Abstract
Vector surveillance is an essential component of vector-borne disease prevention, but many communities lack resources to support extensive surveillance. The Great Arizona Mosquito Hunt (GAMH) was a collaborative citizen science project conducted during 2015–17 to enhance surveillance for Aedes aegypti in Arizona. Citizen science projects engage the public in scientific research in order to further scientific knowledge while improving community understanding of a specific field of science and the scientific process. Participating schools and youth organizations across the state conducted oviposition trapping for 1–4 wk during peak Ae. aegypti season in Arizona and returned the egg sheets to collaborating entomologists for identification. During the 3-year program, 120 different schools and youth organizations participated. Few participants actually collected Aedes eggs in their traps in 2015 or 2017, but about one-third of participants collected eggs during 2016, including 3 areas that were not previously reported to have Ae. aegypti. While relatively few new areas of Ae. aegypti activity were identified, GAMH was found to be a successful method of engaging citizen scientists. Future citizen science mosquito surveillance projects might be useful to further define the ecology and risk for vector-borne diseases in Arizona.
Keywords: Aedes aegypti, citizen science, invasive species, public health, surveillance
INTRODUCTION
Aedes aegypti (L.) is the primary vector of important viral diseases, including dengue fever, yellow fever, and Zika. These arboviruses are a significant threat to human health around the world. For example, the World Health Organization estimates that almost half of the world’s human population live at risk of dengue virus infection transmitted by Ae. aegypti or Ae. albopictus (Skuse) (WHO 2017). Aedes aegypti is an efficient vector of human diseases because the female preferentially feeds on humans and often bites several times before completing oogenesis; additionally, it lives around human dwellings and thrives in urban environments (Christophers 1960, Service 1992, Wilder-Smith et al. 2017).
Aedes aegypti is not only an important disease vector but a highly invasive species as well. Its close association with humans and drought-resistant eggs have facilitated its range expansion even into arid regions (Kraemer et al. 2015). Originally native to Africa (Tabachnick and Powell 1979), Ae. aegypti has been established in the Americas since the 1600s (Brathwaite et al. 2012) and was reported in Arizona in the 1930s (Bequaert 1946, Murphy 1953). Curiously, the mosquito was not found during surveys in the 1950s (Hayes and Tinker 1958, McDonald et al. 1973), but reappeared in 1994 (Engelthaler et al. 1997, Fink et al. 1998). While several accidental introductions of Ae. albopictus have occurred, that species is not known to be established in the state (ADHS 2017a). Since Ae. aegypti’s reappearance in Arizona, there are anecdotal reports of its range expanding across the state. Targeted surveillance for Ae. aegypti has not been systematic, however. No locally acquired chikungunya, dengue, or Zika virus cases have been documented in Arizona, but all 3 arboviruses are transmitted across the southern border in Sonora, Mexico, and the risk of virus introduction is high (Ravel et al. 2001, Martínez-Medina and Cañedo-Dorame 2017).
Surveillance for invasive mosquitoes such as Ae. aegypti is limited in Arizona and other states in part due to the types of traps typically used. Most mosquito surveillance in Arizona uses carbon dioxide (CO2)-baited traps that attract Culex spp. mosquito vectors of St. Louis encephalitis and West Nile virus. These traps are not considered effective for assessing Ae. aegypti density, although mosquito control professionals in several Arizona counties have reported significant numbers of Ae. aegypti in some CO2 traps (Monaghan et al. 2016). Oviposition traps (ovitraps) may be a more sensitive as well as economical method for detecting Ae. aegypti populations. They can be particularly useful for identification of areas with new or expanding Ae. aegypti populations (Fay and Eliason 1966), although egg counts in ovitraps may not be accurate indicators of adult mosquito densities (Reiter and Gubler 1997). While the traps themselves are inexpensive, they do require a considerable investment of labor (CDC 2017).
