Abstract
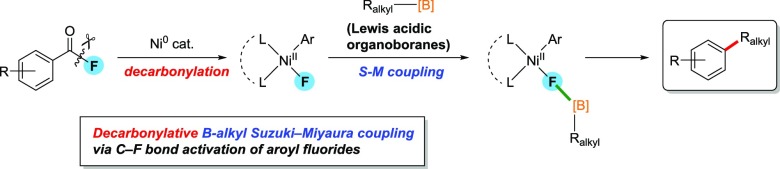
Herein, nickel-catalyzed decarbonylative C–F bond alkylation of aroyl fluorides with organoboron reagents is reported. Aroyl fluorides are more chemically stable than the corresponding aroyl chlorides and can be readily synthesized from the corresponding carboxylic acids. The fluoronickel intermediate formed via oxidative addition interacts with Lewis-acidic trialkylboranes, and the subsequent decarbonylative alkylation proceeds. This new synthetic methodology allows 1,2-bifunctionalization of aromatic carboxylic acids via palladium-catalyzed ortho-C–H arylation. In addition, an unprecedented 1,4-nickel migration on ortho-arylated aroyl fluorides was observed. As a demonstration of the synthetic utility of the present reaction, the sequential 1-alkyl-2-arylation of 3-hydroxy-2-naphthoic acid was accomplished via chemoselective alkylation at a fluorocarbonyl moiety and the subsequent C–O bond arylation at an acetoxy group.
Introduction
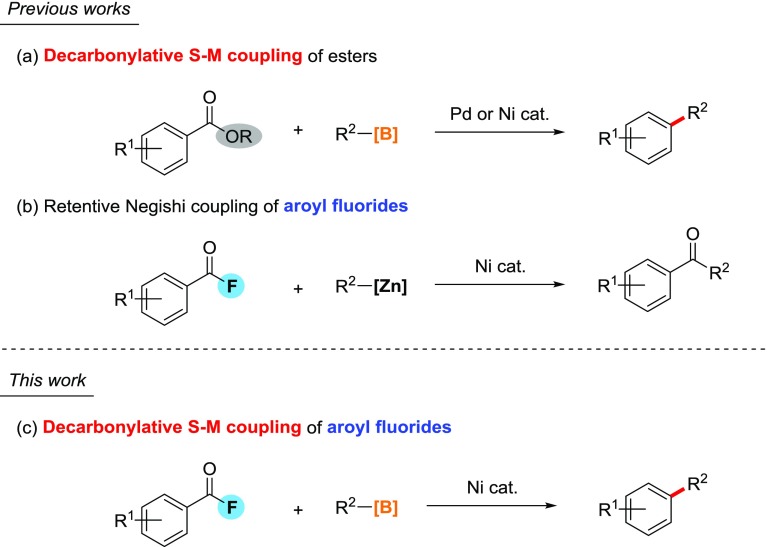
In the past three decades, palladium-catalyzed alkylation of aroyl chlorides and anhydrides with organozinc,1 aluminum,2 tin,3 and lead compounds4 in a retentive manner (i.e., where the reaction proceeds without decarbonylation) has been utilized to afford unsymmetrical alkyl aryl ketones. In 2000, the first palladium-catalyzed Suzuki–Miyaura (S–M) alkylation reaction of aroyl chlorides with alkylboranes was reported.5 Since then, transition-metal-catalyzed S–M alkylation reaction has been widely applied as one of the most reliable and versatile synthetic methods to construct C(sp2)–C(sp3) bonds.6 An example of the utility of these reactions is found in the total synthesis of epothilones.7 In general, S–M alkylation requires a special palladium complex bearing a sterically hindered bidentate ligand because the target C(sp2)–C(sp3) bond formation should occur prior to a competing β-hydride elimination.8
Decarbonylative cross-coupling of carboxylic acid derivatives has been developed as an alternative class of transition-metal-catalyzed cross-coupling reactions. Hence, development of S–M coupling reaction of carboxylic acid derivatives with alkylboron reagents is expected to be a new sustainable synthetic strategy of C–C bond formation because naturally abundant carboxylic acid derivatives could readily be synthesized and then be utilized as aryl electrophile equivalents. In general, decarbonylative coupling reaction requires relatively harsh conditions to overcome the high activation barrier to decarbonylation. Malanga reported a Ni-catalyzed decarbonylative reduction of aroyl chlorides with tin hydride into the corresponding simple arenes,9 where decarbonylation of a (aroyl)(chloro)nickel complex predominates above 50 °C. In addition, previously reported density functional theory (DFT) studies indicated that a relatively high activation barrier of 20–30 kcal/mol exists for decarbonylation.10
Recent development of decarbonylative cross-couplings of aromatic carboxylic acid derivatives have been extensively reported. For example, Yu and co-workers reported Rh(I)-catalyzed decarbonylative C–H functionalization of aroyl chlorides.11 Sanford demonstrated widespread functionalizations via decarbonylative chlorination of aroyl chlorides.12 In the viewpoint of utilization of aromatic esters, Itami–Yamaguchi, Love, and Rueping independently have demonstrated a practical Ni- or Pd-catalyzed decarbonylative arylation of phenyl esters with organoboron or zinc compounds through C(aroyl)–OPh bond cleavages and sequential decarbonylation (Scheme 1a).13 On the other hand, regarding the use of aroyl fluorides as substrates, there have been only one example, which is a catalytic Negishi coupling in a retentive manner, affording unsymmetrical alkyl aryl ketones, reported by Rovis (Scheme 1b),14 but no precedent decarbonylative transformation of aroyl fluorides have been disclosed. Accordingly, we paid much attention to develop an unprecedented decarbonylative alkylation of aroyl fluorides. Herein, we report a first example of contrasting alkylation of aroyl fluorides with Lewis-acidic alkylboranes. In previous studies on alkylation in S–M coupling reaction, Lewis-acidic trialkylboranes have been applied to accelerate transmetalation of alkyl groups via interaction of oxygen in alkoxypalladium complexes with a boron center.8,15 We herein describe a novel C–F bond functionalization strategy to construct alkyl–aryl scaffold starting from naturally abundant carboxylic acid (Scheme 1c).
Scheme 1. Ni-Catalyzed Retentive vs Decarbonylative C–F Bond Functionalization.
Results and Discussion
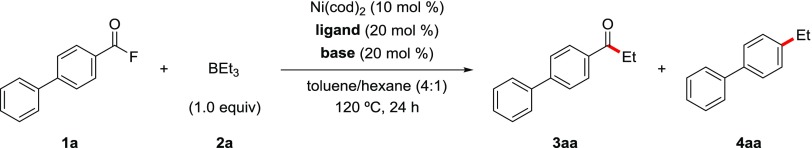
In an initial study, we optimized reaction conditions for the nickel-catalyzed C(aroyl)–F bond ethylation of biphenyl-4-carbonyl fluoride (1a) with BEt3 (2a, 1.0 equiv) (Table 1). We first elucidated the ligand effect (entries 1–5). To our delight, the reaction with an air-stable and moderately electron-donating 1,2-bis(diphenylphosphino)ethane (DPPE) ligand selectively delivered the target decarbonylative cross-coupled product 4-ethylbiphenyl (4aa), in 83% yield (entry 3). Very recently, Rueping and co-workers have reported decarbonylative coupling of aromatic ethers, and the related nickel/bidentate phosphine catalysis could accelerate this decarbonylative coupling.13d,13e In contrast, results with a strong σ-donating N-heterocyclic carbene, ICy·HCl, ligand gave the retentive product of 4-phenylacetophenone (3aa) in 27% yield (entry 5). This result is notable because the reaction proceeds in a completely retentive manner in spite of the fast oxidative addition obtained with the strong σ-donating ICy·HCl ligand. We also optimized reaction temperature; 4aa was obtained in 85% yield at 130 °C (entry 6), but in only 28% at 110 °C (entry 8). We finally performed the reaction without any ligand, but no product was formed (entry 9).
Table 1. Optimization of Reaction Conditions in Nickel(0)-Catalyzed C(aroyl)–F Bond Ethylation of Biphenyl-4-Carbonyl Fluoride (1a) with BEt3 (2a)a.
| yield (%)b |
||||
|---|---|---|---|---|
| entry | ligand | base | 3aa | 4aa |
| 1 | PPh3 | none | 0 | 21 |
| 2 | PCy3 | none | 22 | 26 |
| 3c | DPPE | none | 0 | 83 (78) |
| 4c | DPPBz | none | 0 | 45 |
| 5 | ICy·HCl | pyridine | 27 | trace |
| 6c,d | DPPE | none | 0 | 85 (85) |
| 7c,e | DPPE | none | 0 | 77 (73) |
| 8c,f | DPPE | none | 0 | 28 |
| 9 | none | none | 0 | 0 |
Reaction conditions: 1a (0.125 mmol, 1.0 equiv), 2a (0.125 mmol, 1.0 equiv), Ni(cod)2 (0.0125 mmol, 10 mol %), ligand (0.025 mmol, 20 mol %), base (0.025 mmol, 20 mol %), solvent (0.5 mL, 0.25 M of 1a), 120 °C, 24 h.
Determined by 1H NMR analysis of the crude mixture, using dibromomethane as an internal standard. An isolated yield was shown in parentheses.
10 mol % of ligand was used.
At 130 °C for 24 h.
At 140 °C for 24 h.
At 110 °C for 24 h. DPPE, 1,2-bis(diphenylphosphino)ethane; DPPBz, 1,2-bis(diphenylphosphino)benzene; ICy·HCl, 1,3-dicyclohexylimidazolium chloride; Ni(cod)2, bis(1,5-cyclooctadiene)nickel(0); PCy3, tricyclohexylphosphine.
We then also examined the reaction with an array of catalysts and aromatic carboxylic acid derivatives (see Supporting Information). As a result, we identified that the current decarbonylative alkylation was possible with the economical and air-stable Ni(OAc)2·4H2O precatalyst (80% NMR yield of 4aa at 130 °C), while NiCl2 showed no activity (Table S4). Notably, alternative carboxylic acid derivatives, such as aroyl chlorides, esters, and thioesters, did not participate in the reaction (Table S5). These results clearly indicate that the nickel-catalyzed decarbonylative alkylation is specific transformation for aroyl fluorides.
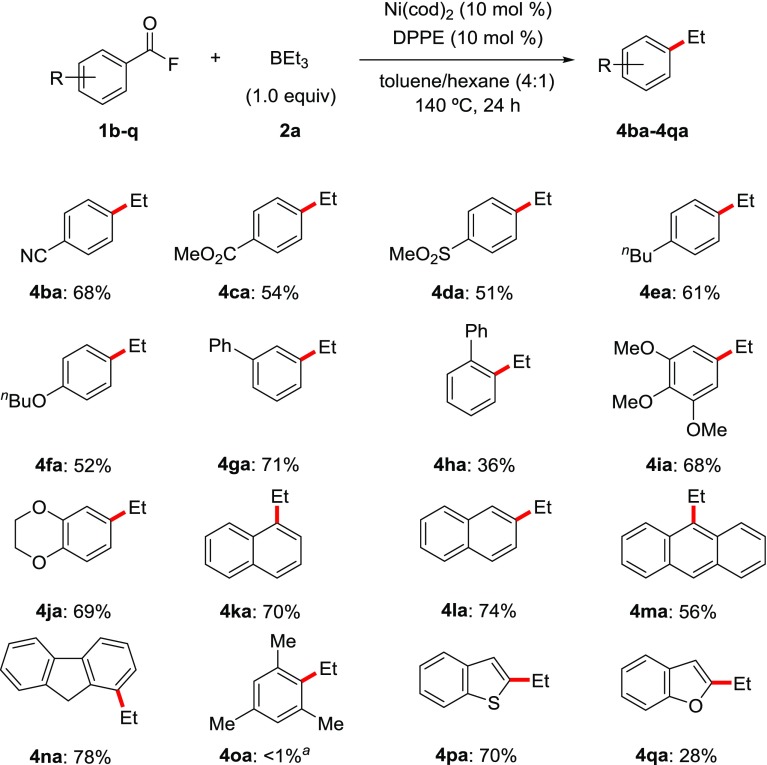
Our investigations on the scope of aroyl fluorides substrates for decarbonylative C(aroyl)–F bond ethylation are presented in Table 2. For this screen, we employed a higher reaction temperature of 140 °C to obtain better results. We established that products bearing electron-withdrawing 4-cyano (4ba, 68%), 4-methoxycarbonyl (4ca, 54%), 4-methylsulfonyl (4da, 51%), and electron-donating 4-butyl (4ea, 61%) and 4-butoxy (4fa, 52%) groups were formed in moderate to good yields. Biphenyl-3-carbonyl fluoride, in which phenyl group is installed in the meta-position, was also readily transformed to 3-ethylbiphenyl (4ga) as the target product in 71% yield. However, aroyl fluorides bearing a substituent in the ortho-position, such as biphenyl-2-carbonyl fluoride, gave lower yields (4ha, 36%). Decarbonylative ethylation of aroyl fluorides bearing multiple substituents (4ia, 68% and 4ja, 69%) or a fused ring (4ka–na; 56–78%) at the meta- and para-positions were also demonstrated. However, a substrate having a sterically hindered mesityl group did not afford any corresponding product at all (4oa, <1%), as predicted by a previous DFT study.9 Finally, we evaluated heterocyclic substituents. Benzo[b]thiophene-substituted aroyl fluoride afforded a good yield of the target product 4pa in 70% yield. However, benzo[b]furan-substituted aroyl fluoride gave low yield (4qa, 28%). During this substrate screening process, protonated products from β-hydride elimination were also detected (4/β-hydride elimination >10:1).
