Abstract
Introduction:
Fibromyalgia affects more than 5 million people in the United States and has a detrimental impact on individuals’ quality of life. Current pharmacological treatments provide limited benefits to relieve fibromyalgia’s pain, along with risks of adverse effects, a scenario that explains the increasing interest for multimodal approaches. A tailored strategy to focus on this dysfunctional endogenous pain inhibitory system is transcranial direct current stimulation (tDCS) of the primary motor cortex. By combining tDCS with aerobic exercise, the effects can be optimized.
Areas covered:
The relevant literature was reviewed and discussed the methodological issues for designing a mechanistic clinical trial to test this combined intervention. Also, we reviewed the neural control of different pathways that integrate the endogenous pain inhibitory system, as well as the effects of tDCS and aerobic exercise both alone and combined. In addition, potential neurophysiological assessments are addressed: Conditioned Pain Modulation, Temporal Slow Pain Summation, Transcranial Magnetic Stimulation and Electroencephalography in the context of fibromyalgia.
Expert Commentary:
By understanding the neural mechanisms underlying pain processing and potential optimized interventions in fibromyalgia with higher accuracy, the field has an evident potential of advancement in the direction of new neuromarkers and tailored therapies.
Keywords: aerobic exercise, conditioned pain modulation, electroencephalography, endogenous pain control system, fibromyalgia, temporal slow pain summation, transcranial direct current stimulation, transcranial magnetic stimulation
1. Introduction
Fibromyalgia affects an upwards of 5 million people in the United States [1] and has a considerable impact on individuals’ quality of life [2]. Pain, which is the primary symptom in fibromyalgia, may be extremely hard to quantify and correspondingly hard to treat, since most of the limited treatment options are often associated with adverse effects. Common pharmacological interventions in fibromyalgia involve the use of nonsteroidal anti-inflammatory drugs, antidepressants, and/or anticonvulsants, all with considerably high risks and poor rates of success [3]. Although the etiology of fibromyalgia is still unknown, evidence suggests that the widespread pain experienced by these patients is related to deficits in an endogenous pain control system [4] that involves several structures, from primary sensory areas to the limbic system, from midbrain to medullary and spinal sites.
When this endogenous pain control system is dysfunctional, the excessive response can lead to hyperalgesia, allodynia and ultimately chronic pain. A decreased postsynaptic inhibition in somatosensory neurons of the dorsal horn can lead to central pain sensitization [5] or to a dysfunction in descending pain modulating pathways which include the periaqueductal gray (PAG) and the rostroventromedial medulla [6]. The understanding of chronic pain as a phenomenon resulting from this imbalance has led to the development of interventions aimed at novel targets in the central nervous system [7].
In this context, enhancing sensory processing in order to trigger an inhibitory endogenous response, instead of just aiming to block pain as analgesic drugs do, can reach these targets. For instance, Transcutaneous Electrical Nerve Stimulation (TENS) has shown successful anti-hyperalgesia responses both in animal [8] and human [9] studies. Its effects can last long after the stimulation (particularly in low-frequency TENS) and hypoalgesia can be achieved even when electrodes are placed on the contralateral side of pain, providing support to the idea that a central modulation of the endogenous pain control system is happening as a consequence of the peripheral stimulation.
Using this rationale, transcranial direct current stimulation (tDCS) may offer interesting advantages, modulating pain-processing circuits involved in the endogenous pain inhibitory system. tDCS leads to a change in neural membrane thresholds, enhancing or decreasing excitability of brain areas according to the polarity of stimulation. For instance, it has been documented that when excitability-enhancing anodal tDCS is applied to the primary motor cortex, there is an increase in thalamic activity [10] related to the activation of endogenous pain inhibitory pathways [11].
Aerobic exercises can also elicit responses in the endogenous pain system, modulating intracortical excitability even in areas corresponding to non-exercised muscles [12]. Animal models for neuropathic pain [13] have also provided evidence that moderate intensity exercise can reverse hypersensitivity and lead to significant changes in endogenous pain inhibitory activity. Comparable outcomes have also been found in fibromyalgia patients, resulting in what is known as exercise-induced hypoalgesia.
