Abstract
The valorization of olive oil mill solid wastes (OMW) has been addressed by considering it as a possible source of humic-like substances (HLSs), to be used as auxiliary substances for photo-Fenton, employing caffeine as a target pollutant to test the efficiency of this approach. The OMW-HLS isolation encompassed the OMW basic hydrolysis, followed by ultrafiltration and drying. OMW-HLS structural features have been investigated by means of laser light scattering, fluorescence, size exclusion chromatography, and thermogravimetric analysis; moreover, the capability of OMW-HLS to generate reactive species under irradiation has been investigated using spin-trap electronic paramagnetic resonance. The caffeine degradation by means of photo-Fenton process driven at pH = 5 was significantly increased by the addition of 10 mg/L of OMW-HLS. Under the mechanistic point of view, it could be hypothesized that singlet oxygen is not playing a relevant role, whereas other oxidants (mainly OH• radicals) can be considered as the key species in promoting caffeine degradation.
Introduction
Food wastes has become a serious environmental concern in developed countries. For instance, according to the FUSION project,1 it was estimated that 88 million tonnes of food are wasted annually in the EU, with associated costs estimated at 143 billion euros, mainly because of households (53%) and production and processing (30%). Hence, valorization of these residues is an important goal to be accomplished, in line with European polices leading to a circular economy.2 As a consequence, important efforts are being devoted to isolate valuable chemicals from these wastes, to be employed in different industrial processes or as a source of energy, as recently reviewed.3
In this context, olive oil mill wastes is a serious concern in the Mediterranean Basin countries, where olive oil production is among the most important activities related to agriculture and food processing. Commonly, in addition to oil, two different residues are generated in the process: (a) the olive oil mill wastewater, containing the water added to the process, the vegetation water of the olives and water-soluble products (e.g., phenols and polyphenols) and (b) a solid waste (pomace or olive cake) that contains the solid parts of the olive fruit.4 In order to decrease the generation of the highly polluting olive mill wastewaters, the production process has been modified in some countries, among them Spain, into a two-phase process that only generates olive oil and a wet solid residue, called olive wet husk or wet pomace.5 In both cases, the high amount of residues that are produced is an inconvenience. However, the oil mill wastes could be considered as a potential source of valuable substances such as phenols and polyphenols, dietary fibers, animal feed, biofuel, biogas, or enzymes.4,6,7 Hence, strategies for their reuse are a hot research topic in the Mediterranean countries.8−11
Humic-like substances (HLSs) are among the products that might be isolated from olive oil mill wastes. HLSs are macromolecules that share important characteristics with humic substances (HSs), which are one of the major constituents of natural organic matter, being its origin decomposition of vegetable or animal.12 Recently, soluble bioorganic substances (SBO) exhibiting humic-like properties were isolated from urban wastes13 and employed in different fields such as agriculture,14 textile industry,15 or pollution remediation.16
Among the environmental applications of SBO it can be found the wastewater treatment. Some research has been performed about the use of these substances as photoactivators because of the ability of HS and HLS to generate highly reactive species under irradiation. Some interesting results have been achieved, although high amounts of these substances are needed (on the order of g/L).17 Another approach was to employ SBO for the synthesis of hybrid materials, which can be employed as absorbents and/or heterogeneous photocatalysts.18 Finally, the behavior of SBO as iron complexing agents has been exploited to drive the photo-Fenton process at mild pH conditions;19 in this context, HLSs extracted from the compost have been recently used in the Fenton process for soil remediation.20
Regarding these last uses, photo-Fenton is a very efficient photochemical process for wastewater treatment that employs hydrogen peroxide as an oxidizing agent, which is catalytically decomposed by iron salts into more reactive species, such as hydroxyl radical21 or superoxidized iron species (e.g., FeO2+, Fe5+, and Fe6+).22 The relative role of OH• versus FeO2+ is still debated, and a definite conclusion seems far to be obtained.23−25 The Fenton process is enhanced by light, and solar irradiation can be employed for this purpose, with a further enhancement of the overall sustainability of the process.26 However, a major drawback is the highly acidic pH (2.8) that is required to maximize the stoichiometric efficiency of the process. One promising strategy to extend photo-Fenton to milder pH is to form complexes with iron, which are photoactive and stable at neutral or slightly acidic pH.27 SBO accomplishes both conditions, and relatively efficient photo-Fenton can be driven at pH = 5 as recently studied.19,28−30
As far as we know, there is no information on the isolation of HLSs from the olive wet husk. Hence, the aim of this work is to explore the possibility of obtaining HLSs from olive oil mill solid wastes (OMW-HLS) and to employ them as auxiliary to drive a photo-Fenton process at mild pH under sunlight. This result would be very significant as a hazardous waste very abundant in the Mediterranean Basin would be valorized to be used in the remediation of polluted water under solar irradiation. For this purpose, a method of isolation, adapted from that used for SBO, will be employed. It involves basic digestion of the solid waste and nanofiltration of the digestate. The obtained OMW-HLS will be characterized and tested in solar-simulated photo-Fenton at pH = 5, using caffeine as a probe molecule; this compound has been chosen because it can be widely found in municipal sewages and even in aqueous ecosystems.31 Finally, the ability of OMW-HLS to generate reactive species under irradiation (hydroxyl radicals or high reactive species that mimic the OH• behavior and singlet oxygen) will also be investigated, in order to gain further insight into the mechanistic issues of the photochemical process.
Results and Discussion
Isolation of the OMW-HLS
OMW-HLSs were isolated from olive OMW subjected to no aging process. The percentage of humidity of the initial sample was approximately 60%, whereas the amount of volatile solids was 95%, confirming the predominance of the organic matter in the sample. Substances were digested at the basic medium in order to solubilize the HLS. For this purpose, 125 g of dry sample (around 300 g of humid product) was introduced in a 2 L open reactor and a solution of KOH (500 mL, pH = 13 or 11.7) was added. Three different conditions were used: (a) digestion at pH = 13.0 for one day, (b) digestion at pH = 13.0 for 4 h, and (c) digestion at pH = 11.7 for 1 day.
