Abstract
Introduction:
Quitlines are effective in helping smokers quit, but pediatrician quitline referral rates are low, and few parents who smoke use the service. This study compared enrollment of parents who smoke in the quitline using electronic referral with that using manual referral.
Study design:
The study was designed as a pragmatic RCT.
Setting/participants:
Participants were recruited from one large, urban pediatric primary care site in Philadelphia, Pennsylvania with a high percentage of low-income families. Participants included adult parents who smoked and were present at their child’s healthcare visit.
Intervention:
Pediatricians screened for tobacco use; smokers were given brief advice to quit and, if interested in quitting, were referred to the quitline. The eReferral (“warm handoff”) involved electronically sending parent information to the quitline (parent received a call within 24–48 hours). Control group procedures were identical to eReferral, except the quitline number was provided to the parent. Data were collected between March 2017 and February 2018 and analyzed in 2018.
Main outcome measures:
The primary outcome was the proportion of parents enrolled in quitline treatment. Secondary outcomes included parent factors (e.g., demographics, nicotine dependence, and quitting motivation) associated with successful enrollment. Number of quitline contacts was also explored.
Results:
During the study period, in the eReferral group, 10.3% (24 of 233) of parents who smoked and were interested in quitting enrolled in the quitline, whereas only 2.0% (5 of 251) of them in the control group enrolled in the quitline—a difference of 8.3% (95% CI=4.0, 12.6). Parents aged ≥50 years enrolled in the quitline more frequently. Although more parents in the eReferral group connected to the quitline, among parents who had at least one quitline contact, there was no significant difference in the mean number of quitline contacts between eReferral and control groups (mean, 2.04 vs 2.40 calls; difference, 0.36 [95% CI=0.35, 1.06]).
Conclusions:
Smoking parent eReferral from pediatric primary care may increase quitline enrollment and could be adopted by practices interested in increasing rates of parent treatment.
INTRODUCTION
Secondhand smoke (SHS) exposure affects approximately 40% of the U.S. pediatric population and increases the risk for acute respiratory infections, sudden infant death syndrome, and premature death, and exacerbates chronic respiratory diseases such as asthma.1,2 When parents quit smoking, they eliminate most of their children’s SHS exposure,1 decrease the risk of their children becoming smokers as adults,3 and increase their own life expectancy by an average of 10 years.4
Pediatricians are uniquely positioned to protect children from tobacco and SHS exposure by educating, motivating, and initiating tobacco dependence treatment for parents who smoke. Parents who smoke are often underserved medically, without regular contact with an adult healthcare clinician,5 but they see their child’s doctor an average of three to four times each year.6,7 More than 20% of parents who are smokers accompany their child to a healthcare visit.8,9 Parents expect pediatricians to ask about their smoking status and are receptive to their intervention, including prescribing cessation medications and connecting to treatment resources.10
There is strong evidence that advice and support delivered by healthcare professionals to smokers achieve quit rates of 5%–10% with minimal interventions and 15%–30% with more intensive interventions.11,12 Thus, the American Academy of Pediatrics, as detailed in Bright Futures: Guidelines for Health Supervision for Infants, Children and Adolescents,13 recommends that pediatricians provide smoking-cessation strategies to parents who smoke, including giving information about or telling them to call state tobacco quitlines—publicly funded and scalable programs that have shown impressive efficacy and real-world effectiveness in helping smokers quit.14,15 Further, limited research in pediatric settings suggests proactive referral to the quitline (offering enrollment in the quitline to a parent who smokes at the time of their child’s visit) is associated with greater quitline use.16,17 Despite evidence that parents are receptive to such intervention during routine pediatric care,10 pediatric healthcare settings deliver effective tobacco dependence assistance to parents <3% of the time, with 0% of parents reporting tobacco quitline enrollment at the visit.9
Electronic health records (EHRs) and clinical decision support (CDS) systems—tools that enhance decision making in the clinical workflow—may improve the quality and standardization of clinical interventions for tobacco use.15 EHR-based interventions have improved clinical care in numerous domains,18,19 and CDS systems have been shown to improve pediatricians’ adherence to national asthma guidelines, increase vaccination, and promote screening for maternal depression and developmental disorders.20–24 Further, CDS systems show potential to help pediatricians engage in treatment parents who smoke.25–28 Electronic quitline referral systems for parents who smoke that fit into the clinical workflow may reduce the burden of providing smoking-cessation services and thus increase the likelihood of parents engaging in treatment.25 In adult settings, CDS systems that connect interested patients to quitlines lead to a significant increase in the proportion of smokers who successfully enroll in treatment.29,30 Despite their promise, no pediatric-based studies have evaluated automated electronic referral (eReferral, where parent information is sent electronically to the quitline, with the quitline calling the parent—a “warm handoff”) in comparison with the manual “cold handoff” process (i.e., giving the parent the quitline phone number) for enrollment in the quitline program by parents who smoke. The study hypothesis was that eReferral, compared with the current process, would increase enrollment with the quitline.
