Abstract
Background and Aims:
Pecs block and its variations provides perioperative analgesia, reduce PONV and other opioid related side effects. We hypothesized that COMIBPES block in addition to general anaesthesia will provide better postoperative analgesia when compared to general anaesthesia alone in breast cancer surgery patients.
Methods:
After obtaining permission from the institutional review board and registering the trial with Clinical Trials Registry of India (CTRI), we conducted a double blinded randomized controlled trial of 100 patients posted for elective breast surgery with axillary dissection. Patients were divided into two groups, P (Pecs block) and C (control). Intraoperative analgesia, postoperative analgesia, postoperative nausea vomiting (PONV) and shoulder mobility on first postoperative day (POD1) were noted. Primary outcomes were the pain scores measured by visual analog scale (VAS) and cumulative intravenous morphine consumption from patient controlled analgesia (PCA) pump at measurement intervals of 0, 1, 4, 8, 12 and 24 hours postoperatively.
Results:
Intraoperatively, Group P patients did not require any additional analgesia, whereas all the patients in Group C required additional intraoperative morphine (mean, SD: 5.12, 2.63 mg, compared to nil in group P, P< 0.01). COMBIPECS block group had lower pain scores and PCA morphine requirements, less PONV and better shoulder mobility on POD1.
Conclusion:
COMBIPECS block is a valuable addition to general anaesthesia for breast cancer surgery as it reduces pain and PONV while allowing better postoperative shoulder mobility.
Key words: Breast surgery, chest wall, regional anesthesia, ultrasound
INTRODUCTION
Breast cancer is the most common cancer among women. Acute postoperative pain originates from the skin, subcutaneous tissue or pectoral muscles, and is an integral risk factor in the development of chronic post-mastectomy pain.[1] Regional anaesthesia techniques have provided better quality acute pain control which reduced chronic pain. Effective acute pain control preserves immune function by suppressing the surgical stress response and by decreasing the need for general anaesthetics and opioids. Opioids—especially morphine—inhibit cellular and humoral immune functions which may contribute to higher rates of postsurgical local recurrence and/or metastasis.[2] Postoperative nausea and vomiting (PONV) is a serious concern in female patients, and use of morphine or other opioid analgesia may contribute to this.
Thoracic epidural block, thoracic paravertebral block (TPVB), intrapleural block, intercostal nerve block, interscalene block, and wound infiltration have all been used in anaesthesia and/or analgesia for breast cancer surgery.[3] The pectoral nerve (Pecs) block, a less invasive, novel technique,[4] is an interfascial plane block where local anaesthetic (LA) is deposited in the plane between the pectoralis major muscle (PMm) and the pectoralis minor muscle (Pmm) (Pecs 1 block) and above the serratus anterior (SA) muscle at level of the third rib (Pecs 2 block). These injections attempt to block the pectoral, intercostobrachial, intercostals III, IV, V, VI and long thoracic nerves.[5,6]
The modified Pecs block is a two-needle approach covering both territories, Pecs 1 (10 ml of LA between the pectoralis muscles) and Pecs2 (20 ml of local anaesthetic between the Pmm and SA). The long thoracic and intercostal nerves are reached by breaking through the 'axillary door' and aims to block the region over SA where significant amount of pain is experienced by patients undergoing axillary dissection.
Following this, we described and published a one-injection technique which combined both Pecs 1 and Pecs 2 in a single needle pass and named it COMBIPECS.[7]
We hypothesized that COMBIPECS block, when administered as part of multimodal analgesia would provide better postoperative pain control in patients undergoing breast cancer surgery involving axillary dissection, when compared to a conventional analgesic regime, with lower requirement of opioid in the postoperative period improving overall outcome. We also wanted to look at the effect of COMBIPECS block on postoperative limitation of shoulder mobility on the affected side.
METHODS
This study was approved by the Institutional Review Board (IRB) of Tata Medical Center (Approval number EC/TMC/55/15, dated 08/10/2015) and was registered with Clinical Trials Registry India (CTRI/2017/10/010131). The study was conducted between 1st November 2017 to 15th November 2018. Written informed consent was obtained from all subjects before recruitment in the study. Our study was preceded by a pain audit in breast surgery and a published case series where we established the safety of the COMBIPECS block.[7]
We included American Society of Anesthesiologists (ASA) physical status1 and 2 patients of age 18 to 65 years, having elective unilateral breast surgery requiring axillary dissection. Patients with drug allergies, coagulopathy, local infection at the injection site, a history of psychiatric disorders, chronic pain, previous breast surgery, COPD/asthma and unable to use PCA were excluded.
