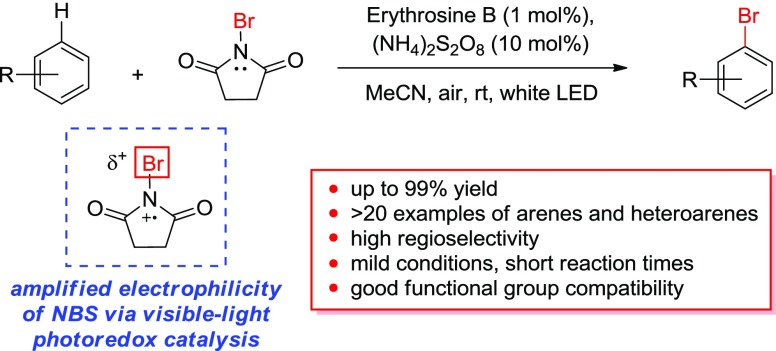
Abstract
A variety of arenes and heteroarenes are brominated in good to excellent yields using N-bromosuccinimide (NBS) under mild and practical conditions. According to mechanistic investigations described within, the reaction is speculated to proceed via activation of NBS through a visible-light photoredox pathway utilizing erythrosine B as a photocatalyst. A photo-oxidative approach effectively amplifies the positive polarization on the bromine atom of the NBS reagent. This increase in the electrophilic nature of NBS results in drastically reduced reaction times and diversion from competing light-promoted reactive pathways.
Introduction
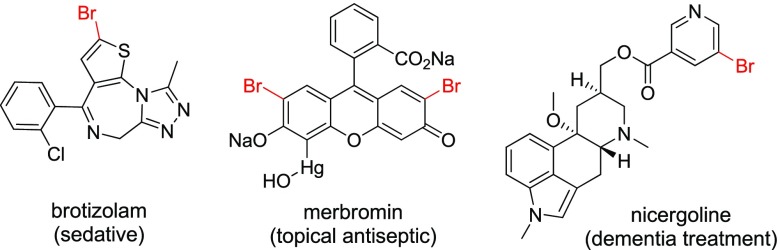
Aryl bromides are found as valuable end-targets in a variety of synthetic applications including pharmaceuticals, natural products, agrochemicals, and advanced materials (Figure 1).1−3 In addition, brominated arenes function as important precursors in a variety of synthetic transformations including SNAr-type halogen displacements4 and cross-coupling reactions (Suzuki–Miyaura,5 Heck,6 Stille,7 Sonogashira,8 and Buchwald–Hartwig9,10).
Figure 1.
Representative examples of pharmaceutical compounds containing aryl bromide functionality.
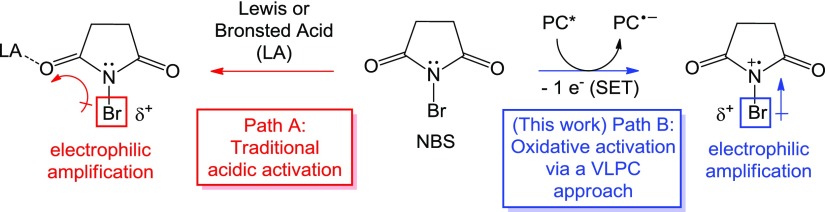
Because of the ubiquity of these synthetic building blocks, the predictable and selective installation of bromine into aromatic scaffolds under mild and practical reaction conditions has received constant attention from the synthetic community. Traditionally, bromination of arenes is accomplished through pathways that include Friedel–Crafts-type electrophilic aromatic halogenation,11,12 organometallic-mediated or transition metal-catalyzed approaches,1,13 or Sandmeyer-type reactions of diazonium salts.14 The most convenient and popular approach, electrophilic aromatic halogenation, typically involves either the use of elemental bromine under harsh conditions, an electrophilic “Br+” species (including N-halo reagents), or a source of anionic bromide in conjunction with a potentially hazardous and/or corrosive oxidizing agent.11,12,15−19N-Bromosuccinimide (NBS) is a widely used example of an N-halo brominating agent because of its practicality, low cost, and relative stability. Within the family of N-halosuccinimides, NBS is a more reactive electrophilic halogen source than either N-chlorosuccinimide (NCS) or N-iodosuccinimide and can be used to brominate highly electron-rich aromatics under relatively mild conditions in polar solvents.20−22 Although NBS is more reactive in electrophilic aromatic substitution reactions than analogous succinimide reagents, it is also more capricious with regard to reaction conditions. Long reaction times may be required because of precautions (dark environments, low temperatures) that are necessary to discourage other competing light-activated, radical pathways (including radical oxybromination at benzylic positions and C–H bonds α to a ketone carbonyl).18,19,23 In addition, for aromatic substrates that are less electron-rich, activation of the N-halosuccinimide reagent by an external acid may be required. In such an example, a strong Bronsted or Lewis acid is used to coordinate the O atom of the succinimide, which draws the electron density away from the halogen, thus amplifying the electrophilicity of the halogen by increasing the N–X bond polarization (Scheme 1, Path A).24−27
Scheme 1. General Scheme of Traditional Acidic Activation of NBS and Oxidative Activation by Photoredox Catalysis (PC = Photocatalyst; SET = Single Electron Transfer).
As an alternative approach to electrophilic amplification via acidic activation, we have found inspiration in recent reports in which halogenating reagents are activated under visible-light photoredox catalysis (VLPC) conditions.28−32 König’s Ru(II)-catalyzed photoredox approach to NCS activation relies upon the oxidation of an N-chloro reagent to produce an activated cationic N-radical, thus inducing strong positive polarization (δ+) that amplifies the electrophilicity of the halogen atom.29 The use of a similar VLPC approach toward bromination would offer a mild catalytic alternative for N-bromo reagent activation without the requirement of an external acid (Scheme 1, Path B). Therefore, in following our research group’s interest in developing synthetic methodologies that utilize N-centered radical species,33−42 we turned our attention toward the production of a cationic N-radical species from NBS using a visible-light photoredox catalyst.
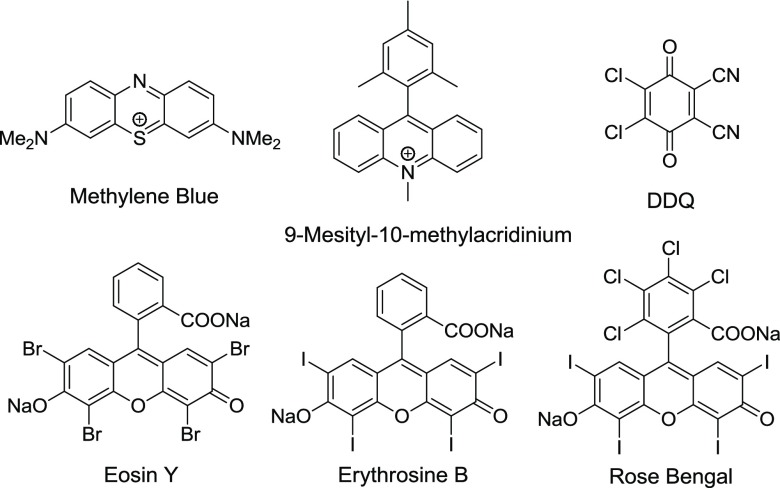
VLPC has attracted intense attention from the synthetic community as a mild means of initiating transformations involving electron-transfer pathways.43−46 In the recent expansion in the field of VLPC, the majority of synthetic applications have been dominated by the use of transition metal complexes such as Ru(II) and Ir(III) bipyridyl complexes. Transition metal complexes offer the ability to electronically tune a complex by control of the coordinating ligand and are generally well-understood with regard to electrochemical, kinetic, and spectroscopic information.43,45 However, these widely employed transition metal complexes may require synthesis or are costly if commercially available. As an attractive complement to metal-catalyzed VLPC systems, a number of organic dyes have been shown to exhibit efficient, and in some cases, superior reactivity to the transition metal catalysts at a fraction of the cost.44,47−54 To date, the majority of organic dyes employed as visible-light photocatalysts for synthetic applications belong to the azine (methylene blue), acridinium (9-mesityl-10-methylacridinium salts), quinone (DDQ), or xanthene (eosin Y, erythrosine B, and rose bengal) structural classes (Figure 2).44
Figure 2.
Selected examples of organic dye visible-light photoredox catalysts.
Herein, we report a new pathway for mild, electrophilic bromination of arenes and heteroarenes that utilizes the organic dye, erythrosine B, as a visible-light photoredox catalyst to amplify the electrophilicity of NBS. The method complements existing strategies and offers practical advantages in examples in which either nonacidic activation of NBS or the prevention of byproducts from unwanted light-promoted radical pathways is desired.
Results and Discussion
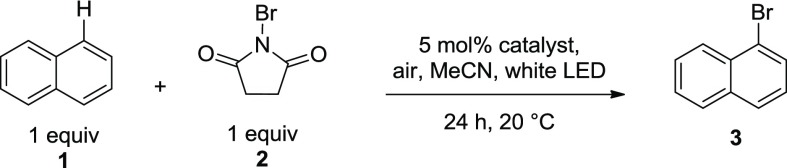
To begin our investigation, a representative aromatic substrate (naphthalene 1) was selected for bromination using NBS 2, and a number of organic dyes were screened as catalysts. Initial settings were chosen that offered operationally convenient and practical conditions (ambient temperature, open to air, 24 h). To our delight, several catalysts promoted the formation of 1-bromonaphthalene 3 more effectively than the uncatalyzed background reaction (Table 1).
