Abstract
Purpose
Wee1 kinase inhibitors are effective radiosensitizers in cells lacking a G1 checkpoint. In this study we examined the potential effect of Wee1 kinase inhibition on inducing replication stress in hepatocellular carcinoma (HCC).
Methods
Five independent datasets from the Oncomine Database comparing gene expression in HCC compared to normal tissue were combined and specific markers associated with Wee1 sensitivity were analyzed. We then performed a series of in vitro experiments to study the effect of Wee1 inhibition on irradiated HCC cell lines with varying p53 mutational statuses. Clonogenic survival assays and flow cytometry using anti-γH2AX and phospho-histone H3 antibodies with propidium iodide were performed to study the effect of AZD1775 on survival, cell cycle, and DNA repair. Additionally, nucleoside enriched media was used to examine the effect of altering nucleotide pools on Wee1 targeted radiosensitization.
Results
Our analysis of the Oncomine Database found high levels of CDK1 and other cell cycle regulators indicative of Wee1 sensitivity in HCC. In our in vitro experiments, treatment with AZD1775 radiosensitized and chemosensitized Hep3B, Huh7, and HepG2 cell lines and was associated with delayed resolution of γH2AX foci and the induction of pan-nuclear γH2AX staining. Wee1 inhibition attenuated radiation induced G2 arrest in the Hep3B (TP53 null) and Huh7 (TP53 mutant) cell lines but not in the TP53 wild type cell line HepG2. Supplementation with nucleosides reversed the radiosensitizing effect of AZD1775 and reduced the amount of cells with pan-nuclear γH2AX staining after radiation.
Conclusions
Radiosensitization with Wee1 inhibition occurs in cells regardless of their p53 mutational status. In this study we show for the first time that replication stress via the overconsumption of nucleotides plays an important role in AZD1775 induced radiosensitization.
Keywords: Hepatocellular carcinoma, Wee1, AZD1775, radiosensitizer
Summary
Wee1 inhibition with AZD1775 has the potential to be an effective strategy of radiosensitization in hepatocellular carcinoma given the high prevalence of CDK1 overexpression in this disease. In the current study, we tested the ability of AZD1775 to radiosensitize HCC cell lines with different TP53 mutation statuses. AZD1775 was found to be an effective radiation sensitizer in all cell lines. Both checkpoint abrogation and induced replication stress play an important role in AZD1775 induced radiosensitization.
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer related death worldwide (1). External beam radiation therapy and transarterial radioembolization are commonly used in patients unable to undergo resection or transplantation (2–4). More recently, liver stereotactic body radiation therapy (SBRT) has shown promising results in clinical trials (5–7); however, its effectiveness in larger tumors is limited by the radiosensitivity of normal tissue including the liver and small bowel (2, 8–10). A major challenge in the management of patients with HCC is that cytotoxic chemotherapy has had disappointing results in clinical trials (11, 12). Sorafenib has a modest effect on overall survival but no effect on time to symptomatic progression (13). Novel agents that preferentially sensitize HCC versus normal tissue to the cytotoxic effects of radiation therapy and chemotherapy are greatly needed.
Targeting the response of cancer cells to DNA damaging agents is an attractive strategy for chemosensitization and radiosensitization (14). Wee1 is a serine-threonine kinase that regulates the G2 checkpoint through the inhibitory phosphorylation of CDK1 (15–18). Since many cancers have an aberrant G1 checkpoint due to abnormal p53, p21, Rb, or other G1 regulators, they are dependent on the G2 checkpoint to repair radiation induced DNA damage (19–21). Drugs that alter the G2 checkpoint in cells with a deficient G1 checkpoint promote premature entry into mitosis after DNA damage leading to mitotic catastrophe (22). As normal cells have an intact G1 checkpoint they can arrest in G1 to repair DNA damage, potentially resulting in tumor cell selectivity with G2 checkpoint abrogation (23). Prior studies examining inhibitors of the G2 checkpoint regulator Wee1 and the related kinase Chk1 in combination with radiation therapy or chemotherapy have shown promise in preclinical models (24–28).
In addition to regulating the transition from G2 to M phase, Wee1 also regulates replication initiation through its suppression of CDK1 activity. This action protects cells by preventing aberrant replication origin firing, nucleotide overconsumption, and replication stress (29, 30). Promoting replication stress as a strategy of radiosensitization is potentially tumor cell specific as tumor cells have high baseline levels of replication stress due to the presence of oncogenic drivers and higher rates of replication than non-malignant cells (31).
Replication stress is a complex cellular process that affects genomic stability and cell survival. It is characterized by stalling of replication fork progression and DNA synthesis. The replication stress response is mediated through ATR and ATM in response to aberrant replication forks containing ssDNA (31). There are several markers of replication stress including pan-nuclear γH2AX staining and phosphorylation of CHK1 or RPA, although, none of these markers are specific for this process. In the current study, our aim was to explore the role of induced replication stress on radiosensitivity in cell lines with aberrant and intact cell cycle checkpoints.
Targeting Wee1 is an attractive strategy in patients with hepatocellular carcinoma. TP53 is commonly mutated or underexpressed in several human hepatocellular carcinoma cell lines (32), suggesting that many liver tumors are dependent on the G2 checkpoint to repair DNA damage. Additionally, abnormal expression of cell cycle regulators is believed to be a major contributor to hepatocellular carcinogenesis and progression (33–38). This vulnerability of HCC may be effectively targeted with cell cycle checkpoint inhibitors.