Citizen science, defined as research activities in which general citizens contribute their knowledge or time and resources, may be a valuable tool for extending mosquito surveillance (Maki and Cohnstaedt 2015, Broeder et al. 2016, Hamer et al. 2018). Ovitraps can be used safely by the general public and do not require specialized training to deploy (WHO 2009), making them ideal for citizen science mosquito surveillance. Previously, ovitrapping by community members was used to enhance Ae. aegypti surveillance in Santa Cruz County, AZ, a small county on the USA-Mexico border (Casai et al. 2016). To better describe Ae. aegypti distribution in Arizona, the Great Arizona Mosquito Hunt (GAMH) was launched in a collaboration between the Arizona Partnership in Science Program, the Arizona Department of Health Services (ADHS), University of Arizona Department of Entomology, and Maricopa County Department of Public Health Office of Epidemiology. This project aimed to assess the utility and feasibility of enlisting citizen scientists to contribute to Ae. aegypti surveillance efforts with a particular focus on increasing surveillance capacity in more rural regions of the state. The GAMH engaged schools as well as youth organizations in Ae. aegypti surveillance, providing participants with kits to make ovitraps as well as educational materials on mosquito biology and disease transmission.
MATERIALS AND METHODS
The GAMH was initiated in 2015 and continued through 2017. Each year, the program involved 3 phases: recruitment, trapping, and evaluation. Participants were asked to place ovitraps for 1–4 wk during peak mosquito season in Arizona. After the trapping period, participants dried the egg sheets and mailed them to collaborating entomologists who verified whether eggs were present and reared a selection of the eggs to adults to verify species. Public health officials used the trapping results from the GAMH to supplement ongoing, routine mosquito surveillance to better define risk areas for viruses vectored by Ae. aegypti.
Recruitment
During 2015, the inaugural year of the GAMH, ovitrap kits were sent to every high school in the state (n = 415), when possible to heads of science departments. An email announcing the project was sent to all high school principals and science teachers in Arizona during the 2nd week of August 2015. All high schools were automatically enrolled unless they specifically opted out of the project. Ovitrap kits were mailed on in mid-August, and data were collected through the end of September. A project website (https://www.azdhs.gov/preparedness/ epidemiology-disease-control/mosquito-borne/index. php) was developed, which included a PowerPoint® (Microsoft Corporation, Redmond, WA) curriculum that could be used by teachers and youth leaders to introduce mosquito biology, disease transmission, and the concept of citizen science (ADHS 2017b). PowerPoint presentations and/or how-to demonstrations were presented to students and faculty at several schools. As an incentive, all participating schools were entered in a drawing to win $ 100 for classroom supplies, with 5 schools randomly selected as winners.
In 2016 and 2017, all schools that returned egg sheets the previous years were automatically enrolled. Schools and youth groups that did not participate before were invited to register using an online enrollment form. Enrollment was open to all interested schools and youth/community organizations throughout the summer. The project was promoted through electronic mailing lists, newsletters, and the ADHS website. As in 2015, kits in 2016 were mailed in mid-August and returned by the end of September, while kits in 2017 were mailed slightly later. A live webinar was hosted by University of Arizona on September 7, 2017. The webinar discussed mosquito biology and the importance of mosquito surveillance, and allowed for real-time questions from participants. No prize incentives were offered in 2016 or 2017.
Trapping
Kits included instructions for creating the trap, a fact sheet about mosquito-borne disease, a plastic bag containing approximately 1 teaspoon of an alfalfa-based rabbit food (Kaytee® Supreme Rabbit Food; Kaytee Products Inc., Chilton, WI) to serve as an attractant, several sheets of seed germination paper (5 in 2015, 10 in 2016 and 2017) (Regular Weight Seed Germination Paper; Anchor Paper Company®, Saint Paul, MN), a plastic bag, and a stamped, preaddressed envelope to return the egg sheets. Kits did not include the water-holding containers. Participants were advised to use a sturdy glass or plastic container, approximately 1 quart in size, and covered in dark paint or dark paper. Each kit cost a total of between $3.50 and $3.94, most of which was postage.
In 2015, participants were instructed to mix the rabbit pellets with water and allow the mixture to sit for 5–7 days, then dilute the mixture by adding 4 parts water to 1 part alfalfa infusion. Kits included written instructions with pictures, as well as links to Microsoft PowerPoint slides on trap assembly and placement. Participants were instructed to place the traps on school grounds (or at home, for youth groups) for 1 wk then remove them to avoid actively breeding mosquitoes. Pictures of mosquito eggs were supplied to assist in determining if eggs were present on their egg sheets. Egg sheets were dried and mailed in prepaid return envelopes provided in the kits.
