Abstract
OBJECTIVE:
The present study aims to investigate the role of caveolin-1 in dementia of Alzheimer's type using intracerebroventricular streptozotocin (ICV-STZ)-induced neurodegeneration model in rats.
MATERIALS AND METHODS:
Male Wistar rats (220–260 g) were employed. STZ 3 mg/kg via ICV route was given once to cause neuronal injury. Daidzein – a caveolin inhibitor at 0.2, 0.4, and 0.6 mg/kg s.c. were given daily whereas minoxidil – a caveolin activator was given at 0.45 mg/kg, i.p. on alternate days for 28 days. STZ was also given at its submaximal dose 1.5 mg/kg to minoxidil group only.
RESULTS:
ICV-STZ control animals exhibited cognitive and neurological deficits on the Morris water maze, elevated plus maze, and balance beam tests (P < 0.0001). Treatment with daidzein significantly restored memory impairments and decreased oxidative damage whereas minoxidil potentiates the effect of STZ causing significant impairment in memory. Significant oxidative stress such as lipid peroxidation and glutathione (P < 0.0001) were also observed due to ICV-STZ administration resulting in neuronal damage which was significantly prevented by treatment with daidzein in brain tissues.
CONCLUSION:
The findings from the present investigation may conclude that the caveolin-1 from caveolae at the cell membrane induces memory deficits and oxidative stress phenotype that resemble the neurological phenotype of Alzheimer's disease. Further studies are warranted to gauge the effect of caveolin dyshomeostasis on the amyloidogenic cascade.
Keywords: Alzheimer's, amyloid, caveolin, memory, streptozotocin
Introduction
Alzheimer's disease (AD) is characterized by memory deficit or dementia in advancing age reflected as difficulty in thinking, cognition, decision-making, abrupt behavior, and speech and makes every task of a day-to-day life complex. The molecular triggers for memory and cognitive debility associated with AD are still elusive. However, there is compelling evidence supporting the involvement of increased amyloid beta (Aβ) production due to genetic polymorphisms, aging, stress, and poor lifestyle.[1] Aβ is produced by a misbalance in two competing pathways, amyloidogenic and non-amyloidogenic including metabolism of the mature amyloid precursor protein (APP).[2] The risk of the AD is heightened by the amyloidogenic microprocessing of the APP.[3] Aβ exhibits microheterogeneity in amino acid sequence. Excessive sequential cleavage of the APP by β- and γ-secretase results in pathological oligomerization of Aβ that ensues degeneration of neurons of hippocampus and cortex regions, resulting in cognitive deficits and memory dysfunctions.[4] The molecular studies evidence that the trafficking of APP is controlled by insulin signaling and insulin receptor tyrosine kinase.[5] Insulin activity affects complex brain functions such as cognition and the memory in vivo.[6]
Systemic administration of streptozotocin (STZ) induces experimental type 1 diabetes in animals. STZ targets a glucose transporter protein and disrupts the insulin homeostasis and integrity of beta cells in the pancreas.[7] However, intracerebroventricular (ICV) administration of low doses of STZ does not induce diabetes but disrupts the insulin and energy homeostasis in the brain. Although ICV-STZ is reported to cause dementia in rodents, it was debatable whether the pathology caused by ICV-STZ is an appropriate model for the AD in rats. This question is addressed by various studies which report that the pathological states caused by ICV-STZ in animal brain resemble Alzheimer's like characteristics in neuronal morphology of Aβ plaques and tangles' accumulation and gene expression.[8]
Caveolin, inherent membrane protein is a major structural component of nonclathrin, flask-shaped invaginations named Caveolae.[9] The physiological functions of caveolin include vesicular trafficking, cholesterol homeostasis, cell adhesion, and apoptosis.[10,11] Caveolins interact with several signaling executioners such as G-a subunit, protein kinase C tyrosine kinase receptors, and endothelial nitric oxide synthase.[12,13] Hence, it serves as the scaffolding protein for the integration of signal transduction.[14] An emerging role of caveolin and cholesterol biosynthesis in the proteolytic processing of APP is also established.[15] The generation of Aβ has been linked with membrane regions of high cholesterol content, such as lipid rafts or caveolae. In contrast, the sites of APPsα generation are recognized as membranes with low cholesterol content and high fluidity.[16] Caveolin-1 physically associates with APP, which further supports the idea that caveolae and caveolin may play a role in the proteolysis of APP in vivo and further be responsible for the formation of Aβ with activation of β-secretase.[17] Lipid rafts associated with APP and colocalization of β-secretase with caveolae influence the production of Aβ. The present literature survey may draw a view to hypothesize that a role of caveolin activity at caveolae may influence the neurological status possibly through an oxidative stress-dependent mechanism in ICV-STZ model of neurodegeneration in rats. Daidzein – a well-noted inhibitor of caveolin-1[18,19,20] and minoxidil – an activator for caveolin-1 were used as modulators for caveolin activity in vivo.