In this study we investigated AZD1775, a highly specific small molecule inhibitor of Wee1, as a radiosensitizing strategy for hepatocellular carcinoma. We found that AZD1775 is associated with significant radiosensitization and chemosensitization in p53 mutant, null, and wild type HCC cell lines. Therefore, we went on to investigate potential novel mechanisms of Wee1 targeted radiosensitization involving the induction of replication stress.
Methods and Materials
Cell Culture
The human hepatocellular carcinoma cell lines HepG2 (ATCC), Hep3B (ATCC), and Huh7 were maintained in F-12 or RPMI media supplemented with 10% fetal bovine serum (Life Technologies) and penicillin/streptomycin as previously described (41). AZD1775 (previously known as MK1775) was provided by the Cancer Therapy Evaluation Program (NCI). It was dissolved in dimethyl sulfoxide (Sigma) and stored in aliquots at −20°C.
Radiation Technique
Radiation was delivered using a Philips RT250 orthovoltage unit (Kimtron Medical) at a dose rate of approximately 2 Gy per minute. Dosimetry was carried out using an ionization chamber connected to an electrometer system directly traceable to a National Institute of Standards and Technology calibration.
Clonogenic Survival Assays
Unless otherwise specified, AZD1775 was added to cell culture plates 1 hour prior to radiation therapy and was left on for 24 hours, at which time the media was changed. After treatment was complete, cells were suspended and counted then plated at set densities based on the dose of radiation received. Cells were incubated until visible colonies were present. Colonies were fixed with methanol/acetic acid (7:1) and stained with crystal violet. The number of colonies containing ≥50 cells was determined. Enhancement ratios were calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose with drug treatment. For our cytotoxicity experiments with AZD1775 +/− 5-FU, cell were treated with 5-FU in dialyzed media for 24 hours (39), the media was changed and AZD1775 was added for 24 additional hours.
Flow Cytometry
After treatment, cells were trypsinized at the indicated time points, washed with PBS, and fixed in 70% ethanol at a concentration of 1–2 × 10^6 cells/ml. Cells were stained with rabbit anti-phosho-Histone H3 (S10, Millipore) or mouse anti-γH2AX antibody (JBW301, Millipore) overnight at 4°C followed by incubation with a FITC conjugated secondary antibody (Sigma) and propidium iodide. Cells were analyzed using a FACScan flow cytometer (Becton Dickinson) with FlowJo software (Tree Star).
Immunoblotting
Whole cell lysates were treated with SDS lysis buffer (10 mM Tris, 2% SDS) supplemented with phosphatase inhibitor (PhosSTOP) and a protease inhibitor (Roche) as previously described (26). Antibodies for Cdk1, p-Cdk1 (Y15), and GAPDH (Cell Signaling Technology) were used with the appropriate HRP labeled secondary antibodies.
Immunofluorescence
Cells were cultured and treated on coverslips in a 12-well chamber then fixed with 70% ethanol and stained with 4’,6-diamidino-2-phenylinodole (DAPI) and anti-γH2AX antibody. Images were obtained using an Olympus IX71 FluoView confocal microscope with a 60x oil objective. γH2AX was scored by determining the percentage of cells with >10 foci or with pan-nuclear staining.
Statistics
The mean, median, and standard error were calculated using Microsoft Excel software. A Student’s t test (paired when applicable) was used to compare treatment groups. All experiments were produced in at least triplicate to ensure reproducibility.
Results
CDK1 expression in human HCC samples
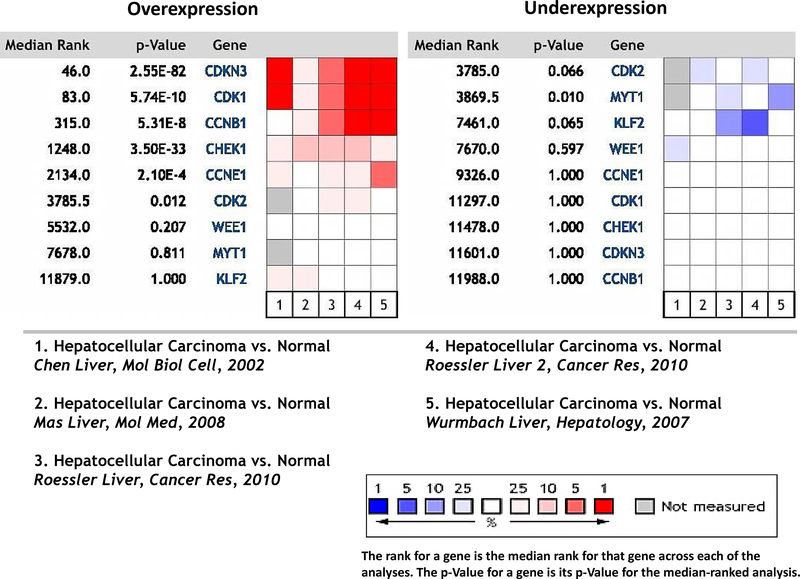
To determine the potential of Wee1 inhibition as a therapeutic strategy in hepatocellular carcinoma, we analyzed the Oncomine™ (www.oncomine.com) Database for potential predictors of Wee1 kinase inhibition sensitivity. The five available datasets for gene expression in HCC versus normal tissue (40–43) were combined and analyzed for genes involved in G1 and G2 checkpoint regulation. Significant overexpression of CDK1 (p=5.74E-10), CDKN3 p=2.55E-82), CCNB1 (p=5.31E-8) and CHEK1 (3.50E-33) were present in a combined analysis of all five datasets. WEE1 gene expression levels were not elevated compared to normal tissue. Additionally, decreased expression of MYT1 (p=0.01) and to a lesser extent CDK2 (p=0.066) and KLF2 (p=0.065) were present (Figure 1). These findings suggest hepatocellular carcinoma tumors have altered expression of several proteins associated with the G2 checkpoint compared to normal tissue.
