Abstract
A novel luminescent azo-linked polymer (ALP) has been constructed from 1,3,6,8-tetra(4-aminophenyl)pyrene using a copper(I)-catalyzed oxidative homocoupling reaction. The polymer displays high porosity with a Brunauer–Emmett–Teller surface area of 1259 m2 g–1 and narrow pore size distribution (1.06 nm) and is able to take up a significant amount of CO2 (2.89 mmol g–1) at 298 K and 1.00 bar with a high isosteric heat of adsorption of 27.5 kJ mol–1. Selectivity studies applying the ideal adsorbed solution theory revealed that the novel polymer has moderately good selectivities for CO2/N2 (55.1) and CO2/CH4 (10.9). Furthermore, the ALP shows fluorescence quenching in the presence of Hg2+, Pb2+, Tl+, and Al3+ ions. Compared with these ions, the ALP showed no sensitivity to light metal ions such as Na+, K+, and Ca2+ in ethanol–water solution, clearly indicating the high selectivity of the ALP toward heavy metal ions. The exceptional physiochemical stability, high porosity, and strong luminescence make this polymer an excellent candidate as a fluorescent chemical sensor for the detection of heavy metal ions.
1. Introduction
Porous organic polymers (POPs) are an emerging class of materials that have witnessed increasing interest because of their applications in gas storage and separation,1−4 catalysis,5−10 sensing,11−15 and optoelectronics.16,17 Such diverse applications stem from the numerous available building blocks and various synthetic routes that allow the design and construction of different POPs tailored for specific applications. Because of their exceptional physiochemical stability, high surface area, and structure/function tunability, POPs have been widely explored for the capture and separation of carbon dioxide (CO2), a gas that is linked directly to climate change and global warming.1−4,18,19 It is well documented that POPs containing polar chemical functionalities and Lewis basic sites show enhanced CO2 binding energy, presumably via hydrogen bonding, dipole–quadrupole interactions, and Lewis acid–base interactions, thus leading to an improvement in CO2 uptake and selectivity over gases such as nitrogen and methane.20−22 Introduction of polar functional groups such as amines into the POP frameworks such as amine, which provide CO2-philic sites, has been explored using both post- and presynthetic modification of sorbents.23−27 However, such synthetic routes, in general, suffer from the use of expensive transition metal-based catalysts, multistep reactions, and extensive purification steps and hence are not practical for large-scale preparation. In addition, amine-decorated POPs show limited chemical stability because of their proneness to oxidative degradation in air, whereas imine-linked POPs suffer from poor CO2/N2 selectivity, especially at elevated temperatures.27 To avoid such drawbacks, others and we have recently reported a low-cost and efficient synthetic route for the preparation of a series of azo-linked polymers (ALPs).2,28−30 The nitrogen–nitrogen double bonds (azo-bonds) in such porous materials provide the Lewis basic sites that are needed for the CO2 molecules to interact with the framework without compromising the chemical stability as the case in amine-based POPs. Not surprisingly, these ALPs showed remarkable physiochemical stability, high CO2 uptake capacities, and good CO2/N2 selectivity at ambient conditions.2,29
Besides CO2 capture, the detection of heavy metal ions also poses another environmental and health challenge addressed in this work. Natural resource pollution is a huge problem especially water contaminated with heavy metals such as mercury, lead, and thallium, which are extremely toxic to the environment as well for human consumption.31 A recent example of such contamination of drinking water by the lead metal from industrial sources was reported in Flint, Michigan in 2015.32 The incident had a devastating effect on public health, where many people have reported lead-poisoning symptoms and a huge economic cost. Such incidents impose a challenging problem to the scientific community to develop fast-response sensor systems for continuous monitoring of drinking water sources and for remediation of contaminations. Luminescent sensors represent potential solutions for sensitive and selective detection of heavy metal contaminants of drinking water; a recent example has shown detection limits as low as parts per billion levels for Ag+ ions and parts per million levels for lead and thallium by a phosphorescent molecular/polymeric composite chemosensor.33
The synergistic functionalities and properties of POPs containing rich π-conjugated building blocks decorated with heteroatoms, and high surface areas provide docking sites and broad interface for metals’ interaction, which allow enhancement in the signaling sensitivity.13,15,34,35 In addition, the recyclability of POP sensors can be easily achieved because of the weak interaction (physisorption) of the analytes and the frameworks of POPs. Another added advantage of POPs as sensors is the plentiful apertures’ availability, leading to increase in the diffusion rates and thereby faster response times. Despite such attractive properties that POPs offer, their use as chemical sensors is not well-developed and mostly limited to the detection of nitroaromatic compounds.36−38
With the above considerations and challenges in mind, herein, we report on the synthesis and characterization of a new azo-bond-functionalized polymer bearing a pyrene moiety, Azo-Py. The novel azo-linked porous polymer was synthesized using a 1,3,6,8-tetra(4-aminophenyl)pyrene monomer and a CuBr-catalyzed oxidative homocoupling reaction.39 We have shown that the electron-rich and rigid pyrene core of 1,3,6,8-tetra(4-aminophenyl)pyrene leads to a highly porous polymer and exhibits a solid-state packing behavior, resulting in a nanofiber morphology.21 This was facilitated by π–π stacking of the highly conjugated pyrene group. The CO2 uptake and selectivity of Azo-Py were studied for flue gas and landfill gas mixtures. Furthermore, because of its excellent fluorescence properties, the potential utility of Azo-Py as a selective chemosensor for the detection of heavy metal ions (such as mercury, lead, and thallium, among others) was also investigated.
2. Experimental Section
2.1. General Techniques and Materials
All solvents, starting materials, and reagents were purchased from Acros Organics and used without any further purification unless otherwise noted. 4-Aminophenylboronic acid pinacol ester and tetrakis(triphenylphosphine)palladium(0) were purchased from Aldrich. Air-sensitive materials and reactions were handled or carried out in an inert atmosphere (nitrogen gas) using a glovebox or Schlenk line techniques. 1,3,6,8-Tetra(4-aminophenyl)pyrene was prepared according to literature procedures.40 Elemental analysis was performed for carbon, nitrogen, and hydrogen on an Euro EA3000 Series CHN elemental analyzer (EuroVector Instruments). 1H NMR was conducted using a Varian Mercury-400 MHz NMR spectrometer. Fourier-transform infrared (FTIR) spectra were obtained using a Nicolet Nexus 670 spectrometer equipped with an attenuated total reflectance accessory. The thermal stability of the synthesized polymers was assessed using a Perkin thermo-gravimetric analyzer with a temperature ramp rate of 5 °C/min under N2 flow. Scanning electron microscopy (SEM) images were obtained to the morphology of the polymers using a Hitachi SU-70 FE-SEM. The samples were prepared by dispersion onto a double-sided carbon tape attached to the sample holder, followed by a platinum coating for 70 s at 1 × 10–6 bar. Powder X-ray diffraction data were collected using a PANalytical X’Pert PRO multipurpose diffractometer by Cu Kα radiation with a 2θ range of 2–30.
2.2. Gas Uptake Studies
Porosity and gas uptake studies were conducted using a Quantachrome Autosorb iQ volumetric analyzer using gases of ultrahigh-purity grade. Typically, an Azo-Py sample (∼40–50 mg) was loaded into a preweighed 9 mm large bulb Quantachrome cell, which was then hooked up to the degassing station of the gas analyzer. The sample was then degassed at 120 °C and 1.0 × 10–5 bar for 12 h. The degassed sample and cell were refilled with helium, weighed, and hooked up to the analysis station of the gas analyzer. Adsorption temperatures were attained using a temperature-controlled water/ethylene glycol mixture bath for 273 and 298 K and a refrigerated bath of liquid nitrogen for 77 K.