Table 2. Scope of (Hetero)aroyl Fluorides for Decarbonylative C((hetero)aroyl)–F Bond Alkylationa.
NMR yield based on 1,4-dioxane as an internal standard.
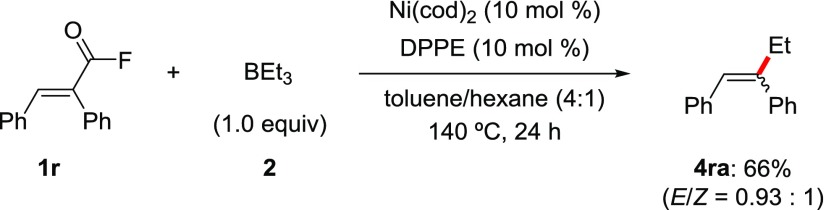
To expand the scope of the reaction, we examined the reaction with α-phenylcinnamoyl fluoride (1r), which proceeds via C(alkenyl)–F bond cleavage. Under the same reaction conditions, an E/Z mixture of products (4ra, 66% combined yield, E/Z = 0.93:1) was obtained. This result implies that decarbonylation and E/Z isomerization sequences are much faster than reductive elimination after the initial oxidative addition (Scheme 2).
Scheme 2.
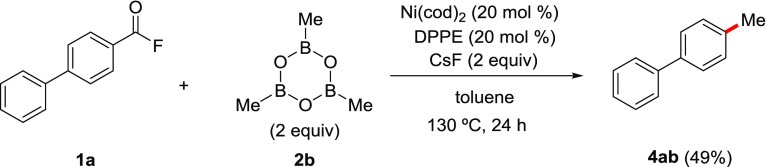
In the field of pharmaceuticals and natural product synthesis, methylation is the most common alkylation. However, trimethylborane is difficult to handle because it is a highly flammable gaseous substance at room temperature (rt). We thus evaluated the other reaction with alternative methyl-containing organoboranes (see Supporting Information, Table S3). Pleasingly, we show that use of trimethylboroxine (2b) in the presence of an equimolar amount of CsF efficiently afforded the target 4-methylbiphenyl (4ac), whereas excess CsF retarded the reaction. Finally, by employing a 2-fold greater amount of all of the reagents, we succeeded in obtaining 4ac in 49% yield (Scheme 3). Other methylating reagents such as SnMe4 and SiMe4 proved unsuitable for the present reaction.
Scheme 3.
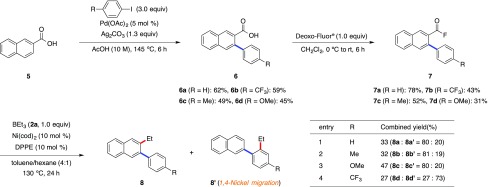
To apply this decarbonylative C–F bond alkylation, we developed a novel ortho-arylation/ipso-alkylation sequences since aroyl fluorides can be directly synthesized from the corresponding carboxylic acids. Several research groups have independently reported the palladium-catalyzed ortho-C–H bond arylation of carboxylic acids.16 We thus combined the ortho-C–H arylation protocol of carboxylic acid with the sequential fluorination17 and the present decarbonylative C–F bond alkylation (Scheme 4). As the first step, we synthesized 3-aryl-2-naphthoic acids (6a–d) in 45–62% yields under modified conditions of Daugulis’ procedure.16b Next, the corresponding aroyl fluorides (7a–d) were readily prepared by fluorination with nucleophilic fluorinating reagent (Deoxo-Fluor) in 31–78% yields. Finally, we carried out the nickel-catalyzed decarbonylative alkylation of the C–F bond. Notably, we detected a mixture of the target 2-ethyl-3-arylnaphtalenes (8a–d) and 2-(2-ethyl-4-substituted phenyl)naphthalenes (8a′–d′). As the possible reaction mechanism for the formation of unexpected regioisomers of 8a′–d′, we herein propose the unprecedented 1,4-nickel migration whose analogous phenomena have been well known for palladium,18 rhodium,19 cobalt,20 iridium,19m,21 platinum,18j and ruthenium.22 Only one example of 1,4-nickel migration has been reported, albeit in a bimetallic system.23 However, to the best of our knowledge, a monometallic 1,4-nickel migration has not been reported to date.
Scheme 4. ortho-Arylation, Fluorination, and ipso-Alkylation Sequences, Starting from 2-Naphthoic Acid (5).
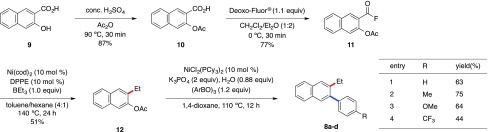
As an additional application, we planned more straightforward alkylation and arylation sequences. Previously, Itami reported the orthogonal couplings of different esters; decarbonylative coupling of phenyl ester containing C(aroyl)–O bond cleavage predominantly occurred prior to C(aryl)–O/C–H coupling.13a Hence, we next examined whether the present decarbonylative coupling can proceed prior to C(aryl)–O bond cross-coupling to realize a straightforward 1-alkyl-2-arylation. To investigate this strategy, we conducted the nickel-catalyzed C(aryl)–O bond arylation with triarylboroxines under the standard conditions reported by Shi.24 On the basis of the synthetic procedure, we started the chemoselective functionalization of the substrate having both fluorocarbonyl and acetoxy groups with organoboron under nickel catalysis (Scheme 5). 3-Acetoxy-2-(fluorocarbonyl)naphthalene (11) was readily synthesized in 62% overall yield through acid-catalyzed acetoxylation of 3-hydroxy-2-naphthoic acid (9) and the subsequent nucleophilic fluorination with Deoxo-Fluor. Subsequently, we examined the nickel-catalyzed decarbonylative alkylation of 11. To our delight, the desired 3-acetoxy-2-ethylnaphthalene (12) could be obtained in 51% yield without any C(aryl)–O bond-cleaved byproduct. Finally, we conducted the nickel-catalyzed arylation via C(aryl)–O bond cleavage and isolated 8a–d as the target products in 44–75% yield. Through this synthetic scheme, we accomplished a straightforward 1-alkyl-2-arylation sequence of a naturally abundant hydroxy carboxylic acid scaffold.
Scheme 5. Chemoselective Alkylation–Arylation Sequences, Starting from ortho-Hydroxy Carboxylic Acid 9.
Conclusions
In summary, we have developed a new protocol for decarbonylative C(aroyl)–F bond alkylation with Lewis-acidic organoboranes. Lewis acidity of organoboranes is required due to the acceleration of transmetalation in S–M reaction. Synthetically significant methylation was also demonstrated by applying trimethylboroxine. A thermodynamic driving force derived from B–F bond formation provides a nexus for the specific transformation of aroyl fluorides over aroyl halides. We are currently further investigating the mechanism of this novel monometallic 1,4-nickel migration.
Experimental Section
General
All of the reactions were carried out under Ar atmosphere using standard Schlenk techniques. Glassware was dried in an oven (150 °C) and heated under reduced pressure prior to use. Solvents were employed as eluents for all other routine operations, and dehydrated solvents were purchased from commercial suppliers and employed without any further purification. For thin-layer chromatography (TLC) analyses throughout this work, Merck precoated TLC plates (silica gel 60 GF254, 0.25 mm) were used. Silica gel column chromatography was carried out using silica gel 60 N (spherical, neutral, 40–100 μm) from Kanto Chemicals Co., Ltd. The 1H, 11B{1H}, 13C{1H}, and 19F{1H} NMR spectra were recorded on Varian INOVA-600 (600 MHz) spectrometers. Infrared spectra were recorded on a Shimadzu IR Prestige-21 spectrophotometer. Gas chromatography (GC) analyses were performed on a Shimadzu GC-14A equipped with a flame ionization detector using Shimadzu Capillary Column (CBP1-M25-025) and Shimadzu C-R6A-Chromatopac integrator. GC–mass spectrometry (MS) analyses were carried out on a SHIMADZU GC-17A equipped with a Shimadzu QP-5050 GC–MS system. Elemental analyses were carried out with a PerkinElmer 2400 CHN elemental analyzer at Okayama University. Compounds 1b,25,261c,271k,281l,27 and 1o,29 were prepared according to the reported literature methods.
Representative Procedure for the Synthesis of Aroyl Fluorides from Acid Chlorides
To a 20 mL of Schlenk tube charged with a magnetic stirrer bar, aroyl chlorides 1-Cl (2.0 mmol), 18-crown-6 (26.4 mg, 0.1 mmol, 5 mol %), KF (1.16 g, 20 mmol, 10 equiv), and tetrahydrofuran (10 mL) were successively added. After the reaction was stirred at 40 °C for 24 h, the insoluble inorganic solid (KF or KCl) was filtered and the volatiles were concentrated using a rotary evaporator. The crude product was purified by bulb-to-bulb distillation to afford the corresponding aroyl fluorides 1 in 30–72% yields.
Biphenyl-4-carbonyl Fluoride (1a)
Yield was 64%. mp: 42–45 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.43–7.46 (m, 1H), 7.48–7.51 (m, 2H), 7.63–7.65 (m, 2H), 7.77 (d, J = 7.8 Hz, 2H), 8.11–8.13 (m, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 123.6 (d, 2JC–F = 61 Hz), 127.5, 127.8, 128.9, 129.2, 132.1 (d, 3JC–F = 3 Hz), 139.4, 148.3, 157.5 (d, 1JC–F = 343 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 18.1. Fourier transform infrared (FT-IR) (neat, cm–1): 1803 (s), 1607 (s), 1450 (w), 1408 (m), 1256 (s), 1180 (w), 1030 (s), 1003 (s), 853 (m), 743 (s), 691 (m). Anal. Calcd for C13H9FO: C, 77.99; H, 4.53%. Found: C, 77.91; H, 4.48%.
4-Butylbenzoyl Fluoride (1e)
Yield was 30%. bp: 140 °C/80 mmHg. 1H NMR (CDCl3, 600 MHz, rt): δ 0.94 (t, J = 7.2 Hz, 3H), 1.36 (sext, J = 7.6 Hz, 2H), 1.60–1.65 (m, 2H), 2.70 (t, J = 7.8 Hz, 2H), 7.32 (d, J = 7.8 Hz, 2H), 7.95 (d, J = 8.4 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 14.0, 22.4, 33.2, 36.0, 122.4 (d, 2JC–F = 60 Hz), 129.3, 131.7 (d, 3JC–F = 5 Hz), 151.6, 157.7 (d, 1JC–F = 343 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 17.4. FT-IR (neat, cm–1): 2959 (s), 2932 (s), 2866 (m), 1807 (s), 1773 (m), 1609 (s), 1462 (w), 1418 (w), 1256 (s), 1177 (m), 1107 (m), 1007 (s), 878 (m), 845 (m), 737 (m), 694 (w). Anal. Calcd for C11H13FO: C, 73.31; H, 7.27%. Found: C, 73.29; H, 7.41%.
4-Butoxylbenzoyl Fluoride (1f)
Yield was 44%. bp: 140 °C/80 mmHg. 1H NMR (CDCl3, 600 MHz, rt): δ 0.98 (t, J = 7.5 Hz, 3H), 1.50 (sext, J = 7.4 Hz, 2H), 1.77–1.82 (m, 2H), 4.04 (t, J = 6.6 Hz, 2H), 6.95 (d, J = 8.4 Hz, 2H), 7.96 (d, J = 8.4 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 13.9, 19.2, 31.1, 68.3, 114.9, 116.6 (d, 2JC–F = 62 Hz), 133.8 (d, 3JC–F = 5 Hz), 157.4 (d, 1JC–F = 339 Hz), 165.0; 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 15.7. FT-IR (neat, cm–1): 2961 (s), 2938 (s), 2873 (m), 1803 (s), 1605 (s), 1510 (s), 1470 (m), 1319 (m), 1254 (s), 1171 (s), 1109 (m), 1024 (s), 999 (s), 968 (m), 846 (s), 691 (m). Anal. Calcd for C11H13FO2: C, 67.33; H, 6.68%. Found: C, 66.96; H, 6.89%.
1,2,3-Trimethoxy-5-benzoyl Fluoride (1i)
Yield was 34%. mp: 77–79 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 3.88 (s, 6H), 3.92 (s, 3H) 7.24 (s, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 56.4, 61.2, 108.7 (d, 3JC–F = 2 Hz), 119.4 (d, 2JC–F = 62 Hz), 144.4, 153.4, 157.3 (d, 1JC–F = 342 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 16.5. FT-IR (neat, cm–1): 1809 (s), 1591 (s), 1503 (m), 1462 (s), 1416 (s), 1339 (s), 1238 (s), 1167 (s), 1126 (s), 1070 (s), 995 (m), 891 (s), 858 (w), 748 (m), 681 (m). Anal. Calcd for C10H11FO4: C, 56.08; H, 5.18%. Found: C, 56.11; H, 5.24%.
Benzo[b]thiophene-2-carbonyl Fluoride (1p)
Yield was 46%. mp: 79–81 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.48 (ddd, J = 8.1, 7.2, 0.9 Hz, 1H), 7.56 (ddd, J = 8.4, 7.2, 1.2 Hz, 1H), 7.91 (dd, J = 8.4, 0.6 Hz, 1H), 7.95 (d, J = 8.4 Hz, 1H), 8.21 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 123.0, 125.8, 126.5, 126.9 (d, 2JC–F = 75 Hz), 128.6, 135.3 (d, 3JC–F = 2 Hz), 138.3, 143.8 (d, 3JC–F = 2 Hz), 153.3 (d, 1JC–F = 335 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 25.1. FT-IR (neat, cm–1): 1796 (s), 1680 (m), 1512 (s), 1165 (m), 962 (m), 756 (m), 714 (m). Anal. Calcd for C9H5FOS: C, 59.99; H, 2.80%. Found: C, 60.00; H, 2.55%.