Patients were randomized into either Group P: (Pecs block group) or Group C (control group). All patients were educated about the use of PCA morphine. Group P patients received COMBIPECS block immediately after induction of general anaesthesia, and group C patients received general anaesthesia with conventional intravenous analgesics according to the study protocol. Postoperatively, all patients had free use of intravenous (IV) morphine via PCA.
A computer-generated random series was placed in numbered, sealed envelopes which were allocated to patients in order of recruitment. Before induction, the anaesthetist opened the numbered allocation envelope, and followed the allocated protocol for induction and analgesia. Patients remained blinded as blocks were given after induction of general anaesthesia. Post-operative pain scores, PONV scores and shoulder mobility scores were recorded by trained ward nurses, who were blinded to study allocation. Data was entered in a standardised case report form and a customized REDcap[8] database. After recruitment was completed, records were exported from Redcap to SPSS v23 for statistical analysis.
General anaesthesia (GA) was induced in all patients with IV propofol 2 mgKg-1, fentanyl 2 mcgKg-1 and rocuronium 0.9 mgKg-1. Airway was secured with endotracheal tube of adequate size, anaesthesia maintained with inhaled desflurane at one minimum alveolar concentration (MAC) in oxygen and air mixture with an inspired oxygen fraction (FIO2) of 40%. After induction, patients in Group P received ultrasound guided COMBIPECS block. All the blocks were performed by either of the 2 senior anaesthesiologists in the study team. 15 minutes' time interval was allowed in all patients from the block to surgical incision. All patients received IV dexamethasone 8 mg at the beginning of the surgery, slow infusion of paracetamol 1 gm and IV ondansetron 4 mg at the end of the operation. During surgery the need for additional analgesia was judged clinically using standard blood pressure, heart rate, sweating and temperature (PRST) criteria (see supplementary: Evan's score PRST) and monitoring of bispectral index (BIS). An increase of blood pressure and heart rate more than 20% from baseline, visible sweating and lacrimation indicated need for additional analgesia which was titrated to keep PRST score up to 3 and BIS between 40-60, was provided with IV morphine, 3 mg initially and additional aliquots of 0.5 mg.
For the COMBIPECS block, after sterile preparation, the breast was retracted to the opposite side. Baseline anatomy of the infraclavicular and axillary area was assessed with a high frequency (12 MHz) linear array ultrasound transducer (UST) (GE Logiqe™, Milwaukee, Wisconsin, USA), placed transversely below the lateral third of the clavicle to identify PMm, Pmm and the vessels lying in between. UST was moved caudally and laterally to the third rib to identify the margin of Pmm and serratus anterior (SA). Pleura was identified as a shining white line and the rib as a hyperechoic bar just above it [Figure 1]. After visualizing the two muscle planes—superficially between the two pectorals and deep between Pmm and SA—a 19G Tuohy needle was directed laterally towards the anterior axillary line to touch the rib, then withdrawn to place the tip in the plane between SA and Pmm. The needle position was confirmed by injecting 2mL saline. After confirming negative aspiration, 20 mL of 0.25% levo-bupivacaine was injected and the needle then withdrawn to the space between the two pectorals where 10 mL of 0.25% levo-bupivacaine was injected.
Figure 1.
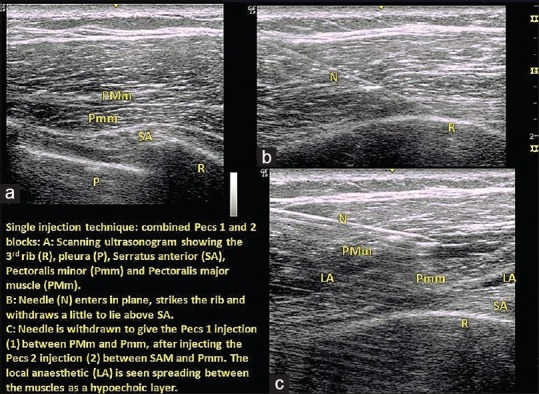
Ultrasound images of COMBIPECS block showing (a) Initial ultrasonogram, (b) Needle entry and rib strike, (c) LA injected at the intended planes while withdrawing
At the end of the surgery and reversal of anaesthesia, patients were shifted to post-anesthesia care unit (PACU), where they were provided with IV PCA (Graseby 3300 Pump; Smith Medical International, Ashford, Kent, UK) with morphine (1 mg/ml, 1 ml/bolus, no basal infusion, 5 minutes lockout time and maximum 10 doses in an hour), until the morning after surgery. Patients also received i.v. Paracetamol infusion 1 gm 8 hourly till discharge.
