Abstract
Nitrophenols (NPs) and related derivatives are industrially important chemicals, used notably to synthesize pharmaceuticals, insecticides, herbicides, and pesticides. However, NPs and their metabolites are highly toxic and mutagenic. They pose a serious threat to human health and ecosystem. Current work was undertaken to develop a suitable visible-light active catalyst for the sustainable and efficient mineralization of NPs in an aqueous environment. Nanocrystalline cellulose (NCs)-based nitrogen-doped titanium dioxide and carbonaceous material (N-TiO2/C) was synthesized by pyrolysis and sol–gel methods using NCs, polydopamine, and TiO2. The synthesized N-TiO2/C was characterized using different analytical techniques. Photocatalytic degradation of NPs under visible light indicated that acidic pH (3) was most suitable for the optimal degradation. 4-NP degradation followed both pseudo-first-order (R2 = 0.9985) and Langmuir–Hinshelwood adsorption kinetic models (adsorption constant, KLH = 1.13 L mg–1). Gas chromatography–mass spectrometry and ion chromatography analysis confirmed the total mineralization of 4-NP into smaller molecular fragments such as acids, alcohols, and nitrates. The total organic carbon showed that 67% of total carbon present in 4-NP was mineralized into CO2 and CO. The catalyst was recycled for five consecutive cycles without losing its catalytic activities. The degradation mechanism of NPs with N-TiO2/C was also explored.
Introduction
Water pollution has become a global issue in today’s world. Modern societies and industries are responsible for the utilization and subsequent release of hazardous chemicals such as heavy metals, organic halogens, dyes, nitroaromatic compounds, and so forth into the environment.1 Among them, nitrophenols (NPs) and their derivatives have been detected in water and atmosphere in significant levels.2 Inappropriate waste disposal practice, medical, agricultural, and industrial uses are the causes for NP contamination in water. Microbial degradation or photodegradation of industrial products such as pesticides, synthetic dyes, and pharmaceuticals (Figure 1a) enables the NPs to be released into the atmosphere.3,4 Thus, NPs are found in all places and humans are most likely to get exposed to environmental NPs through breathing, drinking, eating, and skin contact.5 Human exposure to NPs causes eye and skin irritation and damage of functionally important organelles such as kidney, liver, central nervous system, and so forth.6,7 The U.S. Environmental Protection Agency (EPA) has listed 4-NP as one of the “priority environmental pollutants” because of its toxicity, carcinogenic, and mutagenic effects.8 According to the EPA report, the maximum permissible concentration of 4-NP in water is restricted to be <10 ng L–1. This impels researchers to develop some new techniques where complete removal of NPs could be achievable. Over the several decades, a variety of techniques such as adsorption, biodegradation, chemical oxidation, and so forth have been identified for the NP decontamination. However, they are always associated with some sorts of disadvantages.9−11 For example, the adsorption process is insufficient to provide complete decontamination of NPs. It simply transforms NPs into the other secondary forms. On the other hand, the chemical partial oxidation process may generate intermediates of NPs which are more toxic than the original compound. Therefore, developing a new remediation method which can remove NPs completely and economically is an attractive area of research in wastewater treatment.
Figure 1.
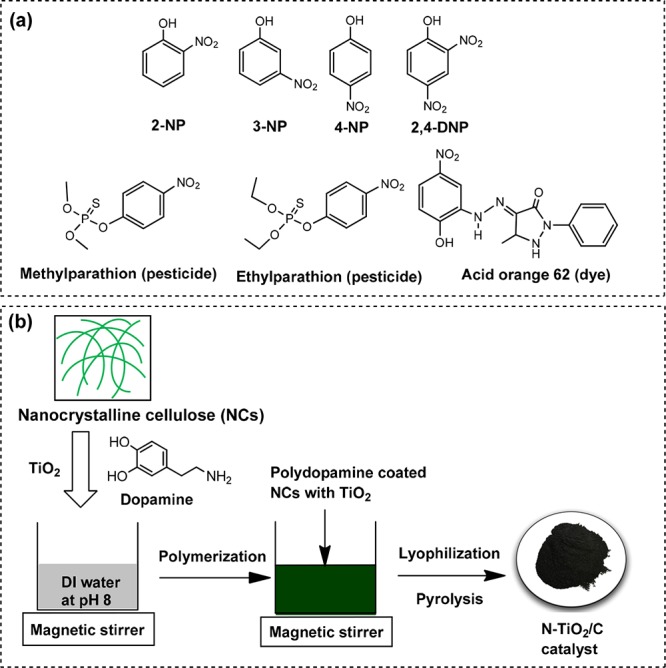
Schematic diagram of (a) structures of different NPs and NP-containing organic pollutants and (b) synthesis of the N-TiO2/C catalyst.
Recently, photocatalytic treatment of NPs using TiO2 has gained a lot of attention. TiO2 have several advantages such as high thermal and chemical stability, low cost, and nontoxicity.12 TiO2-based nanomaterials are highly photoactive and result in complete mineralization of the pollutants.13,14 However, because of the large band gap (∼3.3 eV), TiO2 requires high energy ultraviolet (UV) light for the photocatalytic activation.15 This limits the practical and large scale application of TiO2 as a photocatalyst material for effective remediation of NPs from the environment. An effort has been made to extend the light absorption of TiO2 in the visible light region making it more economical and safe.16 Dye sensitization, ion-implantation, and doping of metal atoms or ions have been applied to increase the optical response of the TiO2 catalyst.15 These methods have been found to be effective in retarding the rate of the e–h recombination and increasing the overall lifetime of photoinduced oxidation and reduction process.16 Metal-doped TiO2 photocatalysis is quite popular but major drawbacks are the high cost, toxicity of metals, and photocorossion.17 One of the most feasible ways to enhance visible light photocatalytic activity of TiO2 is heteroatom doping, such as nitrogen (N).18 This method obviates the use of expensive and toxic metals. N-doping improves the charge transfer process by introducing a large electron donor state near the Fermi level of TiO2.3 Furthermore, TiO2 with different nanostructures such as a nanotubes, nanospheres, and nanowires are possible also through N-doping.19 As a result, N-doped TiO2 possesses unique optical and electronic properties than bulk TiO2 and improves the overall photocatalytic activity toward degradation of pollutants. To further enhance the catalytic activity of N-doped TiO2, carbonaceous nanomaterials are useful because of their high specific surface area, improved thermal and electrical conductivity, porosity, and mechanical strength.19,20 Because of these characteristics properties, they help in enhancing the loading capacity of catalysts, lowering the aggregation, and increasing the chances of separation and recovery of catalysts for consecutive uses.21 So far, carbon nanotubes,22 graphene,23 and reduced graphene oxide24 have been used to generate various types of N-doped TiO2 nanocomposites. However, because of probable inherent nanotoxicity, their use for sustainable and environment-friendly processes appear to be limited. In this context, nanocrystalline cellulose (NCs) seems to be a promising candidate.
NCs is a new class of nanomaterial derived from cellulose, which is a naturally abundant, biodegradable, biocompatible, and renewable source.25,26 NCs have garnered much attention in recent years owing to its unique physicochemical properties and myriad applications.27,28 NCs possess high specific surface area, high crystallinity, superior mechanical strength, and tunable chemistry.26 These properties make NCs a fascinating material for various applications.29 However, limited work has been done on NCs for making carbonaceous material-doped metal nanocomposites.27 The aim of the present investigation is to exploit the unique physicochemical property of NCs for developing a sustainable and efficient, visible light active N-doped TiO2 and carbon nanocomposites catalyst (N-TiO2/C). For the synthesis of our catalyst (N-TiO2/C), NCs and dopamine were selected as a source of carbon and N dopant, respectively. Dopamine undergoes self-polymerization in alkaline pH in the open air to form polydopamine, a bioinspired polymer, which forms a strong coating on any surface easily.30 Therefore, when TiO2 and NCs were mixed in the presence of dopamine under alkaline condition, polydopamine forms in situ and it helps to immobilize TiO2 on the NCs.31 Furthermore, when mixed together TiO2 alone is capable of forming weak co-ordinate covalent bond with the hydroxyl (−OH) groups present in NCs. However, in the presence of polydopamine, TiO2 is immobilized strongly to the surfaces of NCs. Therefore, such a design may furnish better N-doping to both TiO2 and carbonaceous material. Finally, the synthesis of desired N-TiO2/C photocatalyst was achieved by the pyrolysis of a lyophilized sample containing polydopamine-immobilized TiO2 on NCs. The structure morphology, chemical composition, and elemental mapping of N-TiO2/C were analyzed by scanning electron microscopy (SEM), Brunauer–Emmett–Teller analysis (BET), transmission electron microscopy (TEM), energy dispersive X-ray analysis (EDAX), thermal gravimetric analysis (TGA), Fourier transform infrared (FT-IR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). The photocatalytic activity of N-TiO2/C on NPs degradation was studied using both UV and visible light sources. We demonstrated that N-TiO2/C showed excellent reactivity in NP degradation under visible light. To the best of our knowledge, this is the first report of synthesis, characterization, and applications of N-TiO2/C which was produced using NCs, dopamine, and TiO2.