Representative Procedure for the Synthesis of Aroyl Fluorides from Carboxylic Acids
To a 20 mL of Schlenk tube charged with a magnetic stirrer bar, carboxylic acids 1-OH (3.0 mmol) and CH2Cl2 (15 mL) were successively added. After the mixture was stirred at 0 °C, Deoxo-Fluor reagent (1.1 equiv, 608 μL, 3.3 mmol) was slowly added to the reaction mixture. After the reaction mixture was stirred at 0 °C for 30 min, the solution was slowly poured into saturated NaHCO3, and after CO2 evolution ceased, it was extracted into CH2Cl2 (3 × 15 mL) and dried over MgSO4. The crude product was purified by flash chromatography (CH2Cl2 (1d), Hex/Et2O = 10:1 (others)) to afford the corresponding aroyl fluorides 1 in 39–91% yields.
4-(Methylsulfonyl)benzoyl Fluoride (1d)
Yield was 50%. mp: 124–125 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 3.11 (s, 3H), 8.12 (d, J = 7.8 Hz, 2H), 8.25 (d, J = 8.4 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 44.3, 128.3, 129.8 (d, 2JC–F = 62 Hz), 132.5 (d, 3JC–F = 3 Hz), 146.6, 155.9 (d, 1JC–F = 347 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 20.9. FT-IR (neat, cm–1): 1802 (s), 1406 (m), 1325 (m), 1296 (m), 1252 (s), 1153 (s), 1040 (m), 1007 (w), 957 (w), 737 (s), 681 (s). Anal. Calcd for C8H7FO3S: C, 47.52; H, 3.49%. Found: C, 47.46; H, 3.10%.
Biphenyl-3-carbonyl Fluoride (1g)
Yield was 50%. mp: 43–45 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.42 (t, J = 7.8 Hz, 1H), 7.49 (t, J = 7.8 Hz, 2H), 7.60–7.62 (m, 3H), 7.92–7.94 (m, 1H), 8.03 (d, J = 7.8 Hz, 1H), 8.28 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 125.6 (d, 2JC–F = 60 Hz), 127.3, 128.4, 129.2, 129.7, 130.1 (d, 3JC–F = 5 Hz), 130.2 (d, 3JC–F = 3 Hz), 134.0, 139.3, 142.5, 157.6 (d, 1JC–F = 345 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 18.6. FT-IR (neat, cm–1): 1809 (s), 1477 (w), 1452 (w), 1416 (w), 1294 (s), 1217 (s), 1064 (m), 1036 (m), 1007 (m), 816 (m), 770 (m), 733 (s), 692 (m), 644 (m). Anal. Calcd for C13H9FO: C, 77.99; H, 4.53%. Found: C, 77.99; H, 4.50%.
Biphenyl-2-carbonyl Fluoride (1h)
Yield was 90%. 1H NMR (CDCl3, 600 MHz, rt): δ 7.34 (d, J = 7.8 Hz, 2H), 7.41–7.46 (m, 4H), 7.51 (t, J = 7.5 Hz, 1H), 7.68 (td, J = 7.5, 1.4 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 124.3 (d, 2JC–F = 57 Hz), 127.8, 128.0, 128.4, 128.5, 131.9 (d, 3 or 4JC–F = 2 Hz), 132.3 (d, 3 or 4JC–F = 3 Hz), 134.0, 140.2, 145.6 (d, 3 or 4JC–F = 2 Hz), 159.2 (d, 1JC–F = 348 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 34.9. FT-IR (neat, cm–1): 3063 (m), 3028 (m), 1821 (s), 1597 (s), 1568 (m), 1475 (s), 1450 (m), 1261 (s), 1225 (s), 1126 (m), 1067 (s), 995 (s), 777 (s), 748 (s), 698 (s). Anal. Calcd for C13H9FO: C, 77.99; H, 4.53%. Found: C, 78.27; H, 4.30%.
1,4-Benzodioxane-6-carbonyl Fluoride (1j)
Yield was 89%. mp: 88–89 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 4.29–4.30 (m, 2H), 4.34–4.36 (m, 2H), 6.95 (dd, J = 8.4, 1.2 Hz, 1H), 7.55–7.57 (m, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 64.1, 64.9, 117.7 (d, 2JC–F = 62 Hz), 118.0, 120.9 (d, 3JC–F = 5 Hz), 125.7 (d, 3JC–F = 3 Hz), 143.8, 150.0, 157.2 (d, 1JC–F = 340 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 16.5. FT-IR (neat, cm–1): 1792 (s), 1609 (m), 1585 (m), 1506 (m), 1456 (m), 1436 (m), 1336 (m), 1304 (s), 1180 (m), 1123 (m), 1064 (m), 1040 (s), 1005 (m), 895 (s), 878 (m), 826 (w), 748 (s), 716 (m), 660 (w). Anal. Calcd for C9H7FO3: C, 59.35; H, 3.87%. Found: C, 59.38; H, 3.71%.
9-Anthracenecarbonyl Fluoride (1m)
Yield was 57%. mp: 106–108 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.55–7.57 (m, 2H), 7.65–7.68 (m, 2H), 8.08 (d, J = 8.4 Hz, 2H), 8.32 (dd, J = 9.0, 0.6 Hz, 2H), 8.69 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 120.2 (d, 2JC–F = 54 Hz), 120.8 (d, 3JC–F = 3 Hz), 126.0, 128.7, 129.1, 130.3, 130.9, 133.3, 158.6 (d, 1JC–F = 354 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 59.6. FT-IR (neat, cm–1): 2361 (w), 1819 (s), 1798 (s), 1159 (m), 1115 (m), 1096 (s), 941 (m), 922 (m), 901 (m), 735 (s), 719 (m). Anal. Calcd for C15H9FO: C, 80.35; H, 4.05%. Found: C, 80.11; H, 3.91%.
9H-Fluorene-1-carbonyl Fluoride (1n)
Yield was 39%. mp: 88–90 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 4.21 (s, 2H), 7.37–7.43 (m, 2H), 7.54 (t, J = 7.8 Hz, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.81 (d, J = 7.2 Hz, 1H), 7.96 (dd, J = 7.8, 0.6 Hz, 1H), 8.07 (d, J = 7.2 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 38.5 (d, 4JC–F = 2 Hz), 120.2, 121.7 (d, 2JC–F = 60 Hz), 125.2, 126.3, 127.2, 127.8, 128.0, 129.8 (d, 3 or 4JC–F = 2 Hz), 139.8, 143.3 (d, 3 or 4JC–F = 2 Hz), 143.8 (d, 3 or 4JC–F = 2 Hz), 147.8 (d, 3 or 4JC–F = 6 Hz), 157.0 (d, 1JC–F = 346 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 25.2. FT-IR (neat, cm–1): 1794 (s), 1582 (w), 1427 (w), 1254 (s), 1171 (w), 1119 (s), 1092 (m), 1065 (w), 1024 (m), 1005 (s), 856 (m), 766 (w), 739 (s). Anal. Calcd for C14H9FO: C, 79.23; H, 4.27%. Found: C, 79.14; H, 4.18%.
Benzofuran-2-carbonyl Fluoride (1q)
Yield was 72%. mp: 94–96 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.38 (td, J = 7.5, 1.0 Hz, 1H), 7.56 (td, J = 7.8, 1.2 Hz, 1H), 7.63 (dd, J = 8.4, 0.6 Hz, 1H), 7.75–7.77 (m, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 112.8, 119.5, 123.7, 124.7, 126.4, 129.6, 140.1 (d, 2JC–F = 90 Hz), 149.6 (d, 1JC–F = 330 Hz), 157.0 (d, 3JC–F = 2 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 17.3. FT-IR (neat, cm–1): 1801 (s), 1612 (w), 1556 (s), 1477 (w), 1329 (w), 1298 (s), 1163 (s), 1134 (s), 1049 (s), 928 (m), 883 (w), 750 (s), 731 (m), 700 (w). Anal. Calcd for C9H5FO2: C, 65.86; H, 3.07%. Found: C, 65.93; H, 2.85%.
α-Phenylcinnamoyl Fluoride (1r)
Yield was 91%. mp: 47–50 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.14 (d, J = 7.2 Hz, 2H), 7.23 (t, J = 7.8 Hz, 2H), 7.30–7.33 (m, 3H), 7.44–7.46 (m, 3H), 7.99 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 127.4 (d, 2JC–F = 54 Hz), 128.6, 128.8, 129.2, 129.7, 130.6, 131.2, 133.5, 133.9, 146.5 (d, 3JC–F = 2 Hz), 158.3 (d, 1JC–F = 346 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 18.9. FT-IR (neat, cm–1): 1790 (s), 1620 (m), 1493 (w), 1445 (m), 1223 (s), 1207 (m), 1138 (s), 1105 (w), 1074 (w), 949 (m), 907 (m), 781 (m), 762 (m), 698 (s). Anal. Calcd for C15H11FO: C, 79.63; H, 4.90%. Found: C, 79.55; H, 4.96%.
Representative Procedure for Decarbonylative Ethylation of 1a
Synthesis of 4-Ethylbiphenyl (4aa)30
To a 20 mL Schlenk tube, Ni(cod)2 (10 mol %, 3.4 mg, 0.0125 mmol), DPPE (10 mol %, 5.0 mg, 0.0125 mmol), and toluene (500 μL) were added. After stirring for a short time, BEt3 (2a) (1.0 M hexane solution, 125 μL, 0.125 mmol) and biphenyl-4-carbonyl fluoride (1a) (25.0 mg, 0.125 mmol) were added, and resulting mixture was heated at 130 °C. After 24 h, the reaction was quenched with 1 M HCl solution, extracted with EtOAc, washed by brine, and dried over MgSO4. After the volatiles were removed under vacuum, the crude product was purified by column chromatography (hexane/Et2O = 10:1) to afford the desired product of 4-ethylbiphenyl (4aa) (17.7 mg, 0.0971 mmol) in 78% yield. 1H NMR (CDCl3, 600 MHz, rt): δ 1.29 (t, J = 7.8 Hz, 3H), 2.71 (q, J = 7.8 Hz, 2H), 7.29 (d, J = 7.8 Hz, 2H), 7.32–7.35 (m, 1H), 7.42–7.45 (m, 2H), 7.52–7.54 (m, 2H), 7.59–7.60 (m, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.7, 28.7, 127.10, 127.16, 127.22, 128.4, 128.8, 138.8, 141.3, 143.5.
4-Ethylbenzonitrile (4ba)31
Yield was 68% (11.1 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.25 (t, J = 7.8 Hz, 3H), 2.71 (q, J = 7.6 Hz, 2H), 7.29 (d, J = 9.0 Hz, 2H), 7.57 (d, J = 9.0 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.2, 29.2, 109.6, 119.3, 128.8, 132.3, 149.9.
Methyl 4-Ethylbenzoate (4ca)31
Yield was 54% (11.0 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.25 (t, J = 7.8 Hz, 3H), 2.70 (q, J = 7.4 Hz, 2H), 3.90 (s, 3H), 7.26 (d, J = 8.4 Hz, 2H), 7.96 (d, J = 8.4 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.4, 29.1, 52.1, 127.6, 128.0, 129.8, 149.9, 167.4.
1-Ethyl-4-(methylsulfonyl)benzene (4da)32
Yield was 51% (11.8 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.27 (t, J = 7.8 Hz, 3H), 2.75 (q, J = 7.6 Hz, 2H), 3.04 (s, 3H), 7.39 (d, J = 9.0 Hz, 2H), 7.85 (d, J = 8.4 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.3, 29.0, 44.8, 127.6, 129.0, 138.0, 150.9.
1-n-Butyl-4-ethylbenzene (4ea)33
Yield was 61% (12.4 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 0.92 (t, J = 7.2 Hz, 3H), 1.23 (t, J = 7.5 Hz, 3H), 1.36 (sext, J = 7.4 Hz, 2H), 1.55–1.63 (m, 2H), 2.58 (t, J = 7.8 Hz, 2H), 2.62 (q, J = 7.6 Hz, 2H), 7.09–7.12 (m, 4H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 14.1, 15.8, 22.6, 28.6, 33.9, 35.4, 127.8, 128.5, 140.2, 141.5.
1-n-Butoyl-4-ethylbenzene (4fa)34
Yield was 52% (11.6 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 0.97 (t, J = 7.5 Hz, 3H), 1.21 (t, J = 7.8 Hz, 3H), 1.43–1.51 (m, 2H), 1.74–1.77 (m, 2H), 2.59 (q, J = 7.6 Hz, 2H), 3.94 (t, J = 6.6 Hz, 2H), 6.83 (d, J = 9.0 Hz, 2H), 7.10 (d, J = 9.0 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 14.0, 16.0, 19.4, 28.1, 31.6, 67.8, 114.5, 128.8, 136.3, 157.3.
3-Ethylbiphenyl (4ga)30
Yield was 71% (16.2 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.30 (t, J = 7.8 Hz, 3H), 2.73 (q, J = 7.6 Hz, 2H), 7.20 (d, J = 7.2 Hz, 1H), 7.33–7.38 (m, 2H), 7.41–7.46 (m, 4H), 7.60 (dd, J = 8.4, 1.2 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.8, 29.1, 124.7, 127.0, 127.28, 127.31, 127.35, 127.39, 128.83, 128.87, 128.89, 141.4, 141.6, 144.9.
