Abstract
Lignin is a byproduct of agricultural industries and only has limited applications. In this study, lignin was investigated for use in sustainable biopolymeric packaging film. Alkali lignin (AL) and lignosulfonate (LSS) were added to enzymatically modified soy protein isolate (SPI) biopolymeric film with different concentrations with the goal of improvement of film physical and functional properties. A radical scavenging activity test revealed that films containing LSS had values 28 and 6% higher than control and AL-based films, respectively; AL itself (not in films) had significantly higher radical scavenging activity than LSS. This indicates the activity of lignin is affected by interaction with SPI. The higher compatibility between LSS and enzymatically modified SPI resulted in a positive effect on surface smoothness, water absorption, and mechanical properties of LSS-based films. Films containing AL showed a high light absorption range in the UV region, and this UV-blocking ability increased with increasing level of lignin. Deconvoluted Fourier transform infrared spectra confirmed that the addition of lignin resulted in some changes in the secondary structure of the protein matrix, which were aligned with X-ray diffraction results. The addition of lignin improved tensile strength (TS) and thermal stability of films compared to the lignin-free control. This improvement in TS and thermal stability was probably a result of new intermolecular interactions between lignin and SPI. Water vapor permeability of the films containing lignin decreased to 50% of the control because lignin played a role as a filler in the matrix. On the basis of our observations, the incorporation of lignin into biopolymeric film is capable of providing additional benefits and solutions to various industries, such as food, packaging, agriculture, and pharmaceuticals.
Introduction
Active packaging is one of many types of smart packaging that offers extra functionality for food preservation other than providing an inert barrier.1 Because of the customer demand and market trends, the area of active packaging is becoming increasingly important. Incorporating antioxidants to food packaging, especially for products sensitive to oxidation, is designed to provide additional safety and security by inhibiting the initiation or propagation of oxidizing chain reactions.2 Lipid oxidation is associated with coronary heart disease, atherosclerosis, cancer, and the aging process.3 Antioxidants prevent oxidation by three main mechanisms: UV-light blocking, radical scavenging, and chelation.4−6 In general, there are two categories of antioxidants: natural and synthetic. Common synthetic antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), have some restrictions in application to food products due to their carcinogenicity.7
Lignin, as a natural antioxidant, contains phenolic compounds that prevent lipid oxidation with a similar mechanism to that of BHT or BHA.8 According to Pan et al.,9 the characteristics of lignin due to phenolic hydroxyl groups, aliphatic hydroxyl groups, low molecular weight, and narrow polydispersity result in significant antioxidant activity. Lignin has an aromatic and highly cross-linked structure and is quite reactive due to its functional groups. Therefore, lignin is able to interact with many polymers and change their wettability, fire resistance, and mechanical properties.8 Oliviero et al.8 revealed that the addition of lignin to zein protein matrix caused some changes in the secondary structure of the zein protein, α-helix, β-sheet, and β-turn. Therefore, lignin can modify physicochemical properties (other than antioxidant activity) in some films.
On the basis of the type, source of lignin, and its extraction procedure, lignin has different structures, purity, and corresponding properties.10 Alkali lignin (AL) and lignosulfonate (LSS) are derived from pulping processes in papermaking. Lignosulfonates are water soluble and possess higher molecular weight than AL. Alkali lignin is soluble at high pH. Both AL and LSS are commercially available.10
The effect of different types of lignin on the functional and physicochemical properties of natural polymers at different film preparation conditions has been extensively studied.4,8,11−13 However, the interaction between soy protein isolate (SPI) and lignin under transglutaminase enzyme treatment, which may potentially cross-link them, has not been explored yet.
Transglutaminase was applied in the SPI structure to catalyze cross-linking between ε-amine group in lysine and δ-carboxyamide group on protein-bound glutamine residues leading to the improvement of covalent cross-linking of proteins. Soy protein isolate is a rich source of lysine (6.4%) and glutamine (19%) residues.14
The ultimate objective of this study is to investigate the effect of the enzyme treatment on both physicochemical properties and cross-linking possibility of biopolymeric films fabricated with SPI and lignin types and concentrations, as well as processing conditions.
Results and Discussion
Rheological Behaviors of Film-Forming Solutions
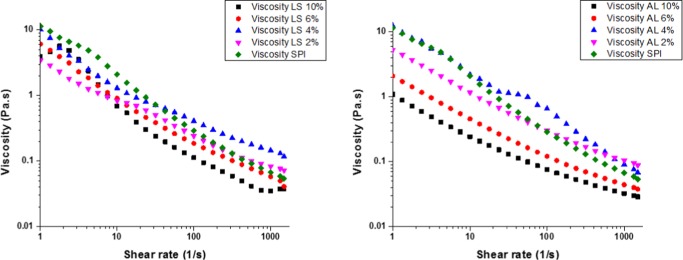
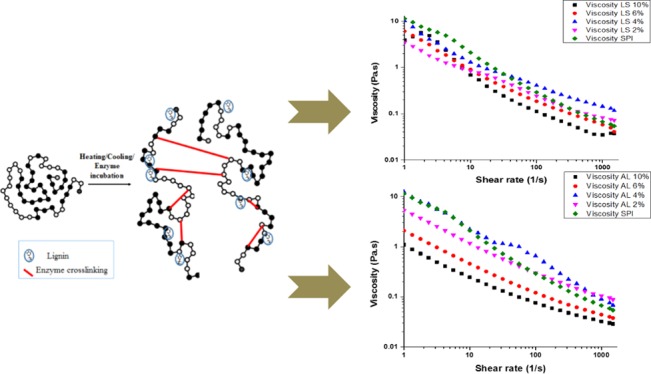
It was expected that the enzyme may catalyze cross-linking between soy protein isolate (SPI) and lignin due to many functional groups, such as abundant free amino groups, in both materials and it may bring in increased viscosity of the solution (Figure 1). However, as observed in Figure 2, the addition of lignin reduced the viscosity of the film forming SPI solution comparing to the control. This reduction in the viscosity suggests that lignin played a role of a lubricant. When the concentration of AL increased, the viscosity decreased by approximately 10-fold compared to the control. The viscosity of the protein solution containing 4 g/100 g was higher than that of 2 g/100 g SPI–lignin solution and similar to the control. This may result from the possible formation of intermolecular secondary bonds between SPI and AL. As shown in Figure 2 (left), modifying the structure of the protein matrix with LSS showed the very complicated rheological profile upon the shear rate. This is clearly indicating that there is molecular level interaction between SPI and water-soluble lignosulfonate (LSS), which has more reactive functional groups. At a higher shear rate (more than 10) and a higher concentration of both AL and LSS (more than 6 g/100 g), the interaction might be easily disrupted, which was due to the hindrance effect of high concentration of lignin material in the protein matrix that caused lignin to act like a lubricant. In a previous study reported by Mu et al.,15 a mixture of gelatin and lignin was applied as an environmentally friendly (green) lubricant due to the abundance of hydrogen bonding sites and their interchain interactions. Our observations agree well with other experiments, such as Fourier transform infrared (FTIR) and X-ray diffraction (XRD) results, which will be explained in the following sections. In conclusion, incorporation of lignin into SPI solution followed by enzymatic reaction may form physicochemical interactions between components instead of enzymatic cross-linking.