Results and Discussion
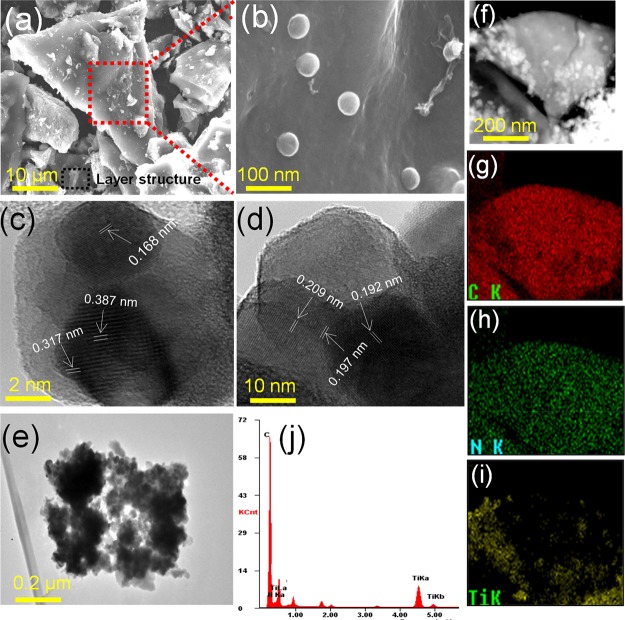
The morphology of the N-TiO2/C was observed by SEM and TEM analysis (Figure 2). Figure 2a,b shows the SEM images of N-TiO2/C at different magnifications. As shown in Figure 2a, it can be observed that the N-TiO2/C surface was rough and composed of stacked graphitic carbon layers whose size falls in the micrometer range. Several nanosized TiO2 nanoparticles in aggregated forms were also observed on the surface. For better clarity, the N-TiO2/C surface was visualized under higher magnifications, which clearly showed that the spherical-shaped TiO2 nanoparticles are anchored on the surface of graphitic layer (Figure 2b). The average diameter of the spherical TiO2 nanoparticles was measured using ImageJ software and it was found to be around 21.7 nm. Furthermore, TEM analysis of N-TiO2/C was also performed and the images obtained at different magnifications are shown in Figure 2c–e. As seen in Figure 2c,d, the average interlayer distance measured between the lattice planes of TiO2 was 0.247 nm. TEM micrographs also showed the dense agglomerated sheetlike structure with TiO2 nanoparticles maintaining their spherical shape (Figure 2e). EDAX conducted for the sample ensured the presence of carbon, nitrogen, and titanium in the sample (Figure 2j). The scanning TEM (STEM) image (Figure 2f) with corresponding elemental mapping further confirmed the presence of graphitic carbon (Figure 2g), nitrogen (Figure 2h), and titanium (Figure 2i) in N-TiO2/C.
Figure 2.
SEM images (a) ×2000 and (b) ×100 000 magnifications, TEM images (c–e), EDAX spectra (j), STEM image (f), and corresponding elemental mapping images for carbon (g), nitrogen (h), and titanium (i) of N-TiO2/C.
To determine the crystal structure of N-TiO2/C, powder X-ray diffraction (XRD) was conducted and the experimental data are presented in Figure S1. N-TiO2/C displayed diffraction peaks (2θ) at 25°, 38°, 48°, 53°, 55°, 63°, 69°, 71°, and 73°, which can be correlated with the (hkl) indices (101), (004), (200), (105), (211), (204), (116), (220), and (215) of anatase TiO2, respectively.24,32 The XRD pattern of N-TiO2/C showed that the major peaks were identical in shape and peak position to that of standard anatase TiO2. Because of the overlapping of adjacent (103), (004), and (112) diffraction peaks, a broadened diffraction peak centered at around 42° was observed. This property is the indication that TiO2 has nanometer particle size, which is one of the most influencing factors in determining the catalytic activity of any photocatalyst.32 The formation of graphitic carbon was difficult to distinguish in the XRD patterns of N-TiO2/C because of peak overlapping and widening. To further characterize the material, FT-IR was performed and the results are shown in Figure S2. In the spectrum of N-TiO2/C, broad peaks belonging to C–N stretching bonds were observed in the region 1000–1300 cm–1, which were not found in undoped TiO2.33 Similar peaks were present in the spectra of N doped carbonaceous (N-C) material. The results indicated the formation of graphite C–N structures along with the growth of TiO2 during the pyrolysis. The peak at around 500 cm–1 (Figure S2b,c) represented vibration of the Ti–O–Ti bond,34 which was found in undoped TiO2 and N-TiO2/C but not in N-C materials.
To probe the elemental state, the N-TiO2/C was characterized by XPS (Figure 3). The elemental compositions of C, N, Ti, and O atoms were examined from XPS survey scan (Figure 3a) with their characteristic peaks centered at around 284.00, 400.00, 459.00, and 532.00 eV, respectively. The contents of C, N, Ti, and O in N-TiO2/C were found to be 82.2, 3.6, 11.5, and 1.1%, respectively (Table S1). The narrow scan C 1s spectrum (Figure 3b) reveals that it was resolved into four different peaks with binding energies of 284.00, 284.80, 286.58, and 288.78 eV, which were attributed to sp2 carbon, sp3 carbon, carbon with hydroxy or ethers (C–OH, C–O–C), and carbonyl carbons (C=O), respectively. Figure 3c shows the narrow scan N 1s spectrum for N-TiO2/C, which were fitted with three peaks at about 398.48, 400.48, and 404.08 eV, corresponding to pyridinic N, graphitic N, and pyridinic N+–O–, respectively.35 However, in some literature studies, it was noted that the peak at 398.48 eV might be attributed to the N bonded in O–Ti–N linkage.36−38 The peak at 400.48 eV might be due to the presence of N in the Ti–N–O environment.36 Therefore, the broad peak at 398.38 eV could be due to both pyridinic N and O–Ti–N linkage N, and another broad peak at 400.48 eV could be due to the mixture of both graphitic N and N in Ti–N–O linkage. The narrow scan Ti 2p spectrum is shown in Figure 3d. The spectrum can be fitted with two peaks at 458.68 and 464.28 eV corresponding to the N-doped TiO2 containing Ti4+ (2p1/2) and Ti4+ (2p3/2), respectively. The decrease in binding energy of pure TiO2 from 459.54 to 458.68 eV is due to the N-doping to TiO2.18
Figure 3.
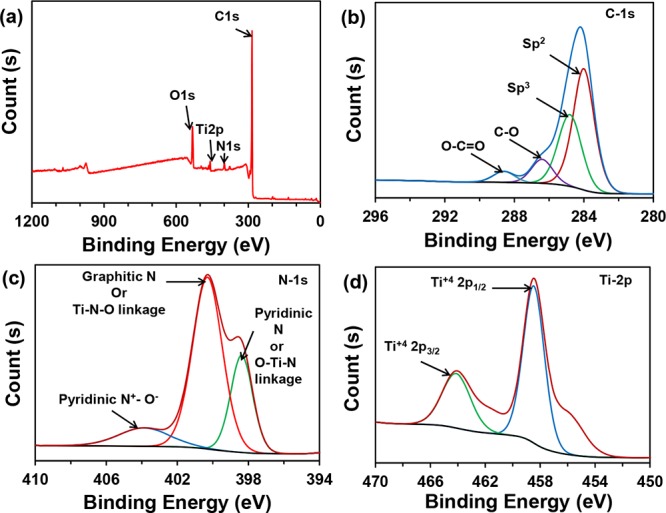
(a) Survey XPS spectrum and narrow scans of (b) C 1s, (c) N 1s, and (d) Ti 2p, XPS spectra of the N-TiO2/C.
The amount of TiO2 present in N-TiO2/C was more precisely estimated by performing TGA of the N-TiO2/C catalyst. TGA of the sample was performed three times using an air flow rate of 50 mL min–1 and a heating ramp of 5 °C min–1 up to 850 °C to get the more accuracy of the data. TGA curve (Figure S3a) shows the initial weight loss of 4% from room temperature to 150 °C, which was assigned to physically adsorbed water. The pronounced second weight loss of 64% from 330 to 530 °C was attributed to the decomposition of nitrogen-doped carbonaceous material. The residual material remaining after 850 °C was 30% of initial total weight of the sample, which was attributed to TiO2. Additionally, the residual material was further tested by EDAX (Figure S3b) to confirm the presence of TiO2. The EDAX spectrum shows that most of the residual sample was TiO2. The inset SEM image of residual material showed the presence of crystalline needlelike structure of TiO2. Furthermore, the surface area and porosity of N-TiO2/C were determined using BET analysis. The BET linear isotherm plot for adsorption/desorption cycle (Figure S4a) and Barrett, Joyner and Halenda (BJH) pore size distribution plot (Figure S4b) proved the presence of abundant micropores along with some mesopores. The BET surface area and BJH average pore volume of N-TiO2/C were found to be 21.97 m2/g and 0.024 cm3/g, respectively, which enhanced the adsorption of NPs and aided for their degradation.