Figure 1.
Five independent datasets from Oncomine were analyzed for potential predictors on AZD1775 sensitivity in hepatocellular carcinoma compared to normal tissue. Shown is a heat map of gene expression and the p value for each gene analyzed across all five datasets.
We also used the Oncomine database to examine a previously reported gene signature for AZD1775 responsiveness (44). Genes predictive of response included MCM10, CCNE1, CCNE2, FBXO5, and CLSPN. In our analysis, four of these five genes were overexpressed in hepatocellular carcinoma versus normal tissue including MCM10 (p=2.2E-5), CCNE1 (p=2.1E-4), and CCNE2 (p=0.004) (Figure S1). Overall, these findings suggest that Wee1 is an attractive target in hepatocellular carcinoma.
Wee1 inhibitor AZD1775 sensitizes hepatocellular carcinoma cell lines to the cytotoxic effects of radiation therapy
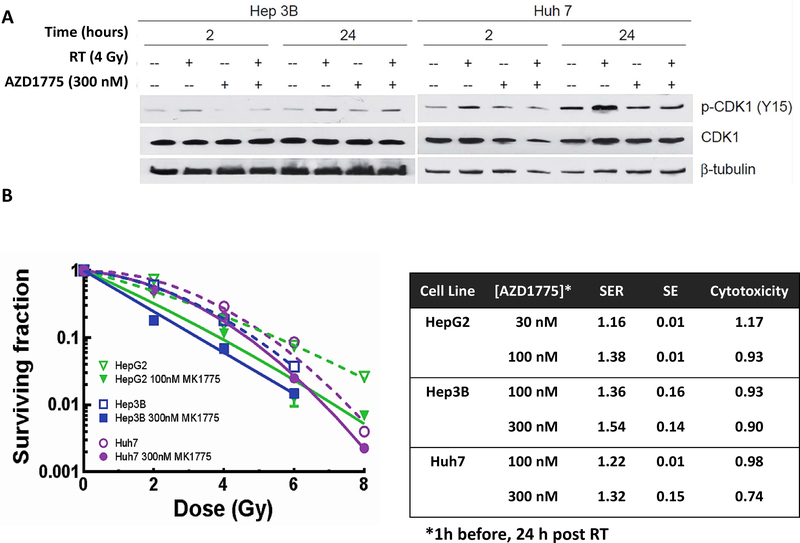
To start our evaluation of the potential radiosensitizing efficacy of Wee1 inhibition in HCC, we first determined the ability of AZD1775 to inhibit Wee1 kinase activity in response to radiation using CDK1 phosphorylation (Y15) as a surrogate. As anticipated, radiation induced CDK1 (Y15) phosphorylation in both Huh7 and Hep3B cells, consistent with CDK1 inactivation and G2 arrest (26–27). Treatment with AZD1775 attenuated radiation-induced CDK1 phosphorylation at multiple time points (Figure 2A).
Figure 2.
Radiosensitization with AZD1775. A, Treatment with the Wee1 kinase inhibitor AZD1775 one hour prior to radiation attenuates radiation induced phosphorylation of CDK1 at 2 and 24 hours. B, Clonogenic survival with AZD1775 and radiation. AZD1775 sensitized three HCC cells lines to radiation therapy at non-cytotoxic doses.
Given the effectiveness of AZD1775 to attenuate radiation induced CDK1 phosphorylation, we next studied the ability of AZD1775 to radiosensitize HCC cell lines. Cell lines were treated with clinically achievable concentrations of AZD1775 1 hour prior to and 24 hours after treatment with 0 to 8 Gy (Figure 2B). The 24 h time point was used as this is most consistent with clinical dosing and our prior work with this class of drug. Sensitizer enhancement ratios up to 1.54 were seen in the Hep3B cell line, 1.38 in the HepG2 cell line, and 1.32 in the Huh7 cell line. There was minimal cytotoxicity at 100 or 300 nM AZD1775 in the Hep3B or Huh7 cell lines. The HepG2 cell line was sensitive to treatment with 300 nM AZD1775 alone (survival 0.30) but had minimal cytotoxicity with 30 or 100 nM AZD1775. To further study the effect of drug alone, cell lines were treated with AZD1775 for 24, 48, and 72 hours (Figure S2). Increased exposure time to AZD1775 results in increased cytotoxicity.
We next examined the effect of combining AZD1775 with the anti-metabolite 5-FU given its widespread use in gastrointestinal cancers and its ability to deplete nucleotide pools. We have previously shown that treatment with 5-FU induces S-phase arrest in Hep3B, HepG2, and Huh7 cell lines (39). In this current study, Wee1 kinase inhibition with AZD1775 effectively sensitized HCC cell lines to treatment with 5-FU. HCC cells treated with 1 μM 5-FU and non-cytotoxic concentrations of AZD1775 had decreased clonogenic survival compared to treatment with 5-FU alone (figure S3).