Table 1. Screening of VLPC Catalysts in the Bromination of Naphthalene with NBS.
| entrya | catalyst | % 3b | entrya | catalyst | % 3b |
|---|---|---|---|---|---|
| 1 | none | 48 | 11 | methylene blue | 18 |
| 2 | acridine orange | 15 | 12 | methylene green | 77 |
| 3 | azure A | 31 | 13 | Nile blue A | 67 |
| 4 | brilliant cresyl blue | 27 | 14 | pyronin Y | 64 |
| 5 | brilliant green | 55 | 15 | rhodamine 6G | 61 |
| 6 | DDQ | 19 | 16 | rhodamine B | 60 |
| 7 | eosin Y | 0 | 17 | rose bengal | 87 |
| 8 | erythrosine B | 94 | 18 | safranin O | 54 |
| 9 | fluorescein | 58 | 19 | thionin | 71 |
| 10 | 9-mesityl-10-methylacridinium | 75 | 20 | [Ru(bpy)3]Cl2 | 0 |
Conditions: 0.25 mmol naphthalene, 0.25 mmol NBS, 0.0125 mmol catalyst, 3 mL MeCN, air, 20 °C, white light-emitting diode (LED), 24 h.
Gas chromatography (GC) yields calculated using adamantane as the internal standard.
From the results of the screening, erythrosine B was selected as the most efficient catalyst for production of 1-bromonaphthalene 3. Erythrosine B is a member of the xanthene family of dyes and is widely used as a food colorant (FD&C Red no. 3) and painting ink, but thus far, the chemical applications of erythrosine B have remained remarkably limited55−62 relative to other xanthene dyes such as eosin Y, fluorescein, and rose bengal.
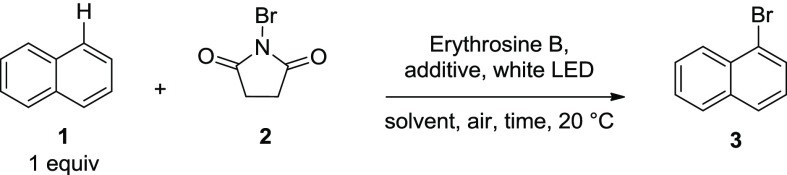
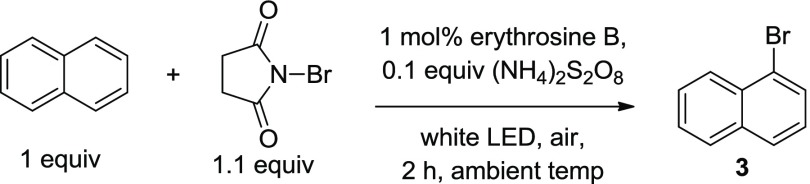
Employing erythrosine B as a VLPC catalyst, a series of experiments were conducted to develop an optimized set of reaction conditions (Table 2). First, the stoichiometric ratio of naphthalene and NBS substrate was examined (Table 2, entries 1–3). An excess of brominating agent had little to no effect on the product yield, so a 1:1 ratio of NBS/naphthalene was chosen for further experiments. The catalyst loading of erythrosine B was then investigated (entries 4–10). A low catalyst concentration is necessary to achieve full conversion as the catalyst is deactivated under a high concentration, which may be due to radical collisional quenching.63 Next, a survey of solvents and reaction concentrations was conducted (entries 11–16). It was observed that [1.7 mM] of erythrosine B in acetonitrile was optimal for the reaction conditions (entry 16). The use of an external oxidizing agent such as oxone (entry 17) had little effect on the reaction. However, when ammonium peroxodisulfate was added to the reaction mixture, a considerable increase in the rate of the reaction was observed (entries 18–20). Full conversion of naphthalene was observed using 1.1 equivalents of NBS; therefore, entry 19 represents the optimized conditions for the reaction.
Table 2. Optimization of Reaction Conditions for Erythrosine B-Catalyzed Production of 1-Bromonaphthalene.
| entrya | equiv 2 | eryth B (mol %) | time (h) | solvent (mL) | additive (equiv) | % 3b |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 6 | MeCN (3) | none | 67 |
| 2 | 1.1 | 1 | 6 | MeCN (3) | none | 68 |
| 3 | 2 | 1 | 6 | MeCN (3) | none | 69 |
| 4 | 1 | 0 | 6 | MeCN (3) | none | 26 |
| 5 | 1 | 0.5 | 6 | MeCN (3) | none | 44 |
| 6 | 1 | 2 | 6 | MeCN (3) | none | 73 |
| 7 | 1 | 3 | 6 | MeCN (3) | none | 62 |
| 8 | 1 | 5 | 6 | MeCN (3) | none | 41 |
| 9 | 1 | 10 | 6 | MeCN (3) | none | 0 |
| 10 | 1 | 20 | 6 | MeCN (3) | none | 0 |
| 11 | 1 | 2 | 6 | MeOH (3) | none | 0 |
| 12 | 1 | 2 | 6 | DCM (3) | none | 12 |
| 13 | 1 | 2 | 6 | 4:1 MeCN/H2O (3) | none | 42 |
| 14 | 1 | 2 | 6 | MeCN (6) | none | 18 |
| 15 | 1 | 2 | 6 | MeCN (1.5) | none | 76 |
| 16 | 1 | 1 | 6 | MeCN (1.5) | none | 80 |
| 17 | 1 | 2 | 6 | MeCN (3) | oxone (1) | 78 |
| 18 | 1 | 2 | 2 | MeCN (3) | (NH4)2S2O8 (1) | 88 |
| 19 | 1.1 | 1 | 2 | MeCN (1.5) | (NH4)2S2O8(0.1) | 90 |
| 20 | 1.1 | 1 | 2 | MeCN (1.5) | (NH4)2S2O8 (0.02) | 78 |
Reaction conditions: 1 equiv = 0.25 mmol naphthalene.
GC yields calculated using adamantane as the internal standard.
A series of control reactions were conducted, and the results are displayed in Table 3. Light plays a key role in the reaction, as exhibited by the substantial decrease in product formation when the reaction is performed in the dark (Table 3, entry 3; Table 4, entry 5). Though ammonium peroxodisulfate is an important component in the promotion of the reaction, it is not solely responsible for activation of NBS, as observed in entries 3–5. The exclusion of catalytic erythrosine B results in a significant decrease in the formation of 3 (entries 4 and 5). In addition, oxygen (air) appears to serve as an oxidizing agent throughout the duration of the reaction. Ammonium peroxodisulfate can be used as a stoichiometric oxidant in the absence of O2 (entry 6), but when no oxidant (such as (NH4)2S2O8 or O2) was present (entry 7), the reaction was severely impacted, resulting in a 7% yield. Finally, known radical inhibitors BHT (butylated hydroxytoluene) and TEMPO were separately tested under standard reaction conditions to determine if the presence of a radical intermediate might exist in the potential mechanistic pathway, and in both examples, product formation was completely halted (entries 8 and 9).
Table 3. Control Reactions and Mechanistic Experiments.
| entry | deviation from standard conditions | % yield 3a |
|---|---|---|
| 1 | none | 90 |
| 2 | 0.4 equiv (NH4)2S2O8 | 90 |
| 3 | 0.4 equiv (NH4)2S2O8, reaction in darkb | 32 |
| 4 | 0.4 equiv (NH4)2S2O8, no erythrosine B | 56 |
| 5 | no erythrosine B, no (NH4)2S2O8 | 14 |
| 6 | anaerobic conditionsc | 68 |
| 7 | anaerobic conditionsc, no (NH4)2S2O8 | 7 |
| 8 | no (NH4)2S2O8, 1 equiv BHT added | 0 |
| 9 | no (NH4)2S2O8, 1 equiv TEMPO added | 0 |
GC yields calculated using adamantane as the internal standard.
Reaction vessel was covered in an aluminum foil, and the reaction was carried out in a laboratory with the lights turned off.
Solvent was degassed, and the reaction was conducted under argon.
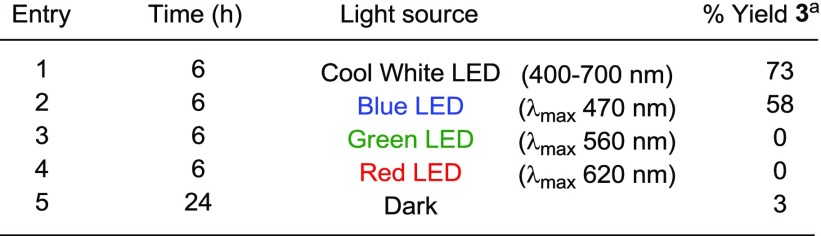
Table 4. Variation of Light Source in the VLPC Bromination of Naphthalene.
GC yields calculated using adamantane as the internal standard.