To study the photocatalytic efficacy of N-TiO2/C, 4-NP was chosen for the detailed photodegradation study. All other NPs were studied under the similar conditions. Before testing the degradation of 4-NP in the presence of visible light, various control studies were performed in the presence and absence of UV light as shown in Figure S5. It can be observed that using N-TiO2/C, 85% of 4-NP was degraded in the presence of UV light compared to 10% removal in the absence of UV light after 150 min of treatment. For the control study, the effect of UV light without N-TiO2/C, anatase TiO2, and N-C was tested separately on the degradation of 4-NP. In the absence of N-TiO2/C, UV light alone could not degrade 4-NP. The result clarified the fact that the presence of catalyst was essential for the degradation of NPs. Similarly, the difference in the percentage degradation of 4-NP (25%) using N-TiO2/C and anatase TiO2 under similar conditions indicated that the doping of N on carbon played a significant role in the degradation of 4-NP. Furthermore, the role of N-doped TiO2 in N-TiO2/C for 4-NP degradation is also shown in Figure S5. The presence of N-doped TiO2 showed better performance for the 4-NP degradation. 4-NP was degraded more efficiently using the N-TiO2/C sample (85%) compared to N-C sample (26%) after 150 min of UV light exposure. After analyzing the data, it was confirmed that the N-TiO2/C was photocatalytically active and an efficient catalyst.
However, the use of UV light can be costly and physically hazardous.39 Therefore, we evaluated the degradation of NPs in the presence of less expensive and harmless visible light. Figure 4a represents the efficacy of N-TiO2/C for the degradation of 4-NP under visible light. Additionally, the control studies were performed (Figure 4b) for the comparison of 4-NP degradation under different conditions using (i) TiO2 in the presence of visible light, (ii) TiO2 and carbon nanocomposite (TiO2/C) in the presence of visible light, and (ii) N-TiO2/C in the absence of visible light. After 7 h, 80% of 4-NP with an initial concentration of 10 mg L–1 was degraded in the presence of N-TiO2/C under visible light. In the absence of visible light, N-TiO2/C removed only 30% of the 4-NP under similar conditions. It also confirmed that N-TiO2/C was porous in nature. It was also observed that TiO2/C removed 54% of 4-NP under similar reaction conditions. The noticeable difference (26%) for the degradation of 4-NP using N-TiO2/C material compared to TiO2/C was due to the effect of possible N-doping in the N-TiO2/C material. As the control experiment showed that TiO2 alone as the catalyst degraded only 42% of 4-NP after 7 h of reaction. When TiO2 alone was used to degrade 4-NP, the degradation rate was found to be slow initially. However, after 3 h of visible light exposure, the degradation profile was similar to that of the N-TiO2/C catalyst. The similar degradation profile indirectly verifies the possible role of N-doped TiO2 for degradation of 4-NP. Additionally, the photocatalytic activity of N-TiO2/C material was extended by performing an experiment for the degradation of 2-chlorophenol (2-CP) (Figure S6) under similar conditions. After 5 h of visible light irradiation, 88% of 2-CP was degraded whereas only 66% 4-NP degradation was obtained under similar conditions. The faster degradation of 2-CP was attributed to the electron donating and withdrawing effect of chlorine, which activates the benzene ring and facilitates the degradation process.40,41 The excellent photocatalytic activity of N-TiO2/C under visible light may be attributed to the fact that during synthesis TiO2 interacts strongly with the hydroxyl groups of nanostructured NCs in the presence of polydopamine, which results in more doping on N-TiO2/C and generation of spherically shaped anchored TiO2 on the surface of graphitic carbonaceous structures. Because of the formation of such structures, it is possible to produce close intimate interfacial interactions between TiO2 at 2D heterojunction of the layers. As a result of this, the charge separation process during photocatalysis may become easier which aid in the overall photocatalytic activity by N-TiO2/C.26 We have recently synthesized a similar carbonaceous material involving TiO2 and polydopamine (NGC-TiO2). However microcrystalline cellulose was used for the synthesis. In this material, TiO2 nanoparticles were found embedded inside the graphitic carbon layers. Because of this, NGC-TiO2 suppressed desire photocatalytic activity but showed adsorption behavior.42 Therefore, another reason for the enhanced photocatalytic activity under visible light could be attributed to the sufficient adsorption of 4-NP on the surface of N-TiO2/C.
Figure 4.
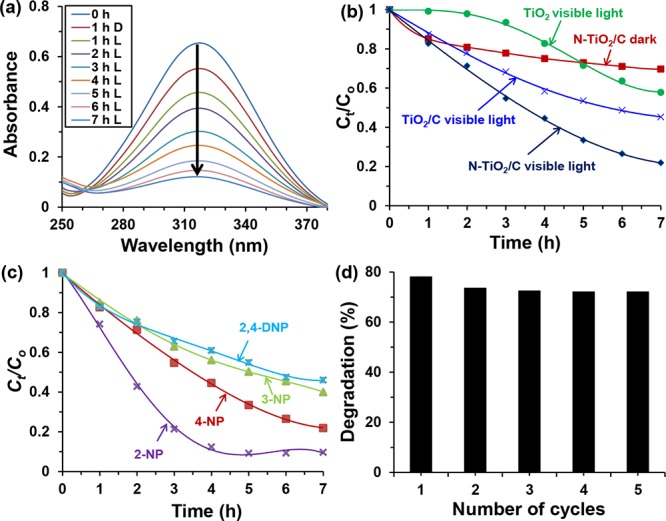
(a) UV–vis spectrum of 4-NP degradation in the presence of visible light, (b) control study for the 4-NP degradation, (c) comparative study of different NPs degradation, and (d) recyclability test of N-TiO2/C for the degradation of 4-NP after 7 h of reaction. Experimental conditions: 10 mg L–1 NPs solution at pH 3, 20 mg of N-TiO2/C at room temperature. *“D” stands for “dark” and “L” stands for “light”.
The pH of the medium is an important factor in catalysis because it determines the interaction between the catalyst and organic impurities.43 Therefore, optimized pH of the NP containing solution is necessary for the effective degradation of NP in the presence of a catalyst. Solution pH ranging from 2 to 10 was selected for the optimization. The effect of solution pH on 4-NP degradation in the presence of visible light is shown in Figure S7. It was observed that the degradation rate increased with increasing pH up to 3 and then decreased with further increasing the solution pH. The optimized pH for 4-NP degradation was found to be 3. 4-NP undergoes dissociation to the phenolate ion if the pH of the solution is greater than its pKa (7.15) value.44 In acidic pH (pH < 7), 4-NP remains in a molecular form. The pH of the solution affects the surface charge of the catalyst and consequently adsorption and degradation of the organic pollutants.45,46 In acidic solution, the surface of the N-TiO2/C is most likely to be positively charged because of the protonation of any pyridinic N present in the catalyst. The point of zero charge (PZC) might be another reason for catalyst being positively charged.47,48 The pHPZC of the TiO2 is reported in the range of 6.25–6.90. Because TiO2 was used for the synthesis, we assume that pHPZC of NTiO2/C is also in the similar range. Therefore, the surface of the catalyst becomes positively charged if pH < pHPZC and negatively charged pH > pHPZC as shown in eqs 1 and 2.48
| 1 |
| 2 |
In the acidic pH solution, electrostatic interaction between the positively charged surface of the catalyst and the lone pairs of electron present on the nitrogen and oxygen atoms of 4-NP brings molecular 4-NP more closely to the surface of the catalyst, which results in higher degradation.49 However, if the pH < 3, the percentage degradation of 4-NP diminished. In this condition, •OH radical produced on the surface of the catalyst may interact with Cl– (due to the addition of strong HCl solution) to produce the •OCl radical which is less reactive than •OH.47 Another possible reason for decreasing degradation efficiency of the catalyst at pH < 3 is due to the repulsion between partially protonated lone pair of electrons of phenolic oxygen atom of 4-NP and positively charged surface of the catalyst.49 In alkaline pH (pH > 7), negative charges are developed on the surface of the catalyst and the phenolate ion formed after the dissociation of 4-NP is repelled from the catalyst surface.50 This reduces the proximity of 4-NP with the reactive oxygen species generated on the surface of the catalyst and decreases the overall degradation.