Treatment with AZD1775 is associated with persistent DNA damage after radiation therapy
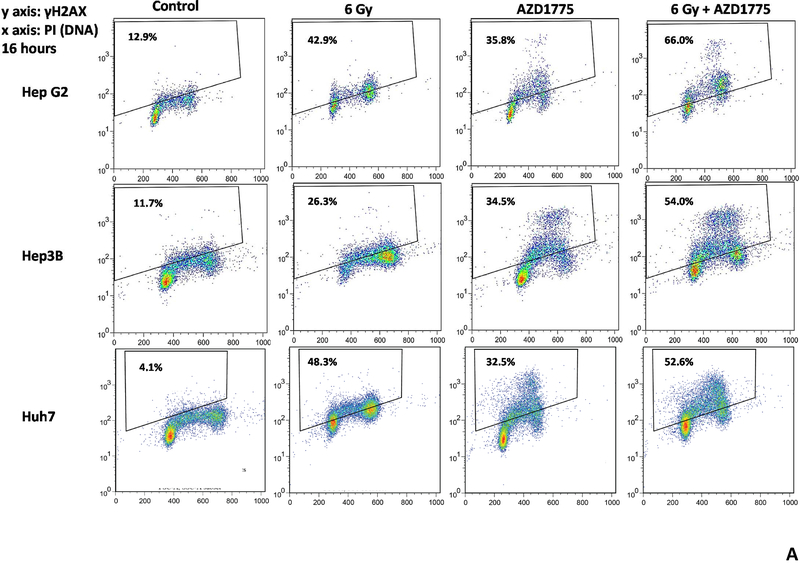
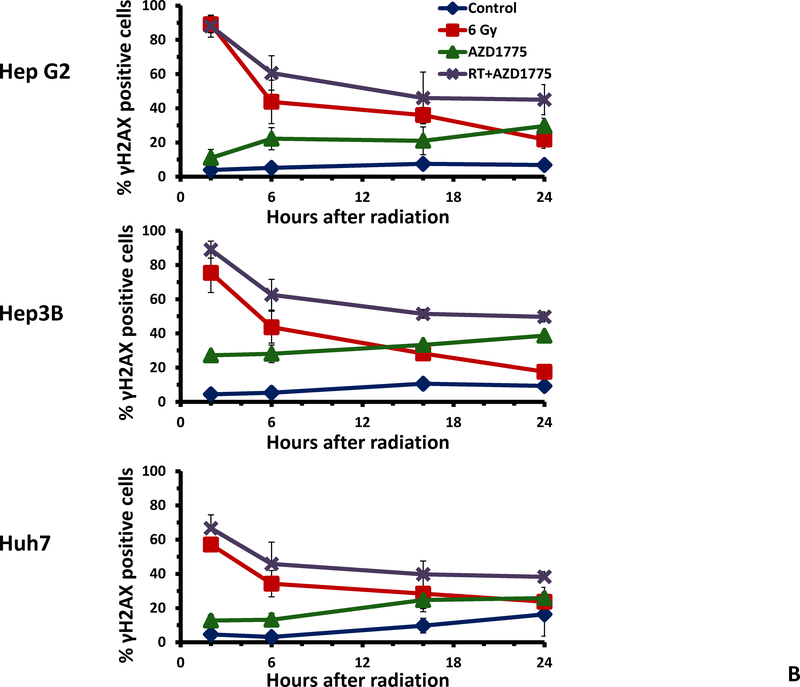
Given the ability of AZD1775 to radiosensitize and chemosensitize HCC cell lines, we next examined the effect of this drug on the repair of radiation induced DNA double strand breaks. The use of AZD1775 delayed resolution of γH2AX and resulted in a higher percentage of γH2AX positive cells 16 hours after radiation (figure 3A). Additionally, treatment with AZD1775 alone and AZD1775 with radiation produced intense pan-γH2AX staining of nuclei, a marker of replication fork collapse (indicated by the cluster of data points above the range of staining with radiation alone). In figure 3B, the time course of γH2AX staining is shown for each cell line. Resolution of DNA double strand breaks was delayed at 6, 16, and 24 hours with AZD1775 + RT compared to RT alone. There also was an increase in γH2AX staining with AZD1775 alone, as expected.
Figure 3.
Effects of AZD1775 on radiation induced DNA damage repair. HCC cell lines were treated with AZD1775 for 1 hour then irradiated with 6 Gy. At the indicated time points, cells were fixed and stained with anti-γH2AX antibody and PI then analyzed by flow cytometry. A, scatter plots at 16 hours after radiation therapy. Combined treatment with AZD1775 followed by radiation resulted in more γH2AX positive cells at 16 hours compared to radiation alone. Additionally, the intensity of γH2AX staining was greater with the combination of RT and AZD1775 in all cell lines. B, Time course of γH2AX positivity after radiation therapy for each cell line.
