Abstract
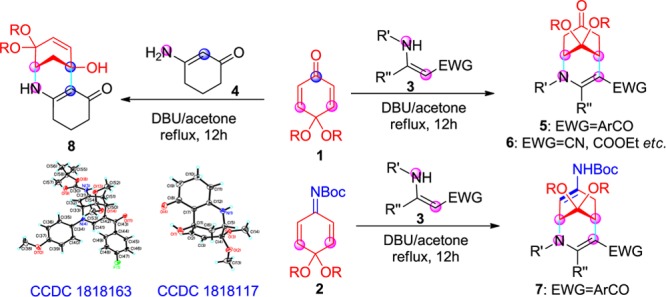
An efficient and concise protocol was developed for the synthesis of diverse morphan derivatives 5–7 by the Michael and aza-Michael reaction of different types of quinone monoketals 1 or quinone imine ketals 2 with enaminones or enamine esters 3 promoted by 1,8-diazabicyclo[5.4.0]undec-7-ene in acetone at reflux. Notably, when cyclic enaminone 4 was used as a substrate in the aza-Michael and 1,2-addition reactions with quinone monoketals 1, they gave another novel morphan 8. This method is suitable for parallel synthesis of bridged ring compounds. As a result, highly diverse morphan derivatives were easily and efficiently prepared by the Michael/aza-Michael or aza-Michael/1,2-addition reactions.
Introduction
The morphan (2-azabicyclo[3.3.1]nonane) skeleton widely exists in biologically active natural and natural-like synthetic products. For example, strychnine1 and morphine2 (Figure 1) are the best known molecules due to their broad biological profile. In addition, some synthetic pharmaceuticals or natural-like biological molecules, such as immune suppressants (FR901483),3 antitumor agents (daphniphyllum alkaloids),4 opioid agonists (compound A),5 and opioid receptor antagonists (e.g., compound B(6) and naloxone), are morphan derivatives.
Figure 1.
Structures of morphans and the targeted compounds.
Available morphan derivatives have been a concern for many years due to their broad spectrum of biological activities. Consequently, in recent years, more and more practical procedures have been developed for the synthesis of various morphan derivatives by the organic chemists.7−12 These procedures have greatly contributed to synthesis of natural-like morphan products. However, most of these approaches involve metal catalysts under strict anhydrous conditions or multistep synthesis. Accordingly, it is necessary and desirable to develop concise, highly site-selective cascade reaction to construct a library of molecularly diverse morphan derivatives.
Quinone monoketals are an important building block that are widely used for the synthesis of various structurally diverse natural or natural-like products.13,14 Quinone monoketals contain two unsaturated C=C bonds, which are often used as 1,4-nucleophilic addition acceptors by one of the two C=C bonds for the synthesis of fused-ring compounds.15,16 Compared with quinone monoketals, quinone imine ketals17,18 have some of their own properties, such as having a more active imino group that is attacked by alkyne or alkyl amino, like the 1,2-addition, or one of the two C=C bonds as in the Diels–Alder reaction. However, the two C=C bonds of quinone imine ketals are hardly used as 1,4-nucleophilic addition acceptors. For many years, only a few successful examples have been completed.18
Enaminones and enamine esters are interesting and versatile building blocks19−24 that are used unparalleled to synthesize various fused heterocycles by the thousands. However, the enaminones and enamine esters used as substrates to construct bridged ring compounds are very limited. To date, incorporation at the two nucleophilic sites (α-C and N) through the Michael/aza-Michael addition or the aza-Michael/1,2-addition reaction of enaminones or enamine esters to form bridged ring frame morphan compounds has been very limited.
For many years, 1,1-enediamines25−35 have been widely used as building blocks. Among them, some morphan derivatives have been designed and synthesized on the basis of 1,1-enediamines.36,37 However, despite being versatile building blocks, enaminones and enamine esters have not been widely used in the synthesis of bridged ring compounds, such as morphan derivatives whose reactivity is usually lower than that of 1,1-enediamines.
To prepare molecularly diverse morphan derivatives starting with the less reactive enaminones and enamine esters substrates and to further investigate the more active immunosuppressant, we study the reactions of different types of quinone monoketals 1 or quinone imine ketals 2 with enaminones or enamine esters 3–4 under special conditions. We obtained novel target morphan compounds 5–7 by the Michael and aza-Michael reaction of quinone monoketals 1 or quinone imine ketals 2 with enaminones or enamine esters 3–4 promoted by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in acetone at reflux. Unexpectedly, when the cyclic enaminone 4 was used as a substrate for the reaction with quinone monoketals 1, they produced special morphans 8 by the aza-Michael reaction and 1,2-addition reaction (Scheme 1).
Scheme 1. Synthesis Routes of Morphans 5–8.
Results and Discussion
Initially, to evaluate the Michael and aza-Michael addition of quinone monoketals 1 and Enaminones 3, the mixture (1a/3a = 1.1:1) was treated in acetonitrile at reflux for 12 h (Table 1, entry 1) and the reaction could not proceed. Then, proton acids, including AgOTf and Cu(OTf)2, were added into the mixture (1a/3a = 1.1:1) at reflux for 12 h (Table 1, entries 2–3). The results demonstrated that the reaction could not proceed at all. Then, K2CO3 was added into the mixture with the same solvent and refluxed for 12 h (Table 1, entry 4); we luckily obtained the product with a 33% yield. Thereafter, different solvents, including acetonitrile, 1,4-dioxane and acetone, were used as solvent and promoted by the inorganic base Cs2CO3. The results showed that Cs2CO3 can promote the reaction to some extent. Then, a stronger alkali t-BuOK was used with 1,4-dioxane at reflux for 12 h. The results indicated that the reaction becomes more complex and cannot achieve a positive result as the alkali is too strong (Table 1, entry 7). Then, the organic base DBU was added with different solvents and the mixture was refluxed for 12 h (Table 1, entries 8–11). Fortunately, we found that the organic base DBU can largely promote the reaction and produce the product with excellent yield in acetone (90%). Subsequently, other organic bases, including Et3N and DABCO, were evaluated in acetone at reflux for 12 h and showed that DABCO and Et3N also could not produce a positive result (Table 1, entries 12–13). On the basis of these results, we concluded that DBU is the optimal promoter and acetone is the best solvent for this cascade reaction (Table 1, entry 10). Finally, the different reaction time was also investigated (Table 1, entries 14–15). The results revealed that the optimal reaction conditions for the synthesis of morphan derivatives were acetone as the solvent and DBU as the base under reflux for 12 h with a 90% yield (Table 1, entry 10).
Table 1. Optimization of the Reaction Conditions for the Model Reactiona.
| entry | solvent | promoter | t (°C) | time (h) | yieldb (%) |
|---|---|---|---|---|---|
| 1 | CH3CN | reflux | 12 | nr | |
| 2 | CH3CN | AgOTf | reflux | 12 | nr |
| 3 | CH3CN | Cu(OTf)2 | reflux | 12 | nr |
| 4 | CH3CN | K2CO3 | reflux | 12 | 33 |
| 5 | CH3CN | Cs2CO3 | reflux | 12 | 62 |
| 6 | 1,4-dioxane | Cs2CO3 | reflux | 12 | 68 |
| 7 | 1,4-dioxane | t-BuOK | reflux | 12 | complex |
| 8 | CH3CN | DBU | reflux | 12 | 82 |
| 9 | 1,4-dioxane | DBU | reflux | 12 | nr |
| 10 | acetonec | DBU | reflux | 12 | 90 |
| 11 | EtOH | DBU | reflux | 12 | 12 |
| 12 | acetone | DABCO | reflux | 12 | nr |
| 13 | acetone | Et3N | reflux | 12 | nr |
| 14 | acetone | DBU | reflux | 8 | 85 |
| 15 | acetone | DBU | reflux | 16 | 89 |
Reaction conditions: 1a (1.1 mmol), 3a (1.0 mmol), base (2.0 mmol), solvent (15 mL).
Isolated yield based on 3a. nr = no reaction.
Bold entry indicates the best condition.
We further explored the scope and limitations of the cascade reaction based on the optimal conditions. Therefore, different quinone monoketals 1 and various enaminones 3 were utilized in this cascade reaction (Table 2, 5a–u). The results showed that various enaminones are suitable for the reaction to obtain the target compounds with moderate to good yields. However, the structure of quinone monoketals 1 has influence on the reaction yield (Table 2, 5a vs f; 5l vs t). Similarly, the structure of enaminones 3 also determines the reaction yield. Usually, the electron-withdrawing (F, Cl) benzoyl group of enaminones is favorable to the yield (Table 2, 5a vs c; 5q vs r).
Table 2. Michael and Aza-Michael Reaction for the Synthesis of Morphans 5a,b.
Reaction conditions: 1 (1.1 mmol), 3 (1.0 mmol), DBU (2.0 mmol), acetone (15 mL).
Isolated yield based on 3.
To further explore the scope and limitations of the cascade reaction, the different enamine esters 3 were used as substrates to react with quinone monoketals 1 in acetone (Table 3). The results showed that many kinds of enamine esters are a good substrate and produce the target compounds with good yields. Among them, on using (E)-3-aminobut-2-enenitrile as a substrate, the reaction can generate the products with excellent yields (Table 3, 6d vs b). On the basis of the reaction of the different quinone monoketals 1 with diverse enaminones and enamine esters 3, the quinone imine ketal 2 were used in this reaction and the scope and limitations of the cascade reaction were evaluated (Table 4). The results showed that the cascade reaction can smoothly proceed under the same conditions and produce the target compounds with good yields. Remarkably, we only obtain one of the isomers of 7a–e (Table 4).
Table 3. Michael and Aza-Michael Addition for the Synthesis of Morphans 6a,b.
Reaction conditions: 1 (1.1 mmol), 3 (1.0 mmol), DBU (2.0 mmol), acetone (15 mL).