In fact, separately, tDCS of the primary motor cortex (M1) and aerobic exercise have demonstrated significant effects in fibromyalgia [14, 15, 16, 17], with both reducing the dysregulation of the inhibitory control system [18]. Other studies have shown that combining tDCS with a behavioral therapy may also optimize its effects [19, 20]. However, this evidence is not unanimous [21].Given that both tDCS and exercise simultaneously target the endogenous pain inhibitory system, their combination would likely induce a greater response than the single interventions alone.
In this article, a combined brain stimulation and exercise strategy to generate a larger impact on the endogenous pain inhibitory system is discussed, followed by a proposal of a study design to test it. Proper assessments will not only help to answer how the interventions improve pain, but they may also provide insights and increase the understanding on how the different areas of the central nervous system are affected by fibromyalgia. In order to pursue this rationale, four non-invasive neurophysiological methods to evaluate the effects of optimized tDCS are addressed: Temporal Slow Pain Summation (TSPS), Conditioned Pain Modulation (CPM), Transcranial Magnetic Stimulation (TMS) and Electroencephalography (EEG).
2. Understanding the neural effects of tDCS combined with aerobic exercises
Optimized tDCS with aerobic exercise can maximize the restoration of the pain endogenous regulatory system by modulating activity in a widespread neural network that includes the primary motor cortex (M1), the dorsolateral prefrontal cortex (DLPFC), the cingulate cortex, the insula, the thalamus, the subnucleus dorsalis reticularis (SDR) and the spinal cord dorsal horn; thus, enhancing pain relief in patients with fibromyalgia. This combination of tDCS with exercise has been demonstrated to decrease pain in patients with fibromyalgia [22]; however, the mechanisms involved are still not clear. The rationale for a combined therapy is discussed below.
2.1. Neural control of the endogenous pain inhibitory system
Endogenous pain control mechanisms have long been known to produce analgesia during “flight or fight” situations and to contribute to cognitively driven pain modulation, such as placebo analgesia [23]. Early inhibition can enhance escape behaviors, while late facilitation may create conditions that are optimal for healing tissue injuries. On this basis, the descending modulation of the dorsal horns of the spinal cord can ensure a protective function, while psychological features strongly influence the inhibitory modulation of noxious peripheral signals that occur at both spinal and supraspinal levels [24].
Sensory activity is one of the main contributors to the modulation of the endogenous pain system. While increased sensory input leads to a feedback inhibitory activity, a loss of afference, such as with limb loss or spinal cord injury, results in neuropathic pain and a defective endogenous inhibitory system [25]. As a consequence, there is a change in cortical and subcortical areas of this neural circuit, leading to a worsening of the pain experience [26, 27].
Endogenous pain modulation is a complex two-step process involving central nervous system (CNS) networks comprehending various brain regions (cingulate cortex, prefrontal cortex and insula), the PAG, the medulla, and the spinal cord [28]. Initially, pain facilitation may take place with painful stimuli. The stimulation of nociceptors induces the release of glutamate from the central terminals of primary afferent neurons terminating in laminae I, II and V of the dorsal horn. During this time, substance P is also discharged from the afferent neurons owing to activation of N-methyl-D-aspartate (NMDA) receptors. Finally, increased intracellular calcium levels induce signaling pathways that lower the firing threshold of dorsal horn neurons [24, 29].
Following a painful or non-painful stimulation, descending inhibition of the nociceptive activation in the spinal cord can be seen. This system is also modulated by the prefrontal cortex, midbrain and brainstem. Studies show the importance of PAG for pain inhibition as well as for the integration of input from the spinal cord, brainstem (pain modulation signals can reach the dorsal horn of the spinal cord from the PAG via rostral ventromedial medulla) and cerebral cortex. It has been shown that spinal inputs to the PAG can induce pain inhibition via a spinal-supraspinal-spinal loop [30, 31, 32].
2.2. Neural effects of tDCS
Recent reviews on studies about motor cortex stimulation for pain syndromes reported increased activation with tDCS both in areas adjacent to the primary motor cortex such as the dorsolateral prefrontal cortex (DLPFC), as well as in distant structures, including the cingulate cortex, thalamus and insula [33, 34]. However, there is some variability in tDCS studies (on modeling of electrical fields) [18] in regard to the regions reached by this tool as differences in cerebral anatomy among subjects are common. Therefore, one cannot draw conclusions about where exactly tDCS is stimulating; nevertheless, a growing body of evidence is contributing to the disentanglement of this phenomenon.
