Abstract
Black liquor (BL) from the kraft pulping process has been treated at elevated temperatures (380 °C) in a batch reactor to give high yields of a bio oil comprising monomeric phenolic compounds that were soluble in organic solvents and mineral oil and a water fraction with inorganic salts. The metal content in the product was <20 ppm after a simple extraction step. A correlation between concentration, temperature, and reaction time with respect to yield of desired product was found. At optimal reaction conditions (treating BL with 16 wt % dry substance at 380 °C for 20 min), the yield of extractable organics was around 80% of the original lignin with less than 7% of char. The product was analyzed by gel permeable chromatography, mass spectroscopy, nuclear magnetic resonance, elemental analysis, and inductively coupled plasma. It was found that a large fraction composed of mainly cresols, xylenols, and mesitols. This process provides a pathway to convert a major waste stream from a pulp mill into a refinery feed for fuel or chemical production, whereas at the same time the inorganic chemicals are recovered and can be returned back to the pulp mill.
Introduction
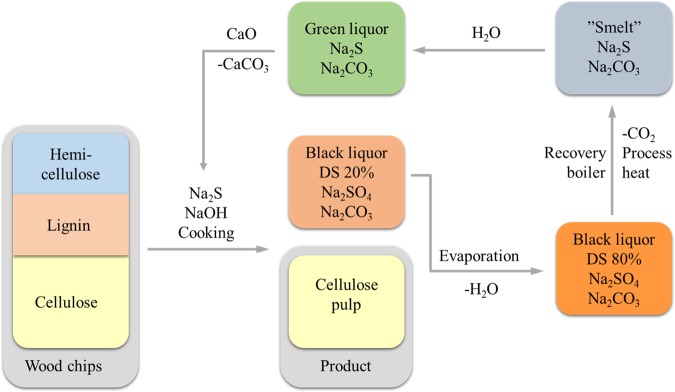
Biomass has received considerable attention as a source of renewable carbon for production of chemicals and fuels.1,2 One challenge is to find a suitable waste stream for upgrading to valorized products. One such potential waste stream is lignin-containing black liquor (BL) that is generated as a low value byproduct in pulp mills (Scheme 1). BL is currently burnt in the recovery boiler to regenerate process chemicals and to produce energy, usually in large excess. As the recovery boiler often is the bottleneck in a pulp mill, the BL is burnt to a low value and at the same time limits the production of high value pulp. To increase pulp production, techniques to extract the lignin part of BL have been developed and implemented.3−5 However, besides a few reports,6−12 the lignin from such extractions has not yet been converted to high value products.
Scheme 1. Illustration of the Kraft Process. DS = Dry Substance.
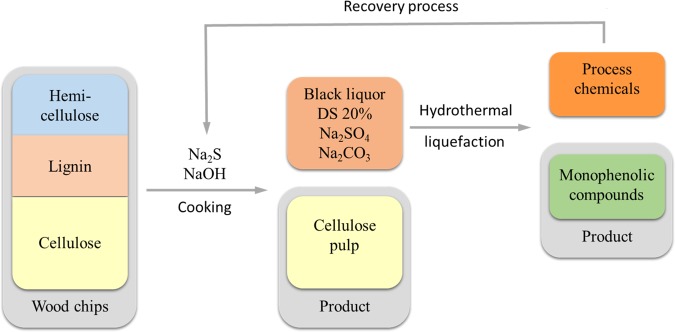
An interesting pathway would be to convert lignin, hemicellulose, and other organic materials present in the aqueous BL to a renewable bio oil, comprising phenolic compounds, vide infra (Scheme 2). It is known that lignin from the kraft process can be transformed in aqueous solutions at temperatures and pressure close to the critical point of water.13−19 Although most reports show good to excellent conversion of the lignin fraction of the cooking liquor, the techniques rely on addition of phenols, hydrogen gas, and catalysts. These additives typically improve product yield and decrease coke formation but bring additional cost and complexity to the process. Several reports have been published on liquefaction of kraft lignin in presence of bases, such as K2CO3 and NaOH as additives.12,13,19−23
Scheme 2. Hydrothermal Liquefaction (HTL) of BL to Produce Monophenolic Compounds.
