Abstract
As the single opportunity for plants to move, seed dispersal has an important impact on plant fitness, species distributions and patterns of biodiversity. However, models that predict dynamics such as risk of extinction, range shifts and biodiversity loss tend to rely on the mean value of parameters and rarely incorporate realistic dispersal mechanisms. By focusing on the mean population value, variation among individuals or variability caused by complex spatial and temporal dynamics is ignored. This calls for increased efforts to understand individual variation in dispersal and integrate it more explicitly into population and community models involving dispersal. However, the sources, magnitude and outcomes of intraspecific variation in dispersal are poorly characterized, limiting our understanding of the role of dispersal in mediating the dynamics of communities and their response to global change. In this manuscript, we synthesize recent research that examines the sources of individual variation in dispersal and emphasize its implications for plant fitness, populations and communities. We argue that this intraspecific variation in seed dispersal does not simply add noise to systems, but, in fact, alters dispersal processes and patterns with consequences for demography, communities, evolution and response to anthropogenic changes. We conclude with recommendations for moving this field of research forward.
Keywords: Global change, interspecific, intraspecific, long-distance dispersal, population, seed dispersal, spread, variability, within species
Seed dispersal—the single opportunity for plants to move—is important for plant fitness, species distributions and patterns of biodiversity. Models that predict extinction risk of species, range shifts and biodiversity tend to rely on average dispersal distances. However, we know that seed dispersal is highly variable even within a single species (e.g. some seeds go very far and some barely move away from their parent plant, some seeds end up in great quality habitats and some end up on roads). This paper looks at the consequences of this variation in seed dispersal for plants and their ability to respond to future global change.
Introduction
For most plants, seed dispersal represents the main opportunity to move and thus has an important impact on plant fitness, species distributions, community composition and patterns of biodiversity (e.g. Merritt et al. 2010; Vellend 2010; Kroiss and Hillerslambers 2015). However, models that predict extinction risk of species, range shifts and biodiversity loss rarely incorporate realistic dispersal mechanisms or distances, and tend to assume either global or no dispersal (e.g. Engler et al. 2011; Bateman et al. 2013). When these models include a more realistic representation of seed dispersal, they tend to rely on mean estimates of dispersal that are assumed to be identical across individuals within a species (e.g. Miller and McGill 2018). By focusing on mean population (or species) estimates, variation among individuals or variability caused by complex spatial and temporal dynamics is ignored. This variation can lead to differences in their seed dispersal effectiveness (sensuSchupp et al. 2010) as well as in their contributions to long-distance dispersal (e.g. Jordano 2017) and gene flow (Saastamoinen et al. 2018). These differences can have important consequences for our ability to understand and predict plant population dynamics, local to regional biogeographic patterns of species and communities, and ecosystem processes.
Individual variation in the seed dispersal process is multifaceted and can include differences in the number of seeds dispersed (e.g. Jordano and Schupp 2000), the specific traits of the dispersed seeds (Wang and Ives 2017), the treatment of the seed during transit (Traveset et al. 2007), the dispersal distance (e.g. Thiede and Augspurger 1996) and the quality of the habitat in which they are deposited (i.e. as described by the seedscape, sensuBeckman and Rogers 2013). The causes of individual variation in dispersal includes both intrinsic traits of plants (e.g. differences in seed crop size, fruit or seed size, plant height, etc.) and extrinsic characteristics of the environment (e.g. fruiting neighbourhood, habitat structure, community of seed-dispersing animals; Schupp et al. 2019). It is also important to recognize that many plant traits affecting seed dispersal, such as fruit diameter, vary not only among individuals, but also within individuals and across years (González-Varo and Traveset 2016; Herrera 2017). Variability in seed dispersal is well documented and is present regardless of the seed dispersal mechanism (see section Seed Dispersal is Influenced by Intrinsic and Extrinsic Variability below, and Schupp, this issue). However, the magnitudes and consequences of intraspecific variation in seed dispersal are poorly understood. We use the term intraspecific variability throughout to capture both inter- and intra-individual variability in the dispersal of seeds within species. We acknowledge that the consequences of such variations may sometimes diverge, especially with respect to the evolution of dispersal, but would generally be similar, regardless of the source of variation.
We propose that intraspecific variation in seed dispersal has important implications for our understanding of plant fitness, as well as population, community and landscape dynamics. This is because dispersal estimates based on population means are not the same as dispersal estimates that consider individual variation (Box 1 and Box 2). Chesson has called this effect non-linear averaging (Chesson 1996), and it is based on the mathematical fact that a non-linear function evaluated at its average input values does not yield the same result as evaluating the function over a distribution of input values and then averaging its conditional results. Jensen’s inequality for convex and concave functions is a specific example of non-linear averaging. For example, if the number of seeds produced by an individual is a concave function of its biomass, then Jensen’s inequality (Jensen 1906) implies that variation among individuals in biomass would reduce the population-level mean seed production compared to seed production predicted from mean biomass. Alternatively, if the mean dispersal distance is a convex function with plant height, then variation among individuals in plant height would increase the population-level mean dispersal distance compared to dispersal distances predicted from mean plant height. In stochastic simulations, models based on species- or population-level average dispersal kernels may yield results that are systematically but unpredictably biased in terms of direction and magnitude. Conservation and management efforts require accurate predictions for how species may respond under different management and global change scenarios. With sufficient time, even small systematic biases that may arise by ignoring variation in dispersal have the potential to compound into large misrepresentations.
Box 1.
Intraspecific variation in dispersal and non-linear averaging.
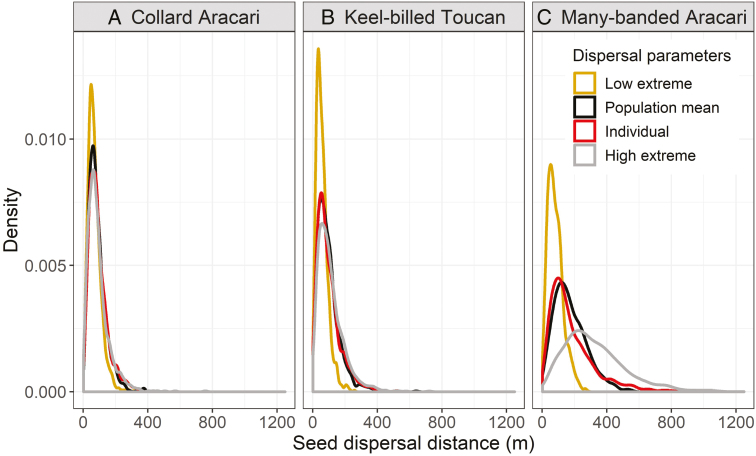
Seed dispersal distances from parent trees for three species of toucans (Ramphastidae) in the New World tropics, A) collared aracari (Pteroglossus torquatus), B) keel-billed toucan (Ramphastos sulfuratus) and C) many-banded aracari (Pteroglossus pluricinctus). Dispersal kernels were generated by combining animal movements from 12 to 23 radio-tracked birds and gut retention times (for Virola koschnyi; A, B, Jones (2017) and Virola flexuosa seeds; C, Holbrook and Loiselle (2007, 2009)). Gut retention time was based on trials with captive birds, so a single distribution was used with the average gut retention time as the mean in a gamma distribution. We used an exponential distribution to simulate animal movement using four scenarios (low extreme, high extreme, population, individual) and then combined those with simulated gut retention times. For animal movement, exponential distributions were fitted to the radio-tracked individual with the lowest average movement (low extreme) and the individual with the highest average movement (high extreme) to illustrate the range in individual variation in movement. The population-level kernel (population) was fitted using movement data pooled from all individual birds, and the individual-level kernel (individual) used data from each individual separately (i.e. each individual had their own fitted distribution to movement prior to combining across individuals). These results highlight the differences in seed dispersal distances generated by variation in animal movement. They also demonstrate that dispersal kernels generated from the mean population data are not the same as those created from individual kernels. In this particular example, the population kernel underestimates the number of long-distance dispersal (LDD) events (the tail of the curve). For example, with V. flexuosa trees in the Ecuadorian Amazon, we defined LDD events as those where seeds were deposited >500 m from their origin by many-banded aracari. The percentage of LDD was 0.6 % under the population-level model, compared to 3.9 % using data that incorporated variation in movement among individuals. For Costa Rican Ramphastids, we defined LDD events as those >200 m. The results were similar, with the population model underestimating LDD events compared to the individual-based model (collared aracari—3.7 % with population model, 6.8 % with individual model; keel-billed toucan—7.3 % with population model, 9.6 % with individual model). Data are available as Supporting Information - Appendix S2.
Box 2.
Intraspecific variation in dispersal and kurtosis.
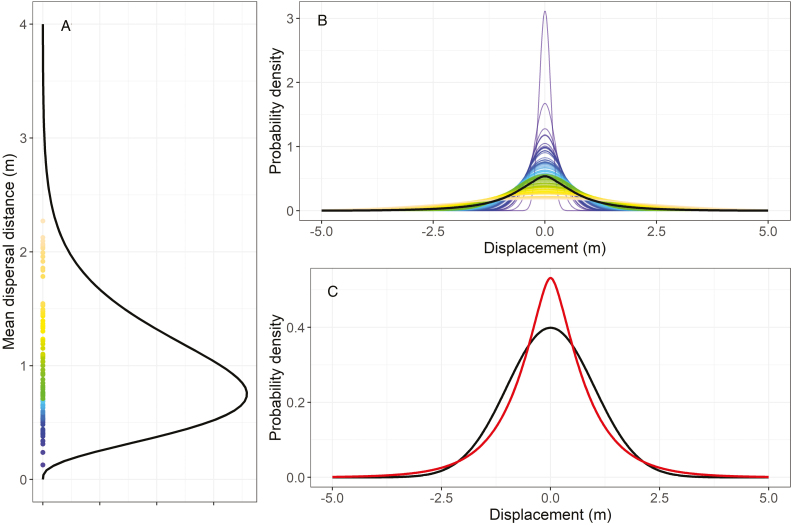
To illustrate that variability in mean dispersal distance creates more leptokurtic dispersal kernels, we can imagine a population of individuals (seeds) each of which exhibits a diffusive movement but whose mean dispersal distance (as determined by their individual diffusion constant) vary. In (A), the distribution among individual mean dispersal distances is gamma distributed with a subsampling of 200 individuals from this distribution shown as coloured points (shape parameter (s) = 4, scale parameter (a) = 0.25, mean dispersal distance = s * a = 1 m). In (B), the Gaussian dispersal kernels for each of these 200 individuals are shown using the same colours, and the Gaussian dispersal kernel of the ‘average’ individual (i.e. assuming the mean dispersal distance of 1 m from the gamma distribution) is shown in black. In (C), the population-level dispersal kernel (red) of the heterogenous population is more leptokurtic than the dispersal kernel of a homogenous population where all individuals have the same mean dispersal distance (black). The population-level dispersal kernel k is calculated by conditioning, i.e. where knorm is the density of a standard Gaussian and p(L) is the density of a gamma distribution. Increasing the amount of individual variation leads to more leptokurtic dispersal kernels [see Supporting Information—Appendix S1]. Simulation data are available as Supporting Information - Appendix S3.