In order to understand the effects of tDCS of M1 on the endogenous control system, it is important to recognize the contribution of the first experiments with motor cortex stimulation. Tsubokawa et al. [35] have shown that chronic epidural stimulation of M1 can be very useful for controlling deafferentation pain secondary to thalamic lesions. The hyperactivity of nociceptive neurons in this kind of central pain is thought to be suppressed by the stimulation of the motor cortex, which not only inhibits burst firing in the vicinity of the damaged thalamus, but also induces neuroplastic effects on the thalamus itself [36].
Following these experiments, Garcia-Larrea et al. [37, 38] showed elegantly that the primary motor cortex is a reliable “entry port” to modulate dysfunctional activity in a pain-related neural network. Up-regulation of motor cortex excitability modulates pain perception through indirect effects on pain-modulating areas, such as thalamic nuclei and the cingulate gyrus. Neuroimaging studies with positron emission tomography (PET) scans in chronic pain patients have shown that motor cortex stimulation leads to changes in the motor (lateral) thalamus activity, which is then transmitted to the sensory (medial nuclei) thalamus and other pain-related structures such as the subthalamic area, insula, brainstem and anterior cingulate. These neuroimaging studies have shown that the effects of motor cortex stimulation are not related to direct cortical activation but rather excitation of white matter axons. Another study using a forward-model analysis compared the neuroanatomic location and strength of the predicted electrical current peaks induced by M1 tDCS, demonstrating that the thalamus is directly stimulated in this type of montage[39]. In addition, changes in spinal reflexes have also been found during cortical stimulation, consistent with the idea that corticospinal axons are able to inhibit nociceptive neurons at the spinal level. Therefore, this second mechanism may also explain how M1 stimulation relieves pain, besides the modulation of thalamic activity [40].
Regarding the specific effects of M1 tDCS in chronic pain, the main mechanism appears to be related to thalamic activity modulation [10, 41, 42]. Indeed, a previous study from this same research group indicated that active M1 tDCS increased sensory and pain thresholds compared to sham tDCS[43]. Single-voxel proton magnetic resonance spectroscopy images [44] of the thalamus have shown that these changes were positively correlated to the ratio between glutamine and creatinephosphocreatine and negatively related to the ratio between glutamate and creatine. Other studies demonstrated the effect of tDCS in pain modulation by increasing motor cortex excitability [45] and directly and indirectly affecting pain-related neural networks which involve the thalamic nuclei [46]. In summary, M1 stimulation may help normalize the marked thalamic hyperactivity related to chronic pain, acting on pyramidal neurons and inhibitory interneurons in the prefrontal, orbitofrontal and cingulate cortices as part of the pain modulation network [41]. The neural effects of tDCS are summarized in Figure 1 by the yellow color (yellow arrows).
Figure 1:
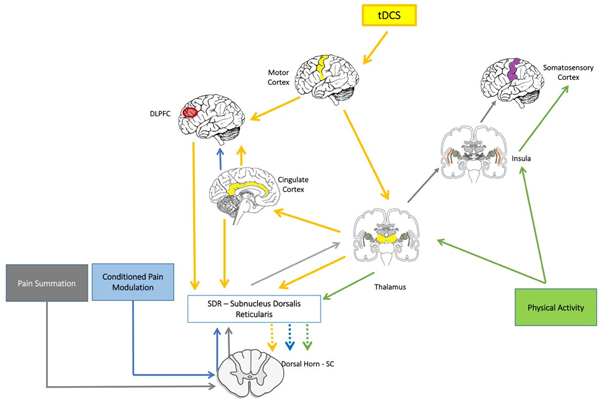
The above-detailed figure shows how two interventions would maximize their effects in the neuromatrix of pain. tDCS is represented by yellow arrows, physical activity by green arrows, conditioned pain modulation by blue arrows and pain summation by grey arrows. The combined intervention would increase inhibitory activity from the thalamic regions to the subnucleus dorsalis reticularis and then to the dorsal horn of the spinal cord, which would increase the CPM response (enhancing descending inhibitory pain systems) and decrease the TSPS response (decreasing central sensitization). Moreover, it is expected that this effect is also mediated and enhanced by important cortical areas such as prefrontal cortex, cingulate cortex, insula, and somatosensory cortex. (SC: Spinal Cord)