Based on results from the 2015 GAMH, the instructions and kits were slightly modified in 2016 and 2017. Rather than brew and then dilute the alfalfa infusion (which often resulted in very strong infusions), participants were instructed to simply add 2 alfalfa pellets to the water in each trap immediately before placing it in a shaded area. Participants were provided more germination papers and asked to trap in the same spot for 2–4 wk, checking the traps and replacing water at least weekly. Each kit cost between $3.80 and $3.94.
Evaluation
Egg sheets were returned to collaborating entomologists at the Maricopa County Department of Public Health and the University of Arizona to verify the presence of Aedes eggs and, if possible, rear out the eggs to adults to allow for species identification. Teachers or youth group leaders were also asked to take a brief online survey to provide information about the location of the ovitraps and report whether their students had identified eggs on the sheets. Final results and a map of identified Aedes locations were shared with participating schools and groups by the end of each year. In 2017, a more detailed online survey asked teachers to evaluate impacts of program participation on student learning and engagement.
Statistical analyses
Chi-square analysis was used to assess whether the proportion of GAMH participants who collected Aedes eggs differed between years. A p-value of <0.05 was considered significant. Analyses were run using JMP (JMP, version 12.1; SAS, Cary, NC).
Ethical considerations
The project involved online questionnaires, but these were used in program evaluation rather than research. Therefore, both the University of Arizona Human Subjects Protection Program and ADHS Human Subject Review Board determined that Human Subjects Review was not required. Two participants were Centers for Disease Control and Prevention (CDC) personnel; therefore, CDC also reviewed this study for human subject protection and deemed the work to be nonresearch.
RESULTS
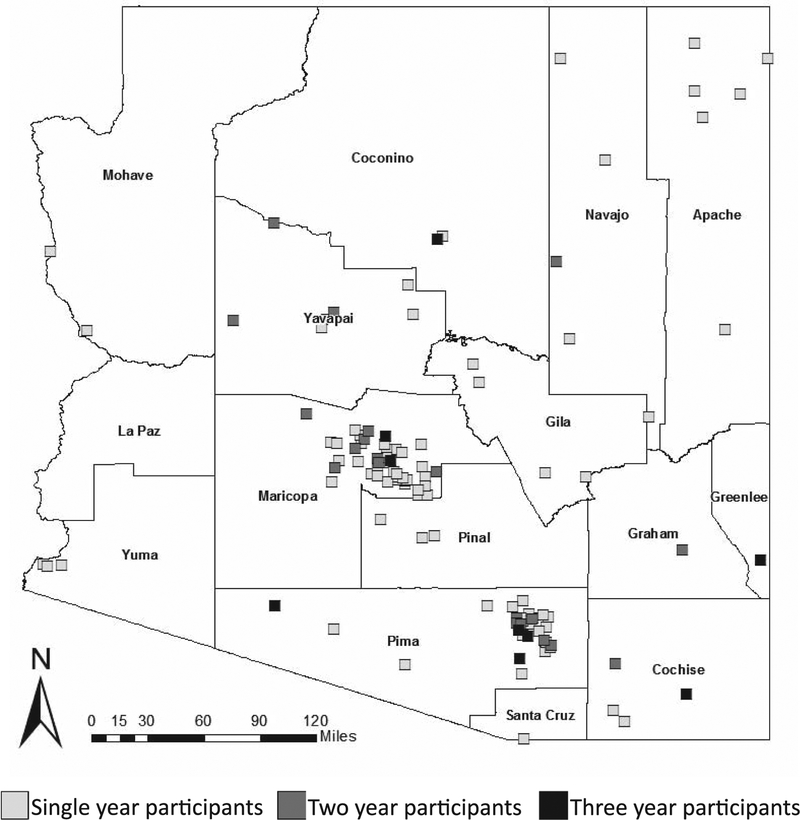
A total of 120 different schools or youth organizations participated by returning egg sheets in the GAMH over the 3 years of the program. Most participants returned more than 1 egg sheet, and a total of 879 egg sheets were sent to entomologists for verification of the presence of Aedes eggs. Participation was higher in the 1st 2 years of the project, with a 50% decrease in participation during 2017 (Table 1). In 2016 and 2017, many more participants enrolled in the project and requested kits than actually participated. A majority of participants were from high school classes (Table 1). Most classes in the GAMH participated for only a single year. Nine classes participated for all 3 years of the project. While participation was higher in the urban centers of Phoenix and Tucson, many participants were from small towns and rural areas, including tribal communities. At least 1 school participated from 14 of the 15 counties in the state (Fig. 1).