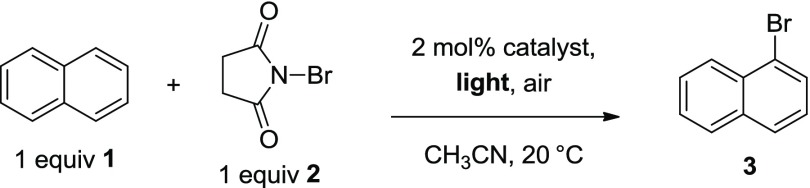
The source of light in the activation of NBS by erythrosine B was then investigated (Table 4). Ammonium peroxodisulfate was excluded from the reaction to eliminate the background production of brominated arene, as observed in the control reactions shown in Table 3. The absorption maximum (λab) of the ground state of erythrosine B is approximately 530 nm in acetonitrile. As shown in Table 3, the use of blue LED resulted in a moderately reduced yield, whereas utilization of a green or red LED light chamber had a severely detrimental effect on the reaction. The use of a white LED photochamber (see Supporting Information for description) displayed the most efficient production of 1-bromonaphthalene 3 and was used for all subsequent studies.
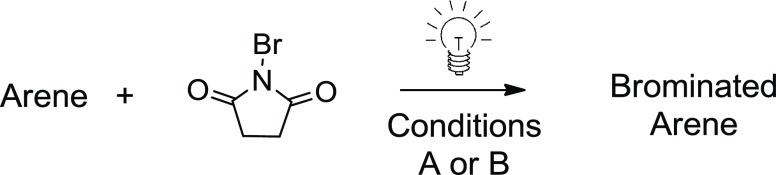
Following the optimization of reaction conditions (1 equiv arene, 1.1 equiv NBS, 0.1 equiv (NH4)2S2O8, 0.01 equiv erythrosine B [0.0017 M], acetonitrile, white LED, air), an exploration of the substrate scope was conducted with respect to the arene reagent (Table 5). In addition to naphthalene, several other arene substrates see dramatic enhancement in both the rate of reaction and the yield of brominated product upon use of catalytic erythrosine B. When 2-methylnaphthalene was employed in the absence of a catalyst, no conversion of arene was observed in 10 min by 1H nuclear magnetic resonance (NMR) spectroscopy. After 2 h, a complex mixture was obtained containing products of benzylic bromination (see the Supporting Information for 1H NMR spectra). In the presence of erythrosine B, however, 2-methylnaphthalene was cleanly converted to 4 within 10 min with no trace of benzylic functionalization. This example illustrates the potential utility of the photocatalyzed system. By oxidizing NBS and increasing the electrophilicity of the bromine atom, a photoredox approach appears to divert a potential side reaction (light-promoted radical bromination of benzylic C–H bonds) to a more reactive electrophilic aromatic bromination (Br+) pathway.
Table 5. Substrate Scope of Arene Bromination.
Conditions A: 1 equiv. arene, 1.1 equiv. NBS, 0.1 equiv. (NH4)2S2O8, 0.01 equiv. erythrosine B [0.0017M], MeCN, air, 20 °C, white LED. All yields are isolated unless otherwise noted.
Conditions B: 2.2 equiv. NBS used. Yields are isolated.
GC yield calculated using adamantane as the internal standard.
Determined by 1H NMR integration using nitrobenzene as the internal standard.
Inseparable mixture of isomers.
Bromination of anisole occurred in a quantitative fashion according to 1H NMR, with the para-substituted isomer being the only observable product (5). The substituted anisole derivative, 4′-methoxyacetophenone, was also efficiently brominated on the aromatic ring with no observable bromination occurring alpha to ketone (6). A variety of aryl ether-containing substrates as well as phenol derivatives were examined (7–14). In examples where a mixture of mono- and dibromination occurred, 2.2 equivalents of NBS was employed to produce a dibrominated major product. Aniline derivatives (15 and 16) along with acetanilide (17) and the anesthetic, lidocaine (18), performed well under the photocatalyzed conditions. Electron-deficient arenes such as chlorobenzene and nitrobenzene were unsuccessful under the optimized reaction conditions. Additionally, aldehyde functionality is not tolerated under the reaction conditions, as 4-methoxybenzaldehyde was oxidized to the carboxylic acid when tested. Arenes that contain electron-withdrawing groups (carbonyl, nitro, and halide) are tolerated as long as they are accompanied by an electron-donating group on the aromatic ring. It is noteworthy that, despite the reactions being conducted in a visible-light photochamber, brominated byproducts were not observed in any example using an arene that contained benzylic C–H bonds (4, 11, 12, and 18) or C–H bonds α to carbonyls (6, 17, 18).
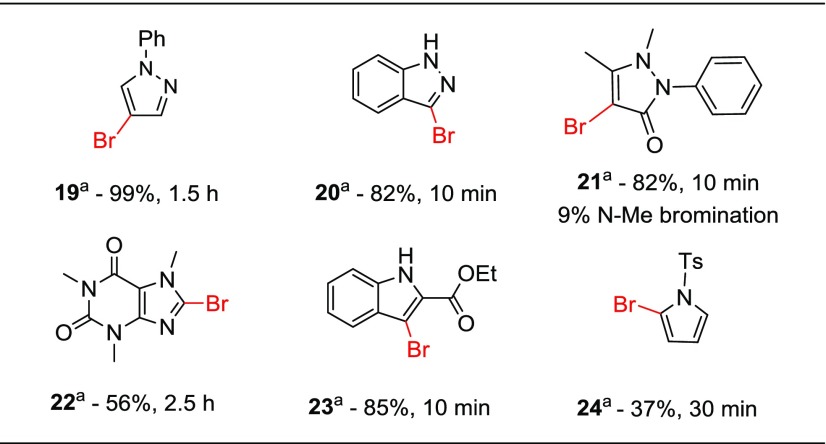
In addition to the variety of brominated arenes shown in Table 5, a series of N-containing heteroarenes were cleanly brominated and are displayed in Table 6. In general, good to excellent yields of brominated products were obtained with high regioselectivity. Heteroarenes that were employed include pyrrole, pyrazole, indole, and indazole cores along with bioactive compounds such as caffeine and the painkiller phenazone. Bromination at the N-methyl position of phenazone was an observed minor product with 21. Products of N-methyl bromination were not observed, however, when using caffeine as a substrate.
Table 6. Substrate Scope of Heteroarene Bromination.
Conditions: 1 equiv heteroarene, 1.1 equiv NBS, 0.1 equiv (NH4)2S2O8, 0.01 equiv erythrosine B [0.0017M], MeCN, air, 20 °C, white LED. All yields are isolated.
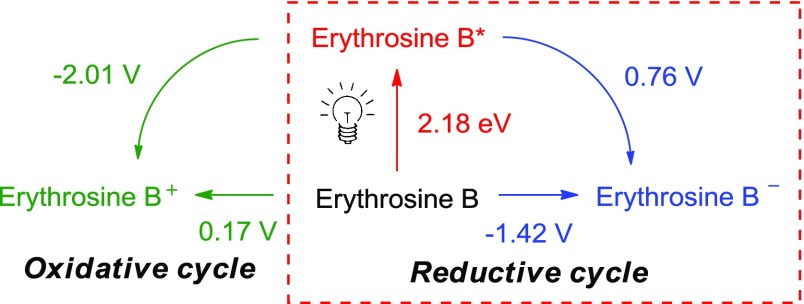
To investigate whether the oxidation potential of NBS was within the range of the photocatalyst erythrosine B, the cyclic voltammograms of NBS and erythrosine B were obtained versus Fc/Fc+ (see the Supporting Information). The fluorescence emission profile of erythrosine B exhibits a peak with a maximum at 568 nm (λem), which correlates with a Eoo value of 2.18 eV. The Stokes shift (Δλ) of erythrosine B is therefore calculated as 38 nm (λem 568 nm – λab 530 nm). This relatively large Stokes shift value for a xanthene dye (most are 20–35 nm) might be attributed to an excited-state intramolecular charge transfer.64−66 On the basis of the redox potentials obtained, the oxidation of NBS (+0.28 vs Fc/Fc+) is feasible by the excited state of erythrosine B; therefore, the catalyst is speculated to proceed through a reductive quenching cycle, as shown in Scheme 2.
Scheme 2. Redox Properties of Erythrosine B.
Potentials are given vs Fc/Fc+.
