Abstract
Acrolein, the simplest α, β-unsaturated aldehyde, is present in relatively large quantities in cigarette smoke and several studies have raised the possibility of it being a major etiological agent for smoking related lung cancer. Acrolein reacts directly with DNA to form primarily Acr-dGuo adducts, which serve as important biomarkers for the assessment of exposure to acrolein and its potential role in smoking related lung cancer. In this study, we developed an ultra-sensitive and low-artifact method using liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry (LC-NSI-HRMS/MS) to quantitate Acr-dGuo adducts in normal lung tissue DNA obtained at surgery from lung cancer patients who never smoked and from those who continued smoking until surgery, as confirmed by urinary total cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL). This provides a direct comparison of Acr-dGuo levels in human lung tissue as a result of cigarette smoking versus other etiological causes. There was no significant difference between the total Acr-dGuo levels in smokers (28.5 ± 14.9 adducts/109 nucleotides) and non-smokers (25.0 ± 10.7 adducts/109 nucleotides), suggesting rapid removal of acrolein by glutathione conjugation and other detoxification mechanisms. Our results do not support the hypothesis that acrolein is a major etiological agent for cigarette smoking related DNA damage.
Graphical Abstract
Introduction:
Lung cancer is the leading cause of cancer incidence and mortality in the world, with 2.1 million new lung cancer cases and 1.8 million deaths predicted in 2018, representing 18.4% of all cancer deaths.1 Cigarette smoking is responsible for over 80% of lung cancer incidence worldwide.2 Comprehensive molecular profiling of lung adenocarcinoma and squamous cell carcinoma showed that the TP53 tumor suppressor gene and multiple other genes were commonly mutated.3, 4 The high rates of somatic mutation seen in these cancers are completely consistent with exposure to the large variety of carcinogens and toxicants in cigarette smoke. The TP53 gene is the most frequently mutated gene in lung cancers and more frequent in smokers than non-smokers with primarily G to T transversions in smokers, but commonly G to A transitions in non-smokers.5, 6 Polycyclic aromatic hydrocarbons (PAHs) produce predominantly G to T transversions in the TP53 gene, and the distribution of the PAH diol epoxide binding sites along the TP53 gene largely mimics the mutational spectrum found in lung cancers arising in smokers.7–9 However, Feng et al. found that acrolein also forms DNA adducts at similar mutational hotspots in TP53 as found in smoking related lung cancers, raising the possibility that acrolein is a relevant etiological agent responsible for cigarette smoking related lung cancer.10
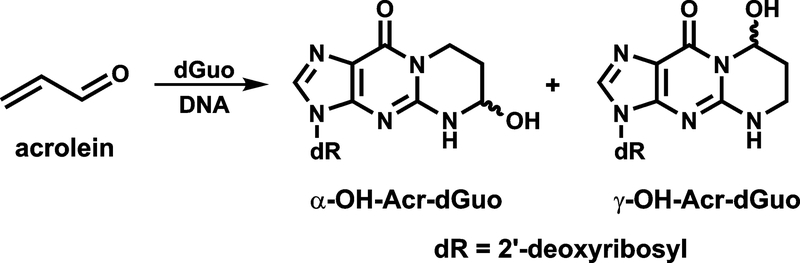
Acrolein is widely distributed in the environment, arising from incomplete combustion, and is also present endogenously from lipid peroxidation.11–13 It occurs at relatively high concentrations in cigarette smoke (~18–98 μg/cigarette),14 and the levels of (3-hydroxypropyl)mercapturic acid (3-HPMA), the major urinary metabolite of acrolein, are 4–5-fold higher in smokers than in non-smokers,15, 16 indicating cigarette smoking is a major source of acrolein exposure in humans. Acrolein reacts primarily with guanines in DNA by Michael addition followed by ring closure to form two pairs of regioisomeric 1,N2-propanodeoxyguanosine adducts: (6R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8,-tetrahydro-6-hydroxypyrimido[1,2-a]purine-10(3H)one (α-Acr-dGuo) and (8R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8,-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one (γ-Acr-dGuo) (Figure 1).17–19
Figure 1.
Structures of Acr-dGuo Adducts
Similar to adducts formed by PAH diol epoxide metabolites such as benzo[a]pyrene diol epoxide-dGuo (BPDE-N2-dGuo), Acr-dGuo also causes predominantly G to T transversions, although less efficiently.7, 20, 21 The level of acrolein is about 1,000 times higher than those of PAH in cigarette smoke.22 However, acrolein is generally believed not carcinogenic (group 3 carcinogen by IARC), while there are multiple highly carcinogenic PAH compounds such as benzo[a]pyrene (BaP), a group 1 carcinogen.23 Taken together, the above evidence encouraged us to further investigate the role of acrolein-DNA adducts in the etiology of tobacco smoking-related lung cancer by analyzing Acr-dGuo levels in DNA of lung tissue obtained from lung cancer patients who were smokers or non-smokers.
We have previously measured Acr-dGuo adducts in lung DNA from smokers and non-smokers using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS).18 However, we only had incomplete information on the smoking status of the subjects in that study. In the current study, we have obtained normal tissue adjacent to tumors from lung cancer patients during surgery, as well as their urine samples and smoking history information. Only small amounts of DNA were available for the analysis of Acr-dGuo in this study. Therefore, we developed a more sensitive assay using liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry (LC-NSI-HRMS/MS). Artifactual formation of Acr-dGuo during sample preparation has been observed recently,24 and no precautionary steps to block artifact formation were included in our earlier study.18 In this study, we have modified our previous method to lower the levels of artifacts formed during sample preparation and minimize their interference in quantitation.
Experimental procedures:
Chemicals.
