Abstract
Klinefelter syndrome (KS, 47,XXY) is the most common sex chromosome aneuploidy in males. A variety of complex clinical needs is associated with KS, including physical, cognitive and psychosocial impairments. Standard treatment for KS consists of androgen replacement therapy in adolescence to offset testosterone deficiency. Such treatment has a beneficial effect on the physical and behavioral manifestations of this syndrome. Whether androgen supplementation has a significant influence on the brain, however, is unknown. In the current study, we examined regional gray matter volume in boys with KS to assess whether treatment with oxandrolone, a synthetic hormone analog of testosterone, was associated with structural changes in the brain. Specifically, we focused our investigation on the hippocampus, given (1) its involvement in KS, and (2) the high concentration of androgen receptors found in this region. Structural magnetic resonance imaging data was acquired from a subsample of boys who completed a 2-year double-blind clinical trial in which patients were randomized to treatment with oxandrolone or to placebo, as well as from a sample of typically developing (TD) boys. Group differences in hippocampal volume were examined. A significant main effect of group was observed. Pairwise comparisons indicated smaller hippocampal volume in the placebo group relative to the oxandrolone group, as well as smaller volume in the placebo group relative to the TD control group. No difference in volume was observed between the treatment and TD groups. Moreover, across KS subgroups, a significant positive association was observed between hippocampus volume and performance on a spatial memory task, indicating treatment-based changes in brain structure may underlie cognitive change. These findings confirm prior reports implicating a role of the hippocampus in KS and are important in extending previous research by demonstrating a significant effect of androgens on brain structure.
Keywords: magnetic resonance imaging, androgens, genetics, cognition, structural magnetic resonance imaging
Introduction
Klinefelter syndrome (KS) is a highly prevalent sex chromosome aneuploidy condition that affects up to 1 in 450 male births (Herlihy et al., 2011). The majority of affected males exhibit a 47,XXY karyotype, although more than one supernumerary X chromosome is sometimes observed (Rey et al., 2011, Boada et al., 2009). The physical phenotype typically includes tall stature, hypogonadism, reduced testosterone, infertility and gynecomastia. Features comprising the behavioral phenotype include mood symptoms and deficits in specific domains of cognitive function, predominantly language, learning and memory, and executive function (Boada et al., 2009, Ross et al., 2009).
Typically, fewer than 10% of patients with KS are diagnosed before puberty or early adulthood (Bojesen et al., 2003) when specific physical manifestations of the disorder, such as incomplete puberty and fertility issues attract the attention of pediatric endocrinologists and fertility specialists. Recent new advances in routine prenatal genetic testing however have significantly improved early detection, permitting the prospective evaluation and study of the emergence of the physical and behavioral phenotype. Importantly, reports from XXY cohorts ascertained at birth have shown that reduced testicular androgen production is present in males with KS as early as infancy and early childhood (Ross et al., 2005, Stewart et al., 1986, Ratcliffe, 1982, Zeger et al., 2008). These deficiencies may have a negative impact on early brain development; androgen receptors densely populate several areas, including the CA1 subregion of the hippocampus (Frankfurt and Luine, 2015, Leranth et al., 2003) and the frontal and temporal cortices (Abdelgadir et al., 1999, Beyenburg et al., 2000, Hajszan et al., 2008, Nunez et al., 2003, Simerly et al., 1990). Binding of testosterone to these receptors, in turn, induces changes in the function and structure of these regions (Neufang et al., 2009, Peper et al., 2009, Bramen et al., 2011, Friederici et al., 2008). Alterations in hippocampal, frontal and temporal lobe gray matter in boys with KS (Giedd et al., 2007, Bryant et al., 2011, Hong et al., 2014) therefore may be caused at least in part by KS-based deficiencies in testosterone.
To better understand the role of testosterone treatment in children with KS, we recently completed a double-blind clinical trial in which we assessed longitudinal changes in cognition and psychosocial function in 93 pre-pubertal boys with KS (aged 4–12 years), 46 of whom were treated with a low dose of a synthetic oral androgen for two years and 47 received a placebo (Ross et al., 2017). Relative to placebo, we found that treatment with androgen was associated with significant improvements in visual-motor functioning, social problems and internalizing symptoms. These findings demonstrate that problematic behaviors in children with KS may be due, at least in part, to testosterone deficiency.
