Abstract

A new water-soluble reactive perylene tetracarboxylic diimide derivative (PDI-pfp) is designed and synthesized that can realize fast imaging of the endoplasmic reticulum in living cells. The PDI-pfp comprises three functional moieties: perylene tetracarboxylic diimide as fluorescent backbone, poly(ethylene glycol) for providing good water disperse ability, and pentafluorophenol active ester as the reactive group under physiological condition. On the basis of covalent reaction between the active ester group of PDI-pfp and amine groups on cytomembrane, PDI-pfp can rapidly interact with cytomembrane, followed by uptake by living MCF-7 cells within 1 min and also exhibit low cell cytotoxicity. Furthermore, it is proved that PDI-pfp acts as a universal imaging agent for other types of cells. This fluorescent probe is of great potential for the application in the rapid imaging of organelles in cells.
Introduction
On account of the properties of intuition, multi-information, high sensitivity, and specificity,1−6 indispensable and emerging fluorescent probes and materials for cell imaging have been greatly developed.7−10 Compared to fluorescent proteins11 and fluorescent nanoparticles,12−16 organic dyes, which have smaller size, higher emission intensity, and broader available spectral range, play an important role in fluorescence imaging of cells.17−20 Furthermore, the design and synthesis of advantageous cell imaging materials with good water solubility, high fluorescence quantum yield, good biocompatibility, and low cytotoxicity are extremely imperative.
Perylene tetracarboxylic diimide (PDI) and its derivatives with excellent redox and optical properties as well as superior photochemical and thermal stabilities21−24 have been extensively applied in organic pigments, electrophotography, and photovoltaic cells.25−28 Owing to the delocalized π-electronic rigid plane structure, PDI is endowed with high fluorescence quantum yield and, recently, has been widely utilized as a chromophore.29 However, the hydrophobic backbone of PDI results in its poor solubility in aqueous solution, which has greatly limited its application in biological systems. Two strategies have been employed so far to enhance the water solubility of PDI: 1) the attachment of hydrophilic chains at the imide nitrogen of PDI;30,31 and 2) the introduction of charge groups at the bay region of PDI.32
Pentafluorophenol active ester exhibits high reactivity with many functional groups,33−35 especially with amino groups under physiological conditions. Therefore, pentafluorophenol ester as an active group could be used to facilitate fluorescent probe to enter cells because of the widely distributed amino groups on the cell surfaces.36 In this work, we report the design and synthesis of a new water-soluble reactive perylene tetracarboxylic diimide derivative (PDI-pfp) with high brightness, good water solubility, and high chemical reactivity. By introducing oxylalkyl chains to PDI, the water solubility is enhanced without affecting its high fluorescence quantum yield because of the inappreciable influence of N-substituent for nodes of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) orbitals of PDI.21 Pentafluorophenol active ester offers PDI-pfp a high reactive group to interact with cells. It was demonstrated that the PDI-pfp exhibited low cytotoxicity and sufficient fluorescence ability for rapid imaging of the endoplasmic reticulum (ER) in living cells.
Results and Discussion
The synthetic procedure of PDI-pfp is shown in Scheme 1. The precursor molecule PDI–COOH was first synthesized by reacting perylene-3,4,9,10-tetracarboxylic dianhydride (PDI) and NH2-PEG10-COOH in imidazole at 130 °C for 5 h with a yield of 70%. PDI-pfp was then prepared by esterification of PDI–COOH with pentafluorophenol in the presence of N,N-dimethylaminopyridine (DMAP) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) in dichloromethane overnight with a yield of 53%. All of the chemical structures were confirmed by the 1H NMR and mass spectra.
Scheme 1. Synthetic Procedure of PDI-pfp.