Acr-dGuo standards and [13C10, 15N5]Acr-dGuo internal standards were synthesized.17 Puregene DNA purification solutions, proteinase K and RNase A were purchased from Qiagen (Valencia, CA). Calf thymus DNA, micrococcal nuclease (from Staphylococcus aureus), and phosphodiesterase II (from bovine spleen) were from Worthington Biochemical (Lakewood, NJ). Alkaline phosphatase (from calf intestine) was obtained from Roche Diagnostics (Indianapolis, IN). Amicon Ultra-0.5 (10 kDa) centrifugal filter units were purchased from Sigma-Aldrich (St. Louis, MO). Strata-X solid phase extraction (SPE) cartridges were from Phenomenex (Torrance, CA). All other chemicals were from Sigma-Aldrich or Fisher Scientific (Fairlawn, NJ).
Human lung and urine samples.
This study was approved by the University of Minnesota Institutional Review Board Human Subjects Committee. The lung samples were obtained during surgery for lung cancer through the Tissue Procurement Facility and stored at −80 °C until DNA isolation. The samples were from the margins of tumors and are considered normal tissue. Urine samples from the same individuals were obtained just prior to surgery and analyzed for total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and cotinine [urinary metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and nicotine] as described previously.25
Sample Preparation for the Analysis of Acr-dGuo.
DNA was isolated and purified from the normal human lung tissues by following the Gentra Puregene protocol (Qiagen, Valencia, CA) with modifications as described previously,18 and then stored at −20 °C in sealed vials. For artifact assessment, lung tissue samples were divided into three portions. One was isolated as usual without any scavenger for acrolein, and the other two portions were isolated with 10 mM glutathione (GSH) or β-mercaptoethanol (BME) present in all reagents.
The isolated human lung DNA (10 – 100 μg) was dissolved in 200 μL of 10 mM succinate buffer (pH 7.0) containing 5 mM CaCl2 and 0.5mM GSH, and 10 fmol of each isomer of [13C10, 15N5]Acr-dGuo were added as internal standards. The sample mixtures were heated at 100 °C for 30 min and then cooled on ice. The DNA was then hydrolyzed using 7.5 units of micrococcal nuclease and 0.045 units of phosphodiesterase II at 37 °C for 5 h, followed by further hydrolysis with 15 units of alkaline phosphatase at 37 °C overnight. The hydrolysate was filtered using an Amicon centrifugal filter unit with membrane NMWL of 10 kDa to remove the enzymes. A 10 μL aliquot of the filtrate was taken for dGuo quantitation by HPLC and then used to calculate the amount of DNA, considering that dGuo accounts for 19.9% of total nucleotides in human DNA.26 The adduct levels were expressed per 109 nucleotides.
The remaining filtrate was loaded onto a Strata-X SPE cartridge (33 μm, 30 mg, 1 mL) after the cartridges had been conditioned with 3 mL of CH3OH and 3 mL of 0.1 mM GSH. The cartridge was extensively washed with 6 mL of 0.1 mM GSH and 1 mL of 5% CH3OH in H2O containing 0.1 mM GSH, followed by elution with 1 mL of 25% CH3OH in H2O containing 0.1 mM GSH into a vial containing 55 nmol of GSH. The eluted fraction was concentrated to dryness in a SpeedVac without heat. The dried residue was reconstituted in 20 μL H2O and analyzed by LC-NSI-HRMS/MS the next day.
Quantitation of Acr-dGuo by LC-NSI-HRMS/MS.
The dried residue was reconstituted in 20 μL H2O the next day, and 2 μL was injected for Acr-dGuo quantitation using LC-NSI-HRMS/MS with accurate mass detection. A nanoHPLC column (75 μm ID, 360 μm OD, 17 cm length, and 10 μm orifice) packed with Luna C18 bonded separation media (Phenomenex, Torrance, CA) in a commercially available fused silica emitter (New Objective, Woburn, MA) was installed on a Dionex UltiMate 3000 RSLCnano HPLC system coupled to an Orbitrap Fusion Tribrid Mass Spectrometer (Thermo Scientific, San Jose, CA) for sample separation and quantitation. The 2 μL sample was first loaded onto the nanoHPLC column through a 5 μL injection loop with 1% CH3CN in 2 mM NH4OAc (pH 6.8) at a flow rate of 1 μL/min for 5.5 min. Then the injection valve was switched to the load position, removing the injection loop from the flow path, and the flow rate was reduced to 300 nL/min in 0.5 min and the gradient was ramped to 8% CH3CN in 2 mM NH4OAc (pH 6.8) over 20 min, followed by 30% CH3CN wash for 5 min and returned to 1% CH3CN for re-equilibration at 1 μL/min for 7 min. The following ion transitions were monitored with accurate mass extracted: m/z 324.1 [M+H]+ to m/z 208.0829 [M+H-dR]+ and 190.0723 [M+H-dR-H2O]+ for both α-Acr-dGuo and γ-Acr-dGuo, and additional product ion m/z 164.0567 [M+H-dR-H2O-C2H2]+ for γ-Acr-dGuo. The corresponding internal standard ion transitions monitored were as follows: m/z 339.1 to m/z 218.0848 and 200.0743 for both α-[13C10, 15N5]Acr-dGuo and γ-[13C10, 15N5]Acr-dGuo, and additional product ion m/z 174.0586 for γ-[13C10, 15N5]Acr-dGuo. The precursor ions were isolated by a quadrupole with an isolation width of m/z 1.5, fragmented by higher-energy collision dissociation (HCD) at 20% or 50% collision energy and the resulting spectra detected by the Orbitrap detector with a resolution of 60,000. The ion source was operated in positive ion mode with a spray voltage of 2200 V and the ion transfer tube at 300 °C. Levels of Acr-dGuo were quantitated using the transitions at 20% collision energy: m/z 324.1 to m/z 208.0829 for Acr-dGuo and m/z 339.1 to m/z 218.0848 for the internal standard [13C10, 15N5]Acr-dGuo. Peak integration was performed at 5 ppm mass tolerance.
Method Characterization.