No noticeable change in the pH was observed in the experiments carried out at pH 13.0, whereas a decrease in this value to 7.8 was observed for the digestion performed at initial pH = 11.7; this is an indication that although there was an excess of hydroxide in the first experimental condition, this was not true for the pH = 11.7 reaction. After digestion, samples were first flown through a 100 μm filter in order to remove the remaining suspended solids. Then, the filtrate was flown consecutively through three different ceramic membranes, with a size of 300, 150, and 50 kg/mol. The retentate of each membrane was collected and dried in an air oven. The weight of each dry sample can be observed in Table 1.
Table 1. Amount of OMW-HLS Isolated from the Retentate of Each Ceramic Membrane (the Cutoff Is Given in the Heading of the Column), for Each Type of Digestiona.
| 300 kg/mol | 150 kg/mol | 50 kg/mol | total | |
|---|---|---|---|---|
| pH = 13.0, 24 h | 32.1 g (39%) | 17.5 g (20%) | 13.0 g (42%) | 62.6 g |
| pH = 13.0, 4 h | 19.9 g (25%) | 7.0 g (20%) | 4.7 g (35%) | 31.6 g |
| pH = 11.7, 24 h | 5.4 g (42%) | 1.9 g (53%) | 1.1 g (40%) | 8.4 g |
The initial amount of starting material was 125 g/L. The percentage of volatile solids is given in brackets. The total amount of OMW-HLS is given in the last column.
As can be observed, the stronger the digestion process, the higher amount of the final product was obtained; this effect is more significant in the fractions obtained from the retentate of membranes with the lower pore size. This is in agreement with the leaching of the macromolecular aggregations present in the starting material to release smaller blocks, among them, the HLS. This process has not been completely accomplished at milder pH or at shorter digestion times; in those cases, most of the organic matter remain as suspended solids and it is removed in the filtration step.
The yield of the process could be calculated from the total amount of dry solid obtained, which was for each experimental condition, 51, 25, and 7% (see results reported in Table 1). However, these results might be misleading, as an important amount of salts, derived from KOH, are expected to be incorporated in the final product. In fact, Table 1 shows that the percentage of volatile solids obtained in each case was in the range 20–50%, in sharp contrast with the 95% found in the untreated material. For this reason, the yield was also calculated from a balance including only volatile solids. In this case, the yields were 18, 7, and 2%. On the basis of these data, it is clear that strongly basic pH and long digestion times are required.
Because of the presence of important amounts of inorganic matter, namely, salts, OMW-HLS was submitted to further purification. For this purpose, the sample was dialyzed. The amount of organic carbon was increased from 12 to 67%. This was also confirmed by thermogravimetric measurements: in the raw sample, 17% of organic matter was detected, as well as 14% of humidity; in sharp contrast, the dialyzed sample showed 80% of organic matter and 6% of humidity.
Characterization of the OMW-HLS
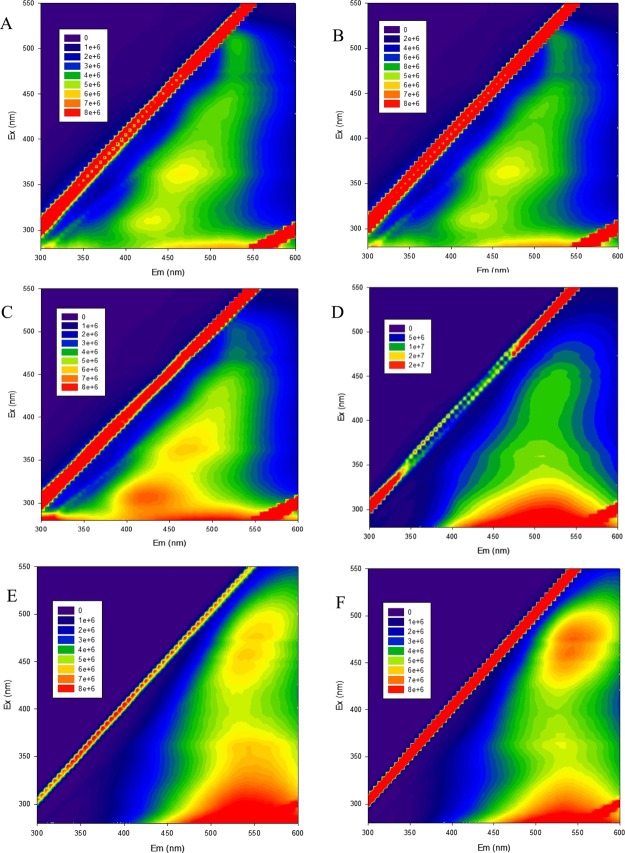
Spectroscopic measurements were performed in order to confirm the nature of the obtained materials. Excitation emission matrixes (EEMs) are good tools for the analysis of complex samples containing organic matter.32Figure 1 shows the EEM recorded for the retentate of the 50 and 150 kDa membranes, as well as for four different humic-(like) substances, namely, commercial HSs supplied by Aldrich, two standard HSs (Pahokee peat humic acid and leonardite) and HLS isolated form urban wastes (CVT230).19 In all cases, very similar matrixes were obtained, with strong signals in the area corresponding to the HSs (emission in the area 380–550 nm and excitation in 250–400 nm).33 The main similarities were observed with the synthetic HLSs isolated from urban wastes, as a parallel procedure was followed to obtain both materials, despite the differences in the starting materials (in both cases vegetal residues).
Figure 1.
EEM of the different humic or humic-like substances: (A) retentate of the 150 kg/mol membrane when olive oil mill wastes were used as staring materials, (B) retentate of the 50 kg/mol membrane when olive oil mill wastes were used as staring materials, (C) HLS isolated from urban wastes, (D) commercial humic acids provided by Aldrich, (E) Pahokee peat humic acid, and (F) leonardite humic acid. X-axis corresponds to the emission wavelength (300–600 nm), whereas Y-axis shows the excitation wavelength (280–550 nm). In the plot, red colors represent higher intensity of fluoresce, whereas blue shows lower fluorescence.