Table 1.
Participation in and egg catches from the Great Arizona Mosquito Hunt (GAMH) by year, 2015–17.
| Participants | 2015 | 2016 | 2017 |
|---|---|---|---|
| GAMH registrants | 4151 | 121 | 80 |
| Total registrants who returned egg sheets | 69 | 58 | 312 |
| Elementary school classes | 5 | 9 | 3 |
| Middle school classes | 5 | 12 | 5 |
| High school classes | 56 | 32 | 21 |
| Other groups | 3 | 5 | 2 |
| Registrants who responded to online survey | 67 | 46 | 21 |
| Registrants who reported collecting eggs | 34 | 31 | 10 |
| Registrants with verified eggs | 3 | 22 | 4 |
| Total egg sheets returned | 226 | 448 | 205 |
In 2015, all high schools in Arizona were automatically enrolled in GAMH, unless the school specifically opted out.
In 2017, 2 egg sheets (both negative for Aedes spp. eggs) were returned but could not be matched with a registrant. These egg sheets were excluded from the analysis.
Fig. 1.
Great Arizona Mosquito Hunt (GAMH) participant locations, by number of years of participation.
The proportion of participants that collected Aedes eggs varied significantly by year (χ2 = 24.48, P <0.001). Few participants collected eggs in their traps in 2015 or 2017, but approximately one-third of participants collected eggs in 2016 (Table 1). All eggs that successfully hatched were identified as Ae. aegypti. In the online surveys, about half of participants reported that students found eggs on the seed germination papers, but the proportion reporting eggs did not vary significantly by year (χ2 = 3.77, P = 0.15). No participants failed to find eggs that were actually present.
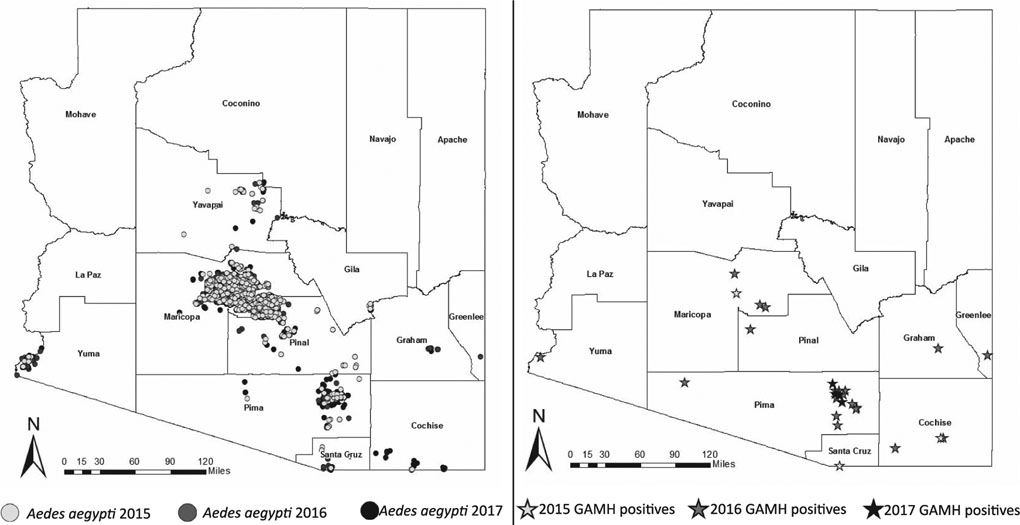
Routine mosquito surveillance by county health departments documented Ae. aegypti in southern and central Arizona between 2015 and 2017, primarily using CO2 traps (Fig. 2a). Figure 2b shows positive ovitraps collected by GAMH participants by project year. In 2015, Aedes eggs were collected in Cochise, Maricopa, and Santa Cruz counties. All 3 counties were known through routine surveillance to have Ae. aegypti, but GAMH participants found Ae. aegypti in a new location within Cochise County. In 2016, Aedes eggs were collected in 7 different counties, including numerous catches from the urban areas in Maricopa and Pima counties as well as 2 new rural sites in Pima and Cochise counties. In 2017, the only Aedes eggs collected were from urban Pima County.