Concentrated black liquor has been gasified to produce synthesis gas. Economical aspects of this process have been evaluated,24,25 and several pilot plants were successfully operated.26−28
Here, we report a simple, fast hydrothermal process for the conversion of lignin in BL into phenolic organic bio oils without any additives. In one case, ethanol was used as an additive to reduce char in HTL of high concentration (51 wt % DS) BL. The process gives significant yields of phenolic compounds that are easily separated from the process chemicals.
Results and Discussion
Given that existing reports of hydrothermal degradation of lignin and BL often apply hydrogen gas, catalysts, capping agents, and long reaction times, we wanted to evaluate a simpler approach. The use of hydrogen is associated with high costs for safety reasons, capping agents, such as phenols, add cost and complexity, and catalysts used in these systems are a subject to fouling and thus require frequent regeneration. Our hope was to instead process dilute BL at near-critical point temperatures and with short reaction times to maximize product yield and minimize the formation of insoluble coke. We investigated a fast hydrothermal decomposition of pure BL (mainly 16 and 19 wt % DS, fir BL) into oil, gas, inorganics, and coke at temperatures around the critical point of water.
Optimization of the Reaction Conditions
Fresh BL (16 wt % DS, of which 5 wt % is acid precipitated lignin) from a kraft pulp mill was used in the experiments. The reactions were performed in 10 mL Swagelok reactors using a fluidized sand bath for heating. We found that heating BL in a closed reactor at temperatures of 340–410 °C lowered the pH in the medium from above 11 to 8–9, enabling the phenolic material to separate from the aqueous phase without the need of acidification. To obtain a total yield of organics with low molecular weight (<700 Da), the product mixtures were directly extracted with organic solvents. In this report, we define the product yield as
Temperature Dependence
As reported by others,29 the temperature has a significant impact on the extractable fraction obtained by hydrothermal treatment of lignin. In the present system, the yield of bio oil, measured as ethyl acetate extractable material obtained in the temperature range of 340–420 °C was studied (Figure 1). The yield of extractable organic material showed a linear correlation between 340 and 370 °C with an increase of yield with temperature, peaking at 370–380 °C. At temperatures above 380 °C, the yield of extractable organics decreased, and the formation of char increased. Interestingly, the yield of low molecular weight phenols, cresols, and mesitols showed a near linear correlation to temperature, where at 340 °C, only 3 wt % monomers were obtained; at 380 °C, 5 wt % monomers were obtained; and at 420 °C, 9 wt % of monomers were obtained. This shows that the monomers are not responsible for the yield of extractable materials obtained.
Figure 1.
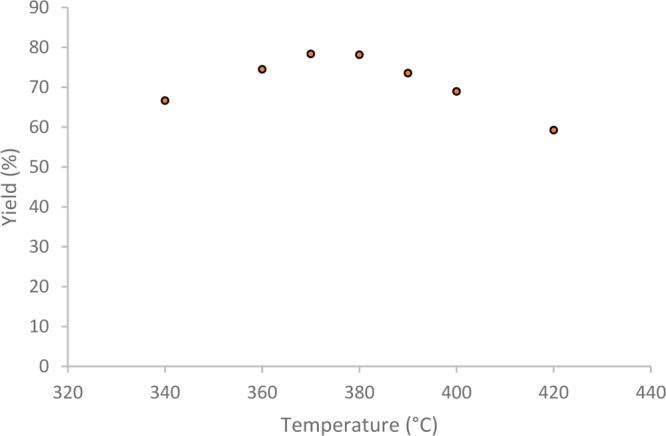
Temperature influence on the yield of bio oil from 16 wt % DS BL, 1 h reaction time. An average yield from two separate attempts is presented.