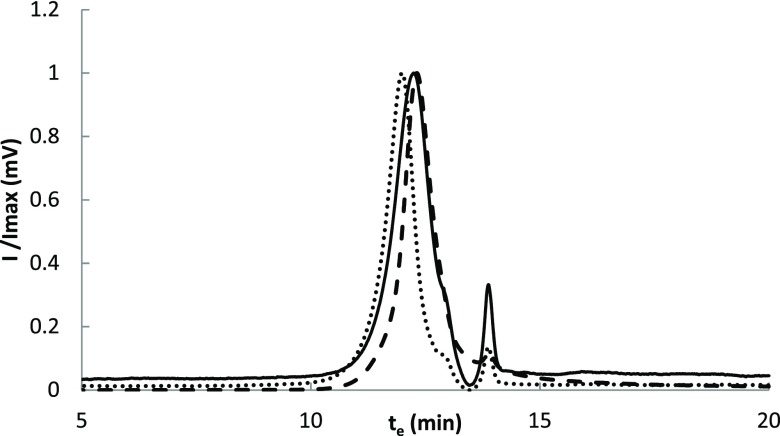
The molecular weight was calculated by size exclusion chromatography (SEC) (see Figure 2 for chromatogram). Retention times were converted into molecular weight by means of a calibration with standards, as indicated in the Experimental Section. The molecular weight distribution showed a maximum at 4600 Da in the case of the OMW-HLS isolated from olive oil mill wastes, and it was increased to 6000 Da for the dialyzed samples. A weight of 4200 Da was calculated for a commercial humic acid supplied by Aldrich, which is in agreement with the data provided in the literature for this substance.34 In general, these results are in line with the molecular weight reported in the literature for aquatic HSs.35 This relatively low molecular weight seems to be contradictory with the size of the membranes employed in the isolation process (50–300 kDa). This apparently anomalous results can be understood, taking into account the ability of HLSs to form supramolecular aggregates.36 Indeed, the size of these aggregates was characterized by dynamic light scattering (DLS) measurements. This method allows to determine the hydrodynamic radii of the particles. For a concentration of 300 mg/L of OMW-HLS, a hydrodynamic radius around 400 nm was measured for both the raw and dialyzed HLSs (420 and 380 nm, respectively). This value was slightly higher than the one measured for soluble biobased substances extracted from urban wastes, which was 135 nm.37
Figure 2.
Size exclusion chromatogram obtained for the OMW-HLS (solid line), for the dialyzed OMW-HLS (points) and for a commercial humic acid, provided from Aldrich (dashes).
HLSs as Auxiliaries for the Photo-Fenton Process
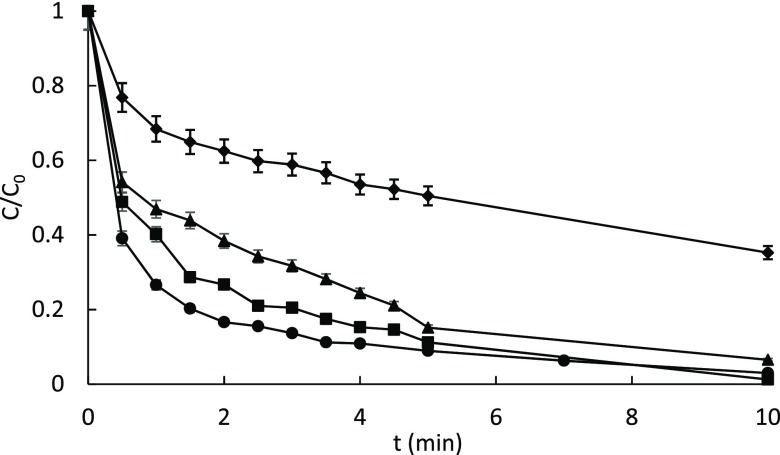
The ability of OMW-HLSs as auxiliaries to drive the photo-Fenton process was tested using caffeine as a target pollutant. Figure 3 shows the profile of relative caffeine concentration versus irradiation time. It can be observed that photo-Fenton at pH = 5 in the absence of HLS (10 mg/L) was able to reach some degradation of caffeine; however, results obtained in the presence of HLSs were systematically more satisfactory. In fact, the photo-Fenton-like process at pH = 5 in the presence of OMW-HLS was able to degrade more than 95% of the initial amount of caffeine in 10 min of irradiation. Very similar results were achieved with the dialyzed and nondialyzed samples, showing that dialysis of the OMW-HLS might not be necessary for practical purposes. These results show that OMW-HLSs are good complexing agents for iron to drive the photo-Fenton process at mild conditions. In fact, their performance is slightly better than that observed for the HLS extracted from urban solid wastes (SBO) (Figure 3), which is the HLS that has been most widely studied as a complexing agent.29 In fact, analysis of EEMs of SBO by PARAFAC at different pHs showed the ability of this HLS to complex iron, giving the highest complexing constant at pH = 5 (log K = 6.55–6.75).33 Finally, dark controls (iron, iron + HLS, and iron + HLS + hydrogen peroxide) featured negligible caffeine degradation, indicating the higher efficiency of photo-Fenton versus the Fenton reaction; these results also allowed ruling out the effect of absorption of caffeine onto the HLS; also, photolysis of caffeine in the absence of HLSs was negligible.
Figure 3.
Photodegradation of caffeine (5 mg/L) in a solar simulator at pH = 5, [Fe2+] = 5 and 60 mg/L of H2O2 under different conditions: in the absence of HLS (⧫), with 10 mg/L of SBO (▲); with 10 mg/L of raw OMW-HLS (■); and with 10 mg/L of dialyzed OMW-HLS (●).