The photophysical properties of PDI–COOH and PDI-pfp were investigated in aqueous solution because the linkage of low toxic and uncharged alkoxy chains extremely enhanced their solubility. UV–vis absorption and fluorescence emission spectra of PDI and PDI-pfp were measured in aqueous solution. As shown in Figure 1, owing to the fact that both PDI–COOH and PDI-pfp consist of the same backbones, they exhibited quite similar absorption with a maximum peak at 498 nm. On the basis of Lambert Beer’s law, molar absorption coefficients of PDI–COOH and PDI-pfp were calculated to be 2.7 × 105 and 1.9 × 105 M–1 cm–1, respectively. Moreover, PDI-pfp has nodes in the orbital HOMO and LUMO at nitrogen atoms, which cause decoupling of single bonds between nitrogen atoms and substitutions.14 Thus, compared to the precursor molecule PDI–COOH, there is less influence on the fluorescence of PDI-pfp. PDI-pfp exhibited a bright green fluorescence with a maximum emission at 548 nm and an absolute fluorescence quantum yield of 0.35 in aqueous solution, which shows good potential for fluorescent imaging.
Figure 1.

UV–vis absorption and fluorescence emission spectra of PDI–COOH and PDI-pfp in aqueous solution. The excited wavelength of 507 nm was used for emission spectra.
Low cytotoxicity is necessary for fluorescent probes to be utilized in cell imaging and biology applications.37 Cytotoxicities of PDI-pfp and PDI–COOH were investigated through a standard MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay, in which optical density values of formazan reduced from MTT by dehydrogenase in mitochondria of viable cells were measured. In this experiment, MCF-7 cells were incubated in 96-well culture plates treated with different concentrations of PDI and PDI-pfp for 30 min. Then, the supernatant was discarded, and MCF-7 cells were continuously cultured in fresh Dulbecco’s modified Eagle’s medium (DMEM) for the next 24 h, and cell viabilities were measured and calculated. As shown in Figure 2, PDI–COOH displayed nearly nontoxic behavior up to 10 μM concentration, whereas PDI-pfp also exhibited less cytotoxicity at concentrations lower than 10 μM, indicating their good biocompatibility for cell imaging.
Figure 2.
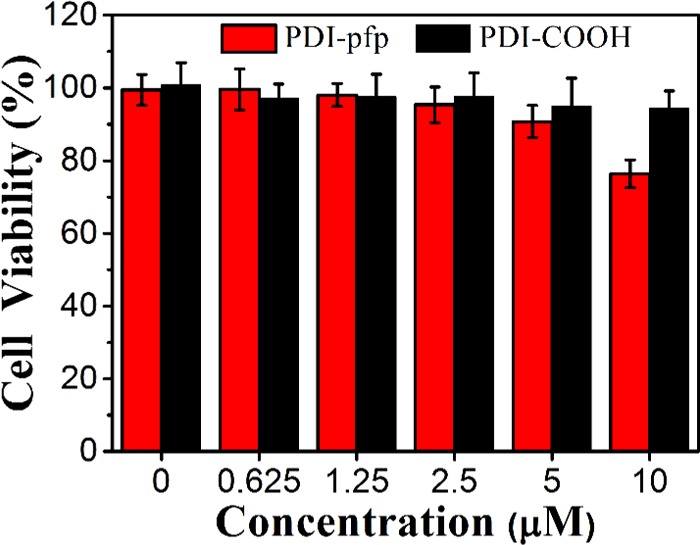
Viability of MCF-7 cells after incubating with PDI-pfp and PDI–COOH for 30 min treatment. [PDI-pfp] or [PDI–COOH] = 0–10 μM.