Concerning the clinical effects of tDCS on fibromyalgia, two recent meta-analyses showed statistically significant results with moderate effect sizes [47, 48] and a recent evidence-based guideline from Lefaucheur and colleagues graded Level B recommendation (probable efficacy) for anodal tDCS of the left primary motor cortex (M1) (with right orbitofrontal cathode) in fibromyalgia [49]. In opposition to this, Fagerlund et al. (2015) evaluating 48 patients with fibromyalgia achieved a small and non-clinically important improvement in the group, which received anodal tDCS directed to the M1 area [50]. Similarly, non-significant results were demonstrated by Riberto and colleagues as well as unclear findings among studies explored in a recent Cochrane review (2018) on chronic pain [21]. Most of the divergent evidence could be attributed to the large variability between the tDCS protocols, such as differences in electrode placement (M1 or DLPFC) and polarity of the stimulation (anodal or cathodal) that can contribute to the significant heterogeneity between the tDCS trials. Most of the tDCS studies in chronic pain used anodal stimulation over the primary motor cortex (M1 area: C3/C4 – International 10–20 system for the electroencephalography (EEG) electrode) of the hemisphere contralateral to the pain location. Other montages have been tested including anodal/cathodal over the left dorsolateral pre-frontal cortex (DLPFC) for fibromyalgia and in most of the studies, the cathode was placed over the contralateral supraorbital region. These controversial findings drive the discussion in this paper and encourage trials focused on mechanistic outcomes in order to understand the complexity of non-invasive electrical brain stimulation treatments in fibromyalgia.
2.3. Neural effects of exercise
Aerobic exercise influences various aspects of bodily function, including a large neural circuit via afferent input (bottom-up) from somatosensory stimulation and a neuroendocrine response [51, 52]. This concept is known as exercise-induced hypoalgesia [53]. Studies with rats show that following exercise training, there is increased efficiency of neural processing over the sensorimotor cortex, striatum and vermis, as well as in the cerebellar-thalamic-cortical circuit [54]. Moreover, in humans, physical exercise has been shown to activate the insular cortex [55], in an intensity dependent manner [56]. Thus, increases in insular cortex activity may actually increase the thalamic down regulation [17].
The complexity of chronic pain syndromes and the innumerous possibilities for exercise regimens have provided conflicting results regarding the benefits of this intervention [57]. However, studies that involved skill training [58] and exercise [59] have clearly shown cortical excitability changes when intracortical inhibition was measured by TMS. These effects are probably the consequence of the activation of inhibitory interneurons under these conditions [60]. In fact, the effect of exercise on the endogenous pain system has been associated with gamma-aminobutyric acid (GABA)- mediated mechanisms [61], modulating cortical activity and ultimately leading to neuroplasticity [62] and improvement in different kinds of chronic pain [17, 63].
In summary, exercises also lead to enhancement of endogenous pain inhibitory systems and decrease of pain sensitization through cortical and thalamic modulation. The effects of exercise are summarized in Figure 1 by the green color (green arrows).
2.4. Understanding the neural mechanisms of combined tDCS and exercises
Both tDCS and exercises affect the endogenous pain control system through complimentary mechanisms. tDCS primes the pain inhibitory system which is subsequently modified by aerobic exercise. Therefore, tDCS acts as an augmentative type of therapy when combined with physical exercise, enhancing effects by targeting similar but different circuits [64].
The main rationale for the optimized clinical effects of the combined intervention is the “top-down” (from cortical: primary sensory motor cortex, cingulate cortex and insula; to subcortical: thalamus) enhancement on neural excitability elicited by tDCS and complemented by a “bottom-up” effect (from subcortical: thalamus, cerebellum to cortical: sensorimotor cortex, insula and DLPFC) elicited by aerobic exercise. These two mechanisms of action strengthen the endogenous pain control system and normalize thalamocortical circuits. Furthermore, at the molecular level, they have an effect on the glutamatergic system and the calcium signalizing as well as on the long-term potentiation.