2-Ethylbiphenyl (4ha)30
Yield was 36% (8.2 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.10 (t, J = 7.8 Hz, 3H), 2.60 (q, J = 7.6 Hz, 2H), 7.18–7.42 (m, 9H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.8, 26.3, 125.7, 126.9, 127.3, 127.4, 127.6, 128.1, 128.7, 128.9, 129.4, 130.1, 141.75, 141.77.
5-Ethyl-1,2,3-trimethoxybenzene (4ia)35
Yield was 68% (16.6 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.24 (t, J = 7.5 Hz, 3H), 2.60 (q, J = 7.6 Hz, 2H), 3.82 (s, 3H), 3.86 (s, 6H), 6.42 (s, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.8, 29.4, 56.2, 61.0, 104.8, 128.9, 136.0, 153.2.
6-Ethyl-1,4-benzodioxane (4ja)36
Yield was 69% (14.2 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.20 (t, J = 7.8 Hz, 3H), 2.55 (q, J = 7.6 Hz, 2H), 4.24 (m, 4H), 6.67 (dd, J = 8.1, 2.1 Hz, 1H), 6.71 (d, J = 1.8 Hz, 1H), 6.78 (d, J = 7.8 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.9, 28.3, 64.5, 64.6, 116.6, 117.1, 120.9, 137.8, 141.6, 143.4.
1-Ethylnaphthalene (4ka)30
Yield was 70% (13.7 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.39 (t, J = 7.5 Hz, 3H), 3.12 (q, J = 7.2 Hz, 2H), 7.34 (d, J = 6.6 Hz, 1H), 7.41 (t, J = 6.9 Hz, 1H), 7.46–7.53 (m, 2H), 7.71 (d, J = 8.4 Hz, 1H), 7.86 (d, J = 7.8 Hz, 1H), 8.06 (d, J = 8.4 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.2, 26.0, 123.9, 125.0, 125.5, 125.8 (2C), 126.5, 128.9, 131.9, 133.9, 140.4.
2-Ethylnaphthalene (4la)30
Yield was 74% (14.5 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.33 (t, J = 7.5 Hz, 3H), 2.82 (q, J = 7.6 Hz, 2H), 7.35 (dd, J = 8.4, 1.8 Hz, 1H), 7.39–7.46 (m, 2H), 7.63 (s, 1H), 7.76–7.81 (m, 3H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.7, 29.2, 123.6, 125.1, 125.7, 126.0, 127.2, 127.5, 127.7, 127.9, 131.0, 133.8, 141.9.
9-Ethylanthracene (4ma)37
Yield was 56% (14.4 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.45 (t, J = 7.8 Hz, 3H), 3.65 (q, J = 7.8 Hz, 2H), 7.45–7.47 (m, 2H), 7.49–7.52 (m, 2H), 8.01 (d, J = 7.8 Hz, 2H), 8.28 (d, J = 8.4 Hz, 2H), 8.34 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.7, 21.3, 124.5, 124.9, 125.5, 125.6, 129.3, 129.4, 131.8, 136.8.
1-Ethyl-9H-fluorene (4na)38
Yield was 78% (19.0 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.32 (t, J = 7.5 Hz, 3H), 2.79 (q, J = 7.6 Hz, 2H), 3.85 (s, 2H), 7.17 (d, J = 7.8 Hz, 1H), 7.30 (td, J = 7.2, 1.2 Hz, 1H), 7.34–7.38 (m, 2H), 7.56 (d, J = 7.2 Hz, 1H), 7.65 (d, J = 7.2 Hz, 1H), 7.78 (d, J = 7.8 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 14.4, 26.4, 35.6, 117.6, 120.1, 125.1, 126.1, 126.7, 126.8, 127.4, 140.5, 141.5, 141.6, 142.2, 143.2.
2-Ethylbenzo[b]thiophene (4pa)39
Yield was 70% (14.1 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.38 (t, J = 7.5 Hz, 3H), 2.94 (qd, J = 7.6, 1.2 Hz, 2H), 7.01 (d, J = 0.6 Hz, 1H), 7.23–7.26 (m, 1H), 7.28–7.32 (m, 1H), 7.66 (d, J = 7.8 Hz, 1H), 7.76 (dd, J = 7.8, 0.6 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.6, 24.3, 119.8, 122.3, 122.8, 123.5, 124.2, 139.3, 140.4, 148.5.
2-Ethylbenzofuran (4qa)36
Yield was 28% (5.1 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.34 (t, J = 7.8 Hz, 3H), 2.80 (qd, J = 7.5, 0.9 Hz, 2H), 6.38 (d, J = 0.6 Hz, 1H), 7.16–7.22 (m, 2H), 7.41 (dd, J = 7.8, 0.6 Hz, 1H), 7.48 (dd, J = 6.3, 0.9 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 12.0, 21.9, 101.1, 110.8, 120.3, 122.5, 123.2, 129.1, 154.8, 161.1.
(E)-40 and (Z)-1,2-Diphenyl-1-butene (4ra)41
Yield was 66% (E/Z = 0.93:1) (17.2 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 1.05–1.08 (m, (E) and (Z)), 2.51 (qd, J = 7.4, 1.5 Hz, (Z)), 2.75 (q, J = 7.6 Hz, (E)), 6.43 (s, (Z)), 6.69 (s, (E)), 6.92 (d, J = 7.2 Hz, (Z)), 7.04–7.10 (m, (Z)), 7.15 (d, J = 7.2 Hz, (Z)), 7.24–7.48 (m, (E) and (Z)); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 13.0 (Z), 13.6 (E), 23.4 (E), 33.7 (Z), 125.2 (Z), 126.2 (Z), 126.7 (E), 126.8 (Z), 126.9 (E), 127.3 (E), 127.7 (Z), 127.9 (E), 128.4 (Z), 128.5 (E), 128.6 (E), 128.7 (Z), 128.9 (E), 129.1 (Z), 137.7 (Z), 138.4 (E), 141.6 (Z), 142.8 (E), 144.6 (E), 145.1 (Z).
Decarbonylative C(aroyl)–F Bond Methylation of 1a with Trimethylboroxine: Synthesis of 4-Methylbiphenyl (4ab)42
To a 20 mL Schlenk tube, Ni(cod)2 (20 mol %, 6.9 mg, 0.025 mmol), DPPE (20 mol %, 10.0 mg, 0.025 mmol), and toluene (500 μL) were added. Subsequently, CsF (38.0 mg, 0.25 mmol, 2 equiv), (MeBO)3 (2b) (34.9 μL, 0.25 mmol, 2 equiv), and biphenyl-4-carbonyl fluoride (1a) (25.0 mg, 0.125 mmol) were added, and the resulting mixture was heated at 130 °C. After 24 h, the reaction mixture was quenched with 1 M HCl, extracted with EtOAc, washed by brine, and dried over MgSO4. After the volatiles were removed under vacuum, the crude product was purified by column chromatography (hexane/Et2O = 10:1) to afford 4ab (10 mg, 0.061 mmol) in 49% yield.
1H NMR (CDCl3, 600 MHz, rt): δ 2.40 (s, 3H), 7.26 (d, J = 7.8 Hz, 2H), 7.31–7.34 (m, 1H), 7.43 (t, J = 7.5 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 7.59 (dd, J = 8.4, 1.2 Hz, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 21.2, 127.12, 127.14, 127.3, 128.9, 129.6, 137.2, 138.5, 141.3.
Representative Procedure for Palladium-Catalyzed ortho-C–H Arylation of 2-Naphthoic Acid (5)
Synthesis of 3-Phenyl-2-naphthoic Acid (6a)43
To an oven-dried 20 mL Schlenk tube, Pd(OAc)2 (11.2 mg, 0.05 mmol, 5 mol %), Ag2CO3 (358 mg, 1.3 mmol, 1.3 equiv), 2-naphthoic acid (5) (172 mg, 1.0 mmol), iodobenzene (336 μL, 3.0 mmol, 3.0 equiv), and AcOH (100 μL) were added under argon atmosphere. After the reaction mixture was stirred at 145 °C for 6 h, it was cooled to room temperature and quenched with 1 M HCl. The mixture was filtrated by Celite, extracted with CH2Cl2, washed with brine, and dried over MgSO4. After the volatiles were evaporated, the crude mixture was purified by column chromatography (CH2Cl2/EtOAc = 10:1) and the high-boiling starting material 2-naphthoic acid (5) was removed by bulb-to-bulb distillation (2 mmHg, 200 °C) to afford 6a in 62% yield.
1H NMR (CDCl3, 600 MHz, rt): δ 7.41–7.46 (m, 5H), 7.56 (td, J = 7.5, 1.2 Hz, 1H), 7.62 (td, J = 7.6, 1.4 Hz, 1H), 7.82 (s, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.96 (d, J = 7.8 Hz, 1H), 8.55 (s, 1H). The signal of carboxylic acid was not observed. 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 126.9, 127.3, 127.5, 127.8, 128.1, 128.78, 128.81, 128.9, 130.3, 131.5, 132.6, 134.8, 139.2, 141.2, 173.9.
3-(p-Tolyl)-2-naphthoic Acid (6b)44
Yield was 49% (129 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 2.44 (s, 3H), 7.25 (d, J = 7.8 Hz, 2H), 7.37 (d, J = 7.8 Hz, 2H), 7.55 (td, J = 7.5, 1.2 Hz, 1H), 7.61 (td, J = 7.5, 1.2 Hz, 1H), 7.82 (s, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.96 (d, J = 8.4 Hz, 1H), 8.56 (s, 1H). The signal of carboxylic acid was not observed. 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 21.4, 126.8, 127.7, 127.9, 128.7, 128.8, 128.9, 129.0, 130.3, 131.5, 132.5, 134.9, 137.0, 138.3, 139.1, 173.9.
3-(p-Anisoyl)-2-naphthoic Acid (6c)44
Yield was 45% (125 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 3.78 (s, 3H), 6.99 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 7.55 (td J = 7.5, 0.8 Hz, 1H), 7.61 (td, J = 7.6, 1.0 Hz, 1H), 7.81 (s, 1H), 7.86 (d, J = 8.4 Hz, 1H), 7.95 (d, J = 8.4 Hz, 1H), 8.56 (s, 1H). The signal of carboxylic acid was not observed. 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 55.3, 113.7, 126.8, 127.7, 127.8, 128.7, 128.9, 129.9, 130.2, 131.3, 132.5, 133.6, 134.9, 138.8, 159.0, 174.0.
3-(p-Trifluoromethylphenyl)-2-naphthoic Acid (6d)44
Yield was 59% (187 mg). 1H NMR (CDCl3, 600 MHz, rt): δ 7.53 (d, J = 7.8 Hz, 2H), 7.60 (td, J = 7.5, 1.2 Hz, 1H), 7.66 (td, J = 8.4 Hz, 1.2 Hz, 1H), 7.68 (d, J = 7.8 Hz, 2H), 7.78 (s, 1H), 7.88 (d, J = 7.8 Hz, 1H), 7.99 (d, J = 8.4 Hz, 1H), 8.64 (s, 1H). The signal of carboxylic acid was not observed. 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 124.5 (q, 1JC–F = 272 Hz), 125.0 (q, 2JC–F = 4 Hz), 126.7, 127.5, 128.0, 129.1, 129.2, 129.3, 129.5, 130.7, 131.9, 133.3, 134.9, 138.1, 145.2, 172.6; 19F{1H} NMR (CDCl3, 564 MHz, rt) δ −62.3.
Representative Procedure for the Direct Fluorination of 3-Aryl-2-naphthoic Acids with Deoxo-Fluor
Synthesis of 3-Phenyl-2-naphthoyl Fluoride (7a)
To a 20 mL Schlenk tube charged with a magnetic stirrer bar, Deoxo-Fluor reagent (32.3 μL, 0.175 mmol, 1.1 equiv) was slowly added to the solution of 3-phenyl-2-naphthoic acid (6a) (39.4 mg, 0.159 mmol) in CH2Cl2 (0.8 mL) at 0 °C. After the addition, the solution was allowed to warm to room temperature. After 30 min, the reaction mixture was quenched with sat. NaHCO3 aq, extracted with CH2Cl2, washed by brine, and dried over MgSO4. After the volatiles were removed under vacuum, the crude product was subjected to column chromatography (Hex/Et2O = 10:1) to afford 3-phenyl-2-naphthoyl fluoride (7a) (31.1 mg) in 78% yield. mp: 76–78 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.41–7.48 (m, 5H), 7.61–7.63 (m, 1H), 7.68–7.71 (m, 1H), 7.86 (s, 1H), 7.90 (d, J = 7.8 Hz, 1H), 8.01 (d, J = 7.8 Hz, 1H), 8.66 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 122.3 (d, 2JC–F = 57 Hz), 127.5, 127.8, 128.0, 128.4, 128.9, 129.3, 130.1, 131.0 (d, 3JC–F = 2 Hz), 131.4, 135.0 (d, 3JC–F = 2 Hz), 135.6, 140.1 (d, J = 2 Hz), 140.4, 157.5 (d, 1JC–F = 347 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 33.4. FT-IR (neat, cm–1): 1801 (s), 1273 (m), 1252 (m), 1186 (s), 1128 (m), 1055 (m), 1011 (m), 949 (s), 916 (m), 893 (m), 764 (s), 698 (m). Anal. Calcd for C17H11FO: C, 81.59; H, 4.43%. Found: C, 81.91; H, 4.64%.
