Abstract
Three-dimensional (3D) biodegradable and biomimetic porous scaffolds are ideal frameworks for skin tissue engineering. In this study, hybrid constructs of 3D scaffolds were successfully fabricated by the freeze-drying method from combinations of the type I collagen (Col) and synthetic poly(lactic acid) (PLLA) or polycaprolactone (PCL). Four different groups of 3D porous scaffolds including PCL, PCL–Col, PCL–PLLA, and PCL–PLLA–Col were fabricated and systematically characterized by hydrogen nuclear magnetic resonance, Fourier transform infrared spectroscopy, and scanning electron microscopy (SEM). Adipose tissue-derived mesenchymal stem cells (AT-MSCs) were seeded in all scaffolds, and the viability, proliferation, and adhesion of the cells were investigated using dimethylthiazol diphenyltetrazolium bromide assay and SEM. The results showed that scaffolds containing Col, particularly PCL–PLLA–Col scaffold, with pore sizes close to 400 nm and being sufficiently interconnected, have significantly greater potential (p < 0.01) for encouraging AT-MSCs adhesion and growth. The PCL–PLLA provided a mechanically stronger mesh support, and the type I Col microsponges encouraged excellent cell adhesion and tissue formation. The scaffold with the best properties could be an appropriate functional candidate for the preparation of artificial skin constructs.
1. Introduction
Tissue engineering (TE) provides a promising alternative approach to repair and regenerate the injured or malfunctioned tissues with biologically and mechanically appropriate autologous replacement organ or tissue.1 In this regard, TE is employed to help the patient’s body and produce materials which can mimic the body’s in vivo tissue conditions. In addition, TE strategies showed promising results for improving the quality of regenerative therapeutics. The master plan for TE is the isolation of specific cells from patient,2−4 then seeding the isolated cells on a desired biomimetic scaffold and administration of growth media, and eventually grafting back the construct into the same patient.5he first item in TE, as mentioned previously, is the cells. Although, a few different cells have been used in TE purposes, stem cells could be the best candidates because of their unique properties such as self-renewal and differentiation. There are three main types of stem cells, including embryonic stem cells, induced pluripotent stem cells (PSCs), and adult stem cells.4 The embryonic stem cells show pluripotency which is ideal for regenerative therapies of all cell types of the body, but ethical issues related to the destruction of embryos, propensity for becoming turmeric after transplantation, and their limited availability are the main factors hampering their routine use. Induced PSCs (iPSCs) are another group, which have pluripotency potential. Because iPSCs originated from reprograming of somatic cells,6 the embryonic stem cells’ moral issues are not important in this case, but their in vivo administration concerns about their becoming tumorogenic. In comparison, adult stem cells, in particular mesenchymal stem cells (MSCs), are the best candidates for TE because they are available in adulthood, without any ethical concerns and are multipotent. Recent in vitro studies have reported that MSCs could be extracted from various sources, including bone marrow, cord blood, adipose, and so on. The second item for a TE process is the use of macro–nano structured porous scaffolds, for supporting initial cell adhesion, and subsequently successful tissue preparation. Six methods were established for the fabrication of three-dimensional (3D) hybrid scaffolds including fiber extrusion and bonding, gas foaming, 3D printing, phase separation, porogen leaching, and emulsion freeze-drying.7 The 3D scaffolds usually are built from synthetic or natural polymers, mimicking extracellular matrix (ECM) for directing the proliferation and spread of seeded cells in vitro and in vivo. It is essential for a scaffold to be biocompatible as well as biodegradable.8
Nowadays, the number of the patients who have been suffering from skin damages such as burning or chronic wounds is dramatically increasing, and thus, there is an unmet need for introducing effective and prompt wound healing strategies.9 In this regard, donor sit limitation and morbidity, reduction of the surgical procedure are main limitations of skin substitution.10 In the last decade, TE as a novel strategy for skin regeneration is holding a great promise because of the excellent design and fabrication of tunable living replacements.