In the current study, we examined the effect of testosterone supplementation on the brain in children with this condition. Using magnetic resonance imaging (MRI), we imaged a subsample of scan-eligible participants at the end of this 2-year clinical trial, as well as a group of typically developing (TD) boys, and tested whether regional gray matter volume in KS varied as a function of treatment. In particular, we focused our investigation on the hippocampus, given evidence that this region (1) contains high concentrations of androgen receptors (Abdelgadir et al., 1999, Beyenburg et al., 2000), (2) is reduced in volume among boys with KS relative to typically developing boys (Lentini et al., 2013), (3) subserves behaviors known to be disrupted in KS (Phelps, 2004, Bannerman et al., 2004) and (4) is implicated in animal studies showing that hippocampal-dependent behaviors change with androgen supplementation (Roof, 1993, Roof and Havens, 1992, Naghdi et al., 2001). We hypothesized, on the basis of these findings as well as findings from animal studies indicating a causal association between testosterone level and hippocampal volume (Leranth et al., 2003, Isgor and Sengelaub, 2003), that boys with KS who received treatment with androgen would exhibit increased volume of the hippocampus relative to boys who received a placebo. Further, given a role for this region in spatial memory (Cohen and Eichenbaum, 2001), we examined in secondary analyses whether treatment-associated changes in hippocampal volume would be associated with scores on a spatial memory test.
Materials and Methods
Participants
The study was approved by the Human Subjects Committee of Thomas Jefferson University (TJU) and was registered in the ClinicalTrials.gov website (NCT00348946). Boys provided written assent, and a parent provided written informed consent. Subjects with KS were recruited through an established referral network comprised of university and community-based pediatricians, pediatric endocrinologists, and medical geneticists, and through advertisements in the local and national chapters of organizations serving individuals with KS and their families. Subjects without KS (henceforth referred to as participants in the TD control group) were recruited through advertisements posted in numerous locations (e.g., internet bulletin boards, supermarkets). To qualify for the study, boys with KS were required to have (1) a karyotype diagnosis of XXY, XXYY, or XXXY, (2) <10% mosaicism for 46,XY cell line, (3) no evidence of spontaneous onset of puberty (testicular size <4 ml), and (4) no treatment with androgen in the preceding year. Exclusion criteria for this study included (1) karyotypes of 46,XX or 47,XYY (2) baseline verbal or nonverbal Differential Ability Scales (DAS) – 2nd edition (Elliott, 1990) cluster standard scores <70, and (3) the inability to complete the cognitive and behavioral evaluation and contraindications for MRI. As previously reported (Ross et al., 2017), participants with KS were randomly assigned to treatment in a 1:1 ratio using a computer-generated randomization table, and study medications were secured until dispensed by the TJU research pharmacy. Both participants and investigators were blinded to treatment group assignment.
Treatment
Oxandrolone dose was administered to participants in the treatment group at 0.06 mg/kg/day, rounded to the nearest 2.5 mg. Oxandrolone or placebo was given for 24 months. A protocol-specified dose reduction schedule was used, as previously described (Ross et al., 2017). Families filled out dosing cards for recording of treatment compliance, and cards and unused study medication were returned at each visit, every 6 months. Safety was evaluated at each visit by adverse event history, physical examination, and laboratory analyses. An independent Data and Safety Monitoring Board (DSMB) reviewed annual interim analyses.
MRI Data Acquisition
All participants were prepared for unsedated MRI scans through scan simulations performed by research staff. Eighty participants were scanned using a Phillips 3T Achieva whole body MRI system with an 8-channel head coil, including 48 boys with KS and 32 TD boys. Three-dimensional T1-weighted images of the brain were acquired axially, anterior to posterior, using a fast gradient echo sequence (repetition time (TR) = 25ms, echo time (TE) = 2.1ms, Flip Angle (FA) = 30o, 1mm3 isotropic voxels, 160 contiguous slices of 1mm thickness, acquisition time: 6:09 minutes). All data were visually inspected to eliminate scans with significant artifact. Of the 48 scanned participants in the KS group, 24 were excluded because of image artifacts; one was excluded due to neuroanatomic abnormality. Of the 32 participants in the TD group, ten were excluded due to image artifacts, one was excluded due to neuroanatomic abnormality, four were excluded due to age, and five were excluded at random to provide group sizes roughly equivalent to the two KS subgroups. The final sample included 35 participants (treatment group, N=10, placebo group, N=13, TD control group, N=12).
Hippocampus delineation
An initial segmentation of the hippocampus was obtained from the T1-weighted structural scan using FreeSurfer (version 5.3, http://surfer.nmr.mgh.harvard.edu), an automated MRI analysis tool used to segment brain structures. Automated hippocampal segmentations were visually inspected and manually corrected by a single rater (LCFR) who was blinded to participant group.
Statistical analysis of a main effect of treatment group
Hippocampal volumes were analyzed using a repeated measures general linear model (GLM). Hippocampal volumes were entered as the dependent variable; hemisphere was entered as a within-subjects factor and treatment group (placebo, oxandrolone, TD control) was entered as a between-subjects factor. Total gray matter volume (TGMV) and age were entered as covariates. Volumetric analyses were conducted within the Statistical Package for Social Sciences (SPSS, version 19). Significance was established based on a two-tailed α level of 0.05.