The photocatalytic efficacy of N-TiO2/C was tested for the degradation of different NPs as well. 2-NP, 3-NP, 4-NP, and 2,4-dinitrophenol (2,4-DNP) were tested for degradation under similar optimized conditions. The decrease in the concentration of NP in the solution was evaluated by measuring the change in absorbance peak at their corresponding λmax. Figure 4c illustrates the decrease in concentration of different NPs with time using N-TiO2/C under visible light condition. The results exhibit that 2-NP was degraded more efficiently compared to other NPs. The degradation efficiency of N-TiO2/C reached 90% in case of 2-NP compared to 80% for 4-NP, 60% for 3-NP, and 54% for 2,4-DNP in the presence of visible light after 7 h of reaction at room temperature. The results were in agreement with those reported by Kavitha and Palanivelu,39 which explained that the position and numbers of −NO2 group in the phenolic compound affect the ability of their degradation on the surface of the catalyst. They also reported that the •OH undergoes electrophilic attack on ortho and para positions of phenolic −OH groups with more preferably to the para position. Therefore, 2-NP being electron-rich in the para position degraded faster than 4-NP being electron-rich in the ortho position. On the other hand, −NO2 group are ortho- and para-deactivating group.14 Therefore, 3-NP was difficult to degrade under similar conditions. In addition to the deactivating effect of −NO2 group, the presence of two −NO2 groups in 2,4-DNP blocks the favorable positions susceptible to •OH attack. This is the reason for the less degradation of 2,4-DNP under similar conditions. On the other hand, the effect of O2•– radical for NPs degradation cannot be neglected. According to the report of Hameed et al.,51 the proper orientation of −NO2 group in 2-NP creates soft site for O2•– radical attack on the benzene ring. In 4-NP, the presence of additional negative inductive effect prevents the approach of O2•–, resulting in less degradation compared to 2-NP. In case of 3-NP, the improper position (meta-position) of −NO2 group causes less degradation. Similarly, in case of 2,4-DNP, the presence of bulkier −NO2 group hinders the sites for attack by O2•– radical.
In terms of economy, the stability and reusability of the catalyst are crucial factors for the removal of organic pollutants. Multiple use of the catalyst remarkably minimizes the cost of the water purification. In the present work, the catalyst was used for five different cycles for 4-NP degradation as shown in Figure 4d. The recyclability experiment was performed in the presence of visible light under optimized conditions. After each cycle of photocatalytic degradation, the catalyst was recovered by centrifuging and repeated washing with methanol and doubly distilled deionized (DI) water to remove the adsorbed 4-NP or its degraded products. The regenerated catalyst was dried completely and used for the next identical batch experiments. The results indicated that the N-TiO2/C was efficient to remove 4-NP. In the 1st cycle, 80% of the 4-NP solution was degraded after 7 h of reaction. The percentage of 4-NP degradation remained almost consistent in the subsequent cycles. The slight decrease in the percentage degradation of 4-NP in the subsequent cycles might be due to the loss of sample during the washing process.
Degradation of organic pollutants was initiated by the reactive species such as •OH or O2•– present on the surface of the catalyst. For the effective degradation of the organic pollutants, the proximity of the oxidizing species should be necessary.39 Therefore, the adsorption of organic pollutants on the surface of catalyst will influence their degradation rate under light illumination. To study the adsorption behavior of 4-NP on the surface of N-TiO2/C, a Langmuir adsorption model was fitted and the adsorption constant under the equilibrium condition was calculated using eq 3.52
| 3 |
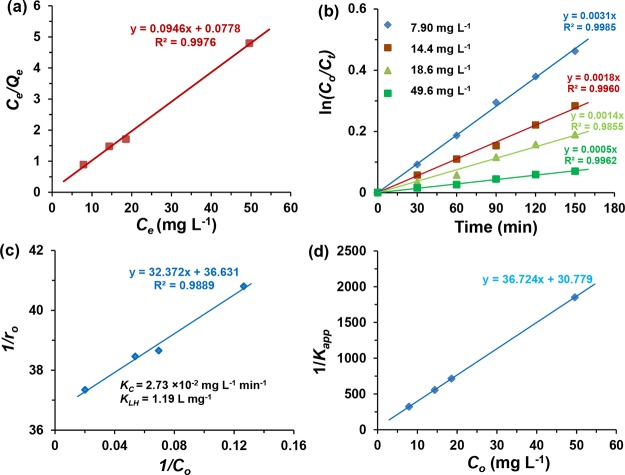
where Ce (mg L–1) is the equilibrium concentration; Kads (L mg–1) is the Langmuir adsorption constant; Qe (mg g–1) is the adsorbed equilibrium quantity, and Qm (mg g–1) is the maximum adsorbed quantity. Qm and Kads can be calculated from the slope and intercept of the straight line, respectively, using the plot of Ce/Qe versus Ce. The 4-different initial 4-NP concentrations of 10, 15, 20, and 50 mg L–1 were taken to study the dark adsorption kinetics. Using the eq 3, the linear plot of Ce/Qe against Ce was drawn as shown in Figure 5a and the values of Kads and Qm were determined as 1.22 L mg–1 and 10.57 mg g–1, respectively.
Figure 5.
Linear plots of (a) Ce/Qe vs Ce based on the Langmuir monolayer adsorption model, (b) ln(Co/Ct) vs time for different initial 4-NP concentrations, (c) 1/ro vs 1/Co, and (d) 1/Kapp vs Co based on the L–H kinetic model. Experimental conditions: 4-NP solution at pH 3, 20 mg of N-TiO2/C at room temperature.
For the kinetic study of the photocatalytic degradation of 4-NP, four different initial 4-NP concentrations of 7.90, 14.4, 18.6, and 49.6 mg L–1 obtained after 1 h of 4-NP adsorption in the dark were considered in this batch experiment. Figure 5b shows the linear plot of ln(Co/Ct) versus time with different initial concentrations of 4-NP solution. The 4-NP degradation followed the pseudo-first-order kinetic given in the following eq 4 as
| 4 |
where kapp (min–1) is the apparent pseudo-first-order reaction rate constant. Also, Co and Ct are 4-NP concentrations (mg L–1) at the initial and at time t, respectively. The kapp values for four different initial concentrations of 4-NP were obtained directly from the regression analysis of the linear curve present in Figure 5b. The kapp values along with initial rates of reaction are given in Table S2. It was observed that the degradation rate constant values decreased with the increase in the initial 4-NP concentrations. To examine the effect of the initial 4-NP concentration on the reaction rate, a Langmuir–Hinshelwood (L–H) adsorption model was applied. The L–H adsorption model52−54 can be expressed in the following eq 5 as
| 5 |
where ro (mg L–1 min–1) is the initial rate of photocatalytic degradation of 4-NP, kapp (min–1) is the apparent rate constant, KLH (L mg–1) is the L–H adsorption constant, and kC (mg L–1 min–1) is the L–H rate constant of the surface reaction, respectively. The linear form of the L–H adsorption model can be expressed as shown in following eq 6.
| 6 |
The linear plot of 1/ro against 1/Co as shown in Figure 5c gives the linear relationship between 1/ro and 1/Co. From the value of intercept (1/kC) and slope (1/kCKLH), kC and KLH values were determined to be 2.73 × 10–2 mg L–1 min–1 and 1.13 L mg–1, respectively.
Equation 5 can also be rearranged to obtain the following eq 7, which gives the linear relationship of 1/Kapp and Co as shown in the Figure 5d.
| 7 |
The linear plot of 1/Kapp versus Co gave the straight line with the slope of 1/KC and intercept of 1/KCKLH. From the slope and intercept, the values of KC and KLH were calculated to be the same as obtained above from the plot of 1/ro against 1/Co.
The constant values obtained from the L–H adsorption model and the Langmuir adsorption isotherm model were found to be close to each other (Kads = 1.08KLH). This result confirmed that photocatalytic degradation of 4-NP at pH 3 followed the L–H model satisfactorily under visible light.55
We believe that during the visible light irradiation of 4-NP in the presence of N-TiO2/C, a number of intermediate products were formed, which were finally mineralized into inorganic products such as CO2, CO, and H2O. To detect the possible compounds formed during the reaction, we used gas chromatography–mass spectrometry (GC/MS), FT-IR, ion-chromatography (IC), and total organic carbon (TOC) analysis. At first, the intermediate products obtained after the degradation of 4-NP were detected by GC/MS analysis. Table 1 shows the list of intermediate products of 4-NP after degradation along with their formula, molecular mass, and the retention time in the gas chromatogram. As it can be seen in Table 1, many of the products are acids and alcohols and they are produced in low concentrations. Particularly, acids and more polar intermediates with high molecular mass are difficult to detect in GC/MS without derivatization. Therefore, after the reaction, the products were extracted in an organic solvent (ethyl acetate) and then reacted with bis(trimethylsilyl)trifluroacetamide (BTSTFA) (a silylating agent) to make them volatile for facile detection in GC/MS. The GC chromatogram of 4-NP degradation with some intermediate products are shown in Figure S8 and mass spectra of some of the trimethylsilyl (TMS) derivative of intermediate products are shown in Figures S9 and S10. The major intermediate products detected after 30 min of 4-NP degradation include low molecular weight alcohols such as isobutanol, 1,3-propendiol, and glycerol with their retention times of 3.41, 11.33 and 18.41 min, respectively. Smaller acids such as formic acid, acetic acid, oxalic acid, and lactic acid with their corresponding retention times of 1.23, 2.73, 6.69, and 11.91 min, respectively, were also detected during the degradation (Figure S8). These intermediate products further degraded to give CO2, CO, and H2O.