To determine the accuracy of Acr-dGuo quantitation by LC-NSI-HRMS/MS and validate the assay, 0.4, 1, 2 and 4 fmol of α-Acr-dGuo, and 0.3, 1.5, 3 and 6 fmol of γ-Acr-dGuo were spiked into 35 μg of calf thymus DNA in succinate buffer with 10 fmol of [13C10, 15N5]Acr-dGuo as internal standard and subjected to the sample preparation procedures described above. Three spiked samples at each concentration level were prepared and analyzed by LC-NSI-HRMS/MS. The concentration was calculated using a calibration curve constructed by varying the concentration of Acr-dGuo (0.02 – 0.5 fmol on-column) and keeping the concentration of [13C10, 15N5]Acr-dGuo constant (0.2 fmol on-column). Background levels of Acr-dGuo in calf thymus DNA were determined by four non-spiked samples and subtracted from each amount detected in the spiked samples. The assay precision was determined by analyzing multiple samples of calf thymus DNA on the same day (intra-day) or on multiple days (inter-day).
Statistical Analyses.
The two-tailed t-test was used to compare the Acr-dGuo levels between smokers and non-smokers at 95% level of confidence.
Results:
Method Development and Validation.
Acr-dGuo can be artificially formed during the sample preparation steps by the reaction of unmodified dGuo in DNA with acrolein in the air and water. In this study, we have carefully monitored and controlled the artifactual formation of Acr-dGuo during DNA isolation, enzymatic hydrolysis, and SPE clean-up processes. Acrolein reacts with thiol-containing compounds quickly and efficiently, and we have compared two thiol compounds - GSH and BME - for their ability to scavenge any acrolein present. During the DNA isolation and purification, there was no difference in the amount of Acr-dGuo from DNA extracted with 10 mM GSH, 10 mM BME or no scavenger present, indicating minimal artifactual formation in this step. However, when scavenger was omitted during the hydrolysis and SPE steps, the amount of Acr-dGuo measured was significantly higher and highly variable, indicating artifactual formation during these two steps. GSH (0.5 mM) was chosen as the scavenger for this assay since it was found to be more effective than BME (2.5 mM). γ-Acr-dGuo was the major artifact formed and it was almost exclusively formed during the SpeedVac drying step; while α-Acr-dGuo was formed to a much lesser extent and was formed during both the hydrolysis and drying steps. Therefore, we have also modified the SPE clean-up step by extensively washing with 6 mL of 0.1 mM GSH and 1 mL of 5% CH3OH in 0.1 mM GSH to remove as much unmodified dGuo as possible (> 99.99%) to prevent further artifact formation during the drying step. Further details of the method development and validation are presented in the Supporting Information.
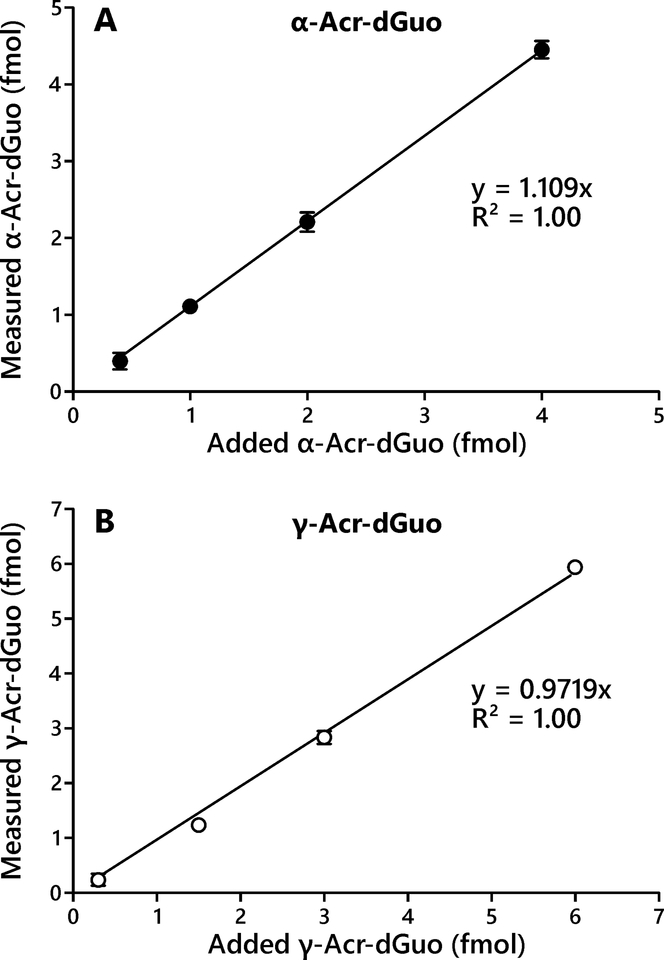
We have previously analyzed Acr-dGuo adducts in human lung and leukocyte DNA samples by LC-ESI-MS/MS using a few hundred micrograms of DNA.18 In the current study, we developed a more sensitive method using LC-NSI-HRMS/MS that requires only a few micrograms of DNA. The limit of detection for the new method was 2 adducts per 109 nucleotides for α-Acr-dGuo and 1 adduct per 109 nucleotides for γ-Acr-dGuo starting from 35 μg of DNA. The improved sensitivity was due to the use of high-resolution mass spectrometry, which has eliminated the background noise from the sample matrix and instrument by using accurate mass detection, and resulted in maximized signal to noise ratio, as well as improved ionization efficiency by reducing the flow rate to nanoliters per minute allowing for the use of NSI.27 The concentration range for the calibration curves and validation experiments were chosen to cover the range of Acr-dGuo levels found in the human lung DNA samples. The calibration curves for both α- and γ-Acr-dGuo showed good linearity within the low concentration range (R2 > 0.99). The assay accuracy was calculated as a percentage of added Acr-dGuo and the average accuracy was 107% for α-Acr-dGuo and 89% for γ-Acr-dGuo (n = 4), and good linearity was observed across the tested concentration ranges (Figure 2A and 2B). The assay precision was determined by analyzing calf thymus DNA (n = 12) and the intra-day and inter-day CV were both within 25% (Table 1). The assay recovery was 41% for α-Acr-dGuo and 27% for γ-Acr-dGuo.