Mendonça et al. [22] obtained positive and larger effects on pain relief, quality of life, depression and anxiety by combining tDCS with aerobic exercise for the treatment of fibromyalgia. In addition, preliminary data regarding non-invasive brain stimulation for fibromyalgia and aerobic exercise have shown that these techniques yield significant results when compared to control interventions and baseline symptoms [16, 21, 65, 66]. Pinto et al. is using mirror therapy with tDCS to treat phantom limb pain, exemplifying a combined therapy with tDCS as a strategy to enhance sensory afference [67]. Moreover, it was demonstrated that the combination between tDCS and visual illusion provides long term effects in pain relief of spinal cord injury patients [68] and leads to significant alterations in contact heat-evoked potential (CHEPS) and pain thresholds [69]. In this context, some studies have shown the activation of sensorimotor cortex after mirror therapy, mirror illusion and visual feedback when combined with M1 tDCS, therefore inferring the potential benefits of this strategy [70, 71]. In addition, for chronic low back pain, the combination of tDCS and peripheral electrical stimulation seems to improve pain and sensitization, in a greater magnitude than when compared with control or when performed alone [72, 73]. It is important to point out that the main characteristic to consider for selecting an optimized combination therapy is to ensure that both interventions target neural networks synergistically, driving the neurophysiological mechanisms of each treatment alone towards similar directions or pathways.
In summary, it is hypothesized that optimized tDCS with aerobic exercise can maximize the restoration of the pain endogenous regulatory system by modulating activity in a widespread neural network enhancing pain relief in patients with fibromyalgia.
2.5. Assessing the effects of optimized tDCS treatment for enhancing endogenous pain inhibitory control
In order to develop and test this proposed optimized tDCS intervention, it is important to understand how to assess its effect and also how to titrate the doses of this treatment. Sometimes referred to as “Diffuse Noxious Inhibitory Control” (DNIC) or “Heterotopic Noxious Conditioned Stimulation”(HNCS) [74], Conditioned Pain Modulation (CPM) is a neurophysiological assessment that has been increasingly used as a marker of the descending inhibitory system [75] (the descending analgesic pathways dampen pain signals). This powerful pain modulating system is involved in mediating the effects of tDCS and exercise. Patients with fibromyalgia exhibit lower CPM inhibitory efficacy and a significant increase in the rate of pain facilitation during the CPM procedure, when compared to non-clinical controls [76]. Recent studies show that the subnucleus dorsalis reticularis (SDR) is one of the main modulators of the descending inhibitory activity and this area is also secondarily modulated by the DLPFC and cingulate cortex [77, 78]. CPM is represented in Figure 1 by the blue color.
Temporal Slow Pain Summation (TSPS) is another assessment related to central sensitization. It can measure enhanced pain severity during and after repetitive stimuli (temporal summation). TSPS seems not to be dependent on increased impulse C-fiber input, suggesting that TSPS is indeed a central nervous system rather than peripheral phenomenon [79]. There are several neural correlates for this central phenomenon such as rostral ventromedial medulla facilitation, as well as ineffective central pain modulation [80]. Again, the thalamus seems to play a key moderator role, as outputs from the dorsal horn of the spinal cord are sent to the thalamus, which in turn are relayed to the insula and somatosensory cortices. TSPS is represented in Figure 1 by the gray color.
Single and paired-pulse TMS can be used to measure excitability and inhibition. These assessments demonstrate changes in cortical excitability indexed by motor evoked potentials and resting motor thresholds as well as changes in short-intracortical inhibition and intracortical facilitation assessed by paired-pulse technique. It has been demonstrated that fibromyalgia is associated with a deficit in inhibitory control and these patients exhibit an increased resting motor threshold and decreased intracortical inhibition and facilitation [81]. Studies with tDCS combined with exercise have demonstrated the synergistic effect with an increase in the motor-evoked potential [82] and given the excitatory nature of both interventions, tDCS combined with exercise may induce a significant effect on the inhibitory circuits of the cortical target: the primary motor cortex.
EEG is a reliable tool to measure electrical activity in the brain and can identify features of cortical excitability and inhibitory activities, as well as thalamocortical rhythm’s abnormalities [83]. The power of different EEG bandwidths has been associated with the intensity of pain experience and patients with fibromyalgia present abnormal thalamo-cortical circuits which can lead to the overproduction of theta oscillatory activity in the cortex. Since thalamo-cortical modules in theta frequency distort lateral inhibition on other cortical modules, these are over-activated at high-frequencies (beta and gamma) which creates the edge-effect [84]. Boosted low and high-frequency activity especially of the prefrontal cortex can be related to the persistent pain in fibromyalgia patients, so interventions that can help normalize the thalamocortical rhythm by changing neural oscillation may be useful to improve the pain [85].