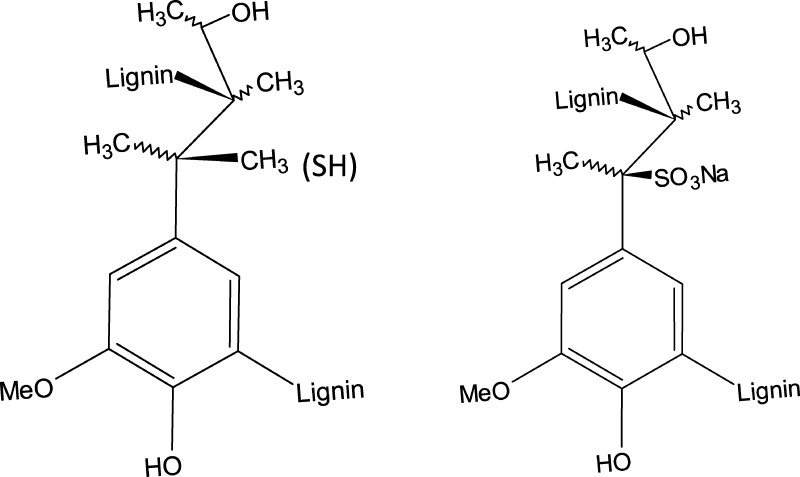
Figure 1.
Chemical structure of alkali lignin (left) and lignosulfonate (right).
Figure 2.
Effect of different concentrations of lignosulfonate (LSS), left, and alkali lignin (AL), right, on apparent viscosity of enzymatically modified SPI film (lignin%: g/100 g of SPI).
Antioxidant Activity of Lignin
Antioxidant activity of lignin was measured by the ability of material to absorb or scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals in a food system. Free radicals accelerate lipid oxidation, which can decrease the shelf life of food products. As shown in Table 1, the radical scavenging activity of alkali lignin was approximately 55%. It was higher than lignosulfonate and some other natural antioxidants extracted from different berries (tested at 1 mg/mL).16 Herein, the DPPH scavenging activity is expressed relative to the testing solution (methanol). Radical scavenging activity of alkali lignin is equivalent to 56% of BHT. It is well known that the radical scavenging activity is affected by many factors, such as structural features, presence of functional groups such as phenolic hydroxyl groups, and molecular weight.9,17 The higher radical scavenging activity of alkali lignin itself resulted from the structural and chemical composition differences compared to lignosulfonate (lower molecular weight10 and higher hydrogen donation ability in the AL, perhaps due to −SH groups; Figure 1).
Table 1. Radical Scavenging Activity of Two Types of Lignin and BHTa,b,c.
| AL material (%) | LSS material (%) | BHT material (%) |
|---|---|---|
| 54.9 ± 2.31 | 12.8 ± 1.23 | 98.9 ± 0.52 |
Results are mean ± standard deviation (SD) in triplicate.
Abbreviations: AL: alkali lignin, LSS: lignosulfonate, BHT: butylated hydroxyltoluene, ND: not detected.
Lignin%: g/100 g of SPI.
DPPH Free Radical Scavenging Test of Films
Radical scavenging activity of SPI films with and without lignin and a control containing BHT are shown in Table 2. SPI films modified with the enzyme did not show powerful antioxidant activity (2.25%). However, after the addition of lignin to SPI films, radical scavenging activity of films in the DPPH methanol solution increased dramatically. Interestingly, films containing LSS showed a slightly higher radical scavenging ability compared to films containing AL, whereas AL material itself showed higher scavenging activity than LSS material itself (Table 1). This discrepancy could be attributed to high compatibility of LSS in the protein matrix. This compatibility is due to the strong intra- and intermolecular interactions between hydrophobic, hydrophilic, and polar groups of lignosulfonate and soy protein side chains.11 Lignosulfonate electrolyte contains a high amount of sulfonate groups (more than 5–8%, based on the supplier datasheet), Ca2+, and many polar groups in their aliphatic side chains, which resulted in high concentration of ionic compounds in the protein matrix11 (Figure 1).
Table 2. Radical Scavenging Activity of Two Types of Lignin and BHT Filma,b,c.
| SPI film (control) (%) | AL film (%) | LSS film (%) | BHT film (%) | |
|---|---|---|---|---|
| radical scavenging activity | 2.2 ± 0.08 | 24.5 ± 0.12 | 30.9 ± 0.09 | 89.7 ± 0.65 |
Results are mean ± SD in triplicate.
Abbreviations: AL: alkali lignin, LSS: lignosulfonate, BHT: butylated hydroxyltoluene, SPI: soy protein isolate.
Lignin%: g/100 g of SPI.
Color, Opacity, and UV-Blocking of Films
The color of the packaging film is important for general appearance and consumer acceptance. The enzymatically modified soy protein isolate film was transparent, unmarked, and had a homogenous texture. After the addition of lignosulfonate (Figure 3, right column), films were still transparent and the visual appearance was well-matched to the L-value measured by the Hunter Lab color meter. Both the greenness and yellowness of these films increased as a function of lignin concentration. This increase showed the same trend with opacity (Figure 3 and Table 3). On the other hand, films incorporating AL (Figure 3, left column) were dark and the darkness increased with the increase of concentration (Figure 3). This trend was also observed for opacity measurements. Consequently, the observations in Figure 3 for both films agreed well with opacity and color measurements. This may indicate that lignin is homogeneously distributed in the SPI matrix.
Figure 3.

Soy protein isolate film modified with different concentrations of lignosulfonate (right) and alkali lignin (left), lignin% w/w.
Table 3. Effect of Different Concentrations of Lignosulfonate (LSS) and Alkali Lignin (AL) on Color (L-Value, a-Value, and b-Value) and Opacity of Enzymatically Modified SPI Filma,b,c,d.
| film | opacity | L-value | a-value | b-value |
|---|---|---|---|---|
| SPI | 0.90 ± 0.06Aa | 89.18 ± 0.10Aa | 0.84 ± 0.21Aa | 2.95 ± 0.84A |
| LSS 2% | 0.88 ± 0.04B | 87.88 ± 0.41A | 0.26 ± 0.12B | 7.06 ± 0.51B |
| LSS 4% | 0.93 ± 0.07A | 86.64 ± 0.19A | –0.47 ± 0.06C | 12.19 ± 0.27C |
| LSS 6% | 1.22 ± 0.08C | 83.5 ± 0.25A | –0.46 ± 0.07C | 20.09 ± 0.34D |
| LSS 10% | 1.05 ± 0.04D | 81.33 ± 0.40A | –0.32 ± 0.12C | 24.85 ± 0.62E |
| AL 2% | 1.73 ± 0.21b | 76.67 ± 0.64b | 2.34 ± 0.24b | 24.39 ± 1.28b |
| AL 4% | 2.24 ± 0.22c | 67.93 ± 1.91c | 5.88 ± 1.00c | 32.48 ± 1.07c |
| AL 6% | 2.67 ± 0.1d | 54.62 ± 1.03d | 12.99 ± 0.47d | 36.76 ± 0.14d |
| AL 10% | 4.01 ± 0.19e | 42.96 ± 1.83e | 17.71 ± 0.40e | 29.57 ± 2.08c |
Results are mean ± SD in triplicate.