Figure 2.
Assay accuracy presented by relationship of measured to added α-Acr-dGuo (A) and γ-Acr-dGuo (B). Various amounts of α- and γ-Acr-dGuo standards (0.3 to 6 fmol) were added to calf thymus DNA (35 μg) and analyzed using the method described here. Background levels from calf thymus DNA were subtracted. Each point represents the average of three replicates. Error bars represent the standard deviations.
Table1.
Assay precision determined by analyzing calf thymus DNA (n = 12)
| CTDNA | Acr-dGuo | CV% | |
|---|---|---|---|
| adducts/109 nucleotides | intra-day | inter-day | |
| α-Acr-dGuo | 6.1 ± 0.7 | 11% | 12% |
| γ-Acr-dGuo | 10.6 ± 2.6 | 14% | 24% |
Acr-dGuo levels in human lung DNA.
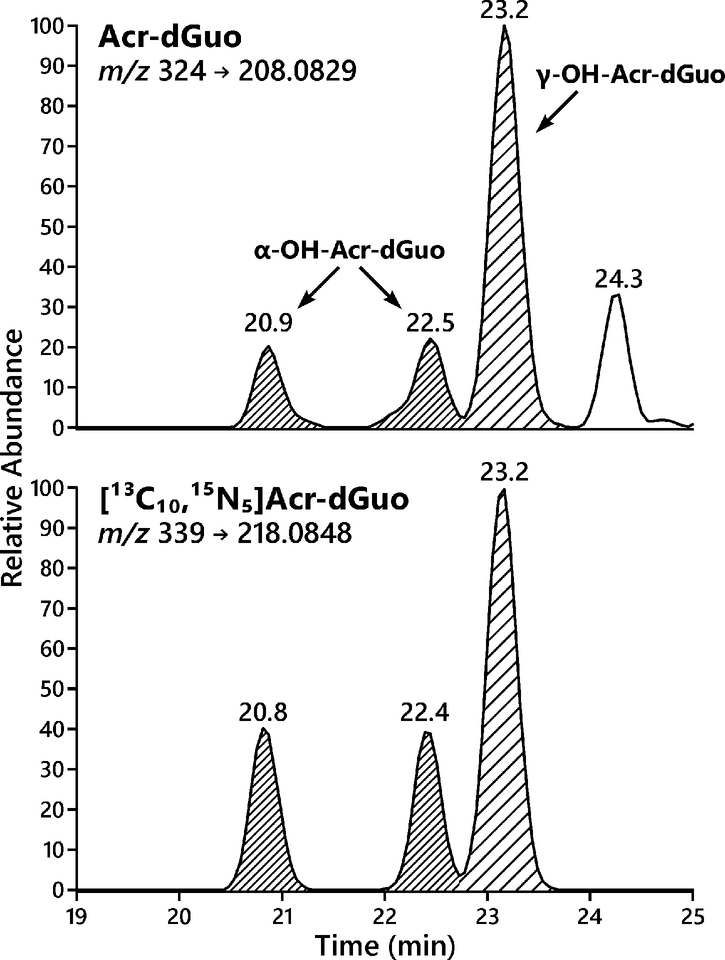
We analyzed the levels of Acr-dGuo in lung DNA of non-smokers (n = 18) and smokers (n = 19). Due to the altered gene expression and metabolism between normal and tumor tissues, DNA extracted from normal tissues adjacent to tumors was analyzed to provide information on adduct levels before tumor formation. Four subjects who reported being current smokers (subjects # 34–37) had urinary total NNAL and cotinine levels close to those of non-smokers, indicating they had already quit smoking by the time of surgery, and thus they were included in the group of non-smokers. Ion transitions at two different collision energy levels were monitored with the lower collision energy (20% HCD) for quantitation and higher collision energy (50% HCD) for confirmation. Typical extracted ion chromatograms for the Acr-dGuo quantitation using LC-NSI-HRMS/MS are shown in Figure 3. Both α-Acr-dGuo and γ-Acr-dGuo were detected in all samples and all peaks co-eluted with their corresponding internal standards [13C10, 15N5]Acr-dGuo. The first two eluting peaks of the same size are the two stereoisomers of the cyclic α-Acr-dGuo, and the third peak is γ-Acr-dGuo.21 The levels of Acr-dGuo in lung DNA of non-smokers and smokers are listed in Table 2, together with the levels of their urinary biomarkers for smoking (total NNAL and total cotinine) and the levels of BPDE-N2-dGuo, a DNA adduct formed by benzo[a]pyrene (BaP), in their lung DNA determined by LC-NSI-HRMS/MS in our earlier study.28 The average levels of α-Acr-dGuo were 10.3 ± 4.7 adducts per 109 in non-smokers and 8.6 ± 2.7 adducts per 109 in smokers, and the average levels of γ-Acr-dGuo were 14.7 ± 6.4 adducts per 109 in non-smokers and 19.9 ± 14.3 adducts per 109 in smokers. There was no significant difference in the levels of α-Acr-dGuo and γ-Acr-dGuo between non-smokers and smokers (p > 0.05). Deletion of subjects #34–37 from the non-smoker data did not affect this conclusion. Levels of BPDE-N2-dGuo adducts averaged 31 ± 22 adducts/1012 nucleotides in the same samples from smokers and 13 ± 8 adducts/1012 nucleotides in non-smokers.28
Figure 3.
Representative LC-NSI-HRMS/MS extracted ion chromatograms (EIC) for Acr-dGuo analysis in DNA extracted from human lung.
Table 2.