Isolated yield based on 3.
Table 4. Michael and Aza-Michael Addition for the Synthesis of Morphans 7a,b.
Reaction conditions: 2 (1.1 mmol), 3 (1.0 mmol), DBU (2.0 mmol), acetone (15 mL).
Isolated yield based on 3.
To achieve the molecular diversity of the products, cyclic enaminone 4 was used as a substrate in reactions with quinone monoketals 1a–b. Surprisingly, we obtained another kind of morphan derivatives 8a–b with moderate yields (63–66%). The reaction was via the aza-Michael reaction and 1,2-addition reaction. First, the amino group of the enaminones binds to one of the C=C bonds of the quinone monoketals 1 through the aza-Michael reaction. Second, the α-C of the enaminones attack the carbonyl of compounds 1 via 1,2-addition to form the target compounds (Table 5, 8a–b). Since the amino group of 4 is more active than that of α-C in the cyclic enaminone 4, it leads to different products via different reactions.
Table 5. Michael and Aza-Michael Addition for the Synthesis of Morphans 8a,b.
Reaction conditions: 1 (1.1 mmol), 4 (1.0 mmol), DBU (2.0 mmol), acetone (15 mL).
Isolated yield based on 4.
To confirm the structure of the morphan derivatives, compounds 5n, 6e, 7b, and 8a were selected as the representative compounds and each was characterized by X-ray crystallography, as shown in Figure 2 (5n: CCDC 1817955; 6e: CCDC 1817956) and Figure 3 (7b: CCDC 1818163; 8a: CCDC 1818117).
Figure 2.
X-ray crystal structure of 5n (left) and 6e (right) (ellipsoids are drawn at the 30% probability level).
Figure 3.
X-ray crystal structure of 7b (left) and 8a (right) (ellipsoids are drawn at the 30% probability level).
A proposed mechanism of how DBU promoted the Michael and aza-Michael addition reactions is presented in Scheme 2. Initially, the quinone imine ketals 2 react with the enaminones 3 via Michael addition to obtain the intermediate 9. Then, intermediate compounds 9 are inverted to give intermediates 10 through imine-enamine tautomerization. Finally, intermediates 10 underwent intramolecular aza-Michael addition to form the target compounds 7.
Scheme 2. Mechanism Hypotheses of the Cascade Reaction.
Conclusions
In conclusion, we developed a procedure for concise synthesis of potentially biologically active morphan derivatives through the Michael and aza-Michael addition or aza-Michael and 1,2-addition reactions. Accordingly, a molecularly diverse morphan library was rapidly constructed in one-pot with good yields by simply refluxing a reaction mixture of quinone monoketals 1 or quinone imine ketals 2 and enaminones or enamine esters 3–4 in acetone and promoted by DBU.
Experimental Section
General Methods
All compounds were fully characterized by spectroscopic data analysis. The NMR spectra were recorded on a Bruker DRX500 or DRX600 NMR spectrophotometer (Bruker Biospin GmbH, Ettlingen, Germany); chemical shifts (δ) are expressed in ppm, J values are given in Hz, and deuterated dimethyl sulfoxide (DMSO)-d6 or CDCl3 was used as solvent. The IR spectra were recorded on a Fourier transform infrared (FT-IR) Avatar 360 spectrophotometer (Thermo Nicolet Corp., Madison WI) using the KBr pellet technique. The reactions were monitored by thin-layer chromatography (TLC) using silica gel GF254 (EMD Millipore, Billerica, MA). The melting points were determined on an XT-4A melting point apparatus and are uncorrected. High-resolution mass spectra time-of-flight (HRMS-TOF) were performed on an AutoSpec Premier P776 mass spectrometer (Waters Corp., Milford,. MA). X-ray diffraction analysis was performed using an Apex Duo diffractometer (Bruker AXS GmbH., Karlsruhe, Germany). All chemicals and solvents were used as received without further purification unless otherwise stated. Column chromatography was performed on silica gel (200–300 mesh).
The materials were purchased from Adamas-β Ltd. (Shanghai China). Compounds 1 were prepared according to the literature.17 Compounds 2 were prepared according to the literature.38
General Procedure for the Synthesis of Compounds 5–6
The quinone monoketals 1 (1.1 mmol), enaminones or enamine esters 3 (1.0 mmol), and acetone (15 mL) were charged into a 25 mL round-bottomed flask. Then, DBU (2.0 mmol) was added into the mixture. The mixture was held at reflux for about 12 h and monitored by TLC until the enaminones or enamine esters 3 substrates were completely consumed. After the reaction was completed, the reaction system was cooled to room temperature. The reaction mixture was then poured into 25 mL of water and 50 mL of ethyl acetate for extraction and separation. Subsequently, the crude product was concentrated by distillation under reduced pressure and then purified by column chromatography (petroleum ether/ethyl acetate = 1: 2) and a series of compounds 5–6 were obtained with a 61–96% yield. The products were further identified by NMR spectroscopy, FT-IR spectroscopy, and HRMS analysis.
(1R,5R)-4-(4-Fluorobenzoyl)-9,9-dimethoxy-2-(p-tolyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5a)
Yellow solid; yield: 368 mg, 90%; mp 187.9 °C; IR (KBr): 2945, 2836, 1720, 1570, 1510, 1251, 817, 602, 419 cm–1; 1H NMR (600 MHz, DMSO-d6): δ = 2.26–2.28 (m, 3H, CH3), 2.28–2.32 (m, 1H, COCH2), 2.46–2.52 (m, 1H, COCH2), 2.87–2.90 (m, 1H, COCH2CHN), 2.95–2.99 (m, 1H, COCH2CHN), 3.15 (s, 3H, CH3O), 3.44 (s, 3H, CH3O), 3.50–3.55 (m, 1H, CH), 4.50–4.55 (m, 1H, CH2CHN), 7.02–7.04 (m, 2H, ArH), 7.16–7.18 (m, 2H, ArH), 7.26 (s, 1H, CHN), 7.27–7.28 (m, 2H, ArH), 7.48–7.51 (m, 2H, ArH); 13C NMR (150 MHz, DMSO-d6): δ = 20.7, 20.7, 33.7, 43.4, 44.2, 49.0, 49.1, 57.4, 97.6, 113.9, 115.7 (d, J = 21.0 Hz), 119.7, 130.7, 131.1 (d, J = 7.5 Hz), 134.6, 136.8, 141.7, 145.2, 163.6 (d, J = 246.0 Hz), 190.8, 208.4; 19F NMR (560 MHz, DMSO-d6): δ = −110.3; HRMS (TOF EI+): m/z calcd. for C24H25FNO4 [M + H]+, 410.1762; found, 410.1761.
(1R,5R)-4-(4-Fluorobenzyl)-9,9-dimethoxy-2-(4-methoxyphenyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5b)
Brown solid; yield: 391 mg, 92%; mp 190.6 °C; IR (KBr): 2834, 1720, 1562, 1509, 1436, 1248, 1147, 829, 600 cm–1; 1H NMR (500 MHz, CDCl3): δ = 2.60–2.67 (m, 2H, COCH2), 2.87–2.91 (m, 2H, COCH2CHN), 3.28 (s, 3H, CH3O), 3.48 (s, 3H, CH3O), 3.77 (s, 3H, OCH3Ar), 3.75–3.79 (m, 1H, CH), 4,20–4.24 (m, 1H, CH2CHN), 6.85–6.87 (m, 2H, ArH), 6.93–6.95 (m, 2H, ArH), 7.03–7.06 (m, 2H, ArH), 7.16 (s, 1H, CHN), 7.48–7.54 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 33.4, 43.4, 43.9, 49.0, 49.0, 55.6, 59.0, 97.6, 113.4, 115.1, 122.6, 130.6 (d, J = 8.8 Hz), 136.4, 137.8, 146.5, 157.7, 162.9, 164.9, 191.4, 208.7; HRMS (TOF EI+): m/z calcd. for C24H25FNO5 [M + H]+, 426.1711; found, 426.1710.
(1R,5R)-4-(4-Chlorobenzoyl)-9,9-dimethoxy-2-(p-tolyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5c)
Yellow solid; yield: 375 mg, 88%; mp 223.5–224.0 °C; IR (KBr): 3446, 2975, 2928, 1715, 1577, 1511, 1399, 1114, 839, 761 cm–1; 1H NMR (500 MHz, DMSO-d6): δ = 2.15–2.10 (s, 3H, CH3), 2.20–2.25 (m, 1H, COCH2), 2.38–2.44 (m, 1H, COCH2), 2.79–2.82 (m, 1H, COCH2CHN), 2.87–2.88 (m, 1H, COCH2CHN), 3.07 (s, 3H, CH3O), 3.36 (s, 3H, CH3O), 3.41–3.46 (m, 1H, CH), 4.41–4.46 (m, 1H, CH2CHN), 6.96–6.97 (m, 2H, ArH), 7.09–7.10 (m, 2H, ArH), 7.21 (s, 1H, CHN), 7.36–7.37 (m, 2H, ArH), 7.42–7.44 (m, 2H, ArH); 13C NMR (125 MHz, DMSO-d6): δ = 20.7, 20.7, 33.6, 43.4, 44.2, 49.0, 49.1, 57.4, 97.6, 113.8, 119.8, 128.9, 130.5, 130.7, 134.7, 135.4, 139.1, 141.7, 145.4, 190.8, 208.4; HRMS (TOF EI+): m/z calcd. for C24H25ClNO4 [M + H]+, 426.1467; found, 426.1467.