Abbreviations: AL: alkali lignin, LSS: lignosulfonate, BHT: butylated hydroxyltoluene, SPI: soy protein isolate.
Small letters show significant differences in different AL samples, and capital letters show significant differences in LS samples.
Lignin%: g/100 g.
Antioxidant activity of lignin in a polymeric matrix is attributed to not only radical scavenging activity but also its UV-blocking ability due to the opacity in the film (Figure 4). Films containing AL are reddish, which is due to the chromophoric nature of lignin, and this characteristic is known to block UV radiation.18,19 As shown in Figure 4, modified films with AL showed a broader range of absorption within the UV region (under 400 nm) compared to films with LSS. The UV absorption region increased as a function of concentration in the films modified with both lignins. The UV scanning results of modified films were in agreement to the results from other methods, such as visual observation (Figure 3), colorimeter, and opacity (Table 3). Modified films with AL 10 g/100 g showed a UV absorption region similar to modified fish gelatin films prepared by Núñez-Flores et al.4 with 15 g/100 g lignin, although their lignin type was different than the one we used. The UV blocking ability of lignin-modified films may be a beneficial property in food packaging, especially when the product is sensitive to the UV-induced oxidation process.
Figure 4.
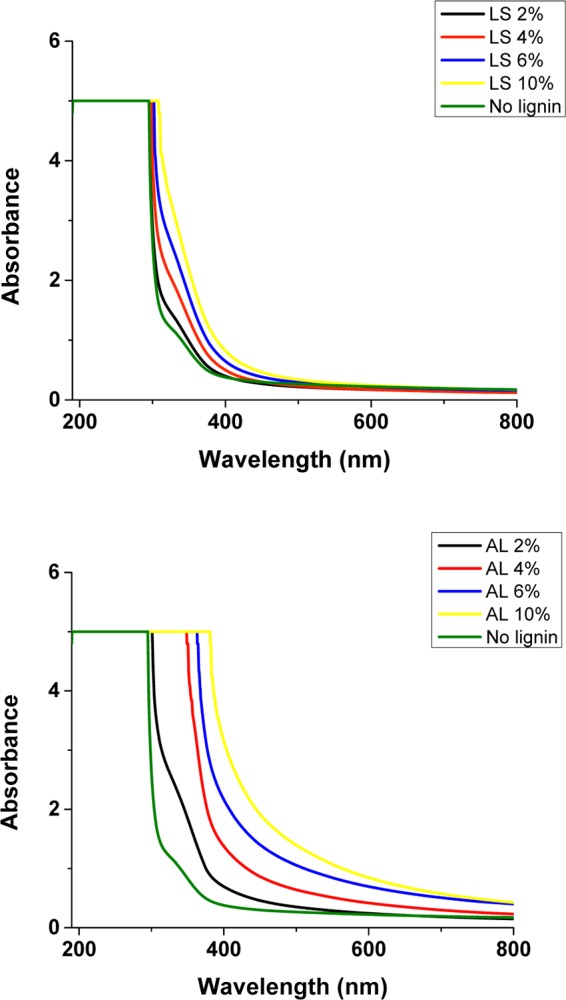
Effect of lignosulfonate (LSS) and alkali lignin (AL) on light absorbance at wavelengths 200–800 nm, lignin% w/w.
FTIR
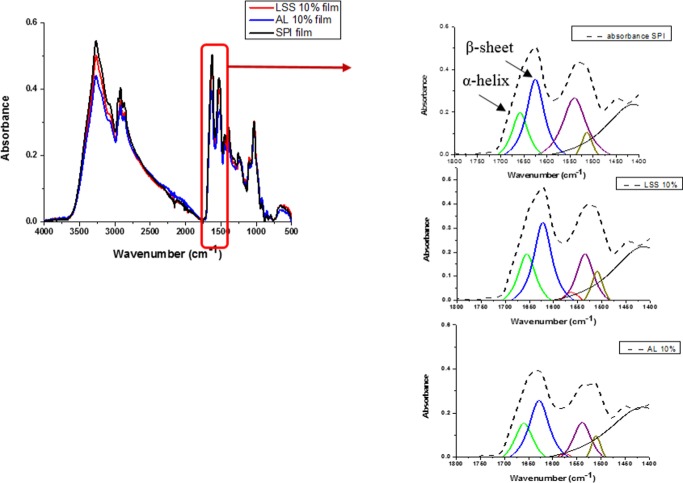
To assess the effect of lignin on the secondary structure of enzymatically modified SPI film, FTIR analysis can be used.20 As shown in Figure 5, FTIR spectra of control film and films containing 10% w/w AL or LSS were not significantly different. According to the rheological behavior observed, secondary structure changes (α-helix and β-sheets) might occur in the enzymatically modified SPI matrix. Therefore, the amide I and amide II regions (1800–1400 cm–1) in FTIR spectra were deconvoluted. Amide I was created from C=O stretching vibration and the sensitive changes in the secondary structure of protein, such as α-helix, β-sheets, and β-turn, where their stretching vibrations were at 1651, 1623, and 1676 cm–1, respectively.21,22 The deconvolutions of the analyzed spectral region of the control film and the modified films with AL and LSS are shown in Figure 5. The addition of AL showed shifts of α-helix and β-sheet peaks to a higher wavenumber, whereas LSS-based film showed shifts to a lower wavenumber. In general, shifting amide I to lower wavenumber values indicates more order in the secondary structure of proteins.20 This suggests that AL caused more disordering of the secondary structure of the protein matrix compared to LSS. This disordering behavior of AL agrees with the rheological behavior, which is observed in Figure 2, and the XRD pattern, which is shown in Figure 6. This behavior also agrees with the finding of Oliviero et al.8 They reported that low concentrations of lignin (1 and 3 g/100 g) in zein protein shifted amide I spectra to lower frequencies due to the formation of hydrogen bonding between amino acids of zein and functional groups of lignin.
Figure 5.
FTIR spectra of enzymatic modified SPI film with lignosulfonate (LSS) and alkali lignin (AL) (left) and deconvoluted spectra in the amide I and II region (wavenumber 1800–1400 cm–1). Abbreviations: AL: alkali lignin, LSS: lignosulfonate, SPI: soy protein isolate, lignin%: g/100 g.
Figure 6.
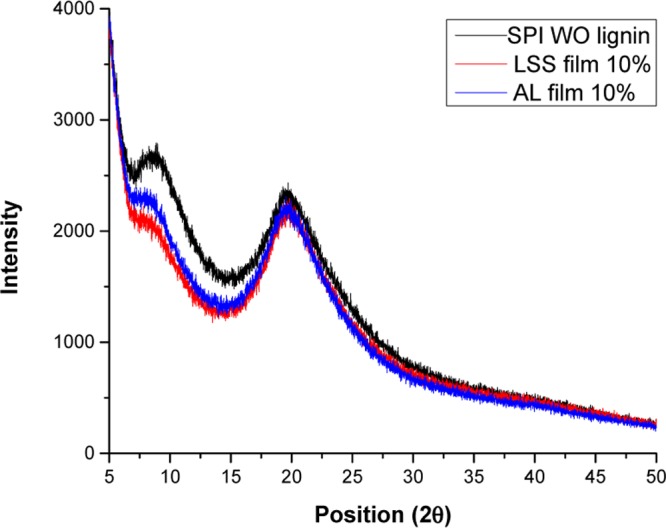
X-ray pattern of enzymatic modified SPI films with lignosulfonate (LSS) and alkali lignin (AL). Abbreviations: AL: alkali lignin, LSS: lignosulfonate, SPI: soy protein isolate, lignin%: g/100 g.