Materials and Methods
Animals
Adult male Wistar albino rats weighing 220–260 g and aged about 13–15 weeks were procured from Animal house facility, School of Pharmaceutical Sciences, Shoolini University of Biotechnology and Management Sciences, Solan, HP, India. Animals were maintained under the controlled temperature at 25°C ± 2°C, humidity 55% ±5% with light/dark cycle (12/12 h), and also provided with standard chow diet procured from Ashirwad Industries Pvt. Ltd., Chandigarh, India and water ad libitum. The experimental study was conducted as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals. The protocol was duly approved from the institutional animal ethical committee of the institution vide protocol number IAEC/SU-PHARM/14/04.
Drugs and chemicals
STZ from Himedia laboratories, Mumbai, India; daidzein from Sigma Aldrich, Bengaluru, India; and minoxidil from United Pharmacies, Hyderabad, India were used. All other chemicals and biochemical reagents of analytical grade were used as freshly prepared solutions.
Methods
Streptozotocin-induced neuronal damage
Procedure
The animal was anesthetized using chloral hydrate at 350 mg/kg i.p., and hairs on the scalp were removed. The animal was placed in Stereotaxic apparatus (incisor bar - 3.3 mm, ear bars positioned symmetrically). The scalp was cleaned with iodine solution, incised on the midline, and a burr hole was drilled through the skull 0.8 mm posterior to bregma, 1.5 mm lateral to sagittal suture, and 3.6 mm beneath the surface of the brain, according to the stereotaxic atlas.[21] STZ was dissolved in citrate buffer (pH 4.5) before administration and injected bilaterally into each ventricle on day 1 (4 μl at 1 μl/min) using a Hamilton Microsyringe.[22]
Experimental protocol
The animals were divided into six groups (n = 8). Normal control; Sham control; STZ control (ICV-STZ, 3 mg/kg); Daidzein (Sigma Aldrich, CAS# 83701-22-8) 0.2, 0.4 and 0.6, STZ (3 mg/kg) on day 1 and daidzein (Sigma Aldrich, CAS# 486-66-8) was dissoved in a 1:10 solution of dimethyl sulfoxide: Phosphate buffered saline (pH 7.2) and administered at (0.2, 0.4 and 0.6 mg/kg/day, s. c. respectively) from 3rd to 28th day; and Minoxidil 0.45, STZ (1.5 mg/kg) on day 1 and minoxidil 0.45 mg/kg, i. p. alternatively from 3rd day to 28th day. The variable frequency and doses of daidzein and minoxidil were chosen based on the pharmacokinetics of respective drugs to maintain the plasma availability.[23,24] Group Minoxidil 0.45 was administered STZ at its submaximal dose, 1.5 mg/kg in the view that the activation of caveolin (using minoxidil) may potentiate the effect of STZ.
Behavioral assessments
Elevated plus maze test
Retention of memory was assessed on the 28th day of the protocol using elevated plus maze as explained by Sharma and Gupta.[22] Rats were placed individually at an end of an open arm facing away from the central square, and latency to enter a closed arm was recorded as initial transfer latency (ITL), and the memory retention for a same behavioral response as retention transfer latency (RTL) was recorded 24 h after evaluation of ITL on the 29th day.