Morphine consumption and pain scores at rest (Visual Analog Scale, VAS) were noted by trained nurses at intervals of 1, 4, 8, 12 and 24 hours.
PONV was measured on a 4-point scale (no nausea, mild nausea, moderate to severe nausea and vomiting) and shoulder mobility using a “Shoulder Mobility Score (SMS)”, which is a composite score designed by the study team and validated by IRB.
After recruitment was completed, records were exported from Redcap to SPSS v23 for statistical analysis. Kolmogorov-Smirnov test was used for finding out normal distribution of data. Continuous variables were analysed using Student's t test (age, BMI, duration of surgery, PCA morphine comsumption, VAS scores) and categorical data (arm movement, type of surgery, PONV) were analysed using Chi square test/Fisher exact testwith a confidence interval of 95% and P value less than 0.05 was considered significant.
Assuming postoperative morphine requirement of 8 mg in the control group and 3 mg in the COMBIPECS group with standard deviation of +/- 1.8 mg, 42 patients in each group (Total = 84) were needed to detect a statistically significant difference at 80% power and alpha = 0.05. We recruited 100 patients to account for drop outs.
RESULTS
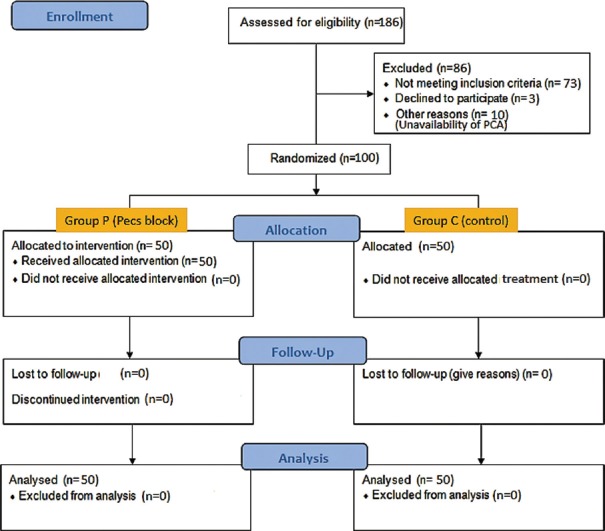
A total of 186 patients were assessed for eligibility in the study, of whom 73 did not meet the inclusion criteria and 13 others could not be recruited due to various reasons. 100 patients were recruited and randomized [Figure 2].
Figure 2.
CONSORT diagram
The median age of patients in Group P was higher than Group C (2.5 years). However no differences were noted for surgery performed (breast conservation vs mastectomy), duration of surgery or baseline shoulder mobility score [Table 1].
Table 1.
Demographic and baseline parameters, duration of surgery, PONV
| Category | Parameter | Group C | Group P | P |
|---|---|---|---|---|
| Demographic | Age (Yrs) mean, SD | 48.14, 8.90 | 52.14, 8.17 | 0.021 |
| BMI: mean, SD | 31.01, 4.49 | 30.87, 4.67 | 0.879 | |
| Baseline | Baseline arm flexion (0,1) | 50, 0 | 49, 1 | 1.0 |
| Baseline arm abduction (0,1) | 50, 0 | 48, 2 | 0.495 | |
| Baseline shoulder mobility score (0,1) | 50, 0 | 47, 3 | 0.242 | |
| Type of surgery | Breast Conservation Surgery/Mastectomy | 28/22 | 28/22 | 1.0 |
| Duration | Duration of surgery (minute) mean, SD | 122, 29.25 | 112.56, 21.65 | 0.07 |
| PONV | PONV, PACU (normal/mild nausea/moderate to severe nausea/vomited) n=50 | 35/4/3/8 | 48/0/1/1 | 0.006 |
| PONV, 1 hr post-op | 29/12/7/2 | 49/0/0/1 | <0.001 | |
| PONV, 4 hr post-op | 36/9/1/4 | 48/0/0/2 | 0.006 | |
| PONV, 8 hr post-op | 40/3/3/4 | 49/0/0/1 | 0.033 | |
| PONV, 12 hr post-op | 45/3/1/1 | 50/0/0/0 | 0.154 | |
| PONV, 24 hr post-op | 49/1/0/0 | 50/0/0/0 | 1.0 |
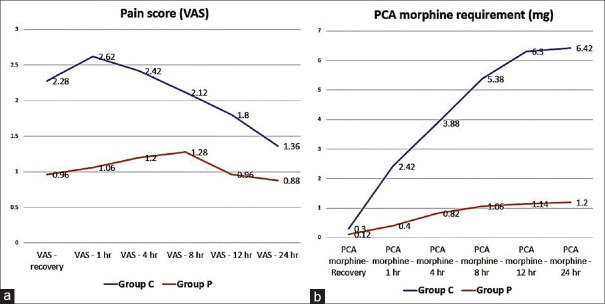
Pain scores were lower for patients in Group P for all time points, the difference being progressively narrower though it remained statistically significant [Figure 3]. Cumulative PCA morphine consumption for the two groups was similar in PACU but increased significantly from 1st hour postoperatively till 24 hours. However, hourly consumption was comparable from 12 hours onwards [Figure 3].