Secondary analyses between hippocampus volume and spatial memory
Associations between hippocampal structure and cognitive variables were examined across the entire KS sample using a repeated measures GLM, with age- and TGMV-adjusted hippocampal volumes entered as the dependent variable. Hemisphere was entered as a within-subjects factor and age-adjusted (scaled) scores on the Recall of Designs subtest of the DAS (Elliott, 1990) were entered as a continuous predictor. This subtest measures a specific aspect of cognition that is dependent on intact hippocampal function: the short-term recall of visual and spatial relationships (Shrager et al., 2007). Significant effects from this model were followed up with individual bivariate correlations between adjusted hippocampal volume and age-normed scores on the Recall of Designs subtest, conducted separately for each hemisphere and for each treatment group.
Results
Participants
There were no significant differences between the three groups in age, F(2,32)=0.552, p=0.581, TGMV, F(2,31)=1.641, p=0.210, or Performance IQ, F(2,31)=2.229, p=0.125 (Table 1). As anticipated, verbal IQ and full scale IQ were lower in the KS subgroups relative to the TD control group (Fs>7.358, ps<0.011).
Table 1:
Clinical characteristics of study participants
| Treatment | Placebo | TD Control | p | |
|---|---|---|---|---|
| N | 10 | 13 | 12 | - |
| Age at scan (years) | 10.9 (1.3) | 11.6 (1.5) | 11.1 (2.0) | 0.581 |
| TGMV (cm3) | 638.0 (37.2) | 611.4 (44.7) | 632.0 (42.5) | 0.210 |
| Performance IQ | 102.0 (14.0) | 104.4 (19.6) | 115.1 (10.0) | 0.125 |
| Verbal IQ | 94.3 (12.2) | 96.2 (17.4) | 116.7 (11.0) | 0.002 |
| FSIQ | 97.5 (12.8) | 98.4 (19.9) | 118.0 (12.6) | 0.010 |
TD, typically developing; TGMV, total gray matter volume; FSIQ, Full-scale intelligence quotient. Values indicate means and standard deviations.
The duration of treatment among the oxandrolone and placebo groups was 2.0 ± 0.1 years. Two boys in the treatment group had received < 3 months treatment with testosterone in the newborn period. One boy in the placebo group had received < 6 months treatment with oxandrolone in early childhood. No participants received treatment with androgen in the year preceding the start of the clinical trial. Karyotypes included 20 47,XXY, 2 low level mosaic 47,XXY/46,XY and 1 KS variant (X;Y translocation).
Statistical analysis of a main effect of treatment group on hippocampus volume
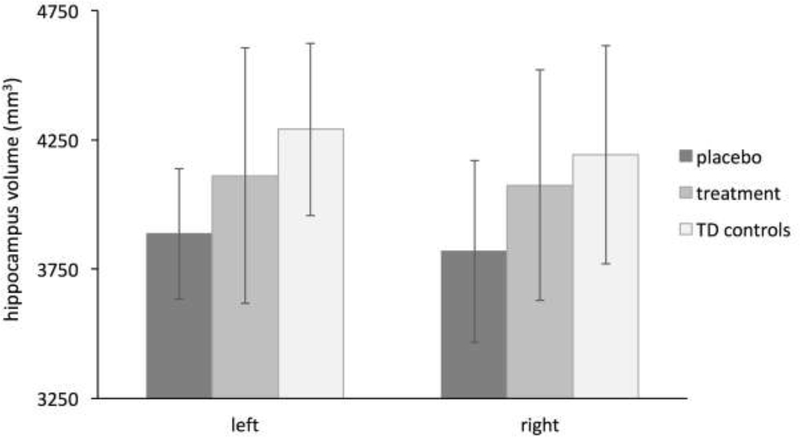
Repeated measures GLM indicated a significant main effect of group after adjusting for TGMV and age, F(2,30)=4.324, p=0.022 (Figure 1). Post-hoc tests showed hippocampal volume was significantly increased in the treatment group relative to the placebo group in the right hemisphere, F(1,19)=7.800, p=0.012. This difference was marginally significant in the left hemisphere, F(1,19)=4.191, p=0.055. Hippocampal volume was significantly increased in the TD group relative to the placebo group, F(1,21)=9.014, p=0.007, in both the left, F(1,21)=11.505, p=0.003, and right hemispheres, F(1,21)=5.103, p=0.035. No difference in hippocampal volume was observed between the treatment group relative to the TD group, F(1,18)=0.668, p=0.425. The interaction between group and age was not significant, F(2,28)=1.430, p=0.256. The main effect of group remained significant when the three participants who received treatment with androgens in infancy or early childhood were excluded from the analyses, F(2,27)=3.364, p=0.050.
Figure 1.
Hippocampus volume for males with KS in the placebo and treatment (oxandrolone) groups and for typically developing males without KS (TD controls). Error bars indicate standard deviation.