There are different types of biopolymers for ECM mimicking; one of them is collagen (Col) which showed promising results in treating skin burns, wounds, and cosmetics.11 Up to now, various hybrid scaffold types have been developed for skin TE. The ultimate goal of these scaffolds is to promote cell adhesion and proliferation; therefore, Col and gelatin are best options for hybrid scaffolds fabrication.12 However, these biopolymers do not have enough mechanical strength supporting cell seeding and post transplantation physical supports. To solve this problem, a number of biodegradable synthetic polymers with appropriate mechanical properties can be used for the fabrication of porous scaffolds such as poly(glycolic acid), poly(dl-lactic-co-glycolic acid), and poly(l-lactic acid) (PLLA).8,13 Additionally, porosity and interconnected architecture of 3D scaffolds is necessary for ideal functionality.14,15 The aim of this study was to design and fabricate Col hybrid scaffolds for skin TE. In this study, four types of 3D porous scaffolds were prepared, using combinations of polycaprolactone (PCL), PLLA, and Col and then characterized by hydrogen nuclear magnetic resonance (1H NMR), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). The cell adhesion, biocompatibility, and cell proliferation of fabricated scaffolds were studied by SEM and dimethylthiazol diphenyltetrazolium bromide (MTT) assay. Our results revealed that scaffolds containing Col in particular PCL–PLLA–Col interconnected with pore sizes close to 400 nm showed significantly greater potential (p < 0.01) for encouraging adipose tissue derived MSCs (AT-MSCs) adhesion and growth. The PCL–PLLA provided a mechanically strong mesh support and the type I Col microsponges encouraged cell adhesion and tissue formation.
2. Results and Discussion
2.1. Characterization of Synthesized Polymers by 1H NMR
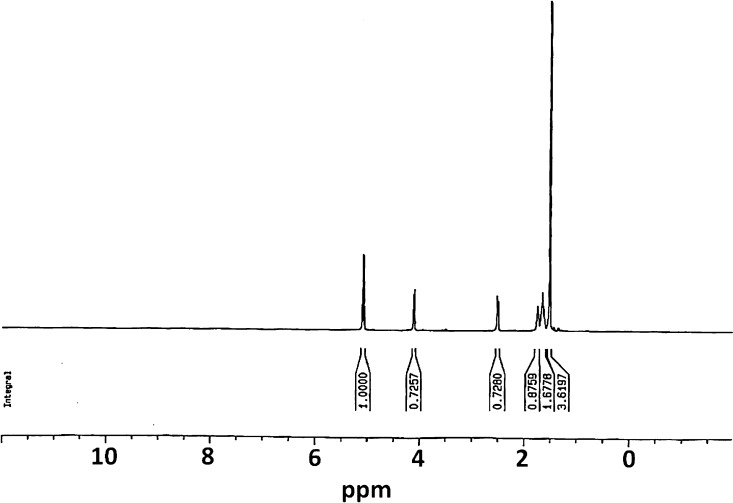
In this work, both PCL polymers and PCL–PLLA copolymers were synthesized using ring-opening polymerization (ROP) of caprolactone in the presence of PLLA. NMR spectroscopy was employed to study the chemical structure of synthesized polymers. Figure 1 shows the corresponding NMR spectra. According to 1H NMR spectrum of PCL–PLLA, those three peaks which are located at 4.10 ppm (O–CH2–(CH2)3–CH2–C=O), 2.26 ppm (O–CH2–(CH2)3–CH2–C=O), and 1.53 ppm (O–CH2–(CH2)3–CH2–C=O) are related to the protons of PCL, and the peaks at 5.1 ppm (O–(CH)CH3–C=O) and 1.6 ppm (O–(CH)CH3–C=O) are attributed to methylene hydrogens in lactide.
Figure 1.
1H NMR analysis of synthesized PCL–PLLA, showing their chemical structure.