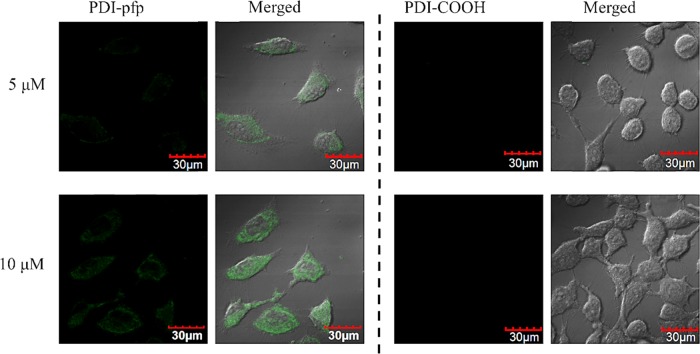
The ability of imaging for living cells of PDI-pfp was then determined using MCF-7 cells. To investigate the effect of pentafluorophenol active esters of PDI-pfp on cell imaging, PDI–COOH without pentafluorophenol active esters was also studied as control. To accomplish this, considering the cell viability in Hank’s balanced salt solution (HBSS), the incubation time was set as 30 min. MCF-7 cells were treated with PDI–COOH and PDI-pfp for 30 min, and the treated cells were washed with Hank’s balanced salt solution (HBSS), followed by the addition of serum-free medium into the plates for confocal laser scanning microscopy (CLSM) characterization. As shown in Figure 3, PDI-pfp with pentafluorophenol active esters as terminal groups could uptake into MCF-7 cells and well stain cells. It is noted that MCF-7 cells internalized by PDI-pfp were observed in a good condition. Compared with PDI-pfp, PDI–COOH with carboxyl groups at the terminal position being negatively charged in aqueous solution is believed to be repelled by the negatively charged membrane of cells, leading to no interaction between PDI–COOH and MCF-7 cells. Furthermore, the imaging results indicated that PDI-pfp with higher concentration (10 μM) stains cells better than that with lower concentration (5 μM).
Figure 3.
CLSM images of MCF-7 cells incubated with PDI–COOH and PDI-pfp (5 and 10 μM) for 30 min. The excitation wavelength was 488 nm, and the fluorescence images were collected at the signals from 500 to 600 nm.
To further confirm the localization of PDI-pfp inside cells, MCF-7 cells were incubated with PDI-pfp for 30 min, and then dyes using specific imaging of organelles in cells were added. It was observed that the location of PDI-pfp was well overlapped with that of ER Tracker Red, which is used for specific staining of the endoplasmic reticulum (ER) in cells (Figure 4a), while poor overlaps were seen with those of other organelle-specific dyes. A white line that was randomly selected in the fluorescent image of MCF-7 cells was chosen for obtaining the Pearson coefficient (Figure 4b). The value of the Pearson coefficient was calculated to be 0.79178, which showed that PDI-pfp was uptaken into cells and mainly located in the endoplasmic reticulum.
Figure 4.
(a) Colocalizing with endoplasmic reticulum-specific ER Tracker Red after treating MCF-7 cells with PDI-pfp for 30 min. (b) Line-series analysis of PDI-pfp with ER Tracker Red. The Pearson coefficients were calculated along the white line on the MCF-7 cells. PDI-pfp and ER Tracker Red were excited at the wavelengths of 488 and 559 nm, respectively.
Rapid imaging of cells is an overwhelming superiority of biomaterials. The incubating times of cells with PDI-pfp at 1, 5, 10, 30, and 60 min were set as a time node to investigate the imaging ability of PDI-pfp toward cells. Figure 5a demonstrates that PDI-pfp could be internalized immediately and distributed in the cytoplasm of MCF-7 cells even within 1 min. With the extension of incubation time, the accumulation amount of PDI-pfp increased and maintained the same distribution in MCF-7 cells. It was noted that the accumulation amount of PDI-pfp did change up to 10 min. To prove the generality of PDI-pfp in rapid imaging toward cells, HeLa cells were chosen as the representative of cancer cells, and 293T cells were chosen as the representative of normal cells for imaging experiments. As shown in Figure 5b,c, similar imaging results for HeLa cells and 293T cells by PDI-pfp were observed as that of MCF-7 cells. Thus, PDI-pfp exhibits great potential for application in the rapid imaging of cells within 1 min. The uptake is the main driving force for the fast internalization of PDI-pfp, and the reaction between PDI-pfp containing pentafluorophenol active ester groups and amino groups on the surface of cells assists the process.31
Figure 5.