METHODS
Study Population
This study used an RCT design to compare eReferral to the quitline with referral through the process of providing the phone number to the parent (control), at a large pediatric primary care site that is part of the Children’s Hospital of Philadelphia Care Network.31 The site was an urban, high-volume clinic in West Philadelphia that cares for a high percentage of low-income, black, non-Hispanic families who have Medicaid insurance, with high rates of tobacco use and SHS exposure. Parents aged ≥18 years who were present for the child’s healthcare visit (either well-child or acute), smoked cigarettes, were interested in quitting, and gave their permission to be referred to the tobacco quitline, were eligible. Interest in quitting was defined as a yes answer to the following suggested prompt and question: One of the best things you can do for your health and the health of your child is to quit smoking. Are you interested in quitting? Those not meeting these criteria were excluded.
The trial was designed to be pragmatic on the basis of Pragmatic Explanatory Continuum Indicator Summary (PRECIS-2) criteria, a tool to help researchers design clinical trials that best align with how the trial results are intended to be used. A pragmatic randomized trial is performed in clinical practice (the “real world”) with usual care as the comparison group, the goal of which is to help guide decisions on whether to deliver an intervention.32 As the goal was to ensure the applicability of the intervention to typical pediatric care, it was delivered to patients/parents who would receive it if it became the standard of care, was embedded within the EHR, and was implemented at a group level within primary care practice by pediatricians and nurse practitioners without direct involvement of the research team in the recruitment of subjects.33 The protocol was approved by the IRB at the Children’s Hospital of Philadelphia. A waiver of informed consent was obtained to maximize the real-world generalizability of this study—meaning the parents enrolled in the study would be most like the parents encountered in clinical practice. Without the waiver, parents referred to the quitline might have changed their behavior if they knew they were being studied, which could have decreased the likelihood that they would be willing to be referred (either electronically or through the manual process) to an evidence-based tobacco treatment service. Parents who reported interest in quitting smoking and gave their permission to be referred (and, if applicable, to have their name and telephone number sent) to the quitline were automatically enrolled into the study. The study was registered with clinicaltrials.gov before enrollment ().
Measures
Parents who smoke were randomized using a random number sequence programmed in Python, version 3.4.3, and automatically implemented, as pediatric clinicians entered parents’ smoking and contact information into the EHR using a previously tested CDS system. Simple randomization was used (rather than block/stratified randomization) as there was no a priori patient or parent characteristic that was felt to be strongly linked to acceptance of the quitline. Pediatric clinicians were not blinded to group allocation, given the pragmatic nature of the study.33
The eReferral (warm handoff) was embedded within a CDS system previously developed by the study team.26 This work was inspired by conceptual approaches from industrial engineering that focus on simplifying processes to maximize service receipt and minimize obstacles between screening and intervention delivery.34 The CDS tool interfaces seamlessly within the EHR (Epic) to prompt the pediatric clinician to ask the parent about smoking status and advise quitting. The tool collects information about parents interested in quitting, facilitates the prescription of nicotine replacement therapy (NRT), and guides appropriate documentation. NRT, with a prescription, is covered by most commercial health insurance plans as well as PA Medicaid. When clinicians entered into the CDS tool the names and telephone numbers of parents who smoke, an eReferral was sent to the state quitline (through a secure electronic communication via ShareFile) for those randomized to eReferral. The quitline then called the parent within 24–48 hours to enroll them in treatment. The tested approach extended an existing workflow for CDS already used for other aspects of pediatric tobacco-cessation treatment in the study practice, previously identified as an acceptable and usable tool, with most pediatric clinicians using the tool at most eligible visits.26 Pediatricians and nurse practitioners received brief, in-person training in use of the CDS tool, e-mails regarding use of the tool for reference, and, when requested, additional training through one-on-one discussion in a clinical setting.
All procedures implemented in the manual referral process were identical to those in the eReferral process (i.e., screening, prescribing, and guided documentation), with the exception that, for control parents, the telephone number for the quitline was provided in the paper form given at the conclusion of the visit, and parents were encouraged to call on their own (with no eReferral).