Amide II band was created from C–N and N–H stretching deformation vibration.22 AL-based film showed shifts of amide II band to higher frequencies, whereas LSS films showed shifts to lower frequencies compared to the control film.
XRD
The XRD patterns of enzymatically modified SPI film with AL and LSS are shown in Figure 6. XRD spectra of films showed a typical pattern for protein. Two peaks at 2θ = 9.5 and 20° positions were related to two d-spacings around 4.6 Å (d0) and 8.96 Å (di). Olivero et al.23 suggested that these two peaks are attributed to the average backbone distance within α-helix structure of the protein and d-spacing of α-interhelix packing, respectively, and they observed these two peaks in thermoplastic zein foam nanocomposite modified with 10 g/100 g AL and LSS. Compared with the control film, the lower intensity of modified films with lignin showed the hierarchical changes in SPI matrix due to the addition of lignin. This reduction may be attributed to the disruption of α-interhelix packing.23 The XRD spectra were well-matched to the FTIR spectra, indicating that the addition of lignin interrupted the secondary structure of the protein matrix according to the reduced intensity. The consistency in the general pattern implies that there is a high compatibility between lignin and enzymatically modified SPI matrix. On the other hand, Huang et al.11 reported that SPI sheets containing LSS exhibited a higher degree of crystallinity compared to the films without lignin due to the physical cross-linking of LSS that favored the ordered structure in the SPI matrix. This discrepancy in their finding and ours might be due to the variation in films processing conditions, while, they used hot-pressed and we applied solvent casting method. In conclusion, XRD spectra showed that films with and without lignin are in the amorphous state.
Environmental Scanning Electron Microscopy (ESEM)
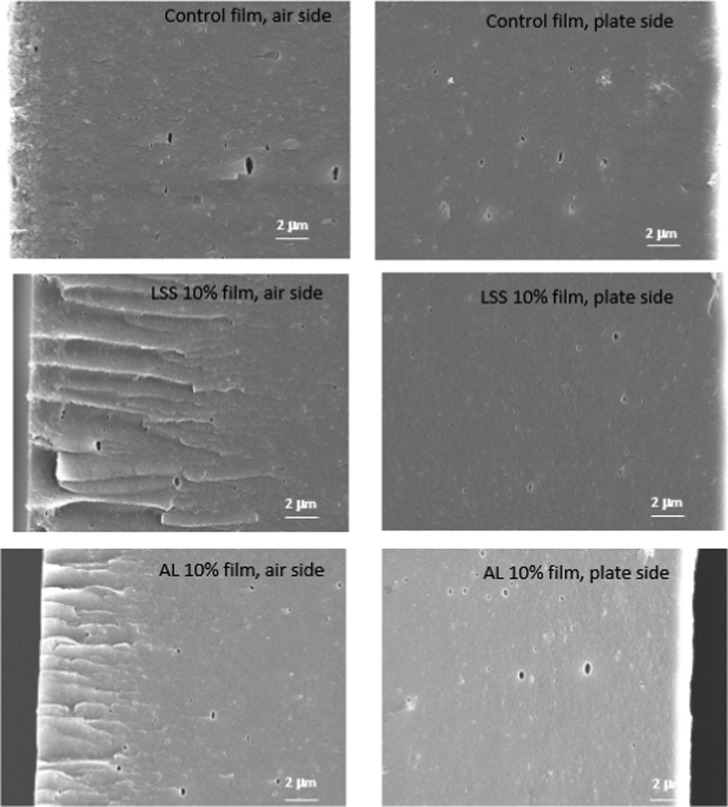
Scanning electron microscopy (SEM) images of both airside and plateside surfaces of films modified with AL and LSS are shown in Figure 7. Our previous research has shown that the enzymatic treatment of SPI film matrix increased surface roughness of the film compared to the nonenzyme treated films, which was due to the aggregation of hydrophobic groups of SPI made by transglutaminase.24 In contrast, enzymatically modified films with lignin have different morphological structures on both airside and plateside surfaces. These differences may have resulted from the thickness (approximately 0.1 mm) of the films. The control film showed smooth surfaces on both the airside and the plateside. However, the roughness on the airside of films increased after modifying the film with lignin and these differences in roughness resulted in decreasing the contact angle of these two types of film. This roughness was only observed on the airside of the film, as shown in Figure 7, which has been subjected to the water evaporation during the drying process.4 The same pattern was also observed by other researchers who modified the structure of SPI and chitosan film with lignin using extrusion and solvent casting, respectively, and the addition of lignin resulted in a fluctuant fracture surface.12,25 Shankar et al.26 created agar films with different lignin contents (3, 5, and 10 g/100 g), and they reported that film surface roughness increased with increasing lignin content. At lower concentrations of lignin (3 and 5 g/100 g), the lignin was dispersed uniformly; however, films containing 10 g/100 g lignin seemed to have more agglomerated lignin on the surface.
Figure 7.
SEM micrographs (at 10× magnification) of airside and plateside surfaces of control and the modified enzyme-treated films with lignosulfonate (LSS) and alkali lignin (AL), lignin%: g/100 g.
Mechanical Properties
Tensile strength (TS) and percent elongation (%E) of enzymatically modified film with two types of lignin are shown in Table 4. The addition of alkali lignin (6 and 10 g/100 g) increased the TS of SPI-modified films to around 2 times higher than the control film, whereas elongation decreased as a function of lignin concentration. Conversely, percent elongation in films containing 6 and 10 g/100 g AL dropped significantly compared to the control film. This finding implies that AL produced more disruption in the protein matrix than LSS, also agreeing with rheological behavior, the FTIR spectra, and XRD patterns. Increasing the concentration of LSS to over 2 g/100 g improved TS of SPI films. The addition of 2 g/100 g LSS did not change TS and %E of the modified films compared to the control film. This consistency might be attributed to the high compatibility and negligible effect of LSS at 2 g/100 g in the protein matrix. According to the technical data sheet from the producer, the glass transition temperature (Tg) of AL is higher than LSS (Tg of AL is 160 °C and LSS is 140 °C), which indicates the films containing AL might be glassier than LSS-based films at testing temperature. Huang et al.,11 who modified the structure of soy protein with different ratios of LSS using a melt extrusion method, reported similar patterns to our observation. Shankar et al.,26 who modified the structure of agar film with different percentages of lignin (1, 3, 5, and 10 g/100 g) with solvent casting reported that the addition of lignin improved TS and reduced %E of films. This group also found that films with 3% lignin showed the highest TS, whereas higher concentration of lignin caused agglomeration and nonuniform dispersion that had a negative effect on the TS of films. Núñez-Flores et al.4 reported that the addition of a low concentration of lignin (0.6 g/100 mL in the solution) dropped TS and improved %E of fish gelatin-based films plasticized with 15% glycerol and 15% sorbitol. They reported that lignin produced plasticizing effects on fish gelatin film and it increased %E and reduced TS. The same pattern was also achieved by Espinoza Acosta et al.,18 who modified wheat starch structures with lignin, and the addition of lignin significantly reduced TS but increased %E. Accordingly, we observed that lignin acts as a reinforcing agent in enzymatically modified SPI matrix.