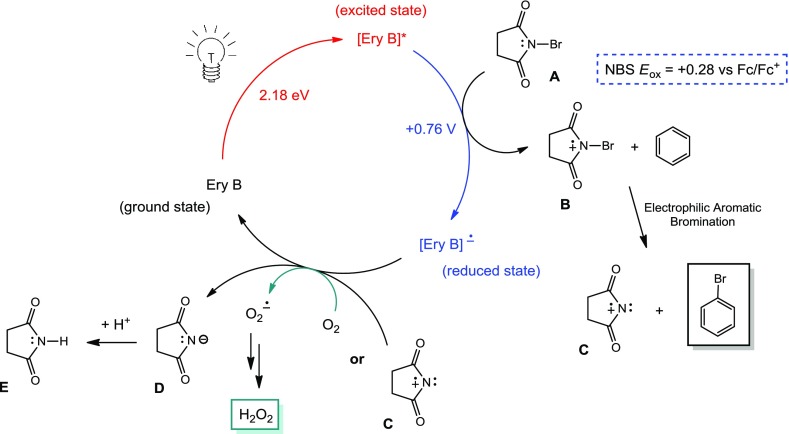
On the basis of the experimental results, a plausible reaction mechanism is proposed in Scheme 3. Ammonium peroxodisulfate has been excluded from the proposed mechanism because it is not a necessary component of the reaction according to control experiments. The photoexcited state of erythrosine B can induce oxidation of the nitrogen atom of NBS to cationic radical species B, thus amplifying the positive polarization on the bromine atom. Following electrophilic aromatic bromination with an arene substrate, an external oxidant [such as O2, (NH4)2S2O8, or H2O2] may serve as the oxidizing agent to return erythrosine B to its ground state. Alternatively, the resulting charged succinimide species C could oxidize the reduced state of the catalytic erythrosine B to form the succinimide anion D.
Scheme 3. Plausible Mechanism.
To determine if the superoxide anion O2–• is formed under the photocatalyzed conditions, the iodide test (NaI in glacial acetic acid) was performed to attempt to observe the presence of H2O2 byproduct, which would ultimately result from the intermediate superoxide anion. Ammonium peroxodisulfate was intentionally excluded from the reaction mixture to prevent the false positive test that would result from the oxidation of iodide by (NH4)2S2O8. As shown in Figure 3, upon addition of the crude reaction mixture at 0 min, the NaI/AcOH mixture appeared as the orange/red color of the erythrosine B crude reaction. Following a 2 h reaction period, the formation of hydrogen peroxide in the reaction mixture was confirmed by the appearance of the dark red/brown color of I2 (Figure 3, panel F).
Figure 3.
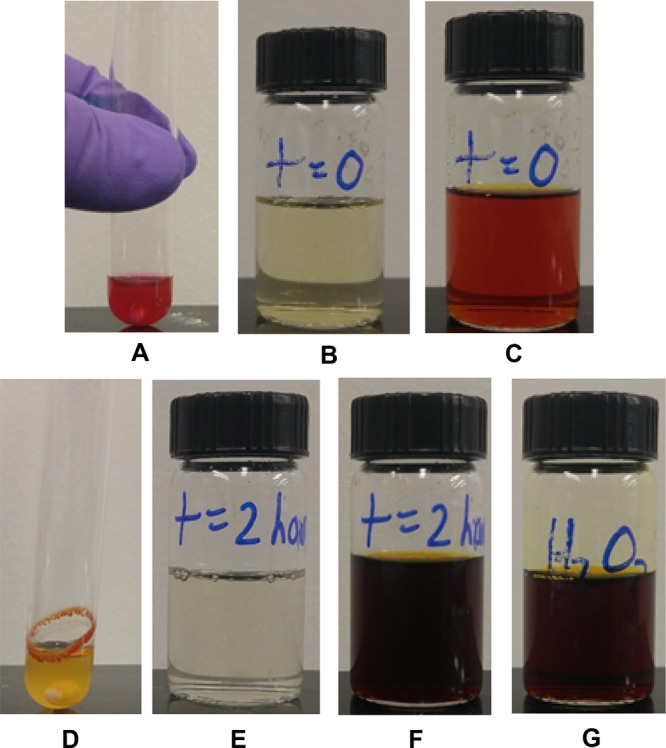
Test for the production of hydrogen peroxide. Bromination reaction mixture = 1 equiv naphthalene, 1.1 equiv NBS, 0.01 equiv erythrosine B, air, and acetonitrile. (A) Reaction at t = 0. (B) NaI in glacial acetic acid. (C) Crude reaction mixture A added to mixture B at t = 0. (D) Crude reaction mixture at t = 2 h. (E) NaI in glacial acetic acid. (F) Crude reaction mixture D added to mixture E at t = 2 h. (G) Positive control experiment in which H2O2 is added to NaI in glacial acetic acid solution.
Conclusions
A new catalytic mode of activation of NBS for bromination of arenes and heteroarenes has been developed using erythrosine B as a visible-light photoredox catalyst. The reaction operates under mild, practical conditions and produces brominated arenes in high regioselectivity and in short reaction times. The approach diverts NBS from competing light-promoted pathways, such as benzylic bromination, toward electrophilic aromatic substitution. A variety of substrates were brominated in good to excellent yields, and many functionalities were tolerated. According to mechanistic studies, the reaction is speculated to operate via a single electron oxidation of NBS by erythrosine B, which is in turn oxidized back to its ground state by air. Finally, attempts to employ a VLPC strategy to activate additional reagents containing N–X bonds for electrophilic transformations are currently underway in our laboratory and will be reported in due course.
Experimental Section
Materials and Instrumentation
All reagents and solvents were purchased from commercial sources and used without further purification. Erythrosine B was purchased from Spectrum as FD&C Red #3. A description of the construction of our light bath (LED) photochamber is provided in the Supporting Information. 1H and 13C NMR spectra were recorded on a Varian 400/100 (400 MHz) spectrometer in deuterated chloroform (CDCl3) or acetonitrile (CD3CN) with the solvent residual peak as the internal reference unless otherwise stated (CDCl3: 1H = 7.26 ppm, 13C = 77.23 ppm; CD3CN: 1H = 1.94 ppm). Data are reported in the following order: Chemical shifts (δ) are reported in ppm, and spin–spin coupling constants (J) are reported in Hz, whereas multiplicities are abbreviated by s (singlet), bs (broad-singlet), d (doublet), dd (doublet-of-doublets), t (triplet), dt (doublet-of-triplets), td (triplet-of-doublets), m (multiplet), and q (quartet). Infrared spectra were recorded on a Nicolet iS50 FT-IR spectrometer, and peaks are reported in reciprocal centimeters (cm–1). Electrochemical experiments were performed using a BioLogic SP-200 Potentiostat using a platinum disk working electrode, a platinum mesh counter electrode, and a silver wire as the quasi-reference electrode that was calibrated using a ferrocene standard. Melting points (mp) were recorded on a Mel-Temp II (Laboratory Devices, USA) apparatus and were uncorrected. Nominal mass spectra (electron ionization) were obtained using a Shimadzu GC-2010 Plus chromatograph with a GCMS-QP2010 mass spectrometer. Relative intensity (in percentage) is shown in parentheses following the fragment peak, where appropriate. Accurate mass spectrum was performed using a Thermo Scientific Exactive spectrometer operating in the positive ion electrospray mode.
General Procedure
To an oven-dried flask was added a magnetic stir bar, erythrosine B (4.4 mg, 0.01 equiv, 0.005 mmol), ammonium peroxodisulfate (11.4 mg, 0.1 equiv, 0.05 mmol), arene/heteroarene (1 equiv, 0.5 mmol), acetonitrile (3 mL), and then NBS (98 mg, 1.1 equiv, 0.55 mmol). The reaction mixture was stirred open to air at room temperature (20 °C) in a white LED chamber for the time specified for each respective arene. Reactions were monitored for completion by thin-layer chromatography (hexanes/ethyl acetate) and/or GC. For substrates that produced a mixture of mono- and dibrominated products upon full conversion, 2.2 equivalents (1.1 mmol) of NBS was employed. Upon completion of the reaction, the crude mixture was evaporated under pressure and the brominated product was isolated via column chromatography on silica gel.
Iodide Test
Test solution preparation: 0.100 g NaI dissolved in 10 mL of glacial acetic acid.
Control Test
Three drops of 3% H2O2 solution were added by a pipette to the NaI/AcOH test solution in a 4-dram vial. The vial was capped and shaken, resulting in an opaque red-brown color.
0 min Test
A test tube was loaded with erythrosine B (0.0022 g, 0.0025 mmol), naphthalene (0.0310 g, 0.25 mmol), 1.5 mL of CH3CN, and NBS (0.0487 g, 0.275 mmol). After addition of all reagents, the test tube was lightly shaken to mix the contents. Fifteen drops of the crude reaction mixture were immediately transferred via a pipette to a freshly prepared NaI/AcOH test solution in a 4-dram vial. The vial was capped and shaken, giving an orange color resembling the color of the reaction mixture.
2 h Test
A test tube was loaded with erythrosine B (0.0022 g, 0.0025 mmol), naphthalene (0.0310 g, 0.25 mmol), 1.5 mL of CH3CN, and NBS (0.0487 g, 0.275 mmol). After addition of all reagents, the test tube was equipped with a stir bar and allowed to react open to air in a white LED photochamber for 2 h. Upon completion of the reaction, 15 drops of the crude reaction mixture were immediately transferred via a pipette to a freshly prepared NaI/AcOH test solution in a 4-dram vial. The vial was capped and shaken, giving an opaque red-brown color resembling the color of the control test.
Compound Characterization
All bromoarenes were isolated according to general procedure, unless otherwise noted, and display the characterizational data shown below (spectra available in the electronic Supporting Information).