In this manuscript, we synthesize recent research that examines intraspecific variation in seed dispersal and its implications for plant ecology to evaluate our current understanding and to recommend avenues for future research to fill remaining knowledge gaps. First, we present a brief overview of how seed dispersal is influenced by intrinsic and extrinsic variability; for more thorough reviews see Schupp (this issue), Côrtes and Uriarte (2012), McConkey et al. (2012), Beckman and Rogers (2013), Zwolak (2018). We do not discuss what causes rapid changes in trait variability in plants in any detail, but instead refer interested readers to Johnson et al. (2019). Then, we discuss the consequences of intraspecific variation in seed dispersal for local population dynamics, spatial spread, community structure and dynamics, and evolution, and argue that this intraspecific variation in dispersal is not simply adding noise, but altering dispersal processes and patterns. To conclude, we discuss intraspecific variation in seed dispersal within the context of anthropogenic global change and suggest directions for future research.
Seed Dispersal Is Influenced by Intrinsic and Extrinsic Variability
Intrinsic and extrinsic factors influence the seed dispersal process and variability in these factors contributes to intraspecific variability in dispersal (Box 1, see Schupp, this issue, for a detailed review). Approximately one quarter of trait variability within plant communities exists within species (i.e. morphological and physiological traits; Siefert et al. 2015). We highlight intraspecific variability in four types of traits that are known to underlie intraspecific variability in dispersal: fruit and seed size, fruit and seed crop size, plant height and dispersal-specific structures. We also briefly introduce several extrinsic factors that can cause intraspecific variation in dispersal. Variation in these intrinsic and extrinsic factors potentially has significant consequences for plant demography and community composition through its impacts on number of seeds dispersed, the seedscape in which seeds land and the frequency of long-distance dispersal events.
Fruit and seed size
Fruit and seed size are highly variable both within and among individual plants (Michaels et al. 1988), and this variability influences seed dispersal in a variety of ways. For abiotic dispersal, size influences dispersal distance as smaller seeds are typically dispersed further by water (e.g. Delefosse et al. 2016) and wind (e.g. Skarpaas et al. 2011). For endozoochorous and synzoochorous (where animals intentionally transport seeds without ingestion) dispersers, variation in fruit diameter and seed size can affect how many and which disperser species are able to feed on an individual plant (Galetti et al. 2013; González-Varo and Traveset 2016), how seeds are processed (e.g. swallowed or regurgitated, Levey 1987; cached or eaten, Jansen et al. 2004; Gómez et al. 2008) and how far seeds are moved (Muñoz and Bonal 2008). Individual variation in fruit and seed size and individual variation in the traits of the dispersal agents interact to mediate the realized disperser assemblages of each fruits. This interaction in intrinsic and extrinsic variability has consequences for seed dispersal (Zwolak 2018).
Fecundity
Individual variation in fecundity has important implications for both long-distance dispersal and number of seeds dispersed, particularly in plants with wind or endozoochorous dispersal (Jordano and Schupp 2000; Norghauer et al. 2011). For wind-dispersed plants, highly fecund individuals tend to have longer maximum dispersal distances because increasing the number of seeds released increases the probability of some seeds catching rare updrafts that result in long-distance dispersal (Nathan et al. 2002; Norghauer et al. 2011; Augspurger et al. 2017). Similarly, larger crop sizes for endozoochorous dispersal may also increase the probability of rare, long-distance dispersal events by animals (e.g. Prunus mahaleb trees, Jordano and Schupp 2000). The consequences of individual variation in fecundity have not, to our knowledge, been explored for other dispersal modes, but are potentially important with any dispersal system since increasing crop size increases the number of dispersal events and thus the probability of a rare long-distance dispersal event.
Plant height
Plant height explains much of the variation in dispersal distance across plant species (Thomson et al. 2011; Tamme et al. 2014; Thomson et al. 2018). Together with diaspore terminal velocity and seed abscission, seed release height is a key phenotypic driver explaining individual variation in dispersal distances of abiotically dispersed plants (Thiede and Augspurger 1996; Wender et al. 2005). For endozoochorous trees, preferential foraging of frugivores at different canopy heights raises the possibility that differences in height may influence the frugivore assemblage to which fruits are exposed (Poulsen et al. 2002; Flörchinger et al. 2010) and consequently impact dispersal outcomes.
Dispersal-specific structures
Intraspecific variation in specialized structures that aid in seed dispersal can also cause intraspecific variation in dispersal kernels. In wind-dispersed plants, pappus and wing morphology can affect seed falling velocity (Riba et al. 2005; Tabassum and Bonser 2017). The quantity of low-density tissues in water-dispersed fruits and seeds can affect buoyancy, which may affect dispersal distances (Guja et al. 2014). For ant-dispersed species, the presence of elaiosomes and the elaiosome/load ratio increased removal rates by ants (Hughes and Westoby 1992). For fleshy-fruited plants, fruits with relatively higher energetic rewards (e.g. pulp to seed ratio or elaisome size) result in higher removal probabilities (Sallabanks 1993; Willson 1994; Mark and Olesen 1996; Stanley and Lill 2002). For fruit dispersed by epizoochory, there is variation in the presence, size and number of appendages that enable mechanical interlocking with animal fur (Gorb and Gorb 2002) or variation in the degree of heterocarpy, in which individual plants produce morphologically distinct diaspores (Monty et al. 2016). However, the impact of this variation on dispersal has not yet been tested for this dispersal mode. Intraspecific studies are also rare among synzoochorous species (e.g. Smallwood et al. 2001; Shimada et al. 2010), although their results consistently suggest that seeds that offer greater rewards and have fewer defences or lower handling times are dispersed further. These same traits also mean that they are consumed at higher rates, with less perishable seeds cached more frequently (reviewed by Vander Wall 2010; Lichti et al. 2017).
Extrinsic factors
Extrinsic factors related to a plant’s local environment and its dispersal vector can cause intraspecific variation in dispersal. For example, the interaction between abiotic dispersal vectors and the landscape structure can cause intraspecific differences in water dispersal due to local flow patterns (Van der Stocken et al. 2015) and in wind dispersal due to local topography, atmospheric conditions and surrounding vegetation (Nathan et al. 2001; Augspurger et al. 2017). Animal-dispersed plant species are impacted by individual variation among seed dispersers (reviewed in Zwolak 2018) and these may interact with intrinsic factors, such as fruit and seed size as discussed above. Animal behaviour and the plants surrounding a focal plant can also interact with the local fruiting neighbourhood impacting dispersal probabilities and distances (Blendinger et al. 2008; Carlo and Morales 2008). Finally, differing impacts of anthropogenic drivers across space also causes within-species variation in dispersal; for example, habitat fragmentation can influence frugivore movement patterns and thus dispersal distances (Levey et al. 2005) and defaunation impacts the composition of the frugivore assemblage and behaviour of remaining frugivores (McConkey and Drake 2006; Holbrook and Loiselle 2009).
Consequences for Local Population Dynamics
Intraspecific variation in seed dispersal can affect demography by influencing vital rates (i.e. germination, growth and survival) as well as dynamics within and among populations (Howe and Miriti 2004). For example, variation in seed dispersal distance can lead to variation in plant survival and growth as some seeds may escape mortality due to natural enemies (Janzen 1970) or experience reduced competition from siblings (Cheplick 1992). Variation in how seeds are dispersed can also lead to variation in survival depending on the time and treatment of seeds passing through the gut for endozoochorous species (Traveset et al. 2007), and the quality of habitat in which seeds are deposited (Beckman and Rogers 2013). It is critical to recognize the effect of this variation, as these vital rates determine population growth. In addition, individual variation in dispersal can affect metapopulation processes by impacting the frequency of movement, genotypes and traits of individuals that move between populations (Cheptou et al. 2008; but see Castorani et al. 2017).
Overlooking intraspecific variation in dispersal can impact conclusions of local population persistence in several ways, particularly in changing environments. First, individual variation in dispersal may impact projections by population matrix and integral projection models. In these models of local population dynamics, dispersal is rarely considered explicitly (see next section for discussion of population spread), but instead subsumed into the various factors affecting the transitions from seed to seedling, or seedling to sapling. Because single individuals can contribute large portions of new recruits in plant communities (Wheelwright 1986; Minor and Kobe 2017), estimated population-level recruitment may change substantially as individual composition changes (e.g. between population, or within populations if the so-called super-producers die). In addition, demographic models that do not explicitly consider dispersal are unable to forecast how altered dispersal processes (i.e. due to defaunation, fragmentation or changing climates) may influence population dynamics and persistence (see section Relevance under Anthropogenic and Global Climate Change below). Models that more mechanistically consider how dispersal and the deposition environment impact growth and survival can incorporate these processes and project population trajectories under altered dispersal (e.g. Caughlin et al. 2014).
A few phenomenological and mechanistic models do explicitly address how dispersal influences local population dynamics (e.g. Godinez-Alvarez and Jordano 2007; Brodie et al. 2009; Loayza and Knight 2010). This is an important first step in understanding the importance of dispersal. Next, researchers should examine how using the mean values related to dispersal (e.g. dispersal distance, fecundity) biases population projections to identify the circumstances when intraspecific variation in dispersal needs to be considered for projecting population dynamics. For example, when estimating dispersal distances based on trait allometries (e.g. Norghauer et al. 2011), the use of mean trait values can under- or overestimate dispersal distances due to Jensen’s inequality. In particular in small populations, individual variation in dispersal can cause population-level patterns of dispersal to differ significantly from expectations based on mean values (Lewis and Pacala 2000). Simulations that explicitly include intraspecific variation are not equal to models that use the mean and variability of the population (Box 3), with the largest consequence for populations occupying habitats located far away from sites suitable for establishment. Overall, there is a need for demographic studies to include dispersal explicitly and to explore how and when intraspecific variation in dispersal affects local population dynamics.
Box 3.
Intraspecific variation in dispersal and demography.
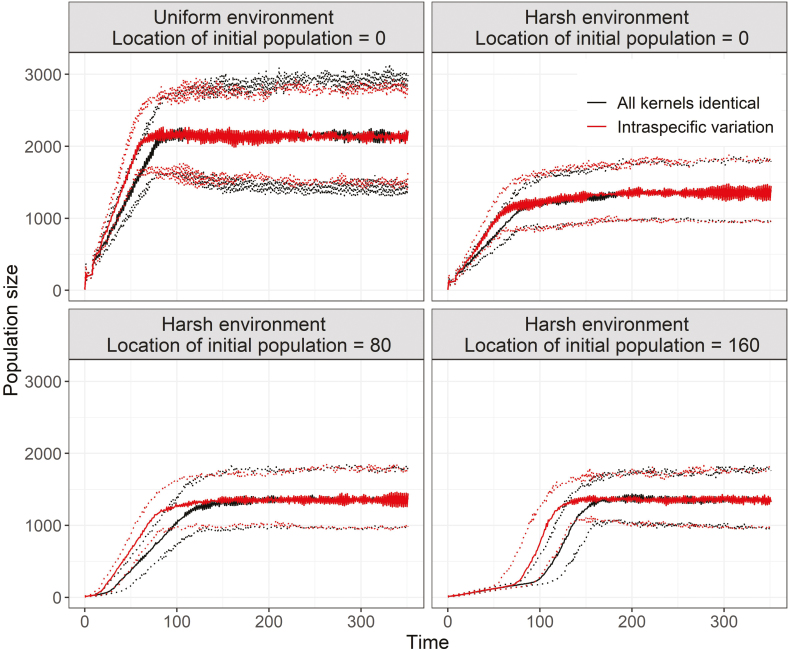
Simulation setup: A 640-m-long wrapped transect (i.e. seeds dispersing off one edge, reappear on the opposite edge). The initial population was restricted to a contiguous 80 m, centred either at 0 (yellow), 80 (grey) or 160 (dark grey). For the ‘Harsh environment’ simulations, half of the transect was made relatively uninhabitable for seedlings (dashed).