2.2. FTIR Results
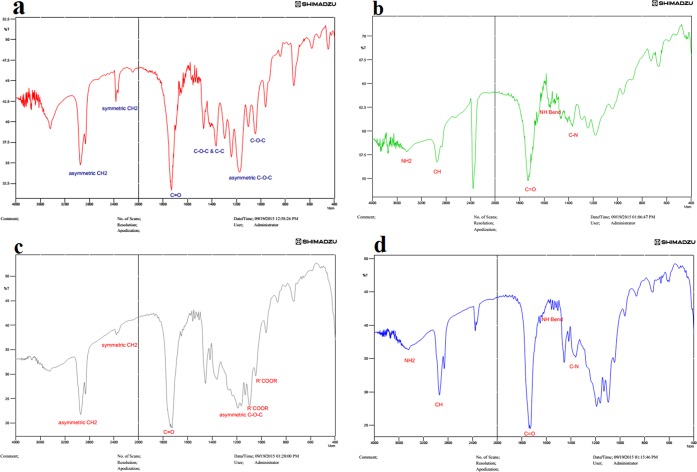
The FTIR spectrum for PCL, PCL–Col, PCL–PLLA, and PCL–PLLA–Col polymers are shown in Figure 2. The Col structure is made of three main amino acids, including proline, glycine, and hydroxyl proline. The peptide bond connects those amino acids, in FTIR spectrums (b,d), the picks in 3600, 3100, 1700–1600, 1600–1500, and 1400–1300 correspond to NH2, C–H, C=O, and NH, C–N, respectively.
Figure 2.
FTIR analysis of (a) PCL, (b) PCL–Col, (c) PCL–PLLA, and (d) PCL–PLLA–Col.
2.3. Fabrication and Properties of 3D Scaffolds
Desired scaffolds in TE are providing a porous structure, in which the porosity ≥70% as well as interconnected pores allowing cell growth is ideal.16 In this work, four scaffold types including two Col blended have been prepared, in the form of 3D.
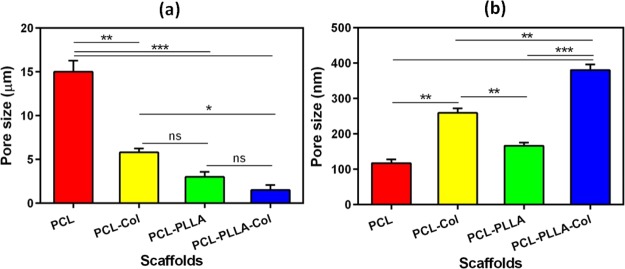
Our scaffolds had porous structures with a heterogeneous pore size. Figure 3a–d shows SEM micrographs of cross section and pore sizes of PCL, PCL–Col, PCL–PLLA, and PCL–PLLA–Col scaffolds, respectively. Their pores were formed applying the freeze-drying technique. According to previous reports, the morphology of the pores is dependent on the freezing temperature of the polymers prior to lyophilization.17 In this study, microporous structures were obtained when a mixture of Col solution and polymer/copolymer were frozen at −50 °C. A desired scaffold for skin TE should present excellent porosity (more than 90%) as well as desired microstructure 100–200 μm mean pore diameters. Previous studies showed that the freeze-drying method has the potential for preparing >90% porosity,18 which was employed in this work. The pore sizes of the obtained scaffolds are summarized in Figure 4a,b. The incorporation of Col into the PCL scaffold resulted in a decrease in the size of micrometric pores, also the insertion of PLLA in PCL–Col and PCL–PLLA scaffolds caused more reduction in micro pore sizes; finally, Col blending forced excessive decrease in micro pore sizes of the PCL–PLLA–Col scaffold (Figure 4).
Figure 3.
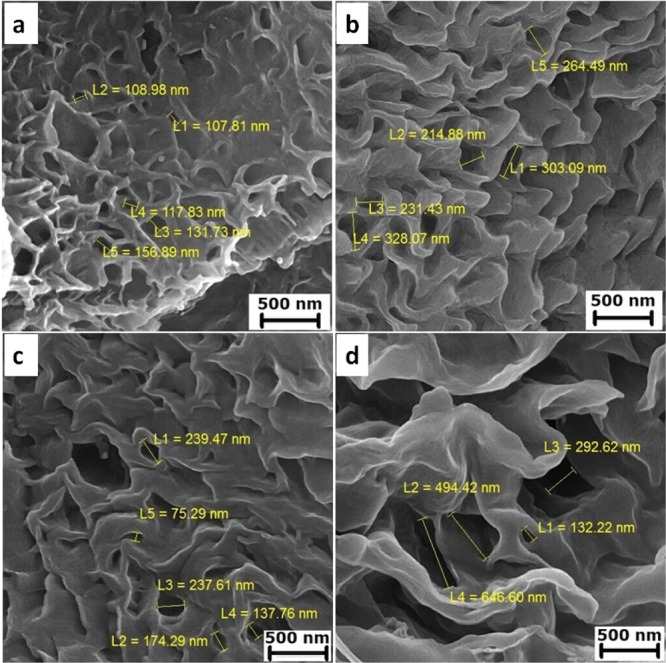
Porous scaffolds with a heterogeneous pore size, (a) PCL, (b) PCL–Col, (c) PCL–PLLA, and (d) PCL–PLLA–Col.