3-(p-Tolyl)-2-naphthoyl Fluoride (7b)
Yield was 52%. mp: 81–83 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 2.44 (s, 3H), 7.27 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 7.60 (m, 1H), 7.68 (m, 1H), 7.84 (s, 1H), 7.89 (d, J = 7.8 Hz, 1H), 7.99 (d, J = 7.8 Hz, 1H), 8.63 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 21.4, 122.4 (d, 2JC–F = 57 Hz), 127.4, 128.0, 128.7, 129.1, 129.2, 130.0, 130.9 (d, 3JC–F = 2 Hz), 131.3, 134.9 (d, 3JC–F = 2 Hz), 135.7, 137.5, 137.6, 140.0, 157.6 (d, 1JC–F = 347 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 33.6. FT-IR (neat, cm–1): 1811 (s), 1626 (m), 1441 (m), 1275 (m), 1258 (s), 1186 (s), 1125 (m), 1055 (s), 1015 (s), 951 (s), 897 (s), 812 (s), 766 (s), 750 (s). Anal. Calcd for C18H13FO: C, 81.80; H, 4.96%. Found: C, 81.55; H, 5.20%.
3-(p-Anisoyl)-2-naphthoyl Fluoride (7c)
Yield was 31%. mp: 97–99 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 3.88 (s, 3H), 7.02 (d, J = 9.0 Hz, 2H), 7.37 (d, J = 9.0 Hz, 2H), 7.60 (m, 1H), 7.68 (m, 1H), 7.83 (s, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.97 (d, J = 8.4 Hz, 1H), 8.62 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 55.3, 113.8, 122.3 (d, 2JC–F = 55 Hz), 127.3, 127.8, 129.1, 129.9, 130.0, 130.8 (d, 3JC–F = 2 Hz), 131.1, 132.6, 134.8 (d, 3JC–F = 2 Hz), 135.6, 139.6, 157.6 (d, 1JC–F = 347 Hz), 159.4; 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 33.8. FT-IR (neat, cm–1): 1811 (s), 1607 (m), 1516 (s), 1458 (m), 1285 (m), 1248 (s), 1182 (s), 1126 (m), 1013 (m), 957 (m), 837 (m), 756 (m). Anal. Calcd for C18H13FO2: C, 77.13; H, 4.68%. Found: C, 77.04; H, 4.43%.
3-(p-Trifluoromethylphenyl)-2-naphthoyl Fluoride (7d)
Yield was 43%. mp: 102–105 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 7.53 (d, J = 7.8 Hz, 2H), 7.65–7.67 (m, 1H), 7.71–7.74 (m, 3H), 7.83 (s, 1H), 7.92 (d, J = 7.8 Hz, 1H), 8.03 (d, J = 7.8 Hz, 1H), 8.71 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 121.6 (d, 2JC–F = 58 Hz), 122.5 (q, 1JC–F = 272 Hz), 125.3 (q, 3JC–F = 4 Hz), 128.0, 128.1, 129.3, 129.4, 130.0 (q, 2JC–F = 32 Hz), 130.5, 131.2 (d, 3JC–F = 2 Hz), 131.7, 135.5, 135.6, 138.7, 144.1, 157.0 (d, 1JC–F = 346 Hz); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ −62.5, 31.7. FT-IR (neat, cm–1): 1807 (s), 1325 (s), 1184 (s), 1109 (s), 1069 (m), 1018 (m), 955 (m), 845 (m), 760 (m). Anal. Calcd for C18H10F4O: C, 67.93; H, 3.17%. Found: C, 67.58; H, 3.04%.
Representative Procedure for Decarbonylative Ethylation of 7a(46)
Synthesis of 8a(45) and 8a′(46)
To a 20 mL Schlenk tube, Ni(cod)2 (10 mol %, 3.4 mg, 0.0125 mmol), DPPE (10 mol %, 5.0 mg, 0.0125 mmol), and toluene (500 μL) were added. Subsequently, BEt3 (2a) (1.0 M hexane solution, 125 μL, 0.125 mmol) and 3-phenyl-2-naphthoyl fluoride (7a) (31.3 mg, 0.125 mmol) were added, and the resulting mixture was heated at 130 °C. After 24 h, the reaction was quenched with 1 M HCl, extracted with EtOAc, washed by brine, and dried over MgSO4. After the volatiles were removed under vacuum, the crude product was purified by column chromatography (hexane/Et2O = 10:1) and high-performance liquid chromatography (eluent: CHCl3) to give a mixture of 8a and 8a′. Combined yield was 33% in an NMR ratio of 83:17 (8a:8a′). 1H NMR (CDCl3, 600 MHz, rt): δ 1.10 (t, J = 7.5 Hz, 8a′), 1.15 (t, J = 7.5 Hz, 8a), 2.64 (q, J = 7.6 Hz, 8a′), 2.75 (q, J = 7.6 Hz, 8a), 7.34–7.90 (m, 8a and 8a′); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.3 (8a), 15.8 (8a′), 26.4 (8a′), 26.7 (8a), 125.6 (8a), 125.7 (8a′), 125.96 (8a′), 125.98 (8a), 126.3 (8a′), 126.6 (8a), 127.0 (8a), 127.3 (8a), 127.6 (8a′), 127.70 (8a), 127.75 (8a′), 127.8 (8a′), 127.9 (8a′), 128.0 (8a′), 128.1 (8a′), 128.2 (8a), 128.7 (8a), 129.0 (8a′), 129.5 (8a), 130.3 (8a′), 131.9 (8a), 132.4 (8a′), 133.1 (8a), 133.4 (8a′), 139.7 (8a′), 140.3 (8a), 140.9 (8a), 141.7 (8a′), 141.96 (8a), 141.97 (8a′).
Synthesis of 8b and 8b′
Combined yield was 32% in an NMR ratio of 81:19 (8b:8b′). 1H NMR (CDCl3, 600 MHz, rt): δ 1.09 (t, J = 7.5 Hz, 8b′), 1.15 (t, J = 7.5 Hz, 8b), 2.41 (s, 8b′), 2.43 (s, 8b), 2.61 (q, J = 7.6 Hz, 8b′), 2.75 (q, J = 7.4 Hz, 8b), 7.08–7.10 (m, 8b′), 7.17 (s, 8b′), 7.19 (d, J = 7.8 Hz, 8b′), 7.24–7.29 (m, 8b and/or 8b′), 7.41–7.51 (m, 8b and/or 8b′), 7.65 (s, 8b), 7.73 (s, 8b), 7.75 (s, 8b′), 7.79 (d, J = 8.4 Hz, 8b), 7.82 (d, J = 7.8 Hz, 8b), 7.84–7.89 (m, 8b and 8b′); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.4 (8b), 15.9 (8b′), 21.4 (8b), 26.3 (8b′), 26.7 (8b), 29.8 (8b′), 125.5 (8b), 125.87 (8b), 125.88 (8b′), 126.2 (8b′), 126.50 (8b), 126.52 (8b′), 127.3 (8b), 127.5 (8b′), 127.7 (8b), 127.8 (8b′), 128.0 (8b′), 128.1 (8b′), 128.2 (8b′), 128.8 (8b), 128.9 (8b), 129.38 (8b), 129.39 (8b′), 129.5 (8b′), 130.3 (8b′), 131.9 (8b), 133.1 (8b), 133.4 (8b′), 136.7 (8b), 137.4 (8b′), 138.1 (8b′), 139.0 (8b), 139.7 (8b′), 140.4 (8b), 140.9 (8b).
Synthesis of 8c and 8c′
Combined yield was 47% in an NMR ratio of 80:20 (8c:8c′). 1H NMR (CDCl3, 600 MHz, rt): δ 1.11 (t, J = 7.5 Hz, 8c′), 1.16 (t, J = 7.5 Hz, 8c), 2.63 (q, J = 7.4 Hz, 8c′), 2.76 (q, J = 7.4 Hz, 8c), 3.87 (s, 8c′), 3.88 (s, 8c), 6.83 (dd, J = 8.4, 2.4 Hz, 8c′), 6.90 (d, J = 2.4 Hz, 8c′), 6.98 (d, J = 9.0 Hz, 8c), 7.23 (d, J = 8.4 Hz, 8c′), 7.32 (d, J = 8.4 Hz, 8c), 7.41–7.51 (m, 8c and/or 8c′), 7.66 (s, 8c), 7.73 (s, 8c), 7.74 (s, 8c′), 7.79 (d, J = 7.8 Hz, 8c), 7.82 (d, J = 7.8 Hz, 8c), 7.84–7.89 (m, 8c′); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.4 (8c), 15.7 (8c′), 26.6 (8c′), 26.8 (8c), 55.4 (8c′), 55.5 (8c), 110.9 (8c′), 113.6 (8c), 114.3 (8c′), 125.5 (8c), 125.8 (8c′), 125.9 (8c), 126.2 (8c′), 126.5 (8c), 127.2 (8c), 127.5 (8c′), 127.6 (8c), 127.8 (8c′), 128.1 (8c′), 128.3 (8c′), 128.8 (8c), 130.5 (8c), 131.3 (8c′), 131.4 (8c′), 131.9 (8c), 132.3 (8c′), 133.1 (8c), 133.4 (8c′), 134.35 (8c), 134.37 (8c′), 139.4 (8c′), 140.53 (8c), 140.58 (8c), 143.4 (8c′), 158.8 (8c), 159.2 (8c′).
Synthesis of 8d and 8d′
Combined yield was 27% in an NMR ratio of 27:73 (8d:8d′). 1H NMR (CDCl3, 600 MHz, rt): δ 1.12 (t, J = 7.8 Hz, 8d′), 1.15 (t, J = 7.5 Hz, 8d), 2.69 (q, J = 7.6 Hz, 8d′), 2.73 (q, J = 7.8 Hz, 8d), 7.38–7.55 (m, 8d and/or 8d′), 7.60 (s, 8d′), 7.65 (s, 8d), 7.70–7.91 (m, 8d and/or 8d′); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.4 (8d), 15.5 (8d′), 26.4 (8d′), 26.7 (8d), 122.5 (q, 3JC–F = 3.8 Hz, 8d′), 123.6 (8d′), 124.5 (q, 1JC–F = 272 Hz, 8d), 125.2 (q, 3JC–F = 3.8 Hz, 8d), 125.5 (q, 3JC–F = 3.8 Hz, 8d′), 125.9 (8d), 126.38 (8d′), 126.40 (8d), 126.6 (8d′), 126.9 (8d), 127.2 (8d′), 127.29 (8d′), 127.34 (8d), 127.7 (8d′), 127.75 (8d), 127.76 (8d′), 127.80 (8d′), 127.9 (8d′), 128.0 (8d′), 128.2 (8d′), 128.8 (8d), 129.2 (8d′), 129.3 (q, 2JC–F = 32 Hz, 8d), 129.87 (8d), 129.9 (q, 2JC–F = 32 Hz, 8d′), 130.7 (8d′), 131.8 (8d), 132.6 (8d′), 133.3 (8d′), 133.4 (8d), 138.3 (8d′), 139.4 (8d), 139.7 (8d), 142.9 (8d′), 145.2 (8d′), 145.7 (8d); 19F{1H} NMR (CDCl3, 564 MHz, rt) δ −62.38 (8d′), −62.37 (8d).
Acetylation of 3-Hydroxy-2-naphthoic Acid (9): Synthesis of 3-Acetoxy-2-naphthoic Acid (10)47
To a 50 mL Schlenk tube, 3-hydroxy-2-naphthoic acid (9) (27 g, 143.5 mmol) and acetic anhydride (Ac2O) (40 mL) were added. Subsequently, a small amount of conc. H2SO4 (20–30 drops) was added, and the resulting mixture was vigorously stirred and heated at 90 °C. After 30 min, the completion of reaction was checked by GC–MS, subsequently quenched by water, extracted with CH2Cl2/acetone (∼10:1), washed by brine, and dried over MgSO4. After the volatiles were removed under vacuum, the crude product was washed with hexane and collected 10 by filtration. Isolated yield of the target 3-acetoxy-2-naphthoic acid (10) was 87% (28.8 g, 125.1 mmol). 1H NMR (acetone-d6, 600 MHz, rt): δ 2.30 (s, 3H), 7.59–7.62 (m, 1H), 7.66–7.69 (m, 2H), 7.96 (d, J = 7.8 Hz, 1H), 8.10 (d, J = 7.8 Hz, 1H), 8.69 (s, 1H). The signal of −COOH was not observed. 13C{1H} NMR (acetone-d6, 151 MHz, rt) δ 21.1, 121.8, 123.5, 127.5, 128.0, 129.8, 129.9, 131.5, 134.4, 136.6, 148.2, 165.9, 170.3.