(1R,5R)-4-(4-Chlorobenzoyl)-9,9-dimethoxy-2-(4-methoxyphenyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5d)
Yellow solid; yield: 380 mg, 86%; mp 197.9 °C; IR (KBr): 3445, 1716, 1572, 1511, 1390, 1249, 1150, 831, 456 cm–1; 1H NMR (500 MHz, CDCl3): δ = 2.59–2.62 (m, 2H, COCH2), 2.87–2.91 (m, 2H, COCH2CHN), 3.28 (s, 3H, CH3O), 3.48 (s, 3H, CH3O), 3.75–3.76 (m, 1H, CH), 3.78 (s, 3H, OCH3Ar), 4.20–4.24 (m, 1H, CH2CHN), 6.85–6.87 (m, 2H, ArH), 6.93–6.95 (m, 2H, ArH), 7.15 (s, 1H, CHN), 7.33–7.34 (m, 2H, ArH), 7.42–7.43 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 33.4, 43.4, 43.9, 49.0, 49.0, 55.6, 59.0, 97.5, 113.4, 115.1, 122.7, 128.3, 129.9, 136.2, 137.7, 138.6, 146.7, 157.7, 191.3, 208.6; HRMS (TOF EI+): m/z calcd. for C24H25ClNO5 [M + H]+, 442.1416; found, 442.1424.
(1R,5R)-9,9-Diethoxy-4-(4-fluorobenzoyl)-2-(4-fluorophenyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5e)
Yellow solid; yield: 353 mg, 80%; mp 185.4 °C; IR (KBr): 3446, 2982, 2930, 1720, 1573, 1509, 1249, 1146, 841, 599 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.13–1.16 (m, 3H, CH3CH2O), 1.31–1.33 (m, 3H, CH3CH2O), 2.59–2.62 (m, 2H, COCH2), 2.91–2.97 (m, 2H, COCH2CHN), 3.41–3.65 (m, 4H, CH2O), 3.69–3.74 (m, 1H, CH), 4.24–4.28 (m, 1H, CH2CHN), 6.94–7.11 (m, 6H, ArH), 7.17 (s, 1H, CHN), 7.49–7.54 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.2, 15.3, 34.3, 43.4, 44.1, 56.9, 56.9, 59.6, 97.1, 115.2 (d, J = 21.3 Hz), 116.7 (d, J = 22.5 Hz), 122.2 (d, J = 8.8 Hz), 130.7 (d, J = 8.8 Hz), 136.2, 140.9, 145.6, 160.2 (d, J = 245.0 Hz), 164.0 (d, J = 248.8 Hz), 191.6, 208.6; HRMS (TOF EI+): m/z calcd. for C25H26F2NO4 [M + H]+, 442.1824; found, 442.1824.
(1R,5R)-9,9-Diethoxy-4-(4-fluorobenzoyl)-2-(p-tolyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5f)
Yellow solid; yield: 328 mg, 75%; mp 79.5 °C; IR (KBr): 3728, 3441, 2975, 2926, 1720, 1598, 1391, 1249, 766, 603 cm–1; H NMR (500 MHz, DMSO-d6): δ = 1.03–1.05 (m, 3H, CH3CH2O), 1.26–1.29 (m, 3H, CH3CH2O), 2.25–2.27 (m, 3H, CH3), 2.28–2.32 (m, 1H, COCH2), 2.45–2.49 (m, 1H, COCH2), 2.87–2.91 (m, 1H, COCH2CHN), 2.97–3.01 (m, 1H, COCH2CHN), 3.30–3.35 (m, 1H, CH), 3.48–3.56 (m, 2H, CH2O), 3.72–3.76 (m, 2H, CH2O), 4.48–4.52 (m, 1H, CH2CHN), 6.99–7.02 (m, 2H, ArH), 7.15–7.20 (m, 2H, ArH), 7.25–7.27 (m, 2H, ArH), 7.28 (s, 1H, CHN), 7.47–7.50 (m, 2H, ArH); 13C NMR (125 MHz, DMSO-d6): δ = 15.6, 15.6, 20.7, 20.7, 34.5, 43.5, 44.4, 56.8, 56.9, 58.0, 97.3, 114.1, 115.7 (d, J = 21.3 Hz), 119.5, 130.7, 131.1 (d, J = 8.8 Hz), 134.5, 136.9 (d, J = 2.5 Hz), 141.9, 145.1, 163.7 (d, J = 246.3 Hz), 190.8, 208.5; 19F NMR (467 MHz, DMSO-d6): δ = −110.3; HRMS (TOF EI+): m/z calcd. for C26H29FNO4 [M + H]+, 438.2075; found, 438.2074.
(1R,5R)-9,9-Diethoxy-4-(4-fluorobenzoyl)-2-(4-methoxyphenyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5g)
Brown solid; yield: 317 mg, 70%; mp 148.3 °C; IR (KBr): 3445, 2980, 1719, 1572, 1511, 1436, 1249, 1147, 836, 558 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.14–1.17 (m, 3H, CH3CH2O), 1.30–1.33 (m, 3H, CH3CH2O), 2.59–2.62 (m, 2H, COCH2), 2.90–2.94 (m, 2H, COCH2CHN), 3.46–3.49 (m, 1H, CH), 3.60–3.63 (m, 2H, CH2O), 3.69–3.74 (m, 2H, CH2O), 3.81 (s, 3H, CH3O), 4,21–4.25 (m, 1H, CH2CHN), 6.84–6.86 (m, 2H, ArH), 6.92–6.94 (m, 2H, ArH), 7.15 (s, 1H, CHN), 7.32–7.34 (m, 2H, ArH), 7.41–7.43 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.3, 15.3, 34.1, 43.5, 44.1, 55.6, 56.8, 56.9, 59.8, 97.2, 113.7, 115.1, 122.6, 128.3, 129.9, 136.1, 137.9, 138.7, 146.7, 157.6, 191.3, 208.9; HRMS (TOF EI+): m/z calcd. for C26H29FNO5 [M + H]+, 454.2024; found, 454.2025.
(1R,5R)-4-(4-Chlorobenzoyl)-9,9-diethoxy-2-(4-methoxyphenyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5h)
Yellow solid; yield: 305 mg, 65%; mp 138.0 °C; IR (KBr): 3445, 1717, 1574, 1511, 1437, 1248, 1148, 832, 555 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.14–1.17 (m, 3H, CH3CH2O), 1.30–1.33 (m, 3H, CH3CH2O), 2.59–2.62 (m, 2H, COCH2), 2.89–2.94 (m, 2H, COCH2CHN), 3.46–3.49 (m, 1H, CH), 3.60–3.63 (m, 2H, CH2O), 3.71–3.74 (m, 2H, CH2O), 3.74 (s, 3H, CH3OAr), 4,20–4.24 (m, 1H, CH2CHN), 6.84–6.86 (m, 2H, ArH), 6.92–6.93 (m, 2H, ArH), 7.03–7.06 (m, 2H, ArH), 7.15 (s, 1H, CHN), 7.47–7.50 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.3, 15.3, 34.2, 43.5, 44.1, 55.6, 56.9, 59.8, 97.2, 113.8, 115.0, 115.2, 122.5, 130.6, 136.5, 138.0, 146.5, 157.6, 162.9, 164.8, 191.4, 209.0; HRMS (TOF EI+): m/z calcd. for C26H29ClNO5 [M + H]+, 470.1729; found, 470.1729.
(1R,5R)-2-Benzyl-4-(4-chlorobenzoyl)-9,9-dimethoxy-2-azabicyclo[3.3.1]non-3-en-7-one (5i)
Yellow liquid; yield: 332 mg, 78%; IR (KBr): 3446, 2943, 1716, 1577, 1398, 1360, 1113, 1009, 698 cm–1; 1H NMR (500 MHz, CDCl3): δ = 2.42–2.56 (m, 2H, COCH2), 2.73–2.85 (m, 2H, COCH2CHN), 3.03 (s, 3H, CH3O), 3.38 (s, 3H, CH3O), 3.61–3.65 (m, 1H, CH), 3.67–3.72 (m, 1H, CH2CHN), 4.27 (s, 2H, ArH), 6.97 (s, 1H, CHN), 7.21–7.37 (m, 9H, ArH); 13C NMR (125 MHz, CDCl3): δ = 33.2, 43.0, 44.1, 48.6, 48.9, 56.2, 58.2, 97.5, 110.3, 128.2, 128.3, 128.6, 129.0, 129.8, 135.0, 135.8, 138.8, 150.1, 190.6, 209.8; HRMS (TOF EI+): m/z calcd. for C24H25ClNO4 [M + H]+, 426.1467; found, 426.1465.
(1R,5R)-2,4-Dibenzyl-9,9-dimethoxy-2-azabicyclo[3.3.1]non-3-en-7-one (5j)
Yellow liquid; yield: 289 mg, 74%; IR (KBr): 3727, 3446, 2922, 1716, 1582, 1439, 1212, 1112, 701, 557 cm–1; 1H NMR (500 MHz, CDCl3): δ = 2.41–2.44 (m, 1H, COCH2), 2.55–2.58 (m, 1H, COCH2), 2.71–2.75 (m, 1H, COCH2CHN), 2.80–2.84 (m, 1H, COCH2CHN), 3.03 (s, 3H, CH3O), 3.36 (s, 3H, CH3O), 3.60–3.65 (m, 1H, CH), 3.70–3.71 (m, 1H, CH2CHN), 4.25 (s, 2H, CH2Ar), 7.03 (s, 1H, CHN), 7.20–7.21 (m, 2H, ArH), 7.29–7.37 (m, 6H, ArH), 7.40–7.42 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 33.1, 43.0, 44.1, 48.6, 48.9, 56.0, 58.1, 97.5, 110.2, 128.0, 128.2, 128.3, 128.5, 129.0, 129.7, 135.2, 140.5, 150.3, 192.0, 209.1; HRMS (TOF EI+): m/z calcd. for C24H26NO4 [M + H]+, 392.1856; found, 392.1864.