Secondary analyses of an association between hippocampus volume and spatial memory
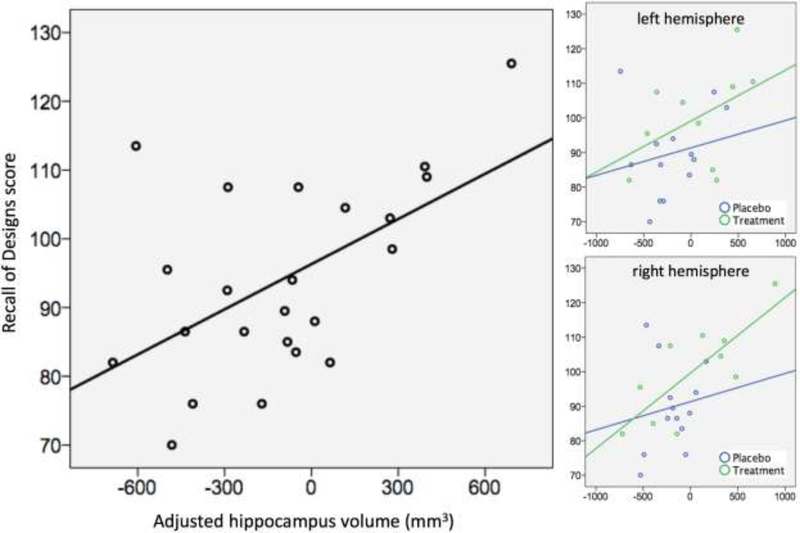
Across KS subgroups, a significant association between hippocampus volume and scores on the DAS Recall of Designs subtest was observed, F(1,21)=8.984, p=0.007 (Figure 2). Planned post-hoc bivariate correlations between age- and TGMV-adjusted hippocampal volumes and age-normed scores on the Recall of Designs subtest indicated that these effects were driven by associations between subtest score and volume of the left hippocampus in the treatment group (Table 2, Figure 2).
Figure 2.
Associations between hippocampal volume and age-normed scores on the Recall of Designs subtest of the Differential Ability Scales. Hippocampal volumes are adjusted for age and total brain volume. Recall of Designs scores are age normed.
Table 2:
Planned post-hoc associations between hippocampal volume and spatial memory scores
| Treatment | Placebo | |
|---|---|---|
| Average hippocampus | r = 0.69, p = 0.03 | r = 0.20, p = 0.51 |
| Left hippocampus | r = 0.78, p < 0.01 | r = 0.14, p = 0.65 |
| Right hippocampus | r = 0.46, p = 0.19 | r = 0.20, p = 0.50 |
Exploratory post-hoc analyses of hippocampus subfields
Post-hoc analyses were performed to explore whether specific subfields drove the reduction in hippocampus volume in the placebo group relative to the TD and treatment groups. Using the automated hippocampus subfield module from FreeSurfer (version 6.0), volumes for the CA1, CA2/3, CA4, granule cell layer of dentate gyrus (GC-DG), parasubiculum, presubiculum, subiculum, hippocampus-amygdala-transition-area (HATA), fimbria, molecular layer, hippocampal fissure, and tail subregions were computed within the manually edited boundaries of the whole hippocampus. A repeated measures GLM was used to compare subfield volumes between the placebo group relative to the TD and treatment groups. Volumes were entered as the dependent variable, group and hemisphere were entered as a within-subjects factors and TGMV and age were entered as covariates. Results of these analyses revealed that the main effect of group was not significant, F(12,20)=0.676, p=0.754.
Discussion
This is the first study, to our knowledge, to examine the influence of androgen therapy on brain structure in boys with KS using a placebo controlled design. We found that treatment with low doses of oxandrolone, a synthetic androgen, is associated with increased volume of the hippocampus. We additionally observed a positive association between volume in this region and participants’ performance on a spatial memory task, suggesting a possible mechanism of action through which androgen replacement therapy may achieve its beneficial effects on cognition and behavior. These findings build upon prior research indicating the involvement of the hippocampus in KS (Bryant et al., 2011, Hong et al., 2014) and are important in demonstrating a significant effect of androgen supplementation on the brain.