Simulation results: In the Uniform environment, the results are the same regardless of the starting location. For simplicity, only one is shown (location = 0).
The median (solid lines) and 95 % quantiles (dashed lines) of population size are shown, from n = 50 replicate simulations per scenario. All simulations used identical rules for individual growth, fecundity and survival. Seeds were dispersed from individual maternal plants according to Gaussian kernels, with a population-level mean dispersal distance of 144 m. For ‘all kernels identical’ scenarios, this mean dispersal distance was applied to all individuals. For scenarios with ‘intraspecific variation’, 20 % of the plants dispersing seeds in a given year were randomly assigned a mean dispersal distance of 464 m, and the remainder had a mean distance of 64 m. Thus, while the population-level mean dispersal distance was identical in both cases, the intraspecific variation scenario had more plants dispersing seeds to short distances while a few plants are dispersing seeds much further. Seedling establishment probabilities and initial population locations also varied by scenario. All simulations included increasing survival with distance from already established plants; however, in the ‘Harsh environment’ scenario, the upper half of the transect was made uninhabitable to new seedlings (e.g. as might occur under severe browsing pressure). In all cases, initial populations were restricted to a contiguous 80 m segment of the transect; however, this segment was centred either at the middle of the transect (location = 0) or closer to the upper half of the transect (location = 80 m, or 160 m). For the ‘Harsh environment’ scenarios, this resulted in different levels of seed limitation as the source population was increasingly isolated from the lower, more habitable portion of the transect. While the final population size was unaffected (i.e. the asymptote), intraspecific variability in dispersal consistently increased population growth rates at the start of the simulations. The greatest difference in population growth rates was found when the source population was the most isolated from suitable habitat (location = 160). Data are available as Supporting Information-Appendix S4.
Consequences for Spatial Spread of Populations
Explaining historical range expansions and predicting future vegetation migration rates is a fundamental question in global change biology and invasion ecology (Clark et al. 1998; Lockwood et al. 2013). As traditional Gaussian dispersal kernels required an unrealistically large mean dispersal distance to match historical spread rates (i.e. ~1000 m, Clark et al. 1998), leptokurtic kernels were proposed as an alternative to describe spatial spread (Mollison 1977; Kot et al. 1996). In contrast to their Gaussian counterparts, these kernels have higher probabilities of short-distance dispersal events, creating a more peaked distribution, and higher probabilities of rare, long-distance dispersal events, creating a fatter or thicker tail (Box 4). These dispersal kernels preserve the mean distance travelled by a seed, but lead to faster rates of either constant or ever-accelerating spatial spread (Box 4).
Box 4.
Rare long dispersal distance events increase rates of spatial spread.
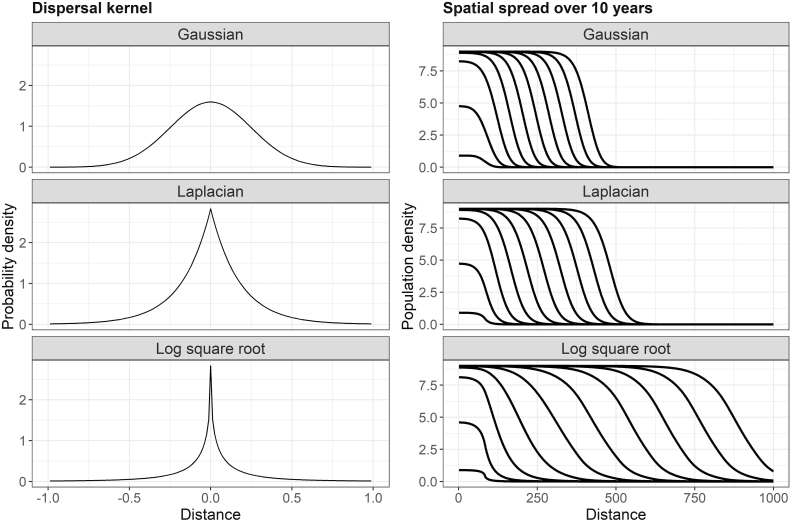
The Gaussian (thin-tailed), Laplacian (leptokurtic) and log square root (leptokurtic and fat-tailed) dispersal kernels plotted on the left-hand side represent the density of seeds dispersed a given distance north or south from its parent. The spatial distribution of the corresponding populations over a 10-year period are shown to the right. Leptokurtic dispersal kernels, such as the Laplacian and log square root, result in faster spread rates than the Gaussian dispersal kernel. Exponentially bounded dispersal kernels, such as the Gaussian and Laplacian, result in asymptotically constant spread rates, whereas distributions that are not exponentially bounded (i.e. fat-tailed, Kot et al. 1996), such as the log square root, result in ever-accelerating spread rates. All dispersal kernels have a mean dispersal distance of 0.25 and local survival and reproduction is given by a Berverton–Holt function 10 * n/(1 + n), where n is the local density.
These population-level leptokurtic kernels could arise due to intraspecific variation in seed dispersal (Box 2), and have been the focus of numerous studies (Neubert and Caswell 2000; Petrovskii and Morozov 2009; Bouin et al. 2012; Stover et al. 2014; Horvitz et al. 2015; Schreiber and Beckman, 2019). For example, Neubert and Caswell (2000) studied spatial spread of the Neotropical Calathea ovandensis due to dispersal by four ant species. The one ant species, Pachycondyla apicalis, that dispersed seeds the furthest (mean dispersal distance > 9 m) only dispersed 7 % of the seeds. However, the inclusion or removal of P. apicalis led to a large change in the rate of spatial spread. Horvitz et al. (2015) reached a similar conclusion with gravity-, catbird-, robin- and raccoon-dispersed seeds in Ardisia elliptica. Using frugivore-stratified spread models, they showed that it was the infrequent but longer-distance dispersal by robins that determined rates of spatial spread. As illustrated in Box 2, continuous variation in the diffusion coefficient D (i.e. the square root of the mean squared displacement) among individuals can generate leptokurtic dispersal kernels at the population level and, thereby, increase rates of spatial spread (Petrovskii and Morozov 2009; Stover et al. 2014; Schreiber and Beckman, 2019). The magnitude of this increase, however, depends subtly on the nature of the distribution underlying the individual variation. For example, gamma-distributed individual variation in D leads to fatter exponentially bounded population-level dispersal kernels (Petrovskii and Morozov 2009). Dispersal kernels that are exponentially bounded result in asymptotically constant spread rates (Box 4). As a first-order approximation, the increase in rate of spatial spread due to individual variation in D is proportional to the variance of this variance (Stover et al. 2014; Schreiber and Beckman, 2019). In contrast, individual variation in D could lead to a population-level dispersal kernel with a power-law tail. As these power-law tails are not exponentially bounded, this form of individual variation would lead to ever-accelerating rates of spatial spread (Box 4; Kot et al. 1996). An example of a process that could lead to a population-level dispersal kernel with a power-law tail arises from individual variation in body size of animal seed dispersers that may affect the movement of seeds—seed dispersers with higher body mass are expected to change movement direction less frequently than seed dispersers with lower body size as it requires a larger force and larger energy expense (Petrovskii and Morozov 2009).
Spread rates are determined by both dispersal and demography at or near the front of an invasion. To understand how individual variation in dispersal affects spread, one must simultaneously assess the role of variation in demography (introduced in the previous section). Variation in dispersal and demography can have a common, external source, which then influences spread through both pathways. In a study in which individual variation was induced externally by a receptacle-feeding weevil that forms cysts inside developing flower head of Carduus nutans, Marchetto et al. (2014) showed that cysts affect the number of seeds produced, the probability that a seed would be dispersed away from the mother plant and the terminal velocity of seeds that do disperse. The consequences for population growth and spread were evaluated with matrix-based travelling wave-speed models (e.g. Neubert and Caswell 2000): the average load of cysts had a greater effect on population spread than local population dynamics of C. nutans in New Zealand (−43 % vs. −17 %), but the reverse was true for the USA (−46 % vs. −64 %). This example further shows that population-level impacts depend not just on the amount of variation in dispersal-related seed traits, but also on the sensitivity of the spread rate to changes in those traits. That sensitivity is jointly shaped by all dispersal, reproduction, survival and growth rates that co-determine the life cycle. While the weevils in the above case study have clear negative effects on both dispersal and fecundity, there may be situations where covariance of dispersal and demography rates can propel population spread. When individual variation in fecundity is positively correlated with mean dispersal distance (e.g. a higher probability of long-distance dispersal for seeds of more fecund parents), this can substantially increase spread rates, as shown in theoretical explorations (Schreiber and Beckman, 2019).
The aforementioned theoretical insights about the effects of individual variation on dispersal are based on the assumption that the populations are experiencing negative density dependence at the population edge. Thus, it is the dispersal traits of the individuals at the leading edge that are ‘pulling’ the population forward. Alternatively, there can be a strong Allee effect at the leading edge, which is a positive density-dependent process. When this occurs, it is the individuals dispersing from the core to the leading edge that are important (i.e. the population is being ‘pushed’ forward). Self-incompatible plants are most likely to experience Allee effects due to mate limitation, thus a potentially important challenge for future work is understanding how individual variation in seed dispersal influences the initiation of spatial spread and the rate of spatial spread. These theoretical insights have also assumed that environmental conditions are relatively homogenous in time and space. While a useful first-order approximation, environmental variation can substantially alter rates of spatial spread (Shigesada and Kawasaki 1997; Ellner and Schreiber 2012). For example, spatial variation in fecundity can reduce spread rates (Shigesada et al. 1986), while temporal variation in dispersal rates can increase spread rates (Ellner and Schreiber 2012). Hence, another important challenge for future work is to identify how the joint effects of environmental and seed dispersal variation impact rates of spatial spread.