Fig. 2.
(a) Aedes aegypti in Arizona identified through routine mosquito surveillance by county health department, by the 1st year of identification at a location during 2015–17; (b) Aedes spp. egg catch locations by Great Arizona Mosquito Hunt (GAMH) participants.
Participant feedback on the program was generally positive (Table 2). Among participating teachers who responded to the online questionnaires, the majority said the project was valuable and they would do it again. The online survey was modified in 2017 to include questions about student interest and engagement. Although the sample size was small, most participating teachers indicated that there was high or moderate interest in the project and increased student engagement in science in 2017.
Table 2.
Participant perceptions of the Great Arizona Mosquito Hunt, as reported by teacher respondents, 2015–17.
| Year, N (%)1 | |||
|---|---|---|---|
| Participant perception | 2015 | 2016 | 2017 |
| Educational value of project | 65 | 43 | 21 |
| Valuable, I would do it again | 37 (57) | 36 (84) | 17 (81) |
| Okay, I might do it if I have time | 28 (43) | 5 (12) | 4 (19) |
| Poor, not worth doing again | 0 (0) | 2 (5) | 0 (0) |
| How would you rate your students’ level of interest in the project?2 | N/A | N/A | 20 |
| High | N/A | N/A | 9 (45) |
| Moderate | N/A | N/A | 9 (45) |
| Low | N/A | N/A | 2 (10) |
| Did this project increase student engagement in classroom science?2 | N/A | N/A | 21 |
| Yes, many students | N/A | N/A | 8 (38) |
| Yes, a few students | N/A | N/A | 12 (57) |
| No | N/A | N/A | 1 (5) |
N/A, not applicable.
Survey question was asked only during 2017.
DISCUSSION
The GAMH engaged Arizona schools in mosquito surveillance, created hands-on educational opportunities in classrooms, and documented Ae. aegypti populations within the state from several areas that were not identified by routine mosquito surveillance during previous years. These results suggest this youth-based citizen science approach could be a useful tool for increasing public awareness of vector-borne diseases while making a modest contribution to vector surveillance. The project also provided insights into components of successful citizen science projects.
Other citizen science-based mosquito surveillance projects have shown similar success in monitoring the expanding ranges of invasive species (Bartumeus et al. 2018). The Muckenatlas (mosquito atlas) project in Germany recruited citizens to capture adult mosquitoes and submit them to research institutes for identification (Kampen et al. 2015). In the 1st 4 years of the project, citizen scientists captured rare species that had not been recorded in decades in addition to submitting specimens that led to the detection of Ae. albopictus in southern Germany (Walther and Kampen 2017). In Spain, “Mosquito Alert,” a mobile phone application, allowed participants to record mosquito sightings, including a brief survey to help participants accurately identify Ae. albopictus mosquitoes (Palmer et al. 2017). During 2014–15, more than 30% of new detections of Ae. albopictus in Spain were reported solely by citizen scientists and were not captured by routine ovitrap surveillance (Palmer et al. 2017).
An important component of citizen science-based mosquito surveillance is participant recruitment (Hamer et al. 2018). Participation in GAMH was highest during 2015, likely due to the intensive recruitment strategy of sending kits to all high schools. This approach was not sustainable, however, as many schools did not use the kits. Recruiting schools through emailing school principals, posting on science education electronic mailing lists, and advertising the event through the ADHS website resulted in higher proportion of registrants actually returning egg sheets in 2016. Middle and elementary school participation also increased in 2016, and some of these students collected eggs, indicating mosquito trapping projects are appropriate for younger students as well as high school students. Another factor supporting school participation may have been media coverage of Zika virus outbreaks in 2015 and 2016. Participation decreased by 50% in 2017 at the same time Zika activity and media coverage also declined. While some citizen science vector surveillance programs have used financial incentives to encourage participation (Bern et al. 2011, Jordan et al. 2017), teachers participating in the GAMH did not report the prize drawings in 2015 as an important motivation.