The Pennsylvania Free Quitline, operated by National Jewish Health, is funded by the Pennsylvania Department of Health and staffed by trained cessation counselors. The quitline operates from 7:00AM to 1:00AM Eastern Time, Monday to Sunday. Outside of these hours, callers hear Quit Tips (pre-recorded cessation information). Services can be accessed online, via pa.quitlogix.org. Counseling is delivered in English, Spanish, and Arabic, as well as in more than 240 additional languages through telephone interpreter services. All smokers who enroll in smoking-cessation treatment receive counseling and support consistent with accepted clinical practice guidelines.11 They can also receive up to 8 weeks of free NRT. The treatment includes as many as five proactive counseling calls, each designed to help develop problem-solving and coping skills, secure social support, provide guidance with NRT use (if applicable), and plan for long-term abstinence. This approach has been shown to be effective in helping smokers quit, including underserved populations comparable to this study population.14
The primary outcome of interest was smoker enrollment in the quitline, defined as the proportion of parents who smoked and were interested in quitting that enrolled in quitline treatment, compared across the intervention (eReferral) and control approaches. Enrollment in the quitline was defined as completing one treatment call with the quitline counselor. The primary analysis was based on an intention-to-treat approach and included all subjects randomized at their referral visit. The secondary objective was to explore patient and parent demographic characteristics and smoking behaviors associated with successful enrollment. The assessed parent characteristics included age, gender, nicotine dependence (measured by number of cigarettes smoked per day, categorized as either ten or fewer cigarettes or ten or more cigarettes per day, to minimize data collection by clinicians, while still creating actionable information for treatment, an approach used by others9), motivation regarding quitting (quit stage, assessed by asking if they were planning to quit in the next 30 days or 6 months), and NRT prescriptions. Pediatric patient characteristics included age (0–18 years), sex, race, ethnicity, insurance category, and asthma diagnosis. Age was organized into traditional developmental visit categories (infant visits, 0−1 year; early childhood, 1–5 years; middle childhood, 6−12 years; adolescence, ≥13 years). Presence of an asthma diagnosis, a highly prevalent chronic condition that is strongly associated with exposure to SHS,1 was selected as an easily captured diagnosis within the EHR to explore the association between child health conditions and parent quitline enrollment. Its prevalence makes it easier to determine whether outcomes differed by the presence of a chronic, smoking-related condition. Differences in the number of calls with the quitline and parent self-reported quit rates (among the subset of parents who actually enrolled in services) were compared across the study arms. The quitline collected a self-reported, 30-day abstinence parent quit rate at 6 months after enrollment for all enrolled parents whom they were able to contact 6 months later. Quit rate information was reported to the study team by the quitline team.
Statistical Analysis
Proportions for parent enrollment in the quitline were calculated and the magnitude and statistical significance of differences between the eReferral and control referral approach (chi-squared analysis) were evaluated. Multivariable logistic regression was used to assess demographic and behavioral factors, specifically patient age, parent age, patient asthma diagnosis, nicotine dependence, and motivation regarding quitting, which independently associated with successful enrollment. Binomial regression was used to compare contacts with the quitline across groups (n=6, for total number of possible calls, enrollment call plus five treatment calls). A priori power calculations demonstrated that enrolling approximately 450–500 parent subjects provided >80% power to detect an absolute difference of at least 6% (3% vs 9% rate of enrollment in the quitline for control vs intervention parents, similar to adult studies) in enrollment between the intervention and control groups, which is a clinically meaningful difference.29 Data were collected between March 2017 and February 2018 and were analyzed in October and November 2018 using R, version 3.4.1, and Stata, version 15.