Figure 4.
Pore sizes of PCL, PCL–Col, PCL–PLLA, and PCL–PLLA–Col scaffolds in (a) micrometer and (b) nanometer scales. The incorporation of Col into PCL and PCL–PLLA scaffolds decreases the size of micrometric pores and increases the size of nanometric pores.
2.4. Characterization of AT-MSCs
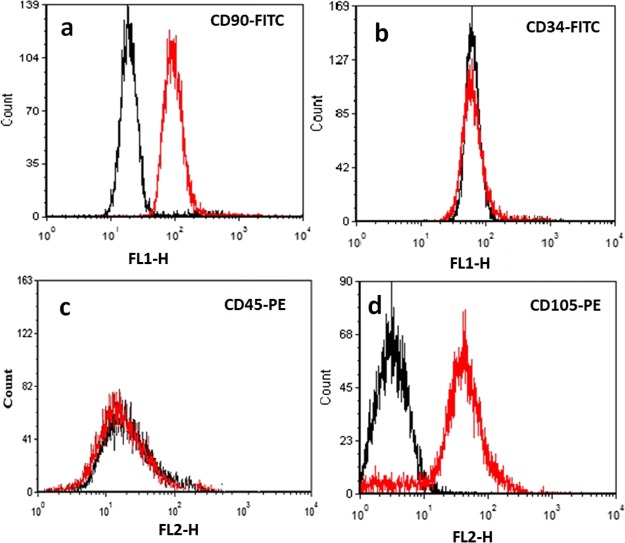
The third important factor in TE is the cell; as previously mentioned, researchers have used keratinocytes or fibroblasts for skin TE. In this study, we have utilized AT-MSCs which were isolated according to our previous works.3 We have utilized flow cytometry analysis of markers CD90, CD105, CD34, and CD45 for characterization of AT-MSCs. It was found that our cells express CD90 and CD105 on their surfaces (positive staining for CD90 and CD105) but did not express CD34 or CD45 (negative for CD34 and CD45) (Figure 5), which confirmed their identity.
Figure 5.
Immunophenotyping of AT-MSCs by fluorescence-activated cell sorting (FACS) which showed positive staining for (a) CD90 and (d) CD105 and negative for (b) CD34 and (c) CD45.
2.5. Cell Viability
The in vitro study of the cytocompatibility of 3D scaffolds was performed by culturing AT-MSCs on the scaffolds. The viability of AT-MSCs cultured on scaffolds were determined 3, 5, and 9 days post seeding by the colorimetric MTT assay; AT-MSCs cultured in tissue cell culture plates (TCPs) were set as the positive control. Figure 6 shows that the cells that survived in each scaffold and all scaffolds showed enough cytocompatibility. Interestingly, the scaffolds containing Col in particular PCL–PLLA–Col showed significant higher cytocompatibility represented by higher ODs in MTT assay (p < 0.01). In the previous studies, fibroblasts and keratinocytes were employed for preparing artificial skin constructs,12,19−21 but in this study, we utilized subcutaneous AT-MSCs which have morphology and properties similar to fibroblasts and also have the potential for keratinocyte differentiation.22 MSCs are recruited into skin wounds and take a part in wound healing by differentiation into different skin cells.23 The AT-MSCs were seeded into four scaffold types, and the MTT results showed that the best cell adhesion encourager may possibly be the PCL–PLLA–Col. Cells on the TCPs grew less, which could be due to the lack of sufficient space for further cell growth, and this illustrates the importance of porous 3D scaffolds in TE.
Figure 6.