Accuracy of the data is a major challenge of citizen science-based vector surveillance (Hamer et al. 2018). Throughout the GAMH, a high proportion of participants incorrectly reported finding eggs; many participants appeared confused by debris that they mistook for eggs. Therefore, confirmation by experts was crucial for accurate interpretation of trap results. This is consistent with results from other citizen science-based mosquito surveillance projects (Kampen et al. 2015, Jordan et al. 2017).
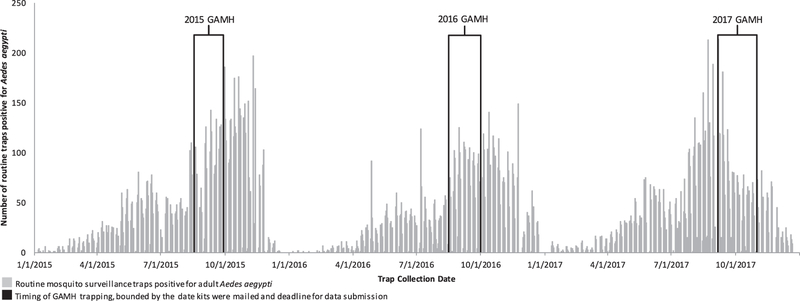
Problems with trapping procedures may also have contributed to errors. The low proportion of traps that collected Aedes eggs in 2015 suggested the 1st trapping protocol needed improvement. Many of the seed papers received were coated with alfalfa residue from highly concentrated infusions, which might discourage Ae. aegypti females from ovipositing (Reiter et al. 1991). The short duration of the 2015 trapping period (1 wk) might also be a factor in the high proportion of negative trap catches. The increased time period of trapping may have allowed mosquitoes to find the traps better in 2016. The low number of positives seen during the 2017 GAMH could be associated with the timing of trapping. Typically, Ae. aegypti activity continues through September and even October in southern Arizona. Routine mosquito surveillance in 2017, however, indicated an earlier than normal peak in Ae. aegypti activity (Fig. 3).
Fig. 3.
Timing of Great Arizona Mosquito Hunt oviposition trapping (bounded by date kits were mailed and deadline for data submission) overlaid on number of routine mosquito surveillance traps positive for Aedes aegypti, by trap collection date.
Public engagement in science and increased awareness of vector-borne diseases is another goal of citizen science-based vector surveillance. Feedback from GAMH teachers was mostly positive, indicating that students were engaged and teachers found educational value in the project, although direct impacts on content learning were not assessed. Throughout the years, many teachers indicated that they would participate in future years, and by 2017, 60% of participants had been active in a previous year. Other school-based citizen science vector surveillance projects have emphasized educational enrichment. The “Invasive Mosquito Project” (IMP) instructs high school classes across the USA in how to create and use ovitraps to enhance mosquito surveillance and assess range expansion by invasive species (Cohnstaedt et al. 2016, Thackrah et al. 2016). The IMP includes extensive online teaching resources, including lesson plans, mosquito identification guides, and an interactive vector database.
Future citizen science mosquito surveillance projects may further define the expanding distribution of Aedes aegypti and other arthropod vectors in Arizona. Protocols should be clear and simple, and project timing should be long enough to minimize temporal variations. School-based citizen science vector surveillance has the added benefit of providing teachers with locally relevant, hands-on science learning opportunities for primary and secondary school students.