RESULTS
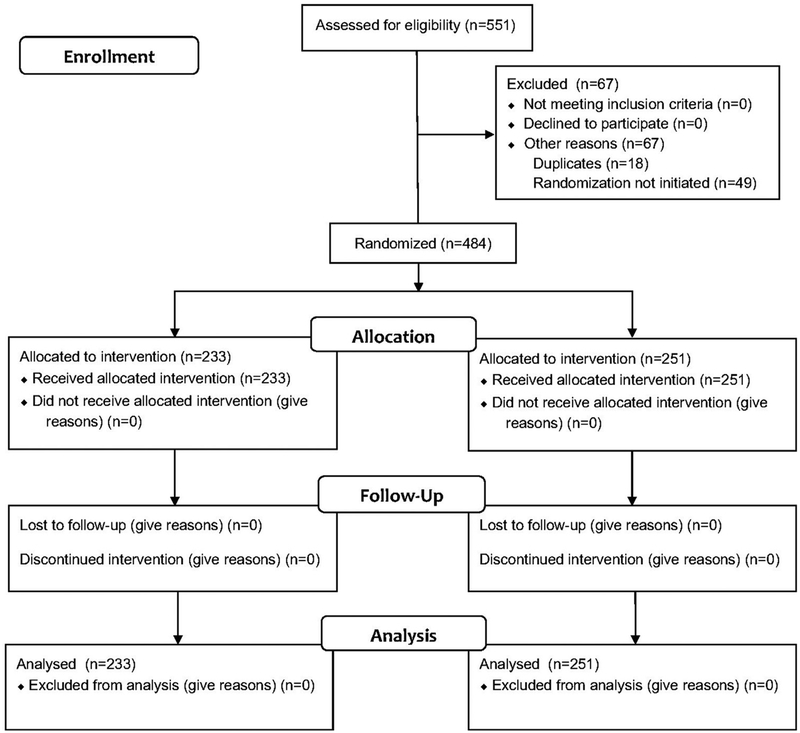
During the study period from March 17, 2017 to February 21, 2018, clinicians screened 6,479 parents for tobacco use. A total of 781 parents/caregivers were identified as smokers, which resulted in a 12.1% overall prevalence of parents who smoked and who were present at their child’s visit. Of the 781 parents who smoked, 551 (70.6%) were interested in quitting and being referred to the quitline, and 484 were successfully randomized: 233 parents to the eReferral group and 251 to the control group (Figure 1 and Appendix Figure 1, available online). Allocation was not exactly 1:1 based on the use of simple randomization. The parent study population was predominantly black, non-Hispanic, and aged 24–49 years. Most of the children whose parents were enrolled in the study had Medicaid insurance, and 29% of children had a diagnosis of asthma. Most participants reported smoking fewer than ten cigarettes per day and were motivated to quit smoking in the next 30 days. The demographic and smoking-related characteristics of the participants were balanced across the study groups (Table 1).
Figure 1.
CONSORT diagram.
Table 1.
Characteristics of Children/Parents According to Study Group
| Characteristic | eReferral n=233 (48.1%)a n (%) |
Control n=251 (51.9%)a n (%) |
p-valueb |
|---|---|---|---|
| Patient age, years | 0.508 | ||
| <1 | 43 (18.5) | 42 (16.9) | |
| 1–5 | 89 (38.2) | 82 (32.9) | |
| 6–12 | 78 (33.5) | 96 (38.6) | |
| ≥13 | 23 (9.9) | 29 (11.6) | |
| Parent age, years | 0.792 | ||
| 18–24 | 20 (8.6) | 23 (9.3) | |
| 25–34 | 107 (46.1) | 122 (49.2) | |
| 35–49 | 87 (37.5) | 82 (33.1) | |
| ≥50 | 18 (7.8) | 21 (8.5) | |
| Patient sex | 0.570 | ||
| Female | 103 (44.2) | 118 (47.2) | |
| Patient race | 0.705 | ||
| Black | 213 (91.4) | 225 (90) | |
| Other | 20 (8.6) | 25 (10) | |
| White | 5 (2.1) | 4 (1.6) | |
| Asian | 2 (0.9) | 3 (1.2) | |
| NHPIc | 0 (0) | 1 (0.4) | |
| Multiple | 4 (1.7) | 2 (0.8) | |
| Other | 9 (3.9) | 15 (6) | |
| Patient ethnicity | 0.442 | ||
| Not Hispanic or Latino | 213 (91.4) | 240 (96.8) | |
| Hispanic or Latino | 20 (8.6) | 8 (3.2) | |
| Insurance status (child) | 0.520 | ||
| Private | 33 (14.2) | 27 (10.8) | |
| Medicaid | 197 (84.5) | 219 (87.6) | |
| Self-pay | 3 (1.3) | 4 (1.6) | |
| Asthma diagnosis (child) | 0.755 | ||
| No asthma | 168 (72.1) | 176 (70.4) | |
| Asthma | 65 (27.9) | 74 (29.6) | |
| Cigarettes smoked perdayd | 0.132 | ||
| <10 | 138 (59.7) | 165 (66.8) | |
| ≥10 | 93 (40.3) | 82 (33.2) | |
| Quit stagee | 0.424 | ||
| 30 days | 124 (53.9) | 133 (54.3) | |
| 6 months | 76 (33) | 71 (29) | |
| >6 months | 30 (13) | 41 (16.7) |
Rows may not add up to total owing to either not reported or missing data.
p-value via Pearson’s chi-squared.
Native American or other Pacific Islander.
Measurement of nicotine dependence.
Motivation regarding quitting, assessed by asking if parent was planning to quit in the next 30 days or 6 months. NHPI, Native Hawaiian and Pacific Islander.
In the primary analysis, in the eReferral group, 10.3% (24 of 233) of parents who smoked enrolled in the quitline, compared with 2.0% (5 of 251) in the control referral group—an absolute difference of 8.3% (95% CI=4.0, 12.6) in enrollment compared across groups (p<0.001). There were no differences between groups in rates of provided NRT prescriptions, with approximately 87% of parents who smoked prescribed NRT through an existing office workflow (Table 2).