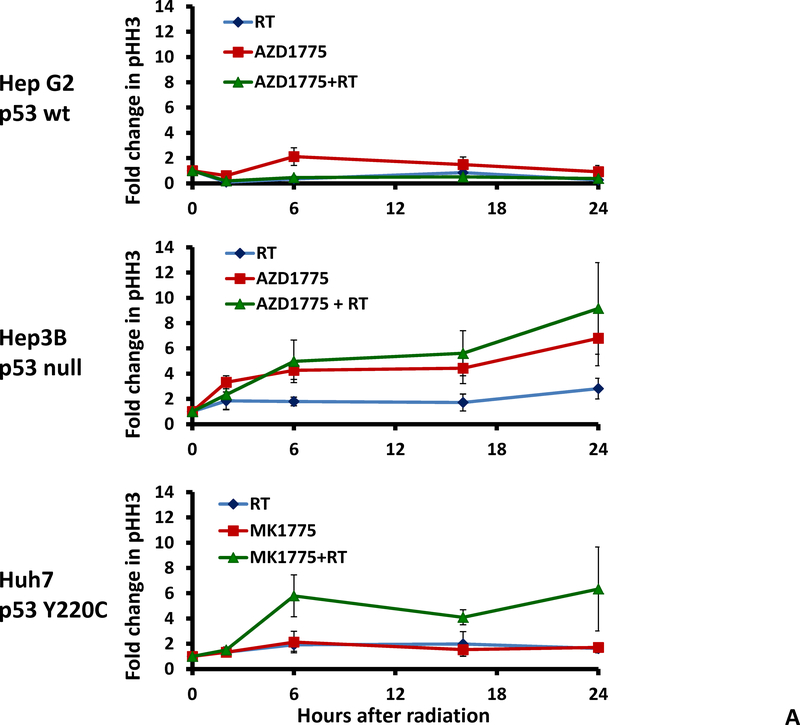
AZD1775 abrogates the radiation induced G2 checkpoint in HCC cell lines
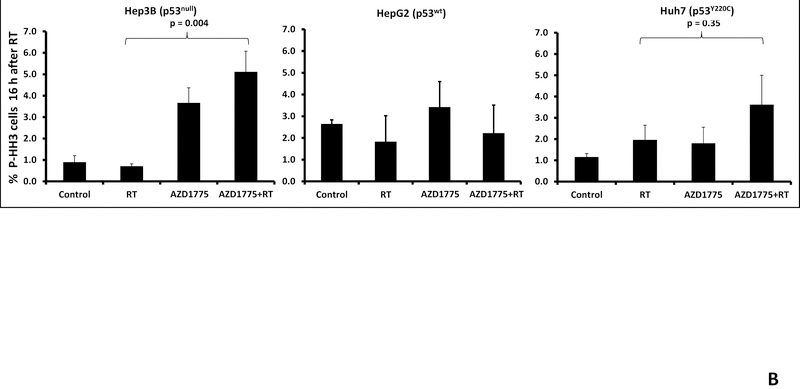
To further examine the effect of Wee1 inhibition on cell cycle checkpoint abrogation we studied phosphorylation of histone H3, a marker of G2 to M transition, using flow cytometry. Treatment with radiation alone resulted in G2 arrest in the Hep3B (p53 null) and Huh7 (p53 mutant) cell lines but not the HepG2 (p53 wild type) cell line. The percentage of cells staining positive for pHH3 increased with time following radiation treatment and Wee1 inhibition with AZD1775 (Figure 4A). The use of AZD1775 led to decreased G2 arrest after radiation therapy and increased pHH3 positivity consistent with G2 checkpoint abrogation and premature mitosis. A statistically significant increase in pHH3 positivity was seen in the p53 null Hep3B cell line but not in the p53 wild type HepG2 cell line (Figure 4B, p=0.004).
Figure 4.
Effect of AZD1775 on cell cycle progression. A, Flow cytometry using anti-phospho-histone H3 antibody was performed to determine the effect of AZD1775 on cell cycle progression from G2 to mitosis. B, In the Hep3B and Huh7 cell lines treatment with AZD1775 and radiation resulted in increased pHH3 staining. This effect was not seen in the HepG2 cell line, likely related to the ability of these cells to arrest in G1.