Consequences for Plant Communities
Dispersal is integral to local community dynamics, as species arrival depends on successful dispersal and establishment from the local and regional species pools (Leibold et al. 2004; Holyoak et al. 2005). Thus, intraspecific variability in dispersal (e.g. variability in dispersal distance, quantity or quality of dispersal, or species composition of the incoming propagules) has the potential to influence community processes such as assembly, composition and species coexistence. To the best of our knowledge, there have been no experiments that directly examine intraspecific variability in dispersal at the community level. However, significant variability in dispersal at the population level across time or space can indeed alter community dynamics, indicating that intraspecific variation in dispersal should affect community dynamics. For example, plant species richness increases with the richness of species’ propagules that arrive (Tilman 1997; Aicher et al. 2011), as well as with the distance from which these species arrive (Germain et al. 2017). Therefore, we can hypothesize that intraspecific variability in dispersal distance and quantity could alter the species richness of a community. Additionally, intraspecific variation in the timing of species arrival can alter community assembly, as some species have strong priority effects that alter overall diversity if they arrive first (e.g. Fukami et al. 2005; Martin and Wilsey 2012).
In theoretical systems, stable coexistence can also be impacted by intraspecific variation in dispersal. High variation among individuals (Clark 2010), or variation in the environmental conditions that in turn create individual variability (Berkley et al. 2010), may promote coexistence through niche partitioning in many dimensions. This individual-level variation can also make coexistence more difficult as it can increase the dominance of superior competitors, reduce species-level niche differentiation and increase the effects of demographic stochasticity (Hart et al. 2016). Currently, we have a limited understanding of the importance of intraspecific variation in dispersal for plant communities. Intraspecific variation could matter for community-level processes (e.g. timing of arrival, diversity of arrival, priority of arrival, etc.) but little is known empirically or theoretically of the consequences for diversity. However, individual-based models and other approaches make these kinds of studies possible. Increased variation in traits can increase or decrease coexistence (Clark 2010; Hart et al. 2016), but studies to date have not explicitly incorporated intraspecific variation in dispersal. The hypotheses outlined here offer a rich area for future studies to test.
Consequences for Evolution
Seed dispersal and pollen dispersal are the primary sources of gene flow in plants. As such, seed dispersal plays a central role in evolutionary biology, driving patterns ranging from population structure (Hamrick and Godt 1996) to inbreeding depression (Roze and Rousset 2005) and local adaptation (Tigano and Friesen 2016). Moreover, the high level of intraspecific variation in seed dispersal provides the foundation for the great evolutionary potential of dispersal itself (Ronce 2007). Natural selection can act on this variation because two conditions are generally fulfilled. First, seed dispersal has a genetic basis with heritability estimates of up to 0.8 (Saastamoinen et al. 2018), although heritability strongly depends on the environment (Donohue et al. 2005; Wender et al. 2005). Second, all elements of dispersal as discussed here can strongly influence maternal fitness. The number of seeds produced determines the potential number of offspring, while subsequent offspring survival and reproductive success depend on where seeds land within the seedscape (Rubio de Casas et al. 2012). Evolution may reduce or maintain intraspecific variation in dispersal. Specific dispersal phenotypes—that either promote or limit dispersal—may be lost if they are selected against (e.g. Cheptou et al. 2008) or through genetic drift (Barrett and Kohn 1991). However, because the evolutionary costs and benefits of dispersal highly depend on environmental conditions and the maternal phenotype (Ronce 2007), contrasting selection pressures in heterogeneous environments can maintain dispersal variation within populations (Mathias et al. 2001; Stevens et al. 2010). Evolution may also promote variation within the seed crop of an individual in temporally variable environments (Snyder 2011; Rubio de Casas et al. 2012). Heterocarpy, where individuals produce fruits with multiple dispersal morphologies, is especially common in the Asteraceae and Chenopodiaceae (Imbert 2002), and has been shown to have a certain degree of phenotypic plasticity (Taghizadeh et al. 2009; Rubio de Casas et al. 2012).
In spreading plant populations, heritable individual variation in dispersal can lead to spatial sorting, a fitness-independent process where highly dispersive genotypes accumulate and reproduce at the leading edge (Shine et al. 2011; Bouin et al. 2012). This results in the spread process itself selecting for the very traits that promote increased dispersal (Travis and Dytham 2002; Perkins et al. 2013) and accelerates the rate of spatial spread (Phillips et al. 2010; Bouin et al. 2012). Williams et al. (2016) showed that populations of Arabidopsis thaliana invading experimental landscapes evolved higher dispersal abilities at the invasion front, and that evolution accelerates the spread velocity up to 200 % in fragmented landscapes. However, mixed evidence for evolutionary change in seed dispersal has been found for range expansions under field conditions (e.g. Bartle et al. 2013; Huang et al. 2015; Monty et al. 2016), which require further study.
Individual variation in dispersal strongly affects maternal fitness, facilitating rapid evolution of dispersal and associated traits with consequences for populations and communities as discussed in the previous sections. Focusing on the mean dispersal value of a population will ignore key standing genetic variation around this mean, which will determine the probability of an adaptive allele reaching fixation and whether the population can rapidly evolve increased or decreased dispersal in the future. The resulting eco-evolutionary dynamics are a major driver of plant population responses to rapid environmental change.
Relevance under Anthropogenic and Global Climate Change
Ongoing and future climate change will lead to increasing temperatures, changes in precipitation regimes, and an increase in the frequency and intensity of extreme events (i.e. drought, floods, heat waves, etc.) (IPCC 2014). This increase in extreme weather events may have a direct effect on the frequency of long-distance dispersal events, in particular for areas affected by hurricanes and storms (Gillespie et al. 2012). In response to changes in climate, species may shift their distributions to stay within their bioclimatic niche, adapt to the new environmental conditions or become locally extinct (Dawson et al. 2011; Hof et al. 2011). As species shift their ranges, changes in community composition (Lloret et al. 2009) and even potentially novel communities (Williams and Jackson 2007) are likely, which can alter ecosystem functioning (e.g. Liu et al. 2018; Morin et al. 2018). Thus, seed dispersal will play a critical role for how plants and ecosystems respond to climate change and there may be additional consequences of ignoring intraspecific variation within this global change context.
As discussed above, ignoring intraspecific variation in seed dispersal can underestimate population spread rates. This is critical for predicting future range shifts, as the ability of plants to track rapid changes in climate remains largely uncertain (e.g. Zhu et al. 2012; Cunze et al. 2013). By not considering dispersal variation, previous estimates may have systematically underestimated plant migration rates (see Box 2; individual variation creates more leptokurtic dispersal kernels at the population level leading to more long-distance dispersal). However, it is important to note that even with faster migration rates, many plant species will still be unable to reach new bioclimatically suitable habitat due to landscape fragmentation and life history constraints (Miller and McGill 2018). While fragmentation can select for increased dispersal ability during population spread (Williams et al. 2016), severe fragmentation may select for reduced dispersal capabilities in metapopulations (Cheptou et al. 2008), further limiting plants’ abilities to persist in fragmented landscapes. Whether dispersal is constrained by life history traits within plant species is an open question. Bonte and Dahirel (2017) propose that dispersal traits evolve independently from other life history traits, but studies for plants documenting intraspecific variation in dispersal in the context of life history strategies are limited. By incorporating intraspecific variability in seed dispersal, it will increase our ability to predict the vulnerability of species to decline or even local extinction (Valladares et al. 2014; Cochrane et al. 2015) and will help inform alternative conservation efforts, such as assisted dispersal (Hallfors et al. 2017). Climate change may also have a direct influence on seed production and seed traits. For example, increasing temperatures reduced both seed set and seed size in kidney beans (Phaseolus vulgaris) (Prasad et al. 2002). As discussed above, variability in seed crop size and seed size affects the probability of seeds reaching favourable habitats which may ultimately alter community composition and ecosystem functioning. To be clear, plants are expected to face challenges in keeping pace with rapid climate change, and even the most optimistic scenarios assume their dispersal kernels remain the same. However, if climate change also alters seed traits (as it appears to), then this induces an additional barrier or benefit for successful seed dispersal.
Climate change is not the only threat that species have to cope with. Anthropogenic activities, such as fragmentation, hunting of animal seed dispersers and harvesting of plants, can also be strong selective forces. Anthropogenic activities have already been shown to alter plant traits and the amount of variation between individuals in plant populations (Hall et al. 2003; Law and Salick 2005; Galetti et al. 2013) as well as disrupting the co-evolutionary dynamics of plant–frugivore interactions, leading to novel selection pressures on dispersal-related seed traits (Fontúrbel and Medel 2017). For example, human harvesting of larger individuals has resulted in a systematic reduction in plant height of a rare plant species (Saussurea laniceps) (Law and Salick 2005). In addition, habitat loss and hunting have contributed to the functional loss of large seed dispersers, resulting in a reduction in seed size in a tropical palm population (Euterpe edulis) (Galetti et al. 2013). This anthropogenically induced reduction of intraspecific variation is often the opposite direction from that which would be driven by natural selection (Carlson et al. 2007). The loss of individual variation may reduce fecundity and recruitment and have consequences under ongoing climate change (Law and Salick 2005; Galetti et al. 2013). Moreover, the reduction of seed size in some plant species may cause increased vulnerability as smaller seeds are more sensitive to desiccation (Galetti et al. 2013; Wyse and Dickie 2017), which will be especially relevant during extended and intensified periods of drought under future climate change. Finally, individual variation in dispersal distance may affect the ability of populations to adapt to these anthropogenic changes, by altering the spatial scale of gene flow. Although gene flow can provide a source of adaptive genetic variation, it may also homogenize populations and prevent local adaptation (reviewed in Tigano and Friesen 2016). The ability to adapt to novel climate conditions or biotic interactions will be critical to the persistence of many populations under rapid environmental change (Gonzalez et al. 2013).
The eco-evolutionary dynamics of dispersal will play a key role for determining species responses to habitat fragmentation, biological invasions and range shifts in response to climate change (Travis et al. 2013; Urban et al. 2016). As noted in previous sections, dispersal-related traits may rapidly evolve during population spread through favourable habitat. However, these evolutionary changes may affect how populations subsequently respond to stressful environments such as those expected during range shifts under climate change. In experimental invasions of A. thaliana, an evolutionary increase in seed size over six generations of spread was associated with a subsequent reduction in population performance under drought stress (Lustenhouwer et al. 2019). Thus, intraspecific variation in dispersal and seed traits and their evolution will influence the ability of plants to respond to anthropogenic and global climate change.
Recommendations for Best Practices and New Approaches for Studying Individual Variation and Its Implications through Combining Empirical and Modelling Studies
As demonstrated throughout this manuscript, ignoring intraspecific variation in dispersal can have important consequences for our understanding of population and community dynamics, spatial spread and evolution, and is especially relevant under future global changes. Below we outline some recommendations that are intended to advance this field of research.