Table 2.
Outcome Variables Across Study Groups
| Characteristic | eReferral n=233 (48.1%)a n (%) |
Control n=251 (51.9%)a n (%) |
p-value |
|---|---|---|---|
| Enrolled in quitline | <0.001b | ||
| No | 209 (89.7) | 245 (98) | |
| Yes | 24 (10.3) | 5 (2) | |
| NRT prescribed | 0.925b | ||
| No | 29 (12.4) | 33 (13.1) | |
| Yes | 204 (87.6) | 218 (86.9) | |
| Number of calls | <0.001c | ||
| No calls | 209 (89.7) | 245 (98) | |
| One call (enrollment only) | 7 (3.0) | 0 (0) | |
| Two calls | 12 (5.2) | 3 (1.2) | |
| Three or more calls | 5 (2.1) | 2 (0.8) |
Note. Boldface indicates statistical significance (p<0.05).
Rows may not add up to total owing to either not reported or missing data.
Via Pearson’s chi-squared.
Via binomial regression.
NRT, nicotine replacement therapy.
In the secondary analysis, the only factor independently associated with quitline enrollment across either group was parent age, with parents aged >50 years being more likely to successfully enroll in treatment. An asthma diagnosis in the child, child age, number of cigarettes smoked by the parent, and parent motivation to quit (quit stage) were not associated with enrollment (Table 3). Upon controlling for these demographic and clinical factors, the eReferral parents remained significantly more likely to enroll in quitline treatment relative to the control group (OR=7.78, 95% CI=2.81, 27.90).
Table 3.
Characteristics Associated With Successful Quitline Enrollment
| Characteristics | OR (95% CI) | p-valuea |
|---|---|---|
| Study arm | <0.001 | |
| Control | 1.00 (ref) | |
| eReferral | 7.78 (2.81, 27.90) | |
| Patient age, years | 0.15 | |
| <1 | 1.00 (ref) | |
| 1–5 | 0.48 (0.13, 1.72) | |
| 6–12 | 0.61 (0.18, 2.12) | |
| ≥13 | 1.82 (0.49, 6.94) | |
| Parent age, years | 0.028 | |
| 18–34 | 1.00 (ref) | |
| 35–49 | 2.38 (0.92, 6.50) | |
| ≥50 | 5.10 (1.46,17.32) | |
| Asthma diagnosis (child) | 0.85 | |
| No asthma | 1.00 (ref) | |
| Asthma | 1.10 (0.40, 2.80) | |
| Cigarettes smoked per dayb | 0.086 | |
| <10 | 1.00 (ref) | |
| ≥10 | 2.07 (0.90, 6.50) | |
| Quitstagec | 0.35 | |
| 30 days | 1.00 (ref) | |
| 6 months | 0.47 (0.15,1.27) | |
| >6 months | 0.67 (0.15, 2.21) |
Note. Boldface indicates statistical significance (p<0.05). ORs from multivariable logistic regression model of characteristics associated with successful quitline enrollment.
Via Wald chi-squared.
Measurement of nicotine dependence.
Motivation regarding quitting, assessed by asking if they were planning to quit in the next 30 days or 6 months.
Among all participants referred to the quitline, parents contacted through the eReferral approach had a greater number of treatment contacts than those who contacted through the control approach (mean, 0.21 vs 0.05 calls; difference, 0.16 [95% CI=0.06, 0.26]). Although more parents in the eReferral group connected to the quitline, among smokers who enrolled in the quitline, there was no significant difference in the mean number of quitline contacts between eReferral and control groups (mean, 2.04 vs 2.40 calls; difference, 0.36 [95% CI=0.35, 1.06]).
For 6-month self-reported parent rates (collected by the quitline), the vast majority of parents were lost to follow-up. The quitline was only able to follow up with four parents total (three in the eReferral group, one in the control group). One parent reported quitting in the eReferral group, compared with zero parents in the control group. Given the pragmatic nature of the study design, the study team did not have consent to separately follow up with the parent participants; thus, quit rates could not be assessed.
DISCUSSION
Directly connecting an urban population of predominantly low-income parents who smoke to the quitline via an eReferral process resulted in an 8-percentage point increase in treatment enrollment compared with providing referral information on a printed form and asking smokers to call on their own. This represents a fivefold increase in successful referrals. Quitline enrollment was associated with parents aged ≥50 years. An asthma diagnosis in the child, child age, number of cigarettes smoked by the parent, and parent motivation to quit were not associated with successful enrollment. Parents in the eReferral group also completed a significantly greater number of calls with the quitline; however, among parents who made initial contact with the quitline, the overall number of calls was not different. Owing to loss to follow-up and the pragmatic study design, quit rates could not be assessed.