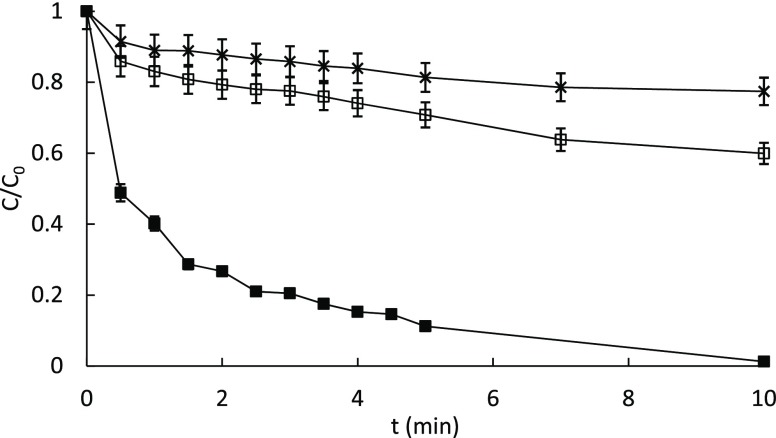
However, when the concentration of OMW-HLS was increased, the reaction rate was observed to decrease (Figure 4). This effect was already observed in the SBO,24 and it was attributed to two different factors: (a) an inner filter effect due to the overlap of the HLS absorption spectrum and the irradiation of the source that was studied in detail with humic acids;38 (b) a competitive effect between the HLS and the pollutants for the generated reactive species; additionally, the Fe chelating ability of HLS might result in decreasing the likelihood of Fe to meet hydrogen peroxide, which is a necessary step in photo-Fenton. Lower amounts of OMW-HLS were inefficient (data not shown), most probably because of the lack of complexing agents to prevent iron inactivation, as also observed with the SBO.28
Figure 4.
Photodegradation of caffeine (5 mg/L) in a solar simulator at pH = 5, [Fe2+] = 5 and 60 mg/L of H2O2 under different concentrations of OMW-HLS: 10 mg/L (■); 30 mg/L (□); and 60 mg/L (×).
In sharp contrast, OMW-HLSs were not able to degrade caffeine under irradiation in the absence of iron and/or hydrogen peroxide (data not shown), which indicates that OMW-HLSs are not good photosensitizers. Similar results have been observed for SBO,30 where very high concentrations of this material and long irradiation times were needed to degrade chlorophenols under UV irradiation.17 The combination of OMW-HLS and hydrogen peroxide was also proven to be inefficient. Although some iron was found in the composition of OMW-HLS (0.011% w/w in the raw product and 0.12% w/w in the dialyzed OMW-HLS), this amount is not able to drive efficiently a photo-Fenton-like process. This values are far from the 0.7% measured in the SBO; this difference can be understood when looking at the starting material that was a complex mixture of urban residues in SBO, more likely to contain iron than the OMW, which consists of a single vegetable residue.
Generation of Reactive Species
In
order to explain
the above-described results, mechanistic experiments based on electronic
paramagnetic resonance (EPR) measurements were performed to investigate
the ability of OMW-HLS to generate reactive oxygen species. The formation
of singlet oxygen was investigated using 2,2,6,6-tetramethylpiperidoxyl
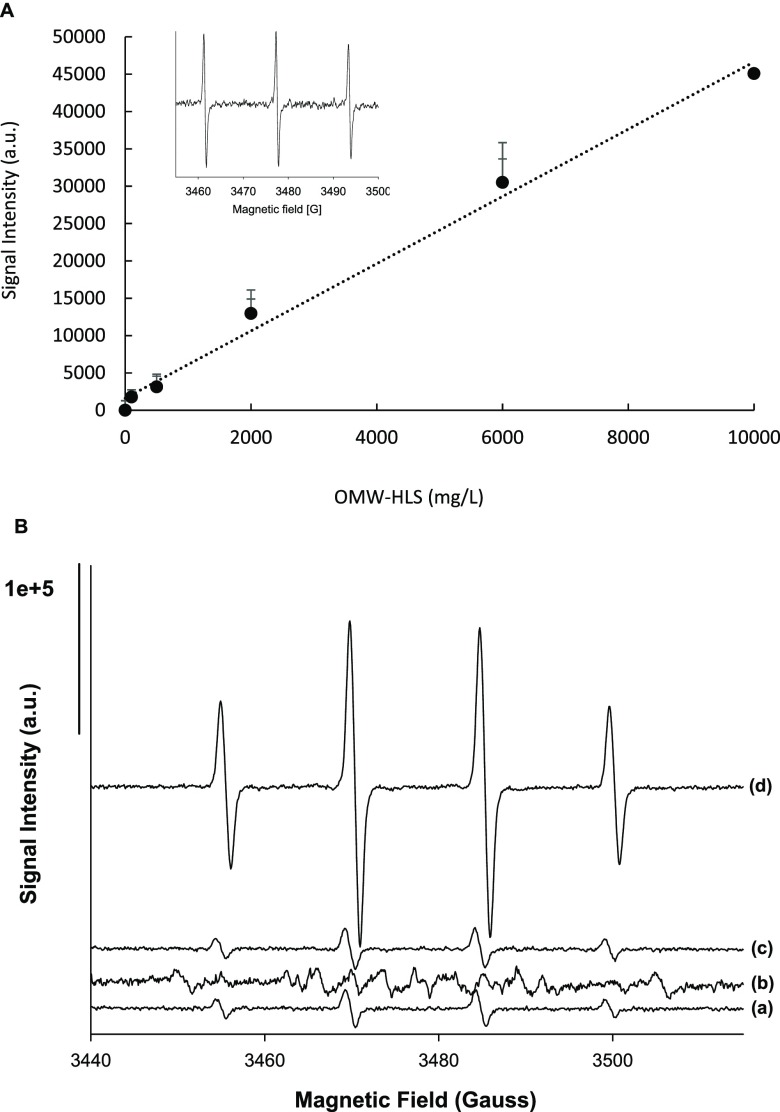
as a probe molecule.39Figure 5 shows that there is a linear
increase in the signal with the concentration of OMW-HLS, indicating
that the formation of this substance is able to sensitize the generation
of singlet oxygen, as previously observed for SBO40 and according to the often reported reactivity of the excited
triplet states of HLS  with dissolved oxygen (3HLS*
+ O2 → HLS + 1O2).41 However, (i) the limited reactivity of singlet
oxygen (the second-order reaction constant of caffeine with 1O2 is kcaffeine,1O2 = 2.9 × 107 M–1 s–1),42 (ii) the scarce photoproduction
of •OH by OMW-HLS alone (vide infra, kcaffeine,•OH = 6.9 × 109 M–1 s–1),42 and (iii) the scarce photoreactivity of the 3HLS* toward caffeine for humic-like compounds under investigation
might explain the bad performance of these substances as photocatalysts.