Androgen receptors are present at high concentrations in the hippocampus (Beyenburg et al., 2000), particularly in pyramidal neurons of the CA1 subregion (Frankfurt and Luine, 2015, Leranth et al., 2003). Consistent with this finding, several reports indicate a fundamental role of testosterone in shaping hippocampal structure and function. Castration of adult male rats for example leads to a roughly 50% reduction of the CA1 pyramidal cell spine synapse pool (Leranth et al., 2003), as well as a decrease in the survival of new hippocampal neurons originating from the dentate gyrus (Spritzer and Galea, 2007). Administration of testosterone (Leranth et al., 2003) or dihydrotestostetone (an androgen that is not readily converted to estradiol) (Spritzer and Galea, 2007, Swift‐Gallant et al., 2018) in turn reverses these changes. The precise mechanisms mediating testosterone-induced changes in synapse density (Leranth et al., 2003), neurogenesis (Hamson et al., 2013) and new neuron survival (Spritzer and Galea, 2007, Swift‐Gallant et al., 2018) have yet to be fully understood but are confirmed to be androgen receptor dependent (Hamson et al., 2013). Studies that pinpoint the precise mechanisms that underlie these and other testosterone-associated changes seen in the human hippocampus, including positive associations between testosterone and hippocampal volume (Neufang et al., 2009) and increased hippocampus function (Moffat and Resnick, 2007) are warranted.
There is one study to our knowledge that has noted an increase in hippocampus volume following gonadectomy (Allen et al., 2014). This contrasts with our findings and with much of the animal and human literature examining testosterone effects on the brain. Such a departure in findings may relate to species-dependent effects; while hippocampal hypertrophy in this prior study was observed in rhesus macaques (Allen et al., 2014), most studies reporting reduced hippocampus structure in gonadectomized animals have been conducted in rodents. Another possibility may relate to pruning. Specifically, gonadectomy in the rhesus macaques may have led to a loss of pruning processes, which normally cause the density of dendritic spines to decrease by half in widespread areas of the CNS (Koss et al., 2014), including in the CA1 region of the hippocampus (Tang et al., 2014). Additional studies that address these and other possibilities would be helpful in reconciling differences in study findings.
Although no investigations, to our knowledge, have tested for an association between testosterone level and hippocampal volume in KS, reduced size of this structure has been documented in this population (Bryant et al., 2011, Hong et al., 2014). Specifically, boys with KS exhibit volume reductions in this area relative to their age-matched typically developing counterparts (Bryant et al., 2011, Hong et al., 2014). We also observed a decrease in hippocampus volume in the current study in untreated boys with KS relative to TD boys. These patterns differ from the available literature on adults with KS, however, which indicate no detectable structural abnormalities in the hippocampus (Warwick et al., 1999, DeLisi et al., 2005, Patwardhan et al., 2002, Itti et al., 2006). The exact reasons for this discrepancy is unknown, but could relate to treatment history. Although standard treatment for KS consists of androgen replacement therapy, none of the published studies examining hippocampus structure in adults with KS, to our knowledge, have controlled for treatment history. Moreover, of the available studies that have examined hippocampal volume in boys with KS, all have sampled from treatment naïve participants. It is therefore tempting to speculate, particularly in the context of the current study findings, that long-term testosterone supplementation (which is typically initiated at puberty) might mitigate volume reductions of the hippocampus. Future studies that directly test this possibility are certainly warranted.
The positive correlation that we observed between hippocampus volume and performance on a spatial memory task suggests treatment-based increases in volume may underlie treatment-based behavioral changes in boys with KS. While further studies are needed to test this possibility in larger samples using more complex statistical approaches (e.g., structural equation modeling), there is wide consensus that spatial memory - a principle aspect of behavior subserved by the hippocampus (Cohen and Eichenbaum, 2001) - is directly modulated by testosterone level (Cherrier et al., 2001). Studies of aging men and men with hypogonadotropic hypogonadism for example show low testosterone is associated with declines in memory and visuospatial performance (Moffat et al., 2002). The initiation of TRT, in turn, mitigates these problems (Lašaitė et al., 2016).
Human lesion and functional neuroimaging studies indicate that spatial processing is primarily lateralized to the right hippocampus whereas episodic memory is lateralized to the left (Burgess et al., 2002). It may be considered surprising, therefore, that the associations we observed between spatial memory and volume in the current study were left-lateralized. Nevertheless, activation of the left hippocampus has been noted in some neuroimaging studies of navigation (e.g., Maguire et al., 2006) and deficits in spatial memory have been found following left medial temporal lobe damage (e.g., Lambrey et al., 2003). Moreover, intracranial recordings from neurosurgical patients indicates spatial memory encoding is associated with low-frequency oscillations in the left (but not right) hippocampus, whereas navigation (movement and orienting) was associated low-frequency oscillations in the right (but not left) hippocampus (Miller et al., 2018). A linkage between spatial memory in the left hippocampus may therefore reflect the left hippocampus’ involvement in forming new episodic memories of stimuli as they relate to their spatial context.