Effect of Concentration of the Black Liquor
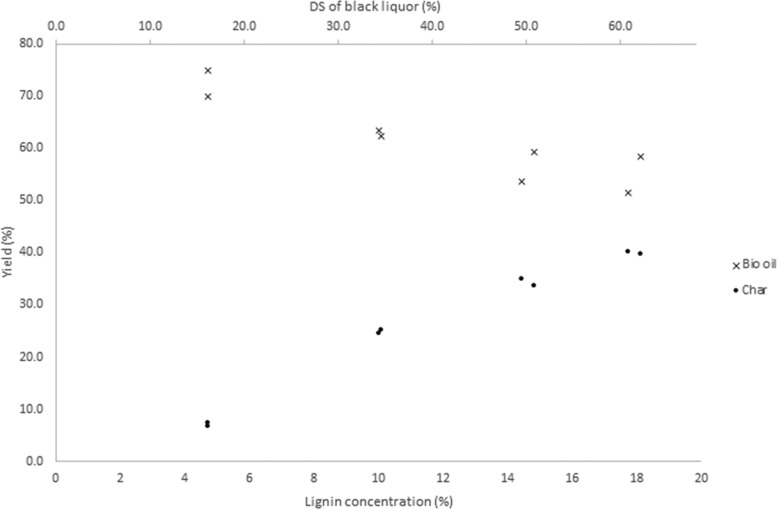
Next, the effect of concentration of the BL in the hydrothermal process was investigated. BL (16 wt % DS corresponding to a lignin concentration of 5 wt %) was evaporated at 25 °C to give more concentrated samples (34–62 wt % DS) for the study without disrupting the lignin structure. The hydrothermal treatment was then performed on samples of different concentrations. We found that the formation of char was proportional to the concentration of lignin in BL (Figure 2), which suggests avoiding high concentrations. A thin liquor, 16 wt % DS (5 wt % lignin), was found to give minimal amounts of char, whereas reactions performed on batches with higher lignin concentrations gave more insoluble material. The addition of aliphatic alcohols (C1–C18) greatly reduced coke formation, analogously to previous reports using phenols.12 In our system, the addition of 10 wt % of ethanol to the hydrothermal treatment of highly concentrated BL (18 wt % lignin, 51 wt % DS) for 1 h reaction reduces char from 27.5 to 9.6%.
Figure 2.
Influence of lignin concentration on yields of bio oil and char. Reactions performed at 380 °C and 1 h.
Influence of Reaction Time
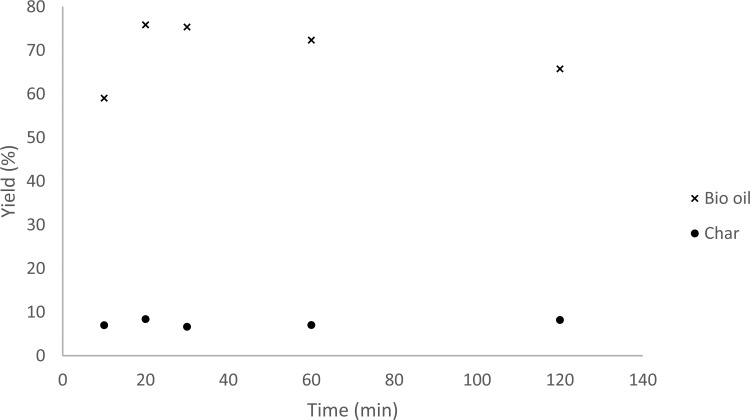
In the present hydrothermal treatment, the reaction time may affect both the depolymerization of the lignin polymer to monomers and also undesired reactions to form char. To find the optimal reaction time, the reactions were performed using the optimized temperature (380 °C) and thin BL (5 wt % lignin). Noteworthy, it was found that the depolymerization reaction is very fast and gives maximum yields of soluble material within 20 min (Figure 3). After 20 min, a slight decrease in extractable organics was observed. An explanation could be that the depolymerized material loses weight by elimination reactions, for example, that methoxy groups on aryls in e.g., guaiacol is converted to cresols. The reaction time had negligible influence on monomer yield where 4 wt % monomer was obtained after 30 min and only increased to 5 wt % after an hour.
Figure 3.
Time influence on yields of bio oil and char at 380 °C. An average yield from two attempts is presented.
Thereby, the optimized reaction condition to generate highest yields of ethyl acetate extractable organics is to perform the reaction at 370–380 °C, using a thin liquor (5 wt % lignin) in less than 30 min. This prompted us to study the extraction with a less polar solvent. One application where the formed extractable organics would be of interest is to produce green gasoline. To process feeds containing high amounts of oxygen in a conventional fuel refinery, it is important that the biofeed is soluble in a hydrocarbon carrier liquid.30,31 A suitable carrier liquid for this feed is light gas oil (diesel type). Gratifyingly, the organics from lignin could efficiently be extracted by the gas oil in 62% yield. Thereby, 62% of the original lignin content could easily be blended in gas oil for hydroprocessing in an oil refinery.
Product Properties
Storage Stability
The obtained bio oil is a black viscous liquid, storage stable at −20 °C under argon. The presence of air and/or at room temperature decreased the solubility and increased the molecular weight at room temperature under air. Most probably, repolymerization reactions of the material are responsible for the weight increase, which is also accompanied by increased viscosity.