2.3. Photoluminescence Studies
The photoluminescence measurements were obtained with a PTI QuantaMaster model QM-4 scanning spectrofluorometer. The instrument has the capabilities to measure near-infrared (IR) luminescence with the attached accessory along with the direct quantum yield using a spherical integration sphere, which is attached to the sample chamber. A suspension of the polymer (0.1 mg/1 mL) in the corresponding solvent was sonicated for 15 min at room temperature and was then placed (3 mL) in a quartz cuvette. Aliquots of the metal solutions (0.1 M) were added, and the fluorescence spectrum (λexc = 360 nm) was recorded after every addition. Mercury salt (HgBr2) was dissolved in acetonitrile, whereas the metal solution (NaNO3, KNO3, Ca(NO3)2, Pb(NO3)2, TlNO3, and AlCl3) was prepared in methanol/water (2:3) solutions. Caution: Thallium compounds are highly toxic in nature and extra caution (e.g., wearing triple gloves) is to be taken while handling them.
2.4. Synthesis of Azo-Py
Azo-Py was synthesized following a procedure established and further optimized by El-Kaderi et al.2,30 A solution of CuBr (25 mg, 0.174 mmol) and pyridine (110 mg, 1.391 mmol) in 11 mL of toluene was stirred at 25 °C for 3 h in a 50 mL round-bottom flask and an open air atmosphere. To this solution, 1,3,6,8-tetra(4-aminophenyl)pyrene (100 mg, 0.176 mmol) dissolved in 11 mL tetrahydrofuran (THF) was added. The mixture was stirred in an open air atmosphere at 25 °C for 24 h and refluxed at 60 °C for 12 h and then at 80 °C for 12 h. A burgundy colored solid was isolated by filtration and subsequently washed with THF and water. The obtained powder was stirred in HCl (100 mL, 2 M) for 12 h, filtered, and then washed with water. The powder was further treated with NaOH (2 M), water, ethanol, THF, and chloroform. The final product was dried at 120 °C under vacuum (150 mTorr) to give Azo-Py as a burgundy colored powder (82 mg, 83%). Anal. Calcd for C40H22N4: C, 86.00%; H, 3.97%; N, 10.03%. Found: C, 70.76%; H, 4.39%; N 8.23%.
3. Results and Discussion
3.1. Synthesis and Characterization of Azo-Py
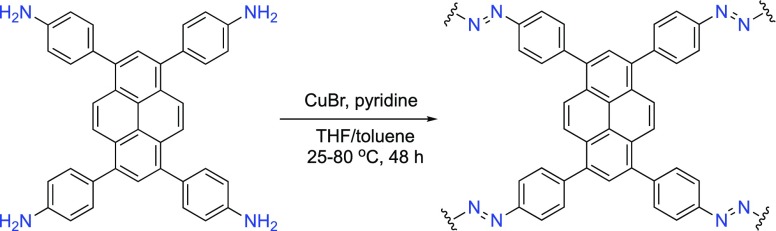
Azo-Py was prepared via an oxidative homocoupling of aniline-functionalized monomer with a pyrene core, yielding robust azo-bonded linkages as presented in Scheme 1.
Scheme 1. Synthetic Route for the Preparation of Azo-Py.
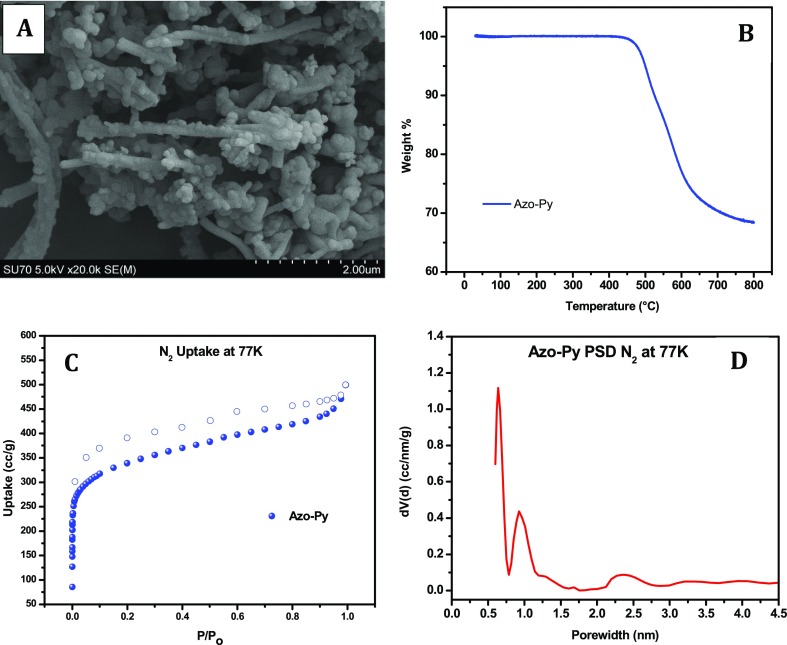
This polymerization reaction was carried out following our recent report, which outlined the optimum molar amount of CuBr, solvent composition, and temperature ramp profile in order to enhance the porosity of the resulting polymer.2 Azo-Py is insoluble in common organic solvents such as water, THF, chloroform, toluene, and ethanol—depicting its structure as a highly cross-linked framework. Polymerization of Azo-Py was confirmed using Fourier transform IR (FTIR) spectroscopy (Figure S1). Vibrational bands in the region of 1415–1400 cm–1 signify the presence of azo bonds expected in Azo-Py. Also, the decrease in intensity of the N–H stretching bands at 3200–3450 cm–1 indicates the consumption of secondary amine groups of the monomers taking part in the azo-bond formation. It should be noted that unreacted terminal amines are seen as expected in the FTIR spectra of Azo-Py. SEM imaging (Figure 1A) shows that Azo-Py formed a nanofibrous morphology similar to previously reported benzimidazole-linked polymers (BILPs) bearing a pyrene core.21,41 It is presumed that these nanofibers, which are about 0.2 μm in thickness and of varying length, result from π–π stacking interactions induced by the rigid and highly-conjugated pyrene core. The thermal stability of Azo-Py was assessed by thermogravimetric analysis (TGA), which showed stability up to near 500 °C (Figure 1B). Powder X-ray diffraction revealed an amorphous material as there were no distinct peaks in the spectra (Figure S2).
Figure 1.
(A) SEM, (B) TGA, (C) N2 sorption–desorption isotherm at 77 K, and (D) PSD of Azo-Py.