Fluorination of 10 with Deoxo-Fluor Reagent: Synthesis of 3-Acetoxy-2-naphthoyl Fluoride (11)
To a 20 mL Schlenk tube equipped with a magnetic stirrer bar, 3-acetoxy-2-naphthoic acid (10) (230 mg, 1.0 mmol), CH2Cl2 (25 mL), and Et2O (10 mL) were successively added. After the mixture was stirred at 0 °C, Deoxo-Fluor reagent (1.1 equiv, 203 μL, 1.1 mmol) was slowly added to the reaction mixture. After the reaction was stirred at 0 °C for 30 min, the solution was slowly poured into saturated NaHCO3. After CO2 evolution ceased, the reaction mixture was extracted into CH2Cl2 (3 × 15 mL) and dried over MgSO4. The crude product was purified by flash chromatography (Hex/Et2O = 10:1) to afford 11 in 77% yield. mp: 77–79 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 2.43 (s, 3H), 7.58 (m, 2H), 7.67–7.70 (m, 1H), 7.85 (d, J = 7.8 Hz, 1H), 7.97 (d, J = 8.4, 0.6 Hz, 1H), 8.65 (s, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 21.1, 116.7 (d, 2JC–F = 60 Hz), 122.0 (d, 3JC–F = 3 Hz), 127.4, 127.6, 129.6, 130.5, 130.6, 136.5, 136.9, 147.2 (d, 3JC–F = 5 Hz), 154.2 (d, 1JC–F = 342 Hz), 170.1; 19F{1H} NMR (CDCl3, 564 MHz, rt) δ 26.8. FT-IR (neat, cm–1): 1809 (s), 1763 (s), 1209 (s), 1153 (m), 1121 (m), 978 (s), 905 (m), 764 (s). Anal. Calcd for C13H9FO3: C, 67.24; H, 3.91%. Found: C, 67.13; H, 3.92%.
Chemoseletive C(aroyl)–F Bond Ethylation of 11: Synthesis of 3-Acetoxy-2-ethylnaphthalene (12)
To a 20 mL Schlenk tube, Ni(cod)2 (10 mol %, 3.4 mg, 0.0125 mmol), DPPE (10 mol %, 5.0 mg, 0.0125 mmol), and toluene (500 μL) were added. Subsequently, BEt3 (2a) (1.0 M hexane solution, 125 μL, 0.125 mmol) and 3-acetoxy-2-naphthoyl fluoride (11) (29.0 mg, 0.125 mmol) were added, and resulting mixture was heated at 130 °C. After 24 h, the reaction mixture was quenched with 1 M HCl, extracted with EtOAc, washed by brine, and dried over MgSO4. After the volatiles were removed under vacuum, the crude product was purified by column chromatography (hexane/Et2O = 10:1) and high-performance liquid chromatography (eluent: CHCl3) to afford 12 (14 mg, 0.065 mmol) in 51% yield. mp: 48–50 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 1.31 (t, J = 7.5 Hz, 3H), 2.39 (s, 3H), 2.71 (q, J = 7.6 Hz, 2H), 7.41–7.45 (m, 2H), 7.50 (s, 1H), 7.71 (s, 1H), 7.75–7.81 (m, 2H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 14.1, 21.1, 23.7, 119.4, 125.8, 125.9, 127.4, 127.9, 132.1, 132.5, 135.3, 147.7, 169.8. FT-IR (neat, cm–1): 1753 (s), 1053 (m), 1439 (m), 1366 (s), 1339 (s), 1211 (s), 1146 (s), 1121 (m), 1092 (s), 1051 (m), 1013 (m), 988 (m), 959 (m), 930 (s), 899 (s), 756 (s). Anal. Calcd for C14H14O2: C, 78.48; H, 6.59%. Found: C, 78.10; H, 6.61%.
C–O Bond Arylation of 12(48)
Synthesis of 2-Ethyl-3-phenylnaphthalene (8a)48
To a 20 mL Schlenk tube, NiCl2(PCy3)2 (10 mol %, 17.3 mg, 0.025 mmol), K3PO4 (2 equiv, 106 mg, 0.5 mmol), (PhBO)3 (1.2 equiv, 94 mg, 0.3 mmol), 3-acetoxy-2-ethylnaphthalene (12) (54 mg, 0.25 mmol), and 1,4-dioxane (2 mL) were added. Subsequently, water (4 μL) was added and resulting mixture was heated at 110 °C. After 12 h, the reaction was quenched with 1 M HCl, extracted with EtOAc, washed by brine, and dried over MgSO4. After the volatiles were removed under vacuum, the crude product was purified by column chromatography (hexane/Et2O = 10:1) and high-performance liquid chromatography (eluent: CHCl3) to afford 8a (36.5 mg, 0.157 mmol) in 63% yield without the formation of any regioisomer. 1H NMR (CDCl3, 600 MHz, rt): δ 1.19 (t, J = 7.5 Hz, 3H), 2.79 (q, J = 7.4 Hz, 2H), 7.40–7.51 (m, 7H), 7.71 (s, 1H), 7.78 (s, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.86 (d, J = 8.4 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.4, 26.7, 125.6, 126.0, 126.6, 127.0, 127.3, 127.7, 128.2, 128.7, 129.5, 131.9, 133.1, 140.3, 140.9, 142.0.
2-Ethyl-3-(p-tolyl)naphthalene (8b)
Yield was 75%. 1H NMR (CDCl3, 600 MHz, rt): δ 1.19 (t, J = 7.5 Hz, 3H), 2.47 (s, 3H), 2.79 (q, J = 7.4 Hz, 2H), 7.28 (d, J = 7.2 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 7.44–7.50 (m, 2H), 7.69 (s, 1H), 7.77 (s, 1H), 7.82 (d, J = 7.8 Hz, 1H), 7.85 (d, J = 7.8 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.4, 21.4, 26.7, 125.5, 125.9, 126.5, 127.2, 127.7, 128.8, 128.9, 129.4, 131.9, 133.1, 136.6, 139.0, 140.4, 140.9. FT-IR (neat, cm–1): 1514 (m), 1493 (s), 1456 (s), 1020 (m), 887 (s), 826 (s), 785 (m), 746 (s), 727 (m). Anal. Calcd for C19H18: C, 92.63; H, 7.37%. Found: C, 92.58; H, 7.42%.
2-Ethyl-3-(4-methoxyphenyl)naphthalene (8c)
Yield was 64%. mp: 85–87 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 1.17 (t, J = 7.5 Hz, 3H), 2.77 (q, J = 7.6 Hz, 2H), 3.89 (s, 3H), 6.99 (d, J = 9.0 Hz, 2H), 7.33 (d, J = 8.4 Hz, 2H), 7.42–7.48 (m, 2H), 7.67 (s, 1H), 7.75 (s, 1H), 7.80 (d, J = 7.8 Hz, 1H), 7.83 (d, J = 7.8 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.4, 26.8, 55.5, 113.6, 125.5, 125.9, 126.5, 127.3, 127.6, 128.8, 130.5, 131.9, 133.1, 134.3, 140.5, 140.6, 158.8. FT-IR (neat, cm–1): 1512 (s), 1493 (m), 1460 (m), 1240 (s), 1179 (m), 1036 (m), 837 (m), 752 (s). Anal. Calcd for C19H18O: C, 86.99; H, 6.92%. Found: C, 86.80; H, 6.93%.
2-Ethyl-3-(4-trifluoromethylphenyl)naphthalene (8d)
Yield was 44%. mp: 98–100 °C. 1H NMR (CDCl3, 600 MHz, rt): δ 1.17 (t, J = 7.8 Hz, 3H), 2.74 (q, J = 7.4 Hz, 2H), 7.46–7.51 (m, 2H), 7.53 (d, J = 8.4 Hz, 2H), 7.67 (s, 1H), 7.72 (d, J = 7.8 Hz, 2H), 7.78 (s, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.85 (d, J = 8.4 Hz, 1H); 13C{1H} NMR (CDCl3, 151 MHz, rt) δ 15.3, 26.6, 124.5 (q, 1JC–F = 272 Hz), 125.2 (q, 3JC–F = 3.5 Hz), 125.9, 126.4, 126.9, 127.3, 127.7, 128.8, 129.3 (q, 2JC–F = 32 Hz), 129.9, 131.8, 133.4, 139.4, 139.7, 145.7; 19F{1H} NMR (CDCl3, 564 MHz, rt) δ −62.35. FT-IR (neat, cm–1): 1323 (s), 1159 (s), 1128 (s), 1105 (s), 1065 (s), 1018 (m), 895 (m), 849 (m), 750 (s). Anal. Calcd for C19H15F3: C, 75.99; H, 5.03%. Found: C, 75.66; H, 4.99%.
Acknowledgments
This work was supported by ACT-C, JST Grant Number JPMJCR12YW, Japan, and a Grant-in-Aid from JSPS (15J00293 to Y.O.). The authors gratefully thank Dr. Xi-Chao Chen for preparation of aroyl fluorides and Megumi Kosaka and Motonari Kobayashi at the Department of Instrumental Analysis, Advanced Science Research Center, Okayama University, for the measurements of elemental analyses and the SC-NMR Laboratory of Okayama University for the NMR spectral measurements.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02155.
More detailed results of nickel-catalyzed reactions and the 1H, 13C{1H}, and 19F{1H} NMR spectra of the products (PDF)
Author Present Address
† Department of Applied Chemistry and Biotechnology, Okayama University of Science, 1-1 Ridai-cho, Kita-ku, Okayama 700-0005, Japan (Y.O.).
The authors declare no competing financial interest.
Supplementary Material
References
- a Negishi E.-i.; Bagheri V.; Chatterjee S.; Luo F.-T.; Miller J. A.; Stoll A. T. Palladium-catalyzed acylation of organozincs and other organometallics as a convenient route to ketones. Tetrahedron Lett. 1983, 24, 5181–5184. 10.1016/S0040-4039(00)88391-4. [DOI] [Google Scholar]; b Grey R. A. A Palladium-Catalyzed Synthesis of Ketones from Acid Chlorides and Organozinc Compounds. J. Org. Chem. 1984, 49, 2288–2289. 10.1021/jo00186a043. [DOI] [Google Scholar]; c Wang D.; Zhang Z. Palladium-Catalyzed Cross-Coupling Reactions of Carboxylic Anhydrides with Organozinc Reagents. Org. Lett. 2003, 5, 4645–4648. 10.1021/ol035801w. [DOI] [PubMed] [Google Scholar]; d Yus M.; Ortiz R. Tandem Intramolecular Carbolithiation-Lithium/Zinc Transmetallation and Applications to Carbon–Carbon Bond-Forming Reactions. Eur. J. Org. Chem. 2004, 3833–3841. 10.1002/ejoc.200400349. [DOI] [Google Scholar]
- Wakamatsu K.; Okuda Y.; Oshima K.; Nozaki H. Palladium-catalyzed Ketone Synthesis from Acyl Chloride and Organoaluminum Reagents. Bull. Chem. Soc. Jpn. 1985, 58, 2425–2426. 10.1246/bcsj.58.2425. [DOI] [Google Scholar]
- Labadie J. W.; Stille J. K. Mechanisms of the palladium-catalyzed couplings of acid chlorides with organotin reagents. J. Am. Chem. Soc. 1983, 105, 6129–6137. 10.1021/ja00357a026. [DOI] [Google Scholar]
- Yamada J.-Y.; Yamamoto Y. Ready coupling of acid chlorides with tetra-alkyl-lead derivatives catalysed by palladium. J. Chem. Soc., Chem. Commun. 1987, 1302–1303. 10.1039/c39870001302. [DOI] [Google Scholar]
- Kabalka G. W.; Malladi R. R.; Tejedor D.; Kelley S. Preparation of ketones via the palladium-catalyzed cross-coupling of acid chlorides with trialkylboranes. Tetrahedron Lett. 2000, 41, 999–1001. 10.1016/S0040-4039(99)02226-1. [DOI] [Google Scholar]
- Miyaura N.; Ishiyama T.; Sasaki H.; Ishikawa M.; Sato M.; Suzuki A. Palladium-catalyzed inter- and intramolecular cross-coupling reactions of B-alkyl-9-borabicyclo[3.3.1]nonane derivatives with 1-halo-1-alkenes or haloarenes. Syntheses of functionalized alkenes, arenes, and cycloalkenes via a hydroboration-coupling sequence. J. Am. Chem. Soc. 1989, 111, 314–321. 10.1021/ja00183a048. [DOI] [Google Scholar]
- Harris C. R.; Kuduk S. D.; Balog A.; Savin K.; Glunz P. W.; Danishefsky S. J. New Chemical Synthesis of the Promising Cancer Chemotherapeutic Agent 12,13-Desoxyepothilone B: Discovery of a Surprising Long-Range Effect on the Diastereoselectivity of an Aldol Condensation. J. Am. Chem. Soc. 1999, 121, 7050–7062. 10.1021/ja991189l. [DOI] [Google Scholar]
- Chemler S. R.; Trauner D.; Danishefsky S. J. The B-Alkyl Suzuki–Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis. Angew. Chem., Int. Ed. 2001, 40, 4544–4568. . [DOI] [PubMed] [Google Scholar]
- Malanga C.; Mannucci S.; Lardicci L. Nickel Mediated Conversion of Acyl Halides in Aldehydes. Tetrahedron Lett. 1997, 38, 8093–8096. 10.1016/S0040-4039(97)10117-4. [DOI] [Google Scholar]
- Lu Q.; Yu H.; Fu Y. Mechanistic Study of Chemoselectivity in Ni-Catalyzed Coupling Reactions between Azoles and Aryl Carboxylates. J. Am. Chem. Soc. 2014, 136, 8252–8260. 10.1021/ja4127455. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Yu Z. Rhodium-Catalyzed Regioselective C–H Functionalization via Decarbonylation of Acid Chlorides and C–H Bond Activation under Phosphine-Free Conditions. J. Am. Chem. Soc. 2008, 130, 8136–8137. 10.1021/ja803154h. [DOI] [PubMed] [Google Scholar]