Levels Acr-dGuo in human lung DNA from smokers (A) and non-smokers (B).
| Subject | NNAL (pmol/mL) |
Cotinine (ng/mL) |
BPDE-N2-dGuo adducts/1012 nt |
DNA (μg) c |
Acr-dGuo (adducts/109 nucleotides) | ||
|---|---|---|---|---|---|---|---|
| α-Acr-dGuo | γ-Acr-dGuo | Total | |||||
| 1 | 1.27 | 733 | 65 | 39 | 6.0 | 9.6 | 15.6 |
| 2 | 1.92 | 765 | 34 | 31 | 9.4 | 41.6 | 51.1 |
| 3 | 0.92 | 855 | 45 | 43 | 6.5 | 19.6 | 26.1 |
| 4 | 1.53 | 1524 | 30 | 34 | 9.0 | 13.4 | 22.4 |
| 5 | 0.48 | 1560 | ND a | 37 | 9.4 | 30.4 | 39.8 |
| 6 | 4.18 | 6822 | 62 | 39 | 10.5 | 26.2 | 36.7 |
| 7 | 5.8 | 15976 | 22 | 41 | 8.5 | 14.9 | 23.4 |
| 8 | 1.12 | 4079 | NA | 38 | 6.9 | 42.2 | 49.1 |
| 9 | 1.10 | 1872 | 24 | 32 | 7.1 | 6.6 | 13.6 |
| 10 | 4.21 | 4276 | ND | 25 | 4.7 | 5.5 | 10.2 |
| 11 | 5.46 | 7269 | 41 | 27 | 3.8 | 4.2 | 8.0 |
| 12 | 0.84 | 647 | ND | 19 | 12.1 | 9.4 | 21.5 |
| 13 | 3.86 | 5660 | NA b | 19 | 6.5 | 55.7 | 62.3 |
| 14 | 0.54 | 2593 | NA | 54 | 8.6 | 14.3 | 22.8 |
| 15 | 0.15 | 515 | NA | 16 | 11.4 | 12.0 | 23.4 |
| 16 | 1.01 | 1917 | NA | 67 | 15.3 | 31.1 | 46.4 |
| 17 | 2.29 | 2532 | NA | 18 | 9.8 | 13.6 | 23.4 |
| 18 | 3.66 | 3955 | NA | 76 | 9.3 | 11.8 | 21.1 |
| 19 | 4.46 | 6722 | NA | 16 | 9.5 | 15.5 | 25.0 |
| Average | 31 ± 22 | 35 ± 17 | 8.6 ± 2.7 | 19.9 ± 14.3 | 28.5 ± 14.9 | ||
| B. Non-smokers | |||||||
| Subject | NNAL (pmol/mL) |
Cotinine (ng/mL) |
BPDE-N2-dGuo adducts/1012 nt |
DNA (μg) c |
Acr-dGuo (adducts/109 nucleotides) | ||
| α-Acr-dGuo | γ-Acr-dGuo | Total | |||||
| 20 | ND a | 1 | ND | 45 | 12.4 | 14.0 | 26.4 |
| 21 | ND | 2 | 13 | 66 | 8.6 | 11.5 | 20.1 |
| 22 | ND | 3 | 31 | 36 | 7.1 | 12.5 | 19.7 |
| 23 | ND | 4 | 17 | 28 | 8.3 | 11.1 | 19.4 |
| 24 | ND | 6 | 22 | 59 | 10.7 | 12.2 | 22.9 |
| 25 | ND | 11 | ND | 41 | 4.7 | 8.4 | 13.1 |
| 26 | ND | 13 | 15 | 51 | 6.6 | 9.8 | 16.4 |
| 27 | ND | 1.9 | 15 | 31 | 17.9 | 26.7 | 44.6 |
| 28 | ND | 5 | ND | 29 | 6.4 | 11.2 | 17.6 |
| 29 | ND | 5 | 14 | 23 | 11.4 | 28.5 | 39.9 |
| 30 | ND | <2.0 | 10 | 14 | 11.6 | 17.9 | 29.5 |
| 31 | ND | 2.7 | ND | 31 | 8.8 | 10.2 | 18.9 |
| 32 | ND | < 2.0 | 11 | 19 | 21.0 | 22.9 | 44.0 |
| 33 | NA b | NA | 28 | 48 | 6.4 | 11.9 | 18.3 |
| 34 | 0.08 | 15 | 20 | 16 | 10.0 | 13.1 | 23.2 |
| 35 | 0.05 | 11 | ND | 7 | 6.7 | 11.2 | 17.9 |
| 36 | ND | 19.2 | 9 | 41 | 19.4 | 23.9 | 43.3 |
| 37 | 0.06 | 5 | ND | 26 | 7.1 | 7.4 | 14.5 |
| Average | 13 ± 8 | 34 ± 16 | 10.3 ± 4.7 | 14.7 ± 6.4 | 25.0 ± 10.7 | ||
D: not detected;
NA: not available;
calculated on the basis of dGuo by HPLC-UV
In each set of samples, a negative control using buffer, a positive control using calf thymus DNA and an artifact control using purified dGuo (~25 nmol) were included and worked up in the same way together with the other samples to ensure data quality. No contamination was observed in the negative controls and good consistency was observed for positive controls on different experimental days. Commercial dGuo contains trace amount of Acr-dGuo and was purified by HPLC before use. The levels of artifactually formed adducts were 2.4 ± 0.9 adducts per 109 nucleotides for α-Acr-dGuo and 2.7 ± 1.2 adducts per 109 nucleotides for γ-Acr-dGuo, accounting for about 25% and 15% of the measured α- and γ-Acr-dGuo, respectively, in the lung DNA samples (Table 2). It should be noted that this artifact monitoring method provides the maximum levels of artifacts formed during the sample work-up since real DNA samples contain other matrix mixtures to further scavenge acrolein and lower artifact formation, as shown by good intra- and inter-day CV% for positive controls.