3.2. Porosity and Textural Properties
The porosity and textural properties of Azo-Py were characterized from its nitrogen isotherm at 77 K (Figure 1C). A steeply-increasing uptake is observed at lower pressure ranges (P/Po < 0.035), followed by a decline in the slope of uptake in higher pressure ranges (P/Po ∼ 0.035–0.9). This trend is indicative of a highly microporous material with a minor mesoporous pore size distribution (PSD).42,43 The textural properties of Azo-Py are summarized in Table 1. The 1259 m2 g–1 Brunauer–Emmett–Teller (BET) surface area of Azo-Py is higher than previously reported azo-bond-functionalized POPs including ALPs (412–1235 m2 g–1),2,30 azo-COPs (493–729 m2 g–1),20 and azo-POFs (439–712 m2 g–1).44 Particularly, the surface area is higher than that in some of the BILPs bearing a pyrene backbone including BILP-10, 11, and 13 (658–787 m2 g–1).21 A direct comparison can be made with BILP-10 (787 m2 g–1) as it is the benzimidazole-linked analogue to Azo-Py. In this case, the superior surface area of Azo-Py is a testament to the optimized synthetic route implemented for achieving high porosity in ALPs that we recently reported.2 However, BILP-12 displays a higher surface area than Azo-Py because of the incorporated triptycene building which has an internal molecular free volume (IMFV).45 A PSD curve was generated by fitting the adsorption branch of the N2 isotherm at 77 K to quenched solid density functional theory (QSDFT) model for slit and cylindrical pores for carbon (Figure 1D). The curve shows a dominant pore size centered at 0.64 nm for Azo-Py. The total pore volume of the polymer was deduced from a single adsorption point, at P/Po = 0.95, and found to be 0.699 cm3 g–1. The micropore volume was also determined to be 0.377 cm3 g–1 making up 53.9% of the total pore volume. Additionally, the profile of ultra-micropores (< 0.7 nm) was elucidated by nonlocalized DFT fitting of the adsorption branch of the CO2 isotherm at 273 K (Figure S3) with the ultra-micropore volume quantified to be 0.016 cm3 g–1.
Table 1. Textural Properties of Azo-Py.
| aSA(BET) (m2 g–1) | bPSD (nm) | cpore vol. (cm–3 g–1) | dvol.(Mic) (cm–3 g–1) | evol.(U. Mic) (cm–3 g–1) | |
|---|---|---|---|---|---|
| Azo-Py | 1259 | 1.06 | 0.699 | 0.377 | 0.0158 |
BET surface area.
Dominant pore size determined by QSDFT fittings of N2 isotherms at 77 K.
Total pore volume at P/Po = 0.95.
Micropore volume determined by DFT (the values in parenthesis are the percentage of micropore volume relative to the total pore volume).
Pore volume of ultra-micropores (< 0.7 nm) determined from CO2 isotherms at 273 K.
3.3. Gas Uptake and Selectivity Studies
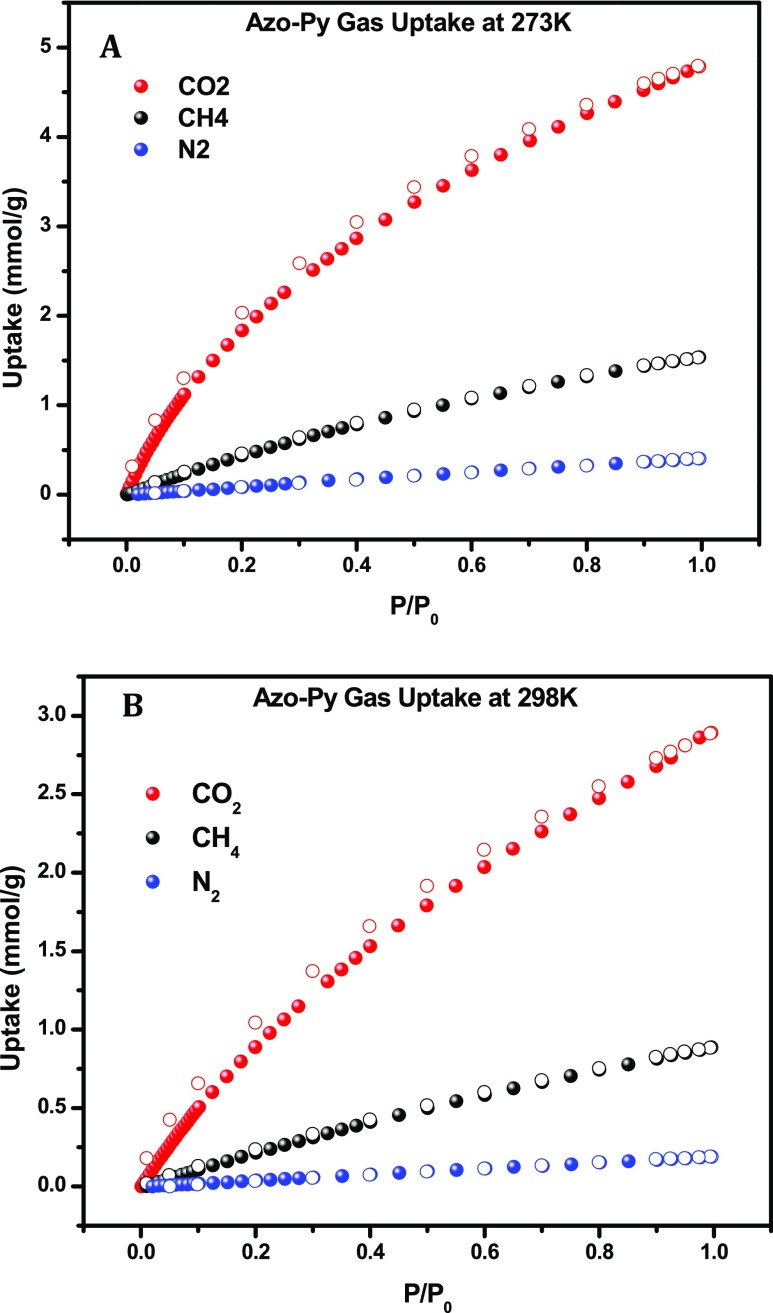
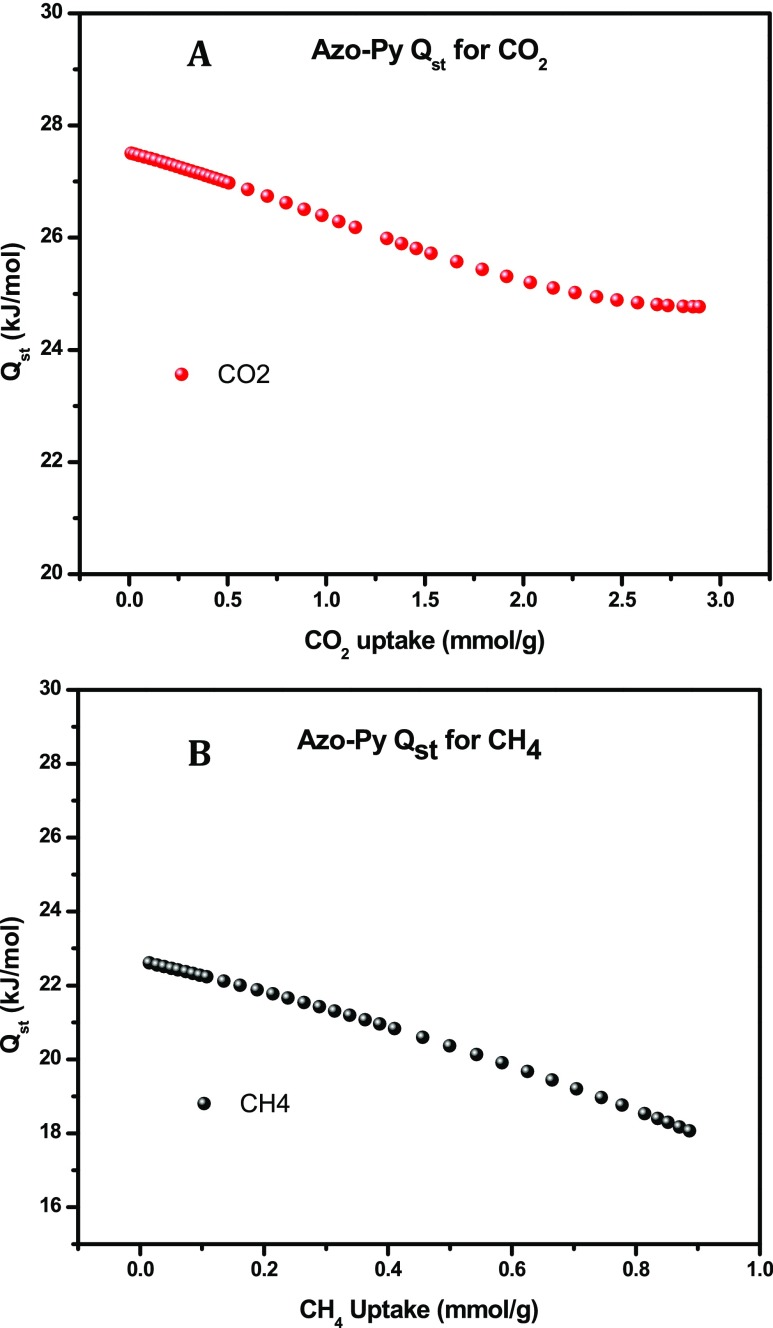
The textural properties discussed above, including the high surface area, microporous nature, and narrow pore size have proven to be effective in enhancing the CO2 uptake in porous frameworks. Additionally, the incorporation of azo-bonds in porous sorbents has been theoretically and experimentally validated to enhance CO2 uptake facilitated via Lewis acid–base interactions between CO2 and the azo functionalities.22 With that in consideration, the gas uptake capacity of Azo-Py was investigated by collecting low-pressure CO2, CH4, and N2 isotherms at 273 and 298 K presented in Figure 2 and summarized in Table 2. The CO2 isotherms display a steep rise in the low-pressure region and no hysteresis in the adsorption and desorption curves characteristic of sorbents that can be regenerated at a low energy penalty. At 273 K and 1 bar, Azo-Py has a CO2 uptake of 4.79 mmol g–1, which is higher than BILP-10, -11, and -13 (2.6–4.0 mmol g–1). Consistent with the surface area difference, Azo-Py had a lower CO2 uptake than BILP-12 (5.1 mmol g–1).41 This is indicative of the direct correlation between the surface area and CO2 uptake that has been observed in most porous polymers. In relation