This is also in line with the recent studies, showing that singlet
oxygen plays only a minor role in the photochemical removal of xenobiotics
in the environment catalyzed by natural organic matter.43
with dissolved oxygen (3HLS*
+ O2 → HLS + 1O2).41 However, (i) the limited reactivity of singlet
oxygen (the second-order reaction constant of caffeine with 1O2 is kcaffeine,1O2 = 2.9 × 107 M–1 s–1),42 (ii) the scarce photoproduction
of •OH by OMW-HLS alone (vide infra, kcaffeine,•OH = 6.9 × 109 M–1 s–1),42 and (iii) the scarce photoreactivity of the 3HLS* toward caffeine for humic-like compounds under investigation
might explain the bad performance of these substances as photocatalysts.
This is also in line with the recent studies, showing that singlet
oxygen plays only a minor role in the photochemical removal of xenobiotics
in the environment catalyzed by natural organic matter.43
Figure 5.
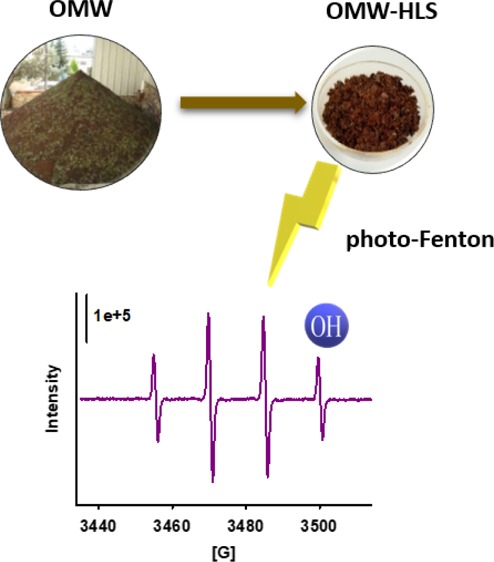
Detection of reactive species in the OMW-HLS system by EPR. (A) Intensity of TMP 45 μm signal, after background subtraction, vs OMW-HLS concentration. Linear fit of data, slope = 4.5, r2 = 0.9913. Inset: the EPR spectrum of the TMP–1O2 adduct. (B) EPR spectra recorded after irradiation of DMPO 17 mM in a solar box during 3 min at pH = 5 under the following experimental conditions: (a) H2O2 (2 mg/L) + Fe2+ (2 mg/L); (b) OMW-HLS (200 mg/L); (c) OMW-HLS (200 mg/L) + H2O2 (2 mg/L) + Fe2+ (2 mg/L); and (d) OMW-HLS (20 mg/L) + H2O2 (2 mg/L) + Fe2+ (2 mg/L).
Experiments using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin trap were performed to investigate the formation of hydroxyl radicals (or high reactive species able to mimic the oxidative reactivity of OH•, e.g., one-electron oxidation of DMPO and successive reaction with water to give the hydroxylated stable adduct).44,45 When irradiating 200 mg/L of OMW-HLS in the presence of DMPO, the signal attributable to the adduct of this substance and OH• was very low, showing that this substance is not able to generate high concentration of OH•. Moreover, also, a low signal ascribable to a C-centered radical is present, probably because of a limited OMW-HLS oxidation in these conditions. However, when Fe2+ (2 mg/L) and H2O2 were added, the signal increased, showing a greatly enhanced generation of reactive species (mainly the hydroxyl radical). As a matter of fact, those results were clearly above those obtained in the experiment involving Fe2+ and H2O2, but without OMW-HLS; this is in agreement with the need of these materials to enhance photo-Fenton at pH = 5, as reported above. Also, the detrimental role of an excess of OMW-HLS can be appreciated: a decrease in the signal is observed when increasing the concentration of this substance from 20 to 200 mg/L that can be due to the quenching of the most reactive species such as OH• by the OMW-HLS or a screen effect of the colored substance that prevents photochemical formation of the reactive species.
Conclusions
HLSs extracted from olive oil mill wastes have been demonstrated as convenient chemical auxiliaries to drive the photo-Fenton process under mild pH conditions. A simple isolation procedure, adapted from that developed to obtain HLS from urban residues, has been followed. This could represent an added value for the management of a residue such as OMW, in the perspective of a transition to the circular economy, therefore contributing to the overall sustainability of the olive oil industry. Furthermore, this result indicate that investigating other food residues as starting materials seems meaningful.
Regarding OMW-HLS, some aspects require further research, such as (a) determining the optimal experimental conditions to drive mild photo-Fenton, (b) to determine the biocompatibility and photostability of these substances, (c) to gain further insight into the performance of the different fractions that have been isolated, and (d) to study mechanistic issues of the process, such as the complexation of iron with the OMW-HLS or the reactive species that are generated. In particular, application of PARAFAC analysis to the EEM will provide relevant information on the composition of the OMW-HLS and differences with other HSs as well as gaining further insight into the speciation of iron and HLS in the system.
Experimental Section
Reagents
Caffeine, employed as a probe substance, was supplied by Sigma-Aldrich. Heptahydrated iron(II) sulfate, hydrogen peroxide (30% w/w), potassium hydroxide, and sulfuric acid (96%) were provided by Panreac. DMPO and 2,2,6,6-tetramethyl-4-piperidone (TMP), employed in EPR, measurements were also supplied by Sigma-Aldrich. Water employed in all solutions was of Milli-Q grade.
The olive oil mill waste was taken from the oil press from Millena, located in the Valencian Community (Spain). The plant worked in a two-phase process, and the sample was taken from the wet pomace. The waste was recent; hence, it was submitted to no composting process. It was immediately frozen until it was used.
Isolation of the HLS
The pomace from the oil press from Millena was first submitted to basic digestion. For this purpose, 0.125 kg (dry weight) of this substance was added to 0.5 L of KOH with the desired pH value (in the range 11–13). The mixture was heated to 65 °C and stirred magnetically for the desired reaction time (4 or 24 h); then, it was left to reach the thermal equilibrium.
The digested sample was filtered to remove the remaining suspended solids through filters with decreasing pore size (100 μm for the smaller one) in order to avoid clogging. The retained solids were washed with 5 L of KOH solution in order to ensure the complete recovery of the solubilized organics.