Table 4. Effect of Different Concentrations of Lignosulfonate (LSS) and Alkali Lignin (AL) on Mechanical and Permeability Properties of Enzymatically Modified SPI Filma,d,e.
| film | TS (MPa)b | %E | WVP (10–13 g cm/(cm2 s Pa))c |
|---|---|---|---|
| control | 4.74 ± 0.34Aa | 126.33 ± 17.9Aa | 7.16 ± 0.83Aa |
| LSS 2% | 4.11 ± 0.33A | 146.17 ± 14.88A | 3.28 ± 0.24B |
| LSS 4% | 7.22 ± 0.41B | 63.99 ± 10.89B | 4.6 ± 0.42C |
| LSS 6% | 7.73 ± 0.13B | 86.93 ± 6.95B | 4.78 ± 0.14C |
| LSS 10% | 8.01 ± 0.89B | 79.95 ± 5.32B | 4.13 ± 0.07C |
| AL 2% | 7.07 ± 0.35b | 57.5 ± 11.8b | 4.33 ± 1.03b |
| AL 4% | 6.49 ± 0.41a | 51.61 ± 6.15b | 4.43 ± 0.4b |
| AL 6% | 10.13 ± 0.73c | 14.7 ± 3.7c | 3.52 ± 0.6b |
| AL 10% | 10.98 ± 1.02c | 7.45 ± 1.24c | 4.23 ± 0.3b |
Abbreviations: TS: tensile strength, %E: percent elongation, WVP: water vapor permeability.
Results are mean ± SD after five times replication.
Results are mean ± SD after three times replication.
Small letters show significant differences in different AL samples, and capital letters show significant differences in LSS samples.
Lignin%: g/100 g.
Thermogravimetric Analysis (TGA) of Films
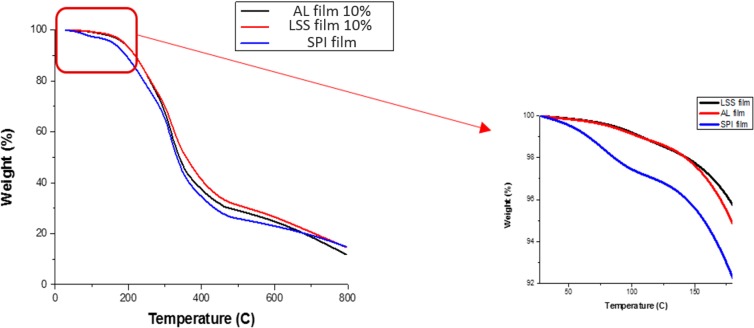
Thermal analysis techniques, such as TGA, provide information on the thermal stability of biopolymer films. As shown in Figure 8, modified films with lignin (both AL and LSS) showed higher thermal stability compared to the control film based on changes in onset temperatures at around 150 and 50 °C, respectively. The first weight loss might be attributed to the evaporation of water from the films. Both AL and LSS showed similar trends in the TGA graph. The second weight loss, which is a result of polymer decomposition, occurred at around 300 °C in films containing lignin, whereas the second weight loss for the control (SPI film) occurred at around 275 °C. The addition of lignin improved the thermal stability of SPI polymer film due to the interaction between lignin and polymer molecules. Espinoza Acosta et al.18 reported that the modified structure of durum wheat starch with lignin showed a second weight loss (decomposition) at a lower temperature than the control due to the discontinuity of the starch matrix after the addition of lignin. This discrepancy may be the result from the miscibility between lignin and the polymer matrix.
Figure 8.
Effect of lignosulfonate (LSS) and alkali lignin (AL) on thermal stability of enzymatically modified SPI film, lignin%: g/100 g.
Contact Angles of the Films
Contact angle is a helpful measurement to determine wettability and surface hydrophobicity of biopolymer films. The contact angles of both airside and plateside of films modified with two types of lignin are shown in Table 5. We expected that the incorporation of lignin may increase the hydrophobicity of SPI films due to the enzymatic reaction between lignin and the polymer. Interestingly, the treatment did not show higher contact angles for both film sides, which would have suggested greater surface hydrophobicity than the control (p > 0.05). In our previous research,24 it was found that the enzymatic modification of SPI film increased surface hydrophobicity due to the influence of hydrophobic groups of SPI, which were initially hidden in the inside of the molecule. However, the addition of lignin reduced the surface hydrophobicity of enzymatically modified SPI films, in both airside and plateside. This reduction in surface hydrophobicity was due to physical interactions of hydrophobic amino acids with the lignin and accelerated the exposure of hydrophilic units of protein on the surface of the film.27 The contact angle of the plateside was lower compared to that of the control and the airside due to the surface roughness induced by molecules arrangement during the drying process. This difference in the roughness of plateside and airside of films coincides with SEM images (Figure 7). There was no significant difference among different concentrations of lignin in the film (p < 0.05). The similar result was reported by Košíková et al.,25 where the addition of lignin reduced surface hydrophobicity and contact angle of PP film as a function of lignin concentration.
Table 5. Effect of Lignosulfonate (LSS) and Alkali Lignin (AL) on Initial Contact Angle and Water Absorption of Enzymatically Modified SPI Filmb,c.
| filma | airside contact angle | plateside contact angle | water absorption (%) |
|---|---|---|---|
| control | 86.14 ± 5.60Aa | 80.21 ± 1.2Aa | 128.73 ± 5.25Aa |
| LSS 2% | 86.65 ± 2.87A | 62.77 ± 0.75B | 145.58 ± 8.05B |
| LSS 4% | 81.87 ± 3.01A | 62.56 ± 1.35B | 120.94 ± 27.3A |
| LSS 6% | 82.63 ± 2.65A | 66.46 ± 4.87B | 132.45 ± 1.76A |
| LSS 10% | 83.15 ± 2.12A | 66.90 ± 3.21B | 119.05 ± 9.79A |
| AL 2% | 77.98 ± 4.25a | 68.79 ± 2.27b | 220.77 ± 65.51b |
| AL 4% | 79.64 ± 0.52a | 67.26 ± 6.23b | 204.73 ± 4.68b |
| AL 6% | 87.07 ± 3.02a | 74.27 ± 4.62a | 235.88 ± 9.08b |
| AL 10% | 81.63 ± 3.21a | 68.23 ± 0.67b | 235.17 ± 4.46b |
Results are mean ± SD after three times replication.
Small letters show significant differences in different AL samples, and capital letters show significant differences in LSS samples.
Lignin%: g/100 g.