First, a paradigm shift is necessary regarding the way we think about dispersal. The default, a priori assumption should be that intraspecific variation in dispersal exists and is biologically relevant. With this in mind, reporting of mean dispersal distance plus some measure of variance (i.e. standard deviation, variance, range) should become standard practice in order to begin quantifying uncertainty due to intraspecific variation. Statistical approaches, such as hierarchical Bayes models, can allow researchers to quantify intraspecific variability arising from different sources (Albert et al. 2011; Nuñez et al. 2019). Due to the disproportionate amount of influence that rare events can cause, explicitly noting the presence of outliers is also important. As the information on fruit and seed traits accessible from publicly available databases continues to grow (TRY Plant Trait Database (Kattge et al. 2011); KEW Seed Information Database (http://data.kew.org/sid/); LEDA (http://www.uni-oldenburg.de/en/biology/landeco/research/projects/leda); FRUBASE (https://doi.org/10.5061/dryad.9tb73)), we recommend researchers make dispersal distance data available along with information on traits, dispersers and environmental context (e.g. (e.g. Tamme et al. 2014; Sullivan et al. 2018). One of the aims of the CoDisperse Network is to create such a centralized dispersal database along with developing standardized protocols to ensure the necessary data are available for simultaneously parameterizing and developing models, and testing model predictions (Beckman et al., 2019).
Second, models that describe the responses of populations, communities or ecosystems need to explicitly account for this variation. This is not a simple task and will require the diverse perspectives of mathematical, computational and statistical ecologists to develop a variety of approaches. We first need to understand how the number of seeds dispersed and the resulting spatial patterns change as a function of parental phenotype, seed phenotype and environmental context (as well as how context changes over space and time). One can take a bottom-up, mechanistic approach for modelling seed dispersal (e.g. Nathan et al. 2011; Côrtes and Uriarte 2012), or one can use a top-down, phenomenological approach by directly fitting dispersal kernels to field data (e.g. Lustenhouwer et al. 2017) as applied to interspecific variation in dispersal. One suggestion would be to sample across the variable space for factors that are known to affect dispersal, ensuring sufficient sampling to estimate a kernel at each combination of predictor values (Box 5).
Box 5.
Developing dispersal kernels that include intraspecific variation.
Averaging over either space or time essentially destroys all rare or location-dependent events that cause long-distance dispersal or location-specific effects (e.g. wind gusts, sharp hill inclines, location-dependent top wind speeds or wind directions, etc.), often resulting in severe underestimation of dispersal distances and significant smoothing of the total distribution. Instead, we can integrate (average) over phenotype heterogeneity instead of space or time. One possibility is to form a composite seed shadow by segmenting space (and if necessary, time) into a raster grid, mapping any conditions that change over space or time (e.g. variable wind fields, parent height), calculating dispersal from each pixel to the other pixels conditional on local conditions and then summing arrivals for each pixel. In doing so, care must be taken to integrate over both the source and destination pixels, as well as any traits or conditions that do not vary over space and time. One must also be aware that the creation of these pixels from the underlying discrete data set is a process of averaging itself. If the pixel grid is overly coarse, this averaging will have all of the same consequences mentioned above. Provided that the fates of individual seeds are conditionally independent (i.e. the movement of one seed provides no information on the movement of other seeds after accounting for the effects of environmental variables) and identical (any two seeds with the same traits that are subject to the same conditions can be exchanged with each other), boa population-level dispersal kernel can be derived by summing the dispersal kernels associated with each combination of traits and environmental conditions, weighted by their probability of occurrence in the population as a whole. There is one important caveat to this approach: some of the phenotypic traits that influence dispersal also influence germination, seedling survival and growth (e.g. seed size; Skarpaas et al. 2011). If the goal is to model population or community processes or trait evolution, we cannot average over traits because we will then lose track of them and their downstream effects.
Both approaches have their strengths and limitations. The phenomenological approach could be a valuable tool to compare the magnitude of variation in dispersal within sets of individuals in various environments. On the other hand, predictions based on top-down statistical approaches are liable to fail when confronted with novel conditions that fall outside the domain of the original data, such as those expected from global change. Mechanistic models would be better suited for simulating population to ecosystem responses under specific future conditions or scenarios that would be difficult to sample. The availability of appropriate data present challenges for both types of approaches, and can limit our ability to choose which variables or processes are important to incorporate during model development (Urban et al. 2016; Lustenhouwer et al. 2017; Beckman et al., 2019). Other relevant, interdisciplinary approaches are discussed by Rogers et al. (this issue) in the context of describing total dispersal kernels and Johnson et al. (this issue) in the context of rapid changes in dispersal.
These updated dispersal kernels then can be utilized in a variety of population and community models to explore the consequences of intraspecific variation of dispersal for plant populations and communities. For an overview of how these dispersal kernels can be integrated with population models, see Beckman et al. (2019) and Jongejans et al. (2008). While the community-level consequences of intraspecific variation in dispersal may be difficult to study in the field, simulation models that integrate empirical studies, evolutionary perspectives and theory can provide a predictive understanding of plant communities and ecosystems in response to variability in dispersal. For example, dynamic vegetation models (DVMs) range from individual-based models that simulate community dynamics using species-specific parameters for establishment, growth, competition and mortality to cohort-based models that simulate biogeochemical cycles and vegetation distributions using plant functional types (Snell et al. 2014). Dynamic vegetation models incorporate information on plant demography, physiology, and simulate interspecific competition for light, water and nutrients (e.g. SORTIE (Pacala et al. 1996), FORMIND (Köhler and Huth 1998), TreeMig (Lischke and Löffler 2006; Meier et al. 2012)), but require mathematical, computational and empirical advances to represent the spatial and temporal scales relevant for seed dispersal (Snell et al. 2014). Although DVMs have not yet been used to simulate intraspecific variability in seed dispersal, this would be a promising research approach to explore. More specifically, significant advancement could be made by performing targeted empirical experiments to determine how various global change factors will alter distributions of dispersal traits, and then incorporate these validated distributions into the above-mentioned individual-based models.
Although we have presented these recommendations separately, we envision an iterative cycle of model building and data collection. Quantitative and empirical ecologists would collaborate to identify the important drivers of individual variation in different dispersal syndromes, characterize their distributions and validate model predictions. Ideally, in the long-term as capabilities increase, studies should characterize not only the marginal variation in drivers, but how those variables change with regard to underlying environmental drivers, genetics, ontology and phenology, and should also characterize covariation in relevant seed or parental traits. There are many exciting research directions that can be pursued with the use of field (e.g. observational, experimental) studies, modelling studies (e.g. statistical, computational, mathematical) and their interface.
Conclusions
We demonstrate that individual variation in seed dispersal is important to consider for responses of populations and communities, especially under global change scenarios. Future studies on intraspecific variation in dispersal are recommended to further elucidate how the dynamics of populations, communities and evolution are affected. More specifically, we suggest (i) measuring and reporting variability in seed dispersal to quantify variance, (ii) incorporating variability in dispersal into models to simulate its effect and (iii) using the results of the models to design experiments to test the predictions about the role of intraspecific variability in seed dispersal.
Supplementary Material
Acknowledgements
We thank all participants of the 2016 Seed Dispersal Workshop for helpful discussions on initial ideas, especially Joy Zhou and Michael Neubert. We thank the staff of the National Socio‐Environmental Synthesis Center for logistical support for the workshop, and two anonymous reviewers for their helpful comments.
Sources of Funding
Ideas for this manuscript initiated during the Seed Dispersal Workshop supported by US National Science Foundation Grant DEB-1548194 to N.G.B. and the National Socio‐Environmental Synthesis Center under the US National Science Foundation Grant DBI‐1052875.
Contributions by the Authors
R.S.S. and N.G.B. led the conceptual development of the manuscript and contributed equally to writing and revising the manuscript. N.G.B., R.S.S., E.F., B.A.L., and E.S. initially developed these ideas during the Seed Dispersal Workshop and a subsequent organized oral session held at the Annual Meeting of the Ecological Society of America in August 2017. N.G.B., C.C., E.F., L.R.J., B.A.L., N.I.L., N.L., E.S., C.S., R.S.S., L.L.S., and S.S. led the writing of different sections. All authors contributed to the development of ideas, writing, and editing the manuscript.
Conflict of Interest
None declared.
Supporting Information
The following additional information is available in the online version of this article—
Appendix S1. Leptokurtic kernels with different amounts of variation.
Appendix S2. Toucan data used for Box 1.
Appendix S3. Simulation data used for Box 2.
Appendix S4. R code for simulating demographic data shown in Box 3.