Then, the liquid sample (filtrate and washing solution) was submitted to ultrafiltration. For this purpose, ceramic membranes, supplied by TAMI Industries (Nyons Cedex, France), were used. The experimental setup consisted of a reservoir from which the sample was flown through the membrane by means of pump (CAT PUMPS 3C91241). The membrane operated in a tangential flux of 4 L/m2 h at a pressure of 2 bars and a temperature of 25 °C. Three different pore sizes were used (molecular weight cutoff equal to 300, 150, and 50 kDa); in all cases, the internal diameter was 10 mm, the length 250 mm, and the permeate area 0.0132 m2. The sample was flown consecutively through all three membranes. The permeate of the first membrane (300 kg/mol) was passed through the next one (150 kg/mol), and again this permeate was flown through the 50 kg/mol membrane. The retentate of each membrane was accounted for approximately 0.5 L. Finally, all retentates were dried in an air oven at 65 °C for 24 h in order to obtain the OMW-HLS.
When a sample nearly free of salts was needed, the raw OMW-HLSs were submitted to dialysis. For this purpose, cellulose membranes, with a pore size of 12 000 Da, supplied by Aldrich were employed. A very concentrated solution of the sample was prepared (2 g in 15 mL), and it was introduced in the membrane, which was sealed and submerged in Milli-Q water. The conductivity of water was monitored, and the accepting solution solvent was changed when this value was constant. The procedure was repeated until low values of conductivity were reached. The desalinated sample was then dried in an oven.
Photochemical Experiments
Photochemical experiments were performed in a cylindrical Pyrex vessel (55 mm i.d.). For each experiment, the reactor was loaded with 250 mL of solution containing caffeine (5 mg/L) and, when needed, OMW-HLS (10 mg/L in most experiments), iron (5 mg/L), and hydrogen peroxide (60 mg/L). The pH was adjusted to 5 by adding diluted sulfuric acid. These experimental conditions are similar to those employed in the experiments involving SBO.28 Samples were irradiated with a solar simulator (Oriel 81160) equipped with a 300 W xenon short arc lamp; a glass filter was employed to cutoff the residual radiation below 300 nm. Samples were periodically withdrawn for analysis; they were diluted 1:1 with methanol to quench further reaction because of the excess of H2O2; then, they were filtered through polypropylene filters with a pore diameter of 0.45 μm (VWR Int., Milan) and analyzed as soon as possible.
Chemical Analyses
SEC was used to determine the distribution of molecular weights of HLS. A Hitachi Chromaster chromatograph (VWR) equipped with a Shodex OHpak SB-805 HQ methacrylate column, an oven to thermostatize the column, an autosampler (Chromaster 5210), pump (Chromaster 5110), and a UV detector (Chromaster 5410). The eluent consisted of an isocratic mixture of acetonitrile (30%) and a phosphate buffer at pH = 7.2 (70%); the flow was 0.8 mL/min. Detection was based on absorbance at 260 nm. The molecular weight of the HLS was calculated based on standards of poly-(sodium 4-styrensulfonate) of well-defined molecular weights, in the range 4230–145 000 Da.
DLS was used to determine the size distribution of the OMW-HLS particles. For this purpose, solutions containing 300 mg/L of this substance were prepared and measured with an ALV-NIBS apparatus, equipped with Ne–He y laser and a digital correlator (tau ALV-5000). Scattered light was measured during at least 20 s at 298 K.
Fluorescence emission–excitation matrices were obtained with a modular fluorimeter QuantaMaster (PTI). Samples were excited in the range 250–550 nm, and emission was recorded in the range 300–600 nm. A 5 nm bandpass was adopted on both excitation and emission. Before measurements, samples were diluted with water to keep absorbance below 1.5 AU at λ = 250 nm.
The concentration of caffeine was followed by liquid chromatography, using a Flexar UHPLC FX-10 chromatograph (PerkinElmer) equipped with a Brownlee Analytical DV C18 column. The apparatus is also equipped with autosampler (S200 Autosampler Comm kit-1022 PUS), pump (Flexar FX 10 UHO PUMP), thermostatic oven for the column, and UV detector (UV/vis KIT-UHPLC Detector Tubing). The mobile phase consisted of a gradient of acetonitrile (A) and formic acid, 0.01 M (B), that was changed from 5% of A to 50% of A in 4 min. The flow was 0.3 mL/min. Detection of caffeine was based on absorption at 275 nm, and the detection limit was below 100 μg/L. Errors were systematically below 5%.
EPR spectra were recorded at room temperature with a Bruker ESR 300E spectrophotometer; DMPO (5,5-dimethyl-1-pirroline-N-oxide) at a concentration of 17 mM was used as a probe for the hydroxyl radical or •OH-like species, and TMP (45 mM) was used for the detection of singlet oxygen. Measurements were carried out in quartz capillary tubes. The following parameters were set: the microwave frequency was 9.78 GHz and the power 5 mW; the modulation frequency was 100 kHz with an amplitude of 0.4 gauss; the time constant was 40 ms. In all experiments, the spin trap (DMPO or TMP) was added to the cell before irradiating. The irradiation time was 3 min for hydroxyl radical determination and 15 min for singlet oxygen, and the spectra were recorded immediately after the end of irradiation.
Organic content in the OML-HLS was estimated from the content of volatile solids. They were determined as the difference of weight of the dry OMW-HLS before and after calcination at 550 °C in an oven.
Thermogravimetric analysis was performed in a TA Q600 apparatus (TA instruments). During the analysis, the temperature was varied from room temperature to 800 °C at a rate of 10 °C/min, in air atmosphere.
Acknowledgments
Authors want to acknowledge the financial support of Spanish Ministerio de Economía y Competitividad (CTQ2015-69832-C04) and European Union (645551-RISE-2014, MAT4TREAT).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- http://www.eu-fusions.org/phocadownload/Publications/ (accessed July 2018).
- Final Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and The Committee of the Regions on the Implementation of the Circular Economy Action Plan, Brussels, 2017.