(1R)-4-(4-Chlorobenzoyl)-9,9-diethoxy-2-(4-fluorobenzyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5k)
Yellow liquid; yield: 330 mg, 70%; IR (KBr): 3446, 2975, 2928, 1715, 1577, 1511, 1399, 1114, 839, 761 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.12–1.14 (m, 3H, CH3CH2O), 1.24–1.27 (m, 3H, CH3CH2O), 2.40–2.44 (m, 1H, COCH2), 2.52–2.55 (m, 1H, COCH2), 2.73–2.77 (m, 1H, COCH2CHN), 2.90–2.95 (m, 1H, COCH2CHN), 3.29–3.32 (m, 1H, CH), 3.45–3.54 (m, 2H, CH2O), 3.57–3.61 (m, 1H, CH2CHN), 3.67–3.72 (m, 2H, CH2O), 4.22–4.25 (m, 2H, CH2Ar), 6.92 (s, 1H, CHN), 7.03–7.06 (m, 2H, ArH), 7.21–7.26 (m, 2H, ArH), 7.31–7.35 (m, 3H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.2, 15.2, 33.8, 43.0, 44.2, 56.5, 56.7, 57.3, 96.9, 110.6, 115.7, 115.9, 128.3, 129.7 (d, J = 6.3 Hz), 131.1 (d, J = 3.8 Hz), 135.9, 138.8, 150.1, 162.7 (d, J = 246.3 Hz), 190.6; HRMS (TOF EI+): m/z calcd. for C26H28ClFNO4 [M + H]+, 472.1685; found, 472.1688.
(1R,5R)-2-Benzyl-4-(4-chlorobenzoyl)-9,9-diethoxy-2-azabicyclo[3.3.1]non-3-en-7-one (5l)
Yellow liquid; yield: 295 mg, 65%; IR (KBr): 3726, 3446, 2973, 2928, 1717, 1576, 1439, 1115, 876 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.10–1.13 (m, 3H, CH3CH2O), 1.23–1.26 (m, 3H, CH3CH2O), 2.42–2.45 (m, 1H, COCH2), 2.52–2.55 (m, 1H, COCH2), 2.73–2.77 (m, 1H, COCH2CHN), 2.88–2.92 (m, 1H, COCH2CHN), 3.23–3.25 (m, 1H, CH), 3.43–3.55 (m, 2H, CH2O), 3.63 (m, 1H, CH2CHN), 3.66–3.71 (m, 2H, CH2Ar), 6.96 (s, 1H, CHN), 7.23–7.37 (m, 9H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.2, 15.2, 33.9, 43.1, 44.2, 56.4, 56.7, 57.3, 58.1, 97.0, 110.4, 127.5, 128.0, 128.4, 128.9, 130.0, 135.2, 135.7, 138.9, 150.3, 190.5, 209.4; HRMS (TOF EI+): m/z calcd. for C24H25ClNO4 [M + H]+, 426.1467; found, 426.1468.
(1R,5R)-4-Benzoyl-2-benzyl-9,9-diethoxy-2-azabicyclo[3.3.1]non-3-en-7-one (5m)
Yellow liquid; yield: 331 mg, 79%; IR (KBr): 3726, 3446, 2974, 2926, 1717, 1583, 1212, 1113, 700, 584 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.11–1.13 (m, 3H, CH3CH2O), 1.23–1.26 (m, 3H, CH3CH2O), 2.41–2.45 (m, 1H, COCH2), 2.55–2.58 (m, 1H, COCH2), 2.72–2.76 (m, 1H, COCH2CHN), 2.89–2.93 (m, 1H, COCH2CHN), 3.23–3.26 (m, 1H, CH), 3.46–3.55 (m, 2H, CH2O), 3.60–3.65 (m, 1H, CH2CHN), 3.68–3.72 (m, 2H, CH2O), 4.26 (s, 2H, CH2N), 7.00 (s, 1H, CHN), 7.23–7.41 (m, 10H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.2, 15.2, 33.8, 43.1, 44.3, 56.4, 56.6, 57.2, 58.0, 97.1, 110.4, 128.0, 128.0, 128.3, 128.3, 128.8, 129.7, 135.4, 140.6, 150.5, 192.0, 209.5; HRMS (TOF EI+): m/z calcd. for C26H30NO4 [M + H]+, 420.2169; found, 420.2170.
(1R,5R)-4-(4-Fluorobenzoyl)-9,9-dimethoxy-2-phenethyl-2-azabicyclo[3.3.1]non-3-en-7-one (5n)
Yellow solid; yield: 300 mg, 71%; mp 200.0–200.5 °C; IR (KBr): 3445, 1717, 1574, 1511, 1437, 1248, 1148, 832, 555 cm–1; 1H NMR (500 MHz, CDCl3): δ = 2.49–2.55 (m, 2H, COCH2), 2.78–2.90 (m, 4H, COCH2CHN, CH2Ar), 3.22–3.24 (m, 1H, CH), 3.28 (s, 3H, CH3O), 3.39–3.43 (m, 1H, CH2CHN), 3.45 (s, 3H, CH3O), 3.66–3.71 (m, 1H, CH2N), 6.51 (s, 1H, CHN), 6.89–6.93 (m, 2H, ArH), 7.10–7.12 (m, 2H, ArH), 7.17–7.19 (m, 2H, ArH), 7.24–7.32 (m, 3H, ArH); 13C NMR (125 MHz, CDCl3): δ = 32.9, 35.4, 43.7, 44.0, 48.7, 48.9, 56.1, 57.4, 97.4, 109.1, 114.8 (d, J = 21.3 Hz), 126.8, 128.7, 129.2, 130.3 (d, J = 7.5 Hz), 136.3, 137.8, 150.2, 163.5 (d, J = 247.5 Hz), 190.1, 209.0; HRMS (TOF EI+): m/z calcd. for C25H27FNO4 [M + H]+, 424.1919; found, 424.1917.
(1R,5R)-9,9-Diethoxy-4-(4-fluorobenzoyl)-2-phenethyl-2-azabicyclo[3.3.1]non-3-en-7-one (5o)
Yellow liquid; yield: 338 mg, 75%; IR (KBr): 3726, 3446, 2975, 2928, 1715, 1600, 1563, 1454, 1114, 701 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.22–1.25 (m, 3H, CH3CH2O), 1.30–1.33 (m, 3H, CH3CH2O), 2.51–2.54 (m, 2H, COCH2), 2.77–2.81 (m, 4H, COCH2CHN, CH2Ar), 3.26–3.48 (m, 2H, CH2O), 3.58–3.64 (m, 3H, CH2O, CH2N), 3.64–3.68 (m, 1H, CH), 3.69–3.73 (m, 1H, CH2CHN), 3.79–3.85 (m, 1H, CH2N), 6.51 (s, 1H, CHN), 6.89–6.92 (m, 2H, ArH), 7.11–7.13 (m, 2H, ArH), 7.20–7.21 (m, 2H, ArH), 7.27–7.32 (m, 3H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.3, 15.4, 33.7, 35.3, 43.7, 44.2, 55.9, 56.7, 56.7, 58.1, 97.1, 114.7, 114.9, 126.8, 128.7, 129.2, 130.4 (d, J = 8.8 Hz), 136.4, 137.9, 150.5, 163.5 (d, J = 247.5 Hz), 190.1, 209.4; 19F NMR (467 MHz, DMSO-d6): δ = −111.1; HRMS (TOF EI+): m/z calcd. for C27H31FNO4 [M + H]+, 452.2232; found, 452.2232.
(1R,5R)-4-(4-Chlorobenzoyl)-2-(4-chlorophenethyl)-9,9-diethoxy-2-azabicyclo[3.3.1]non-3-en-7-one (5p)
Yellow solid; yield: 401 mg, 80%; mp 75.6 °C; IR (KBr): 3725, 3445, 2975, 2927, 1715, 1576, 1399, 1200, 1114, 839 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.16–1.25 (m, 3H, CH3CH2O), 1.30–1.33 (m, 3H, CH3CH2O), 2.47–2.53 (m, 2H, COCH2), 2.75–2.81 (m, 2H, COCH2CHN), 2.93–2.97 (m, 1H, CH2Ar), 3.21–3.26 (m, 1H, CH2Ar), 3.36–3.41 (m, 1H, CH2N), 3.58–3.69 (m, 5H, CH2N, CH2O, CH2O, CH), 3.79–3.83 (m, 1H, CH2CHN), 6.39–6.43 (m, 1H, CHN), 7.04–7.06 (m, 2H, ArH), 7.14–7.15 (m, 2H, ArH), 7.24–7.29 (m, 4H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.3, 15.4, 33.5, 34.7, 43.7, 44.2, 55.7, 56.7, 56.7, 58.2, 97.1, 109.5, 128.2, 128.8, 129.5, 130.7, 132.8, 135.6, 136.3, 138.6, 150.3, 190.2, 209.2; HRMS (TOF EI+): m/z calcd. for C27H30Cl2NO4 [M + H]+, 502.1546; found, 502.1544.
(1R,5R)-4-(4-Chlorobenzoyl)-9,9-diethoxy-2-(4-methoxyphenethyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5q)
Yellow solid; yield: 343 mg, 69%; mp 140.3 °C; IR (KBr): 3445, 1709, 1552, 1511, 1455, 1202, 1103, 826, 775 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.23–1.26 (m, 3H, CH3CH2O), 1.30–1.33 (m, 3H, CH3CH2O), 2.48–2.53 (m, 2H, COCH2), 2.72–2.82 (m, 3H, COCH2CHN, CH2Ar), 2.91–2.95 (m, 1H, CH2Ar), 3.23–3.26 (m, 1H, CH2N), 3.35–3.38 (m, 1H, CH2N), 3.57–3.66 (m, 5H, CH2O, CH2O, CH), 3.80–3.82 (m, 1H, CH2CHN), 3.83 (s, 3H, OCH3), 6.45 (s, 1H, CHN), 6.84–6.85 (m, 2H, ArH), 7.04–7.11 (m, 4H, ArH), 7.19–7.21 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.3, 15.4, 33.5, 34.5, 43.8, 44.2, 55.2, 56.2, 56.7, 56.7, 58.3, 97.1, 109.2, 114.1, 128.0, 129.7, 130.2, 135.4, 138.7, 150.6, 158.6, 190.1, 209.3; HRMS (TOF EI+): m/z calcd. for C28H33ClNO5 [M + H]+, 498.2042; found, 498.2043.