Literature Cited
- Aicher RJ, Larios L, Suding KN. 2011. Seed supply, recruitment, and assembly: quantifying relative seed and establishment limitation in a plant community context. The American Naturalist 178:464–477. [DOI] [PubMed] [Google Scholar]
- Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. 2011. When and how should intraspecific variability be considered in trait-based plant ecology? Perspectives in Plant Ecology Evolution and Systematics 13:217–225. [Google Scholar]
- Augspurger CK, Franson SE, Cushman KC. 2017. Wind dispersal is predicted by tree, not diaspore, traits in comparisons of Neotropical species. Functional Ecology 31:808–820. [Google Scholar]
- Barrett SCH, Kohn JR. 1991. Genetic and evolutionary consequences of small population size. In: Falk DA, Holsinger KE, eds. Genetics and conservation of rare plants. New York: Oxford University Press, 3–30. [Google Scholar]
- Bartle K, Moles AT, Bonser SP. 2013. No evidence for rapid evolution of seed dispersal ability in range edge populations of the invasive species Senecio madagascariensis. Austral Ecology 38:915–920. [Google Scholar]
- Bateman BL, Murphy HT, Reside AE, Mokany K, VanDerWal J. 2013. Appropriateness of full-, partial- and no-dispersal scenarios in climate change impact modelling. Diversity and Distributions 19:1224–1234. [Google Scholar]
- Beckman NG, Rogers HS. 2013. Consequences of seed dispersal for plant recruitment in tropical forests: interactions within the seedscape. Biotropica 45:666–681. [Google Scholar]
- Beckman N, Aslan C, Rogers H, Kogan O, Bronstein J, Bullock J, Hartig F, HilleRisLambers J, Zhou Y, Zurell D, Brodie J, Bruna E, Cantrell S, Decker R, Effiom E, Fricke E, Gurski K, Hastings A, Johnson J, Loiselle B, Miriti M, Neubert M, Pejchar L, Poulsen J, Pufal G, Razafindratsima O, Sandor M, Shea K, Schreiber S, Schupp E, Snell R, Strickland C & Zambrano J. 2019. Advancing an interdisciplinary framework to study seed dispersal ecology. AoB Plants in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley HA, Kendall BE, Mitarai S, Siegel DA. 2010. Turbulent dispersal promotes species coexistence. Ecology Letters 13:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendinger PG, Loiselle BA, Blake JG. 2008. Crop size, plant aggregation, and microhabitat type affect fruit removal by birds from individual melastome plants in the Upper Amazon. Oecologia 158:273–283. [DOI] [PubMed] [Google Scholar]
- Bonte D, Dahirel M. 2017. Dispersal: a central and independent trait in life history. Oikos 126:472–479. [Google Scholar]
- Bouin E, Calvez V, Meunier N, Mirrahimi S, Perthame B, Raoul G, Voituriez R. 2012. Invasion fronts with variable motility: phenotype selection, spatial sorting and wave acceleration. Comptes Rendus Mathematique 350:761–766. [Google Scholar]
- Brodie JF, Helmy OE, Brockelman WY, Maron JL. 2009. Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal-dispersed tree. Ecological Applications 19:854–863. [DOI] [PubMed] [Google Scholar]
- Carlo TA, Morales JM. 2008. Inequalities in fruit-removal and seed dispersal: consequences of bird behaviour, neighbourhood density and landscape aggregation. Journal of Ecology 96:609–618. [Google Scholar]
- Carlson SM, Edeline E, Asbjørn Vøllestad L, Haugen TO, Winfield IJ, Fletcher JM, Ben James J, Stenseth NC. 2007. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius). Ecology Letters 10:512–521. [DOI] [PubMed] [Google Scholar]
- Castorani MCN, Reed DC, Raimondi PT, Alberto F, Bell TW, Cavanaugh KC, Siegel DA, Simons RD. 2017. Fluctuations in population fecundity drive variation in demographic connectivity and metapopulation dynamics. Proceedings of the Royal Society B-Biological Sciences 284:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughlin TT, Ferguson JM, Lichstein JW, Bunyavejchewin S, Levey DJ. 2014. The importance of long-distance seed dispersal for the demography and distribution of a canopy tree species. Ecology 95:952–962. [DOI] [PubMed] [Google Scholar]
- Cheplick GP. 1992. Sibling competition in plants. Journal of Ecology 80:567–575. [Google Scholar]
- Cheptou PO, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proceedings of the National Academy of Sciences of the United States of America 105:3796–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson P. 1996. Matters of scale in the dynamics of populations and communities. In: Floyd RB, Sheppard AW, de Barro PJ, eds. Frontiers of population ecology. Collingwood, VIC, Australia: CSIRO, 353–368.. [Google Scholar]
- Clark JS. 2010. Individuals and the variation needed for high species diversity in forest trees. Science 327:1129–1132. [DOI] [PubMed] [Google Scholar]
- Clark JS, Fastie C, Hurtt G, Jackson ST, Johnson C, King GA, Lewis M, Lynch J, Pacala S, Prentice C, Schupp EW, Webb T, Wyckoff P. 1998. Reid’s paradox of rapid plant migration - dispersal theory and interpretation of paleoecological records. Bioscience 48:13–24. [Google Scholar]
- Cochrane A, Yates CJ, Hoyle GL, Nicotra AB. 2015. Will among-population variation in seed traits improve the chance of species persistence under climate change? Global Ecology and Biogeography 24:12–24. [Google Scholar]
- Côrtes MC, Uriarte M. 2012. Integrating frugivore behavior and animal movement: a review of the evidence and implication for scaling seed dispersal. Biological Reviews of the Cambridge Philosophical Society 88:255–272. [DOI] [PubMed] [Google Scholar]
- Cunze S, Heydel F, Tackenberg O. 2013. Are plant species able to keep pace with the rapidly changing climate? PLoS One 8:e67909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. 2011. Beyond predictions: biodiversity conservation in a changing climate. Science 332:53–58. [DOI] [PubMed] [Google Scholar]
- Delefosse M, Povidisa K, Poncet D, Kristensen E, Olesen B. 2016. Variation in size and chemical composition of seeds from the seagrass Zostera marina-ecological implications. Aquatic Botany 131:7–14. [Google Scholar]
- Donohue K, Polisetty CR, Wender NJ. 2005. Genetic basis and consequences of niche construction: plasticity-induced genetic constraints on the evolution of seed dispersal in Arabidopsis thaliana. The American Naturalist 165:537–550. [DOI] [PubMed] [Google Scholar]
- Ellner SP, Schreiber SJ. 2012. Temporally variable dispersal and demography can accelerate the spread of invading species. Theoretical Population Biology 82:283–298. [DOI] [PubMed] [Google Scholar]
- Engler R, Randin CF, Thuiller W, Dullinger S, Zimmermann NE, Araújo MB, Pearman PB, Le Lay G, Piedallu C, Albert CH, Choler P, Coldea G, De Lamo X, Dirnböck T, Gégout J-C, Gómez-García D, Grytnes J-A, Heegaard E, HøIstad F, Nogués-Bravo D, Normand S, Puşcaş M, Sebastiá M-T, Stanisci A, Theurillat J-P, Trivedi MR, Vittoz P, Guisan A. 2011. 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology 17:2330–2341. [Google Scholar]
- Flörchinger M, Braun J, Böhning-Gaese K, Schaefer HM. 2010. Fruit size, crop mass, and plant height explain differential fruit choice of primates and birds. Oecologia 164:151–161. [DOI] [PubMed] [Google Scholar]
- Fontúrbel FE, Medel R. 2017. Frugivore-mediated selection in a habitat transformation scenario. Scientific Reports 7:45371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami T, Bezemer TM, Mortimer SR, van der Putten WH. 2005. Species divergence and trait convergence in experimental plant community assembly. Ecology Letters 8:1283–1290. [Google Scholar]
- Galetti M, Guevara R, Côrtes MC, Fadini R, Von Matter S, Leite AB, Labecca F, Ribeiro T, Carvalho CS, Collevatti RG, Pires MM, Guimarães PR, Brancalion PH, Ribeiro MC, Jordano P. 2013. Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340:1086–1090. [DOI] [PubMed] [Google Scholar]
- Germain RM, Strauss SY, Gilbert B. 2017. Experimental dispersal reveals characteristic scales of biodiversity in a natural landscape. Proceedings of the National Academy of Sciences of the United States of America 114:4447–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK. 2012. Long-distance dispersal: a framework for hypothesis testing. Trends in Ecology & Evolution 27:47–56. [DOI] [PubMed] [Google Scholar]
- Godinez-Alvarez H, Jordano P. 2007. An empirical approach to analysing the demographic consequences of seed dispersal by frugivores. In: Dennis A, Schupp E, Green R, Westcott DA, eds. Seed dispersal: theory and its application in a changing world. Wallingford, UK: CAB International, 391–406. [Google Scholar]
- Gómez JM, Puerta-Piñero C, Schupp EW. 2008. Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 155:529–537. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Ronce O, Ferriere R, Hochberg ME. 2013. Evolutionary rescue: an emerging focus at the intersection between ecology and evolution. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368:20120404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Varo JP, Traveset A. 2016. The labile limits of forbidden interactions. Trends in Ecology & Evolution 31:700–710. [DOI] [PubMed] [Google Scholar]
- Gorb E, Gorb S. 2002. Contact separation force of the fruit burrs in four plant species adapted to dispersal by mechanical interlocking. Plant Physiology and Biochemistry 40:373–381. [Google Scholar]
- Guja LK, Merritt DJ, Dixon KW, Wardell-Johnson G. 2014. Dispersal potential of Scaevola crassifolia (Goodeniaceae) is influenced by intraspecific variation in fruit morphology along a latitudinal environmental gradient. Australian Journal of Botany 62:56–64. [Google Scholar]
- Hall JS, Harris DJ, Medjibe V, Ashton PMS. 2003. The effects of selective logging on forest structure and tree species composition in a Central African forest: implications for management of conservation areas. Forest Ecology and Management 183:249–264. [Google Scholar]
- Hallfors MH, Aikio S, Schulman LE. 2017. Quantifying the need and potential of assisted migration. Biological Conservation 205:34–41. [Google Scholar]
- Hamrick JL, Godt MJW. 1996. Effects of life history traits on genetic diversity in plant species. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 351:1291–1298. [Google Scholar]
- Hart SP, Schreiber SJ, Levine JM. 2016. How variation between individuals affects species coexistence. Ecology Letters 19:825–838. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 2017. The ecology of subindividual variability in plants: patterns, processes, and prospects. Web Ecology 17:51–64. [Google Scholar]
- Hof C, Levinsky I, Araujo MB, Rahbek C. 2011. Rethinking species’ ability to cope with rapid climate change. Global Change Biology 17:2987–2990. [Google Scholar]
- Holbrook KM, Loiselle BA. 2007. Using toucan-generated seed shadows to estimate seed dispersal in Amazonia Ecuador. In: Dennis A, Green R, Schupp EW, Westcott DA, eds. Seed dispersal: theory and its application in a changing world. Wallingford, UK: CABI, 300–321. [Google Scholar]
- Holbrook KM, Loiselle BA. 2009. Dispersal in a Neotropical tree, Virola flexuosa (Myristicaceae): does hunting of large vertebrates limit seed removal? Ecology 90:1449–1455. [DOI] [PubMed] [Google Scholar]
- Holyoak M, Leibold MA, Holt RD. 2005. Metacommunities: spatial dynamics and ecological communities. Chicago and London: University of Chicago Press. [Google Scholar]