CLSM images of cells treated with PDI-pfp (10 μM) with different incubation times: (a) MCF-7 cells; (b) HeLa cells; and (c) 293T cells. PDI-pfp were excited at the wavelength of 488 nm, and the fluorescence images were collected at the signals from 500 to 600 nm.
Conclusions
In summary, a new water-soluble reactive perylene tetracarboxylic diimide derivative, PDI-pfp, with pentafluorophenol active ester on the ends of the backbone was designed and synthesized. The alkoxy chains efficiently enhanced the solubility of PDI-pfp in aqueous solution. PDI-pfp exhibited low cell cytotoxicity and realized rapid imaging of the endoplasmic reticulum (ER) of living cells. The high reactivity between pentafluorophenol active ester groups and amino group on the cell surface led to the fast uptake of PDI-pfp into cells even within 1 min. PDI-pfp exhibited generality of rapid imaging toward different types of cells, including cancer cells (MCF-7 and HeLa cells) and normal cells (293T cells). This fluorescent probe is of great potential for application in the rapid imaging of organelles in cells.
Experimental Section
Materials and Measurements
All chemicals were procured from Sigma-Aldrich Chemical Company, J&K Chemical Company or AMRESCO and used as received. All organic solvents were purchased from Beijing Chemical Works and used without further purification. NH2-PEG10-COOH were purchased from Yanyi Biotech Company Shanghai. Dulbecco’s modified Eagle’s medium (DMEM) was purchased from HyClone/Thermo Fisher (Beijing, China). (3-(4,5′-Dimethylthiazol-2′yl)-2,5-dipehenyl-2H-tetrazolium hydrobromide) (MTT) was purchased from Xinjingke Biotech (Beijing, China) and dissolved in 1× phosphate-buffered saline (PBS) before use. The 1H NMR and 13C NMR spectra were recorded on Bruker ARX 300 and ARX 400 instruments with tetramethylsilane as the internal standard. High-resolution mass spectra (HRMS) were taken on a Bruker 9.4T Solarix FT-ICR-MS spectrometer. The UV–vis absorption spectrum was measured on a JASCO V-550 spectrophotometer. The fluorescence spectrum was taken on a Hitachi F-4500 fluorometer equipped with a Xenon lamp excitation source. Absolute fluorescence quantum yield was measured on Hamamatsu absolute photoluminescence quantum yield spectrometer C11347. The MTT assay was performed on a BIO-TEK Synergy HT microplate reader. Cell counting was performed on an automated cell counter (Countess, Invitrogen). Cell imaging was recorded by a confocal laser scanning microscope (FV 1000-IX81, Olympus, Japan).
Synthesis of Compound PDI–COOH
3,4,9,10-Perylenetetracareboxylic dianhydride (254.89 mol, 100 mg), imidazole (36 mmol, 2.5 g), and NH2-PEG10-COOH (764.68 mol, 404.99 mg) were mixed together in a 10 mL flask. The solution was heated to 130 °C and refluxed for 5 h. The resulting mixture was cooled down to room temperature. After adding 5 mL of chloroform and 5 mL of 1 M HCl under sonication, the mixture was extracted with chloroform for three times. The combined organic layer was washed by 1 M HCl (5 mL × 3) and dried with anhydrous Na2SO4. The mixture was concentrated and filtrated to remove the solids. Then, the solvent was removed by vacuum, and the residue was a red oily liquid (yield: 254.2 mg, 70%). 1H NMR (400 MHz, CDCl3) δ (ppm) 8.4 (s, 4H), 8.24 (s, 4H), 4.42 (s, 4H), 3.86 (t, J = 4 Hz, 8 Hz, 4H), 3.75 (t, J = 4 Hz, 8 Hz, 4H), 3.63–3.60 (m, 72H), 2.59 (t, J = 8 Hz, 8 Hz, 4H). HRMS (MALDI-TOF) m/z: [M + H]+ calcd 1437.619831, found: 1437.619519.