Effect of nucleoside supplementation on the ability of AZD1775 to sensitize cells to radiation therapy
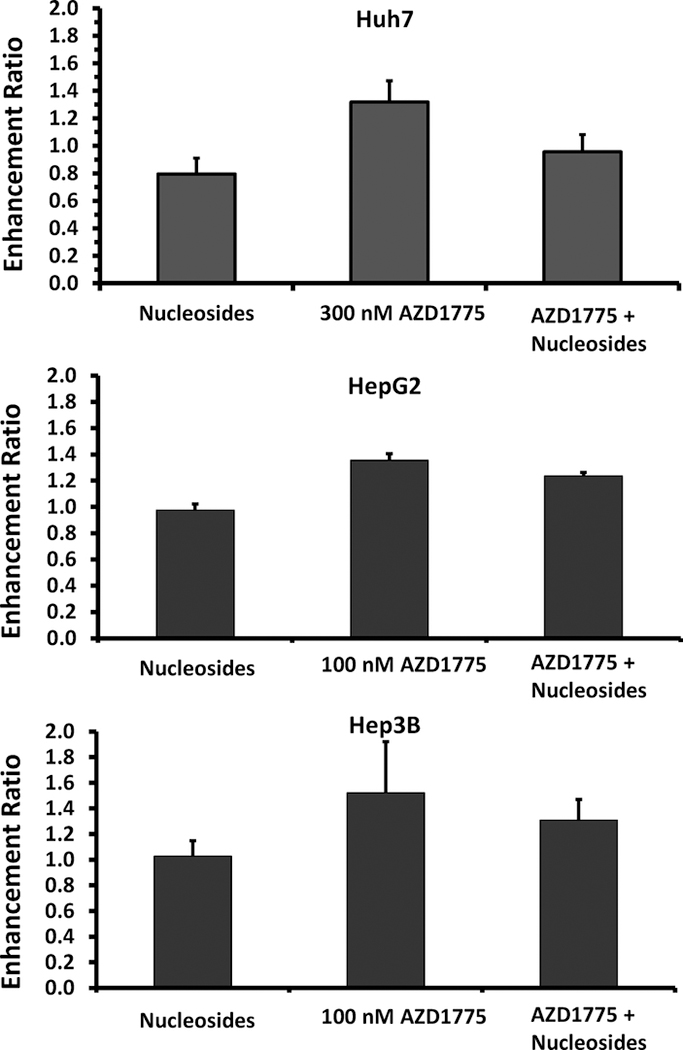
Prior reports have shown that Wee1 kinase inhibition can promote replication stress. To study the effect of AZD1775 on replication stress and nucleotide overconsumption, we examined the ability of nucleoside supplementation to reverse the radiosensitizing effects of Wee1 inhibition. Cell lines were treated with a combination of AZD1775, radiation, and nucleosides. Clonogenic survival assays showed that supplementation with nucleosides reverses the radiosensitizing ability of AZD1775 in Huh7 cells (enhancement ratio decreased from 1.32 +/−0.15 to 0.96 +/−0.13 (p=0.059, figure 5)). A non-significant reduction in enhancement ratio with nucleotide supplementation was seen in the Hep3B and HepG2 cell line.
Figure 5.
Effect of nucleoside rescue on radiosensitization by AZD1775. AZD1775 alone effectively sensitized each cell line to radiation therapy. Treatment with nucleosides alone had a mild radioprotective effect; whereas, treatment with nucleosides reduced the amount of radiosensitization seen with AZD1775 in each cell line.
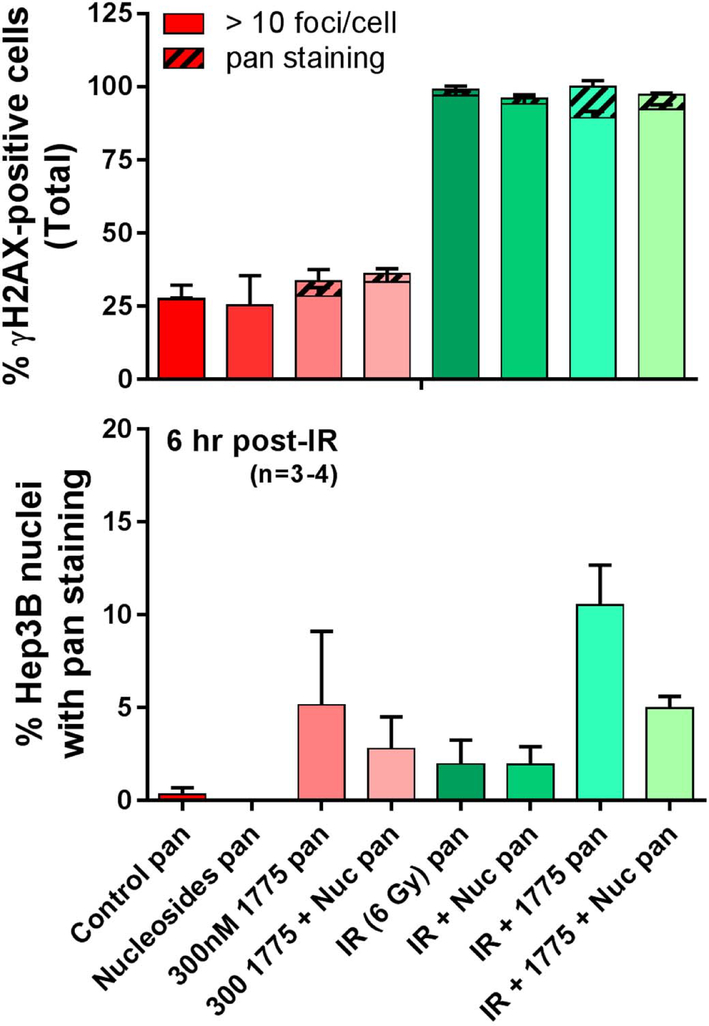
Pan-nuclear γH2AX staining is a surrogate of replication fork collapse. Given the potential of Wee1 inhibition to promote replication stress, we studied this marker using immunofluorescent microscopy in cell lines treated with AZD1775, radiation, and/or nucleosides. Treatment with AZD1775 alone or in combination with radiation resulted in increased γH2AX pan-nuclear staining at 6 hours compared to controls. Supplementation with nucleosides reduced the percentage of cells with pan-nuclear γH2AX staining after treatment with AZD1775 alone or in combination with radiation (Figure 6). These results provide a mechanism to describe the findings seen in the clonogenic assays from figure 5 and suggest that increased replication fork stress is one of the major mechanisms behind AZD1775 induced radiosensitization.
Figure 6.