ACKNOWLEDGMENTS
This work was funded in part by Research Corporation for Science Advancement, AZ Partners in Science Supplemental (Grant ID No. 23831). The authors thank Andrew Strumpf and Ken Komatsu for their expertise in helping design this project and Daniel Williamson, Joshua Arnbrister, and Ben Beal for their evaluation of returned egg sheets. The authors also thank all the teachers and students who participated in the Great Arizona Mosquito Hunt and, without whom, the project would not have occurred. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES CITED
- ADHS [Arizona Department of Health Services]. 2017a. Arbo-AZ: Arizona mosquito activity report, January 1 to December 31, 2017 [Internet]. Phoenix, AZ: Arizona Department of Health Services, Vector-Borne and Zoonotic Disease Program [accessed December 15, 2018]. Available from: http://www.azdhs.gov/documents/preparedness/epidemiology-disease-control/mosquito-borne/arbo-az-2017.pdf.
- ADHS [Arizona Department of Health Services]. 2017b. The Great Arizona Mosquito Hunt! [Internet]. Phoenix, AZ: Arizona Department of Health Services [accessed February 9, 2018]. Available from: http://azdhs.gov//preparedness/epidemiology-disease-control/mosquito-borne/index.php#hunt-home.
- Bartumeus F, Oltra A, Palmer JRB. 2018. Citizen science: a gateway for innovation in disease-carrying mosquito management? Trends Parasitol 34:727–729. [DOI] [PubMed] [Google Scholar]
- Bequaert J 1946. Aedes aegypti, the yellow fever mosquito, in Arizona. Bull Brooklyn Entomol Soc 41:147. [Google Scholar]
- Bern C, Kjos S, Yabsley MJ, Montgomery SP. 2011. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev 24:655–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brathwaite DO, San Martin JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. 2012. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 87:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeder LD, Devilee J, Oers HV, Schuit AJ, Wagemakers A. 2016. Citizen Science for public health. Health Promot Int [Internet] 33:505–514 [accessed February 9, 2018]. Available from: https://academic.oup.com/heapro/advance-article/doi/10.1093/heapro/daw086/2623361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal MG, Arriola J, Erly S, Dent N, Jacobs S, Ernst K, Walker K, Hayden M. 2016. Enhanced mosquito surveillance for Aedes spp. in Santa Cruz County, Arizona [abstract]. In: ISDS Annual Conference Proceedings 2015. 2015 December 10–11; Denver, CO. Online J Public Health Inform 8:e51. [Google Scholar]
- CDC [Centers for Disease Control and Prevention]. 2017. Surveillance and control of Aedes aegypti and Aedes albopictus in the United States [Internet]. Atlanta, GA: Centers for Disease Control and Prevention accessed [December 19, 2018]. Available from: https://www.cdc.gov/chikungunya/pdfs/surveillance-and-control-of-aedes-aegypti-and-aedes-albopictus-us.pdf.
- Christophers SR. 1960. Aedes aegypti (L.), the yellow fever mosquito: its life history, bionomics and structure. Cambridge, United Kingdom: Cambridge Univ. Press. [Google Scholar]
- Cohnstaedt LW, Ladner J, Campbell LR, Busch N, Barrera R. 2016. Determining mosquito distribution from egg data: the role of the citizen scientist. Am Biol Teach 78:317–322. [Google Scholar]
- Engelthaler DM, Fink TM, Levy CE, Leslie MJ. 1997. The reemergence of Aedes aegypti in Arizona. Emerg Infect Dis 3:241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay RW, Eliason DA. 1966. Preferred oviposition sites as a surveillance method for Aedes aegypti. Mosq News 25:531–535. [Google Scholar]
- Fink TM, Hau B, Baird BL, Palmer S, Kaplan S, Ramberg FB, Mead DG, Hagedorn HH. 1998. Aedes aegypti in Tucson, Arizona. Emerg Infect Dis 4:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer S, Curtis-Robles R, Hamer GL. 2018. Contribution of citizen scientists to arthropod vector data in the age of digital epidemiology. Curr Opin Insect Sei 28:98–104. [DOI] [PubMed] [Google Scholar]