Figure 3.
(a) Mean pain scores at studied intervals. X axis: time, Y axis: mean VAS score. (b) PCA morphine cumulative readings at measuring intervals. X axis: time, Y axis: cumulative morphine consumption (mg)
Intraoperatively, Group P patients showed stable haemodynamics and none required any additional analgesia, whereas all the patients in Group C required intraoperative morphine (mean, SD: 0,0 in group P and 5.12, 2.63 mg in group C).
PONV scores were lower in the COMBIPECS group (P) at all the time points, and the difference was statistically significant till 8th postoperative hour [Table 1].
Shoulder mobility for both groups was similar at baseline but significantly better in Group P [Table 2].
Table 2.
Shoulder mobility score (SMS)
| A: Arm flexion | Score | B: Arm abduction | Score | |
|---|---|---|---|---|
| Full range without pain | 0 | Full range without pain | 0 | |
| Full range with pain | 1 | Full range with pain | 1 | |
| More than 90 degrees and less than full range without pain | 2 | More than 90 degrees and less than full range without pain | 2 | |
| More than 90 degrees and less than full range with pain | 3 | More than 90 degrees and less than full range with pain | 3 | |
| <90 degrees without pain | 4 | <90 degrees without pain | 4 | |
| <90 degrees with pain | 5 | <90 degrees with pain | 5 | |
| Shoulder mobility score=A+B (Minimum 0, means best movement, maximum 10, means least movement | ||||
| Group C (n=50) | Group P (n=50) | Total | P (test) | |
| Shoulder Mobility Score POD1 | ||||
| 0 | 5 | 24 | 29 | 0.000 (Chi Sq) |
| 1 | 4 | 8 | 12 | |
| 2 | 8 | 10 | 18 | |
| 3 | 2 | 4 | 6 | |
| 4 | 12 | 2 | 14 | |
| 5 | 5 | 1 | 6 | |
| 6 | 14 | 1 | 15 | |
| Total | 50 | 50 | 100 | |
DISCUSSION
We found that the COMBIPECS block group had reduced postoperative analgesic requirement till 12 hours and pain scores till 24 hours postoperatively. This proved our hypothesis that addition of COMBIPECS block to general anaesthesia would provide better perioperative analgesia compared to general anaesthesia alone.
Following the initial description of pectoral nerve block techniques,[4,6] there have been numerous clinical trials of the technique and its variations, and most showed promising results.[3,7,9,10,11,12,13,14,15] In contrast, another study did not show a significant advantage over conventional analgesia.[16] However in this study 77% of patients had minor surgery, 20% of patients did not have any axillary dissection. They used Pecs 1 alone rather than the modified pecs block, which involves injection in two muscle planes. They also added wound infiltration with LA in both groups, which reduced the difference in post-operative pain intensity. All published trials of pecs block in breast surgery are summarized in Table 3.
Table 3.