(1R,5R)-4-Benzoyl-9,9-diethoxy-2-(4-methoxyphenethyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5r)
Yellow liquid; yield: 402 mg, 87%; IR (KBr): 3424, 3278, 1718, 1625, 1556, 1514, 1360, 1245, 1074, 757 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.22–1.32 (m, 6H, CH3CH2O), 2.47–2.55 (m, 2H, COCH2), 2.71–2.80 (m, 3H, COCH2CHN, CH2Ar), 2.92–2.96 (m, 1H, CH2Ar), 3.20–3.23 (m, 1H, COCH2CHN), 3.31–3.35 (m, 1H, CH), 3.6 (s, 3H, OCH3), 3.67–3.71 (m, 1H, CHN), 3.80–3.83 (m, 4H, CH2O), 3.80–3.83 (m, 4H, CH2O), 6.56 (s, 1H, CHN), 6.83–6.84 (m, 2H, ArH), 7.09–7.27 (m, 6H, ArH), 7.31–7.34 (m, 1H, ArH); 13C NMR (125 MHz, CDCl3): δ = 15.3, 15.4, 33.5, 34.5, 43.8, 44.2, 55.2, 56.1, 56.7, 56.7, 58.1, 97.2, 109.4, 114.1, 127.8, 128.3, 129.5, 129.8, 130.1, 140.4, 150.6, 158.5, 191.4, 209.5; HRMS (TOF EI+): m/z calcd. for C28H34NO5 [M + H]+, 464.2431; found, 464.2432.
(1R,5R)-2-(4-Chlorobenzyl)-4-(4-fluorobenzoyl)-2-azaspiro[bicyclo[3.3.1]nonane-9,2′-[1,3]-dioxolan]-3-en-7-one (5s)
Yellow liquid; yield: 291 mg, 66%; IR (KBr): 3424, 3278, 1718, 1625, 1556, 1514, 1360, 1245, 1074, 757 cm–1; 1H NMR (500 MHz, DMSO-d6): δ = 2.26–2.29 (m, 1H, COCH2), 2.37–2.40 (m, 1H, COCH2), 2.79–2.80 (m, 1H, COCH2CHN), 2.94–2.95 (m, 1H, COCH2CHN), 3.09–3.10 (m, 1H, CH), 3.47–3.51 (m, 1H, CH2CHN), 3.63–3.65 (m, 1H, CH2O), 3.99–4.02 (m, 1H, CH2O), 4.04–4.08 (m, 2H, CH2O), 4.39–4.42 (m, 1H, CH2N), 4.52–4.55 (m, 1H, CH2N), 7.18–7.22 (m, 2H, ArH), 7.27 (s, 1H, CHN), 7.32–7.35 (m, 2H, ArH), 7.44–7.47 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 35.9, 43.8, 45.0, 57.2, 58.3, 65.2, 65.4, 104.9, 110.7, 115.9 (d, J = 22.5 Hz), 128.3, 129.4 (d, J = 8.8 Hz), 129.8, 131.0 (d, J = 2.5 Hz), 135.9, 138.7, 149.6, 162.7 (d, J = 245.0 Hz), 190.7, 208.4; HRMS (TOF EI+): m/z calcd. for C24H22ClFNO4 [M + H]+, 442.1216; found,442.1215.
(1R,5R)-2-Benzyl-4-(4-chlorobenzoyl)-2-azaspiro[bicyclo[3.3.1]nonane-9,2′-[1,3]dioxolan]-3-en-7-one (5t)
Yellow liquid; yield: 296 mg, 70%; IR (KBr): 3424, 3278, 1718, 1625, 1556, 1514, 1360, 1245, 1074, 757 cm–1; 1H NMR (500 MHz, CDCl3): δ = 2.31–2.32 (m, 1H, COCH2), 2.43–2.46 (m, 1H, COCH2), 2.84–2.88 (m, 1H, COCH2CHN), 2.99–3.30 (m, 1H, COCH2CHN), 3.13–3.17 (m, 1H, CH), 3.54–3.58 (m, 1H, CHN), 3.68–3.73 (m, 1H, CH2CH2), 4.03–4.13 (m, 1H, CH2CH2), 4.45–4.48 (m, 1H, CH2Ar), 4.59–4.62 (m, 1H, CH2Ar), 7.32–7.44 (m, 8H, ArH), 7.48–7.52 (m, 1H, ArH), 7.51 (s, 1H, CHN); 13C NMR (125 MHz, CDCl3): δ = 36.0, 43.8, 45.0, 58.0, 58.3, 65.1, 65.4, 104.9, 110.5, 127.8, 128.2, 128.4, 128.9, 129.8, 135.1, 135.8, 138.8, 149.9, 190.7, 208.6; HRMS (TOF EI+): m/z calcd. for C24H23ClNO4 [M + H]+, 424.1310; found, 424.1310.
(1R,5R)-4-(4-Fluorobenzoyl)-9-methoxy-9-methyl-2-(4-methylbenzyl)-2-azabicyclo[3.3.1]non-3-en-7-one (5u)
Yellow liquid; yield: 248 mg, 61%; mp 165.6 °C; IR (KBr): 3445, 2926, 1712, 1571, 1510, 1252, 1154, 765, 504 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.77 (s, 3H, CH3), 2.30 (s, 3H, CH3Ar), 2.66–2.74 (m, 2H, COCH2), 2.75–2.78 (m, 2H, COCH2CHN), 3.25 (s, 3H, CH3), 3.64–3.65 (m, 1H, CH), 4.09–4.13 (m, 1H, CHN), 6.89–6.90 (m, 2H, ArH), 7.03–7.07 (m, 2H, ArH), 7.11–7.13 (m, 2H, ArH), 7.28 (s, 1H, CHN), 7.49–7.52 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 18.5, 20.7, 34.1, 44.2, 44.3, 49.7, 63.1, 71.2, 113.2, 115.1 (d, J = 21.3 Hz), 120.9, 130.3, 130.7 (d, J = 7.5 Hz), 135.4, 136.4, 142.4, 146.4, 163.9 (d, J = 247.5 Hz), 191.7, 208.0; HRMS (TOF EI+): m/z calcd. for C25H27FNO3 [M + H]+, 408.1969; found, 408.1968.
(1R,5R)-Ethyl 9,9-dimethoxy-7-oxo-3-(trifluoromethyl)-2-azabicyclo[3.3.1]non-3-ene-4-carboxylate (6a)
Yellow solid; yield: 269 mg, 80%; mp 77.3 °C; IR (KBr): 3446, 2975, 1691, 1468, 1399, 1187, 1129, 925, 790, 436 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.24–1.27 (m, 3H, CH3), 2.40–2.48 (m, 2H, COCH2), 2.77–2.82 (m, 2H, COCH2CHN), 3.32 (s, 3H, OCH3), 3.42 (s, 3H, OCH3), 3.44–3.48 (m, 1H, CH), 3.89–3.93 (m, 1H, CH2CHN), 4.12–4.21 (m, 2H, CH2O), 5.29 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 13.9, 36.2, 43.1, 46.0, 48.6, 49.0, 50.7, 60.5, 96.0, 101.0, 120.4 (q, J = 274.6 Hz), 138.5 (q, J = 33.8 Hz), 164.9, 208.7; 19F NMR (467 MHz, DMSO-d6): δ = −64.0; HRMS (TOF EI+): m/z calcd. for C14H19F3NO5 [M + H]+, 338.1210; found, 338.1213.
(1R,5R)-Ethyl 9,9-dimethoxy-3-methyl-7-oxo-2-azabicyclo[3.3.1]non-3-ene-4-carboxylate (6b)
Yellow solid; yield: 232 mg, 82%; mp 77.3 °C; IR (KBr): 3446, 2975, 1691, 1468, 1399, 1187, 1129, 925, 790, 436 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.23–1.25 (m, 3H, CH3CH2), 2.19 (s, 3H, CH3), 2.34–2.37 (m, 1H, COCH2), 2.45–2.48 (m, 1H, COCH2), 2.73–2.80 (m, 2H, COCH2CHN), 3.30 (s, 3H, OCH3), 3.40 (s, 3H, OCH3), 3.75–3.79 (m, 1H, CH), 4.06–4.12 (m, 2H, CH2), 4.78–4.79 (m, 2H, CHN); 13C NMR (125 MHz, CDCl3): δ = 14.5, 20.8, 29.6, 35.5, 44.1, 46.8, 48.6, 50.4, 51.4, 59.0, 94.6, 97.0, 129.9, 143.4, 152.0, 167.4, 210.7; HRMS (TOF EI+): m/z calcd. for C14H22NO5 [M + H]+, 284.1492; found, 284.1494.
(1R,5R)-Isopropyl 9,9-dimethoxy-3-methyl-7-oxo-2-azabicyclo[3.3.1]non-3-ene-4-carboxylate (6c)
Yellow liquid; yield: 267 mg, 90%; IR (KBr): 3725, 3386, 2959, 2834, 1715, 1526, 1245, 1087, 976, 816 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.23–1.25 (m, 3H, CH3CH2O), 2.19 (s, 3H, CH3), 2.34–2.37 (m, 1H, COCH2), 2.45–2.48 (m, 1H, COCH2), 2.73–2.80 (m, 2H, COCH2CHN), 3.30 (s, 3H, CH3O), 3.36–3.40 (m, 1H, CH), 3.75–3.79 (m, 1H, CH2CHN), 4.06–4.12 (m, 2H, CH2O), 4.78–4.79 (m, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 14.5, 20.8, 35.5, 44.1, 46.8, 48.6, 48.7, 51.4, 59.0, 94.6, 97.0, 152.0, 167.4, 210.7; HRMS (TOF EI+): m/z calcd. for C15H24NO5 [M + H]+, 298.1649; found, 298.1652.