Balance beam test
Three trials per day were conducted during 4 days' training period starting on the 30th day of the protocol. The ground below the tapered beam was lined with foam to prevent the injury from fall. If an animal failed to progress toward goal box for 20 s, a white-noise signal of 50 dB was recruited at the wider end of the beam. On the day of the testing, each animal was placed on the wider end of the beam with its back toward the goal box. Four parameters were measured: latency to turn around, latency to cross the beam, number of hind paw slips, and total distance covered on beam.[25]
Morris water maze test
Behavioral assessment for task acquisition and retention was done using Morris water maze. A black circular water tank with a diameter of 100 cm was filled to a depth of 20 cm with clear water and maintained at 22°C. The tank was divided into four equal quadrants, and a small platform (19 cm height) was located in the center of any quadrant and kept there throughout the acquisition trials. The animals from all the groups underwent a training period of four consecutive days, four acquisition trials starting from the mid of each platform with animal facing the wall, starting on the 35th day. The trial was considered ended when animals found the platform. Subsequently, the animal was allowed to rest for 30 s on the platform. If an animal had not found the platform for 180 s, it was allowed to rest on the platform for 10 s before retrial.
On day 5 (39th day of the protocol), in a probe trial, the animals were allowed to swim for 180 s with the platform removed. All the rats were started in the east extreme and were tested for one trial. In this trial, animals were tracked for the latency to enter and dwell time in the platform area. The tracking analysis was done using ANY-maze.[26]
Estimation of biochemical parameters
Brain homogenate preparation
All the biochemical parameters were estimated in the brain homogenate on day 28. Animals were sacrificed by decapitation; brains were removed and rinsed with ice-cold isotonic saline. Ten percent homogenized solution of the tissue was prepared using ice-cold 0.1 M phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 × g for 15 min and aliquots of supernatant were separated and used for biochemical estimation.
Estimation of lipid peroxidation
The lipid peroxidation was determined by the method of Wills.[27] Briefly, 500 μl of 10% tissue homogenate was added into 500 μl 0.1 M Tris buffer. The solution was incubated for 2 h at 37°C. 1 ml of 10% trichloroacetic acid (TCA) was added. The solution was then centrifuged at 3000 rpm for 10 min. The supernatant (1 ml) was added to 1 ml of 0.67% of thiobarbituric acid (TBA), boiled for 15 min on a water bath, and cooled under tap water. One milliliter of distilled water was added. The color intensity of this sample was determined in ultraviolet spectrophotometer at a wavelength of 532 nm. The TBA reacting compounds were expressed as malondialdehyde (MDA) as nM/mg of tissue.
Estimation of reduced glutathione
Reduced glutathione (GSH) was determined by the method of Ellman.[28] Briefly, 1 ml of biological sample (10% tissue homogenate in phosphate buffer, 7.4 pH), 1 ml of 20% TCA solution containing 1 mM ethylenediaminetetraacetic acid was mixed for protein aggregation. This solution was incubated for 5 min at room temperature and then centrifuged at 2000 rpm for 10 min. The supernatant (200 μl) was then transferred to a new set of test tubes and added 1.8 ml of the Ellman's reagent (5, 5'-dithio bis-2-nitrobenzoic acid) (0.1 mM) was prepared in 0.3 M phosphate buffer with 1% of sodium citrate solution. Then, all the test tubes were made up to the volume of 2 ml. After completion of the total reaction, the absorbance of the solutions was estimated at 412 nm against blank. The level of GSH was expressed as μmol/mg of tissue.
Statistical analysis
All the results were expressed as mean ± standard deviation (SD). Data were analyzed using one-way and two-way ANOVA (for elevated plus maze only) followed by Bonferroni's multiple comparison tests. Multiple comparisons were carried out if the dataset achieved a significant F value and alpha was set at 0.05. The statistical analysis was carried out using GraphPad Prism (version 5.1, GraphPad Software, San Diego, CA, USA).
Results
Effect of drug treatment on behavioral assessments
Effect of drug treatment on memory retention on Morris water maze
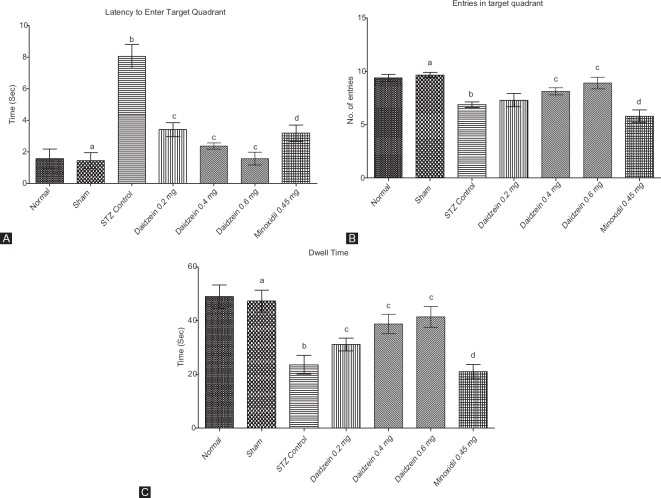
STZ control rats showed a significant decrease in memory retention compared to sham-operated animals. Treatment with daidzein 0.4 and 0.6 mg/kg showed a significant restoration of the memory retention in dose-dependent manner, as shown by reduced latency to enter target quadrant (P < 0.0001), number of entries in target quadrant (P < 0.0001), and dwell time (P < 0.0001) compared to the STZ control. Treatment with minoxidil and ICV-STZ (1.5 mg/kg) cause significant impairment of memory function as compared to the sham group for all indices of water maze (P < 0.0001) [Figure 1].