1-Bromo-2-methylnaphthalene (4)19
The title compound was prepared according to the general procedure. Dark orange oil (86 mg, 78%); purification (EtOAc/hexanes, 20:80) Rf = 0.32. 1H NMR (400 MHz, CDCl3): δ 8.29 (d, J = 8.2 Hz, 1H), 7.79 (d, J = 8.2 Hz, 1H) 7.71 (d, J = 8.6 Hz, 1H) 7.56 (t, J = 8.6 Hz, 1H) 7.46 (t, J = 7.4 Hz, 1H) 7.35 (d, J = 8.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 136.0, 133.0, 132.5, 128.7, 128.0, 127.3, 127.2, 126.9, 125.6, 124.0, 24.2. FT-IR (neat, cm–1) ν: 3051, 2919; MS (EI) m/z: 220 and 222 (M+, 1:1 ratio).
1-(3-Bromo-4-methoxyphenyl)ethan-1-one (6)67
The title compound was prepared according to the general procedure. White solid (94 mg, 82%): mp 79–84 °C; purification (hexanes/EtOAc = 80:20) Rf = 0.25. 1H NMR (400 MHz, CDCl3): δ 8.17 (d, J = 2.3 Hz, 1H), 7.93 (dd, J = 8.6, 2.3 Hz, H) 6.94 (d, J = 8.6 Hz, 1H) 3.97 (s, 3H) 2.56 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 195.6, 159.6, 133.8, 131.2, 129.5, 111.8, 111.0, 56.5, 26.3. FT-IR (neat, cm–1) ν: 2959, 2870, 1500, 1181; MS (EI) m/z: 214 and 216 (M+, 1:1 ratio).
2-Bromodibenzofuran68 and 4-Bromodibenzofuran69 (7)
The title compounds were prepared as an inseparable mixture of isomers (2-bromodibenzofuran/4-bromodibenzofuran = 5:1 ratio by 1H NMR and GC) according to the general procedure. White solid (93 mg, 75% combined): mp 97–100 °C; purification (hexanes/EtOAc = 80:20) Rf = 0.30. Signals corresponding to the major product, 2-bromodibenzofuran, as reported here. Spectra are located within the Supporting Information. 1H NMR (400 MHz, CDCl3): 7.99 (d, J = 2.0 Hz, 1H), 7.83 (d, J = 7.4 Hz, 1H), 7.52–7.47 (m, 2H), 7.44 (dt, J = 7.8, 1.4 Hz, 1H), 7.37 (d, J = 8.6 Hz, 1H), 7.30 (t, J = 7.8 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 155.6, 153.9, 128.9, 126.9, 125.2, 122.7, 122.5, 122.0, 119.8, 112.4, 112.2, 110.8 ppm. IR (neat) ν: 3066, 1437, 841, 751, 688 cm–1. MS (EI) m/z: 246 and 248 (M+, 1:1 ratio).
5-Bromo-1,3-benzodioxole (8)25
The title compound was prepared according to the general procedure. Red oil (78 mg, 78%); purification (hexanes/EtOAc = 90:10) Rf = 0.60. 1H NMR (400 MHz, CDCl3): δ 6.96–6.93 (m, 2H), 6.70–6.67 (m, 1H), 5.97 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 148.6, 147.0, 124.3, 113.0, 112.3, 109.6, 101.2 ppm. IR (neat) ν: 2895, 1712, 1468, 1228, 1033, 932, 874, 795, 573 cm–1. MS (EI) m/z: 200 and 202 (M+, 1:1 ratio).
1-Bromo-2-naphthol (9)19
The title compound was prepared according to the general procedure. Additional information regarding the workup: after completion, the reaction was quenched with 4 mL of saturated Na2CO3 solution. The resulting mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layer was dried over magnesium sulfate, and the filtrate was concentrated under vacuum. Pale orange solid (42 mg, 76%): mp 76–79 °C; Purification (EtOAc/hexanes = 20:80) Rf = 0.67. 1H NMR (400 MHz, CDCl3): δ 8.03 (d, J = 9.0 Hz, 1H), 7.78 (d, J = 9.0 Hz, 1H), 7.74 (d, J = 9.0 Hz, 1H), 7.57 (t, J = 7.4 Hz, 1H) 7.40 (t, J = 7.4 Hz, 1H), 7.27 (d, J = 9.0 Hz, 1H), 5.92 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 150.5, 132.3, 129.7, 129.3, 128.2, 127.8, 125.3, 124.1, 117.1, 106.1. FT-IR (neat, cm–1) ν: 3275, 3056; MS (EI) m/z: 222 and 224 (M+, 1:1 ratio).
2-Bromo-4-(tert-butyl)phenol (10)19
The title compound was prepared according to the general procedure. Oily orange solid (90 mg, 82%); purification (EtOAc/hexanes = 10:90) Rf = 0.20. 1H NMR (400 MHz, CDCl3): δ 7.43 (s, 1H), 7.22 (d, J = 8.6 Hz, 1H) 6.94 (d, J = 8.6 Hz, 1H) 5.33 (s, 1H) 13C NMR (100 MHz, CDCl3): δ 149.8, 145.1, 128.7, 126.2, 115.5, 109.8, 34.2, 31.4. FT-IR (neat, cm–1) ν: 3377, 3135, 2961, 2905, 2863; MS (EI) m/z: 228 and 230 (M+, 1:1 ratio).
2-Bromo-4-(1-methylethyl)-phenol (11)67
The title compound was prepared according to the general procedure. Colorless oil (77 mg, 72% yield); purification (hexanes/EtOAc = 98:2) Rf = 0.67. 1H NMR (400 MHz, CDCl3): δ 7.29 (d, J = 2.0 Hz, 1H), 7.06 (dd, J = 8.2, 2.0 Hz, 2H) 6.93 (d, J = 8.2 Hz, 1H) 5.34 (s, 1H) 2.81 (septet, J = 7.4 Hz, 1H) 1.20 (d, J = 7.4 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 150.1, 142.7, 129.6, 127.2, 115.8, 109.9, 33.2, 24.0. FT-IR (neat, cm–1) ν: 2959, 2870, 1500, 1181; MS (EI) m/z: 214 and 216 (M+, 1:1 ratio).
2-Bromo-4-cyclohexylphenol (12)
The title compound was prepared according to the general procedure. White solid (114 mg, 89% yield): mp 30–32 °C; purification (hexanes/EtOAc = 90:10) Rf = 0.50. 1H NMR (400 MHz, CDCl3): δ 7.28 (d, J = 4.0 Hz, 1H), 7.04 (dd, J = 8.0 Hz, 1H), 6.93 (d, 1H), 5.37 (s, 1H), 2.44–2.38 (m, 1H), 1.84–1.68 (m, 5H), 1.39–1.19 (m, 5H) ppm. 13C NMR (100 MHz, CDCl3): δ 150.2, 142.0, 130.0, 127.6, 115.8, 110.0, 43.5, 34.6, 26.8, 26.1 ppm. IR (neat) ν: 3400, 2922, 2850, 1701, 1606, 1495, 1448, 1279, 1174 cm–1. MS (EI): 257(2), 256(10), 255(2), 254(13), 132(74), 44(100). HRMS (ESI): calcd for C12H15O1Br1 [M]+ requires m/z, 256.02873; found m/z, 256.02835.
2,6-Dibromo-4-chlorophenol (13)70
The title compound was prepared following the general procedure using 2.2 equivalents of NBS (196 mg, 1.1 mmol). Orange solid (76 mg, 53%): mp 79–84 °C; purification (hexanes/EtOAc = 90:10) Rf = 0.25. 1H NMR (400 MHz, CDCl3): δ = 7.46 (s, 2H), 5.97 (br s, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 148.5, 131.5, 126.2, 109.9 ppm. IR (neat) ν: 3406, 3078, 1557, 1455, 1384, 1315, 1213, 1153, 855, 702, 559 cm–1. MS (EI) m/z: 284(45), 286(100), 288(72), 290(12).
2,4-Dibromo-6-chlorophenol (14)70
The title compound was prepared following the general procedure using 2.2 equivalents of NBS (196 mg, 1.1 mmol). Yellow solid (144 mg, 99%): mp 70–74 °C; purification (hexanes/EtOAc, 98:2) Rf = 0.10. 1H NMR (400 MHz, CDCl3): δ = 7.55 (d, J = 2.0 Hz, 1H), 7.44 (d, J = 2.0 Hz, 1H), 5.88 (s, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 148.1, 133.6, 131.4, 121.5, 112.2, 110.8 ppm. IR (neat) ν: 3372, 3073, 1563, 1459, 1384, 1319, 1160, 855, 766, 677, 555 cm–1. MS (EI) m/z: 284(45), 286(100), 288(72), 290(12).
4-Bromo-2-nitroaniline (15)71
The title compound was prepared according to the general procedure. Orange solid (73 mg, 74%): mp 110–112 °C; purification (hexanes/EtOAc = 80:20, 1% NEt3) Rf = 0.30. 1H NMR (400 MHz, CDCl3): δ 8.26 (d, J = 2.3 Hz, 1H), 7.44 (dd, J = 9.0, 2.3 Hz, 1H), 6.74 (d, J = 9.0 Hz, 1H), 6.12 (br s, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ 143.5, 138.4, 128.3, 120.3, 107.8 ppm. IR (neat) ν: 3469, 3344, 3172, 3093, 2922, 2851, 1621, 1557, 1495, 1336, 1239, 813 cm–1. MS (EI) m/z: 216 and 218 (M+, 1:1 ratio).