to other azo-functionalized polymers, Azo-Py has a higher CO2 uptake at 273 K and 1 bar than all ALPs (2.5–4.7 mmol g–1) except for ALP-1 (5.37 mmol g–1).2,30 While at 298 K and 1 bar, Azo-Py has a CO2 uptake of 2.89 mmol g–1 which is higher than azo-COPs (1.2–1.5 mmol g–1) and azo-POFs (1.2–1.5 mmol g–1) but slightly lower than ALP-1 (3.2 mmol g–1) and ALP-5 (2.94 mmol g–1). This is due to the triptycene-based framework of ALP-1 which results in high IMFV, enhancing the total uptake at 1 bar, while ALP-5 has a high binding affinity to CO2 because of its narrow pore size. The CH4 uptake of Azo-Py at 1 bar was recorded at 1.54 and 0.89 mmol g–1 at 273 and 298 K, respectively. This was higher than all pyrene-derived BILPs41 and all ALPs except ALP-1. The binding affinity of Azo-Py for gas molecules was quantified via isosteric heat of adsorption, Qst, by fitting the CO2 and CH4 isotherms at 273 and 298 K, respectively, with the virial equation. The CO2Qst value for Azo-Py at zero surface coverage was quantified to be 27.5 kJ mol–1. From the Qst curves generated (Figure 3), it is evident that the strength of interaction between gas molecules and the polymer pore walls decreases with more loading as the interaction sites and pore volumes are increasingly saturated. The Qst for CH4 was calculated to be 22.6 kJ mol–1 with depreciation in binding affinity observed with increasing gas loading as well.
Figure 2.
CO2, CH4, and N2 uptake isotherms of Azo-Py at 273 K (A) and 298 (B). Open and closed circle symbols denote adsorption and desorption, respectively.
Table 2. Gas Uptakes, Heat of Adsorption, and Selectivity of Azo-Pya.
| CO2 uptake at 1 bar |
CH4 uptake at 1 bar |
N2 uptake at 1 bar |
selectivity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| adsorbent | 273 K | 298 K | Qst | 273 K | 298 K | Qst | 273 K | 298 K | CO2/N2 | CO2/CH4 |
| Azo-Py | 4.79 | 2.89 | 27.5 | 1.54 | 0.89 | 22.6 | 0.40 | 0.19 | 55.1 (42.4) | 10.9 (7.8) |
Gas uptake in mmol g–1, and isosteric heat of adsorption (Qst) at zero coverage in kJ mol–1. Selectivity (mol mol–1, at 1.0 bar) calculated by the IAST method at the mole ratio of 10:90 for CO2/N2 and the mole ratio of 50:50 for CO2/CH4 at 273 and 298 K.
Figure 3.
Azo-Py isosteric heat of adsorption for CO2 (A) and CH4 (B).
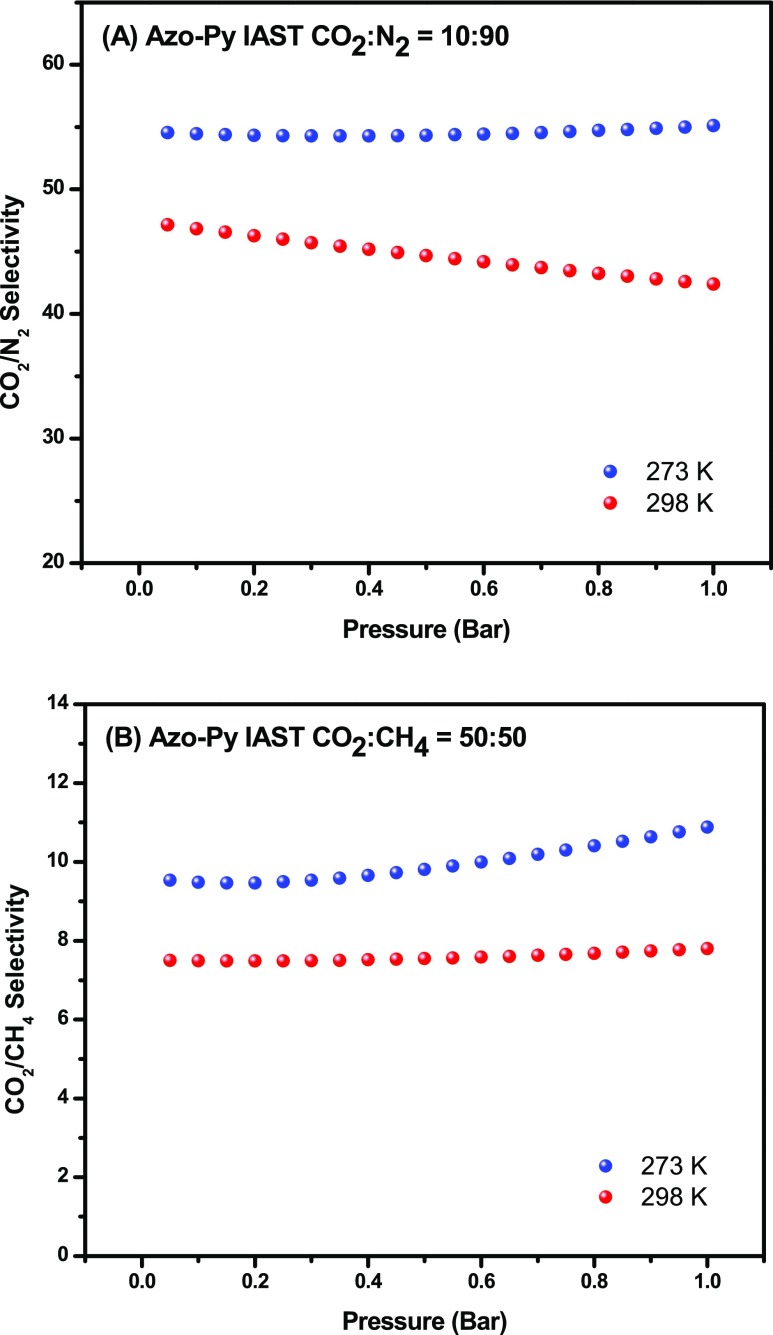
Motivated by the azo-functionality, microporous nature, high CO2 uptake, and suitable binding affinity of Azo-Py, the selective CO2 capture potential of Azo-Py was evaluated from the analysis of single gas isotherms of CO2, CH4, and N2 using two methods: Henry’s law constants and the ideal adsorbed solution theory (IAST). Henry’s law constants were obtained from the initial slope of the adsorption branch of the isotherms (Figure S5a–d). The CO2/N2 selectivities were found to be 39.6 (273 K) and 29.2 (298 K), whereas CO2/CH4 selectivities were 7.04 (273 K) and 4.8 (298 K). IAST is a widely recognized method for deducing the selective gas capture behavior of porous sorbents from single gas isotherms while considering the composition of the targeted gas mixtures at adsorption conditions. In this case, flue gas composed predominantly of CO2 and N2 with ratios of CO2/N2 at 10:90 and landfill gas composed predominantly of CO2 and CH4 with ratios of CO2/CH4 at 50:50 were analyzed (Figure 4). For flue gas compositions, the CO2/N2 IAST selectivity of Azo-Py at 1 bar was calculated at 55.1 and 42.4 at 273 and 298 K, respectively. These values are comparable to other nitrogen-bearing organic polymers ALPs,2,30 BILPs,46 and functionalized NPOFs.24 The decrease in selectivity from 273 to 298 K is a trend that has been observed before in POPs that lack the presence of a large portion of pores in the mesoporous range.47,48 The CO2/CH4 selectivity of Azo-Py at 1 bar was 10.9 and 7.8 for 273 and 298 K, respectively. These values are also comparable to our previously published azo-functionalized polymers.