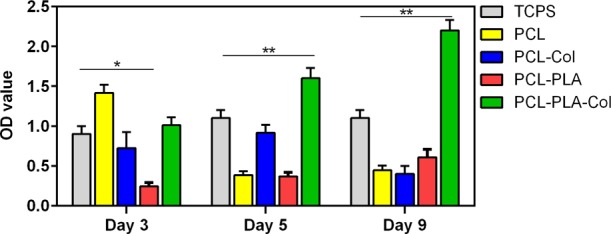
Cell viability and cytocompatibility of PCL, PCL–Col, PCL–PLLA, and PCL–PLLA–Col scaffolds studied by the MTT assay.
2.6. SEM Micrographic Visualization
The surface of 3D scaffolds shows irregular pores with homogeneous distribution, and a range of pore sizes was observed, in which this surface topography could support optimal interaction between cells and scaffold surfaces during the first week of culture (Figure 7a–h). Fibroblastic AT-MSCs adhered on scaffolds and had an elongated spindled morphology with several cytoplasmic extensions, and protrusions are obvious while extending away from the central to the top of nearer pores. These observations confirmed the cytocompatibility and the adhesive potential of our scaffolds for AT-MSCs and their potential for supporting suitable cell growth that could be useful in skin TE applications.
Figure 7.
Scanning electron micrographs of (a,e) PCL, (b,f) PCL–Col, (c,g) PCL–PLLA, and (d,h) PCL–PLLA–Col scaffolds after seeding with AT-MSCs during the first week of the culture.
3. Conclusion
Taken together, the results of this study highlighted the desired porous, cytocompatibility as well as adhesion and growth, encouraging potential of the PCL–PLLA–Col scaffold which could be applicable for preparing artificial skin constructs in skin regenerative therapeutics. The PCL–PLLA provided a mechanically strong mesh support, and the type I Col microsponges because of its water-uptake properties encouraged cell adhesion and tissue formation. Furthermore, in this study, it is shown that using the PCL–PLLA co-polymer with Col led to the production of scaffolds with regular porosity, which are suitable for uniform cellular proliferation on the scaffolds surface, so it is a good structure for future works. These results may help in preparing constructs applicable for skin TE.
4. Materials and Methods
4.1. PCL Polymer and PCL–PLLA Co-polymer Synthesis
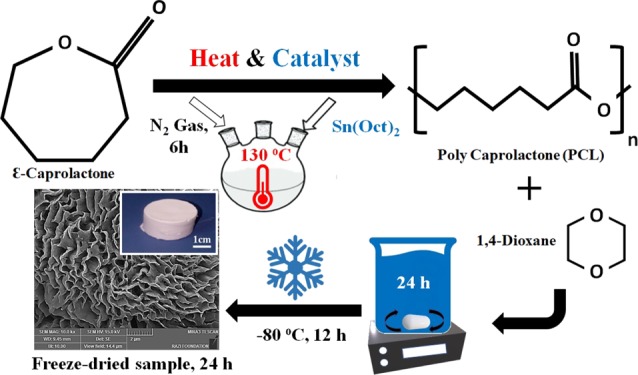
ε-Caprolactone (CL) was purchased from Sigma-Aldrich (Co., Steinheim, Germany). For PCL polymer synthesis, we used the ROP method and stannous octoate (Sn(Oct)2) (Sigma-Aldrich Chemical Co) was added as a catalyst. In the beginning, 10 g of CL was heated up to 130 °C, consequently, 0.1 g (1 wt %) of Sn(Oct)2 was added for starting polymerization and this continued under stirring conditions with N2 gas for 6 h (see Figure 8a). Next, the polymer dissolved in chloroform (Merck Chemical Co) or dichloromethane (Merck Chemical Co), and then the mixture precipitated in an excess of diethyl ether in a cold bath.
Figure 8.