Molecular Weight Distribution
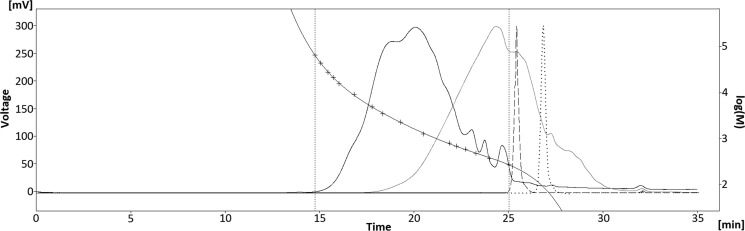
Gel permeable chromatography (GPC) analysis showed an average Mw of 550 Da of the obtained bio oil as compared to the kraft lignin feed that has a molecular weight average of 3000 Da (Figure 4). Although this technique does not provide an exact measurement of molecular weight,32 it gives a clear indication of a severe depolymerization. Simulated distillation analysis confirms this and revealed a boiling point range of 140–737 °C with 68% recovery (Supporting Information). It was also established by matrix-assisted laser desorption ionization that the maxima of the molecule distribution appear to be in the range of 600–700 Da (see Supporting Information).
Figure 4.
Gel permeation chromatogram of kraft lignin (black), HTL bio oil (gray), 2,2′-biphenol (dashed line), and o-cresol (dotted line).
Analysis of Low Boiling Fraction
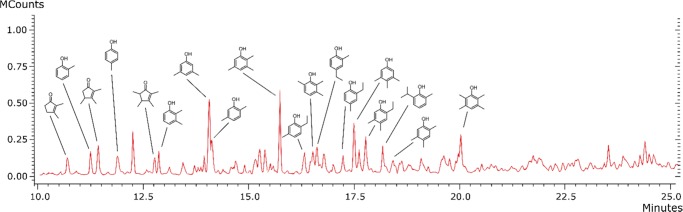
Gas chromatography–mass spectrometry (GC–MS) analysis of product components (bp < 300 °C) confirmed that a majority of the volatiles were methylated phenols, whereas anisoles were completely absent. The most abundant phenols were 2,4-dimethyl-, 2,5-dimethyl-, and 2,4,6-trimethyl-phenol (Figure 5). Phenols containing larger ethyl and iso-propyl substituents were present only in small amounts. This indicates the isomerization of anisoles and/or propyl-phenols into mono, di, and trimethylated phenols during the reaction. Similar results have been reported by others.33
Figure 5.
GC–MS chromatogram of distilled HTL bio oil.
NMR Study of Extractable Organics
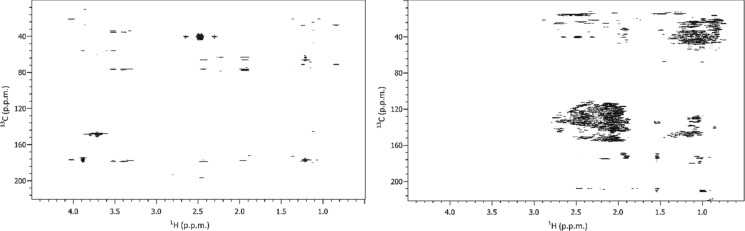
After modification with a phosphorylating agent and 31P NMR analysis34 it could be shown that there are only traces of aliphatic alcohols present. An NMR analysis of the aqueous phase did not show any presence of hydrocarbons. This suggests that all sugars as well as lignin have been transformed and extracted. gHMBCAD suggested disappearance of aromatic methoxy groups and other aliphatic ethers (Figure 6). When comparing bio oil to isolated kraft lignin using 1H NMR, we could observe disappearance of signals at 3.2–3.9 ppm and appearance of signals at 0.8–1.5 and 1.9–3.0 ppm as well as significant shifting of aromatic signals downfield.
Figure 6.
gHMBCAD of dried BL (left) and HTL bio oil (right).
The disappearance of alcohol and ether moieties was in accordance to a low oxygen content obtained from elemental analysis: C 80.47%, H 8.60%, N 0.045%, O 10.45%, and S 0.53%, which is half compared to isolated kraft lignin: C 61.80%, H 6.10%, N 0.31%, O 22.97, and S 2.29%. The inductively coupled plasma (ICP) revealed a severe decrease in metal as well as sulfur content (Table 1).