2,6-Dibromo-4-chloroaniline (16)72
The title compound was prepared following the general procedure using 2.2 equivalents of NBS (196 mg, 1.1 mmol). Dark red solid (120 mg, 86%): mp 84–88 °C; purification (hexanes/EtOAc, 90:10) Rf = 0.50. 1H NMR (400 MHz, CDCl3): δ = 7.38 (s, 2H), 4.54 (br s, 2H) ppm. 13C NMR (100 MHz, CDCl3): δ 140.9, 131.2, 122.6, 108.4 ppm. IR (neat) ν: 3416, 3304, 3075, 1611, 1543, 1455, 1386, 1291, 1055, 850 cm–1. MS (EI) m/z: 283(45), 285(100), 287(73), 289(15).
4′-Bromoacetanilide (17)71
The title compound was prepared according to the general procedure. Additional information regarding the workup: to the crude reaction mixture was added 5 mL of 1 M NaOH upon completion of the reaction. The organic portion was removed and washed with brine, dried with Na2SO4, and evaporated under vacuum. White solid (89 mg, 84%): mp 171–174 °C; purification (hexanes/EtOAc, 50:50) Rf = 0.30. 1H NMR (400 MHz, CDCl3): δ 7.41 (m, 4H), 2.17 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 168.3, 136.9, 131.9, 121.3, 116.8, 24.6 ppm. IR (neat) ν: 3288, 3256, 3185, 3114, 3052, 1666, 1600, 1529, 1392, 1308, 1255, 1007, 812, 737, 501 cm–1. MS (EI) m/z: 213 and 215 (M+, 1:1 ratio).
N-(4-Bromo-2,6-dimethylphenyl)-2-(diethylamino)acetamide (18)32
The title compound was prepared according to the general procedure. Additional information regarding the workup: following evaporation of the reaction solvent, the crude was dissolved in 3 mL of toluene. Acidic extraction from the organic solvent was performed using 3 mL of 4 M HCl. NaOH solution (2 M) was added to the aqueous layer until the pH was greater than 11. The aqueous portion was then extracted with hexanes (3 × 10 mL) and evaporated under vacuum. Light pink oily solid (130 mg, 84%); purification (hexanes/EtOAc = 50:50, 2% NEt3) Rf = 0.45. 1H NMR (400 MHz, CDCl3): δ = 6.90 (br s, 1H), 7.07 (s, 2H), 3.20 (s, 2H), 2.67 (q, J = 7.2 Hz, 4H), 2.22 (s, 6H), 1.12 (t, J = 7.2 Hz, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ 170.2, 135.0, 134.0, 128.2, 127.0, 57.5, 48.9, 18.9, 12.6 ppm. IR (neat) ν: 3284, 2969, 1677, 1492, 730 cm–1. MS (EI) fragmentation m/z: 147(4), 132(2), 119(2), 91(3), 86(100), 58(20).
4-Bromo-1-phenylpyrazole (19)16
The title compound was prepared according to the general procedure. White solid (107 mg, 99%): mp 78–82 °C; purification (hexanes/EtOAc = 85:15) Rf = 0.50. 1H NMR (400 MHz, CDCl3): δ 7.92 (s, 1H), 7.67 (s, 1H), 7.63 (m, 2H), 7.45 (m, 2H), 7.31 (m, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 141.5, 139.6, 129.5, 127.02, 126.99, 119.0, 95.6 ppm. IR (neat) ν: 3110, 1595, 1493, 1381, 1335, 1037, 948, 844, 750, 647 cm–1. MS (EI) m/z: 222 and 224 (M+. 1:1 ratio).
3-Bromo-indazole (20)22
The title compound was prepared according to the general procedure. Pale orange solid (80 mg, 82%): mp 140–143 °C; purification (hexanes/EtOAc = 90:10) Rf = 0.10. 1H NMR (400 MHz, CDCl3): δ 12.22 (br s, 1H), 7.66 (t, J = 8.0 Hz, 2H), 7.46 (t, J = 8.0 Hz, 1H), 7.24 (t, J = 8.0 Hz, 1H) ppm. 13C NMR (100 MHz, CDCl3): δ 141.2, 128.1, 123.0, 122.6, 121.8, 120.1, 110.5 ppm. IR (neat) ν: 3152–2914, 1624, 1479, 1329, 1241, 1024, 900, 769 cm–1. MS (EI) m/z: 196 and 198 (M+, 1:1 ratio).
4-Bromo-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one (21)32
The title compound was prepared as an inseparable mixture of the title compound and brominated isomer (at N-Me position) according to the general procedure. Yellow solid (121 mg, 91% combined): mp 47–52 °C; purification (hexanes/EtOAc, 60:40) Rf = 0.10. Signals corresponding to the major product, 21, as reported here. Spectra are located within the Supporting Information. 1H NMR (400 MHz, CDCl3): δ 7.37 (m, 2H), 7.29 (m, 2H), 7.22 (m, 1H), 3.01 (s, 3H), 2.22 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 162.1, 154.0, 129.4, 129.2, 127.0, 124.1, 90.5, 36.4, 12.5 ppm. IR (neat) ν: 2987, 2927, 1652, 1575, 1496, 1456, 1108, 750, 582 cm–1. MS (EI) m/z: 266 and 268 (1:1 ratio).
8-Bromo-3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione (22)73
The title compound was prepared according to the general procedure. Additional information regarding the workup: to the crude reaction mixture was added 5 mL of 1 M NaOH upon completion of the reaction. The organic portion was removed and washed with brine, dried with Na2SO4, and evaporated under vacuum. White solid (76 mg, 56%): mp 214–216 °C; purification (DCM/EtOAc, 5:3) Rf = 0.45. 1H NMR (400 MHz, CDCl3): δ 3.90 (s, 3H), 3.49 (s, 3H), 3.33 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 154.4, 151.2, 148.0, 128.1, 109.3, 33.9, 29.8, 28.0 ppm. IR (neat) ν: 2954, 1701, 1653, 1540, 1449, 1341, 972, 743, 502 cm–1. MS (EI) m/z: 272 and 274 (M+, 1:1 ratio).
Ethyl 3-Bromo-1H-indole-2-carboxylate (23)16
The title compound was prepared according to the general procedure. White solid (102 mg, 79%): mp 141–144 °C; purification (hexanes/EtOAc, 80:20). Rf = 0.36. 1H NMR (400 MHz, CDCl3): δ 9.12 (br s, 1H), 7.68 (d, J = 8.6 Hz, 1H), 7.41–7.36 (m, 2H), 7.25–7.21 (m, 1H), 4.46 (q, J = 7.3 Hz, 2H), 1.46 (t, J = 7.3 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 161.0, 135.3, 128.0, 126.6, 124.1, 121.5, 121.3, 112.0, 98.3, 61.5, 14.3 ppm. IR (neat) ν: 3291, 2985, 1683, 1619, 1516 cm–1. MS (EI) m/z: 265(2), 263(2), 252(3), 223(4), 221(4), 146(18), 130(6), 114(5), 103(6), 102(6), 44(100).
2-Bromo-1-[(4-methylphenyl)sulfonyl-1H-pyrrole (24)74
The title compound was prepared according to the general procedure. Light brown solid (28 mg, 37%): mp 101–104 °C; purification (hexanes/EtOAc = 80:20) Rf = 0.32. 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 8.6 Hz, 2H), 7.36 (d, J = 8.2 Hz, 2H), 7.32 (dd, J = 5.9, 2.0 Hz, 1H), 6.82 (d, J = 2.0 Hz, 1H), 6.10 (d, J = 5.9 Hz, 1H), 2.45 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 148.8, 145.8, 134.7, 129.6, 128.8, 124.9, 58.1, 21.7 ppm. IR (neat) ν: 3101, 3018, 1738, 1597, 1493, 1367, 1188, 1168 cm–1. MS (EI) m/z: 301(4), 299(4), 155(31), 91(100), 65(38), 44(30), 39(37).
Acknowledgments
The research results discussed in this publication were made possible in part by funding through the award for project number HR18-013 from the Oklahoma Center for the Advancement of Science and Technology (OCAST). We are grateful for the financial support provided by the lab start-up contribution from The University of Tulsa. We would also like to thank the Office of Research and Sponsored Programs for R.B.’s Student Research Grant and the Tulsa Undergraduate Research Challenge (TURC) and the Chemistry Summer Undergraduate Research Program (CSURP) for support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02320.