To study the effect of AZD1775 on replication fork stress, cells were treated with AZD1775 and/or radiation therapy with or without nucleoside supplementation. The Hep3B cell line was used as this cell line demonstrated the greatest intensity γH2AX with combined treatment. Immunofluorescent microscopy was used to quantify cells containing more than 10 γH2AX foci and cells with pan-nuclear γH2AX staining. Six hours after radiation therapy, treatment with AZD1775 increased the percentage of cells with pan-γH2AX staining, and supplementation with nucleosides significantly lowered the amount of pan-γH2AX cells.
Discussion
In this study, we show that Wee1 inhibition with the small molecule AZD1775 radiosensitizes human HCC cell lines. Mechanistically, Wee1 inhibition with AZD1775 abrogates the G2 checkpoint and impairs repair of DNA double strand breaks. Additionally, we show for the first time that the radiosensitizing effect of AZD1775 is attenuated with nucleoside supplementation indicating that the overconsumption of nucleotides due to impaired CDK1 suppression plays a pivotal role in Wee1 inhibitor induced radiosensitization.
Sensitivity to Wee1 and Chk1 inhibition is classically associated with cell lines harboring an aberrant G1 checkpoint due to mutations or deletions of p53, p21, Rb, or other cell cycle regulators (19–21). As predicted, in our study we found effective radiosensitization in cell lines with impaired G1 checkpoints including Hep3B (TP53 null) and Huh7 (TP53 Y220C mutant) (32). Interestingly, AZD1775 also induced radiosensitization in cells with an intact G1 checkpoint (HepG2, TP53 wt). In the Hep3B and Huh7 cells, Wee1 inhibition led to increased histone H3 phosphorylation consistent with G2 checkpoint abrogation and premature mitosis. In the HepG2 cells (TP53 wt), treatment with AZD1775 had minimal effects on histone H3 phosphorylation. This effect is likely related to the ability of this cell line to arrest in G1. The implication of these findings is that Wee1 and Chk1 inhibitors may be effective radiosensitizers in tumors with an intact G1 checkpoint.
Another novel finding from our study is that Wee1 is a potential target in HCC given the high expression of CDK1. Prior reports have shown that replication stress is related to elevated CDK activity (30). CDK1 suppression by Wee1 controls replication initiation and nucleotide consumption, thereby protecting cells from this potentially lethal event (29). In our analysis of the Oncomine datasets, CDK1 was highly overexpressed in HCC compared to normal tissue. Prior reports have illustrated that Wee1 inhibition induces replication stress through the depletion of nucleotide pools (30, 45). In the current study, we found that nucleotide supplementation protected cells from radiosensitization with AZD1775 suggesting nucleotide deficiency/overconsumption is a potential mechanism of AZD1775 induced radiosensitization. Our Oncomine analysis also suggests that hepatocellular carcinoma has a high prevalence MYT1 underexpression. Prior studies show that low MYT1 (a negative regulator of CDK1) levels are predictive of response to drugs which abrogate cell cycle checkpoints (45–46). Given the relatively rapid proliferation of tumor cells compared to normal tissue, treatment with AZD1775 has the potential to selectively enhance the effects of therapy through the induction of replication stress. Therefore, we believe combining a Wee1 inhibitor with radiation therapy and chemotherapy has the potential to improve the therapeutic ratio in patients with hepatocellular carcinoma.
In summary, our study demonstrates the potential use of the Wee1 kinase inhibitor AZD1775 in combination with radiation therapy for patients with hepatocellular carcinoma. We are currently running a phase I dose escalation study with AZD1775 (NCT02037230) in patients with locally advanced pancreatic cancer receiving chemoradiation. Findings from this study will enhance our knowledge of the interaction between AZD1775 and radiation in normal tissue including the liver and small bowel. If this study shows that AZD1775 can be safely administered at radiosensitizing doses to patients receiving abdominal radiation therapy, then clinical trials for other gastrointestinal malignancies including HCC should be performed.
Supplementary Material
Figure S1. A five gene predictor of AZD1775 sensitivity (44) was tested in the Oncomine database containing five independent datasets of human HCC versus normal tissue. Four of the five genes are significantly upregulated in HCC.
Figure S2. Cytotoxicity of AZD1775. Cell lines were exposed to AZD1775 for 24, 48, or 72 hours. The drug was then removed and plates were incubated until colonies formed. Shown is the average surviving fraction and standard error for each condition.
Figure S3. Chemosensitization with AZD1775. HepG2 cells were treated with 5-FU for 24 hours then the media was changed and AZD1775 was added for 24 hours. At non-cytotoxic doses of AZD1775. A significant increase in cell kill was observed with the addition of AZD1775 to 1 μM 5-FU.