Table 1. ICP Results of HTL Bio Oil Obtained at Optimized Conditions (see Experimental Section) and Kraft Lignin (KL)a.
| elements | B | Ca | Fe | K | Mg | Na | S |
|---|---|---|---|---|---|---|---|
| HTL bio oil | 5 | 2 | 1 | 1 | 1 | 10 | 5872 |
| KL | 54.9 | 608 | 15 | 192 | 20.5 | 1797 | 26 651 |
The values are reported in wt ppm.
Conclusions and Outlook
Thermal liquefaction of BL is a promising methodology to incorporate into a kraft process. This gives the possibility to obtain a bio oil suitable for fuel and material purposes as well as making it possible to return inorganic chemicals back to the paper mill. To commercialize an HTL process in the future, there are a number of challenges such as: (1) heat recovery; (2) the discharge of monophenolics, inorganics, and water from the supercritical phase in a continuous flow; (3) materials withstanding corrosive reaction conditions (380 °C, high pH, ionic reaction media). Given that these issues would be solved, the HTL process of BL would be an important strategy to meet the demand of bio-derived monophenolic compounds.
Experimental Section
General
The NMR spectra were recorded on Varian Unity Inova (1H 499.9 MHz, 13C 125.7 MHz, 31P 161.9 MHz) spectrometer. The chemical shifts were referenced to solvent residual peaks or internal standard (1H: CHCl3 at 7.26, DMSO-d5 at 2.49, 13C: CDCl3 at 77.0, 31P: hydrolysis product of 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane at 132.2 ppm). The ICP-atomic emission spectroscopy was performed on a Spectro Ciros CCD system (Kleve, Germany) equipped with an argon gas inlet, a cyclonic spray chamber, modified Lichte nebulizer, and charge-coupled device (CCD) detector. The elemental analysis was done externally by Analytische Laboratorien, Prof. Dr. H. Malissa und G. Reuter GmbH, Industriepark Kaiserau, 51789 Lindlar, Germany. The HTL reactions were performed in a stainless steel (316) reactor built from Swagelok 3/4″ union (SS-1210-6) and two 3/4″ plugs (SS-1210-P), with total volume of the reactor of ca 10 mL. The reactors could be reused at least 50 times at temperatures up to 420 °C without leakage. The heating was done by immersing into a liquid sand bath Omega FSB-3 (with two 750 W heating coils) with one heating coil controlled by a CAL ET2011 thermostat. Gel permeation chromatography (GPC) was performed on YL 9110 HPLC-GPC system with three Styragel columns (HR 0.5, HR 1, HR 3, 7.8 × 300 mm2 each) connected in series (flow rate: 1 mL/min; injection volume: 35 μL; solvent: tetrahydrofuran) and a UV detector (280 nm). A calibration was done against polystyrene standard ReadyCal set with MP 266, 682, 1250, 2280, 3470, 4920, 9130, 15 700, 21 500, 28 000, 44 200, and 66 000 Da. GC–MS was run on a system consisting of a Varian CP-3800 gas chromatograph and a Varian Saturn 2200 GC–MS/MS spectrometer with following settings: injector temperature 280 °C; helium 1 mL/min; program 50 °C, 20 °C/min to 300 °C, 3 min. BL was obtained from Bäckhammar paper mill and used as is. It was stored under argon at 5 °C.
Optimized Procedure for HTL of BL
A 10 mL stainless steel (316) reactor was charged with fir BL (6 g) with 19 wt % DS. The reactor was sealed and heated in a fluidized sand bath for 1 h at 380 °C. After removing from bath and cooling to room temperature the reactor was opened, and the contents were transferred to a flask with small portions of ethyl acetate (20 mL in total) and water (5 mL in total). After removal of organic phase, the aqueous phase was extracted further with ethyl acetate (10 mL). The combined organic fractions were evaporated to give a black viscous oil.
The aqueous phase containing most of the char was filtered, the solids were washed with water and dried in an oven at 50 °C over night.
The products were analyzed with GPC, NMR, and GC–MS.
Distillation of the Bio Oil
The crude bio oil (639 mg) was distilled with a Kugelrohr apparatus to give 348 mg dark yellow oil and 183 mg residue. The distillate was miscible with pentane.