Figure 1.
(A) Latency to enter in target quadrant on probe trial for memory retention, (B) number of entries in target quadrant on probe trial for memory retention, and (C) total time spent in target quadrant on probe trial for memory retention (n = 8); Results: mean ± standard deviation; analyzed by one-way ANOVA followed by Bonferroni's multiple comparison test as post hoc analysis (“a” vs. normal control; “b” vs. sham treated; “c” vs. intracerebroventricular streptozotocin; “d” vs. sham group)
Effect of drug treatment on memory retention on the elevated plus maze
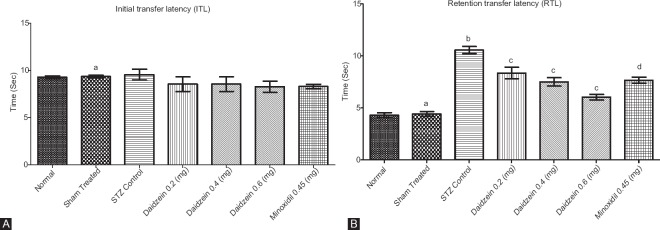
No significant changes were observed in all groups for ITL (P > 0.9999). STZ control rats showed a significant decrease in memory retention (P < 0.0001) compared to sham. Daidzein treatment (0.2, 0.4, and 0.6 mg/kg) significantly reinstated the performance of animals (P < 0.0001) compared to STZ control. Treatment with minoxidil (0.45 mg/kg) with ICV-STZ (1.5 mg/kg) showed a significant increase in the RTL (P < 0.0001) compared to sham [Figure 2].
Figure 2.
(A) Initial transfer latency in rats and (B) retention transfer latency in rats (n = 8); Results: mean ± standard deviation; analyzed by one-way ANOVA followed by Bonferroni's multiple comparison test as post hoc analysis (“a” vs. normal control; “b” vs. sham treated; “c” vs. intracerebroventricular streptozotocin; “d” vs. sham group)
Effect of drug treatment on performance on balance beam
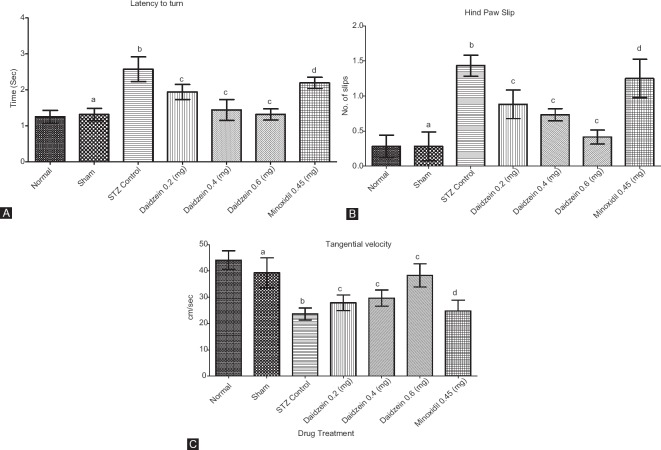
Single trial on the 5th day was conducted to evaluate scores for motor coordination in rats. Three assessments: latency to turn toward goal box, a number of hind paw slips, and tangential velocity were made. STZ-treated animals showed significant high scores on latency to turn (P < 0.0001), paw slips (P < 0.0001), and tangential velocity (P < 0.0001) on the 5th day of the test. The administration of daidzein (0.2, 0.4, and 0.6 mg/kg) caused a highly significant decrease in the scores (P < 0.0001) for all doses and paw slips (P = 0.0001, P < 0.0001, and P < 0.0001) for respective doses. However, daidzein 0.2 and 0.4 showed higher tangential velocity compared to STZ control; however, the difference was not significant (P = 0.6865 and 0.0631), although diazine 0.6 showed a significant increase in tangential velocity compared to STZ control (P < 0.0001). Group minoxidil 0.45 showed increased latency to turn; increased hind paw slips; and decreased tangential velocity due to episodic memory impairment (P < 0.0001) compared to sham [Figure 3].