It remains important to note that the androgen used in this study could not be assayed in blood, and the issue of whether boys with KS are testosterone deficient is an area of debate, since testosterone levels in children are often below the detection limit in most assays. Evidence for androgen deficiency in boys with KS nevertheless comes from reports of small testes and genitalia in infancy and childhood (Stewart et al., 1986, Ratcliffe, 1982, Ross et al., 2005) as well as eunuchoidal body proportions, hypotonia, and decreased muscle mass (Zeger et al., 2008). Adding to these clinical reports are studies showing a beneficial effect of androgen replacement therapy in childhood (Ross et al., 2017, Samango‐Sprouse et al., 2013, Samango‐Sprouse et al., 2015). An important research goal moving forward will involve the development of more specialized testosterone assays that permit the accurate detection of hormone levels in pediatric samples so that associations between hormones, the brain and behavior can be more rigorously tested.
To our knowledge, this is the first study to evaluate the influence of androgen therapy on brain structure in boys with KS. Our findings should be considered in light of some limitations. First, our study used a cross-sectional design. Thus, we cannot exclude the possibility that hippocampus volume was reduced in the placebo group relative to both the TD control and treated groups at baseline assessment. Second, as we note above, because testosterone levels were not assessed in the current study, we cannot determine whether volume differences in this study were linearly associated with androgen level. Third, our sample size was small, which may have led to Type II errors, particularly in our analyses of hippocampal subfields. For these reasons, the findings from this study should be considered preliminary. Neuroimaging studies of boys with KS however are commonly based on small samples; previous reports of brain structure in KS have included as few as 9 (Goddard et al., 2016b), 14 (Brandenburg-Goddard et al., 2014) and 16 (Goddard et al., 2016a) boys. Certainly, future studies that seek to replicate these findings in larger samples are warranted.
In conclusion, in this first-ever randomized, placebo controlled neuroimaging study of treatment effects of androgen therapy in young boys with KS, we found that, relative to boys who were randomized to 2 years of placebo, those treated with oxandrolone exhibited significantly larger volume of the hippocampus. Further, volumes were positively correlated with spatial memory, a domain of cognition that is directly subserved by the hippocampus. Taken together, these findings suggest that treatment with androgen has a significant effect on brain structure, and that these changes affect behavior. Future research designed to assess the influence of hormone supplementation on neural and cognitive development in boys with KS, as well as in adolescents with KS who receive much larger doses of testosterone, are critical for improving our understanding of hormone-responsive aspects of this common genetic disorder.
Highlights.
Androgren replacement therapy was associated with larger hippocampi in boys with Klinefelter Syndrome.
A significant positive association was observed between hippocampus volume and performance on a spatial memory task.
These data suggest androgen replacement therapy influences brain structure and associated behavior in boys with this condition.
Acknowledgements:
We thank the patients and their families for their participation in this study.
Funding. Supported by the National Institute of Neurological Disorders and Stroke (NINDS; NS050597).
Footnotes
Conflict of Interest. The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelgadir SE, Roselli CE, Choate JV & Resko JA 1999. Androgen receptor messenger ribonucleic acid in brains and pituitaries of male rhesus monkeys: studies on distribution, hormonal control, and relationship to luteinizing hormone secretion. Biology of reproduction, 60, 1251–1256. [DOI] [PubMed] [Google Scholar]
- Allen K, Fung S, Rothmond D, Noble P & Shannon Weickert C 2014. Gonadectomy increases neurogenesis in the male adolescent rhesus macaque hippocampus. Hippocampus, 24, 225–238. [DOI] [PubMed] [Google Scholar]
- Bannerman D, Rawlins J, McHugh S, Deacon R, Yee B, Bast T, Zhang W-N, Pothuizen H & Feldon J 2004. Regional dissociations within the hippocampus— memory and anxiety. Neuroscience & Biobehavioral Reviews, 28, 273–283. [DOI] [PubMed] [Google Scholar]
- Beyenburg S, Watzka M, Clusmann H, Blümcke I, Bidlingmaier F, Elger CE & Stoffel-Wagner B 2000. Androgen receptor mRNA expression in the human hippocampus. Neuroscience letters, 294, 25–28. [DOI] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff‐Lee C & Tartaglia N 2009. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Developmental disabilities research reviews, 15, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen A, Juul S & Gravholt CH 2003. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. The Journal of Clinical Endocrinology & Metabolism, 88, 622–626. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM & Sowell ER 2011. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex, 21, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg-Goddard MN, van Rijn S, Rombouts SA, Veer IM & Swaab H 2014. A comparison of neural correlates underlying social cognition in Klinefelter syndrome and autism. Social cognitive and affective neuroscience, 9, 1926–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Hoeft F, Lai S, Lackey J, Roeltgen D, Ross J & Reiss AL 2011. Neuroanatomical phenotype of Klinefelter syndrome in childhood: a voxel-based morphometry study. The Journal of Neuroscience, 31, 6654–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA & O'Keefe J 2002. The human hippocampus and spatial and episodic memory. Neuron, 35, 625–641. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto A, Peskind E, Raskind M, Brodkin K, Bremner W & Petrova A 2001. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology, 57, 80–88. [DOI] [PubMed] [Google Scholar]