Water Absorption Properties
Water sensitivity is one of the main limitations of bioplastics. The effect of different types of lignin on the water absorption properties of enzymatically modified SPI film is shown in Table 5. Films modified with AL had approximately 100% higher water absorption compared to the films modified with LSS. Water absorption results were aligned to what we observed previously in viscosity, FTIR, and XRD graphs (Figures 2, 5, and 6) where AL resulted in more interference in enzymatically modified SPI matrix compared to LSS. The addition of LSS at different concentrations did not have a significant effect (p > 0.05) on the water absorption of the modified films after 2 h swelling in buffer solution at pH 7. Similar results were observed by Oliviero et al.,8 who modified the structure of zein protein with AL and LSS using a melt extrusion method, and they observed that modifying zein protein structure with LSS did not have a significant effect on the water uptake capacity of films. However, AL reduced water absorption of the modified film, which was due to the decrease in the concentration of hydrophilic amino acids regarding the interaction of OH and SH groups of AL with amino acids. Huang et al.12 reported that the addition of LSS to the SPI matrix dramatically reduced water absorption to around 80% in films containing 40 parts LSS; their films were prepared by compression molding. They suggested that sulfonic acid groups of LSS formed a cross-linked network in the SPI matrix. In our experiment, LSS showed good miscibility in enzymatically modified SPI films and LSS did not have a significant effect on the water absorption or contact angle properties of enzymatically modified SPI films (specially at concentration of 2 and 4 g/100 g), which was comparable to what we observed in FTIR spectra.
Water Vapor Permeability
The effect of type and concentration of lignin on water vapor permeability of modified SPI films is shown in Table 4. The addition of lignin to enzymatically modified SPI film improved water vapor barrier properties up to approximately 50% compared to the control film. However, there was not a significant difference between WVP of modified films with AL and LSS (p > 0.05). The composite film of agar/lignin revealed reducing WVP with increasing lignin concentration to 10 g/100 g.26 The lignin may be acting as a filler that provides a high barrier properties to the film. Opposing results were observed by Núñez-Flores et al.,4 who modified the structure of fish gelatin-based film with two types of lignin, that the mixture of gelatin to lignin at the ratio of 85:15 increased water vapor permeability to nearly 50%, which was due to either the water-induced plasticization effect of lignin or poor compatibility of lignin with gelatin.
Conclusions
As raw materials, alkali lignin (AL) and lignosulfonate (LSS) showed significant radical scavenging activity compared to commercial BHT, which carry strong potentials in use for active packaging structure. Films containing AL showed strong UV-blocking ability induced by the natural color of lignin. On the basis of FTIR and XRD spectra, the secondary structures of SPI matrix can be altered by the addition of lignin and transglutaminase treatment. Mechanical properties and thermal stability of films containing lignin were significantly changed compared to the control films without lignin. This implies that the common limitations of biopolymeric film in packaging, such as poor mechanical properties and poor thermal properties, may be minimized or solved by utilizing lignin and enzymatic treatment, while offering an additional potential in functionality, such as antioxidant activity derived from lignin.
Experimental Section
Materials
Soy protein isolate (PROFAM 974) with 90% protein, on a dry basis, was supplied by Archer Daniels Midland company Protein Specialties Division, Netherlands. SPI had 5% moisture, 4% fat, and 5% ash. Transglutaminase (Activa RM) was donated by Ajinomoto, Japan. According to the data sheet provided by the company, Activa RM contains 99.5% sodium caseinate and maltodextrin and 0.5% enzyme. On the basis of our preliminary study, measured activity of the enzyme was 47.8 unit per gram of enzyme.24 Glycerol and alkali lignin (code 370959) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). Calcium lignosulfonate was donated from Green Agrochem, grade one, Jinan, China. Reagents for enzyme activity measurements such as Tris–HCl, Tris-acetate buffer, CaCl2, glutathione, hydroxylamine, CBZ-glutaminylglycine, l-glutamic acid γ-monohydroxamate, and FeCl3-trichloroacetic were purchased from Sigma-Aldrich. Citric acid, ethanol, methanol, BHT, and phosphoric acid were obtained from Fisher Scientific. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was obtained from Sigma Chemical Co.
Antioxidant Activity of Lignin Material
Antioxidant activities of different lignins (AL and LSS) were determined on the basis of the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method and according to radical scavenging ability of lignin material at pH 7. The assay was carried out as described by Brand-Williams et al.28 with some modifications. Six different concentrations of lignin solution in methanol ranging from 0 to 800 μg/mL were prepared, and 0.06 mM DPPH solution in methanol was added to each antioxidant solution and the value of absorbance at 517 nm was measured using a GENESYS 10S UV–vis spectrophotometer (Thermo Fisher Scientific). Butylated hydroxyltoluene (BHT) was used as a reference. Radical scavenging activity of each antioxidant was expressed from the graph by linear regression of absorbance versus concentration.
Film Preparation
Enzymatically modified soy protein isolate-based film was prepared by dissolving 5 g of SPI in 100 mL of distilled water with glycerol 30 g/100 g of SPI, according to the preliminary study. Lignin solution (2, 4, 6, and 10 g/100 g of SPI) and enzyme solution (4 U/g of protein) were prepared separately and added to the final solution under a specific pH condition. Maximum solubility of alkali lignin was at pH 10–11. The pH of water-soluble lignosulfonate was adjusted to pH 7 with sodium hydroxide 50 g/100 g. After heating SPI solution at 80 °C in 30 min, the solution was cooled down to 40 °C and incubated with the lignin for 1 h. Transglutaminase was added to the solution and incubated at the same temperature condition for another 2 h. Solution (100 g) was weighed and casted on Teflon-coated glass plates (20 × 20 cm2) to produce the consistency of thickness in all samples and dried at room temperature for 24 h and carefully peeled off manually. The films were then preconditioned at 25 °C and 50% relative humidity (RH) for 2–3 days before analysis according to ASTM D618-61.29
Rheology of Film-Forming Solutions
The effect of lignin on the rheological properties of enzymatically modified SPI film-forming solution with 7.5 g/100 g of solid content was determined using a rotational rheometer (AR G2, TA Instrument Ltd., New Castle, DE) with a conical concentric cylinder (30.2 mm diameter and 78.4 mm length and a geometry with 28.1 mm diameter and 80.8 mm length). This is according to the method described by Gauche et al.30 The measurements were replicated three times for each sample, and the data were evaluated using the Origin software, version 9.0 (Microcal Software Inc., Northampton, MA). The temperature of rheometer was controlled by Brookfield temperature controller with nitrogen gas to set 25 °C. The sample was preconditioned at 25 °C for 2 min to ensure stability. The shear rate increased linearly from 1 to 1000 s–1 with 8 points per decade in a steady state flow rate. The viscosity of the SPI solution was expressed by Pa s.
DPPH Free Radical Scavenging Test of Films
Radical scavenging activity of film samples with and without lignin was determined according to the method described by Yen et al.31 with a slight modification. A rectangular piece of soy protein-based film (1 cm2) with and without lignin was soaked into 5 mL of DPPH 0.05 mM in methanol. These film samples are insoluble in methanol-based solution; however, lignins are soluble and they change the light absorbency of methanol solution. The solution was shaken at room temperature and in the dark for 2 h. The absorbance of the solution was determined in 517 nm by a GENESYS 10S UV–vis spectrophotometer (Thermo Fisher Scientific). Radical scavenging activity of films was calculated according to the following equation
where Asample is the absorbance of sample and Acontrol is the absorbance of control, which is the film without lignin.
Color and Opacity of Films
The color of film surface was measured using a Konica Minolta (CR-200; Minolta Co. Ltd., Osaka, Japan). Prior to analysis, the colorimeter was calibrated to standard black and white tiles. Five different parts of film samples were measured, and the mean and standard deviation were recorded. The color scale was included L-value, from 0 to 100, a-value, and b-value, from negative to positive scales.