- Horvitz CC, Koop AL, Erickson KD. 2015. Time-invariant and stochastic disperser-structured matrix models: invasion rates of fleshy-fruited exotic shrubs. Discrete and Continuous Dynamical Systems-Series B 20:1639–1662. [Google Scholar]
- Howe HF, Miriti MN. 2004. When seed dispersal matters. Bioscience 54:651–660. [Google Scholar]
- Huang FF, Peng SL, Chen BM, Liao HX, Huang QQ, Lin ZG, Liu G. 2015. Rapid evolution of dispersal-related traits during range expansion of an invasive vine Mikania micrantha. Oikos 124:1023–1030. [Google Scholar]
- Hughes L, Westoby M. 1992. Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73:1300–1312. [Google Scholar]
- Imbert E. 2002. Ecological consequences and ontogeny of seed heteromorphism. Perspectives in Plant Ecology Evolution and Systematics 5:13–36. [Google Scholar]
- IPCC 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Pachauri RK, Meyer LA, eds. Geneva, Switzerland: IPCC. [Google Scholar]
- Jansen PA, Bongers F, Hemerik L. 2004. Seed mass and mast seeding enhance dispersal by a neotropical scatter-hoarding rodent. Ecological Monographs 74:569–589. [Google Scholar]
- Janzen DH. 1970. Herbivores and the number of tree species in tropical forests. American Naturalist 104:501–528. [Google Scholar]
- Jensen JLWV. 1906. Sur les fonctions convexes et les inégalités entre les valeurs moyennes. Acta Math 30:175–193. [Google Scholar]
- Johnson JS, Cantrell RS, Cosner C, Hartig F, Hastings A, Rogers HS, Schupp EW, Shea K, Teller BJ, Yu X, Zurell D, Pufal G. 2019. Rapid changes in seed dispersal traits may modify plant responses to global change. AoB Plants . doi:10.1093/aobpla/plz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LR. 2017. Modeling the effects of animal movements and behavior on spatial patterns of seed dispersal in fragmented landscapes. PhD Dissertation, University of Louisiana at Lafayette, Lafayette, LA. [Google Scholar]
- Jongejans E, Skarpaas O, Shea K. 2008. Dispersal, demography and spatial population models for conservation and control management. Perspectives in Plant Ecology Evolution and Systematics 9:153–170. [Google Scholar]
- Jordano P. 2017. What is long-distance dispersal? And a taxonomy of dispersal events. Journal of Ecology 105:75–84. [Google Scholar]
- Jordano P, Schupp EW. 2000. Seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb. Ecological Monographs 70:591–615. [Google Scholar]
- Kattge J, Diaz S, Lavorel S, Prentice C, Leadley P, Bonisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, Violle C, Harrison SP, van Bodegom PM, Reichstein M, Enquist BJ, Soudzilovskaia NA, Ackerly DD, Anand M, Atkin O, Bahn M, Baker TR, Baldocchi D, Bekker R, Blanco CC, Blonder B, Bond WJ, Bradstock R, Bunker DE, Casanoves F, Cavender-Bares J, Chambers JQ, Chapin FS, Chave J, Coomes D, Cornwell WK, Craine JM, Dobrin BH, Duarte L, Durka W, Elser J, Esser G, Estiarte M, Fagan WF, Fang J, Fernandez-Mendez F, Fidelis A, Finegan B, Flores O, Ford H, Frank D, Freschet GT, Fyllas NM, Gallagher RV, Green WA, Gutierrez AG, Hickler T, Higgins SI, Hodgson JG, Jalili A, Jansen S, Joly CA, Kerkhoff AJ, Kirkup D, Kitajima K, Kleyer M, Klotz S, Knops JMH, Kramer K, Kuhn I, Kurokawa H, Laughlin D, Lee TD, Leishman M, Lens F, Lenz T, Lewis SL, Lloyd J, Llusia J, Louault F, Ma S, Mahecha MD, Manning P, Massad T, Medlyn BE, Messier J, Moles AT, Muller SC, Nadrowski K, Naeem S, Niinemets U, Nollert S, Nuske A, Ogaya R, Oleksyn J, Onipchenko VG, Onoda Y, Ordonez J, Overbeck G, Ozinga WA, Patino S, Paula S, Pausas JG, Penuelas J, Phillips OL, Pillar V, Poorter H, Poorter L, Poschlod P, Prinzing A, Proulx R, Rammig A, Reinsch S, Reu B, Sack L, Salgado-Negre B, Sardans J, Shiodera S, Shipley B, Siefert A, Sosinski E, Soussana JF, Swaine E, Swenson N, Thompson K, Thornton P, Waldram M, Weiher E, White M, White S, Wright SJ, Yguel B, Zaehle S, Zanne AE, Wirth C. 2011. TRY - a global database of plant traits. Global Change Biology 17:2905–2935. [Google Scholar]
- Köhler P, Huth A. 1998. The effects of tree species grouping in tropical rainforest modelling: simulations with the individual-based model FORMIND. Ecological Modelling 109:301–321. [Google Scholar]
- Kot M, Lewis MA, van den Driessche P. 1996. Dispersal data and the spread of invading organisms. Ecology 77:2027–2042. [Google Scholar]
- Kroiss SJ, Hillerslambers J. 2015. Recruitment limitation of long-lived conifers: implications for climate change responses. Ecology 96:1286–1297. [DOI] [PubMed] [Google Scholar]
- Law W, Salick J. 2005. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae). Proceedings of the National Academy of Sciences of the United States of America 102:10218–10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A. 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7:601–613. [Google Scholar]
- Levey DJ. 1987. Seed size and fruit-handling techniques of avian frugivores. American Naturalist 129:471–485. [Google Scholar]
- Levey DJ, Bolker BM, Tewksbury JJ, Sargent S, Haddad NM. 2005. Effects of landscape corridors on seed dispersal by birds. Science 309:146–148. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Pacala S. 2000. Modeling and analysis of stochastic invasion processes. Journal of Mathematical Biology 41:387–429. [DOI] [PubMed] [Google Scholar]
- Lichti NI, Steele MA, Swihart RK. 2017. Seed fate and decision-making processes in scatter-hoarding rodents. Biological Reviews of the Cambridge Philosophical Society 92:474–504. [DOI] [PubMed] [Google Scholar]
- Lischke H, Löffler T. 2006. Intra-specific density dependence is required to maintain diversity in spatio-temporal forest simulations with reproduction. Ecological Modelling 198:341–361. [Google Scholar]
- Liu HY, Mi ZR, Lin L, Wang YH, Zhang ZH, Zhang FW, Wang H, Liu LL, Zhu BA, Cao GM, Zhao XQ, Sanders NJ, Classen AT, Reich PB, He JS. 2018. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proceedings of the National Academy of Sciences of the United States of America 115:4051–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret F, Penuelas J, Prieto P, Llorens L, Estiarte M. 2009. Plant community changes induced by experimental climate change: seedling and adult species composition. Perspectives in Plant Ecology Evolution and Systematics 11:53–63. [Google Scholar]
- Loayza AP, Knight T. 2010. Seed dispersal by pulp consumers, not “legitimate” seed dispersers, increases Guettarda viburnoides population growth. Ecology 91:2684–2695. [DOI] [PubMed] [Google Scholar]
- Lockwood JL, Hoopes MF, Marchetti MP. 2013. Invasion ecology. Oxford, UK: Wiley-Blackwell Publishing. [Google Scholar]
- Lustenhouwer N, Moran EV, Levine JM. 2017. Trait correlations equalize spread velocity across plant life histories. Global Ecology and Biogeography 26:1398–1407. [Google Scholar]
- Lustenhouwer N, Williams JL, Levine JM. 2019. Evolution during population spread affects plant performance in stressful environments. Journal of Ecology 107:396–406. [Google Scholar]
- Marchetto KM, Shea K, Kelly D, Groenteman R, Sezen Z, Jongejans E. 2014. Unrecognized impact of a biocontrol agent on the spread rate of an invasive thistle. Ecological Applications 24:1178–1187. [DOI] [PubMed] [Google Scholar]
- Mark S, Olesen JM. 1996. Importance of elaiosome size to removal of ant-dispersed seeds. Oecologia 107:95–101. [DOI] [PubMed] [Google Scholar]
- Martin LM, Wilsey BJ. 2012. Assembly history alters alpha and beta diversity, exotic-native proportions and functioning of restored prairie plant communities. Journal of Applied Ecology 49:1436–1445. [Google Scholar]
- Mathias A, Kisdi E, Olivieri I. 2001. Divergent evolution of dispersal in a heterogeneous landscape. Evolution 55:246–259. [DOI] [PubMed] [Google Scholar]
- McConkey KR, Drake DR. 2006. Flying foxes cease to function as seed dispersers long before they become rare. Ecology 87:271–276. [DOI] [PubMed] [Google Scholar]
- McConkey KR, Prasad S, Corlett RT, Campos-Arceiz A, Brodie JF, Rogers H, Santamaria L. 2012. Seed dispersal in changing landscapes. Biological Conservation 146:1–13. [Google Scholar]
- Meier ES, Lischke H, Schmatz DR, Zimmermann NE. 2012. Climate, competition and connectivity affect future migration and ranges of European trees. Global Ecology and Biogeography 21:164–178. [Google Scholar]
- Merritt DM, Nilsson C, Jansson R. 2010. Consequences of propagule dispersal and river fragmentation for riparian plant community diversity and turnover. Ecological Monographs 80:609–626. [Google Scholar]
- Michaels HJ, Benner B, Hartgerink AP, Lee TD, Rice S, Willson MF, Bertin RI. 1988. Seed size variation: magnitude, distribution, and ecological correlates. Evolutionary Ecology 2:157–166. [Google Scholar]
- Miller KM, McGill BJ. 2018. Land use and life history limit migration capacity of eastern tree species. Global Ecology and Biogeography 27:57–67. [Google Scholar]
- Minor DM, Kobe RK. 2017. Masting synchrony in northern hardwood forests: super-producers govern population fruit production. Journal of Ecology 105:987–998. [Google Scholar]
- Mollison D. 1977. Spatial contact models for ecological and epidemic spread. Journal of the Royal Statistical Society Series B-Methodological 39:283–326. [Google Scholar]
- Monty A, Maebe L, Mahy G, Brown CS. 2016. Diaspore heteromorphism in the invasive Bromus tectorum L. (Poaceae): sterile florets increase dispersal propensity and distance. Flora 224:7–13. [Google Scholar]
- Morin X, Fahse L, Jactel H, Scherer-Lorenzen M, García-Valdés R, Bugmann H. 2018. Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Scientific Reports 8:5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz A, Bonal R. 2008. Are you strong enough to carry that seed? Seed size/body size ratios influence seed choices by rodents. Animal Behaviour 76:709–715. [Google Scholar]
- Nathan R, Katul GG, Bohrer G, Kuparinen A, Soons MB, Thompson SE, Trakhtenbrot A, Horn HS. 2011. Mechanistic models of seed dispersal by wind. Theoretical Ecology 4:113–132. [Google Scholar]
- Nathan R, Katul GG, Horn HS, Thomas SM, Oren R, Avissar R, Pacala SW, Levin SA. 2002. Mechanisms of long-distance dispersal of seeds by wind. Nature 418:409–413. [DOI] [PubMed] [Google Scholar]
- Nathan R, Safriel UN, Noy-Meir I. 2001. Field validation and sensitivity analysis of a mechanistic model for tree seed dispersal by wind. Ecology 82:374–388. [Google Scholar]
- Neubert MG, Caswell H. 2000. Demography and dispersal: calculation and sensitivity analysis of invasion speed for structured populations. Ecology 81:1613–1628. [Google Scholar]
- Norghauer JM, Nock CA, Grogan J. 2011. The importance of tree size and fecundity for wind dispersal of big-leaf mahogany. PLoS One 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez CL, Clark JS, Clark CJ, Poulsen JR. 2019. Low-intensity logging and hunting have long-term effects on seed dispersal but not fecundity in Afrotropical forests. AoB Plants 11:ply074; doi: 10.1093/aobpla/ply074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacala SW, Canham CD, Saponara J, Silander JA, Kobe RK, Ribbens E. 1996. Forest models defined by field measurements: estimation, error analysis and dynamics. Ecological Monographs 66:1–43. [Google Scholar]
- Perkins TA, Phillips BL, Baskett ML, Hastings A. 2013. Evolution of dispersal and life history interact to drive accelerating spread of an invasive species. Ecology Letters 16:1079–1087. [DOI] [PubMed] [Google Scholar]