Synthesis of Compound PDI-pfp
PDI–COOH (254.2 mg, 179.58 mmol) and pentafluorophenol (99.16 mg, 538.73 mmol) were dissolved in dichloromethane. EDCI (89.51 mg, 466.9 mmol) was then added to the reaction mixture at low temperature using an ice bath, followed by the dropwise addition of DMAP (5.7 mg, 46.69 mmol). The reaction mixture was stirred overnight to ensure the completion of the reaction. Afterward, the reaction mixture was extracted by dichloromethane (20 mL × 3), and the combined organic layer was washed with distilled water (20 mL) and NaCl saturated solution (50 mL) separately. After drying with anhydrous Na2SO4, the solvent was removed, and the residue was purified by silica gel chromatography using dichloromethane/methanol (20:1) as the eluent to afford the red crystal. (Yield: 165.56 mg, 53%) 1H NMR (400 MHz, CDCl3) δ (ppm) 8.658 (d, J = 13.6 Hz, 4H), 8.93 (d, J = 10.8 Hz 4H), 4.47 (t, J = 0.8 Hz, 0.8 Hz 4H), 3.883 (t, J = 3.2 Hz, 5.2 Hz, 4H), 3.744 (t, J = 3.2 Hz, 5.6 Hz, 4H), 3.618–3.607 (m, 72H), 2.939 (t, J = 8 Hz, 8.4 Hz, 4H). HRMS (MALDI-TOF) m/z: [M + H]+ calcd 1769.588213, found: 1769.587103.
Optical Experiment
First, 10 μM PDI-pfp and 10 μM PDI–COOH in aqueous solution (with DMSO less than 1% to improve the water dispersibility) were separately used to investigate the photophysical properties, and the UV–vis absorption spectra and fluorescent emission spectra (excited at 507 nm) were obtained. The absolute fluorescence quantum yield of PDI-pfp in distilled water was measured at an excited wavelength of 507 nm.
Cell Viability Assay
Breast carcinoma cells (MCF-7 cells) were cultured in DMEM with 10% fetal bovine serum (FBS) at 37 °C under 5% CO2 atmosphere. The cells were seeded in a 96-well culture plate at a concentration of 8 × 104 cells per mL and grown for 24 h. After washing with Hank’s balanced salt solution (HBSS), different concentrations of PDI–COOH and PDI-pfp dissolved in HBSS were added separately. Addition of HBSS was considered as the blank group. This was followed by washing with PBS and adding culture medium again. After incubating for 24 h and abandoning the culture medium, 100 μL of 1 mg mL–1 MTT in HBSS was added into each well and incubated at 37 °C under 5% CO2 atmosphere for 4 h. The supernatant was discarded, followed by the addition of 100 μL of DMSO per well to dissolve the formazan. A microplate reader measured the absorbance value of each well at a wavelength of 520 nm. The cell viability rates were calculated with the following equation
where A is the absorbance value of the experimental group to which was added PDI or PDI-pfp. Ab is the absorbance value of the group without cells. A0 is the absorbance value of the control group with cells to which was added HBSS alone.
Localization Analysis of PDI-pfp in MCF-7 Cells
PDI-pfp was dissolved to prepare 10 μM solution in HBSS. First, 1 μM ER Tracker Red solution was prepared with HBSS. MCF-7 cells were incubated in 35 × 35 mm plates. After washing with HBSS twice, MCF-7 cells were incubated with 1 μM ER Tracker Red solution for 30 min. Then, the supernatant was discarded before washing with HBSS again. MCF-7 cells were incubated with 10 μM PDI-pfp solution for 30 min. Cells were washed with HBSS followed by adding serum-free culture medium, and then the fluorescence images of MCF-7 cells were recorded by CLSM; PDI-pfp and ER Tracker was, respectively, excited at the wavelengths of 488 and 559 nm.