1H and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Petrone D. A.; Ye J.; Lautens M. Modern Transition-Metal-Catalyzed Carbon-Halogen Bond Formation. Chem. Rev. 2016, 116, 8003–8104. 10.1021/acs.chemrev.6b00089. [DOI] [PubMed] [Google Scholar]
- Gribble G. W. The diversity of naturally occurring organobromine compounds. Chem. Soc. Rev. 1999, 28, 335–346. 10.1039/a900201d. [DOI] [Google Scholar]
- Tang M. L.; Bao Z. Halogenated Materials as Organic Semiconductors. Chem. Mater. 2011, 23, 446–455. 10.1021/cm102182x. [DOI] [Google Scholar]
- Terrier F.Modern Nucleophilic Aromatic Substitution; Wiley, 2013. [Google Scholar]
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. [DOI] [Google Scholar]
- Heck R. F.; Nolley J. P. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. 10.1021/jo00979a024. [DOI] [Google Scholar]
- Stille J. K. The Palladium-Catalyzed Cross-Coupling Reactions of Organotin Reagents with Organic Electrophiles[New Synthetic Methods(58)]. Angew. Chem., Int. Ed. 1986, 25, 508–524. 10.1002/anie.198605081. [DOI] [Google Scholar]
- Sonogashira K. Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. 10.1016/s0022-328x(02)01158-0. [DOI] [Google Scholar]
- Guram A. S.; Buchwald S. L. Palladium-Catalyzed Aromatic Aminations with in situ Generated Aminostannanes. J. Am. Chem. Soc. 1994, 116, 7901–7902. 10.1021/ja00096a059. [DOI] [Google Scholar]
- Paul F.; Patt J.; Hartwig J. F. Palladium-catalyzed formation of carbon-nitrogen bonds. Reaction intermediates and catalyst improvements in the hetero cross-coupling of aryl halides and tin amides. J. Am. Chem. Soc. 1994, 116, 5969–5970. 10.1021/ja00092a058. [DOI] [Google Scholar]
- De la Mare P. B. D.Electrophilic Halogenation: Reaction Pathways Involving Attack by Electrophilic Halogens on Unsaturated Compounds; Cambridge University Press: London; New York, 1976. [Google Scholar]
- Taylor R.Electrophilic Aromatic Substitution; Wiley: Chichester, U.K., 1990. [Google Scholar]
- Snieckus V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 1990, 90, 879–933. 10.1021/cr00104a001. [DOI] [Google Scholar]
- Hodgson H. H. The Sandmeyer Reaction. Chem. Rev. 1947, 40, 251–277. 10.1021/cr60126a003. [DOI] [PubMed] [Google Scholar]
- Samanta R. C.; Yamamoto H. Selective Halogenation Using an Aniline Catalyst. Chem.—Eur. J. 2015, 21, 11976–11979. 10.1002/chem.201502234. [DOI] [PubMed] [Google Scholar]
- Jiang P.-P.; Yang X.-J. A quick, mild and efficient bromination using a CFBSA/KBr system. RSC Adv. 2016, 6, 90031–90034. 10.1039/c6ra20217a. [DOI] [Google Scholar]
- Xiong X.; Tan F.; Yeung Y.-Y. Zwitterionic-Salt-Catalyzed Site-Selective Monobromination of Arenes. Org. Lett. 2017, 19, 4243–4246. 10.1021/acs.orglett.7b01899. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Liu R.; Xu Q.; Ma L.; Liang X. Sodium Nitrite-Catalyzed Oxybromination of Aromatic Compounds and Aryl Ketones with a Combination of Hydrobromic Acid and Molecular Oxygen under Mild Conditions. Adv. Synth. Catal. 2006, 348, 862–866. 10.1002/adsc.200505495. [DOI] [Google Scholar]
- Podgoršek A.; Stavber S.; Zupan M.; Iskra J. Environmentally benign electrophilic and radical bromination on water: H2O2–HBr system versus N-bromosuccinimide. Tetrahedron 2009, 65, 4429–4439. 10.1016/j.tet.2009.03.034. [DOI] [Google Scholar]
- Carreno M. C.; Garcia Ruano J. L.; Sanz G.; Toledo M. A.; Urbano A. N-Bromosuccinimide in Acetonitrile: A Mild and Regiospecific Nuclear Brominating Reagent for Methoxybenzenes and Naphthalenes. J. Org. Chem. 1995, 60, 5328–5331. 10.1021/jo00121a064. [DOI] [Google Scholar]
- Zysman-Colman E.; Arias K.; Siegel J. S. Synthesis of arylbromides from arenes and N-bromosuccinimide (NBS) in acetonitrile - A convenient method for aromatic bromination. Can. J. Chem. 2009, 87, 440–447. 10.1139/v08-176. [DOI] [Google Scholar]
- Tang R.-J.; Milcent T.; Crousse B. Regioselective Halogenation of Arenes and Heterocycles in Hexafluoroisopropanol. J. Org. Chem. 2018, 83, 930–938. 10.1021/acs.joc.7b02920. [DOI] [PubMed] [Google Scholar]
- Podgoršek A.; Stavber S.; Zupan M.; Iskra J. Free radical bromination by the H2O2–HBr system on water. Tetrahedron Lett. 2006, 47, 7245–7247. 10.1016/j.tetlet.2006.07.109. [DOI] [Google Scholar]
- Prakash G. K. S.; Mathew T.; Hoole D.; Esteves P. M.; Wang Q.; Rasul G.; Olah G. A. N-Halosuccinimide/BF3–H2O, Efficient Electrophilic Halogenating Systems for Aromatics. J. Am. Chem. Soc. 2004, 126, 15770–15776. 10.1021/ja0465247. [DOI] [PubMed] [Google Scholar]
- Mo F.; Yan J. M.; Qiu D.; Li F.; Zhang Y.; Wang J. Gold-catalyzed halogenation of aromatics by N-halosuccinimides. Angew. Chem. 2010, 49, 2028–2032. 10.1002/anie.200906699. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Huang L.; Zhang Y.; Chen X.; Xing W.; Huang J. Silver Catalyzed Bromination of Aromatics with N-bromosuccinimide. Catal. Lett. 2012, 142, 378–383. 10.1007/s10562-011-0764-2. [DOI] [Google Scholar]
- Maibunkaew T.; Thongsornkleeb C.; Tummatorn J.; Bunrit A.; Ruchirawat S. Practical and Metal-Free Electrophilic Aromatic Halogenation by Interhalogen Compounds Generated In Situ from N-Halosuccinimide and Catalytic TMSCl. Synlett 2014, 1769–1775. 10.1055/s-0034-1378225. [DOI] [Google Scholar]
- Ohkubo K.; Mizushima K.; Iwata R.; Fukuzumi S. Selective photocatalytic aerobic bromination with hydrogen bromide via an electron-transfer state of 9-mesityl-10-methylacridinium ion. Chem. Sci. 2011, 2, 715. 10.1039/c0sc00535e. [DOI] [Google Scholar]
- Hering T.; König B. Photocatalytic activation of N-chloro compounds for the chlorination of arenes. Tetrahedron 2016, 72, 7821–7825. 10.1016/j.tet.2016.06.028. [DOI] [Google Scholar]
- Hering T.; Mühldorf B.; Wolf R.; König B. Halogenase-Inspired Oxidative Chlorination Using Flavin Photocatalysis. Angew. Chem. 2016, 55, 5342–5345. 10.1002/anie.201600783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Wang Z. J.; Wang L.; Ma B. C.; Ghasimi S.; Lu H.; Landfester K.; Zhang K. A. I. Photocatalytic Selective Bromination of Electron-Rich Aromatic Compounds Using Microporous Organic Polymers with Visible Light. ACS Catal. 2016, 6, 1113–1121. 10.1021/acscatal.5b02490. [DOI] [Google Scholar]
- Petzold D.; König B. Photocatalytic Oxidative Bromination of Electron-Rich Arenes and Heteroarenes by Anthraquinone. Adv. Synth. Catal. 2018, 360, 626–630. 10.1002/adsc.201701276. [DOI] [Google Scholar]
- Brueckner A. C.; Hancock E. N.; Anders E. J.; Tierney M. M.; Morgan H. R.; Scott K. A.; Lamar A. A. Visible-light-mediated, nitrogen-centered radical amination of tertiary alkyl halides under metal-free conditions to form α-tertiary amines. Org. Biomol. Chem. 2016, 14, 4387–4392. 10.1039/c6ob00616g. [DOI] [PubMed] [Google Scholar]
- Hopkins M. D.; Scott K. A.; DeMier B. C.; Morgan H. R.; Macgruder J. A.; Lamar A. A. Formation of N-sulfonyl imines from iminoiodinanes by iodine-promoted, N-centered radical sulfonamidation of aldehydes. Org. Biomol. Chem. 2017, 15, 9209–9216. 10.1039/c7ob02120h. [DOI] [PubMed] [Google Scholar]
- Zard S. Z. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 2008, 37, 1603–1618. 10.1039/b613443m. [DOI] [PubMed] [Google Scholar]
- Höfling S. B.; Heinrich M. R. Nitrogen-Centered Radical Scavengers. Synthesis 2011, 173–189. 10.1055/s-0030-1258305. [DOI] [Google Scholar]
- Chen J.-R.; Hu X.-Q.; Lu L.-Q.; Xiao W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. 10.1039/c5cs00655d. [DOI] [PubMed] [Google Scholar]