- Malapit C. A.; Ichiishi N.; Sanford M. S. Pd-Catalyzed Decarbonylative Cross-Couplings of Aroyl Chlorides. Org. Lett. 2017, 19, 4142–4145. 10.1021/acs.orglett.7b02024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Muto K.; Yamaguchi J.; Musaev D. G.; Itami K. Decarbonylative organoboron cross-coupling of esters by nickel catalysis. Nat. Commun. 2015, 6, 7508 10.1038/ncomms8508. [DOI] [PMC free article] [PubMed] [Google Scholar]; b LaBerge N. A.; Love J. A. Nickel-Catalyzed Decarbonylative Coupling of Aryl Esters and Arylboronic Acids. Eur. J. Org. Chem. 2015, 5546–5553. 10.1002/ejoc.201500630. [DOI] [Google Scholar]; c Muto K.; Hatakeyama T.; Itami K.; Yamaguchi J. Palladium-Catalyzed Decarbonylative Cross-Coupling of Azinecarboxylates with Arylboronic Acids. Org. Lett. 2016, 18, 5106–5109. 10.1021/acs.orglett.6b02556. [DOI] [PubMed] [Google Scholar]; d Liu X.; Jia J.; Rueping M. Nickel-Catalyzed C–O Bond-Cleaving Alkylation of Esters: Direct Replacement of the Ester Moiety by Functionalized Alkyl Chains. ACS Catal. 2017, 7, 4491–4496. 10.1021/acscatal.7b00941. [DOI] [Google Scholar]; e Chatupheeraphat A.; Liao H.-H.; Srimontree W.; Guo L.; Minenkov Y.; Poater A.; Cavallo L.; Rueping M. Ligand-Controlled Chemoselective C(acyl)–O Bond vs C(aryl)–C Bond Activation of Aromatic Esters in Nickel Catalyzed C(sp2)–C(sp3) Cross-Couplings. J. Am. Chem. Soc. 2018, 140, 3724–3735. 10.1021/jacs.7b12865. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Rovis T. A Unique Catalyst Effects the Rapid Room-Temperature Cross-Coupling of Organozinc Reagents with Carboxylic Acid Fluorides, Chlorides, Anhydrides, and Thioesters. J. Am. Chem. Soc. 2004, 126, 15964–15965. 10.1021/ja044113k. [DOI] [PubMed] [Google Scholar]
- Barbero M.; Cadamuro S.; Dughera S. Palladium-Catalyzed Cross-Coupling Alkylation of Arenediazonium o-Benzenedisulfonimides. Synthesis 2008, 474–478. 10.1055/s-2008-1032027. [DOI] [Google Scholar]
- a Giri R.; Maugel N.; Li J.-J.; Wang D.-H.; Breazzano S. P.; Saunders L. B.; Yu J.-Q. Palladium-Catalyzed Methylation and Arylation of sp2 and sp3 C–H Bonds in Simple Carboxylic Acids. J. Am. Chem. Soc. 2007, 129, 3510–3511. 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; b Chiong H. A.; Pham Q.-N.; Daugulis O. Two Methods for Direct ortho-Arylation of Benzoic Acids. J. Am. Chem. Soc. 2007, 129, 9879–9884. 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]; c Xu Z.; Yang T.; Lin X.; Elliott J. D.; Ren F. A mild and efficient carboxylate-directed C–H arylation of aryl carboxylic acids with iodobenzenes in water. Tetrahedron Lett. 2015, 56, 475–477. 10.1016/j.tetlet.2014.12.001. [DOI] [Google Scholar]
- Lal G. S.; Pez G. P.; Pesaresi R. J.; Prozonic F. M.; Cheng H. Bis(2-methoxyethyl)aminosulfur Trifluoride: A New Broad-Spectrum Deoxofluorinating Agent with Enhanced Thermal Stability. J. Org. Chem. 1999, 64, 7048–7054. 10.1021/jo990566+. [DOI] [Google Scholar]
- a Tian Q.; Larock R. C. Synthesis of 9-Alkylidene-9H-fluorenes by a Novel Palladium-Catalyzed Rearrangement. Org. Lett. 2000, 2, 3329–3332. 10.1021/ol000220h. [DOI] [PubMed] [Google Scholar]; b Larock R. C.; Tian Q. Synthesis of 9-Alkylidene-9H-fluorenes by a Novel, Palladium-Catalyzed Cascade Reaction of Aryl Halides and 1-Aryl-1-alkynes. J. Org. Chem. 2001, 66, 7372–7379. 10.1021/jo010561o. [DOI] [PubMed] [Google Scholar]; c Campo M. A.; Larock R. C. Novel 1,4-Palladium Migration in Organopalladium Intermediates Derived from o-Iodobiaryls. J. Am. Chem. Soc. 2002, 124, 14326–14327. 10.1021/ja027548l. [DOI] [PubMed] [Google Scholar]; d Karig G.; Moon M.-T.; Thasana N.; Gallagher T. C–H Activation and Palladium Migration within Biaryls under Heck Reaction Conditions. Org. Lett. 2002, 4, 3115–3118. 10.1021/ol026426v. [DOI] [PubMed] [Google Scholar]; e Campo M. A.; Huang Q.; Yao T.; Tian Q.; Larock R. C. 1,4-Palladium Migration via C–H Activation, Followed by Arylation: Synthesis of Fused Polycycles. J. Am. Chem. Soc. 2003, 125, 11506–11507. 10.1021/ja035121o. [DOI] [PubMed] [Google Scholar]; f Huang Q.; Fazio A.; Dai G.; Campo M. A.; Larock R. C. Pd-Catalyzed Alkyl to Aryl Migration and Cyclization: An Efficient Synthesis of Fused Polycycles via Multiple C–H Activation. J. Am. Chem. Soc. 2004, 126, 7460–7461. 10.1021/ja047980y. [DOI] [PubMed] [Google Scholar]; g Zhao J.; Campo M.; Larock R. C. Consecutive Vinylic to Aryl to Allylic Palladium Migration and Multiple C–H Activation Processes. Angew. Chem., Int. Ed. 2005, 44, 1873–1875. 10.1002/anie.200462327. [DOI] [PubMed] [Google Scholar]; h Masselot D.; Charmant J. P. H.; Gallagher T. Intercepting Palladacycles Derived by C–H Insertion. A Mechanism-Driven Entry to Heterocyclic Tetraphenylenes. J. Am. Chem. Soc. 2006, 128, 694–695. 10.1021/ja056964d. [DOI] [PubMed] [Google Scholar]; i Zhao J.; Larock R. C. Synthesis of Substituted Carbazoles, Indoles, and Dibenzofurans by Vinylic to Aryl Palladium Migration. J. Org. Chem. 2006, 71, 5340–5348. 10.1021/jo060727r. [DOI] [PubMed] [Google Scholar]; j Singh A.; Sharp P. R. Pt and Pd 1,4-Shifts at the Edge of Dibenz[a,c]anthracene. J. Am. Chem. Soc 2006, 128, 5998–5999. 10.1021/ja060159x. [DOI] [PubMed] [Google Scholar]; k Zhao J.; Yue D.; Campo M. A.; Larock R. C. An Aryl to Imidoyl Palladium Migration Process Involving Intramolecular C–H Activation. J. Am. Chem. Soc. 2007, 129, 5288–5295. 10.1021/ja070657l. [DOI] [PubMed] [Google Scholar]; l Campo M. A.; Zhang H.; Yao T.; Ibdah A.; McCulla R. D.; Huang Q.; Zhao J.; Jenks W. S.; Larock R. C. Aryl to Aryl Palladium Migration in the Heck and Suzuki Coupling of o-Halobiaryls. J. Am. Chem. Soc. 2007, 129, 6298–6307. 10.1021/ja069238z. [DOI] [PubMed] [Google Scholar]; m Chaumontet M.; Piccardi R.; Audic N.; Hitce J.; Peglion J.-L.; Clot E.; Baudoin O. Synthesis of Benzocyclobutenes by Palladium-Catalyzed C–H Activation of Methyl Groups: Method and Mechanistic Study. J. Am. Chem. Soc. 2008, 130, 15157–15166. 10.1021/ja805598s. [DOI] [PubMed] [Google Scholar]; n Kesharwani T.; Verma A. K.; Emrich D.; Ward J. A.; Larock R. C. Studies in Acyl C–H Activation via Aryl and Alkyl to Acyl “Through Space” Migration of Palladium. Org. Lett. 2009, 11, 2591–2593. 10.1021/ol900940k. [DOI] [PubMed] [Google Scholar]; o Piou T.; Bunescu A.; Wang Q.; Neuville L.; Zhu J. Palladium-Catalyzed Through-Space C(sp3)–H and C(sp2)–H Bond Activation by 1,4-Palladium Migration: Efficient Synthesis of [3,4]-Fused Oxindoles. Angew. Chem., Int. Ed. 2013, 52, 12385–12389. 10.1002/anie.201306532. [DOI] [PubMed] [Google Scholar]; p Iwasaki M.; Araki Y.; Iino S.; Nishihara Y. Synthesis of Multisubstituted Triphenylenes and Phenanthrenes by Cascade Reaction of o-Iodobiphenyls or (Z)-β-Halostyrenes with o-Bromobenzyl Alcohols through Two Sequential C–C Bond Formations Catalyzed by a Palladium Complex. J. Org. Chem 2015, 80, 9247–9263. 10.1021/acs.joc.5b01693. [DOI] [PubMed] [Google Scholar]; q Bhunia S. K.; Polley A.; Natarajan R.; Jana R. Through-Space 1,4-Palladium Migration and 1,2-Aryl Shift: Direct Access to Dibenzo[a,c]carbazoles through a Triple C–H Functionalization Cascade. Chem. – Eur. J. 2015, 21, 16786–16791. 10.1002/chem.201503474. [DOI] [PubMed] [Google Scholar]; r Hu T.-J.; Zhang G.; Chen Y.-H.; Feng C.-G.; Lin G.-Q. Borylation of Olefin C–H Bond via Aryl to Vinyl Palladium 1,4-Migration. J. Am. Chem. Soc. 2016, 138, 2897–2900. 10.1021/jacs.5b11990. [DOI] [PubMed] [Google Scholar]; s Hu T.-J.; Li M.-Y.; Zhao Q.; Feng C.-G.; Lin G.-Q. Highly Stereoselective Synthesis of 1,3-Dienes through an Aryl to Vinyl 1,4-Palladium Migration/Heck Sequence. Angew. Chem., Int. Ed. 2018, 57, 5871–5875. 10.1002/anie.201801963. [DOI] [PubMed] [Google Scholar]
- a Hayashi T.; Inoue K.; Taniguchi N.; Ogasawara M. Rhodium-Catalyzed Hydroarylation of Alkynes with Arylboronic Acids: 1,4-Shift of Rhodium from 2-Aryl-1-alkenylrhodium to 2-Alkenylarylrhodium Intermediate. J. Am. Chem. Soc. 2001, 123, 9918–9919. 10.1021/ja0165234. [DOI] [PubMed] [Google Scholar]; b Shintani R.; Hayashi T. Rhodium-Catalyzed Addition of Arylzinc Reagents to Aryl Alkynyl Ketones: Synthesis of β,β-Disubstituted Indanones. Org. Lett. 2005, 7, 2071–2073. 10.1021/ol0506819. [DOI] [PubMed] [Google Scholar]; c Miura T.; Sasaki T.; Nakazawa H.; Murakami M. Ketone Synthesis by Intramolecular Acylation of Organorhodium(I) with Ester. J. Am. Chem. Soc. 2005, 127, 1390–1391. 10.1021/ja043123i. [DOI] [PubMed] [Google Scholar]; d Shintani R.; Okamoto K.; Hayashi T. Rhodium-Catalyzed Isomerization of α-Arylpropargyl Alcohols to Indanones: Involvement of an Unexpected Reaction Cascade. J. Am. Chem. Soc. 2005, 127, 2872–2873. 10.1021/ja042582g. [DOI] [PubMed] [Google Scholar]; e Shintani R.; Takatsu K.; Hayashi T. Rhodium-Catalyzed Asymmetric Synthesis of 3,3-Disubstituted 1-Indanones. Angew. Chem., Int. Ed. 2007, 46, 3735–3737. 10.1002/anie.200700226. [DOI] [PubMed] [Google Scholar]; f Shintani R.; Takatsu K.; Katoh T.; Nishimura T.; Hayashi T. Rhodium-Catalyzed Rearrangement of Aryl Bis(alkynyl) Carbinols to 3-Alkynyl-1-indanones. Angew. Chem., Int. Ed. 2008, 47, 1447–1449. 10.1002/anie.200704818. [DOI] [PubMed] [Google Scholar]; g Zhang W.; Liu M.; Wu H.; Ding J.; Cheng J. Phosphine-free rhodium-catalyzed hydroarylation of diaryl acetylenes with boronic acids. Tetrahedron Lett. 2008, 49, 5214–5216. 10.1016/j.tetlet.2008.05.140. [DOI] [Google Scholar]; h Shintani R.; Isobe S.; Takeda M.; Hayashi T. Rhodium-Catalyzed Asymmetric Synthesis of Spirocarbocycles: Arylboron Reagents as Surrogates of 1,2-Dimetalloarenes. Angew. Chem., Int. Ed. 2010, 49, 3795–3798. 10.1002/anie.201000937. [DOI] [PubMed] [Google Scholar]; i Sasaki K.; Hayashi T. Asymmetric conjugate addition of cis-2-arylethenylboronic acids catalyzed by chiral diene/rhodium complexes: 1,4-rhodium shift from alkenylrhodium to arylrhodium intermediates. Tetrahedron: Asymmetry 2012, 23, 373–380. 