Footnotes
Conflict of Interest: No conflicts of interest exist for any of the authors listed on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence TS. The role of radiation in liver cancer. Clin Adv Hematol Oncol 2006;4:113–115. [PubMed] [Google Scholar]
- 3.Dawson LA, McGinn CJ, Lawrence TS. Conformal chemoradiation for primary and metastatic liver malignancies. Semin Surg Oncol 2003;21:249–255. [DOI] [PubMed] [Google Scholar]
- 4.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010; 138:52–64. [DOI] [PubMed] [Google Scholar]
- 5.Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013; 31:3980–3986. [DOI] [PubMed] [Google Scholar]
- 6.Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol 2006; 45:856–864. [DOI] [PubMed] [Google Scholar]
- 7.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013; 31:1631–1639. [DOI] [PubMed] [Google Scholar]
- 8.Ten Haken RK, Lawrence TS, Dawson LA. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys 2006;65:189–195. [DOI] [PubMed] [Google Scholar]
- 9.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010; 76:S10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010; 76:S94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003; 37:429–442. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015; 12:408–424. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. [DOI] [PubMed] [Google Scholar]
- 14.Morgan MA, Parsels LA, Maybaum J, et al. Improving the efficacy of chemoradiation with targeted agents. Cancer Discov 2014;4:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 1992; 257:1955–1957. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi M, Nagata A, Jinno S, et al. Wee1(+)-like gene in human cells. Nature 1991; 353:80–83. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J 1995; 14:1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGowan CH, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J 1993; 12:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawabe T G2 checkpoint abrogators as anticancer drugs. Mol Cancer Ther 2004; 3:513–519. [PubMed] [Google Scholar]
- 20.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer 2008; 98:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res 2007; 13:1955–1960. [DOI] [PubMed] [Google Scholar]
- 22.De Witt Hamer PC, Mir SE, Noske D, et al. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res 2011; 17:4200–4207. [DOI] [PubMed] [Google Scholar]
- 23.Hirai H, Iwasawa Y, Okada M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther 2009; 8:2992–3000. [DOI] [PubMed] [Google Scholar]
- 24.Bridges KA, Hirai H, Buser CA, et al. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin Cancer Res 2011; 17:5638–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarcar B, Kahali S, Prabhu AH, et al. Targeting radiation-induced G(2) checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines. Mol Cancer Ther 2011; 10:2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karnak D, Engelke CG, Parsels LA, et al. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin Cancer Res 2014;20:5085–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan MA, Parsels LA, Zhao L, et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res 2010;70:4972–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarts M, Sharpe R, Garcia-Murillas I, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov 2012; 2:524–539. [DOI] [PubMed] [Google Scholar]
- 29.Beck H, Nahse V, Larsen MS, et al. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J Cell Biol 2010; 188:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck H, Nahse-Kumpf V, Larsen MS, et al. Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol Cell Biol 2012; 32:4226–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaillard H, Garcia-Muse T, Aguilera A. Replication stress and cancer.Nat Rev Cancer 2015;15:276–289. [DOI] [PubMed] [Google Scholar]
- 32.Bressac B, Galvin KM, Liang TJ, et al. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci U S A 1990; 87:1973–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Chenivesse X, Henglein B, et al. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature 1990; 343:555–557. [DOI] [PubMed] [Google Scholar]
- 34.Nishida N, Fukuda Y, Komeda T, et al. Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer Res 1994; 54:3107–3110. [PubMed] [Google Scholar]
- 35.Zhang YJ, Jiang W, Chen CJ, et al. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res Commun 1993; 196:1010–1016. [DOI] [PubMed] [Google Scholar]
- 36.Yamagata M, Masaki T, Okudaira T, et al. Small hyperechoic nodules in chronic liver diseases include hepatocellular carcinomas with low cyclin D1 and Ki-67 expression. Hepatology 1999; 29:1722–1729. [DOI] [PubMed] [Google Scholar]
- 37.Chao Y, Shih YL, Chiu JH, et al. Overexpression of cyclin A but not Skp 2 correlates with the tumor relapse of human hepatocellular carcinoma. Cancer Res 1998; 58:985–990. [PubMed] [Google Scholar]
- 38.Masaki T, Shiratori Y, Rengifo W, et al. Cyclins and cyclin-dependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology 2003; 37:534–543. [DOI] [PubMed] [Google Scholar]
- 39.Cuneo KC, Davis MA, Feng MU, et al. Low dose rate radiosensitization of hepatocellular carcinoma in vitro and in patients. Transl Oncol 2014;7:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Cheung ST, So S, et al. Gene expression patterns in human liver cancers. Mol Biol Cell 2002;13:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mas VR, Maluf DG, Archer KJ, et al. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol Med 2009; 15:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roessler S, Jia HL, Budhu A, et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res 2010; 70:10202–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wurmbach E, Chen YB, Khitrov G, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 2007; 45:938–947. [DOI] [PubMed] [Google Scholar]
- 44.Mizuarai S, Yamanaka K, Itadani H, et al. Discovery of gene expression-based pharmacodynamic biomarker for a p53 context-specific anti-tumor drug Wee1 inhibitor. Mol Cancer 2009; 8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guertin AD, Li J, Liu Y, et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy. Mol Cancer Ther 2013; 12:1442–1452. [DOI] [PubMed] [Google Scholar]
- 46.Chow JP, Poon RY. The CDK1 inhibitory kinase MYT1 in DNA damage checkpoint recovery. Oncogene 2013; 32:4778–4788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A five gene predictor of AZD1775 sensitivity (44) was tested in the Oncomine database containing five independent datasets of human HCC versus normal tissue. Four of the five genes are significantly upregulated in HCC.
Figure S2. Cytotoxicity of AZD1775. Cell lines were exposed to AZD1775 for 24, 48, or 72 hours. The drug was then removed and plates were incubated until colonies formed. Shown is the average surviving fraction and standard error for each condition.
Figure S3. Chemosensitization with AZD1775. HepG2 cells were treated with 5-FU for 24 hours then the media was changed and AZD1775 was added for 24 hours. At non-cytotoxic doses of AZD1775. A significant increase in cell kill was observed with the addition of AZD1775 to 1 μM 5-FU.