Figure 4.
IAST selectivity of CO2/N2 (10:90) (A) and CO2/CH4 (50:50) (B) at 273 and 298 K, respectively.
3.4. Photoluminescence and Metal-Sensing Studies
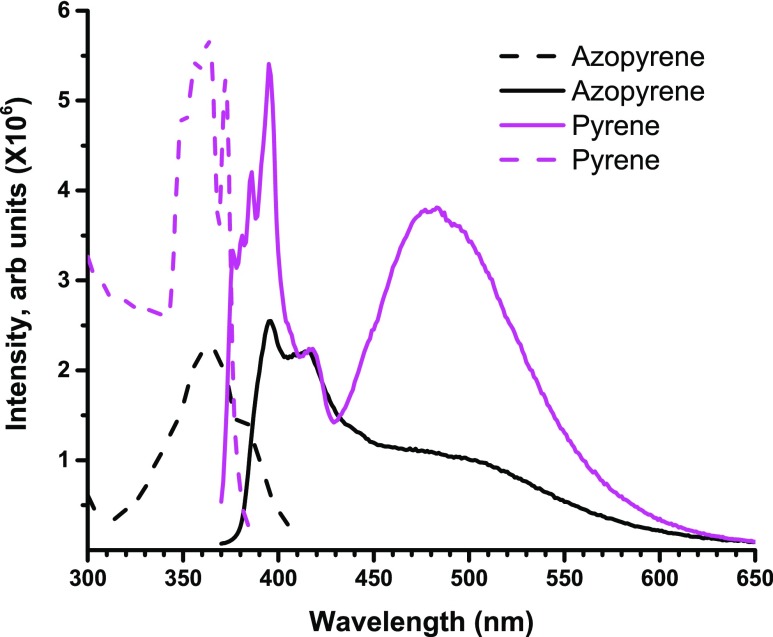
The structural architecture of Azo-Py consists of a two-dimensional -network of pyrene-based backbone linked through the azo-group moieties. This provides a π-electron-rich conjugated system with strong emission intensities, similar to that of pyrene. Figure 5 shows the fluorescence profile of Azo-Py, which is similar to that of pure pyrene in acetonitrile. Azo-Py exhibits mainly a monomer emission peak at ∼415 nm with a less significant excimer emission at ∼490 nm.36,49 Perturbations of the electronic distribution of the π-electrons in this conjugated system through the electrostatic interaction of metal ions would change the emission profile of the polymer;50,51 hence, the azopyrene polymers can be utilized as a fluorescent chemical sensor for metal ions.
Figure 5.
Excitation (dotted lines) and the emission (solid lines) spectra for pyrene (λexc = 360 nm and λemi = 395 nm) and Azo-Py (λexc = 360 nm and λemi = 415 nm), respectively.
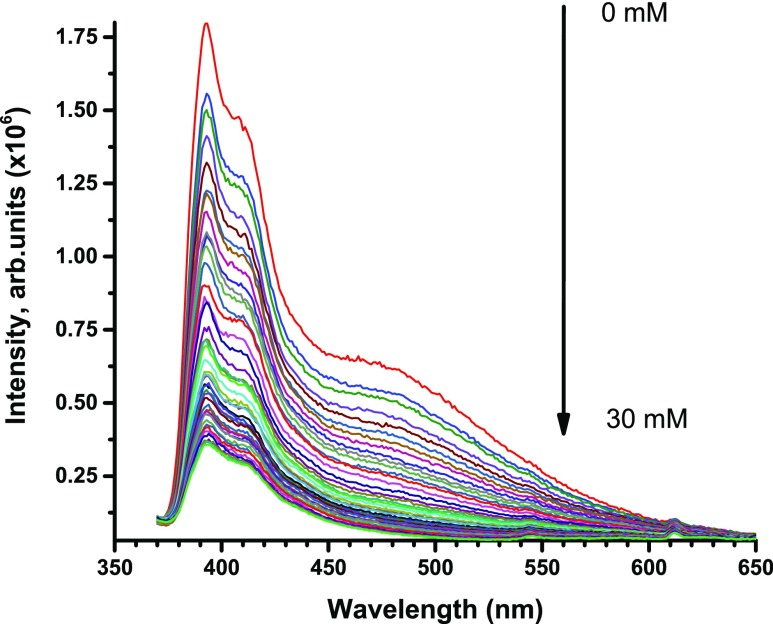
To test the efficacy of Azo-Py as a sensor for metal ions, we investigated the effect of the presence of sodium, potassium, calcium, mercury, lead, thallium, and aluminum ions on the emission of the polymer in solution. Millimolar concentrations of each metal’s salt solution were titrated into a uniform suspension of the azopyrene polymer in acetonitrile (0.1 mg/mL), and the fluorescence spectrum of the polymer was monitored after every addition. Figure 6 shows the change in the fluorescence profile of the polymer upon addition of mercuric bromide solution; as the concentration of the metal increased, the emission of the polymer decreased. Similar trends of emission quenching of the polymer were also observed with the other metals (Figures S6–S8 in the Supporting Information document) but to different extents.
Figure 6.
Emission spectra of Azo-Py (1.0 mg/mL) with successive additions of mercuric bromide (0.1 M) in acetonitrile.