Table 1. List of 4-NP Degraded Intermediate Products Obtained from the GC/MS Studya.
| name of intermediate products | retention time (min) | molecular weight (g mol–1) | molecular formula |
|---|---|---|---|
| formic acid | 1.23 | 46 | CH2O2 |
| acetic acid | 2.73 | 60 | C2H4O2 |
| isobutanol | 3.41 | 74 | C4H10O |
| oxalic acid | 6.69 | 90 | C2H2O4 |
| 2-methyl-1-butanol | 7.43 | 88 | C5H12O |
| 1,3-propendiol | 11.33 | 76 | C3H8O2 |
| lactic acid | 11.91 | 90 | C3H6O3 |
| hydroquinone | 16.99 | 110 | C6H6O2 |
| glycerol | 18.41 | 92 | C3H8O3 |
All of the above compounds are TMS derivative of intermediate products.
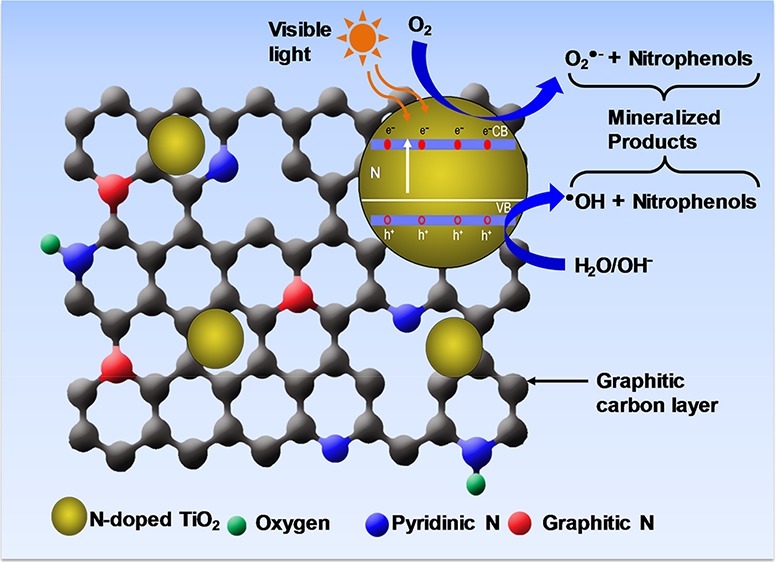
The mechanism of 4-NP degradation is still not fully understood in general. The possible mode of 4-NP degradation initiated by reactive oxygen species such as •OH and O2•– radicals is schematically represented in Figure S11. •OH being a neutral radical and having similar reactivity as of •F initiates the degradation of 4-NP forming different aromatic intermediates.56 On the other hand, the O2•– radical bearing free radical along with an additional negative charge initiates the degradation reaction by attacking and cleaving the aromatic benzene ring to furnish directly aliphatic intermediates without forming any aromatic intermediates.51 No aromatic intermediate products such as p-nitrocatechol, 4-nitropyrogallol, 1,2,4-trihydoxybenzene, and so forth other than hydroquinone were detected in the GC/MS analysis. The possible reason might be either due to the rapid degradation of these intermediate products into smaller aliphatic intermediates or inability to be detected in GC/MS because of their high molecular weight. Furthermore, the aliphatic intermediate products might be formed quickly by the attack of O2•– radical to give the nitrite ion, which can be further oxidized to the nitrate ion.51 The aliphatic intermediates were further attacked by the O2•– or •OH radical to produce mineralized products.
Additional information on the mineralization of 4-NP was obtained from TOC and IC analyses. From the TOC result, it was confirmed that 4-NP was degraded to smaller fragments such as CO and CO2. Before degradation, the theoretical TOC value for 4-NP was calculated to be 5.2 mg L–1 (for 10 mg L–1 of 4-NP), which changed to 1.7 mg L–1 after 7 h of reaction with N-TiO2/C in the presence of visible light. The result showed that 67% of 4-NP was mineralized into species such as CO2 and CO. We believe some intermediates (e.g., acetic acid), which formed after degradation of 4-NP, are recalcitrant and remained for a longer time without further degradation.57 Therefore, percentage of TOC removal was found to be less as compared to the percentage degradation of 4-NP, which was earlier estimated by UV–vis analysis. The TOC removal percentage achieved using N-TiO2/C was higher than TOC removal efficiency (42%) by radiation-induced degradation of 4-NP using TiO2.58 Wang et al. reported the activity of the TiO2 nanocomposite for degradation of 4-NP, where 47% decrease of TOC was observed after 400 min.59 This is much lower than the TOC efficiency that was achieved in 420 min using N-TiO2/C. In some cases, higher TOC removal of 4-NP has been reported using doped TiO2 catalyst but required a longer time (more than 7 h) of light irradiation and a high dose of the catalyst.60 Similarly, the mineralization of 4-NP can be detected by measuring the concentration of ammonium and nitrate ions formed using the IC technique.61 The theoretical concentrations of ammonium and nitrate ion in the 4-NP solution for the complete mineralization process were calculated to be 1.29 and 4.46 mg L–1 (for 10 mg L–1 of 4-NP), respectively. For the blank studies, their concentrations were found to be 0.1 and <0.05 mg L–1, respectively. After 7 h of photocatalytic degradation of 4-NP, the concentration of mineralized products such as ammonium and nitrate ions reached to 0.51 and 1.2 mg L–1, respectively, exhibiting the 32 and 26% of mineralization into respective species. From the literature, it can be found that the ammonium ion takes time to oxidize into the nitrate ion.62 During the degradation process, some of the N atoms of 4-NP are released as nitrogen (N2) gas, while some other N atoms remain in the intermediate products.60 Therefore, the percentage of mineralization into nitrate and ammonium ions was found to be lesser than the percentage of degradation. Total nitrogen percentage conversion in the form of nitrate and ammonium ion achieved is comparable to the previously reported study. However, in their study, TiO2 was used along with other metal oxides containing expensive and rare metals.60
Further insights in the degradation mechanism can be gained by FT-IR analysis of the solids obtained before and after degradation of 4-NP at certain intervals of time using N-TiO2/C under visible light irradiation. The changes in the FT-IR spectra before and after 4 and 7 h of degradations are depicted in Figure S12. The broad and intense peak at around 3400 cm–1 can be attributed to the phenolic OH stretching peak of 4-NP before degradation (Figure S12a). The OH stretching peak intensity decreased with increasing the irradiation time from 4 to 7 h (Figure S12b,c). The characteristic peaks of 4-NP as shown in Figure S10a at 1600 cm–1 (aromatic C=C stretching), 1400 cm–1 (asymmetric N=O stretching), 750–690 cm–1 (aromatic C–H bending), and 3070 cm–1 (aromatic C–H stretching) disappeared after 4 and 7 h of degradation as shown in Figure S12b,c, respectively. The formation of smaller acids after degradation of 4-NP was verified by the appearance of new peak at around 1700 cm–1 (Figure S12b,c). The peak at around 1700 cm–1 was due to the carboxylic C=O bond stretching. The sharp peak at 1070 cm–1 assigned (Figure S12a,b) to alcoholic C–O stretching was detected till 4 h of degradation, which became less intense after 7 h of degradation. The sharp characteristic peak at 1330 cm–1 assigned to stretching frequency of nitrate ion was clearly observed in Figure S12b,c. This also supported the fact of 4-NP mineralization into the nitrate ion.63
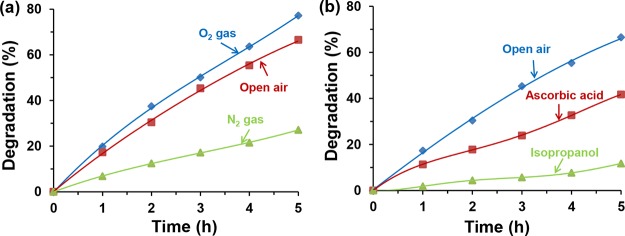
The oxygen species either in the form of •OH or O2•– radicals, which were generated on the surface of the catalyst during the e–h transfer, play an important role in the degradation of NPs.64 To verify that, the effect of the oxygen was studied and the results are depicted in Figure 6a. It was presumed that the dissolved oxygen may have a role in the generation of the reactive species and eventually degradation of NPs. When solution was purged and saturated with oxygen gas (O2), 77% of 4-NP was degraded. On the other hand, only 27% degradation was detected when the solution was saturated with N2 gas. The degradation occurred in the presence of N2 gas was due to the presence of oxygen in water. This indicates that oxygen species played a significant role for the 4-NP degradation. From Figure 6a, it is evident that only 10% more degradation of 4-NP was observed in the case of O2 saturated condition compared to open air condition. This finding indicates that oxygen required for 4-NP degradation was sufficient from open air, which is more economic and beneficial for waste treatment.
Figure 6.
Effect of (a) oxygen and (b) radical quenchers on the percentage degradation of 4-NP solution in the presence of N-TiO2/C under visible light. Experimental conditions: 10 mg L–1 4-NP solution at pH 3, 20 mg of N-TiO2/C at room temperature.