- Xiong T.; Zhang Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 2016, 45, 3069–3087. 10.1039/c5cs00852b. [DOI] [PubMed] [Google Scholar]
- Hopkins M.; Brandeburg Z.; Hanson A.; Lamar A. Visible-Light, Iodine-Promoted Formation of N-Sulfonyl Imines and N-Alkylsulfonamides from Aldehydes and Hypervalent Iodine Reagents. Molecules 2018, 23, 1838. 10.3390/molecules23081838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärkäs M. D. Photochemical Generation of Nitrogen-Centered Amidyl, Hydrazonyl, and Imidyl Radicals: Methodology Developments and Catalytic Applications. ACS Catal. 2017, 7, 4999–5022. 10.1021/acscatal.7b01385. [DOI] [Google Scholar]
- Luo J.; Wei W.-T. Recent Advances in the Construction of C-N Bonds Through Coupling Reactions between Carbon Radicals and Nitrogen Radicals. Adv. Synth. Catal. 2018, 360, 2076–2086. 10.1002/adsc.201800205. [DOI] [Google Scholar]
- Zhao Y.; Xia W. Recent advances in radical-based C-N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 2018, 47, 2591–2608. 10.1039/c7cs00572e. [DOI] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Day J. I.; Teegardin K.; Weaver J.; Chan J. Advances in Photocatalysis: A Microreview of Visible Light Mediated Ruthenium and Iridium Catalyzed Organic Transformations. Org. Process Res. Dev. 2016, 20, 1156–1163. 10.1021/acs.oprd.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angnes R. A.; Li Z.; Correia C. R. D.; Hammond G. B. Recent synthetic additions to the visible light photoredox catalysis toolbox. Org. Biomol. Chem. 2015, 13, 9152–9167. 10.1039/c5ob01349f. [DOI] [PubMed] [Google Scholar]
- Ravelli D.; Fagnoni M. Dyes as Visible Light Photoredox Organocatalysts. ChemCatChem 2012, 4, 169–171. 10.1002/cctc.201100363. [DOI] [Google Scholar]
- Fukuzumi S.; Ohkubo K. Selective photocatalytic reactions with organic photocatalysts. Chem. Sci. 2013, 4, 561–574. 10.1039/c2sc21449k. [DOI] [Google Scholar]
- Ravelli D.; Fagnoni M.; Albini A. Photoorganocatalysis. What for?. Chem. Soc. Rev. 2013, 42, 97–113. 10.1039/c2cs35250h. [DOI] [PubMed] [Google Scholar]
- Fukuzumi S.; Ohkubo K. Organic synthetic transformations using organic dyes as photoredox catalysts. Org. Biomol. Chem. 2014, 12, 6059–6071. 10.1039/c4ob00843j. [DOI] [PubMed] [Google Scholar]
- Hari D. P.; König B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014, 50, 6688–6699. 10.1039/c4cc00751d. [DOI] [PubMed] [Google Scholar]
- Nicewicz D. A.; Nguyen T. M. Recent Applications of Organic Dyes as Photoredox Catalysts in Organic Synthesis. ACS Catal. 2014, 4, 355–360. 10.1021/cs400956a. [DOI] [Google Scholar]
- Joshi-Pangu A.; Lévesque F.; Roth H. G.; Oliver S. F.; Campeau L.-C.; Nicewicz D.; DiRocco D. A. Acridinium-Based Photocatalysts: A Sustainable Option in Photoredox Catalysis. J. Org. Chem. 2016, 81, 7244–7249. 10.1021/acs.joc.6b01240. [DOI] [PubMed] [Google Scholar]
- Pitre S. P.; McTiernan C. D.; Scaiano J. C. Library of Cationic Organic Dyes for Visible-Light-Driven Photoredox Transformations. ACS Omega 2016, 1, 66–76. 10.1021/acsomega.6b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller W. H.; Lawrence R. V. Photodehydrogenation of Resin Acids. J. Org. Chem. 1963, 28, 1386–1387. 10.1021/jo01040a503. [DOI] [Google Scholar]
- Bosanac T.; Wilcox C. S. Precipiton Reagents: Precipiton Phosphines for Solution-Phase Reductions. Org. Lett. 2004, 6, 2321–2324. 10.1021/ol049369+. [DOI] [PubMed] [Google Scholar]
- Wood S.; Metcalf D.; Devine D.; Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J. Antimicrob. Chemother. 2006, 57, 680–684. 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- Ams M. R.; Wilcox C. S. Benzil-Tethered Precipitons for Controlling Solubility: A Round-Trip Energy-Transfer Mechanism in the Isomerization of Extended Stilbene Analogues. J. Am. Chem. Soc. 2007, 129, 3966–3972. 10.1021/ja068211c. [DOI] [PubMed] [Google Scholar]
- Uddin M. M.; Hasnat M. A.; Samed A. J. F.; Majumdar R. K. Influence of TiO2 and ZnO photocatalysts on adsorption and degradation behaviour of Erythrosine. Dyes Pigm. 2007, 75, 207–212. 10.1016/j.dyepig.2006.04.023. [DOI] [Google Scholar]
- Jain R.; Sikarwar S. Semiconductor-mediated photocatalyzed degradation of erythrosine dye from wastewater using TiO2 catalyst. Environ. Technol. 2010, 31, 1403–1410. 10.1080/09593331003758789. [DOI] [PubMed] [Google Scholar]
- Hamri S.; Bouchaour T.; Maschke U. Erythrosine/Triethanolamine System to Elaborate Crosslinked Poly(2-hydroxyethylmethacrylate): UV-Photopolymerization and Swelling Studies. Macromol. Symp. 2014, 336, 75–81. 10.1002/masy.201300018. [DOI] [Google Scholar]
- Gonda Z.; Béke F.; Tischler O.; Petró M.; Novák Z.; Tóth B. L. Erythrosine B Catalyzed Visible-Light Photoredox Arylation-Cyclization of N-Alkyl-N-aryl-2-(trifluoromethyl)acrylamides to 3-(Trifluoromethyl)indolin-2-one Derivatives. Eur. J. Org. Chem. 2017, 2112–2117. 10.1002/ejoc.201601493. [DOI] [Google Scholar]
- Studer A.; Curran D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem. 2016, 55, 58–102. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- Tian Z.; Tian B.; Zhang J. Synthesis and characterization of new rhodamine dyes with large Stokes shift. Dyes Pigm. 2013, 99, 1132–1136. 10.1016/j.dyepig.2013.06.013. [DOI] [Google Scholar]
- Eisenthal K. B. Intermolecular and Intramolecular Excited State Charge Transfer. Laser Chem. 1983, 3, 145–162. 10.1155/lc.3.145. [DOI] [Google Scholar]
- He B.; Nie H.; Chen L.; Lou X.; Hu R.; Qin A.; Zhao Z.; Tang B. Z. High Fluorescence Efficiencies and Large Stokes Shifts of Folded Fluorophores Consisting of a Pair of Alkenyl-Tethered, π-Stacked Oligo-p-phenylenes. Org. Lett. 2015, 17, 6174–6177. 10.1021/acs.orglett.5b03152. [DOI] [PubMed] [Google Scholar]
- Kirste A.; Schnakenburg G.; Waldvogel S. R. Anodic Coupling of Guaiacol Derivatives on Boron-Doped Diamond Electrodes. Org. Lett. 2011, 13, 3126–3129. 10.1021/ol201030g. [DOI] [PubMed] [Google Scholar]
- Wang C.; Piel I.; Glorius F. Palladium-Catalyzed Intramolecular Direct Arylation of Benzoic Acids by Tandem Decarboxylation/C–H Activation. J. Am. Chem. Soc. 2009, 131, 4194–4195. 10.1021/ja8100598. [DOI] [PubMed] [Google Scholar]
- Unger Y.; Meyer D.; Molt O.; Schildknecht C.; Münster I.; Wagenblast G.; Strassner T. Green–Blue Emitters: NHC-Based Cyclometalated [Pt(C^C*)(acac)] Complexes. Angew. Chem., Int. Ed. 2010, 49, 10214–10216. 10.1002/anie.201001316. [DOI] [PubMed] [Google Scholar]
- Suresh P.; Annalakshmi S.; Pitchumani K. Regioselective monobromination of substituted phenols in the presence of β-cyclodextrin. Tetrahedron 2007, 63, 4959–4967. 10.1016/j.tet.2007.03.137. [DOI] [Google Scholar]
- Kavala V.; Naik S.; Patel B. K. A New Recyclable Ditribromide Reagent for Efficient Bromination under Solvent Free Condition. J. Org. Chem. 2005, 70, 4267–4271. 10.1021/jo050059u. [DOI] [PubMed] [Google Scholar]
- Khare R.; Sharma J.; Sharma A. Synthesis, characterization, and antibacterial activity of some thiazoles derived from allyl thioureas. Russ. J. Gen. Chem. 2016, 86, 702–707. 10.1134/s1070363216030312. [DOI] [Google Scholar]
- Popov I.; Do H.-Q.; Daugulis O. In Situ Generation and Trapping of Aryllithium and Arylpotassium Species by Halogen, Sulfur, and Carbon Electrophiles. J. Org. Chem. 2009, 74, 8309–8313. 10.1021/jo9015369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D. W.; Fujitsuka M.; Ryu J. H.; Lee M. H.; Kim H. K.; Majima T.; Im C. S2 emission from chemically modified BODIPYs. Chem. Commun. 2012, 48, 3424–3426. 10.1039/c2cc30569k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.