Figure 7A displays Stern–Volmer plots for the relative change in the monomer emission of the polymer versus the increase in the concentration of the metal ions. The observed linear trends for the quenching of the fluorescence by the metal ions demonstrate that the strongest effects (Table 3) are for aluminum (KSV = 152 M–1) and mercury (KSV = 143 M–1) ions versus weaker effects for lead (KSV = 32 M–1) and thallium (KSV = 19 M–1) ions, whereas sodium, potassium, and calcium ions exhibit insignificant effects on the emission of Azo-Py.36 Similar linear trends (Figure 7B) are also attainable for Benesi–Hildebrand plots, where the reciprocal of the change in the fluorescence intensity of the polymer is plotted versus the reciprocal of the metal concentrations. The linear fitting of these plots has provided the binding constants (Table 3) of the polymer to the metal ions, assuming a 1:1 binding ratio between the metal ion and each pyrene unit in the polymer. The obtained values indicated that interaction of aluminum (KS = 376 M–1) and mercury (KS = 183 M–1) ions with the polymer is stronger than that of lead (KS = 121 M–1) and thallium (KS = 112 M–1) ions, similar to conclusions based upon Stern–Volmer plots. We attribute this strong interaction and quenching effect of aluminum ions to their strong electrostatic attraction to the polymer (through azo-metal binding) because of the high concentration of charge, whereas for mercury ions it is due to the heavy atom effect resulting from spin–orbit coupling associated with cation-π coordination that disrupts excimer formation, consistent with the fluorescence spectral profile change, and quenches both the monomer and excimer fluorescence bands to different extents. Meanwhile, the low quenching effect of lead and thallium ions could be due to the hydration effect of the metal, which decreases the electrostatic metal-π binding.50,51 Unlike Hg(II), these ions include valence electrons in the 6th shell, which could adversely impact the cation-π coordination via an electrostatic repulsive component. Finally, the hard ions of sodium, potassium, and calcium have very low affinity to both nitrogen atoms (azo group) and π-conjugated surfaces.
Figure 7.
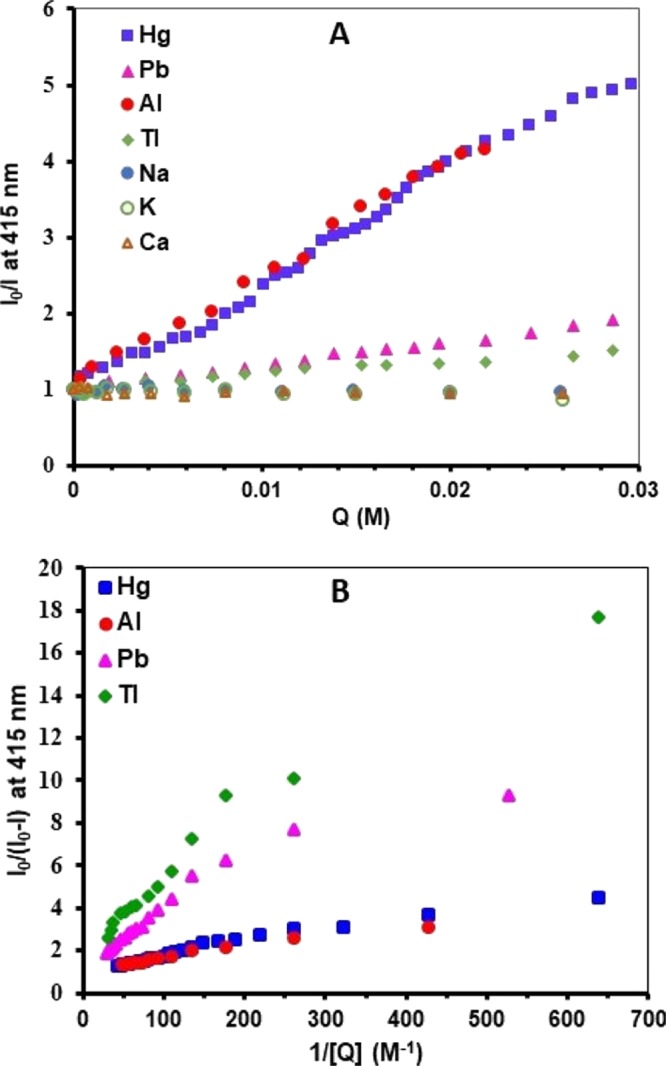
Stern–Volmer (A) and Benesi–Hildebrand (B) plots for the change in the emission (λemi = 415 nm) of Azo-Py upon titrating with the different metal ions.
Table 3. Stern–Volmer (KSV) Constants and Binding Constants (KS) of Azo-Py with Various Metal Cations.
| metal cation | KSV (M–1) | KS (M–1) |
|---|---|---|
| Al3+ | 152 | 376 |
| Hg2+ | 143 | 183 |
| Pb2+ | 31.9 | 120 |
| Tl+ | 19.1 | 112 |
4. Conclusions
In conclusion, a new azo-functionalized porous organic polymer, Azo-Py, was synthesized by incorporating a highly conjugated pyrene core into the polymer framework. The influence of the pyrene moiety in the solid-state packing of the polymer is evident by the formation of nanofibers that are assembled by strong π–π stacking interactions manifest by excimer bands in the fluorescence spectra. Azo-Py displays high porosity with a surface area of 1259 m2 g–1 and high microporosity as a result of the rigid pyrene core. These characteristics induce high CO2 uptakes of 4.79 mmol g–1 (273 K) and 2.89 mmol g–1 (298 K) at 1 bar along with binding affinities conducive for easy regeneration, Qst = 27.5 kJ mol–1. Furthermore, Azo-Py because of its highly conjugated electron density has the ability to sense the electropositive heavy metal cations more selectively than harder cations. The ionic charge, atomic weight, and binding constant of the metal cation play a role in determining the extent of fluorescence quenching of the polymer’s monomeric and excimeric pyrene-based emission bands.
Acknowledgments
T.-D.T. thanks Altria for graduate assistantship. O.M.E.-K. thanks the American University of Sharjah, grant# FRG17-R-12 and EFRG18-GER-CAS-67, for supporting this research project. M.A.O. acknowledges supporting aspects of his group’s contributions by the Robert A. Welch Foundation (grant B-1542), National Science Foundation (CHE-1413641), and the Shenzhen Peacock Plan (no. 1208040050847074).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01920.