Comparison of published of RCTs on regional anaesthesia for breast surgery
| Sr. no. (ref no.) | Authors | Journal, year | Sample size, groups (control, block) | Blinded? | Type of block | Intra-op analgesia measured? | Rescue analgesic | PCA? | PONV measured? | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (9) | Bashandy et al | RegAnesth Pain Med, 2015 | 120; 60,60 | No | Two injection, modified pecs block | Clinically (BP, HR) | Loading dose of Morphine 5 mg, when VAS >3 | Morphine PCA given only when VAS >3 | No | Pecs block provides excellent analgesia |
| 2 (12) | Kulhari S et al | BJA.2016 | 40;20,20 | No | TPVB, PecsII | No | none | Morphine PCA | No | Pecs II block provided superior analgesia |
| 3 (3) | Syal K et al | Indian J Anaesth. 2017 | 65;22, 22,21 | No | Two injection, modified pecs block | No | Fentanyl 1 mcg/Kg when VAS >4 | No | No | TPVB > Pecs > LA infiltration |
| 4 (14) | Kamiya H et al | EJA, 2018 | 60; 30,30 | Yes | Pecs 2, two injection | BIS 40-50, Intra-op propofol requirement reduced but not Remifentanyl | No | No | Pecs block improved post-op pain | |
| 5 (13) | Matsumoto M | Sci Rep, 2018 | 49; 24,25 | No | Pecs 1 + Serratus anterior plane block | Yes, criteria not mentioned | Not mentioned | Morphine PCA | yes | Pecs 1 + SAP block improved analgesia |
| 6 (11) | Kim DH et al | Pain res manag, 2018 | 80; 40,40 | No | Pecs-II, single injection | No | Fentanyl 0.4 mcg/kg in PACU, when VAS >4; Meperidine 25 or Tramadol 50 mg in ward | No | Side effects of analgesics measured as whole | Pecs II block reduced pain intensity and opioid requirement till 24 h |
| 7 (10) | Kumar S et al | Indian J of Anaesth. 2018 | 50; 25, 25 | No | Single injection, modified pecs block | Clinically, Fentanyl bolus 0.25 mcg/kg | I.v. Tramadol 2 mg/Kg when VAS >4 | No | yes | Pecs block provides better post-op analgesia, reduced PONV |
| 8 (16) | J Cross et al | RegAnesth Pain Med, 2018 | 128; 66,62 | Yes | Pec 1 block | No | Intermittent morphine 3 mg bolus | No | No | Pecs 1 block does not improve peri-operative analgesia |
We work in a setting where many patients with breast cancer present with relatively advanced disease, where axillary dissection is required, and all patients included in our study had axillary dissection. The study design also addressed some of the shortcomings from previous trials. All blocks were given after induction of general anaesthesia, and all postoperative assessments were performed by ward based nurses who were blinded to group allocation. This ensured double blinding. We gave access to PCA to all patients from PACU until discharge, measured PONV at each time point, and measured shoulder mobility on POD1. To assess intraoperative analgesia, we used objective clinical criteria as well as BIS monitoring, which is more accurate than use of either alone.[17]
Our technique (COMBIPECS) was validated previously through institutional audits and published as a case series[7] but had not been evaluated in the setting of a randomised trial. Our results showed that COMBIPECS block provided excellent intraoperative analgesia and did not require any opioid supplementation. We also found that the block group had reduced postoperative analgesic requirement till 12 hours and pain scores till 24 hours postoperatively. The reduction of opioid requirement is particularly important in cancer patients as recent evidence suggests a role of morphine in cancer local recurrence and metastasis.[2]
Breast surgery patients are known to have frequent PONV,[18] which can be attributed to the surgery or to opioid analgesics in perioperative period. In our study, the COMBIPECS group had less PONV compared to the control group till 8th postoperative hour.
Shoulder mobilization is a routine part of postoperative rehabilitation for all patients having breast surgery. Patients undergoing axillary dissection may have limited shoulder mobility due to pain and muscle guard, and this may contribute to reduced lymphatic drainage and even long term lymphedema. Also in elder age group and in patients predisposed to joint diseases, a brief period of immobilization may lead to frozen shoulder,[19] which may further complicate the situation.
Most joint mobility scores were designed for patients with orthopedic problems. We devised a simple shoulder mobility score (SMS) [Table 2] which was approved by independent clinical researchers appointed by our IRB. Using this score, we found that shoulder mobility in Group P was greater than Group C on POD1.
A limitation of our study was that we could not blind the anaesthetists to group allocation, which might have influenced their intraoperative analgesic management. However postoperative pain assessments were performed by PACU nurses and ward based nurses who were blinded to group allocations.
CONCLUSIONS
We conclude that COMBIPECS block when combined with general anaesthesia, reduces perioperative opioid requirement and provides analgesia compared to control in patients undergoing breast cancer surgery with axillary dissection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank Mr. Subir Sinha for helping us with the study design (sample size calculation and preliminary audits).