Discussion:
Acr-dGuo has been quantitated in a number of human tissues, including lung,18, 29 leukocytes,19, 30, 31 placenta,30 brain,32 salivary and oral tissues,11, 33, 34 but only two studies have taken precautions to prevent artifact formation during the sample preparation.19, 24 The Chung group first reported the formation of Acr-dGuo as an artifact during the analysis, and added GSH to block acrolein reaction with DNA.24 We later adopted the same scavenger to inhibit artifacts for the analysis of Acr-dGuo in human leukocytes.19 The measured Acr-dGuo levels were 20–50% lower when GSH was used during the sample preparation. In the current study, we have modified our method to include extensive washing during the SPE clean-up step to remove as much dGuo as possible to further prevent artifact formation during the SpeedVac drying step. We have also included an artifact control using purified dGuo to monitor the amount of artifacts formed during the analysis to ensure our data quality. With these precautions, the measured levels should represent the true levels of Acr-dGuo in human lung DNA.
Compared to our earlier study on Acr-dGuo in human lung DNA,18 the present study is unique in confirming the current smoking status in lung cancer patients by analyzing their matched urine samples. All the non-smokers chosen were those who have reported never smoking according to their self-report smoking history. The smokers chosen in this study were long-term smokers and were still smoking at the time of their surgery. Both groups’ current smoking status was confirmed by their urinary biomarkers. Therefore, we have a direct comparison of Acr-dGuo levels in lung DNA between cigarette smoking induced lung cancers and lung cancers caused by other factors. The hypothesis that has been presented is that if acrolein is a major etiological agent for smoking related lung cancer, then the signature mutations found in smoking related lung cancer are at least partly due to Acr-dGuo adducts.35 Therefore, we expected to observe higher levels of Acr-dGuo adducts in lung DNA from smokers than non-smokers. However, our results showed no difference in Acr-dGuo levels in smokers versus non-smokers, thus do not support the hypothesis that acrolein is a major etiological agent for smoking related DNA damage.
Acrolein is metabolized by conjugation with glutathione (GSH) and excreted in the urine as the mercapturic acid 3-HPMA, an important biomarker for acrolein exposure.11, 13, 36 Levels of urinary 3-HPMA are 4–5-fold higher in smokers than non-smokers, and the levels are reduced by about 80% after smoking cessation.15, 16 This evidence indicates that cigarette smoking is a significant source of exposure to acrolein in smokers. However, we did not observe a significant difference in Acr-dGuo levels in lung DNA from smokers and non-smokers. This suggests that acrolein from external exposure is rapidly conjugated and effectively detoxified by cellular GSH. Other than environmental exposures, acrolein is also produced endogenously from lipid peroxidation, and amino acid and polyamine metabolism.12, 13 The Acr-dGuo we observed here is most likely the background basal level from endogenous processes.
Since both acrolein and BaP have been shown to induce the same signature mutations and similar mutational distributions in the TP53 gene,8, 10 lung DNA from the same sets of subjects were also analyzed for BPDE-N2-dGuo by LC-NSI-HRMS/MS (reported elsewhere).28 We observed higher levels of BPDE-N2-dGuo in lung DNA from smokers than from non-smokers, suggesting that BaP rather than acrolein is a more important contributor to smoking related lung cancer, although full quantitation of BPDE-N2-dGuo was not attempted in that study due to its low levels.28 The overall levels of BPDE-N2-dGuo were about 1,000-fold lower than total Acr-dGuo levels, which is consistent with the levels of the corresponding carcinogens in tobacco smoke. However, in a direct comparison study of mutagenicity in the cII transgene in Big Blue mouse embryonic fibroblasts, BPDE at a concentration of 200 nM showed powerful mutagenicity, whereas acrolein at 100 μM, the highest dose tested due to its toxicity, did not elicit a mutagenic response in the same system.37 Therefore, even the small amounts of elevated BPDE-N2-dGuo levels found in smokers’ lung tissue may play an important role leading to more mutations and elevated lung cancer risk in smokers. Recently, Weng et al have reported higher levels of Acr-dGuo, but not BPDE-N2-dGuo adducts, in lung tissue DNA of smokers than non-smokers using an immunoassay/32P-postlabeling method.29 However, our results using LC-NSI-HRMS/MS suggest opposite conclusions. Our measured levels for Acr-dGuo and BPDE-N2-dGuo were also 10- and 1000- fold lower than theirs, respectively.
There has been great focus on BaP and its ultimate carcinogenic metabolite BPDE when considering the potential roles of PAH vs. acrolein in lung carcinogenesis in smokers. This has obscured to some extent the earlier research of Snook et al and others who characterized more than 500 PAH in mainstream cigarette smoke.38, 39 Fractions of cigarette smoke condensate enriched in PAH are tumorigenic on mouse skin and when implanted in the rat lung,40, 41 but most individual PAH in cigarette smoke have not been studied with respect to their carcinogenic activities, metabolic pathways, or DNA interactions. Among those that have been characterized, 14 PAH including benz[a]anthracene, benzofluoranthenes, chrysene, 5-methylchrysene, and dibenz[a,h]anthracene have been evaluated by the International Agency for Research on Cancer as having sufficient evidence for carcinogenicity in laboratory animals.42 Several PAH diol epoxides other than BPDE induce similar signature mutations in the TP53 gene.43 Thus, when evaluating the role of different compounds in lung cancer induced by cigarette smoke, the potential effects of PAH as a class should be considered.