Results of this RCT show that eReferral to the quitline is effective in helping parents engage in treatment. When using an eReferral process as a “warm handoff” to the quitline, treatment enrollment rates increased to one of ten parents. The pragmatic study design—conducted in a real-world clinical setting as a part of routine care—exposes how using the current process of referring parents to the quitline (telling them to connect with the resource on their own) led to only one of 50 parents successfully enrolling in treatment. Helping parents who smoke quit helps improve their individual health and protects their children from the harms of SHS exposure.1 Quitline-delivered counseling is convenient and more acceptable than in-person counseling, entails no childcare costs, eliminates transportation time and costs, and has demonstrated strong effectiveness.14,35 Using simple clinical tools to guide referral to the smoker quitline by pediatricians for parents who smoke, creates a bridge between pediatric healthcare settings and adult treatment programs and decreases logistic barriers to parents receiving services. This simple approach could be generalized to other settings.
Although eReferral to the quitline led to 10% of parents successfully enrolling, 90% of parents still did not engage in evidence-based tobacco treatment counseling. Quitline enrollment was associated with older parent age but not with other factors associated with parents successfully quitting, such as quitting motivation.36 Older parents may be more receptive to tobacco dependence interventions, or this may be an incidental finding. Not one individual demographic or behavioral factor may have as strong an influence as having a pediatrician advise quitting as a means to improve the health of the child and offer treatment.36 Individuals do not move through predictable stages of behavioral change,37 and a proactive approach to tobacco treatment in adult contexts increases the likelihood that smokers use the treatment and successfully quit (compared with usual care).38 Next steps could involve referring all parent who smoke with any interest in quitting to the quitline regardless of their level of motivation to quit. With current trial results indicating that eReferral to the quitline should serve as a baseline, more work is still needed in pediatric healthcare settings to increase parent engagement in treatment.39
Though pediatric healthcare settings can serve as a catalyst for change of behavior for parents who smoke, additional efforts are needed to support parents in achieving the ultimate outcome of quitting. Follow-up with the quitline after 6 months was low, preventing the ability to collect more robust smoking outcome data. Because of the limitations of the study design—receiving a waiver of parental consent to evaluate a method to increase enrollment in the quitline—the study team did not have consent to follow up with the parents outside of their interactions with the quitline. Tobacco dependence is a chronic disease that requires ongoing assessment and repeated intervention.11,40 Leveraging existing EHR technologies to streamline connections to easily accessible and freely available services is an important first step. According to one meta-analysis, quit rates were higher for smokers who receive multiple proactive counseling calls after the smoker called the quitline, increasing the relative success of quitting by 25% to 50%; three or more proactive calls increased the chances of quitting, with some evidence of a dose-response relationship.14 Given the widespread benefits of tobacco cessation, longer-term follow-up that supports continued parent engagement could include reminders to discuss smoking at the next office visit, text messages to check in with parents and reinforce behavioral change, telephone outreach, additional in-person services available at the office, or even home visits by a community health worker.
Limitations
Several additional limitations should be noted. This study was conducted at only one large, urban pediatric clinic, serving a predominantly Medicaid patient population. Though not nationally representative of the U.S. pediatric population, this study targeted a patient population most at risk for SHS exposure and its health consequences— black, non-Hispanic children living in poverty.2 Further, study participants may not be representative of the typical smoker, for example, as the majority smoked fewer than ten cigarettes per day. Although national estimates suggest that most daily smokers smoke more than ten cigarettes per day,41 black adult smokers smoke fewer cigarettes per day than their white counterparts.1 Overall, these limitations are mitigated by the following: (1) the pragmatic nature of the study—participants were the individuals who would receive the intervention if it became usual care; and (2) the use of a widely available community-based resource (the quitline). Because of the pragmatic nature of the trial, pediatricians were not blinded to the intervention and the randomly assigned group was not masked to the providers. The lack of blinding may have influenced pediatrician smoking-cessation counseling in unanticipated ways. Additionally, the pragmatic nature of the study design with a waiver of informed consent limited the collection of additional data, including additional information about the parent (such as gender, education level, or actual use of NRT). Finally, the CDS software used for this study is not widely available. However, for healthcare settings lacking this particular software, EHRs increasingly allow for the standardized transmission of information to external resources through, for example, new automated eReferral programs to state quitlines (https://pa.quitlogix.org/en-US/Just-Looking/Health-Professional/How-to-Refer-Patients/eReferral). The current results provide evidence to promote this functionality more broadly.