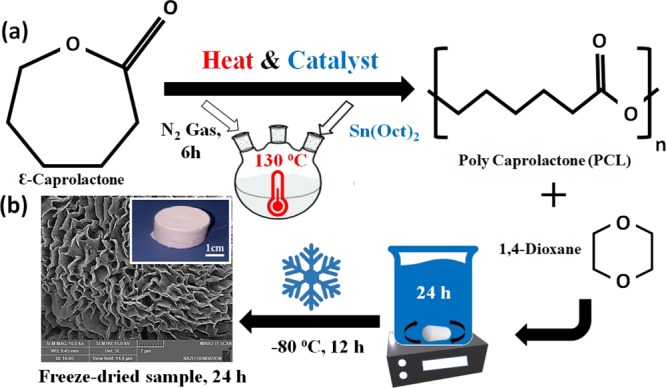
(a) ROP of CL to PCL using heat and catalyst. (b) Fabrication process of porous scaffolds.
Like PCL polymer, the ROP method was employed using lactic acid and CL monomers. dl-lactide monomers were purchased from Sigma-Aldrich (Co., Steinheim, Germany). For PCL–PLLA co-polymer synthesis, lactic acid and CL monomers with a ratio of 2.5 to 97.5 were added to a 50 mL round-bottom flask. After increasing the temperature to 140 °C under stirring in a nitrogen atmosphere, 0.1 g (1 wt %) of Sn(Oct)2 was added for catalyzing the reaction. After 6 h, the obtained co-polymer was dissolved in chloroform or dichloromethane, and then to remove excess catalyst and the unreacted monomers, the mixture precipitated in an excess of diethyl ether in a cold bath and left at room temperature for solvent evaporation.
4.2. Porous PCL and PCL–PLLA Scaffolds Synthesis
We utilized the emulsion freeze-drying technique for the fabrication of highly porous PCL, PLLA scaffolds by creating a homogenized emulsion of a PCL, PLLA polymer solution in 1,4-dioxane solvent and water mixture. The PCL polymer solution and the PCL–PLLA co-polymer solution (10–20 w/v %) was prepared by dissolving the polymeric blend in 1,4-dioxane. The solutions were magnetically stirred at room temperature for 24 h. Then, the emulsion was rapidly cooled to lock in the liquid state structure. For this purpose, the polymer solution is transferred to a suitable mold and placed at −80 °C for 12 h. In the next step, the frozen emulsion is placed in a freeze-dryer system (Telstar-LyoQuest) for 24 h under a vacuum of 12 Torr and a temperature of −50 °C. Next, the solvent and water were removed by this method, and porous PCL and PCL–PLLA scaffolds are obtained (see Figure 8b).
4.3. Porous PCL–Col and PCL–PLLA–Col Scaffolds Synthesis
PCL polymer and PCL–PLLA co-polymer were dissolved in 1,4-dioxane solvent (in a final concentration of 10%). After dissolving PCL polymer and PCL–PLLA co-polymer under magnetic stirring at room temperature for 24 h, Col type I with a concentration of 10 mg/mL was dissolved in aqueous solution of acetic acid and added to polymer solutions in 99.9:0.1 ratio, next 20 μL of glutaraldehyde was added as a cross-linker under stirring conditions, and then the emulsion was replaced in a proper frame and froze at −80 °C for 12 h and next were placed in a freeze-dryer system for 24 h for evaporation of solvents and pores formation. Finally, porous PCL–Col and PCL–PLLA–Col scaffolds are obtained.
4.4. Hydrogen Nuclear Magnetic Resonance
To study the chemical structure of synthesized polymers (PCL–PLLA), NMR spectroscopy was studied using the 1H NMR system (Bruker spectra spin 400 MHz).
4.5. Fourier Transform Infrared Spectroscopy
The structure of the fabricated scaffolds was determined by utilizing the FTIR spectroscopy. Each four types of the scaffolds including PCL, PCL–PLLA, PCL–Col, and PCL–PLLA–Col was mixed with potassium bromide and then pressed to disk. An FTIR spectrometer (Equinox 55 LS 101, Bruker, Germany) was employed in taking infrared spectra of aforementioned scaffolds in a certain range (400–4000 cm–1).
4.6. In Vitro Culturing and Characterization of AT-MSCs
The desired stem cells for this study were isolated from adipose tissue, according to our previous work.4 After isolation, the AT-MSCs were cultivated in Dulbecco’s modified Eagle’s medium (Gibco) medium containing 10% fetal bovine serum (FBS) (Gibco), dexamethasone (Sigma), PenStrep (Gibco), and other supplements; the media were replaced twice weekly. The AT-MSCs (third passage) were plated at a density of 1 × 106 in six-well plates. After reaching the confluence the cells were trypsinized, harvested and seeded into the scaffolds, and subsequently stained with fluorescein isothiocyanate or phycoerythrin conjugated antibodies against CD90, CD73, CD34, and CD45 antibodies at different time points of culture. The fluorescence of samples was studied using a FACS flow cytometer (BD, Bioscience).