2.6. Understanding the specific effects of combined intervention
Ultimately, both interventions when combined are expected to have a final effect on thalamic activity (supporting the combined therapy and its synergistic effects). The thalamus is also a key area mediating the effects measured by CPM and TSPS; however, there seems to be some differences as exercise may have a larger effect on the insula and somatosensory cortex (which is indexed by TSPS) while tDCS seems to have a greater effect on prefrontal and cingulate cortex (which is indexed by CPM). Finally, decreases in the inhibitory system indexed by TMS and theta EEG band power are good markers of the normalization of intracortical inhibition and thalamic dysrhythmia, respectively.
Five potential interpretations of the results of the combined intervention on pain processing using these neurophysiological markers are plausible; however, they provide indirect measures of the endogenous pain control system as a more direct evaluation of cortical and subcortical structures would require neuroimaging techniques such as: a positron emission tomography (PET) or PET/magnetic resonance imaging. The results of these indirect assessments of descending inhibitory system (indexed by CPM), central sensitization (indexed by TSPS) and neural inhibitory control (indexed by TMS and EEG) can not only help to improve the interpretation of these interventions but also may be important clinical predictors of response, holding promise to guide treatment in the future.
Scenario 1 – CPM, TSPS, TMS and EEG show improvement.
Combined treatment led to an overall impact in the main areas of the pain control system such as: prefrontal, cingulate and somatosensory cortex as well as thalamus and insula. The synergistic effect of the combined intervention on modulating pain inhibitory system would enhance CPM, TSPS, TMS and EEG analysis. Although evidence shows a higher positive impact on pain intensity lead by tDCS + exercises compared to either intervention alone [22], the synergistic mechanistic effect of both interventions on the endogenous pain regulatory control system, are not fully clear. This positive scenario will allow us to determine specific biomarkers (e.g., TSPS, CPM, TMS TSPS results may indicate that both interventions and EEG) that could help to predict treatment response in future clinical trials. Preliminary data suggested a positive effect of tDCS and exercise alone in TSPS [15, 86, 87] and CPM [88], however, so far, these specific markers have not been used to guide specific interventions. Furthermore, with these results regarding TMS and EEG, it is possible to indirectly assess how changes in the endogenous pain regulatory control are related to alterations in abnormal cortical excitability [81] as indexed by TMS and thalamic dysrhythmia [83, 89, 90] as indexed by EEG.
Scenario 2 – CPM improves; TSPS does not improve; TMS and EEG improve:
The relative lack of TSPS results may indicate that both interventions have a common target (with normalization of thalamic dysrhythmia and improving intra-cortical inhibition) and tDCS might have a predominant effect on prefrontal and cingulate cortex. The interpretation of such results might be particularly interesting, because although the effects of tDCS on healthy individuals have been associated with CPM improvement [91], this has yet to be demonstrated in individuals with fibromyalgia [14]. Chang et al. [88] demonstrated improvement of CPM in a group of patients with knee osteoarthritis following active tDCS associated with exercise. Here a comparison with tDCS without exercise could show whether it is the combination of tDCS and exercise that can lead to CPM improvement in patients with fibromyalgia, and also if this is clinically relevant. Another explanation can be that the exercise led to a worsening of pain summation, as observed by Vierck (2001) [92] during strenuous exercise in fibromyalgic patients.
Scenario 3 – CPM results negative; TSPS results positive; TMS and EEG positive:
This would be the opposite scenario (to scenario 2) in which the main effects may be driven by exercises and thus having more impact on TSPS. This result may indicate mainly an effect in insula and somatosensory cortex rather than a common target of both interventions (would include prefrontal and cingulate cortex); thus, suggesting that exercise had a predominant effect. This conclusion may be based on the uncertainty of central pain processing of patients with FM. Although it was shown that CPM responses can be modulated by tDCS in healthy subjects [91], no changes were seen in patients with FM [14]. Hereby, the abnormalities in pain processing that these patients present may be the cause of these differences in results.