Porosity, gas uptake measurements, and emission spectra of Azo-Py with successive additions of metal ions in acetonitrile (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zou L.; Sun Y.; Che S.; Yang X.; Wang X.; Bosch M.; Wang Q.; Li H.; Smith M.; Yuan S.; Perry Z.; Zhou H.-C. Porous Organic Polymers for Post-Combustion Carbon Capture. Adv. Mater. 2017, 29, 1700229. 10.1002/adma.201700229. [DOI] [PubMed] [Google Scholar]
- Arab P.; Verlander A.; El-Kaderi H. M. Synthesis of a Highly Porous Bis(imino)pyridine-Linked Polymer and Its Postsynthetic Modification with Inorganic Fluorinated Ions for Selective CO2 Capture. J. Phys. Chem. C 2015, 119, 8174–8182. 10.1021/acs.jpcc.5b00690. [DOI] [Google Scholar]
- Ben T.; Pei C.; Zhang D.; Xu J.; Deng F.; Jing X.; Qiu S. Gas storage in porous aromatic frameworks (PAFs). Energy Environ. Sci. 2011, 4, 3991–3999. 10.1039/c1ee01222c. [DOI] [Google Scholar]
- Popp N.; Homburg T.; Stock N.; Senker J. Porous imine-based networks with protonated imine linkages for carbon dioxide separation from mixtures with nitrogen and methane. J. Mater. Chem. A 2015, 3, 18492–18504. 10.1039/c5ta02504d. [DOI] [Google Scholar]
- Fang Q.; Gu S.; Zheng J.; Zhuang Z.; Qiu S.; Yan Y. 3D Microporous Base-Functionalized Covalent Organic Frameworks for Size-Selective Catalysis. Angew. Chem., Int. Ed. 2014, 53, 2878–2882. 10.1002/anie.201310500. [DOI] [PubMed] [Google Scholar]
- Lin S.; Diercks C. S.; Zhang Y.-B.; Kornienko N.; Nichols E. M.; Zhao Y.; Paris A. R.; Kim D.; Yang P.; Yaghi O. M.; Chang C. J. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. 10.1126/science.aac8343. [DOI] [PubMed] [Google Scholar]
- Ding S.-Y.; Gao J.; Wang Q.; Zhang Y.; Song W.-G.; Su C.-Y.; Wang W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki-Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. 10.1021/ja206846p. [DOI] [PubMed] [Google Scholar]
- Huang Y.-B.; Wang Q.; Liang J.; Wang X.; Cao R. Soluble Metal-Nanoparticle-Decorated Porous Coordination Polymers for the Homogenization of Heterogeneous Catalysis. J. Am. Chem. Soc. 2016, 138, 10104–10107. 10.1021/jacs.6b06185. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Dai Z.; Liu X.; Sheng N.; Deng F.; Meng X.; Xiao F.-S. Highly Efficient Heterogeneous Hydroformylation over Rh-Metalated Porous Organic Polymers: Synergistic Effect of High Ligand Concentration and Flexible Framework. J. Am. Chem. Soc. 2015, 137, 5204–5209. 10.1021/jacs.5b02122. [DOI] [PubMed] [Google Scholar]
- Tong W.; Li W.-H.; He Y.; Mo Z.-Y.; Tang H.-T.; Wang H.-S.; Pan Y.-M. Palladium-Metalated Porous Organic Polymers as Recyclable Catalysts for the Chemioselective Synthesis of Thiazoles from Thiobenzamides and Isonitriles. Org. Lett. 2018, 20, 2494–2498. 10.1021/acs.orglett.8b00886. [DOI] [PubMed] [Google Scholar]
- Dalapati S.; Jin S.; Gao J.; Xu Y.; Nagai A.; Jiang D. An Azine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. 10.1021/ja4103293. [DOI] [PubMed] [Google Scholar]
- Das G.; Biswal B. P.; Kandambeth S.; Venkatesh V.; Kaur G.; Addicoat M.; Heine T.; Verma S.; Banerjee R. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 2015, 6, 3931–3939. 10.1039/c5sc00512d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Li H.; Xia H.; Ding X.; Luo X.; Liu X.; Mu Y. Triarylboron-Linked Conjugated Microporous Polymers: Sensing and Removal of Fluoride Ions. Chem.—Eur. J. 2015, 21, 17355–17362. 10.1002/chem.201502241. [DOI] [PubMed] [Google Scholar]
- Xiang L.; Zhu Y.; Gu S.; Chen D.; Fu X.; Zhang Y.; Yu G.; Pan C.; Hu Y. A Luminescent Hypercrosslinked Conjugated Microporous Polymer for Efficient Removal and Detection of Mercury Ions. Macromol. Rapid Commun. 2015, 36, 1566–1571. 10.1002/marc.201500159. [DOI] [PubMed] [Google Scholar]
- Xiang Z.; Cao D. Synthesis of Luminescent Covalent-Organic Polymers for Detecting Nitroaromatic Explosives and Small Organic Molecules. Macromol. Rapid Commun. 2012, 33, 1184–1190. 10.1002/marc.201100865. [DOI] [PubMed] [Google Scholar]
- Sanders S. N.; Kumarasamy E.; Pun A. B.; Trinh M. T.; Choi B.; Xia J.; Taffet E. J.; Low J. Z.; Miller J. R.; Roy X.; Zhu X.-Y.; Steigerwald M. L.; Sfeir M. Y.; Campos L. M. Quantitative Intramolecular Singlet Fission in Bipentacenes. J. Am. Chem. Soc. 2015, 137, 8965–8972. 10.1021/jacs.5b04986. [DOI] [PubMed] [Google Scholar]
- Dogru M.; Handloser M.; Auras F.; Kunz T.; Medina D.; Hartschuh A.; Knochel P.; Bein T. A Photoconductive Thienothiophene-Based Covalent Organic Framework Showing Charge Transfer Towards Included Fullerene. Angew. Chem., Int. Ed. 2013, 52, 2920–2924. 10.1002/anie.201208514. [DOI] [PubMed] [Google Scholar]
- Raupach M. R.; Marland G.; Ciais P.; Le Quere C.; Canadell J. G.; Klepper G.; Field C. B. Global and regional drivers of accelerating CO2 emissions. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 10288–10293. 10.1073/pnas.0700609104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islamoglu T.; Behera S.; Kahveci Z.; Tessema T.-D.; Jena P.; El-Kaderi H. M. Enhanced Carbon Dioxide Capture from Landfill Gas Using Bifunctionalized Benzimidazole-Linked Polymers. ACS Appl. Mater. Interfaces 2016, 8, 14648–14655. 10.1021/acsami.6b05326. [DOI] [PubMed] [Google Scholar]
- Patel H. A.; Je S. H.; Park J.; Chen D. P.; Jung Y.; Yavuz C. T.; Coskun A. Unprecedented high-temperature CO2 selectivity in N-2-phobic nanoporous covalent organic polymers. Nat. Commun. 2013, 4, 1357. 10.1038/ncomms2359. [DOI] [PubMed] [Google Scholar]
- Rabbani M. G.; Sekizkardes A. K.; El-Kadri O. M.; Kaafarani B. R.; El-Kaderi H. M. Pyrene-directed growth of nanoporous benzimidazole-linked nanofibers and their application to selective CO2 capture and separation. J. Mater. Chem. 2012, 22, 25409–25417. 10.1039/c2jm34922a. [DOI] [Google Scholar]
- Nagaraja C. M.; Haldar R.; Maji T. K.; Rao C. N. R. Chiral Porous Metal Organic Frameworks of Co(II) and Ni(II): Synthesis, Structure, Magnetic Properties, and CO2 Uptake. Cryst. Growth Des. 2012, 12, 975–981. 10.1021/cg201447c. [DOI] [Google Scholar]
- Islamoglu T.; Kim T.; Kahveci Z.; El-Kadri O. M.; El-Kaderi H. M. Systematic Postsynthetic Modification of Nanoporous Organic Frameworks for Enhanced CO2 Capture from Flue Gas and Landfill Gas. J. Phys. Chem. C 2016, 120, 2592–2599. 10.1021/acs.jpcc.5b12247. [DOI] [Google Scholar]
- İslamoğlu T.; Rabbani M. G.; El-Kaderi H. M. Impact of post-synthesis modification of nanoporous organic frameworks on small gas uptake and selective CO2 capture. J. Mater. Chem. A 2013, 1, 10259–10266. 10.1039/c3ta12305g. [DOI] [Google Scholar]