REFERENCES
- 1.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–46. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Cho JS, Lee MH, Kim SI, Park S, Park HS, Oh E, et al. The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: A prospective randomized study. Int J Med Sci. 2017;14:970–6. doi: 10.7150/ijms.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syal K, Chandel A. Comparison of the postoperative analgesic effect of paravertebral block, pectoral nerve block and local infiltration in patients undergoing modified radical mastectomy: A randomised double-blind trial. Indian J Anaesth. 2017;61:643–8. doi: 10.4103/ija.IJA_81_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco R. The 'pecs block': A novel technique for providing analgesia after breast surgery. Anesthesia. 2011;66:847–8. doi: 10.1111/j.1365-2044.2011.06838.x. [DOI] [PubMed] [Google Scholar]
- 5.Blanco R, Fajardo M, Parras MT. Ultrasound description of Pecs II (modified Pecs I): A novel approach to breast surgery. Rev EspAnestesiolReanim. 2012;59:470–5. doi: 10.1016/j.redar.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Pérez MF, Miguel JG, de la Torre PA. A new approach to pectoralis block. Anesthesia. 2013;68:430. doi: 10.1111/anae.12186. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty A, Khemka R, Datta T, Mitra S. COMBIPECS, the single-injection technique of pectoral nerve blocks 1 and 2: A case series. J ClinAnesth. 2016;35:365. doi: 10.1016/j.jclinane.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashandy GMN, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery- A randomized clinical trial. RegAnesth Pain Med. 2015;40:68–74. doi: 10.1097/AAP.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Goel D, Sharma SK, Ahmad S, Dwivedi P, Deo N, et al. A randomised controlled study of the postoperative analgesic efficacy of ultrasound-guided pectoral nerve block in the first 24 h after modified radical mastectomy. Indian J Anaesth. 2018;62:436–42. doi: 10.4103/ija.IJA_523_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DH, Kim S, Kim CS, Lee S, Lee IG, Kim HJ, et al. Efficacy of pectoral nerve block type II for breast-conserving surgery and sentinel lymph node biopsy: A prospective randomized controlled study. Pain Res Manag. 2018;2018:4315931. doi: 10.1155/2018/4315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulhari S, Bharti N, Bala I, Arora S, Singh G. Efficacy of pectoral nerve blockversusthoracic paravertebral block for postoperative analgesia after radical mastectomy: A randomized controlled trial. Br J Anaesth. 2016;117:382–6. doi: 10.1093/bja/aew223. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, Flores EM, Kimachi PP, Gouveia FV, Kuroki MA, Barros ACSD, et al. Benefits in radical mastectomy protocol: A randomized trial evaluating the use of regional anesthesia. Sci Rep. 2018;8:7815. doi: 10.1038/s41598-018-26273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamiya Y, Hasegawa M, Yoshida T, Takamatsu M, Koyama Y. Impact of pectoral nerve block on postoperativepainand quality of recovery in patients undergoing breast cancer surgery: A randomised controlled trial. Eur J Anaesthesiol. 2018;35:215–23. doi: 10.1097/EJA.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 15.Pérez MF, Duany O, de la Torre PA. Redefining PECS blocks for postmastectomy analgesia. RegAnesth Pain Med. 2015;40:729–30. doi: 10.1097/AAP.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 16.Cros J, Sengès P, Kaprelian S, Desroches J, Gagnon C, Labrunie A, et al. Pectoral I block does not improve postoperative analgesia after breast cancer surgery a randomized, double-blind, dual-centered controlled trial. RegAnesth Pain Med. 2018;43:596–604. doi: 10.1097/AAP.0000000000000779. [DOI] [PubMed] [Google Scholar]
- 17.Panousis P, Heller AR, Koch T, Litz RJ. Epidural ropivacaine concentrations for intraoperative analgesia during major upper abdominal surgery: A prospective, randomized, double-blinded, placebo-controlled study. AnesthAnalg. 2009;108:1971–6. doi: 10.1213/ane.0b013e3181a2a301. [DOI] [PubMed] [Google Scholar]
- 18.Fujii Y. Prophylaxis of postoperative nausea and vomiting in patients scheduled for breast surgery. Clin Drug Investig. 2006;26:427–37. doi: 10.2165/00044011-200626080-00001. [DOI] [PubMed] [Google Scholar]
- 19.Nagy MT, Macfarlane RJ, Khan Y, Waseem M. The frozen shoulder: Myths and realities. Open Orthop J. 2013;7:352–5. doi: 10.2174/1874325001307010352. [DOI] [PMC free article] [PubMed] [Google Scholar]