI] [PubMed] [Google Scholar]
- 33.Cofre Lizama LE, Pijnappels M, Faber GH, Reeves PN, Verschueren SM, van Dieen JH: Age effects on mediolateral balance control. PLoS One 2014;9:e110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maki BE, Holliday PJ, Topper AK: A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol 1994;49:M72–M84. [DOI] [PubMed] [Google Scholar]
- 35.Aminian K, Najafi B, Büla C, Leyvraz P-F, Robert P: Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech 2002;35:689–699. [DOI] [PubMed] [Google Scholar]
- 36.Borm GF, Fransen J, Lemmens WA: A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol 2007;60:1234–1238. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J: Statistical power analysis for the behavioral sciencies New York, Routledge, 1988. [Google Scholar]
- 38.Schwenk M, Howe C, Saleh A, Mohler J, Grewal G, Armstrong D, Najafi B: Frailty and technology: A systematic review of gait analysis in those with frailty. Gerontology 2014;60:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins JJ, De Luca CJ: Open-loop and closed-loop control of posture: A random-walk analysis of center-of-pressure trajectories. Exp Brain Res 1993;95:308–318. [DOI] [PubMed] [Google Scholar]
- 40.Winter DA: Human balance and posture control during standing and walking. Gait Posture 1995;3:193–214. [Google Scholar]
- 41.Chou L-S, Kaufman KR, Hahn ME, Brey RH: Medio-lateral motion of the center of mass during obstacle crossing distinguishes elderly individuals with imbalance. Gait Posture 2003;18:125–133. [DOI] [PubMed] [Google Scholar]
- 42.Young W, Ferguson S, Brault S, Craig C: Assessing and training standing balance in older adults: A novel approach using the ‘nintendo wii’balance board. Gait Posture 2011;33:303–305. [DOI] [PubMed] [Google Scholar]
- 43.Sihvonen SE, Sipilä S, Era PA: Changes in postural balance in frail elderly women during a 4-week visual feedback training: A randomized controlled trial. Gerontology 2004;50:87–95. [DOI] [PubMed] [Google Scholar]
- 44.Gerling KM, Schild J, Masuch M: Exergaming for elderly: Analyzing player experience and performance: Mensch & Computer 2011: 11 fachübergreifende Konferenz für interaktive und kooperative Medien Oldenbourg Verlag, 2011, pp 401.
- 45.Hanneton S, Varenne A: Coaching the wii. Haptic Audio visual Environments and Games HAVE 2009 IEEE International Workshop on 2009:54–57. [Google Scholar]
- 46.Schwenk M, Zieschang T, Englert S, Grewal G, Najafi B, Hauer K: Improvements in gait characteristics after intensive resistance and functional training in people with dementia: A randomised controlled trial. BMC Geriatr 2014;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wall C 3rd: Application of vibrotactile feedback of body motion to improve rehabilitation in individuals with imbalance. J Neurol Phys Ther 2010;34:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