Figure 3.
(A) Time taken by rats to turn around and face the goal box once placed on the beam, (B) number of slips on the trial run, and (C) tangential velocity of rats in cm/sec (n = 8); Results: mean ± standard deviation; analyzed by one-way ANOVA followed by Bonferroni's multiple comparison test as post hoc analysis (“a” vs. normal control; “b” vs. sham treated; “c” vs. intracerebroventricular streptozotocin; “d” vs. sham group)
Effect of drug treatment on brain tissue biochemical parameters
Tissue lipid peroxidation level
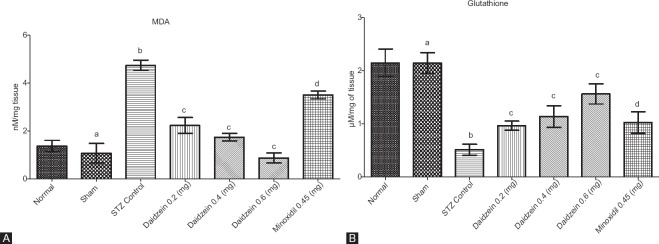
The levels of MDA rose significantly STZ control rats compared to the sham (P < 0.0001). Treatment with daidzein (0.2, 0.4, and 0.6 mg/kg) decreased MDA level significantly (P < 0.0001) for all groups. However, treatment with minoxidil (0.45 mg/kg) with ICV-STZ (1.5 mg/kg) showed a significant increase in the level of MDA compared to the sham group (P < 0.0001) [Figure 4A].
Figure 4.
(A) Effect of drug treatment on brain malondialdehyde level and (B) effect of drug treatment on brain glutathione level (n = 8); Results: mean ± standard deviation; analyzed by one-way ANOVA followed by Bonferroni's multiple comparison test as post hoc analysis (“a” vs. normal control; “b” vs. sham treated; “c” vs. intracerebroventricular streptozotocin; “d” vs. sham group)
Tissue reduced glutathione level
The level of GSH was found to be significantly depleted in ICV-STZ injected rats compared to the sham (P < 0.0001) whereas daidzein treatment group (0.2, 0.4, and 0.6 mg/kg) showed restored levels of GSH (P = 0.0003, P < 0.0001, and P < 0.0001, respectively). Predictably, minoxidil 0.45 group showed a decrease in levels of GSH (P < 0.0001) [Figure 4B].
Discussion
ICV administration of STZ at a subdiabetogenic dose (3 mg/kg) in rats impairs task acquisition and memory on water maze by exerting a profound and protracted effect on brain biochemistry, metabolism, and thereby on the physiology of behavior. Accumulation of Aβ and hyperphosphorylation of tau are the long-term consequences of STZ administration.[29] Caveolin plays a physiological role in the transport of cholesterol from extracellular space into the cell that is required to maintain physiological and metabolic energy requirements for cell survival.[30] In addition, caveolins also participate in the modulation of APP trafficking. An interaction of high cholesterol content in lipid raft of caveolae and the proteolytic processing of APP has been reported.[16]3-Hydroxy-3-methylglutaryl-coenzyme A inhibitors, Statins decreased the production/aggregation of Aβ by reduced cholesterol synthesis.[31] Hence, it may be deduced that the production of Aβ may also be reduced with activation of caveolin-induced cholesterol transport system. An extensive research revealed an undisputed role of caveolin 1 in the oxidative stress-induced damage. Briefly, reactive oxygen species can modulate the expression, degradation, posttranslational modifications, and membrane trafficking of caveolin 1.[32]
The findings herein support the conclusion that the administration of daidzein, a caveolin inhibitor at 0.4 and 0.6 mg/kg, produced significant restoration of memory dysfunction caused by ICV-STZ treatment. This is evidenced by significantly low neurological deficits compared to ICV-STZ control. In addition, no significant differences were observed between groups of a low and high dose of daidzein, 0.4 mg/kg and 0.6 mg/kg (P > 0.9999). Conversely, activation of caveolin using minoxidil with concomitant STZ (1.5 mg/kg) resulted in significantly more deficits compared to STZ control. In this case, low doses of STZ were administered to avoid lethality. The low dose of STZ, which otherwise causes no cognitive deficits, resulted in significantly more deficits compared to sham in the present study. These deficits may directly relate to overproduction of Aβ, due to a combined effect of insulin dysfunction caused by STZ and caveolin activation caused by minoxidil.