- Petrovskii S, Morozov A. 2009. Dispersal in a statistically structured population: fat tails revisited. The American Naturalist 173:278–289. [DOI] [PubMed] [Google Scholar]
- Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91:1617–1627. [DOI] [PubMed] [Google Scholar]
- Poulsen JR, Clark CJ, Connor EF, Smith TB. 2002. Differential resource use by primates and hornbills: implications for seed dispersal. Ecology 83:228–240. [Google Scholar]
- Prasad PVV, Boote KJ, Allen LH, Thomas JMG. 2002. Effects of elevated temperature and carbon dioxide on seed-set and yield of kidney bean (Phaseolus vulgaris L.). Global Change Biology 8:710–721. [Google Scholar]
- Riba M, Mignot A, Freville H, Colas B, Imbert E, Vile D, Virevaire M, Olivieri I. 2005. Variation in dispersal traits in a narrow-endemic plant species, Centaurea corymbosa Pourret (Asteraceae). Evolutionary Ecology 19:241–254. [Google Scholar]
- Ronce O. 2007. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annual Review of Ecology Evolution and Systematics 38:231–253. [Google Scholar]
- Roze D, Rousset F. 2005. Inbreeding depression and the evolution of dispersal rates: a multilocus model. The American Naturalist 166:708–721. [DOI] [PubMed] [Google Scholar]
- Rogers H, Beckman N, Hartig F, Johnson JS, Pufal G, Shea K, Zurell D, Bullock JM, Cantrell S, Loiselle B, Pejchar L, Razafindratsima O, Sandor M, Schupp EW, Strickland C, and Zambrano J. 2019. The total dispersal kernel: a review and future directions. AoB Plants in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio de Casas R, Willis CG, Donohue K. 2012. Plant dispersal phenotypes: a seed perspective of maternal habitat selection. In: Clobert J, Baguette M, Benton TG, Bullock JM, eds. Dispersal ecology and evolution. Oxford, UK: Oxford University Press, 119–138. [Google Scholar]
- Saastamoinen M, Bocedi G, Cote J, Legrand D, Guillaume F, Wheat CW, Fronhofer EA, Garcia C, Henry R, Husby A, Baguette M, Bonte D, Coulon A, Kokko H, Matthysen E, Niitepõld K, Nonaka E, Stevens VM, Travis JMJ, Donohue K, Bullock JM, Del Mar Delgado M. 2018. Genetics of dispersal. Biological Reviews of the Cambridge Philosophical Society 93:574–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallabanks R. 1993. Hierarchical mechanisms of fruit selection by an avian frugivore. Ecology 74:1326–1336. [Google Scholar]
- Schreiber SJ and Beckman NG. 2019. Individual variation in dispersal and fecundity increases rates of spatial spread. AoB Plants in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp EW, Jordano P, Gómez JM. 2010. Seed dispersal effectiveness revisited: a conceptual review. The New Phytologist 188:333–353. [DOI] [PubMed] [Google Scholar]
- Schupp EW, Zwolak R, Jones L, Snell RS, Beckman N, Aslan C, Cavazos B, Effiom E, Fricke E, Montaño-Centellas F, Poulsen J, Razafindratsima O, Sandor M, Shea K. 2019. Intrinsic and extrinsic drivers of intraspecific variation in seed dispersal are diverse and pervasive. AoB Plants in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigesada N, Kawasaki K. 1997. Biological invasions: theory and practice. Oxford, UK: Oxford University Press. [Google Scholar]
- Shigesada N, Kawasaki K, Teramoto E. 1986. Traveling periodic waves in heterogeneous environments. Theoretical Population Biology 30:143–160. [Google Scholar]
- Shimada T, Takahashi A, Shibata M. 2010. Effects of seed size and chemical variation on seed fates in a deciduous oak species Quercus serrata. Journal of Integrated Field Science 7:15–18. [Google Scholar]
- Shine R, Brown GP, Phillips BL. 2011. An evolutionary process that assembles phenotypes through space rather than through time. Proceedings of the National Academy of Sciences of the United States of America 108:5708–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefert A, Violle C, Chalmandrier L, Albert CH, Taudiere A, Fajardo A, Aarssen LW, Baraloto C, Carlucci MB, Cianciaruso MV, de L Dantas V, de Bello F, Duarte LD, Fonseca CR, Freschet GT, Gaucherand S, Gross N, Hikosaka K, Jackson B, Jung V, Kamiyama C, Katabuchi M, Kembel SW, Kichenin E, Kraft NJ, Lagerström A, Bagousse-Pinguet YL, Li Y, Mason N, Messier J, Nakashizuka T, Overton JM, Peltzer DA, Pérez-Ramos IM, Pillar VD, Prentice HC, Richardson S, Sasaki T, Schamp BS, Schöb C, Shipley B, Sundqvist M, Sykes MT, Vandewalle M, Wardle DA. 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology Letters 18:1406–1419. [DOI] [PubMed] [Google Scholar]
- Skarpaas O, Silverman EJ, Jongejans E, Shea K. 2011. Are the best dispersers the best colonizers? Seed mass, dispersal and establishment in Carduus thistles. Evolutionary Ecology 25:155–169. [Google Scholar]
- Smallwood PD, Steele MA, Faeth SH. 2001. The ultimate basis of the caching preferences of rodents, and the oak-dispersal syndrome: tannins, insects, seed germination. American Zoologist 41:840–851. [Google Scholar]
- Snell RS, Huth A, Nabel J, Bocedi G, Travis JMJ, Gravel D, Bugmann H, Gutiérrez AG, Hickler T, Higgins SI, Reineking B, Scherstjanoi M, Zurbriggen N, Lischke H. 2014. Using dynamic vegetation models to simulate plant range shifts. Ecography 37:1184–1197. [Google Scholar]
- Snyder RE. 2011. Leaving home ain’t easy: non-local seed dispersal is only evolutionarily stable in highly unpredictable environments. Proceedings of the Royal Society B-Biological Sciences 278:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley MC, Lill A. 2002. Does seed packaging influence fruit consumption and seed passage in an avian frugivore? Condor 104:136–145. [Google Scholar]
- Stevens VM, Pavoine S, Baguette M. 2010. Variation within and between closely related species uncovers high intra-specific variability in dispersal. PLoS One 5:e11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover JP, Kendall BE, Nisbet RM. 2014. Consequences of dispersal heterogeneity for population spread and persistence. Bulletin of Mathematical Biology 76:2681–2710. [DOI] [PubMed] [Google Scholar]
- Sullivan LL, Clark AT, Tilman D, Shaw AK. 2018. Mechanistically derived dispersal kernels explain species-level patterns of recruitment and succession. Ecology 99:2415–2420. [DOI] [PubMed] [Google Scholar]
- Tabassum S, Bonser SP. 2017. Allometry in the terminal velocity - dispersal architecture relationship explains variation in dispersal and offspring provisioning strategies in wind dispersed Asteraceae species. Australian Journal of Botany 65:149–156. [Google Scholar]
- Taghizadeh MS, Crawford S, Nicolas ME, Cousens RD. 2009. Water deficit changes the anatomy of the fruit abscission zone in Raphanus raphanistrum (Brassicaceae). Australian Journal of Botany 57:708–714. [Google Scholar]
- Tamme R, Götzenberger L, Zobel M, Bullock JM, Hooftman DA, Kaasik A, Pärtel M. 2014. Predicting species’ maximum dispersal distances from simple plant traits. Ecology 95:505–513. [DOI] [PubMed] [Google Scholar]
- Thiede DA, Augspurger CK. 1996. Intraspecific variation in seed dispersion of Lepidium campestre (Brassicaceae). American Journal of Botany 83:856–866. [Google Scholar]
- Thomson FJ, Letten AD, Tamme R, Edwards W, Moles AT. 2018. Can dispersal investment explain why tall plant species achieve longer dispersal distances than short plant species? The New Phytologist 217:407–415. [DOI] [PubMed] [Google Scholar]
- Thomson FJ, Moles AT, Auld TD, Kingsford RT. 2011. Seed dispersal distance is more strongly correlated with plant height than with seed mass. Journal of Ecology 99:1299–1307. [Google Scholar]
- Tigano A, Friesen VL. 2016. Genomics of local adaptation with gene flow. Molecular Ecology 25:2144–2164. [DOI] [PubMed] [Google Scholar]
- Tilman D. 1997. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 78:81–92. [Google Scholar]
- Traveset A, Robertson A, Rodriguez-Perez J. 2007. A review on the role of endozoochory in seed germination. In: Dennis A, Green R, Schupp EW, Westcott DA, eds. Seed dispersal: theory and its application in a changing world. Wallingford, UK: CAB International. [Google Scholar]
- Travis JMJ, Delgado M, Bocedi G, Baguette M, Bartoń K, Bonte D, Boulangeat I, Hodgson JA, Kubisch A, Penteriani V, Saastamoinen M, Stevens VM, Bullock JM. 2013. Dispersal and species’ responses to climate change. Oikos 122: 1532–1540. [Google Scholar]
- Travis JMJ, Dytham C. 2002. Dispersal evolution during invasions. Evolutionary Ecology Research 4:1119–1129. [Google Scholar]
- Urban MC, Bocedi G, Hendry AP, Mihoub JB, Pe’er G, Singer A, Bridle JR, Crozier LG, De Meester L, Godsoe W, Gonzalez A, Hellmann JJ, Holt RD, Huth A, Johst K, Krug CB, Leadley PW, Palmer SCF, Pantel JH, Schmitz A, Zollner PA, Travis JMJ. 2016. Improving the forecast for biodiversity under climate change. Science 353:1113. [DOI] [PubMed] [Google Scholar]
- Valladares F, Matesanz S, Guilhaumon F, Araújo MB, Balaguer L, Benito-Garzón M, Cornwell W, Gianoli E, van Kleunen M, Naya DE, Nicotra AB, Poorter H, Zavala MA. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecology Letters 17:1351–1364. [DOI] [PubMed] [Google Scholar]
- Van der Stocken T, De Ryck DJR, Vanschoenwinkel B, Deboelpaep E, Bouma TJ, Dahdouh-Guebas F, Koedam N. 2015. Impact of landscape structure on propagule dispersal in mangrove forests. Marine Ecology Progress Series 524:95–106. [Google Scholar]
- Vander Wall SB. 2010. How plants manipulate the scatter-hoarding behaviour of seed-dispersing animals. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 365:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellend M. 2010. Conceptual synthesis in community ecology. The Quarterly Review of Biology 85:183–206. [DOI] [PubMed] [Google Scholar]
- Wang B, Ives AR. 2017. Tree-to-tree variation in seed size and its consequences for seed dispersal versus predation by rodents. Oecologia 183:751–762. [DOI] [PubMed] [Google Scholar]
- Wender NJ, Polisetty CR, Donohue K. 2005. Density-dependent processes influencing the evolutionary dynamics of dispersal: a functional analysis of seed dispersal in Arabidopsis thaliana (Brassicaceae). American Journal of Botany 92:960–971. [DOI] [PubMed] [Google Scholar]
- Wheelwright NT. 1986. A seven-year study of individual variation in fruit production in tropical bird-dispersed tree species in the family Lauraceae. In: Estrada A, Fleming TH, eds. Frugivores and seed dispersal. Tasks for vegetation science. Dordrecht, The Netherlands: Springer, 19–35. [Google Scholar]
- Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Frontiers in Ecology and the Environment 5:475–482. [Google Scholar]
- Williams JL, Kendall BE, Levine JM. 2016. Rapid evolution accelerates plant population spread in fragmented experimental landscapes. Science 353:482–485. [DOI] [PubMed] [Google Scholar]
- Willson MF. 1994. Fruit choices by captive American Robins. Condor 96:494–502. [Google Scholar]
- Wyse SV, Dickie JB. 2017. Predicting the global incidence of seed desiccation sensitivity. Journal of Ecology 105:1082–1093. [Google Scholar]
- Zhu K, Woodall CW, Clark JS. 2012. Failure to migrate: lack of tree range expansion in response to climate change. Global Change Biology 18:1042–1052. [Google Scholar]
- Zwolak R. 2018. How intraspecific variation in seed-dispersing animals matters for plants. Biological Reviews of the Cambridge Philosophical Society 93:897–913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.