- Cohen N & Eichenbaum H (2001). From conditioning to conscious recollection. New York: Oxford University Press. [Google Scholar]
- DeLisi LE, Maurizio AM, Svetina C, Ardekani B, Szulc K, Nierenberg J, Leonard J & Harvey PD 2005. Klinefelter's syndrome (XXY) as a genetic model for psychotic disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 135, 15–23. [DOI] [PubMed] [Google Scholar]
- Elliott C (1990). Differential ability scales: Introductory and technical handbook. New York: Psychological Corporation. Harcourt Brace Jovanovich, Inc. [Google Scholar]
- Frankfurt M & Luine V 2015. The evolving role of dendritic spines and memory: interaction (s) with estradiol. Hormones and behavior, 74, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Pannekamp A, Partsch C-J, Ulmen U, Oehler K, Schmutzler R & Hesse V 2008. Sex hormone testosterone affects language organization in the infant brain. Neuroreport, 19, 283–286. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, Blumenthal JD, Nelson JE, Tossell JW & Stayer C 2007. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics, 119, e232–e240. [DOI] [PubMed] [Google Scholar]
- Goddard MN, Swaab H, Rombouts SA & van Rijn S 2016a. Neural systems for social cognition: gray matter volume abnormalities in boys at high genetic risk of autism symptoms, and a comparison with idiopathic autism spectrum disorder. European archives of psychiatry and clinical neuroscience, 266, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard MN, van Rijn S, Rombouts SA & Swaab H 2016b. White matter microstructure in a genetically defined group at increased risk of autism symptoms, and a comparison with idiopathic autism: an exploratory study. Brain imaging and behavior, 10, 1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ & Leranth C 2008. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Hormones and behavior, 53, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Wainwright SR, Taylor J, Jones B, Watson NV & Galea LA 2013. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology, 154, 3294–3304. [DOI] [PubMed] [Google Scholar]
- Herlihy AS, Halliday JL, Cock ML & McLachlan RI 2011. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Medical Journal of Australia, 194, 24. [DOI] [PubMed] [Google Scholar]
- Hong DS, Hoeft F, Marzelli MJ, Lepage J-F, Roeltgen D, Ross J & Reiss AL 2014. Influence of the X-chromosome on neuroanatomy: evidence from Turner and Klinefelter syndromes. The Journal of Neuroscience, 34, 3509–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C & Sengelaub DR 2003. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. Journal of neurobiology, 55, 179–190. [DOI] [PubMed] [Google Scholar]
- Itti E, Gaw Gonzalo I, Pawlikowska-Haddal A, Boone K, Mlikotic A, Itti L, Mishkin F & Swerdloff R 2006. The structural brain correlates of cognitive deficits in adults with Klinefelter’s syndrome. The Journal of Clinical Endocrinology & Metabolism, 91, 14231427. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD & Juraska JM 2014. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse, 68, 61–72. [DOI] [PubMed] [Google Scholar]
- Lambrey S, Samson S, Dupont S, Baulac M & Berthoz A (2003). Reference frames and cognitive strategies during navigation: is the left hippocampal formation involved in the sequential aspects of route memory? International Congress Series. (pp. 261–274). Elsevier. [Google Scholar]
- Lašaitė L, Čeponis J, Preikša R & Žilaitienė B 2016. Effects of two‐year testosterone replacement therapy on cognition, emotions and quality of life in young and middle‐aged hypogonadal men. Andrologia. [DOI] [PubMed] [Google Scholar]
- Lentini E, Kasahara M, Arver S & Savic I 2013. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cerebral Cortex, 23, 2322–2336. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O & MacLusky NJ 2003. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. The Journal of Neuroscience, 23, 1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Woollett K & Spiers HJ 2006. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus, 16, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Miller J, Watrous AJ, Tsitsiklis M, Lee SA, Sheth SA, Schevon CA, Smith EH, Sperling MR, Sharan A & Asadi-Pooya AA 2018. Lateralized hippocampal oscillations underlie distinct aspects of human spatial memory and navigation. Nature communications, 9, 2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD & Resnick SM 2007. Long-term measures of free testosterone predict regional cerebral blood flow patterns in elderly men. Neurobiology of aging, 28, 914–920. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM & Resnick SM 2002. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. The Journal of Clinical Endocrinology & Metabolism, 87, 5001–5007. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Nafisy N & Majlessi N 2001. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain research, 897, 44–51. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR & Konrad K 2009. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex, 19, 464–473. [DOI] [PubMed] [Google Scholar]