4.7. Scaffolds Treatments before Cell Seeding
Each scaffold was placed in six-well plates and then washed two times with phosphate-buffered saline (PBS); next, ethanol 70% was added to each well and incubated overnight and then incubated with sterile PBS for 1 h at room temperature. Finally, the scaffolds were pretreated with cultivation medium for 24 h in 37 °C, 5% CO2 humid atmospheres.
4.8. Cell Viability Investigation by MTT Assay
The scaffolds’ biocompatibility, and the potential for supporting cell viability, was assessed using MTT assay. The MTT can be metabolized by the mitochondrial enzymes (dehydrogenases) of viable cells to formazan (purple), which can be solubilized in dimethyl sulfoxide, and then its observance can be measured by a spectrophotometer. About 2 × 104 AT-MSCs were seeded onto the 3D scaffolds in six-well cell culture plates and were grown at 37 °C in 5% CO2 humid incubator. At days 3, 5, and 9, 0.5 mL of MTT solution was added to 1.5 mL of cultivation medium, and then incubated for 4 h in the incubator. After about 15 min, shaking the dissolved formazan was transferred to a 96-well plate. The absorbances were measured by a plate reader at 570 nm. Also, as control, the cells were grown on TCPs without any scaffolds.
4.9. SEM Observations
Microscopic structures of the PCL, PCL–PLLA, and their Col hybrid scaffolds were studied by a SEM (KYKY 3200). First, the scaffolds were sectioned, and then the top, bottom, and cross section surfaces were coated with a thin layer of gold (5–10 nm) and visualized. To view the cell growth in the scaffolds, the scaffolds seeded with cells were harvested from the six-well plates, then washed with PBS, and fixed with 2.5% glutaraldehyde in PBS at room temperature for 2 h. Then, the scaffolds were washed with PBS and were let to dry at room temperature. Finally, the samples were coated with gold and visualized by SEM with a voltage of 25 kV.
4.10. Statistical Analysis
All experiments were performed in triplicate, and data are represented as mean ± SD. For statistical analysis, the statistical method analysis of variance was employed using SPSS software. In our outputs, p values less than 0.05 were interpreted significant.
Acknowledgments
The authors thank Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University for all supports provided. This work is funded by 2016 Drug Research Center, Tabriz University of Medical Sciences Grant.
Author Contributions
A.R.D.B. conceived the study and participated in its design and coordination. All authors helped in drafting the manuscript. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
References
- Hoseinzadeh S.; Atashi A.; Soleimani M.; Alizadeh E.; Zarghami N. MiR-221-inhibited adipose tissue-derived mesenchymal stem cells bioengineered in a nano-hydroxy apatite scaffold. In Vitro Cell. Dev. Biol. 2016, 52, 479–487. 10.1007/s11626-015-9992-x. [DOI] [PubMed] [Google Scholar]
- Alizadeh E.; Akbarzadeh A.; Eslaminejad M. B.; Barzegar A.; Hashemzadeh S.; Nejati-Koshki K.; et al. Up regulation of liver-enriched transcription factors HNF4a and HNF6 and liver-specific microRNA (miR-122) by inhibition of Let-7b in mesenchymal stem cells. Chem. Biol. Drug Des. 2015, 85, 268–279. 10.1111/cbdd.12398. [DOI] [PubMed] [Google Scholar]
- Alizadeh E.; Eslaminejad M. B.; Akbarzadeh A.; Sadeghi Z.; Abasi M.; Herizchi R.; et al. Upregulation of MiR-122 via trichostatin a treatments in hepatocyte-like cells derived from mesenchymal stem cells. Chem. Biol. Drug Des. 2016, 87, 296–305. 10.1111/cbdd.12664. [DOI] [PubMed] [Google Scholar]
- Alizadeh E.; Zarghami N.; Eslaminejad M. B.; Akbarzadeh A.; Barzegar A.; et al. The effect of dimethyl sulfoxide on hepatogenic differentiation of mesenchymal stem cells. Artif Cells Nanomed Biotechnol. 2016, 44, 157. 10.3109/21691401.2014.928778. [DOI] [PubMed] [Google Scholar]