Opacity of films was measured by the GENESYS 10S UV–vis spectrophotometer (Thermo Fisher Scientific) and according to a method described by Núñez-Flores et al.4 The films were cut into a rectangle piece and directly placed into a cuvette. An empty cuvette was used as the reference. The opacity of films was calculated by the equation O = Abs600/X, where Abs600 is the value of absorbance at 600 nm and X is the thickness of the film. Results were obtained in triplicate.
Film UV-Blocking Measurements
UV absorbance of the films was measured by the UV–vis spectrometer (Shimadzu 2550 UV–vis spectrometer spectrum, Kyoto, Japan) with a wavelength range of 200–800 nm.
Fourier Transform Infrared (FTIR) Spectroscopy
Fourier Transform Infrared (FTIR) spectroscopy was performed with an 8700 Nicolet Thermo Electron, with transmission mode and a resolution of 4 cm–1, in the range of 4000–500 cm–1. The number of scans for background was 32 and for the sample was 96. The samples were preconditioned in a dissector containing P2O5 for a week to achieve the lowest moisture content. To see the effect of lignin on secondary structure of protein matrix, the absorbance region between 1800 and 1400 cm–1 was deconvoluted by OriginPro, version 9.0 (Microcal Software Inc., Northampton, MA) using the best fit by R2 > 99% to specify the position of the peaks to correspond to the different secondary conformations of protein matrix.8
X-ray Diffraction
To analyze the crystalline structure of enzymatically modified SPI-based film with and without the lignin, X-ray diffraction was performed by XRD (Bruker D8 Discover) from 2θ values of 5–50° at the rate of 0.1 s/step and equipped with Cu Kα radiation (λ = 0.1542 nm).
Environmental Scanning Electron Microscopy (ESEM)
The surface of film samples was coated with gold 60:40 gold–palladium mixture deposited by Cressington 208HR coater. The samples were tested with FEI Quanta 600 FEG, Oregon.
Mechanical Properties
For determination of tensile strength (TS) and percent elongation (%E), the ASTM D88232 method was used and evaluated by TA.XTPLUS texture analyzer (Stable Micro Systems, Surrey, U.K.) with 50 kg loading capacity. The films were cut in rectangles 2.5 × 8 cm2 and put in the environmental chamber (50% RH and 23 °C) for 2 days before the analysis. The samples were fixed between the grips with an initial grip separation of 5 cm and pulled apart at cross head speed of 50 mm/min. Tensile strength was determined by dividing the peak load (N) to the cross-sectional area (m2), and the results were expressed using MPa. The percent elongation was calculated by dividing the extended length by the initial length multiplied by 100.
Surface Hydrophobicity
The surface hydrophobicity of enzymatically modified films activated with different levels of lignin was assessed by measuring the contact angle (FTA 200; Dynamic Contact Angle Analyzer, Portsmouth, VA). A drop of 1 μL of distilled water at the rate of 0.3 μL/s was placed on the surface of the film with an automatic piston syringe (100 μL) and photographed. Initial contact angle was measured after 0.3 s to minimize the noise in the picture. The method is based on image processing and curve fitting for contact angle according to measuring the contact angle between the baseline of the drop and the tangent of drop boundary. The contact angles were measured on both sides of the drop and the average recorded.
Water Absorption Properties
Water absorption property of the modified films at pH 7 was determined according to the ASTM D57033 method. The films were cut to 25 × 25 mm2 and preconditioned at environmental chamber for 2 days and dried in an oven at 50 °C for 24 h before the analysis. Dry samples were weighted (w1) by AG104 Mettler Toledo with 0.1 mg precision. Then, they were immersed in 0.1 M sodium phosphate dibasic solution and the pH was adjusted with NaOH 50 g/100 g to 7 at 25 °C for 2 h. The wet samples were then wiped with filter paper to remove extra water and weighed (w2). Swelling (%) calculated by the following equation
Thermogravimetric Analysis (TGA)
Thermal decomposition of modified SPI-based films with lignin was measured using a TA Q500 thermogravimetric analyzer (TA Instrument Inc., New Castle, DE) at a heating rate of 10 °C/min from 25 to 800 °C under nitrogen gas. Film samples were preconditioned in a desiccator containing P2O5 for a week to achieve the lowest moisture content. A palladium pan was used for the experiment.
Water Vapor Permeability (WVP)
Water vapor permeability coefficients (WVP, g cm/(cm2 s Pa)) of the films were measured with i-Hydro 7500 Water Vapor Transmission Rate Testing System (Labthink Instruments Co., Ltd., China) according to an internal standard ISO 15106-3 with the electrolytic detection sensor method.34 The thicknesses of the three parts of each film were measured by the micrometer and then averaged by thickness of each film. The WVP coefficient of each film was calculated using the equation
where WVTR is water vapor transmission rate (g/m2·24 h) through the film and x indicates the mean thickness of each films (μm). Δp (Pa) is the difference in water vapor partial pressure between the two sides (a dry chamber and controlled humidity chamber) of the film. Each film (7 cm × 7 cm) was attached between two sides of the punched aluminum plates. The diameter of the punched area on the plates was 5.08 cm. The actual test area of WVP was 3.5 cm × 3.5 cm based on the exposed punched area of the plates. The aluminum plate containing the film was inserted in the cell chamber and closed tight. The flow rate was adjusted to 100 mL/min, and the test relative humidity and temperature were controlled at 50 ± 2% and at 23.5 °C, receptively. The standard testing mode was programmed with four cycles testing mode for each cell chamber. Each test time interval per a cycle was 30 min. Before four cycles of each test were started, the zero purging for 60 min was conducted.
Factorial Design and Statistical Analysis
Enzymatically modified soy protein isolate was activated with lignin in different concentrations. Two factors: lignin type (AL and LSS) and lignin concentration (0, 2, 4, 6, and 10 g/100 g) were determined. Therefore, there were 10 treatments. Each type of film was prepared three times, and each film replicated five times for mechanical test and three times for surface hydrophobicity and swelling properties. In total, 15 replicates for the mechanical test and 9 replicates for the others were done.
All data were analyzed by one-way analysis of variance to determine the difference between samples. The Minitab Software model 14.12.0 (Minitab Inc., State College, PA) was applied. Tukey test was used to carry out the difference of means between pairs with 95% confidence interval.
The authors declare no competing financial interest.