STZ spares basal ganglia and ventral striatum from degeneration, parts of the brain responsible for motor coordination and development of skill memory.[33] The balance beam test is conventionally used to assess motor coordination of animals; however, we employed this test to assess the dysregulation of memory retention in the present study. Any deficits in the motor coordination were ruled out before the balance beam test by measuring spontaneous locomotor activity using an actophotometer, therefore affirming that any abnormality in the behavior on balance beam would result from memory deficits. Briefly, we used the balance beam apparatus to assess animals' ability to memorize various unfavorable conditions: elevation, bright light, and loud noise (buzzer). These unfavorable conditions drive the animal away from the wider end of the beam to the goal box in addition to the animal's instinct to reach dark goal box in anticipation of reward. In addition, the memory of tapered beam, if consolidated, results in lesser hind paw slips compared to animals that show deficits in memory retention. STZ-treated animals showed more deficits; latency to turn around and paw slips compared to the sham on the final day of the experiment. The administration of daidzein decreased the latency to turn and cross the beam with decreased hind paw slips. Animals treated with minoxidil and a sublethal dose of STZ resulted in increased latency to turn and increased hind paw slips compared to the sham (deficits were comparable to STZ control). Assessments for memory retention on Morris water maze and elevated plus maze revealed a similar pattern of scores. STZ control animals exhibited significantly low memory retention, evidenced by lesser dwell time and the number of entries into the target quadrant, and greater RTL. These deficits were restored with inhibition of caveolin with daidzein, although high doses of daidzein did not cause any significant change compared to lower doses. Activation of caveolin with minoxidil exaggerated the pathology of an otherwise sublethal dose of STZ, which indicates the involvement of caveolin in the progression of Alzheimer's like dementia.
Malondialdehyde is a product of lipid peroxidation of membrane phospholipids due to oxidative stress whereas reduced GSH is an antioxidant in the body; these two are deemed as biomarkers to measure the severity of oxidative stress. Oxidative stress might be indicated by a marked decrease in GSH and an increase in MDA levels.[22] In the present study, STZ produced a significant increase in MDA level and a decrease in the GSH level, as compared to the sham group. However, administration of caveolin inhibitor daidzein resulted in reduced levels of MDA and elevated levels of GSH in the brain tissue. Daidzein is an isoflavone and has been reported for antioxidant properties. Moreover, daidzein produced a protection to the rats from ischemic injury by reducing the expression of MDA and other markers of oxidative stress.[34] Administration of minoxidil with a low dose of STZ caused an increase in MDA and decrease in GSH levels further supporting the findings of behavioral assessments. The results from the present study support the hypothesis that Alzheimer's like pathology in rats caused by ICV administration of STZ may be restored by inhibition of the activity of caveolin. This finding may open new arena for drug discovery research to combat AD. Conclusively, a typical STZ pathology regardless of nonpathological dose of STZ along with activation of caveolin-1 using minoxidil was observed; however, neurological deficits were restored by inhibition of caveolin-1.
Conclusion
The findings from the present investigation may conclude that the caveolin-1 from caveolae at the cell membrane induces memory deficits and oxidative stress phenotype that resemble the neurological phenotype of AD. Further studies are warranted to gauge the effect of caveolin dyshomeostasis on the amyloidogenic cascade.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to the management of Shoolini University, Solan, HP, India, for providing research facilities.