Cell Imaging with PDI-pfp and PDI–COOH
Human cervical carcinoma cells (HeLa cells) and human epithelial cells (293T) were cultured in DMEM with 10% FBS at 37 °C under 5% CO2 atmosphere. Then, 5 μM and 10 μM of PDI–COOH and PDI-pfp solutions were prepared with HBSS, respectively. MCF-7 cells, HeLa cells, and 293T cells incubated in 35 × 35 mm plates were washed with HBSS twice before using. Washed MCF-7 cells were incubated with 5 and 10 μM of PDI–COOH and PDI-pfp solutions at 37 °C for 30 min. In addition, washed MCF-7 cells, HeLa cells, and 293T cells were incubated with the addition of 10 μM of PDI-pfp solutions at 37 °C for 1, 5, 10, 30, and 60 min. Discarding the supernatant and washing with HBSS twice was followed by adding serum-free culture medium, and then the fluorescence images of cells were recorded by CLSM using 488 nm as excitation wavelength.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (Nos 21473220, 91527306, and 21661132006).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Luo C. J.; Stoyanov S. D.; Stride E.; Pelan E.; Edirisinghe M. Electrospinning versus fibre production methods: from specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. 10.1039/c2cs35083a. [DOI] [PubMed] [Google Scholar]
- Stender A. S.; Marchuk K.; Liu C.; Suzanne S.; Meyer M. W.; Smith E. A.; Neupane B.; Wang G. F.; Li J. J.; Cheng J. X.; Huang B.; Fang N. Single cell optical imaging and spectroscopy. Chem. Rev. 2013, 113, 2469–2527. 10.1021/cr300336e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R.Emerging biomedical applications of time-resolved fluorescence spectroscopy. In Topics in Fluorescence Spectroscopy, 4th ed.; Lakowicz J. R., Ed.; Plenum Press: New York, 1995; pp 1–19. [Google Scholar]
- Ge X.; Tolosa L.; Rao G. Dual-labeled glucose binding protein for ratiometric measurement of glucose. Anal. Chem. 2004, 76, 1403–1410. 10.1021/ac035063p. [DOI] [PubMed] [Google Scholar]
- Weiss S. Fluorescence spectroscopy of single biomolecules. Science 1999, 283, 1676–1683. 10.1126/science.283.5408.1676. [DOI] [PubMed] [Google Scholar]
- Fernández-Suárez M.; Ting A. Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 929–943. 10.1038/nrm2531. [DOI] [PubMed] [Google Scholar]
- Edetsberger M.; et al. Fluorescence spectroscopy: an emerging excellent diagnostic tool in medical sciences. Appl. Spectrosc. Rev. 2010, 45, 1–11. 10.1080/05704920903435375. [DOI] [Google Scholar]
- Böhmer M.; Enderlein J. Fluorescence spectroscopy of single molecules under ambient conditions: methodology and technology. ChemPhysChem 2003, 4, 792–808. 10.1002/cphc.200200565. [DOI] [PubMed] [Google Scholar]
- Gillenwater A.; Jacob R.; Richards-Kortum R. Fluorescence spectroscopy: a technique with potential to improve the early detection of aerodigestive tract neoplasia. Head Neck 1998, 20, 556–562. . [DOI] [PubMed] [Google Scholar]
- Hao Q.; Qiu T.; Chu P. K. Surfaced-enhanced cellular fluorescence imaging. Prog. Surf. Sci. 2012, 87, 23–45. 10.1016/j.progsurf.2012.03.001. [DOI] [Google Scholar]
- Nienhaus K.; Nienhaus G. U. Fluorescent protein for live-cell imaging with super-resolution. Chem. Soc. Rev. 2014, 43, 1088–1106. 10.1039/C3CS60171D. [DOI] [PubMed] [Google Scholar]