References
- Gómez-Estaca J.; López-de-Dicastillo C.; Hernández-Muñoz P.; Catalá R.; Gavara R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. 10.1016/j.tifs.2013.10.008. [DOI] [Google Scholar]
- Vallverdú-Queralt A.; Regueiro J.; Martínez-Huélamo M.; Alvarenga J. F. R.; Leal L. N.; Lamuela-Raventos R. M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. 10.1016/j.foodchem.2013.12.106. [DOI] [PubMed] [Google Scholar]
- Abbasi A. M.; Shah M. H.; Li T.; Fu X.; Guo X.; Liu R. H. Ethnomedicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J. Ethnopharmacol. 2015, 162, 333–345. 10.1016/j.jep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Núñez-Flores R.; Giménez B.; Fernández-Martín F.; López-Caballero M. E.; Montero M. P.; Gómez-Guillén M. C. Physical and functional characterization of active fish gelatin films incorporated with lignin. Food Hydrocolloids 2013, 30, 163–172. 10.1016/j.foodhyd.2012.05.017. [DOI] [Google Scholar]
- Shahidi F.; Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Schreiber S. B.; Bozell J. J.; Hayes D. G.; Zivanovic S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocolloids 2013, 33, 207–214. 10.1016/j.foodhyd.2013.03.006. [DOI] [Google Scholar]
- Mushtaq N.; Schmatz R.; Pereira L. B.; Ahmad M.; Stefanello N.; Vieira J. M.; Abdalla F.; Rodrigues M. V.; Baldissarelli J.; Pelinson L. P.; et al. Rosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2014, 32, 287–293. 10.1002/cbf.3014. [DOI] [PubMed] [Google Scholar]
- Oliviero M.; Verdolotti L.; Di Maio E.; Aurilia M.; Iannace S. Effect of supramolecular structures on thermoplastic Zein–Lignin bionanocomposites. J. Agric. Food Chem. 2011, 59, 10062–10070. 10.1021/jf201728p. [DOI] [PubMed] [Google Scholar]
- Pan X.; Kadla J. F.; Ehara K.; Gilkes N.; Saddler J. N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. 10.1021/jf0605392. [DOI] [PubMed] [Google Scholar]
- Laurichesse S.; Averous L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. 10.1016/j.progpolymsci.2013.11.004. [DOI] [Google Scholar]
- Huang J.; Zhang L.; Chen F. Effects of lignin as a filler on properties of soy protein plastics. I. Lignosulfonate. J. Appl. Polym. Sci. 2003, 88, 3284–3290. 10.1002/app.12185. [DOI] [Google Scholar]
- Huang J.; Zhang L.; Chen P. Effects of lignin as a filler on properties of soy protein plastics. II. Alkaline lignin. J. Appl. Polym. Sci. 2003, 88, 3291–3297. 10.1002/app.12184. [DOI] [Google Scholar]
- Kun D.; Pukánszky B. Polymer/lignin blends: interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. 10.1016/j.eurpolymj.2017.04.035. [DOI] [Google Scholar]
- Gaspar A. L. C.; de Góes-Favoni S. P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. 10.1016/j.foodchem.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Mu L.; Shi Y.; Hua J.; Zhuang W.; Zhu J. Engineering Hydrogen Bonding Interaction and Charge Separation in Bio-Polymers for Green Lubrication. J. Phys. Chem. B 2017, 121, 5669–5678. 10.1021/acs.jpcb.7b03194. [DOI] [PubMed] [Google Scholar]
- Nakajima J.-i.; Tanaka I.; Seo S.; Yamazaki M.; Saito K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. J. Biomed. Biotechnol. 2004, 2004, 241–247. 10.1155/S1110724304404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai D.; Tan M. J.; Chee P. L.; Chua Y. K.; Yap Y. L.; Loh X. J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. 10.1039/C5GC02616D. [DOI] [Google Scholar]
- Espinoza Acosta J. L.; Torres Chávez P. I.; Ramírez-Wong B.; Bello-Pérez L. A.; Vega Ríos A.; Carvajal Millán E.; Plascencia Jatomea M.; Ledesma Osuna A. I. Mechanical, thermal, and antioxidant properties of composite films prepared from durum wheat starch and lignin. Starch/Stärke 2015, 67, 502–511. 10.1002/star.201500009. [DOI] [Google Scholar]
- Qian Y.; Qiu X.; Zhong X.; Zhang D.; Deng Y.; Yang D.; Zhu S. Lignin reverse micelles for UV-absorbing and high mechanical performance thermoplastics. Ind. Eng. Chem. Res. 2015, 54, 12025–12030. 10.1021/acs.iecr.5b03360. [DOI] [Google Scholar]
- Turasan H.; Kokini J. L. Advances in Understanding the Molecular Structures and Functionalities of Biodegradable Zein-Based Materials Using Spectroscopic Techniques: A Review. Biomacromolecules 2017, 18, 331–354. 10.1021/acs.biomac.6b01455. [DOI] [PubMed] [Google Scholar]
- Oliviero M.; Di Maio E.; Iannace S. Effect of molecular structure on film blowing ability of thermoplastic zein. J. Appl. Polym. Sci. 2010, 115, 277–287. 10.1002/app.31116. [DOI] [Google Scholar]
- Pelton J. T.; McLean L. R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000, 277, 167–176. 10.1006/abio.1999.4320. [DOI] [PubMed] [Google Scholar]
- Oliviero M.; Verdolotti L.; Nedi I.; Docimo F.; Di Maio E.; Iannace S. Effect of two kinds of lignins, alkaline lignin and sodium lignosulfonate, on the foamability of thermoplastic zein-based bionanocomposites. J. Cell. Plast. 2012, 48, 516–525. 10.1177/0021955X12460043. [DOI] [Google Scholar]
- Zadeh E. M.; O’Keefe S. F.; Kim Y.-T.; Cho J.-H. Evaluation of Enzymatically Modified Soy Protein Isolate Film Forming Solution and Film at Different Manufacturing Conditions. J. Food Sci. 2018, 946–955. 10.1111/1750-3841.14018. [DOI] [PubMed] [Google Scholar]
- Košíková B.; Revajová A.; Demianova V. The effect of adding lignin on modification of surface properties of polypropylene. Eur. Polym. J. 1995, 31, 953–956. 10.1016/0014-3057(95)00052-6. [DOI] [Google Scholar]
- Shankar S.; Reddy J. P.; Rhim J.-W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biol. Macromol, 2015, 81, 267–273. 10.1016/j.ijbiomac.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Salas C.; Rojas O. J.; Lucia L. A.; Hubbe M. A.; Genzer J. On the surface interactions of proteins with lignin. ACS Appl. Mater. Interfaces 2013, 5, 199–206. 10.1021/am3024788. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W.; Cuvelier M.-E.; Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- ASTM D618-61 . Standard Practice for Conditioning Plastics and Electrical Insulating Materials for Testing. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, 1995; Vol. 8.01. [Google Scholar]
- Gauche C.; Vieira J. T.; Ogliari P. J.; Bordignon-Luiz M. T. Crosslinking of milk whey proteins by transglutaminase. Process Biochem. 2008, 43, 788–794. 10.1016/j.procbio.2008.04.004. [DOI] [Google Scholar]
- Yen G. C.; Hsieh P. P. Antioxidative activity and scavenging effects on active oxygen of xylose-lysine maillard reaction products. J. Sci. Food Agric. 1995, 67, 415–420. 10.1002/jsfa.2740670320. [DOI] [Google Scholar]
- ASTM D 882-97 . Standard Test Method for Tensile Properties of Thin Plastic Sheeting. Standard Designation. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, 1997. [Google Scholar]
- ASTM E 96-80 . Standard Test Method for Water Absorption Plastic. Standard Designation. In Annual Book of ASTM standards; American Society for Testing and Materials: Philadelphia, PA, 1997. [Google Scholar]
- ASTM E 96-80 . Standard Test Method for Water Vapor Transmission of Materials. Standards Designation: E96-80. In Annual Book of ASTM; ASTM: Philadelphia, PA, 1980; pp 771–778. [Google Scholar]