(1R,5R)-9,9-Dimethoxy-3-methyl-7-oxo-2-azabicyclo[3.3.1]non-3-ene-4-carbonitrile (6d)
Yellow liquid; yield: 226 mg, 96%; IR (KBr): 3362, 2920, 2184, 1716, 1612, 1531, 1401, 1101, 420 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.99 (s, 3H, CH3), 2.35–2.38 (m, 1H, COCH2), 2.49–2.52 (m, 1H, COCH2), 2.74–2.78 (m, 2H, COCH2CHN), 2.88–2.92 (m, 1H, CH), 3.34 (s, 3H, OCH3), 3.38 (s, 3H, OCH3), 3.80–3.84 (m, 1H, CHN), 5.10–5.11 (m, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 18.9, 36.7, 43.7, 46.3, 48.8, 50.4, 51.1, 75.4, 96.1, 121.5, 152.4, 208.8; HRMS (TOF EI+): m/z calcd. for C12H17N2O3 [M + H]+, 237.1234; found, 237.1234.
(1R,5R)-Ethyl 9,9-diethoxy-7-oxo-3-(trifluoromethyl)-2-azabicyclo[3.3.1]non-3-ene-4-carboxylate (6e)
Yellow solid; yield: 321 mg, 88%; mp 116.5 °C; IR (KBr): 3370, 2983, 2936, 1720, 1671, 1612, 1518, 1404, 1167, 830 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.17–1.20 (m, 3H, CH3), 1.22–1.30 (m, 6H, CH3CH2O), 2.40–2.48 (m, 2H, COCH2), 2.79–2.85 (m, 2H, COCH2CHN), 3.45–3.49 (m, 1H, CH), 3.56–3.72 (m, 4H, CH2O), 3.85–3.89 (m, 1H, CH2CHN), 4.12–4.20 (m, 2H, CH2), 5.01 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 13.9, 15.2, 15.2, 36.8, 43.1, 46.1, 51.5, 51.5, 56.9, 60.5, 95.7, 101.5, 120.5 (q, J = 273.8 Hz), 138.4 (q, J = 33.8 Hz), 164.9, 208.7; HRMS (TOF EI+): m/z calcd. for C16H23F3NO5 [M + H]+, 366.1523; found, 366.1524.
(1R,5R)-9,9-Diethoxy-3-methyl-7-oxo-2-azabicyclo[3.3.1]non-3-ene-4-carbonitrile (6f)
Yellow liquid; yield: 242 mg, 92%; IR (KBr): 3375, 2927, 2930, 2184, 1716, 1612, 1528, 1401, 1101, 420 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.21–1.23 (m, 3H, CH3CH2O), 1.25–1.28 (m, 3H, CH3CH2O), 1.98 (s, 3H, CH3), 2.33–2.36 (m, 1H, COCH2), 2.49–2.52 (m, 1H, COCH2), 2.76–2.81 (m, 1H, COCH2CHN), 2.88–2.92 (m, 1H, CH), 3.60–3.67 (m, 4H, CH2O), 3.77–3.81 (m, 1H, CHN), 4.78 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 15.2, 15.3, 18.9, 37.5, 43.8, 46.5, 52.1, 56.6, 56.7, 75.9, 95.8, 121.5, 152.1, 208.9; HRMS (TOF EI+): m/z calcd. for C14H21N2O3 [M + H]+, 265.1547; found, 265.1548.
General Procedure for the Synthesis of Compounds 7
The quinone monoketals 1 (1.1 mmol), enamine esters 3 (1.0 mmol), and acetone (15 mL) were charged into a 25 mL round-bottomed flask. Then DBU (2.0 mmol) was added into the mixture. The mixture was at reflux for about 12 h. After the reaction was completed, the reaction system was cooled to room temperature. The reaction mixture was poured into 25 mL of water and 50 mL of ethyl acetate for extraction and separation. Then, the crude product was concentrated by distillation under reduced pressure, which was purified by column chromatography (petroleum ether/ethyl acetate = 1: 2) and a series of compounds 7 with 87–92% yields was obtained.
tert-Butyl-((1R,5R)-4-(4-fluorobenzoyl)-2-(4-fluorophenyl)-9,9-dimethoxy-2-azabicyclo[3.3.1]-nona-3,6-dien-7-yl)carbamate (7a)
Yellow solid; yield: 455 mg, 89%; mp 222.7–223.2 °C; IR (KBr): 3430, 3254, 3056, 1729, 1562, 1506, 1230, 1145, 770 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.46 (s, 9H, CH3), 2.26–2.29 (m, 1H, CH2CHN), 2.71–2.72 (m, 1H, CH2CHN), 3.11 (s, 3H, OCH3), 3.35 (s, 3H, OCH3), 3.52–3.53 (m, 1H, CH), 4.32–4.33 (m, 1H, CHN), 5.95–5.99 (m, 1H, CHCH), 6.4 (br, 1H, NH), 7.01–7.02 (m, 4H, ArH), 7.05–7.09 (m, 2H, ArH), 7.22 (s, 1H, CHC), 7.52–7.56 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 28.3, 31.5, 35.2, 48.6, 49.6, 56.0, 60.4, 80.6, 97.4, 100.4, 115.1 (d, J = 21.3 Hz), 115.6, 116.5 (d, J = 22.5 Hz), 120.8 (d, J = 8.75 Hz), 130.7 (d, J = 7.5 Hz), 136.7 (d, J = 3.8 Hz), 137.6, 141.0, 145.6, 152.2, 159.8 (d, J = 243.8 Hz), 163.9 (d, J = 248.8 Hz), 192.1. HRMS (TOF EI+): m/z calcd. for C28H31F2N2O5 [M + H]+, 513.2196; found, 513.2194.
tert-Butyl((1R,5R)-4-(4-fluorobenzoyl)-9,9-dimethoxy-2-(4-methoxyphenyl)-2-azabicyclo[3.3.1]nona-3,6-dien-7-yl)carbamate (7b)
Yellow solid; yield: 471 mg, 90%; mp 238.2–238.7 °C; IR (KBr): 3261, 2965, 2837, 1732, 1509, 1326, 1233, 1070, 768 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.46 (s, 9H, CH3), 2.27–2.30 (m, 1H, CH2CHN), 2.69–2.73 (m, 1H, CH2CHN), 3.12 (s, 3H, OCH3), 3.36 (s, 3H, OCH3), 3.51–3.52 (m, 1H, CH), 3.78 (s, 3H, OCH3Ar), 4.31–4.32 (m, 1H, CHN), 6.06–6.10 (m, 1H, CHCH), 6.35 (br, 1H, NH), 6.83–6.86 (m, 2H, ArH), 6.99–7.07 (m, 2H, ArH), 7.23 (s, 1H, CHC), 7.52–7.55 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 28.5, 31.4, 34.8, 48.4, 49.4, 55.4, 55.8, 79.3, 97.5, 101.2, 115.0, 115.4, 115.6 (d, J = 21.3 Hz), 120.6, 131.0 (d, J = 8.8 Hz), 137.5, 138.0, 138.5, 145.3, 152.9, 156.6, 163.4 (d, J = 245.0 Hz), 190.9. HRMS (TOF EI+): m/z calcd. for C29H34FN2O6 [M + H]+, 525.2395; found, 525.2392.
tert-Butyl((1R,5R)-4-(4-chlorobenzoyl)-9,9-dimethoxy-2-phenyl-2-azabicyclo[3.3.1]nona-3,6-dien-7-yl)carbamate (7c)
Yellow liquid; yield: 456 mg, 87%; IR (KBr): 3425, 3062, 1727, 1539, 1497, 1240, 1147, 1051, 760 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.46 (s, 9H, CH3), 2.27–2.30 (m, 1H, CH2CHN), 2.71–2.72 (m, 1H, CH2CHN), 3.10 (s, 3H, OCH3), 3.36 (s, 3H, OCH3), 3.51–3.52 (m, 1H, CH), 4.42–4.43 (m, 1H, CHN), 5.96–6.00 (m, 1H, CHCH), 6.38 (br, 1H, NH), 7.04–7.06 (m, 2H, ArH), 7.27 (s, 1H, CHC), 7.31–7.37 (m, 4H, ArH), 7.48–7.49 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 28.3, 31.7, 35.1, 48.6, 49.6, 55.4, 80.5, 97.4, 100.7, 115.6, 118.7, 124.5, 128.3, 129.8, 130.0, 136.1, 137.5, 139.0, 144.4, 145.6, 152.2, 192.2. HRMS (TOF EI+): m/z calcd. for C28H32ClN2O5 [M + H]+, 511.1994; found, 511.1994.