- Asghari F.; Salehi R.; Agazadeh M.; Alizadeh E.; Adibkia K.; Samiei M.; et al. The Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydroxyapatite-coated Biodegradable Nanofibrous Scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 720. 10.1080/00914037.2016.1163564. [DOI] [Google Scholar]
- Takahashi K.; Tanabe K.; Ohnuki M.; Narita M.; Ichisaka T.; Tomoda K.; et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007, 131, 861–872. 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Loh Q. L.; Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng., Part B 2013, 19, 485–502. 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Del Bakhshayesh A.; Annabi N.; Khalilov R.; Akbarzadeh A.; Samiei M.; Alizadeh E.; et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. 10.1080/21691401.2017.1349778. [DOI] [PubMed] [Google Scholar]
- Yannas I. V.; Burke J. F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res., Part A 1980, 14, 65–81. 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- Lu H.; Oh H. H.; Kawazoe N.; Yamagishi K.; Chen G. PLLA-collagen and PLLA-gelatin hybrid scaffolds with funnel-like porous structure for skin tissue engineering. Sci. Technol. Adv. Mater. 2012, 13, 064210. 10.1088/1468-6996/13/6/064210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafaya P. B.; Silva G. A.; Reis R. L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Trasciatti S.; Podestà A.; Bonaretti S.; Mazzoncini V.; Rosini S. In vitro effects of different formulations of bovine collagen on cultured human skin. Biomaterials 1998, 19, 897–903. 10.1016/S0142-9612(97)00175-0. [DOI] [PubMed] [Google Scholar]
- Asghari F.; Samiei M.; Adibkia K.; Akbarzadeh A.; Davaran S. Biodegradable and biocompatible polymers for tissue engineering application: a review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. 10.3109/21691401.2016.1146731. [DOI] [PubMed] [Google Scholar]
- Mostafavi E.; Ataie A. Destructive Interactions between Pore Forming Agents and Matrix Phase during the Fabrication Process of Porous BiFeO3 Ceramics. J. Mater. Sci. Technol. 2015, 31, 798–805. 10.1016/j.jmst.2015.05.002. [DOI] [Google Scholar]
- Mostafavi E.; Ataie A. Fabrication and Characterization of Nanostructured Ba-doped BiFeO3 Porous Ceramics. Mat. Sci. 2016, 34, 148–156. 10.1515/msp-2016-0017. [DOI] [Google Scholar]
- Chen G.; Kawazoe N. Collagen-based porous scaffolds for tissue engineering. Biomater. Nat. Adv. Devices Ther. 2016, 1–15. 10.1002/9781119126218.ch1. [DOI] [Google Scholar]; Chapter 1.
- Tsourouflis S.; Flink J. M.; Karel M. Loss of structure in freeze-dried carbohydrates solutions: effect of temperature, moisture content and composition. J. Sci. Food Agric. 1976, 27, 509–519. 10.1002/jsfa.2740270604. [DOI] [Google Scholar]
- O’Brien F. J.; et al. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. 10.1016/S0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- Ojeh N.; et al. Stem cells in skin regeneration, wound healing, and their clinical applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. 10.3390/ijms161025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.; Kawazoe N.; Tateishi T.. Collagen-Based Scaffolds; ScienceDirect, 2008; pp 396–415. [Google Scholar]
- Groeber F.; Holeiter M.; Hampel M.; Hinderer S.; Schenke-Layland K. Skin tissue engineering - In vivo and in vitro applications. Adv. Drug Delivery Rev. 2011, 63, 352–366. 10.1016/j.addr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Paunescu V.; Deak E.; Herman D.; Siska I. R.; Tanasie G.; Bunu C.; et al. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J. Cell. Mol. Med. 2007, 11, 502–508. 10.1111/j.1582-4934.2007.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M.; Abe R.; Fujita Y.; Ando S.; Inokuma D.; Shimizu H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]