CONCLUSIONS
For parents who smoke, eReferral to quitlines yielded an 8-percentage point increase, representing a fivefold improvement, in evidence-based cessation treatment enrollment compared with the current recommended standard of care among parents seen in a pediatric primary care clinic. Smoking parent eReferral from pediatric primary care may increase quitline engagement and could be adopted by practices interested in increasing the rates of enrollment. These findings may help inform clinical and policy decisions regarding incorporating a simple screening and treatment approach for parents who smoke in pediatric settings by providing evidence for adoption of this intervention into real-world clinical practice. This provides support for vendors to integrate this functionality into EHRs and for quitlines to accept these eReferrals.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the network of primary care clinicians, their patients, and families for their contribution to this project and clinical research facilitated through the Pediatric Research Consortium at Children’s Hospital of Philadelphia. They also thank the coaches at National Jewish Health for providing services to parents. The authors thank Mark Ramos for his help with data collection and Thomas Ylioja for his help with data collection and review of the manuscript. All phases of the study were supported by an Academic Pediatric Association Bright Futures Young Investigator Award to Dr. Jenssen, funded by the Health Resources and Services Administration/Maternal and Child Health Bureau in cooperation with the American Academy of Pediatrics (Federal Grant U04MC07853–10) The research presented in this paper is that of the authors and does not reflect the official policy of these organizations. IRB: Children’s Hospital of Philadelphia, IRB 16–012868.
Dr. Fiks and Dr. Grundmeier are co-inventors of the Care Assistant software that was used to provide clinical decision support in this study. Ms. Baca was an employee of National Jewish Health, the service provider for the Pennsylvania Quitline, during the study period. Dr. Muthu, Ms. Kelly, and Dr. Shults have no relevant conflicts of interest to disclose. The study sponsor did not have any role in the study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
No financial disclosures were reported by the authors of this paper.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2019.03.005.
Trial registration: This study is registered at www.clinicaltrials.gov .
REFERENCES
- 1.HHS. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Atlanta, GA: HHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Tsai J, Homa DM, Gentzke AS, et al. Exposure to secondhand smoke among nonsmokers − United States, 1988−2014. MMWR Morb Mortal Wkly Rep. 2018;67(48):1342–1346. 10.15585/mmwr.mm6748a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Exter Blokland EAW, Engels RCME, Hale WW, Meeus W, Willemsen MC. Lifetime parental smoking history and cessation and early adolescent smoking behavior. Prev Med. 2004;38(3):359–368. 10.1016/j.ypmed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 5.Ku L, Bruen BK, Steinmetz E, Bysshe T. Medicaid tobacco cessation: big gaps remain in efforts to get smokers to quit. Health Aff (Millwood). 2016;35(1):62–70. 10.1377/hlthaff.2015.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newacheck PW, Stoddard JJ, Hughes DC, Pearl M. Health insurance and access to primary care for children. N Engl J Med. 1998;338(8): 513–519. 10.1056/NEJM199802193380806. [DOI] [PubMed] [Google Scholar]
- 7.Hatch B, Angier H, Marino M, et al. Using electronic health records to conduct children’s health insurance surveillance. Pediatrics. 2013; 132(6):e1584–e1591. 10.1542/peds.2013-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winickoff JP, Tanski SE, McMillen RC, et al. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics. 2005;115(4):1013–1017. 10.1542/peds.2004-1372. [DOI] [PubMed] [Google Scholar]
- 9.Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics. 2013;132(1):109–117. 10.1542/peds.2012-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen LJ, Noach MB, Winickoff JP, Hovell MF. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. 2012;129(1):141–152. 10.1542/peds.2010-3209. [DOI] [PubMed] [Google Scholar]
- 11.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update Clinical Practice Guideline. Rockville, MD: HHS, Public Health Service; 2008. [Google Scholar]
- 12.Siu AL US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement for interventions for tobacco smoking cessation. Ann Intern Med. 2015;163(8):622–634. 10.7326/M15-2023. [DOI] [PubMed] [Google Scholar]
- 13.Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children and Adolescents. 4th ed Elk Grove Village, IL: American Academy of Pediatrics; 2017. [Google Scholar]
- 14.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013;8: CD002850 10.1002/14651858.CD002850.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database Syst Rev. 2014;12: CD008743 10.1002/14651858.CD008743.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winickoff JP, Buckley VJ, Palfrey JS, Perrin JM, Rigotti NA. Intervention with parental smokers in an outpatient pediatric clinic using counseling and nicotine replacement. Pediatrics. 2003;112(5): 1127–1133. 10.1542/peds.112.5.1127. [DOI] [PubMed] [Google Scholar]