tert-Butyl((1R,5R)-4-(4-chlorobenzoyl)-9,9-dimethoxy-2-(p-tolyl)-2-azabicyclo[3.3.1]nona-3,6 -dien-7-yl)carbamate (7d)
Yellow solid; yield: 456 mg, 87%; mp 243.5–244.0 °C; IR (KBr): 3265, 2969, 1726, 1512, 1430, 1391, 1245, 1150, 762 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.38 (s, 9H, CH3), 2.25 (s, 3H, CH3), 2.25 (s, 1H, CH2CHN), 3.00 (s, 3H, OCH3), 3.23–3.27 (m, 1H, CH), 3.36 (s, 3H, OCH3), 4.44–4.46 (m, 1H, CHN), 6.15–6.19 (m, 1H, CHCH), 7.02–7.04 (m, 2H, ArH), 7.15–7.17 (m, 2H, ArH), 7.23 (br, 1H, NH), 7.44–7.48 (m, 4H, ArH), 8.58 (s, 1H, CHC); 13C NMR (125 MHz, CDCl3): δ = 20.7, 28.5, 31.5, 34.7, 48.4, 49.4, 55.0, 79.3, 97.4, 101.2, 115.5, 118.7, 128.8, 130.5, 130.7, 133.8, 135.1, 138.5, 139.6, 142.0, 145.0, 152.9, 191.1. HRMS (TOF EI+): m/z calcd. for C29H34ClN2O5 [M + H]+, 525.2151; found, 525.2150.
tert-Butyl-((1R,5R)-4-(4-chlorobenzoyl)-9,9-dimethoxy-2-(4-methoxyphenyl)-2-azabicyclo[3.3.1]nona-3,6-dien-7-yl)carbamate (7e)
Yellow solid; yield: 496 mg, 92%; mp 136.2 °C; IR (KBr): 3351, 2833, 1732, 1509, 1434, 1389, 1238, 1148, 762 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.46 (s, 9H, CH3), 2.27–2.30 (m, 1H, CH2CHN), 2.70–2.73 (m, 1H, CH2CHN), 3.12 (s, 3H, OCH3), 3.36 (s, 3H, OCH3), 3.51–3.52 (m, 1H, CH), 3.78 (s, 3H, OCH3Ar), 4.31–4.32 (m, 1H, CHN), 6.03–6.07 (m, 1H, CHCH), 6.35 (br, 1H, NH), 6.84–6.85 (m, 2H, ArH), 6.99–7.01 (m, 2H, ArH), 7.27 (s, 1H, CHC), 7.33–7.35 (m, 2H, ArH), 7.46–7.48 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3): δ = 28.3, 31.5, 35.2, 48.6, 49.6, 55.6, 56.2, 80.5, 97.5, 100.8, 114.7, 114.9, 121.0, 128.3, 130.0, 135.9, 137.5, 138.2, 139.2, 146.4, 152.3, 157.0, 191.8. HRMS (TOF EI+): m/z calcd. for C29H34ClN2O6 [M + H]+, 541.2100; found, 541.2101.
General Procedure for the Synthesis of Compounds 8
The quinone monoketals 1 (1.1 mmol), enaminones 4 (1.0 mmol), and acetone (15 mL) were charged into a 25 mL round-bottomed flask. Then, DBU (2.0 mmol) was added into the mixture. The mixture was at reflux for about 12 h. After the reaction was completed, the reaction system was cooled to room temperature. The reaction mixture was poured into 25 mL of water and 50 mL of ethyl acetate for extraction and separation. Then, the crude product was concentrated by distillation under reduced pressure, which was purified by column chromatography (petroleum ether/ethyl acetate = 1: 2) and a series of compounds 8 with 63–66% yields were obtained.
(2S,6S)-6-Hydroxy-3,3-dimethoxy-2,3,6,8,9,10-hexahydro-2,6-methanobenzo[b]azocin-7(1H)-one (8a)
Yellow liquid; yield: 175 mg, 66%; IR (KBr): 3414, 3233, 2943, 1617, 1528, 1428, 1343, 1121, 770 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.63–1.66 (m, 1H, CH2), 1.72–1.73 (m, 1H, CH2), 1.78–1.79 (m, 1H, CH2NH), 2.09–2.11 (m, 1H, CH2NH), 2.13–2.15 (m, 1H, CH2NH), 2.35–2.40 (m, 2H, CH2C), 3.11 (s, 3H, OCH3), 3.21 (s, 3H, OCH3), 3.72–3.76 (m, 1H, CHN), 5.43–5.46 (m, 1H, CH3C), 5.89–5.92 (m, 1H, CHC), 7.70 (br, 1H, NH), 7.93 (br, 1H, OH); 13C NMR (125 MHz, CDCl3): δ = 21.7, 27.9, 33.6, 36.5, 47.9, 48.9, 52.0, 69.0, 99.9, 106.7, 121.3, 140.9, 160.6, 194.1. HRMS (TOF EI+): m/z calcd. for C14H18NO4 [M – H+], 264.1241; found, 264.1241.
(2S,6S)-3,3-Diethoxy-6-hydroxy-2,3,6,8,9,10-hexahydro-2,6-methanobenzo[b]azocin-7(1H)-one (8b)
Yellow liquid; yield: 184 mg, 63%; IR (KBr): 3414, 3240, 2971, 1617, 1379, 1191, 1010, 605 cm–1; 1H NMR (500 MHz, CDCl3): δ = 1.01–1.11 (m, 3H, CH3CH2O), 1.12–1.15 (m, 3H, CH3CH2O), 1.62–1.65 (m, 1H, CH2), 1.71–1.80 (m, 2H, CH2, CH2CH), 2.07–2.17 (m, 3H, CH2CH, CH2CO), 2.32–2.43 (m, 2H, CH, CHC), 3.37–3.50 (m, 3H, CH2O, CH2O), 3.59–3.62 (m, 1H, CH2O), 5.41–5.43 (s, 1H, CH), 5.85–5.87 (s, 1H, CH), 7.69 (br, 1H, OH), 7.85 (br, 1H, NH); 13C NMR (125 MHz, CDCl3): δ = 15.8, 15.8, 21.7, 27.9, 33.7, 36.5, 52.8, 55.3, 56.5, 69.0, 99.8, 106.8, 122.4, 140.3, 160.6, 194.1. HRMS (TOF EI+): m/z calcd. for C16H22NO4 [M – H+], 292.1554; found, 292.1555.
Acknowledgments
This work was supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT17R94), the National Natural Science Foundation of China (nos. 21662042, 81760621, 21362042, and U1202221), the Natural Science Foundation of Yunnan Province (2017FA003), and Donglu Scholars of Yunnan University (WX173602).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00726.
Spectroscopic and analytical data as well as the original copy of 1H and 13C NMR spectra of all new compounds (PDF)
X-ray crystallographic data of compounds 5n (CCDC 1817955) (CIF)
X-ray crystallographic data of compounds 6e (CCDC 1817956) (CIF)
X-ray crystallographic data of compounds 7b (CCDC 1818163) (CIF)
X-ray crystallographic data of compounds 8a (CCDC 1818117)(CIF)
Author Contributions
§ X.-M.H. and B.Z. contributed equally to this article.
The authors declare no competing financial interest.
Supplementary Material
References
- Cannon J. S.; Overman L. E. Is There No End to the Total Syntheses of Strychnine? Lessons Learned in Strategy and Tactics in Total Synthesis. Angew. Chem., Int. Ed. 2012, 51, 4288–4311. 10.1002/anie.201107385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer B. L. Drug discovery: Designing the ideal opioid. Nature 2016, 537, 170–171. 10.1038/nature19424. [DOI] [PubMed] [Google Scholar]
- Huo H.-H.; Xia X.-E.; Zhang H.-K.; Huang P.-Q. Enantioselective Total Syntheses of (−)-FR901483 and (+)-8-epi-FR901483. J. Org. Chem. 2013, 78, 455–465. 10.1021/jo302362b. [DOI] [PubMed] [Google Scholar]
- Yao Y.; Liang G. Rapid Construction of the ABC Ring System in the Daphniphyllum Alkaloid Daphniyunnine C. Org. Lett. 2012, 14, 5499–5501. 10.1021/ol3026395. [DOI] [PubMed] [Google Scholar]
- Gates M.; Montzka T. A. Some Potent Morphine Antagonists Possessing High Analgesic Activity1. J. Med. Chem. 1964, 7, 127–131. 10.1021/jm00332a002. [DOI] [PubMed] [Google Scholar]
- Carroll F. I.; Melvin M. S.; Nuckols M. C.; Mascarella S. W.; Navarro H. A.; Thomas J. B. N-Substituted 4β-Methyl-5-(3-hydroxyphenyl)-7α-amidomorphans Are Potent, Selective κ Opioid Receptor Antagonists. J. Med. Chem. 2006, 49, 1781–1791. 10.1021/jm058264p. [DOI] [PubMed] [Google Scholar]
- Palmer D. C.; Strauss M. J. Benzomorphans: synthesis, stereochemistry reactions, and spectroscopic characterizations. Chem. Rev. 1977, 77, 1–36. 10.1021/cr60305a003. [DOI] [Google Scholar]
- Bosch J.; Bonjoch J. Synthesis of 2-Azabicyclo[3.3.1]nonanes. Heterocycles 1980, 14, 505 10.3987/R-1980-04-0505. [DOI] [Google Scholar]
- Bonjoch J.; Diaba F.; Bradshaw B. Synthesis of 2-Azabicyclo[3.3.1]nonanes. Synthesis 2011, 7, 993–1018. 10.1055/s-0030-1258420. [DOI] [PubMed] [Google Scholar]
- Kang B.; Jakubec P.; Dixon D. J. Strategies towards the synthesis of calyciphylline A-type Daphniphyllum alkaloids. Nat. Prod. Rep. 2014, 31, 550–562. 10.1039/C3NP70115H. [DOI] [PubMed] [Google Scholar]
- Gammack Yamagata A. D.; Datta S.; Jackson K. E.; Stegbauer L.; Paton R. S.; Dixon D. J. Enantioselective Desymmetrization of Prochiral Cyclohexanones by Organocatalytic Intramolecular Michael Additions to α,β-Unsaturated Esters. Angew. Chem., Int. Ed. 2015, 54, 4899–4903. 10.1002/anie.201411924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L.; Tian X.-C.; Wang C.; Cui Q.; Li W.-H.; Huang S.-H.; Yu Z.-X.; Hong R. Construction of Morphan Derivatives by Nitroso–Ene Cyclization: Mechanistic Insight and Total Synthesis of (±)-Kopsone. Angew. Chem., Int. Ed. 2017, 56, 11599–11603. 10.1002/anie.201706018. [DOI] [PubMed] [Google Scholar]