Scenario 4 – CPM results negative; TSPS results negative; TMS and EEG positive:
In this scenario, both interventions would make the largest impact in excitability and thalamic activity thus showing a greater reduction in inhibitory function and thalamic dysrhythmia. This scenario would reinforce that CNS changes in FM are associated with a deficit in the inhibitory control, namely abnormal cortical excitability as expressed by decreased short intracortical inhibition (SICI) and facilitation (SICF) and increased resting motor threshold [81]. This is thought to reflect an inhibitory deficit that is associated with altered thalamic anatomy and activity [93], which may result in abnormal thalamocortical circuits, as evidenced by the association between central pain and thalamic dysrhythmia [83, 89, 90]. Therefore, the unravelling of inhibitory deficits underlying pain maintenance mechanisms in patients with FM can also help the development of novel interventions for this pathology, as well as for other chronic pain conditions associated with a deficit in the regulatory pain system.
Unfortunately, one consequence of this scenario would be the indication that TSPS and CPM are not reliable tools for pain assessment in fibromyalgia. One of the reasons may be related to the several mechanisms that contribute to TSPS and CPM, including ineffective central pain modulation and central sensitization [94] and rostral ventromedial medulla facilitation [7] which are not well understood. Another main explanation for the negative results could be selection bias since there is strong evidence that the endogenous pain regulatory system is impaired in some FM patients but not necessarily in all FM patients [47, 48]. Moreover, the result could be attributed to the time of the measurement concerning the disease episode, as CPM efficacy has already been related to pain development 6 months after surgery [95], and TSPS related to the maintenance of several chronic pain conditions [96, 97]. They may not be sensitive enough to detect initial recurrence of FM pain episodes.
Scenario 5 – CPM results negative; TSPS results negative; TMS and EEG negative:
This would be the a less likely scenario in which pain decrease would not be correlated with any of the above-mentioned mechanisms and in this case a placebo effect would be more likely. However, a run-in period and a comparison with a sham group would be helpful to understand whether this effect is mediated by placebo or another alternative mechanism.
3. Design of a combined treatment
Based on the extensive literature discussed previously, a combined treatment and four neurophysiological markers (CPM, TSPS, TMS and EEG) are addressed in this study design. The above-described hypothesis will be tested in a 2×2 factorial mechanistic trial dividing subjects with fibromyalgia (n=148) randomly into four groups: active-tDCS and aerobic exercise; active tDCS and non-aerobic exercise; sham-tDCS and aerobic exercise or sham-tDCS and non-aerobic exercise. With this trial design, it is possible to demonstrate separately the efficacy of tDCS and exercise as well as an adjunctive treatment.
The graph below describes the study design with the strategy of performing 16 sessions of tDCS and 12 sessions of exercise as well as the assessments and questionnaires (Figure 2). Participants will undergo a pre-training exercise program for pre-screening (exclusion criteria if failed) and a conditioning exercise program as explained below. The protocol has a total of 18 visits over a 3-month duration.
Figure 2:
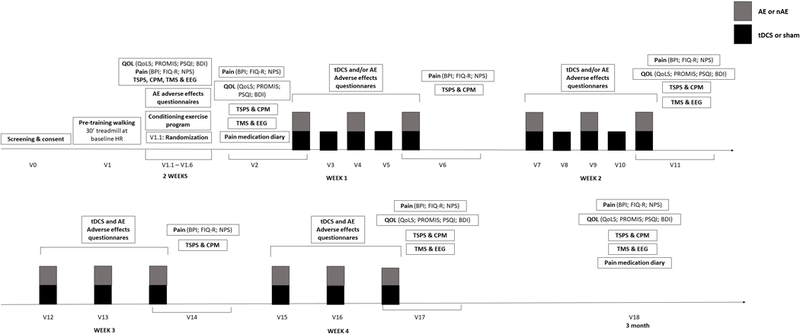
Pre-Training exercise: If the subject passes the screening for exercise, the subject will undergo the pre-training walking (30’ treadmill at baseline HR). If he/she completes the pre-training walking successfully as described above, then he/she will be randomized during visit 1.1; Conditioning exercise program: participants will be randomized to a different conditioning exercise program for two weeks according to their group assignment (as to ensure gradual increase of intensity); tDCS: Transcranial direct current stimulation; AE: Aerobic Exercise or Non aerobic exercise depending on the randomization, each session will last 30 minutes. CPM: conditioned pain modulation; TSPS: Temporal Slow Pain Summation; Pain assessments: Includes the evaluation of average pain intensity as assessed by Modified Brief Pain Inventory (BPI) and by the Numerical Pain Scale (NPS) and the Revised Fibromyalgia Impact Questionnaire (FIQ-R); Quality of life assessments: questionnaires to evaluate changes in quality of life including the Quality of Life Scale (QoLS), Patient Reported Outcomes Measurement Information System (PROMIS), Pittsburgh Sleep Quality Index (PSQI) and Beck Depression Inventory (BDI). TMS: transcranial magnetic stimulation; EEG: electroencephalogram; tDCS Adverse Effects Questionnaire: At the end of each stimulation session, subjects will complete a 5-point scale questionnaire that evaluates potential common adverse effects of tDCS; Exercise Adverse Effects: During each AE session, subjects will be monitored (Heart Rate range); Medication Use Questionnaire and Diary: Patient’s medications will be monitored throughout the course of the study using a subject Medication Diary.