- Biswal B. P.; Chandra S.; Kandambeth S.; Lukose B.; Heine T.; Banerjee R. Mechanochemical Synthesis of Chemically Stable Isoreticular Covalent Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. 10.1021/ja4017842. [DOI] [PubMed] [Google Scholar]
- Gao X.; Zou X.; Ma H.; Meng S.; Zhu G. Highly Selective and Permeable Porous Organic Framework Membrane for CO2 Capture. Adv. Mater. 2014, 26, 3644–3648. 10.1002/adma.201400020. [DOI] [PubMed] [Google Scholar]
- Lu W.; Sculley J. P.; Yuan D.; Krishna R.; Wei Z.; Zhou H.-C. Polyamine-Tethered Porous Polymer Networks for Carbon Dioxide Capture from Flue Gas. Angew. Chem., Int. Ed. 2012, 51, 7480–7484. 10.1002/anie.201202176. [DOI] [PubMed] [Google Scholar]
- Buyukcakir O.; Je S. H.; Park J.; Patel H. A.; Jung Y.; Yavuz C. T.; Coskun A. Systematic Investigation of the Effect of Polymerization Routes on the Gas-Sorption Properties of Nanoporous Azobenzene Polymers. Chem.—Eur. J. 2015, 21, 15320–15327. 10.1002/chem.201501233. [DOI] [PubMed] [Google Scholar]
- Patel H. A.; Je S. H.; Park J.; Jung Y.; Coskun A.; Yavuz C. T. Directing the Structural Features of N2-Phobic Nanoporous Covalent Organic Polymers for CO2 Capture and Separation. Chem.—Eur. J. 2014, 20, 772–780. 10.1002/chem.201303493. [DOI] [PubMed] [Google Scholar]
- Arab P.; Rabbani M. G.; Sekizkardes A. K.; İslamoğlu T.; El-Kaderi H. M. Copper(I)-Catalyzed Synthesis of Nanoporous Azo-Linked Polymers: Impact of Textural Properties on Gas Storage and Selective Carbon Dioxide Capture. Chem. Mater. 2014, 26, 1385–1392. 10.1021/cm403161e. [DOI] [Google Scholar]
- Wu X.; Cobbina S. J.; Mao G.; Xu H.; Zhang Z.; Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- Masten S. J.; Davies S. H.; McElmurry S. P. Flint Water Crisis: What Happened and Why?. J.—Am. Water Works Assoc. 2016, 108, 22–34. 10.5942/jawwa.2016.108.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay P. K.; Marpu S. B.; Benton E. N.; Williams C. L.; Telang A.; Omary M. A. A Phosphorescent Trinuclear Gold(I) Pyrazolate Chemosensor for Silver Ion Detection and Remediation in Aqueous Media. Anal. Chem. 2018, 90, 4999–5006. 10.1021/acs.analchem.7b04334. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S.; Pallavi P.; Anil A. G.; Patra A. Fabrication of porous organic polymers in the form of powder, soluble in organic solvents and nanoparticles: a unique platform for gas adsorption and efficient chemosensing. Polym. Chem. 2015, 6, 3775–3780. 10.1039/c5py00235d. [DOI] [Google Scholar]
- Kreno L. E.; Leong K.; Farha O. K.; Allendorf M.; Van Duyne R. P.; Hupp J. T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. 10.1021/cr200324t. [DOI] [PubMed] [Google Scholar]
- Gupta S. K.; Kaleeswaran D.; Nandi S.; Vaidhyanathan R.; Murugavel R. Bulky Isopropyl Group Loaded Tetraaryl Pyrene Based Azo-Linked Covalent Organic Polymer for Nitroaromatics Sensing and CO2 Adsorption. ACS Omega 2017, 2, 3572–3582. 10.1021/acsomega.7b00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Ren H.; Sun F.; Jing X.; Cai K.; Zhao X.; Wang Y.; Wei Y.; Zhu G. Sensitive detection of hazardous explosives via highly fluorescent crystalline porous aromatic frameworks. J. Mater. Chem. 2012, 22, 24558–24562. 10.1039/c2jm35341e. [DOI] [Google Scholar]
- Räupke A.; Palma-Cando A.; Shkura E.; Teckhausen P.; Polywka A.; Görrn P.; Scherf U.; Riedl T. Highly sensitive gas-phase explosive detection by luminescent microporous polymer networks. Sci. Rep. 2016, 6, 29118. 10.1038/srep29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Jiao N. Copper-Catalyzed Aerobic Oxidative Dehydrogenative Coupling of Anilines Leading to Aromatic Azo Compounds using Dioxygen as an Oxidant. Angew. Chem., Int. Ed. 2010, 49, 6174–6177. 10.1002/anie.201001651. [DOI] [PubMed] [Google Scholar]
- Jin S.; Sakurai T.; Kowalczyk T.; Dalapati S.; Xu F.; Wei H.; Chen X.; Gao J.; Seki S.; Irle S.; Jiang D. Two-Dimensional Tetrathiafulvalene Covalent Organic Frameworks: Towards Latticed Conductive Organic Salts. Chem.—Eur. J. 2014, 20, 14608–14613. 10.1002/chem.201402844. [DOI] [PubMed] [Google Scholar]
- Sekizkardes A. K.; İslamoğlu T.; Kahveci Z.; El-Kaderi H. M. Application of pyrene-derived benzimidazole-linked polymers to CO2 separation under pressure and vacuum swing adsorption settings. J. Mater. Chem. A 2014, 2, 12492–12500. 10.1039/c4ta01281j. [DOI] [Google Scholar]
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S. W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- Zhang X.; Lu J.; Zhang J. Porosity Enhancement of Carbazolic Porous Organic Frameworks Using Dendritic Building Blocks for Gas Storage and Separation. Chem. Mater. 2014, 26, 4023–4029. 10.1021/cm501717c. [DOI] [Google Scholar]
- Lu J.; Zhang J. Facile synthesis of azo-linked porous organic frameworks via reductive homocoupling for selective CO2 capture. J. Mater. Chem. A 2014, 2, 13831–13834. 10.1039/c4ta03015j. [DOI] [Google Scholar]
- Rabbani M. G.; Reich T. E.; Kassab R. M.; Jackson K. T.; El-Kaderi H. M. High CO2 uptake and selectivity by triptycene-derived benzimidazole-linked polymers. Chem. Commun. 2012, 48, 1141–1143. 10.1039/c2cc16986j. [DOI] [PubMed] [Google Scholar]
- Sekizkardes A. K.; Altarawneh S.; Kahveci Z.; İslamoğlu T.; El-Kaderi H. M. Highly Selective CO2 Capture by Triazine-Based Benzimidazole-Linked Polymers. Macromolecules 2014, 47, 8328–8334. 10.1021/ma502071w. [DOI] [Google Scholar]
- Saleh M.; Baek S. B.; Lee H. M.; Kim K. S. Triazine-Based Microporous Polymers for Selective Adsorption of CO2. J. Phys. Chem. C 2015, 119, 5395–5402. 10.1021/jp509188h. [DOI] [Google Scholar]
- Lee J. H.; Lee H. J.; Lim S. Y.; Kim B. G.; Choi J. W. Combined CO2-philicity and Ordered Mesoporosity for Highly Selective CO2 Capture at High Temperatures. J. Am. Chem. Soc. 2015, 137, 7210–7216. 10.1021/jacs.5b03579. [DOI] [PubMed] [Google Scholar]
- Beyazkilic P.; Yildirim A.; Bayindir M. Formation of pyrene excimers in mesoporous ormosil thin films for visual detection of nitro-explosives. ACS Appl. Mater. Interfaces 2014, 6, 4997–5004. 10.1021/am406035v. [DOI] [PubMed] [Google Scholar]
- Dougherty D. A. The Cation-π Interaction. Acc. Chem. Res. 2013, 46, 885–893. 10.1021/ar300265y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevi A. S.; Sastry G. N. Cation – π Interaction : Its Role and Relevance in Chemistry , Biology , and Material Science. Chem. Rev. 2013, 113, 2100–2138. 10.1021/cr300222d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.