- Walling M. A.; Novak J. A.; Shepard J. R. E. Quantum dots for live cell and in vivoimaging. Int. J. Mol. Sci. 2009, 10, 441–491. 10.3390/ijms10020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.; Yang Y.; Zhang C. Y. Toward biocompatible semicoductor quantum dots: from biosynthesis and bioconjugation to biomedical application. Chem. Rev. 2015, 115, 11669–11717. 10.1021/acs.chemrev.5b00049. [DOI] [PubMed] [Google Scholar]
- Lan M. H.; Zhang J. F.; Zhu X. Y.; Wang P. F.; Chen X. F.; Lee C. S.; Zhang W. Highly stable organic fluorescent nanorods for living-cell imaging. Nano Res. 2015, 8, 2380–2389. 10.1007/s12274-015-0748-4. [DOI] [Google Scholar]
- Miao Q.; Xie C.; Zhen C.; Lyu Y.; Duan H. W.; Liu X. G.; Jokerst J. V.; Pu K. Y. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102–1110. 10.1038/nbt.3987. [DOI] [PubMed] [Google Scholar]
- Zhen X.; Zhang J. J.; Huang J. G.; Xie C.; Miao Q. Q.; Pu K. Y. Macrotheranostic probe with disease-actived near-infrared fluorescence, photoacoustic, and photothermal signals for imaging-guided therapy. Angew. Chem., Int. Ed. 2018, 57, 7804–7808. 10.1002/anie.201803321. [DOI] [PubMed] [Google Scholar]
- Rombouts K.; Braeckmans K.; Remaut K. Fluorescent labeling of plasmid DNA and mRNA: gains and losses of current labeling strategies. Bioconjugate Chem. 2016, 27, 280–297. 10.1021/acs.bioconjchem.5b00579. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Fischer M. K. R.; Bauerle P. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem., Int. Ed. 2009, 48, 2474–2499. 10.1002/anie.200804709. [DOI] [PubMed] [Google Scholar]
- Miao Q.; Yeo D. C.; Wiraja C.; Zhang J. J.; Ning X. Y.; Xu C. J.; Pu K. Y. Near-Infrared Fluorescent Molecular Probe for Sensitive Imaging of Keloid. Angew. Chem., Int. Ed. 2018, 57, 1256–1260. 10.1002/anie.201710727. [DOI] [PubMed] [Google Scholar]
- Li J.; Rao J. H.; Pu K. Y. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235. 10.1016/j.biomaterials.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würthner F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 1564–1579. 10.1039/B401630K. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xu Z.; Cai L. Z.; Lai G. Q.; Qiu H. X.; Shen Y. J. Highly soluble perylene tetracarboxylic diimides and tetrathiafulvalene–perylene tetracarboxylic diimide–tetrathiafulvalene triads. J. Photochem. Photobiol., A 2008, 200, 334–345. 10.1016/j.jphotochem.2008.08.011. [DOI] [Google Scholar]
- Margineanu A.; Hofkens J.; Cotlet M.; Habuchi S.; Stefan A.; Qu J. Q.; Kohl C.; Müllen K.; Vercammen J.; Engelborghs Y.; Gensch T.; De Schryver F. C Photophysics of a water–soluble rylene dye: comparison with other fluorescent molecules for biological applications. J. Phys. Chem. B 2004, 108, 12242–12251. 10.1021/jp048051w. [DOI] [Google Scholar]
- Wu J. J.; Yang Z.; Jiao J. M.; Sun P. F.; Fan Q. L.; Huang W. The synthesis and biological application of water-soluble perylene diimides. Prog. Chem. 2017, 29, 216–230. 10.7536/PC160717. [DOI] [Google Scholar]