Acknowledgments
We would like to thank the Swedish Energy Agency for financial support. The authors thank Nordic Paper for providing black liquor.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00854.
NMR data and simulated distillation curves (PDF)
The authors declare the following competing financial interest(s): J.S.M.S. is Professor at Stockholm University and owner and CRO at RenFuel AB.
Supplementary Material
References
- Huber G. W.; Iborra S.; Corma A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- Corma A.; Iborra S.; Velty A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- Öhman F.; Theliander H.; Tomani P.; Axegard P.. Method for Separating Lignin from Black Liquor. EP1794363B1, 2005.
- Lake M. A.; Blackburn J. C.. Process for Recovering Lignin. U.S. Patent US20110294991A1, 2011.
- Jönsson A.-S.; Wallberg O. Cost estimates of kraft lignin recovery by ultrafiltration. Desalination 2009, 237, 254–267. 10.1016/j.desal.2007.11.061. [DOI] [Google Scholar]
- Goheen D. W.Hydrogenation of Lignin by the Noguchi Process. In Lignin Structure and Reactions; Marton J., Ed.; American Chemical Society, 1966; pp 205–225. [Google Scholar]
- Oshima M.; Maeda Y.; Kashima K.. Process of Liquefaction of Lignin. U.S. Patent US3105095A, 1963.
- Villar J. C.; Caperos A.; García-Ochoa F. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci. Technol. 2001, 35, 245–255. 10.1007/s002260100089. [DOI] [Google Scholar]
- Shabtai J. S.; Zmierczak W. W.; Chornet E.. Process for Conversion of Lignin to Reformulated Hydrocarbon Gasoline. U.S. Patent US5959167A, 1999.
- Yoshikawa T.; Yagi T.; Shinohara S.; Fukunaga T.; Nakasaka Y.; Tago T.; Masuda T. Production of phenols from lignin via depolymerisation and catalytic cracking. Fuel Process. Technol. 2013, 108, 69–75. 10.1016/j.fuproc.2012.05.003. [DOI] [Google Scholar]
- Löfstedt J.; Dahlstrand C.; Orebom A.; Meuzelaar G.; Sawadjoon S.; Galkin M. V.; Agback P.; Wimby M.; Corresa E.; Mathieu Y.; Sauvanaud L.; Eriksson S.; Corma A.; Samec J. S. M. Green diesel from kraft lignin in three steps. ChemSusChem 2016, 9, 1392–1396. 10.1002/cssc.201600172. [DOI] [PubMed] [Google Scholar]
- Nguyen T. D. H.; Maschietti M.; Åmand L.-E.; Vamling L.; Olausson L.; Andersson S. I.; Theliander H. The effect of temperature on the catalytic conversion of kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. 10.1016/j.biortech.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Belkheiri T.; Vamling L.; Nguyen T. D. H.; Maschietti M.; Olausson L.; Andersson S.-I.; Åmand L.-E.; Theliander H. Kraft lignin depolymerization in near-critical water: effect of changing co-solvent. Cellulose Chem. Technol. 2014, 48, 813–818. [Google Scholar]
- Ma R.; Hao W.; Ma X.; Tian Y.; Li Y. Catalytic ethanolysis of kraft lignin into high-value small-molecular chemicals over a nanostructured α-molybdenum carbide catalyst. Angew. Chem., Int. Ed. 2014, 53, 7310–7315. 10.1002/anie.201402752. [DOI] [PubMed] [Google Scholar]
- Yuan Z.; Tymchyshyn M.; Xu C. Reductive depolymerisation of kraft and organosolv lignin in supercritical acetone for chemicals and materials. ChemCatChem 2016, 8, 1968–1976. 10.1002/cctc.201600187. [DOI] [Google Scholar]
- Goudriaan F.; Peferoen D. G. R. Liquid fuels from biomass via a hydrothermal process. Chem. Eng. Sci. 1990, 45, 2729–2734. 10.1016/0009-2509(90)80164-A. [DOI] [Google Scholar]
- Johansson A. A new chemical recovery system for the kraft process. Results from continuous bench-scale operations. Biomass 1984, 4, 155–160. 10.1016/0144-4565(84)90064-7. [DOI] [Google Scholar]