This study as well as our previous LC-MS/MS study of acrolein-DNA adducts in human lung found α-Acr-dGuo in all samples tested whereas our study of human leukocyte DNA, which also found no difference in acrolein-DNA adduct levels between smokers and non-smokers, detected mainly γ-Acr-dGuo (50 out of 50 samples) but rarely α-Acr-dGuo (3/50 samples).19 Two other studies of human leukocyte DNA, carried out by LC-MS/MS methods, also found higher levels of γ-Acr-dGuo than α-Acr-dGuo.30, 31 The more frequent detection of α-Acr-dGuo in lung DNA than in leukocyte DNA suggests that there could be a DNA repair deficiency for α-Acr-dGuo in the human lung. α-Acr-dGuo has stronger mutagenic properties than γ-Acr-dGuo.21
Our study focused on DNA adduct formation by acrolein because of the central role of adducts and mutations in lung cancer etiology. We found no evidence for differences in adduct levels between smokers and non-smokers, leading to the conclusion that DNA damage by acrolein plays no role in lung cancer etiology in smokers. However, acrolein has powerful toxic effects including irritation, inflammation, cell proliferation, oxidative stress, protein damage, mitochondrial disruption, membrane damage, and immune dysfunction among others.11, 44, 45 The theoretical non-cancer hazard index for cigarette smoke is dominated by acrolein.46 Any of acrolein’s toxic effects could play a role in lung cancer induction by exacerbating the effects of DNA damage through tumor promotion, co-carcinogenesis or related non-DNA damage dependent mechanisms.
In summary, we have developed a sensitive LC-NSI-HRMS/MS method, with minimal artifact formation, for quantitation of Acr-dGuo in small amounts of human lung DNA from both smoking and non-smoking lung cancer patients. Our results demonstrate that there is no difference in Acr-dGuo levels between smokers and non-smokers, consistent with our previous study of leukocyte DNA. Our results suggest that BaP and other PAH are more important DNA damaging agents than acrolein in lung cancer caused by cigarette smoking.
Supplementary Material
Acknowledgements:
The authors thank Lei Meng for extracting DNA from human lung tissues, Makenzie Pillsbury and Steven Carmella for the analysis of NNAL and cotinine in urine, and Bob Carlson for editorial assistance.
Funding:
This study was supported by grant P01-CA138338 from the National Cancer Institute. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, funded in part by Cancer Center Support Grant P30 CA-077598. Salary support for P.W.V. was provided by the U.S. National Institutes of Health and National Cancer Institute [Grant R50 CA-211256].
Footnotes
Supporting Information:
The Supporting Information is available free of charge at the ACS Publications website. Further details of method validation for analysis of acrolein-DNA adducts.
The authors declare no competing financial interest.
References:
- (1).Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin DOI: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- (2).International Association for the Study of Lung Cancer. (September 7, 2015) IASLC 2015 Statement on Tobacco Control and Smoking Cessation. [Google Scholar]
- (3).Cancer Genome Atlas Research Network. (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature 511, 543–550. PMCID: PMC4231481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Cancer Genome Atlas Research Network. (2012) Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525. PMCID: PMC3466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Le Calvez F, Mukeria A, Hunt JD, Kelm O, Hung RJ, Taniere P, Brennan P, Boffetta P, Zaridze DG, and Hainaut P (2005) TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: Distinct patterns in never, former, and current smokers. Cancer Res 65, 5076–5083. [DOI] [PubMed] [Google Scholar]
- (6).Toyooka S, Tsuda T, and Gazdar AF (2003) The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat 21, 229–239. [DOI] [PubMed] [Google Scholar]
- (7).Jelinsky SA, Liu TM, Geacintov NE, and Loechler EL (1995) The Major, N2-gua adduct of the (+)-anti-benzo[a]pyrene diol epoxide is capable of inducing G→A and G→C, in addition to G→T, mutations. Biochemistry 34, 13545–13553. [DOI] [PubMed] [Google Scholar]
- (8).Denissenko MF, Pao A, Tang MS, and Pfeifer GP (1996) Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 274, 430–432. [DOI] [PubMed] [Google Scholar]
- (9).Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, and Hainaut P (2002) Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 21, 7435–7451. [DOI] [PubMed] [Google Scholar]
- (10).Feng Z, Hu W, Hu Y, and Tang MS (2006) Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA 103, 15404–15409. PMCID: PMC1592536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).International Agency for Research on Cancer. (1995) Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals, In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v. 63 pp 393–407, IARC, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- (12).Chung FL, Chen HJC, and Nath RG (1996) Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis 17, 2105–2111. [DOI] [PubMed] [Google Scholar]
- (13).Stevens JF, and Maier CS (2008) Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 52, 7–25. PMCID: PMC2423340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen T, Reininghaus W, Carchman RA, Gaworski CL, and Podraza KF (2004) Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology 195, 31–52. [DOI] [PubMed] [Google Scholar]
- (15).Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, and Hecht SS (2007) Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem Res Toxicol 20, 986–990. PMCID: PMC2556963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Alwis KU, deCastro BR, Morrow JC, and Blount BC (2015) Acrolein exposure in U.S. tobacco smokers and non-tobacco users: NHANES 2005–2006. Environ Health Perspect 123, 1302–1308. PMCID: PMC4671235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Chung FL, Young R, and Hecht SS (1984) Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res 44, 990–995. [PubMed] [Google Scholar]
- (18).Zhang S, Villalta PW, Wang M, and Hecht SS (2007) Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol 20, 565–571. PMCID: PMC2518976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zhang S, Balbo S, Wang M, and Hecht SS (2011) Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem Res Toxicol 24, 119–124. PMCID: PMC3064499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Yang IY, Chan G, Miller H, Huang Y, Torres MC, Johnson F, and Moriya M (2002) Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry 41, 13826–13832. [DOI] [PubMed] [Google Scholar]