- Hayes GR, Tinker ME. 1958. The 1956–1957 status of Aedes aegypti in the United States. Mosq News 18:253–257. [Google Scholar]
- Jordan RC, Sorensen AE, Ladeau S. 2017. Citizen science as a tool for mosquito control. J Am Mosq Control Assoc 33:241–245. [DOI] [PubMed] [Google Scholar]
- Kampen H, Medlock JM, Vaux A, Koenraadt CJ, van Vliet AJ, Bartumeus F, Oltra A, Sousa CA, Chouin S, Werner D. 2015. Approaches to passive mosquito surveillance in the EU. Parasit Vectors 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, More CG, Carvalho RG, Coelho GE, Van Bortel W, Hendrickx G, Schaffner F, Elyazar IRF, Teng H-J, Brady OJ, Messina JP, Pigott DM, Scott TW, Smith DL, William Wint GR, Golding N, Hay SI. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4:eo837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki E, Cohnstaedt L. 2015. Crowdsourcing for large-scale mosquito (Diptera: Culicidae) sampling. Can Entomol 147:118–123. doi: 10.4039/tce.2014.27 [DOI] [Google Scholar]
- Martínez-Medina MA, Cañedo-Dorame IA. 2017. First case of chikungunya fever in Hermosillo, Sonora, Mexico. Rev Med Inst Mex Seguro Soc 55:123–127. [PubMed] [Google Scholar]
- McDonald JL, Sluss TP, Lang JD, Roan CC. 1973. Mosquitoes of Arizona. Technical Bulletin 205. Tucson, AZ: Univ. of Arizona Agricultural Experiment Station. [Google Scholar]
- Monaghan AJ, Morin CW, Steinhoff DF, Wilhelmi O, Hayden M, Quattrochi DA, Reiskind M, Lloyd AL, Smith K, Schmidt CA, Scalf PE, Ernst K. 2016. On the seasonal occurrence and abundance of the Zilca virus vector mosquito Aedes aegypti in the contiguous United States. PLoS Curr 1:1–30. doi: 10.1371/currents.outbreaks.50dfc7f46798675fc63e7d7da563da76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DR. 1953. Collection records of some Arizona mosquitoes. Entomol News 64:233–238. [Google Scholar]
- Palmer JRB, Oltra A, Collantes F, Delgado JA, Lucientes J, Delacour S, Bengoa M, Eritja R, Bartumeus F. 2017. Citizen science provides a reliable and scalable tool to track disease-carrying mosquitoes. Nat Commun 8:916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel S, Monteny N, Velasco Olmos D, Escalante Verdugo J, Cuny G. 2001. A preliminary study of the population genetics of Aedes aegypti (Diptera: Culicidae) from Mexico using micro satellite and AFLP markers. Acta Trop 78:241–250. [DOI] [PubMed] [Google Scholar]
- Reiter P, Amador MA, Colon N. 1991. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc 7:52–55. [PubMed] [Google Scholar]
- Reiter P, Gubler DJ. 1997. Surveillance and control of urban dengue vectors In: Gubler DJ, Kuno G, eds. Dengue and dengue hemorrhagic fever. Fort Collins, CO: CAB International, p 425–53. [Google Scholar]
- Service MW. 1992. Importance of ecology in Aedes aegypti control. Southeast Asian J Trop Med Public Health 23:681–690. [PubMed] [Google Scholar]
- Tabachnick WJ, Powell JR. 1979. A world-wide survey of genetic variation in the yellow fever mosquito, Aedes aegypti. Genet Res 34:215–229. [DOI] [PubMed] [Google Scholar]
- Thackrah A, Cemicchiaro N, Cohnstaedt LW. 2016. The invasive mosquito project: a public education tool. Wing Beats 27:23–24. [Google Scholar]
- Walther D, Kämpen H. 2017. The citizen science project ‘Muechkenatlas’ helps monitor the distribution and spread of invasive mosquito species in Germany. J Med Entomol 54:1790–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO [World Health Organization]. 2009. Dengue: guidelines for diagnosis, treatment, prevention, and control [Internet]. Geneva, Switzerland: World Health Organization; [accessed February 9, 2018]. Available from: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. [PubMed] [Google Scholar]
- WHO [World Health Organization]. 2017. What is dengue? [Internet]. Geneva, Switzerland: World Health Organization; [accessed December 19, 2018]. Available from: http://www.who.int/denguecontrol/disease/en/. [Google Scholar]
- Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. 2017. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis 17:el01–el06. [DOI] [PubMed] [Google Scholar]