- 17.Drehmer JE, Hipple B, Nabi-Burza E, et al. Proactive enrollment of parents to tobacco quitlines in pediatric practices is associated with greater quitline use: a cross-sectional study. BMC Public Health. 2016;16:520 10.1186/s12889-016-3147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shojania KG, Jennings A, Mayhew A, et al. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009;3:CD001096 10.1002/14651858.CD001096.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157(1):29–43. 10.7326/0003-4819-157-1-201207030-00450. [DOI] [PubMed] [Google Scholar]
- 20.Bell LM, Grundmeier R, Localio R, et al. Electronic health record-based decision support to improve asthma care: a cluster-randomized trial. Pediatrics. 2010;125(4):e770–e777. 10.1542/peds.2009-1385. [DOI] [PubMed] [Google Scholar]
- 21.Fiks AG, Grundmeier RW, Mayne S, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114–1124. 10.1542/peds.2012-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiks AG, Grundmeier RW, Biggs LM, Localio AR, Alessandrini EA. Impact of clinical alerts within an electronic health record on routine childhood immunization in an urban pediatric population. Pediatrics. 2007;120(4):707–714. 10.1542/peds.2007-0257. [DOI] [PubMed] [Google Scholar]
- 23.Carroll AE, Biondich P, Anand V, Dugan TM, Downs SM. A randomized controlled trial of screening for maternal depression with a clinical decision support system. J Am Med Inform Assoc. 2013;20(2): 311–316. 10.1136/amiajnl-2011-000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll AE, Bauer NS, Dugan TM, et al. Use of a computerized decision aid for developmental surveillance and screening: a randomized clinical trial. JAMA Pediatr. 2014;168(9):815–821. 10.1001/jamapediatrics.2014.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharifi M, Adams WG, Winickoff JP, et al. Enhancing the electronic health record to increase counseling and quit-line referral for parents who smoke. Acad Pediatr. 2014;14(5):478–484. 10.1016/j.acap.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Jenssen BP, Bryant-Stephens T, Leone FT, Grundmeier RW, Fiks AG. Clinical decision support tool for parental tobacco treatment in primary care. Pediatrics. 2016;137(5):e20154185 10.1542/peds.2015-4185. [DOI] [PubMed] [Google Scholar]
- 27.Jenssen BP, Shelov ED, Bonafide CP, et al. Clinical decision support tool for parental tobacco treatment in hospitalized children. Appl Clin Inform. 2016;7(2):399–411. 10.4338/ACI-2015-12-RA-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahabee-Gittens EM, Dexheimer JW, Tabangin M, et al. An electronic health record-based strategy to address child tobacco smoke exposure. Am J Prev Med. 2018;54(1):64–71. 10.1016/j.amepre.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173(6):458–464. 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidrine JI, Shete S, Li Y, et al. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med. 2013;45(6):737–741. 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiks AG, Grundmeier RW, Margolis B, et al. Comparative effectiveness research using the electronic medical record: an emerging area of investigation in pediatric primary care. J Pediatr. 2012;160(5): 719–724. 10.1016/j.jpeds.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 33.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454–463. 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 34.Womack J, Daniel J. Lean thinking: Banish Waste and Create Wealth in Your Corporation. New York, NY: Simon & Schuster; 2003. [Google Scholar]
- 35.Kruger J, O’Halloran A, Rosenthal AC, Babb SD, Fiore MC. Receipt of evidence-based brief cessation interventions by health professionals and use of cessation assisted treatments among current adult cigarette-only smokers: National Adult Tobacco Survey, 2009−2010. BMC Public Health. 2016;16:141 10.1186/s12889-016-2798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahabee-Gittens EM, Collins BN, Murphy S, et al. The parent−child dyad and risk perceptions among parents who quit smoking. Am J Prev Med. 2014;47(5):596–603. 10.1016/j.amepre.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cahill K, Lancaster T, Green N. Stage-based interventions for smoking cessation. Cochrane Database Syst Rev. 2010;11:CD004492 10.1002/14651858.CD004492.pub4. [DOI] [PubMed] [Google Scholar]
- 38.Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: a randomized clinical trial. JAMA Intern Med. 2015;175(2):218–226. 10.1001/jamainternmed.2014.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baxi R, Sharma M, Roseby R, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2014;3:CD001746 10.1002/14651858.CD001746.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg MB, Schmelzer AC, Richardson DL, Foulds J. The case for treating tobacco dependence as a chronic disease. Ann Intern Med. 2008;148(7):554–556. 10.7326/0003-4819-148-7-200804010-00012. [DOI] [PubMed] [Google Scholar]
- 41.Jamal A, Phillips E, Gentzke AS, et al. Current cigarette smoking among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(2):53–59. 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.