- Lin C. S. K.; Pfaltzgraff L. A.; Herrero-Davila L.; Mubofu E. B.; Abderrahim S.; Clark J. H.; Koutinas A. A.; Kopsahelis N.; Stamatelatou K.; Dickson F.; Thankappan S.; Mohamed Z.; Brocklesby R.; Luque R. Food waste as a valuable resource for the production of chemicals, materials and fuels: Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. 10.1039/c2ee23440h. [DOI] [Google Scholar]
- Dermeche S.; Nadour M.; Larroche C.; Moulti-Mati F.; Michaud P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. 10.1016/j.procbio.2013.07.010. [DOI] [Google Scholar]
- Roig A.; Cayuela M. L.; Sánchez-Monedero M. A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. 10.1016/j.wasman.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Scoma A.; Bertin L.; Zanaroli G.; Fraraccio S.; Fava F. A physicochemical–biotechnological approach for an integrated valorization of olive mill wastewater. Bioresour. Technol. 2011, 102, 10273–10279. 10.1016/j.biortech.2011.08.080. [DOI] [PubMed] [Google Scholar]
- Ntaikou I.; Kourmentza C.; Koutrouli E. C.; Stamatelatou K.; Zampraka A.; Kornaros M.; Lyberatos G. Exploitation of olive oil mill wastewater for combined biohydrogen and biopolymers production. Bioresour. Technol. 2009, 100, 3724–3730. 10.1016/j.biortech.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Castello E.; Cassano A.; Criscuoli A.; Conidi C.; Drioli E. Recovery and concentration of polyphenols from olive mil wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. 10.1016/j.watres.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Pattara C.; Cappelletti G. M.; Cichelli A. Recovery and use of olive stones: Commodity, environmental and economic assessment. Renewable Sustainable Energy Rev. 2010, 14, 1484–1489. 10.1016/j.rser.2010.01.018. [DOI] [Google Scholar]
- El-Abbassi A.; Saadaoui N.; Kiai H.; Raiti J.; Hafidi A. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total Environ. 2017, 576, 10–21. 10.1016/j.scitotenv.2016.10.032. [DOI] [PubMed] [Google Scholar]
- Lafka T.-I.; Lazou A. E.; Sinanoglou V. J.; Lazos E. S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. 10.1016/j.foodchem.2010.08.041. [DOI] [Google Scholar]
- Engebretson R. R.; von Wandruszka R. Microorganization in dissolved humic acids. Environ. Sci. Technol. 1994, 28, 1934–1941. 10.1021/es00060a026. [DOI] [PubMed] [Google Scholar]
- Montoneri E.; Mainero D.; Boffa V.; Perrone D. G.; Montoneri C. A project to turn an urban wastes treatment plant into biorefinery for the production of energy, chemicals and consumer’s products with friendly environmental impact. Int. J. Global Environ. Issues 2011, 11, 170–196. 10.1504/ijgenvi.2011.043528. [DOI] [Google Scholar]
- Fascella G.; Montoneri E.; Ginepro M.; Francavilla M. Effect of urban biowaste derived soluble substances on growth, photosynthesis and ornamental value of Euphorbia x lomi. Sci. Hortic. 2015, 197, 90–98. 10.1016/j.scienta.2015.10.042. [DOI] [Google Scholar]
- Negueroles P. G.; Bou-Belda E.; Santos-Juanes L.; Amat A. M.; Arques A.; Vercher R. F.; Monllor P.; Vicente R. Treatment and reuse of textile wastewaters by mild solar photo-Fenton in the presence of humic-like substances. Environ. Sci. Pollut. Res. 2017, 24, 12664–12672. 10.1007/s11356-016-7889-1. [DOI] [PubMed] [Google Scholar]
- Soluble Bio-Based Substances Isolated from Urban Wastes: Environmental Applications; Arques A., Bianco-Prevot A., Eds.; Springer, 2015. [Google Scholar]
- Avetta P.; Bella F.; Prevot A. B.; Laurenti E.; Montoneri E.; Arques A.; Carlos L. Waste cleaning waste: Photodegradation of monochlorophenols in the presence of waste-derived photosensitizer. ACS Sustainable Chem. Eng. 2013, 1, 1545–1550. 10.1021/sc400294z. [DOI] [Google Scholar]
- Magnacca G.; Allera A.; Montoneri E.; Celi L.; Benito D. E.; Gagliardi L. G.; Gonzalez M. C.; Mártire D. O.; Carlos L. Novel magnetite nanoparticles coated with waste-sourced biobased substances as sustainable and renewable adsorbing materials. ACS Sustainable Chem. Eng. 2014, 2, 1518–1524. 10.1021/sc500213j. [DOI] [Google Scholar]
- Gomis J.; Prevot A. B.; Montoneri E.; González M. C.; Amat A. M.; Mártire D. O.; Arques A.; Carlos L. Waste sourced bio-based substances for solar-driven wastewater remediation: Photodegradation of emerging pollutants. Chem. Eng. J. 2014, 235, 236–243. 10.1016/j.cej.2013.09.009. [DOI] [Google Scholar]
- Zingaretti D.; Lombardi F.; Baciocchi R. Soluble organic substances extracted from compost as amendments for Fenton-like oxidation of contaminated sites. Sci. Total Environ. 2018, 619–620, 1366–1374. 10.1016/j.scitotenv.2017.11.178. [DOI] [PubMed] [Google Scholar]
- Pignatello J. J.; Oliveros E.; MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. 10.1080/10643380500326564. [DOI] [Google Scholar]
- Wink D. A.; Nims R. W.; Saavedra J. E.; Utermahlen W. E.; Ford P. C. The Fenton oxidation mechanism: reactivities of biologically relevant substrates with two oxidizing intermediates differ from those predicted for the hydroxyl radical. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 6604–6608. 10.1073/pnas.91.14.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossmann S. H.; Oliveros E.; Göb S.; Siegwart S.; Dahlen E. P.; Payawan L.; Straub M. Jr.; Wörner M.; Braun A. M. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J. Phys. Chem. A 1998, 102, 5542–5550. 10.1021/jp980129j. [DOI] [Google Scholar]