- Tang K.; Zhou X.-F. The degradation of kraft lignin during hydrothermal treatment for phenolics. Pol. J. Chem. Technol. 2015, 17, 24–28. 10.1515/pjct-2015-0045. [DOI] [Google Scholar]
- Miller J. E.; Evans L.; Littlewolf A.; Trudell D. E. Batch microreactor studies of lignin and lignin model compound depolymerisation by bases and alcohol solvents. Fuel 1999, 78, 1363–1366. 10.1016/S0016-2361(99)00072-1. [DOI] [Google Scholar]
- Beauchet R.; Monteil-Rivera F.; Lavoie J. M. Conversion of lignin to aromatic-based chemicals (L-chems) and biofuels (L-fuels). Bioresour. Technol. 2012, 121, 328–334. 10.1016/j.biortech.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Lavoie J.-M.; Baré W.; Bilodeau M. Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour. Technol. 2011, 102, 4917–4920. 10.1016/j.biortech.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Pińkowska H.; Wolak P.; Złocińska A. Hydrothermal decomposition of alkali lignin in sub- and supercritical water. Chem. Eng. J. 2012, 187, 410–414. 10.1016/j.cej.2012.01.092. [DOI] [Google Scholar]
- Yong T. L.-K.; Matsumura Y. Kinetic analysis of lignin hydrothermal conversion in sub- and supercritical water. Ind. Eng. Chem. Res. 2013, 52, 5626–5639. 10.1021/ie400600x. [DOI] [Google Scholar]
- Naqvi M.; Yan J.; Dahlquist E. Black liquor gasification integrated in pulp and paper mills: A critical review. Bioresour. Technol. 2010, 101, 8001–8015. 10.1016/j.biortech.2010.05.013. [DOI] [PubMed] [Google Scholar]
- de Freitas Ferreira E. T.; Balestieri J. A. P. Black liquor gasification combined cycle with CO2 capture – technical and economic analysis. Appl. Therm. Eng. 2015, 75, 371–383. 10.1016/j.applthermaleng.2014.09.026. [DOI] [Google Scholar]
- Landälv I.; Gebart R.; Marke B.; Granberg F.; Furusjö E.; Löwnertz P.; Öhrman O. G. W.; Sørensen E. L.; Salomonsson P. Two years experience of the BioDME project – a complete wood to wheel concept. Environ. Prog. Sustainable Energy 2014, 33, 744–750. 10.1002/ep.11993. [DOI] [Google Scholar]
- Jafri Y.; Furusjö E.; Kirtania K.; Gebart R. Performance of a pilot-scale entrained-flow black liquor gasifier. Energy Fuels 2016, 30, 3175–3185. 10.1021/acs.energyfuels.6b00349. [DOI] [Google Scholar]
- Jafri Y.; Furusjö E.; Kirtania K.; Gebart R.; Granberg F. A study of black liquor and pyrolysis oil co-gasification in pilot scale. Biomass Convers. Biorefin. 2018, 8, 113–124. 10.1007/s13399-016-0235-5. [DOI] [Google Scholar]
- Kong J.; He M.; Lercher J. A.; Zhao C. Direct production of naphthenes and paraffins from lignin. Chem. Commun. 2015, 51, 17580–17583. 10.1039/C5CC06828B. [DOI] [PubMed] [Google Scholar]
- Karatzos S.; McMillan J. D.; Saddler J. N.. The Potential and Challenges of Drop-in Biofuels, IEA Report T39-T1, 2014.
- Egeberg R.; Knudsen K.; Nyström S.; Lind-Grennfelt E.; Efraimsson K. Industrial-scale production of renewable diesel. Pet. Technol. Q. 2011, 16, 59–65. [Google Scholar]
- Lange H.; Rulli F.; Crestini C. Gel permeation chromatography in determining molecular weights of lignins: critical aspects revisited for improved utility in the development of novel materials. ACS Sustainable Chem. Eng. 2016, 4, 5167–5180. 10.1021/acssuschemeng.6b00929. [DOI] [Google Scholar]
- Kang S.; Li X.; Fan J.; Chang J. Classified separation of lignin hydrothermal liquefied products. Ind. Eng. Chem. Res. 2011, 50, 11288–11296. 10.1021/ie2011356. [DOI] [Google Scholar]
- Sen S.; Sadeghifar H.; Argyropoulos D. S. Kraft lignin chain extension chemistry via propargylation, oxidative coupling, and Claisen rearrangement. Biomacromolecules 2013, 14, 3399–3408. 10.1021/bm4010172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.