References
- 1.Marsh JL, Lukacsovich T, Thompson LM. Animal models of polyglutamine diseases and therapeutic approaches. J Biol Chem. 2009;284:7431–5. doi: 10.1074/jbc.R800065200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–11. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Goyal R. Amyloid beta plaque: A culprit for neurodegeneration. Acta Neurol Belg. 2016;116:445–50. doi: 10.1007/s13760-016-0639-9. [DOI] [PubMed] [Google Scholar]
- 4.Golde TE, Koo EH, Felsenstein KM, Osborne BA, Miele L. Γ-secretase inhibitors and modulators. Biochim Biophys Acta. 2013;1828:2898–907. doi: 10.1016/j.bbamem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer's disease? Trends Pharmacol Sci. 2002;23:288–93. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 6.Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. The effect of intrahippocampal insulin microinjection on spatial learning and memory. Horm Behav. 2006;50:748–52. doi: 10.1016/j.yhbeh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer's disease: In search of a relevant mechanism. Mol Neurobiol. 2016;53:1741–52. doi: 10.1007/s12035-015-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos TO, Mazucanti CH, Xavier GF, Torrão AS. Early and late neurodegeneration and memory disruption after intracerebroventricular streptozotocin. Physiol Behav. 2012;107:401–13. doi: 10.1016/j.physbeh.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG, et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 10.Fielding CJ, Fielding PE. Cholesterol and caveolae: Structural and functional relationships. Biochim Biophys Acta. 2000;1529:210–22. doi: 10.1016/s1388-1981(00)00150-5. [DOI] [PubMed] [Google Scholar]
- 11.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–11. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “Spreassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 13.Nomura R, Fujimoto T. Tyrosine-phosphorylated caveolin-1: Immunolocalization and molecular characterization. Mol Biol Cell. 1999;10:975–86. doi: 10.1091/mbc.10.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volonté D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr(14)) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem. 2001;276:8094–103. doi: 10.1074/jbc.M009245200. [DOI] [PubMed] [Google Scholar]
- 15.Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 1996;271:4436–40. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 16.Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha – Secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–20. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikezu T, Trapp BD, Song KS, Schlegel A, Lisanti MP, Okamoto T. Caveolae, plasma membrane microdomains for alpha-secretase-mediated processing of the amyloid precursor protein. J Biol Chem. 1998;273:10485–95. doi: 10.1074/jbc.273.17.10485. [DOI] [PubMed] [Google Scholar]
- 18.Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta-estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol. 2004;44:155–63. doi: 10.1097/00005344-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Ajmani P, Yadav HN, Singh M, Sharma PL. Possible involvement of caveolin in attenuation of cardioprotective effect of ischemic preconditioning in diabetic rat heart. BMC Cardiovasc Disord. 2011;11:43. doi: 10.1186/1471-2261-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma S, Singh M, Sharma PL. Ameliorative effect of daidzein: A caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. Indian J Exp Biol. 2012;50:28–34. [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed. San Diego: Academic Press; 1996. pp. 579–628. [Google Scholar]
- 22.Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–9. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 23.Lowenthal DT, Affrime MB. Pharmacology and pharmacokinetics of minoxidil. J Cardiovasc Pharmacol. 1980;2(Suppl 2):S93–106. doi: 10.1097/00005344-198000022-00002. [DOI] [PubMed] [Google Scholar]
- 24.Rüfer CE, Bub A, Möseneder J, Winterhalter P, Stürtz M, Kulling SE. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: A randomized, double-blind, crossover study. Am J Clin Nutr. 2008;87:1314–23. doi: 10.1093/ajcn/87.5.1314. [DOI] [PubMed] [Google Scholar]
- 25.Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–77. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- 26.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM, et al. Intracerebral streptozotocin model of type 3 diabetes: Relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 30.Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG. Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162:328–38. doi: 10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparks DL, Scheff SW, Hunsaker JC, 3rd, Liu H, Landers T, Gross DR, et al. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Wang N, Zheng Y, Zhang J, Zhang F, Wang Z. Caveolin-1: An oxidative stress-related target for cancer prevention. Oxid Med Cell Longev. 2017;2017:7454031. doi: 10.1155/2017/7454031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohsen K, Zahra K, Batol R. Behavioral and histological analysis of crocus sativus effect in intracerebro-ventricular streptozotocin model of AD in rats. Iran J Pathol. 2010;5:234–56. [Google Scholar]
- 34.Aras AB, Guven M, Akman T, Ozkan A, Sen HM, Duz U, et al. Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats. Neural Regen Res. 2015;10:146–52. doi: 10.4103/1673-5374.150724. [DOI] [PMC free article] [PubMed] [Google Scholar]