- Nunez J, Huppenbauer CB, McAbee M, Juraska J & DonCarlos LL 2003. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. Developmental Neurobiology, 56, 293–302. [DOI] [PubMed] [Google Scholar]
- Patwardhan AJ, Brown WE, Bender BG, Linden MG, Eliez S & Reiss AL 2002. Reduced size of the amygdala in individuals with 47, XXY and 47, XXX karyotypes. American journal of medical genetics, 114, 93–98. [DOI] [PubMed] [Google Scholar]
- Peper JS, Schnack HG, Brouwer RM, Van Baal GCM, Pjetri E, Szekely E, Van Leeuwen M, Van Den Berg SM, Collins DL & Evans AC 2009. Heritability of regional and global brain structure at the onset of puberty: a magnetic resonance imaging study in 9‐year‐old twin pairs. Human brain mapping, 30, 2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA 2004. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current opinion in neurobiology, 14, 198–202. [DOI] [PubMed] [Google Scholar]
- Ratcliffe SG 1982. 6 The sexual development of boys with the chromosome constitution 47, XXY (Klinefelter's syndrome). Clinics in endocrinology and metabolism, 11, 703–716. [DOI] [PubMed] [Google Scholar]
- Rey RA, Gottlieb S, Pasqualini T, Bastida MG, Grinspon RP, Campo SM & Bergadá I 2011. Are Klinefelter boys hypogonadal? Acta Paediatrica, 100, 830–838. [DOI] [PubMed] [Google Scholar]
- Roof RL 1993. The dentate gyrus is sexually dimorphic in prepubescent rats: testosterone plays a significant role. Brain research, 610, 148–151. [DOI] [PubMed] [Google Scholar]
- Roof RL & Havens MD 1992. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain research, 572, 310–313. [DOI] [PubMed] [Google Scholar]
- Ross JL, Kushner H, Kowal K, Bardsley MZ, Davis S, Reiss AL, Tartaglia N & Roeltgen D 2017. Androgen Treatment Effects on Motor Function, Cognition, and Behavior in Boys with Klinefelter Syndrome. Journal of pediatrics, 185, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Samango-Sprouse C, Lahlou N, Kowal K, Elder FF & Zinn A 2005. Early androgen deficiency in infants and young boys with 47, XXY Klinefelter syndrome. Hormone Research in Paediatrics, 64, 39–45. [DOI] [PubMed] [Google Scholar]
- Ross JL, Zeger MP, Kushner H, Zinn AR & Roeltgen DP 2009. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47, XYY syndrome or 47, XXY Klinefelter syndrome. Developmental disabilities research reviews, 15, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samango‐Sprouse C, Stapleton EJ, Lawson P, Mitchell F, Sadeghin T, Powell S & Gropman AL (2015). Positive effects of early androgen therapy on the behavioral phenotype of boys with 47, XXY. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. (pp. 150–157). Wiley Online Library. [DOI] [PubMed] [Google Scholar]
- Samango‐Sprouse CA, Sadeghin T, Mitchell FL, Dixon T, Stapleton E, Kingery M & Gropman AL 2013. Positive effects of short course androgen therapy on the neurodevelopmental outcome in boys with 47, XXY syndrome at 36 and 72 months of age. American Journal of Medical Genetics Part A, 161, 501–508. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Bayley PJ, Bontempi B, Hopkins RO & Squire LR 2007. Spatial memory and the human hippocampus. Proceedings of the National Academy of Sciences, 104, 2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly R, Swanson L, Chang C & Muramatsu M 1990. Distribution of androgen and estrogen receptor mRNA‐containing cells in the rat brain: An in situ hybridization study. Journal of Comparative Neurology, 294, 76–95. [DOI] [PubMed] [Google Scholar]
- Spritzer MD & Galea LA 2007. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Developmental Neurobiology, 67, 1321–1333. [DOI] [PubMed] [Google Scholar]
- Stewart D, Bailey J, Netley C, Rovet J & Park E 1986. Growth and development from early to midadolescence of children with X and Y chromosome aneuploidy: the Toronto Study. Birth defects original article series, 22, 119. [PubMed] [Google Scholar]
- Swift‐Gallant A, Duarte‐Guterman P, Hamson DK, Ibrahim M, Monks DA & Galea LA 2018. Neural androgen receptors affect the number of surviving new neurones in the adult dentate gyrus of male mice. Journal of neuroendocrinology, 30, e12578. [DOI] [PubMed] [Google Scholar]
- Tang G, Gudsnuk K, Kuo S-H, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C & Yamamoto A 2014. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron, 83, 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick MM, Doody GA, Lawrie SM, Kestelman JN, Best JJ & Johnstone EC 1999. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. Journal of Neurology, Neurosurgery & Psychiatry, 66, 628632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger MP, Zinn AR, Lahlou N, Ramos P, Kowal K, Samango-Sprouse C & Ross JL 2008. Effect of ascertainment and genetic features on the phenotype of Klinefelter syndrome. The Journal of pediatrics, 152, 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]