- Quante H.; Müllen K. Quaterrylenebis(dicarboximides). Angew. Chem., Int. Ed. 1995, 34, 1323–1325. 10.1002/anie.199513231. [DOI] [Google Scholar]
- Law K. Y. Organic photoconductive materials: recent trends and developments. Chem. Rev. 1993, 93, 449–486. 10.1021/cr00017a020. [DOI] [Google Scholar]
- Schmidt-Mende L.; Fechtenkötter A.; Müllen K.; Moons E.; Friend R. H.; MacKenzie J. D. Self-organized discotic liquid crystals for high-efficiency organic photovoltaics. Science 2001, 293, 1119–1122. 10.1126/science.293.5532.1119. [DOI] [PubMed] [Google Scholar]
- Yakimov A.; Forrest S. R. High photovoltage multiple-heterojunction organic solar cells incorporating interfacial metallic nanoclusters. Appl. Phys. Lett. 2002, 80, 1667–1669. 10.1063/1.1457531. [DOI] [Google Scholar]
- Qu J.; Kohl C.; Pottek M.; Müllen K. Ionic perylenetetracarboxdiimides: highly fluorescent and water-soluble dyes for biolabeling. Angew. Chem., Int. Ed. 2004, 43, 1528–1531. 10.1002/anie.200353208. [DOI] [PubMed] [Google Scholar]
- Icli S.; Icil H. A thermal and photostable reference probe for Qf measurements: chloroform soluble perylene 3,4,9,10-tetracarboxylic acid-bis-N,N-dodecyl diimide. Spectrosc. Lett. 1996, 29, 1253–1257. 10.1080/00387019608007119. [DOI] [Google Scholar]
- Sun P.; Yuan P. C.; Wang W. X.; Deng W. X.; Tian S. C.; Wang C.; Lu X. M.; Huang W.; Fan Q. L. High density glycopolymers functionalized perylene diimide nanoparticles for tumor-targeted photoacoustic imaging and enhanced photothermal therapy. Biomacromolecules 2017, 18, 3375–3386. 10.1021/acs.biomac.7b01029. [DOI] [PubMed] [Google Scholar]
- Schlichting P.; Rohr U.; Müllen K. New synthetic routes to alkyl-substituted and functionalized perylenes. Liebigs Ann. 1997, 1997, 395–407. 10.1002/jlac.199719970218. [DOI] [Google Scholar]
- Wiss K. T.; Krishna O. D.; Roth P. J.; Klick K. L.; Theato P. A versatile grafting-to approach for the bioconjugation of polymers to collagen-like peptides using an activated ester chain transfer agent. Macromolecules 2009, 42, 3860–3863. 10.1021/ma900417n. [DOI] [Google Scholar]
- Zhang X. A.; Qin A.; Tong L.; Zhao H.; Zhao Q.; Sun J. Z.; Tang B. Z. Synthesis of functional disubstituted polyacetylenes bearing highly polar functionalities via activated ester strategy. ACS Macro Lett. 2012, 1, 75–79. 10.1021/mz200024a. [DOI] [PubMed] [Google Scholar]
- Engler A. C.; Chan J. M. W.; Coady D. J.; O’Brient J. M.; Sardon H.; Nelson A.; Sanders D. P.; Yang Y. Y.; Hedrick J. L. Accessing New Materials through Polymerization and Modification of a Polycarbonate with a Pendant Activated Ester. Macromolecules 2013, 46, 1283–1290. 10.1021/ma4001258. [DOI] [Google Scholar]
- Nie C.; Li S. L.; Wang B.; Liu L. B.; Hu R.; Chen H.; Lv F. T.; Dai Z. H.; Wang S. Preparation of reactive oligo (p-phenylene vinylene) materials for spatial profiling of chemical reactivity of intracellular compartments. Adv. Mater. 2016, 28, 3749–3754. 10.1002/adma.201600106. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Campbell R. E.; Ting A. Y.; Tsien R. Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002, 3, 906–918. 10.1038/nrm976. [DOI] [PubMed] [Google Scholar]