10.1016/j.tetasy.2012.03.004. [DOI] [Google Scholar]; j Matsuda T.; Suda Y.; Takahashi A. Double 1,4-rhodium migration cascade in rhodium-catalysed arylative ring-opening/spirocyclisation of (3-arylcyclobutylidene)acetates. Chem. Commun. 2012, 48, 2988–2990. 10.1039/c2cc18098g. [DOI] [PubMed] [Google Scholar]; k Sasaki K.; Nishimura T.; Shintani R.; Kantchev E. A. B.; Hayashi T. Rhodium/diene-catalyzed tandem 1,4-shift/1,4-addition of (E)-1,2-diphenylethenylboronic acid to enones: density functional theory modeling and asymmetric catalysis. Chem. Sci. 2012, 3, 1278–1283. 10.1039/c2sc01093c. [DOI] [Google Scholar]; l Ikeda Y.; Takano K.; Kodama S.; Ishii Y. 1,4-Metal migration in a Cp*Rh(III) complex. Chem. Commun. 2013, 49, 11104–11106. 10.1039/c3cc46700g. [DOI] [PubMed] [Google Scholar]; m Ikeda Y.; Takano K.; Waragai M.; Kodama S.; Tsuchida N.; Takano K.; Ishii Y. Reversibility of 1,4-Metal Migration in Cp*RhIII and Cp*IrIII Complexes. Organometallics 2014, 33, 2142–2145. 10.1021/om5003232. [DOI] [Google Scholar]; n Shintani R.; Iino R.; Nozaki K. Rhodium-Catalyzed Polymerization of 3,3-Diarylcyclopropenes Involving a 1,4-Rhodium Migration. J. Am. Chem. Soc. 2014, 136, 7849–7852. 10.1021/ja5032002. [DOI] [PubMed] [Google Scholar]; o Matsuda T.; Watanuki S. Rhodium-catalysed arylative annulation of 1,4-enynes with arylboronic acids. Org. Biomol. Chem. 2015, 13, 702–705. 10.1039/C4OB02210F. [DOI] [PubMed] [Google Scholar]; p Guo S.; Yuan K.; Gu M.; Lin A.; Yao H. Rh(III)-Catalyzed Cascade Annulation/C–H Activation of o-Ethynylanilines with Diazo Compounds: One-Pot Synthesis of Benzo[a]carbazoles via 1,4-Rhodium Migration. Org. Lett. 2016, 18, 5236–5239. 10.1021/acs.orglett.6b02534. [DOI] [PubMed] [Google Scholar]; q Ming J.; Hayashi T. Aryloxymethyltrifluoroborates for Rhodium-Catalyzed Asymmetric Conjugate Arylation. o-Methoxyarylation through 1,4-Rhodium Shift. Org. Lett. 2016, 18, 6452–6455. 10.1021/acs.orglett.6b03347. [DOI] [PubMed] [Google Scholar]; r Wang J.-X.; Tan Y.-X.; Liao W.-W.; Tian P.; Lin G.-Q.; Zhao W. Efficient Access to cis-Hydrobenzo[b]oxepines: Rhodium(I)-Catalyzed Cyclization of Cyclohexadienone-Tethered o-Tolyl-Substituted Alkynes. Synlett 2018, 29, 1223–1228. 10.1055/s-0036-1591956. [DOI] [Google Scholar]
- a Tan B.-H.; Dong J.; Yoshikai N. Cobalt-Catalyzed Addition of Arylzinc Reagents to Alkynes to Form ortho-Alkenylarylzinc Species through 1,4-Cobalt Migration. Angew. Chem., Int. Ed. 2012, 51, 9610–9614. 10.1002/anie.201204388. [DOI] [PubMed] [Google Scholar]; b Wu B.; Yoshikai N. Versatile Synthesis of Benzothiophenes and Benzoselenophenes by Rapid Assembly of Arylzinc Reagents, Alkynes, and Elemental Chalcogens. Angew. Chem., Int. Ed. 2013, 52, 10496–10499. 10.1002/anie.201304546. [DOI] [PubMed] [Google Scholar]; c Tan B.-H.; Yoshikai N. Cobalt-Catalyzed Addition of Arylzinc Reagents to Norbornene Derivatives through 1,4-Cobalt Migration. Org. Lett. 2014, 16, 3392–3395. 10.1021/ol501449j. [DOI] [PubMed] [Google Scholar]; d Yoshikai N. Development of Cobalt-Catalyzed C–H Bond Functionalization Reactions. Bull. Chem. Soc. Jpn. 2014, 87, 843–857. 10.1246/bcsj.20140149. [DOI] [Google Scholar]; e Yan J.; Yoshikai N. Cobalt-Catalyzed Arylative Cyclization of Acetylenic Esters and Ketones with Arylzinc Reagents through 1,4-Cobalt Migration. ACS Catal. 2016, 6, 3738–3742. 10.1021/acscatal.6b01039. [DOI] [Google Scholar]
- Partridge B. M.; Gonzalez J. S.; Lam H. W. Iridium-Catalyzed Arylative Cyclization of Alkynones by 1,4-Iridium Migration. Angew. Chem., Int. Ed. 2014, 53, 6523–6527. 10.1002/anie.201403271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K.; Ikeda Y.; Kodama S.; Ishii Y. Remote rearrangement of the metal center in a (η6-C6Me6)Ru(II) complex. Chem. Commun. 2015, 51, 4981–4984. 10.1039/C4CC09699A. [DOI] [PubMed] [Google Scholar]
- Keen A. L.; Doster M.; Johnson S. A. 1,4-Shifts in a Dinuclear Ni(I) Biarylyl Complex: A Mechanistic Study of C–H Bond Activation by Monovalent Nickel. J. Am. Chem. Soc. 2007, 129, 810–819. 10.1021/ja067112w. [DOI] [PubMed] [Google Scholar]
- Guan B.-T.; Wang Y.; Li B.-J.; Yu D.-G.; Shi Z.-J. Biaryl Construction via Ni-Catalyzed C–O Activation of Phenolic Carboxylates. J. Am. Chem. Soc. 2008, 130, 14468–14470. 10.1021/ja8056503. [DOI] [PubMed] [Google Scholar]
- Chambers R. D.; Sandford G.; Trmcic J.; Okazoe T. Elemental Fluorine. Part 21.1 Direct Fluorination of Benzaldehyde Derivatives. Org. Process Res. Dev. 2008, 12, 339–344. 10.1021/op700194r. [DOI] [Google Scholar]
- Okano T.; Harada N.; Kiji J. Catalytic Acid Fluoride Synthesis via Carbonylation of Organic Bromides in the Presence Potassium Fluoride. Bull. Chem. Soc. Jpn. 1992, 65, 1741–1743. 10.1246/bcsj.65.1741. [DOI] [Google Scholar]
- Birrell J. A.; Desrosiers J.-N.; Jacobsen E. N. Enantioselective Acylation of Silyl Ketene Acetals through Fluoride Anion-Binding Catalysis. J. Am. Chem. Soc. 2011, 133, 13872–13875. 10.1021/ja205602j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T.; Konishi H.; Manabe K. Palladium-Catalyzed Fluorocarbonylation Using N-Formylsaccharin as CO Source: General Access to Carboxylic Acid Derivatives. Org. Lett. 2013, 15, 5370–5373. 10.1021/ol4026815. [DOI] [PubMed] [Google Scholar]
- Arisawa M.; Yamada T.; Yamaguchi M. Synthesis of acylphosphine sulfides by rhodium-catalyzed reaction of acid fluorides and diphosphine disulfides. Tetrahedron Lett. 2010, 51, 4957–4958. 10.1016/j.tetlet.2010.07.038. [DOI] [Google Scholar]
- Guan B.-T.; Xiang S.-K.; Wang B.-Q.; Sun Z.-P.; Wang Y.; Zhao K.-Q.; Shi Z.-J. Direct Benzylic Alkylation via Ni-Catalyzed Selective Benzylic sp3 C–O Activation. J. Am. Chem. Soc. 2008, 130, 3268–3269. 10.1021/ja710944j. [DOI] [PubMed] [Google Scholar]
- Kondolff I.; Doucet H.; Santelli M. Palladium–Tetraphosphine as Catalyst Precursor for High-Turnover-Number Negishi Cross-Coupling of Alkyl- or Phenylzinc Derivatives with Aryl Bromides. Organometallics 2006, 25, 5219–5222. 10.1021/om060605p. [DOI] [Google Scholar]
- Yuan G.; Zheng J.; Gao X.; Li X.; Huang L.; Chen H.; Jiang H. Copper-catalyzed aerobic oxidation and cleavage/formation of C–S bond: a novel synthesis of aryl methyl sulfones from aryl halides and DMSO. Chem. Commun. 2012, 48, 7513–7515. 10.1039/c2cc32964f. [DOI] [PubMed] [Google Scholar]
- Alonso N.; Miller L. Z.; Muñoz J. d. M.; Alcázar J.; McQuade D. T. Continuous Synthesis of Organozinc Halides Coupled to Negishi Reactions. Adv. Synth. Catal. 2014, 356, 3737–3741. 10.1002/adsc.201400243. [DOI] [Google Scholar]
- NMR data of this product has not been reported, CAS Registry Number 1338999-54-40.
- Olsen E. P. K.; Madsen R. Iridium-Catalyzed Dehydrogenative Decarbonylation of Primary Alcohols with the Liberation of Syngas. Chem. – Eur. J. 2012, 18, 16023–16029. 10.1002/chem.201202631. [DOI] [PubMed] [Google Scholar]
- Kalutharage N.; Yi C. S. Scope and Mechanistic Analysis for Chemoselective Hydrogenolysis of Carbonyl Compounds Catalyzed by a Cationic Ruthenium Hydride Complex with a Tunable Phenol Ligand. J. Am. Chem. Soc. 2015, 137, 11105–11114. 10.1021/jacs.5b06097. [DOI] [PubMed] [Google Scholar]
- Chauhan B. P. S.; Rathore J. S.; Bandoo T. “Polysiloxane-Pd” Nanocomposites as Recyclable Chemoselective Hydrogenation Catalysts. J. Am. Chem. Soc. 2004, 126, 8493–8500. 10.1021/ja049604j. [DOI] [PubMed] [Google Scholar]
- NMR data of this product has not been reported, but synthesis was reported;Cairns J. F.; Hickinbottom W. J. Alkylation of the aromatic nucleus. Part VI. Fluorene. J. Chem. Soc. 1962, 867–870. 10.1039/jr9620000867. [DOI] [Google Scholar]
- Bryan C. S.; Braunger J. A.; Lautens M. Efficient Synthesis of Benzothiophenes by an Unusual Palladium-Catalyzed Vinylic C–S Coupling. Angew. Chem., Int. Ed. 2009, 48, 7064–7068. 10.1002/anie.200902843. [DOI] [PubMed] [Google Scholar]
- McKinley N. F.; O’Shea D. F. Carbolithiation of Diphenylacetylene as a Stereoselective Route to (Z)-Tamoxifen and Related Tetrasubstituted Olefins. J. Org. Chem. 2006, 71, 9552–9555. 10.1021/jo061949s. [DOI] [PubMed] [Google Scholar]
- Tanaka R.; Sanjiki H.; Urabe H. Yttrium-Mediated Conversion of Vinyl Grignard Reagent to a 1,2-Dimetalated Ethane and Its Synthetic Application. J. Am. Chem. Soc. 2008, 130, 2904–2905. 10.1021/ja077992u. [DOI] [PubMed] [Google Scholar]
- Bandari R.; Höche T.; Prager A.; Dirnberger K.; Buchmeiser M. R. Ring-Opening Metathesis Polymerization Based Pore-Size-Selective Functionalization of Glycidyl Methacrylate Based Monolithic Media: Access to Size-Stable Nanoparticles for Ligand-Free Metal Catalysis. Chem. – Eur. J. 2010, 16, 4650–4658. 10.1002/chem.200902654. [DOI] [PubMed] [Google Scholar]
- Chiong H. A.; Pham Q.-N.; Daugulis O. Two Methods for Direct ortho-Arylation of Benzoic Acids. J. Am. Chem. Soc. 2007, 129, 9879–9884. 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Yang T.; Lin X.; Elliott J. D.; Ren F. A mild and efficient carboxylate-directed C–H arylation of aryl carboxylic acids with iodobenzenes in water. Tetrahedron Lett. 2015, 56, 475–477. 10.1016/j.tetlet.2014.12.001. [DOI] [Google Scholar]
- Jagdale A. R.; Park J. H.; Youn S. W. Cyclization Reaction for the Synthesis of Polysubstituted Naphthalenes in the Presence of Au(I) Precatalysts. J. Org. Chem. 2011, 76, 7204–7214. 10.1021/jo201339z. [DOI] [PubMed] [Google Scholar]
- Solorio-Alvarado C. R.; Echavarren A. M. Gold-Catalyzed Annulation/Fragmentation: Formation of Free Gold Carbenes by Retro-Cyclopropanation. J. Am. Chem. Soc. 2010, 132, 11881–11883. 10.1021/ja104743k. [DOI] [PubMed] [Google Scholar]
- Souza B. S.; Nome F. Importance of Equilibrium Fluctuations between Most Stable Conformers in the Control of the Reaction Mechanism. J. Org. Chem. 2010, 75, 7186–7193. 10.1021/jo101366m. [DOI] [PubMed] [Google Scholar]
- Guan B.-T.; Wang Y.; Li B.-J.; Yu D.-J.; Shi Z.-J. Biaryl Construction via Ni-Catalyzed C–O Activation of Phenolic Carboxylates. J. Am. Chem. Soc. 2008, 130, 14468–14470. 10.1021/ja8056503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.