- Deguillaume L.; Leriche M.; Chaumerliac N. Impact of radical versus non-radical pathway in the Fenton chemistry on the iron redox cycle in clouds. Chemosphere 2005, 60, 718–724. 10.1016/j.chemosphere.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Minero C.; Lucchiari M.; Maurino V.; Vione D. A qauntitative assessment of the production of ·OH and additional oxidants in the dark Fenton reaction: Fenton degradation of aromatic amines. RSC Adv. 2013, 3, 26443–26450. 10.1039/c3ra44585b. [DOI] [Google Scholar]
- Malato S.; Fernández-Ibáñez P.; Maldonado M. I.; Blanco J.; Gernjak W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. 10.1016/j.cattod.2009.06.018. [DOI] [Google Scholar]
- Santos-Juanes L.; Amat A. A.; Arques A. Strategies to drive photo-Fenton process at mild conditions for the removal of xenobiotics from aqueous systems. Curr. Org. Chem. 2017, 21, 1074–1083. 10.2174/1385272821666170102150337. [DOI] [Google Scholar]
- Gomis J.; Carlos L.; Prevot A. B.; Teixeira A. C. S. C.; Mora M.; Amat A. M.; Vicente R.; Arques A. Bio-based substances from urban waste as auxiliaries for solar photo-Fenton treatment under mild conditions: optimization of operational variables. Catal. Today 2015, 240, 39–45. 10.1016/j.cattod.2014.03.034. [DOI] [Google Scholar]
- Gomis J.; Gonçalves M. G.; Vercher R. F.; Sabater C.; Castillo M.-A.; Prevot A. B.; Amat A. M.; Arques A. Determination of photostability, biocompatibility and efficiency as photo-Fenton auxiliaries of three different types of soluble bio-based substances (SBO). Catal. Today 2015, 252, 177–183. 10.1016/j.cattod.2014.10.015. [DOI] [Google Scholar]
- Gomis J.; Vercher R. F.; Amat A. M.; Mártire D. O.; González M. C.; Prevot A. B.; Montoneri E.; Arques A.; Carlos L. Application of soluble bio-organic substances (SBO) as photocatalysts for wastewater treatment: sensitizing effect and photo-Fenton-like process. Catal. Today 2013, 209, 176–180. 10.1016/j.cattod.2012.08.036. [DOI] [Google Scholar]
- Khetan S. K.; Collins T. J. Human pharmaceuticals in the aquatic environment: A challenge to green chemisty. Chem. Rev. 2007, 107, 2319–2364. 10.1021/cr020441w. [DOI] [PubMed] [Google Scholar]
- Her N.; Amy G.; McKnight D.; Sohn J.; Yoon Y. Characterization of DOM as a function of MW by fluorescenceEEM and HPLC-SEC using UVA, DOC, and fluorescence detection. Water Res. 2003, 37, 4295–4303. 10.1016/s0043-1354(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Ballesteros S. G.; Costante M.; Vicente R.; Mora M.; Amat A. M.; Arques A.; Carlos L.; Einschlag F. S. G. Humic-like substances from urban waste as auxiliaries for photo-Fenton treatment: a fluorescence EEM-PARAFAC study. Photochem. Photobiol. Sci. 2017, 16, 38–45. 10.1039/c6pp00236f. [DOI] [PubMed] [Google Scholar]
- Chin Y.-P.; Aiken G.; O’Loughlin E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858. 10.1021/es00060a015. [DOI] [PubMed] [Google Scholar]
- Perminova I. V.; Frimmel F. H.; Kudryavtsev A. V.; Kulikova N. A.; Abbt-Braun G.; Hesse S.; Petrosyan V. S. Molecular weight characteristics of humic substances from different environments as determined by size exclusion chromatography and their statistical evaluation. Environ. Sci. Technol. 2003, 37, 2477–2485. 10.1021/es0258069. [DOI] [PubMed] [Google Scholar]
- Sutton R.; Sposito G. Molecular structure in soil humic substances: the new view. Environ. Sci. Technol. 2005, 39, 9009–9015. 10.1021/es050778q. [DOI] [PubMed] [Google Scholar]
- Avetta P.; Berto S.; Prevot A. B.; Minella M.; Montoneri E.; Persico D.; Vione D.; Gonzalez M. C.; Mártire D. O.; Carlos L.; Arques A. Photoinduced transformation of waste-derived soluble bio-based substances. Chem. Eng. J. 2015, 274, 247–255. 10.1016/j.cej.2015.03.126. [DOI] [Google Scholar]
- Carlos L.; Mártire D. O.; Gonzalez M. C.; Gomis J.; Bernabeu A.; Amat A. M.; Arques A. Photochemical fate of a mixture of emerging pollutants in the presence of humic substances. Water Res. 2012, 46, 4732–4740. 10.1016/j.watres.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Alia; Mohanty P.; Matysik J. Effect of proline on the production of singlet oxygen. Amino Acids 2001, 21, 195–200. 10.1007/s007260170026. [DOI] [PubMed] [Google Scholar]
- Prevot A. B.; Avetta P.; Fabbri D.; Laurenti E.; Marchis T.; Perrone D. G.; Montoneri E.; Boffa V. Waste derived bio-organic substances for light induced generation of reactive oxygenated species. ChemSusChem 2011, 4, 85–90. 10.1002/cssc.201000237. [DOI] [PubMed] [Google Scholar]
- Vione D.; Minella M.; Maurino V.; Minero C. Indirect photochemistry in sunlit surface waters: photoinduced production of reactive transient species. Chem.—Eur. J. 2014, 20, 10590–10606. 10.1002/chem.201400413. [DOI] [PubMed] [Google Scholar]
- Kesavan P. C. Oxygen effect in radiation biology: caffeine and serendipity. Curr. Sci. 2005, 89, 318–328. [Google Scholar]
- Passananti M.; Temussi F.; Iesce M. R.; Previtera L.; Mailhot G.; Vione D.; Brigante M. Photoenhanced transformation of nicotine in aquatic environments: involvement of naturally occurring radical sources. Water Res. 2014, 55, 106–114. 10.1016/j.watres.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Reszka K.; Bilski P.; Chignell C. F. EPR spectra of DMPO spin adducts of superoxide and hydroxyl radicals in pyridine. Free Radical Res. Commun. 1992, 17, 377–385. 10.3109/10715769209083142. [DOI] [PubMed] [Google Scholar]
- Takayanagi T.; Kimiya H.; Ohyama T. Formation of artifactual DMPO-OH spin adduct in acid solutions containing nitrite ions. Free Radical Res. 2017, 51, 739–748. 10.1080/10715762.2017.1369536. [DOI] [PubMed] [Google Scholar]