To further understand the generation of •OH or O2•– radicals during the photocatalysis, radical quenching reactions were performed. Ascorbic acid, which is a quencher of O2•– radical, and isopropanol, an •OH radical quencher, were used to indirectly detect the radicals and confirm their possible roles on degradation.65,66 The concentration of quenchers used in the experiment was 10 times higher than the 4-NP concentration in the reaction mixture. From the Figure 6b, it is evident that the use of radical quenchers suppressed the degradation rate of 4-NP. After 5 h reaction, the catalyst alone was found to degrade only 67% of 4-NP. Isopropanol, on the other hand, showed a more retarding effect in the degradation. In the presence of isopropanol, only 12% 4-NP degradation was observed indicating the possible and major role of •OH in the degradation. In the presence of ascorbic acid, N-TiO2/C degraded 42% of 4-NP after 5 h of reaction. The less degradation observed in the presence of isopropanol compared to ascorbic acid might be due to the smaller size of isopropanol that might block the active sites by its adsorption along with the quenching effect. The results indicated that both radicals were found to be crucial for effective photocatalytic degradation of 4-NP.
Conclusions
In conclusion, photocatalytic degradation of NPs was successfully studied using N-TiO2/C under visible light. The photocatalyst N-TiO2/C was prepared by the sol–gel method followed by pyrolysis at 500 °C and characterized via SEM, TEM, EDAX, XRD, XPS, TGA, and BET techniques, which confirmed the N-doping in N-TiO2/C nanocomposites. Photocatalytic activities of the material were tested by the degradation of 4-NP using visible and UV light. Acidic pH was found to be more suitable for 4-NP degradation with optimum pH of 3. Recyclability experiment showed that the N-TiO2/C can be used multiple cycles without loss of activity. Photocatalytic efficacy of N-TiO2/C was further extended by testing successful degradation of different NPs. The kinetic study revealed that the 4-NP degradation followed the pseudo-first-order kinetics. The KLH and Kads values obtained from the L–H adsorption kinetic model and the Langmuir adsorption isotherm model, respectively, confirmed that the degradation study of 4-NP followed the L–H adsorption kinetic model satisfactorily. GC/MS and FT-IR studies confirmed that 4-NP degraded into smaller molecular fragments. TOC analysis showed that 67% of TOC in 4-NP mineralized into inorganic molecules such as CO2 and CO after 7 h of reaction. Furthermore, IC analysis was performed to determine and confirm mineralization of 4-NP into less toxic products. Different radical quenchers were used to detect the possible role of reactive oxygen species in the photocatalytic degradation process. The synthesized NCs and dopamine-based catalyst has a promising potential for the degradation of other hazardous organic pollutants from wastewater.
Experimental Section
General
All chemicals were analytical grade reagents and were used as received without further purification. NCs (8 w/w %) was obtained from Blue Goose Biorefineries Inc. (Canada). Dopamine hydrochloride, anatase TiO2 (size < 25 nm), 4-NP, 2,4-DNP, 2-CP, l-ascorbic acid, BTSTFA, potassium dihydrogen phosphate (KH2PO4), and dipotassium hydrogen phosphate (K2HPO4) were obtained from Sigma-Aldrich (USA). 2-NP and 3-NP were obtained from Acros Organics (USA). DI water was used to prepare all solutions. Ultrahigh purity N2 and O2 were obtained from NLR Welding Company, North Little Rock, Arkansas (USA). The details of the as-prepared N-TiO2/C characterization instrumentations and techniques, the procedure for the degradation of NPs, and kinetic studies have been described in the Supporting Information.
Synthesis of the N-TiO2/C Photocatalyst
N-TiO2/C was synthesized by mixing NC, dopamine, and anatase TiO2 in the ratio of 5:5:1 by weight in 25 mL of DI water and the mixture was stirred for 24 h in open air at room temperature. The pH of the mixture was maintained at 8 throughout the reaction. During this period, the reaction mixture turned black. After 24 h, the resulting mixture was filtered and the precipitate was washed with copious amounts of DI water. The residual black slurry was frozen at −20 °C and lyophilized using a Labconco FreeZone 1 L benchtop freeze dry system (Cole-Parmer, USA) to obtain a powdery black solid. The freeze-dried sample was then pyrolyzed at 500 °C using a tube furnace (model GSL-1100X, MTI Corporation, USA) for 2 h under continuous N2 gas flow. The heating and cooling rates during pyrolysis were maintained at 10 °C min–1. After pyrolysis, the sample was cooled to room temperature. Finally, a black carbonaceous material was obtained, which was further grounded into a fine powder using a mortar and pestle to generate N-TiO2/C. The material was stored in a desiccator for further use. The synthesis of N-TiO2/C is schematically represented in Figure 1b. N-C was synthesized following the same procedure but no TiO2 was used during the synthesis. Similarly for the control experiment, TiO2 and TiO2/C material was synthesized using anatase TiO2 and NCs. Additionally, anatase TiO2 was treated at 500 °C for 2 h prior to use for any control reaction.
Acknowledgments
This research was financially supported by the National Science Foundation (grant no. IIA-1457888) and the Arkansas EPSCoR Program, ASSET III (CASE).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01020.
Details of characterization techniques, photocatalytic studies and kinetics studies, degradation analyses, XRD of N-TiO2/C; FT-IR of bare TiO2, N-C, N-TiO2/C; TGA of N-TiO2/C; BET analysis of N-TiO2/C; photocatalytic activity of N-TiO2/C under UV light; comparative degradation study of 4-NP with 2-CP; effect of solution pH; GC chromatogram and MS spectra of 4-NP degraded intermediates; possible mechanism of 4-NP degradation; FT-IR of 4-NP degradation products; elemental analysis of N-TiO2/C; and pseudo-first-order apparent rate constant (Kapp) and initial rate of reaction (ro) for 4-NP degradation (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mahy J. G.; Tasseroul L.; Zubiaur A.; Geens J.; Brisbois M.; Herlitschke M.; Hermann R.; Heinrichs B.; Lambert S. D. Highly Dispersed Iron Xerogel Catalysts for P-Nitrophenol Degradation by Photo-Fenton Effects. Microporous Mesoporous Mater. 2014, 197, 164–173. 10.1016/j.micromeso.2014.06.009. [DOI] [Google Scholar]
- Wu D.; Tao X.; Chen Z.-P.; Han J.-T.; Jia W.-J.; Zhu N.; Li X.; Wang Z.; He Y.-X. The Environmental Endocrine Disruptor P-Nitrophenol Interacts with FKBP51, a Positive Regulator of Androgen Receptor and Inhibits Androgen Receptor Signaling in Human Cells. J. Hazard. Mater. 2016, 307, 193–201. 10.1016/j.jhazmat.2015.12.045. [DOI] [PubMed] [Google Scholar]
- Parida K.; Das D. P. Photo-Oxidation of 4-Nitrophenol in Aqueous Suspensions, Catalysed by Titania Intercalated Zirconium Phosphate (ZrP) and Titanium Phosphate (TiP). J. Photochem. Photobiol. A 2004, 163, 561–567. 10.1016/j.jphotochem.2004.02.016. [DOI] [Google Scholar]
- Kowalczyk A.; Eyice Ö.; Schäfer H.; Price O. R.; Finnegan C. J.; van Egmond R. A.; Shaw L. J.; Barrett G.; Bending G. D. Characterization ofpara-Nitrophenol-Degrading Bacterial Communities in River Water by Using Functional Markers and Stable Isotope Probing. Appl. Environ. Microbiol. 2015, 81, 6890–6900. 10.1128/aem.01794-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.; Wang B.; Hu X. Effect of Inoculation of Burkholderia Sp. Strain SJ98 on Bacterial Community Dynamics and Para-Nitrophenol, 3-Methyl-4-Nitrophenol, and 2-Chloro-4-Nitrophenol Degradation in Soil. Sci. Rep. 2017, 7, 5983. 10.1038/s41598-017-06436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidouche S.; Bouras O.; Zermane F.; Cheknane B.; Houari M.; Debord J.; Harel M.; Bollinger J.-C.; Baudu M. Simultaneous Sorption of 4-Nitrophenol and 2-Nitrophenol on a Hybrid Geocomposite Based on Surfactant-Modified Pillared-Clay and Activated Carbon. Chem. Eng. J. 2015, 279, 964–972. 10.1016/j.cej.2015.05.012. [DOI] [Google Scholar]
- Yao Y.-X.; Li H.-B.; Liu J.-Y.; Tan X.-L.; Yu J.-G.; Peng Z.-G. Removal and Adsorption ofp-Nitrophenol from Aqueous Solutions Using Carbon Nanotubes and Their Composites. J. Nanomater. 2014, 2014, 1–9. 10.1155/2014/571745. [DOI] [Google Scholar]
- Ambient Water Quality Criteria for Nitrophenols; United States Environmental Protection Agency, Office of Water Regulations and Standards, Criteria and Standards Division: Washington, DC, 1980.