- Wu W.-T.; Zhang L.; You S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 2016, 45, 1570–1580. 10.1039/C5CS00356C. [DOI] [PubMed] [Google Scholar]
- Liu P.; Fukui Y.; Tian P.; He Z.-T.; Sun C.-Y.; Wu N.-Y.; Lin G.-Q. Cu-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Enynes. J. Am. Chem. Soc. 2013, 135, 11700–11703. 10.1021/ja404593c. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C.; Hale C. R. H.; Nilewski C.; Loannidou H. A.; Elmarrouni A.; Nilewski L. G.; Beabout K.; Wang T. T.; Shamoo Y. Total Synthesis of Viridicatumtoxin B and Analogues Thereof: Strategy Evolution, Structural Revision, and Biological Evaluation. J. Am. Chem. Soc. 2014, 136, 12137–12160. 10.1021/ja506472u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T.; Washimi N.; Kamitanaka T.; Fukushima K.-I.; Kita Y. Coupling of Quinone Monoacetals Promoted by Sandwiched Brønsted Acids: Synthesis of Oxygenated Biaryls. Angew. Chem., Int. Ed. 2011, 50, 6142–6146. 10.1002/anie.201101646. [DOI] [PubMed] [Google Scholar]
- Chen K.; Liu S.; Wang D.; Hao W.-J.; Zhou P.; Tu S.-J.; Jiang B. Silver/Scandium-Cocatalyzed Bicyclization of β-Alkynyl Ketones Leading to Benzo[c]xanthenes and Naphtho[1,2-b]benzofurans. J. Org. Chem. 2017, 82, 11524–11530. 10.1021/acs.joc.7b02134. [DOI] [PubMed] [Google Scholar]
- Hashimoto T.; Gálvez A. O.; Maruoka K. Boronic Acid-Catalyzed, Highly Enantioselective Aza-Michael Additions of Hydroxamic Acid to Quinone Imine Ketals. J. Am. Chem. Soc. 2015, 137, 16016–16019. 10.1021/jacs.5b11518. [DOI] [PubMed] [Google Scholar]
- Wang L.; Shi L.-X.; Liu L.; Li Z.-X.; Xu T.; Hao W.-J.; Li G.-G.; Tu S.-J.; Jiang B. Synthesis of Diastereoenriched Oxazolo[5,4-b]indoles via Catalyst-Free Multicomponent Bicyclizations. J. Org. Chem. 2017, 82, 3605–3611. 10.1021/acs.joc.7b00129. [DOI] [PubMed] [Google Scholar]
- Wan J.-P.; Jiang Y.; Hu C.; Sheng S. Metal-Free Synthesis of Fully Substituted Pyridines via Ring Construction Based on the Domino Reactions of Enaminones and Aldehydes. J. Org. Chem. 2016, 81, 6826–6831. 10.1021/acs.joc.6b01149. [DOI] [PubMed] [Google Scholar]
- Wan J.-P.; Cao S.; Liu Y.-Y. Base-Promoted Synthesis of N-Substituted 1,2,3-Triazoles via Enaminone–Azide Cycloaddition Involving Regitz Diazo Transfer. Org. Lett. 2016, 18, 6034–6037. 10.1021/acs.orglett.6b02975. [DOI] [PubMed] [Google Scholar]
- Wan J.-P.; Gao Y. Domino Reactions Based on Combinatorial Bond Transformations in Electron-Deficient Tertiary Enamines. Chem. Rec. 2016, 16, 1164–1177. 10.1002/tcr.201500296. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Hu C.-F.; Wen C.-P.; Wan J.-P. Tunable Synthesis of Disulfide-Functionalized Enaminones and 1,4-Thiazines via the Reactions of Enaminones and β-Aminoethanethiol. ACS Omega 2017, 2, 7784–7789. 10.1021/acsomega.7b01422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J.-P.; Lin Y.-F.; Huang Q.; Liu Y.-Y. Diastereoselective Construction of Tetrahydropyridine Fused Bicyclic Structures via Three-Component Domino Reaction. J. Org. Chem. 2014, 79, 7232–7238. 10.1021/jo501292q. [DOI] [PubMed] [Google Scholar]
- Wang K.-M.; Yan S.-J.; Lin J. Heterocyclic Ketene Aminals: Scaffolds for Heterocycle Molecular Diversity. Eur. J. Org. Chem. 2014, 6, 1129–1145. 10.1002/ejoc.201300929. [DOI] [Google Scholar]
- Li M.; Zhou Z.-M.; Wen L.-R.; Qiu Z.-X. Chemistry of Heterocyclic Ketene Aminals: Construction of Imidazo(pyrido)[1,2-a]pyridines and Imidazo(pyrido)[3,2,1-ij][1,8]naphthy-ridines via DABCO-Catalyzed Tandem Annulations. J. Org. Chem. 2011, 76, 3054–3063. 10.1021/jo102167g. [DOI] [PubMed] [Google Scholar]
- Chen L.; Huang R.; Kong L.-B.; Lin J.; Yan S.-J. Facile Route to the Synthesis of 1,3-Diazahetero-Cycle-Fused[1,2-a]Quinoline Derivatives via Cascade Reactions. ACS Omega 2018, 3, 1126–1136. 10.1021/acsomega.7b01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Shao P.; Wang S.-W.; Kong W.; Wen L.-R. Four-Component Cascade Heteroannulation of Heterocyclic Ketene Aminals: Synthesis of Functionalized Tetrahydro-imidazo[1,2-a]pyridine Derivatives. J. Org. Chem. 2012, 77, 8956–8967. 10.1021/jo3013836. [DOI] [PubMed] [Google Scholar]
- Bao H.; Shao X.; Zhang Y.; Deng Y.; Xu X.; Liu Z.; Li Z. Specific Synergist for Neonicotinoid Insecticides: IPPA08, a cis-Neonicotinoid Compound with a Unique Oxabridged Substructure. J. Agric. Food Chem. 2016, 64, 5148–5155. 10.1021/acs.jafc.6b01512. [DOI] [PubMed] [Google Scholar]
- Cui S.-S.; Huang R.; Luo D.-Y.; Lin J.; Yan S.-J. Selective Synthesis of Highly Functionalized Bicyclic Pyridinone and 1,3-Oxazinane Derivatives. Eur. J. Org. Chem. 2017, 24, 3442–3450. 10.1002/ejoc.201700283. [DOI] [Google Scholar]
- Zeng C.-C.; Liu F.-J.; Ping D.-W.; Hu L.-M.; Cai Y.-L.; Zhong R.-G. One-Pot Electrochemical Synthesis of Fused Indole Derivatives Containing Active Hydroxyl Groups in Aqueous Medium. J. Org. Chem. 2009, 74, 6386–6389. 10.1021/jo901091s. [DOI] [PubMed] [Google Scholar]
- Du X.-X.; Huang R.; Yang C.-L.; Lin J.; Yan S.-J. Synthesis and evaluation of the antitumor activity of highly functionalised pyridin-2-ones and pyrimidin-4-ones. RSC Adv. 2017, 7, 40067–40073. 10.1039/C7RA06466G. [DOI] [Google Scholar]
- Wang B.-Q.; Zhang C.-H.; Tian X.-X.; Lin J.; Yan S.-J. Cascade Reaction of Isatins with 1,1-Enediamines: Synthesis of Multisubstituted Quinoline-4-carboxamides. Org. Lett. 2018, 20, 660–663. 10.1021/acs.orglett.7b03803. [DOI] [PubMed] [Google Scholar]
- Wang B.-Q.; Luo Q.; Xiao Q.; Lin J.; Yan S.-J. Synthesis of Quinone Methide Substituted Neonicotinoid Derivatives via 1,6-Conjugate Addition of N-Benzyl Nitro Ketene Aminals with para-Quinone Methides Accompanying Oxidation. ACS Sustainable Chem. Eng. 2017, 5, 8382–8389. 10.1021/acssuschemeng.7b02166. [DOI] [Google Scholar]
- Yu F.-C.; Lin X.-R.; Liu Z.-C.; Zhang J.-H.; Liu F.-F.; Wu W.; Ma Y.-L.; Qu W.-W.; Yan S.-J.; Lin J. Beyond the Antagonism: Self-Labeled Xanthone Inhibitors as Modeled “Two-in-One” Drugs in Cancer Therapy. ACS Omega 2017, 2, 873–889. 10.1021/acsomega.6b00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.-L.; Wang K.-M.; Huang R.; Lin J.; Yan S.-J. An environmentally benign double Michael addition reaction of heterocyclic ketene aminals with quinone monoketals for diastereoselective synthesis of highly functionalized morphan derivatives in water. Green Chem. 2017, 19, 3574–3584. 10.1039/C7GC01435J. [DOI] [Google Scholar]
- Hu X.-M.; Luo D.-Y.; Zi Q.-X.; Lin J.; Yan S.-J. Diastereoselective Synthesis of Morphan Derivatives by Michael and Aza-Michael Addition of 1,1-Enediamines to Quinone Monoketals. ACS Omega 2018, 3, 8–21. 10.1021/acsomega.7b01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Zhou B.; Zhou P.; Zhou J.; Shen Y.-H.; Yu F.-C.; Lu L.-L. Insights into the unexpected chemoselectivity in Brønsted acid catalyzed cyclization of isatins with enaminones: convenient synthesis of pyrrolo[3,4-c]quinolin-1-ones and spirooxindoles. Chem. Commun. 2016, 52, 8002–8005. 10.1039/C6CC02659A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.