- (21).Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, and Stone MP (2009) Chemistry and biology of DNA containing 1,N2-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem Res Toxicol 22, 759–778. PMCID: PMC2685875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hecht SS (2012) Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res 14, 18–28. PMCID: PMC3242967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).International Agency for Research on Cancer. (2018) Agents Classified by the IARC Monographs, Volumes 1–122, In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, IARC, Lyon, France: https://monographs.iarc.fr/wp-content/uploads/2018/09/ClassificationsAlphaOrder.pdf [Google Scholar]
- (24).Emami A, Dyba M, Cheema AK, Pan J, Nath RG, and Chung FL (2008) Detection of the acrolein-derived cyclic DNA adduct by a quantitative 32P-postlabeling/solid-phase extraction/HPLC method: blocking its artifact formation with glutathione. Anal Biochem 374, 163–172. PMCID: PMC2275120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kotandeniya D, Carmella SG, Ming X, Murphy SE, and Hecht SS (2015) Combined analysis of the tobacco metabolites cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine. Anal Chem 87, 1514–1517. PMCID: PMC4315695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Snustad DP, Simmons MJ, and Jenkins JB (1997) Principles of genetics. pp. John Wiley, New York. [Google Scholar]
- (27).Yang J, Villalta PW, Upadhyaya P, and Hecht SS (2016) Analysis of O6-[4-(3-pyridyl)-4-oxobut-1-yl]-2’-deoxyguanosine and other DNA adducts in rats treated with enantiomeric or racemic N’-nitrosonornicotine. Chem Res Toxicol 29, 87–95. PMCID: PMC5168933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Villalta PW, Hochalter JB, and Hecht SS (2017) Ultrasensitive high-resolution mass spectrometric analysis of a DNA adduct of the carcinogen benzo[a]pyrene in human lung. Anal Chem 89, 12735–12742. PMCID: PMC6027747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Weng MW, Lee HW, Park SH, Hu Y, Wang HT, Chen LC, Rom WN, Huang WC, Lepor H, Wu XR, Yang CS, and Tang MS (2018) Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc Natl Acad Sci USA 115, E6152–E6161. PMCID: PMC6142211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chen HJC, and Lin WP (2009) Simultaneous quantification of 1,N2-propano-2’-deoxyguanosine adducts derived from acrolein and crotonaldehyde in human placenta and leukocytes by isotope dilution nanoflow lc nanospray ionization tandem mass spectrometry. Analytical Chem 81, 9812–9818. [DOI] [PubMed] [Google Scholar]
- (31).Yin R, Liu S, Zhao C, Lu M, Tang MS, and Wang H (2013) An ammonium bicarbonate-enhanced stable isotope dilution UHPLC-MS/MS method for sensitive and accurate quantification of acrolein-DNA adducts in human leukocytes. Anal Chem 85, 3190–3197. [DOI] [PubMed] [Google Scholar]
- (32).Liu X, Lovell MA, and Lynn BC (2005) Development of a method for quantification of acrolein-deoxyguanosine adducts in DNA using isotope dilution-capillary LC/MS/MS and its application to human brain tissue. Anal Chem 77, 5982–5989. [DOI] [PubMed] [Google Scholar]
- (33).Nath RG, Ocando JE, Guttenplan JB, and Chung FL (1998) 1,N2-propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res 58, 581–584. [PubMed] [Google Scholar]
- (34).Chen HJ, and Lin WP (2011) Quantitative analysis of multiple exocyclic DNA adducts in human salivary DNA by stable isotope dilution nanoflow liquid chromatography-nanospray ionization tandem mass spectrometry. Anal Chem 83, 8543–8551. [DOI] [PubMed] [Google Scholar]
- (35).Wang HT, Zhang S, Hu Y, and Tang MS (2009) Mutagenicity and sequence specificity of acrolein-DNA adducts. Chem Res Toxicol 22, 511–517. PMCID: PMC4606861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Parent RA, Paust DE, Schrimpf MK, Talaat RE, Doane RA, Caravello HE, Lee SJ, and Sharp DE (1998) Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats: II. Identification of urinary and fecal metabolites. Toxicol Sci 43, 110–120. [DOI] [PubMed] [Google Scholar]
- (37).Kim SI, Pfeifer GP, and Besaratinia A (2007) Lack of mutagenicity of acrolein-induced DNA adducts in mouse and human cells. Cancer Res 67, 11640–11647. [DOI] [PubMed] [Google Scholar]
- (38).Snook ME, Severson RF, Arrendale RF, Higman HC, and Chortyk OT (1978) Multi-alkyated polynuclear aromatic hydrocarbons of tobacco smoke: separation and identification. Beiträge Tabakforsch 9, 222–247. [Google Scholar]
- (39).Rodgman A, and Perfetti T (2009) The Chemical Components of Tobacco and Tobacco Smoke. pp. CRC Press, Boca Raton, FL. [Google Scholar]
- (40).Hoffmann D, Schmeltz I, Hecht SS, and Wynder EL (1978) Tobacco carcinogenesis, In Polycyclic Hydrocarbons and Cancer (Gelboin H, and Ts’o POP, Eds.) pp 85–117, Academic Press, New York. [Google Scholar]
- (41).Stanton MF, Miller E, Wrench C, and Blackwell R (1972) Experimental induction of epidermoid carcinoma in the lungs of rats by cigarette smoke condensate. J Natl Cancer Inst 49, 867–877. [PubMed] [Google Scholar]
- (42).International Agency for Research on Cancer. (2010) Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures, In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v. 92 pp 35–818, IARC, Lyon, FR. [PMC free article] [PubMed] [Google Scholar]
- (43).Smith LE, Denissenko MF, Bennett WP, Li H, Amin S, Tang M, and Pfeifer GP (2000) Targeting of lung cancer mutational hotspots by polycyclic aromatic hydrocarbons. J Natl Cancer Inst 92, 803–811. [DOI] [PubMed] [Google Scholar]
- (44).Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, and Joshi-Barve S (2015) Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci 143, 242–255. PMCID: PMC4306719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yeager RP, Kushman M, Chemerynski S, Weil R, Fu X, White M, Callahan-Lyon P, and Rosenfeldt H (2016) Proposed mode of action for acrolein respiratory toxicity associated with inhaled tobacco smoke. Toxicol Sci 151, 347–364. [DOI] [PubMed] [Google Scholar]
- (46).Haussmann HJ (2012) Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol 25, 794–810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.