- Tewari B. B. Removal of P-Aminophenol and P-Nitrophenol from Aqueous Solution through Adsorption on Bismuth, Lead, and Manganese Ferrocyanides and Their Relevance to Environmental Issues. Russ. J. Phys. Chem. A 2014, 88, 1564–1568. 10.1134/s0036024414090076. [DOI] [Google Scholar]
- Tomei M. C.; Annesini M. C.; Prpich G. P.; Daugulis A. J. Biodegradation of 4-Nitrophenol in a Two-Phase System Operating with Polymers as the Partitioning Phase. Environ. Sci. Technol. 2009, 43, 7105–7110. 10.1021/es9010042. [DOI] [PubMed] [Google Scholar]
- Ayral C.; Lebigue C. J.; Stüber F.; Wilhelm A.-M.; Delmas H. Catalytic Wet Air Oxidation of Phenolic Compounds and Mixtures over Activated Carbon: Conversion, Mineralization, and Catalyst Stability. Ind. Eng. Chem. Res. 2010, 49, 10707–10714. 10.1021/ie100240n. [DOI] [Google Scholar]
- Chen X.; Burda C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. 10.1021/ja711023z. [DOI] [PubMed] [Google Scholar]
- Luc W.; Jiao F. Synthesis of Nanoporous Metals, Oxides, Carbides, and Sulfides: Beyond Nanocasting. Acc. Chem. Res. 2016, 49, 1351–1358. 10.1021/acs.accounts.6b00109. [DOI] [PubMed] [Google Scholar]
- Priya M. H.; Madras G. Photocatalytic degradation of nitrobenzenes with combustion synthesized nano-TiO2. J. Photochem. Photobiol. A 2006, 178, 1–7. 10.1016/j.jphotochem.2005.06.012. [DOI] [Google Scholar]
- Linsebigler A. L.; Lu G.; Yates J. T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. 10.1021/cr00035a013. [DOI] [Google Scholar]
- Kumar S. G.; Devi L. G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. 10.1021/jp204364a. [DOI] [PubMed] [Google Scholar]
- Naraginti S.; Stephen F. B.; Radhakrishnan A.; Sivakumar A. Zirconium and silver co-doped TiO2 nanoparticles as visible light catalyst for reduction of 4-nitrophenol, degradation of methyl orange and methylene blue. Spectrochim. Acta, Part A 2015, 135, 814–819. 10.1016/j.saa.2014.07.070. [DOI] [PubMed] [Google Scholar]
- Nassoko D.; Li Y.-F.; Wang H.; Li J.-L.; Li Y.-Z.; Yu Y. Nitrogen-doped TiO2 nanoparticles by using EDTA as nitrogen source and soft template: Simple preparation, mesoporous structure, and photocatalytic activity under visible light. J. Alloys Compd. 2012, 540, 228–235. 10.1016/j.jallcom.2012.06.085. [DOI] [Google Scholar]
- Ansari S. A.; Khan M. M.; Ansari M. O.; Cho M. H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. 10.1039/c5nj03478g. [DOI] [Google Scholar]
- Kaushik B. K.; Majumder M. K.. Carbon Nanotube Based VLSI Interconnects: Analysis and Design, 1st ed.; Kaushik B. K., Ed.; SpringerBriefs in Applied Sciences and Technology; Springer India: New Delhi, 2015; pp 1–14. [Google Scholar]
- Dong H.; Zeng G.; Tang L.; Fan C.; Zhang C.; He X.; He Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. 10.1016/j.watres.2015.04.038. [DOI] [PubMed] [Google Scholar]
- Lee W. J.; Lee J. M.; Kochuveedu S. T.; Han T. H.; Jeong H. Y.; Park M.; Yun J. M.; Kwon J.; No K.; Kim D. H.; et al. Biomineralized N-Doped CNT/TiO2 Core/Shell Nanowires for Visible Light Photocatalysis. ACS Nano 2012, 6, 935–943. 10.1021/nn204504h. [DOI] [PubMed] [Google Scholar]
- Ong W.-J.; Tan L.-L.; Chai S.-P.; Yong S.-T.; Mohamed A. R. Self-assembly of nitrogen-doped TiO2 with exposed {001} facets on a graphene scaffold as photo-active hybrid nanostructures for reduction of carbon dioxide to methane. Nano Res. 2014, 7, 1528–1547. 10.1007/s12274-014-0514-z. [DOI] [Google Scholar]
- Zhang Y.; Yang H. M.; Park S.-J. Synthesis and characterization of nitrogen-doped TiO2 coatings on reduced graphene oxide for enhancing the visible light photocatalytic activity. Curr. Appl. Phys. 2018, 18, 163–169. 10.1016/j.cap.2017.12.001. [DOI] [Google Scholar]
- Wayland H. A.; Boury S. N.; Chhetri B. P.; Brandt A.; Proskurnin M. A.; Filichkina V. A.; Zharov V. P.; Biris A. S.; Ghosh A. Advanced Cellulosic Materials for Treatment and Detection of Industrial Contaminants in Wastewater. ChemistrySelect 2016, 1, 4472–4488. 10.1002/slct.201600653. [DOI] [Google Scholar]
- Lin N.; Dufresne A. Nanocellulose in Biomedicine: Current Status and Future Prospect. Eur. Polym. J. 2014, 59, 302–325. 10.1016/j.eurpolymj.2014.07.025. [DOI] [Google Scholar]
- Henry A.; Plumejeau S.; Heux L.; Louvain N.; Monconduit L.; Stievano L.; Boury B. Conversion of Nanocellulose Aerogel into TiO2 and TiO2@C Nano-thorns by Direct Anhydrous Mineralization with TiCl4. Evaluation of Electrochemical Properties in Li Batteries. ACS Appl. Mater. Interfaces 2015, 7, 14584–14592. 10.1021/acsami.5b00299. [DOI] [PubMed] [Google Scholar]
- Sinha A.; Martin E. M.; Lim K.-T.; Carrier D. J.; Han H.; Zharov V. P.; Kim J.-W. Cellulose Nanocrystals as Advanced “Green” Materials for Biological and Biomedical Engineering. J. Biosyst. Eng. 2015, 40, 373–393. 10.5307/jbe.2015.40.4.373. [DOI] [Google Scholar]
- Kaushik M.; Moores A. Review: Nanocelluloses as Versatile Supports for Metal Nanoparticles and Their Applications in Catalysis. Green Chem. 2016, 18, 622–637. 10.1039/c5gc02500a. [DOI] [Google Scholar]
- Liu Y.; Ai K.; Lu L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- Dyke J. C.; Hu H.; Lee D. J.; Ko C.-C.; You W. The Role of Temperature in Forming Sol-Gel Biocomposites Containing Polydopamine. J. Mater. Chem. B 2014, 2, 7704–7711. 10.1039/c4tb00884g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Chen N.; Li Y.; Deng D.; Xing X.; Wang Y. A General Nonaqueous Sol-Gel Route to G-C3N4-Coupling Photocatalysts: The Case of Z-Scheme G-C3N4/TiO2 with Enhanced Photodegradation toward RhB under Visible-Light. Sci. Rep. 2016, 6, 39531. 10.1038/srep39531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. L.; Williams R.; Khabashesku V. N.; Margrave J. L. Synthesis of Spherical Carbon Nitride Nanostructures. Nano Lett. 2001, 1, 731–734. 10.1021/nl015626h. [DOI] [Google Scholar]
- Chainarong S.; Sikong L.; Pavasupree S.; Niyomwas S. Synthesis and Characterization of Nitrogen-doped TiO2 Nanomaterials for Photocatalytic Activities under Visible Light. Energy Procedia 2011, 9, 418–427. 10.1016/j.egypro.2011.09.046. [DOI] [Google Scholar]
- Wei J.; Hu Y.; Liang Y.; Kong B.; Zhang J.; Song J.; Bao Q.; Simon G. P.; Jiang S. P.; Wang H. Nitrogen-Doped Nanoporous Carbon/Graphene Nano-Sandwiches: Synthesis and Application for Efficient Oxygen Reduction. Adv. Funct. Mater. 2015, 25, 5768–5777. 10.1002/adfm.201502311. [DOI] [Google Scholar]
- Sathish M.; Viswanathan B.; Viswanath R. P. Characterization and photocatalytic activity of N-doped TiO2 prepared by thermal decomposition of Ti-melamine complex. Appl. Catal. B 2007, 74, 307–312. 10.1016/j.apcatb.2007.03.003. [DOI] [Google Scholar]
- Gole J. L.; Stout J. D.; Burda C.; Lou Y.; Chen X. Highly Efficient Formation of Visible Light Tunable TiO2-xNxPhotocatalysts and Their Transformation at the Nanoscale. J. Phys. Chem. B 2004, 108, 1230–1240. 10.1021/jp030843n. [DOI] [Google Scholar]
- Li H.; Li J.; Huo Y. Highly Active TiO2N Photocatalysts Prepared by Treating TiO2 Precursors in NH3/Ethanol Fluid under Supercritical Conditions. J. Phys. Chem. B 2006, 110, 1559–1565. 10.1021/jp055830j. [DOI] [PubMed] [Google Scholar]
- Kavitha V.; Palanivelu K. Degradation of Nitrophenols by Fenton and Photo-Fenton Processes. J. Photochem. Photobiol. A 2005, 170, 83–95. 10.1016/j.jphotochem.2004.08.003. [DOI] [Google Scholar]
- Hou Y.; Yang J.; Lei C.; Yang B.; Li Z.; Xie Y.; Zhang X.; Lei L.; Chen J. Nitrogen Vacancy Structure Driven Photoeletrocatalytic Degradation of 4-Chlorophenol Using Porous Graphitic Carbon Nitride Nanosheets. ACS Sustainable Chem. Eng. 2018, 6, 6497–6506. 10.1021/acssuschemeng.8b00279. [DOI] [Google Scholar]
- Jawad A.; Li Y.; Guo L.; Khan A.; Chen Z.; Wang J.; Yang J.; Liu W.; Yin G. Bimetallic synergistic degradation of chlorophenols by CuCoOx-LDH catalyst in bicarbonate-activated hydrogen peroxide system. RSC Adv. 2016, 6, 72643–72653. 10.1039/c6ra10402a. [DOI] [Google Scholar]
- RanguMagar A. B.; Chhetri B. P.; Parnell C. M.; Parameswaran-Thankam A.; Watanabe F.; Mustafa T.; Biris A. S.; Ghosh A. Removal of Nitrophenols from Water Using Cellulose Derived Nitrogen Doped Graphitic Carbon Material Containing Titanium Dioxide. Part. Sci. Technol. 2018, 1–9. 10.1080/02726351.2017.1391906. [DOI] [Google Scholar]
- Yang L.; Luo S.; Li Y.; Xiao Y.; Kang Q.; Cai Q. High Efficient Photocatalytic Degradation of p-Nitrophenol on a Unique Cu2O/TiO2 p-n Heterojunction Network Catalyst. Environ. Sci. Technol. 2010, 44, 7641–7646. 10.1021/es101711k. [DOI] [PubMed] [Google Scholar]
- Tasseroul L.; Pirard S. L.; Lambert S. D.; Páez C. A.; Poelman D.; Pirard J.-P.; Heinrichs B. Kinetic study of p-nitrophenol photodegradation with modified TiO2 xerogels. Chem. Eng. J. 2012, 191, 441–450. 10.1016/j.cej.2012.02.050. [DOI] [Google Scholar]
- Schneider J.; Matsuoka M.; Takeuchi M.; Zhang J.; Horiuchi Y.; Anpo M.; Bahnemann D. W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- Shokri A.; Mahanpoor K.; Soodbar D. Evaluation of a modified TiO2 (GO B TiO2) photo catalyst for degradation of 4-nitrophenol in petrochemical wastewater by response surface methodology based on the central composite design. J. Environ. Chem. Eng. 2016, 4, 585–598. 10.1016/j.jece.2015.11.007. [DOI] [Google Scholar]
- Nezamzadeh-Ejhieh A.; Khorsandi S. Photocatalytic Degradation of 4-Nitrophenol with ZnO Supported Nano-Clinoptilolite Zeolite. J. Ind. Eng. Chem. 2014, 20, 937–946. 10.1016/j.jiec.2013.06.026. [DOI] [Google Scholar]
- Sun J.; Qiao L.; Sun S.; Wang G. Photocatalytic degradation of Orange G on nitrogen-doped TiO2 catalysts under visible light and sunlight irradiation. J. Hazard. Mater. 2008, 155, 312–319. 10.1016/j.jhazmat.2007.11.062. [DOI] [PubMed] [Google Scholar]
- Nezamzadeh-Ejhieh A.; Khodabakhshi-Chermahini F. Incorporated ZnO onto Nano Clinoptilolite Particles as the Active Centers in the Photodegradation of Phenylhydrazine. J. Ind. Eng. Chem. 2014, 20, 695–704. 10.1016/j.jiec.2013.05.035. [DOI] [Google Scholar]
- J A. I.; A N. M. A Study on Removal Characteristics of Para-Nitrophenol from Aqueous Solution by Fly Ash. J. Environ. Chem. Ecotoxicol. 2011, 3, 32–36. [Google Scholar]
- Hameed A.; Aslam M.; Ismail I. M. I.; Chandrasekaran S.; Kadi M. W.; Gondal M. A. Sunlight assisted photocatalytic mineralization of nitrophenol isomers over W6+ impregnated ZnO. Appl. Catal., B 2014, 160–161, 227–239. 10.1016/j.apcatb.2014.05.023. [DOI] [Google Scholar]
- Khezrianjoo S.; Revanasiddappa H. Langmuir-Hinshelwood Kinetic Expression for the Photocatalytic Degradation of Metanil Yellow Aqueous Solutions by ZnO Catalyst. Chem. Sci. J. 2012, CSJ-85, 1–7. [Google Scholar]
- Barka N.; Assabbane A.; Nounah A.; Ichou Y. A. Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres. J. Hazard. Mater. 2008, 152, 1054–1059. 10.1016/j.jhazmat.2007.07.080. [DOI] [PubMed] [Google Scholar]
- Chhetri B. P.; Soni D.; RanguMagar A. B.; Parnell C. M.; Wayland H.; Watanabe F.; Kannarpady G.; Biris A. S.; Ghosh A. Synthesis, Characterization, and Photocatalytic Activity of N-Doped Carbonaceous Material Derived from Cellulose in Textile Dye Remediation. J. Environ. Chem. Eng. 2017, 5, 2586–2596. 10.1016/j.jece.2017.05.010. [DOI] [Google Scholar]
- Guettaï N.; Ait Amar H. Photocatalytic Oxidation of Methyl Orange in Presence of Titanium Dioxide in Aqueous Suspension. Part II: Kinetics Study. Desalination 2005, 185, 439–448. 10.1016/j.desal.2005.04.049. [DOI] [Google Scholar]
- Yu S.; Hu J.; Wang J. Gamma radiation-induced degradation of p-nitrophenol (PNP) in the presence of hydrogen peroxide (H2O2) in aqueous solution. J. Hazard. Mater. 2010, 177, 1061–1067. 10.1016/j.jhazmat.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Vautier M.; Guillard C.; Herrmann J.-M. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. 10.1006/jcat.2001.3232. [DOI] [Google Scholar]
- Shaoqing Y.; Jun H.; Jianlong W. Radiation-induced catalytic degradation of p-nitrophenol (PNP) in the presence of TiO2 nanoparticles. Radiat. Phys. Chem. 2010, 79, 1039–1046. 10.1016/j.radphyschem.2010.05.008. [DOI] [Google Scholar]
- Wang C.; Li J.; Mele G.; Yang G.-M.; Zhang F.-X.; Palmisano L.; Vasapollo G. Efficient degradation of 4-nitrophenol by using functionalized porphyrin-TiO2 photocatalysts under visible irradiation. Appl. Catal. B 2007, 76, 218–226. 10.1016/j.apcatb.2007.05.028. [DOI] [Google Scholar]
- Chu Y. Y.; Qian Y.; Wang W. J.; Deng X. L. A Dual-Cathode Electro-Fenton Oxidation Coupled with Anodic Oxidation System Used for 4-Nitrophenol Degradation. J. Hazard. Mater. 2012, 199–200, 179–185. 10.1016/j.jhazmat.2011.10.079. [DOI] [PubMed] [Google Scholar]
- Kavitha V.; Palanivelu K. Degradation of Nitrophenols by Fenton and Photo-Fenton Processes. J. Photochem. Photobiol. A 2005, 170, 83–95. 10.1016/j.jphotochem.2004.08.003. [DOI] [Google Scholar]
- Stylidi M.; Kondarides D. I.; Verykios X. E. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions. Appl. Catal., B 2004, 47, 189–201. 10.1016/j.apcatb.2003.09.014. [DOI] [Google Scholar]
- Goebbert D. J.; Garand E.; Wende T.; Bergmann R.; Meijer G.; Asmis K. R.; Neumark D. M. Infrared Spectroscopy of the Microhydrated Nitrate Ions NO3–(H2O)1–6. J. Phys. Chem. A 2009, 113, 7584–7592. 10.1021/jp9017103. [DOI] [PubMed] [Google Scholar]
- Divband B.; Khatamian M.; Eslamian G. R. K.; Darbandi M. Synthesis of Ag/ZnO Nanostructures by Different Methods and Investigation of Their Photocatalytic Efficiency for 4-Nitrophenol Degradation. Appl. Surf. Sci. 2013, 284, 80–86. 10.1016/j.apsusc.2013.07.015. [DOI] [Google Scholar]
- Wang D.; Guo L.; Zhen Y.; Yue L.; Xue G.; Fu F. AgBr quantum dots decorated mesoporous Bi2WO6 architectures with enhanced photocatalytic activities for methylene blue. J. Mater. Chem. A 2014, 2, 11716–11727. 10.1039/c4ta01444h. [DOI] [Google Scholar]
- Nandi A.; Chatterjee I. B. Scavenging of Superoxide Radical by Ascorbic Acid. J. Biosci. 1987, 11, 435–441. 10.1007/bf02704692. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.