In order to provide enough power for the clinical trial mentioned here, the sample size was calculated using information from trials measuring the effects of tDCS and aerobic exercise on CPM and TSPS according to three different strategies. Strategy I: effects of tDCS on CPM in patients with chronic pain - effect size (ES) of 0.79[98]. Strategy II: effects of tDCS on CPM in healthy volunteers - pooled ES of 1.02 [87, 91, 99, 100] and Strategy III: effect of exercise on CPM in chronic pain - ES of 0.78[86]. Based on this analysis, a conservative approach with the lowest ES was chosen using an ES of 0.78. It is expected that tDCS + aerobic exercise will have a higher effect and therefore more power than each intervention alone (tDCS, exercise, or placebo). Finally, also to be conservative, a type I error of 5% (alpha), power of 85% and a dropout rate of 20% were selected resulting in a sample size of 148.
4. Conclusion
This current article discusses how pain control mechanisms can be disturbed in FM, with the expectation to increase this knowledge when the proposed clinical trial is conducted. It is anticipated that the obtained results may improve upon the understanding of treatment in fibromyalgia patients, hoping to ultimately change clinical practice.
5. Expert commentary
TDCS has been associated with pain reduction in fibromyalgia supported by several trials. However, the effect size is small to moderate and there is significant variability within studies. In addition, the mechanism of tDCS to reduce pain in fibromyalgia is not fully understood, therefore in order to enhance effect size of tDCS and decrease variability of these effects, it is important to understand the neural mechanism of pain reduction in fibromyalgia. In pursuance of this goal, a model is proposed to assess the descending inhibitory system with CPM and the central sensitization with TSPS as to further learn the effects of tDCS in fibromyalgia. By clarifying the mechanism, some tDCS parameters will be manipulated in order to investigate how they change central sensitization and descending inhibitory system.
Regarding the mechanism of tDCS, the field might advance in stimulation intensity (current dosage and density); number of sessions and duration, electrode montage and combined interventions focusing on complementary target engagement. For instance, evidence suggests that 15 sessions are more suitable to decrease pain in fibromyalgia when compared to a lower number of sessions [15] and that combination of tDCS with aerobic exercises (or other sort of behavioral and cognitive therapy) is more effective than tDCS alone for different conditions [34].
In terms of novel neuro-markers and neurophysiological predictors, neuroimaging techniques are of high importance, especially functional MRI that maps brain activity in rest and when performing tasks, promising to have a fundamental role in the short future. As important are the neurophysiological measures of excitability and inhibition indexed by TMS (motor threshold, motor evoked potential and paired pulse), which also measures brain plasticity and integrity of neural tracts. In addition, the quantitative EEG has an optimal temporal resolution and coupled to novel software increases its applicability and gives also spatial resolution as well as event-related potential. In summary, these neurophysiological approaches will definitely help finding better responders, stratifying the target population and the disease status with more accuracy and ultimately giving solid basis for a tailored and more individualized medicine trans diagnostically. A good example is the NIH initiative RDoc Matrix that integrates many levels of approach (from genomics and circuits to behavior and cognition) aiming to explore basic dimension of symptoms correlating with neurobiological processes and not to diagnostic criteria.
6. Five-year view
Novel devices and techniques may be developed in the next 5 years. The field has tried to find better systems in order to enhance focality, although it is still unclear whether they will add any clinical benefits; hopefully future trials will address this issue. Another important challenge for the advancement of this technique is the home-based tDCS. There are several novel potential strategies for home-based tDCS that may also help the widespread testing and to make it more feasible.
Finally, the pharmacological combination is being assessed with mixed results and is important to understand the effects of different drugs combined with tDCS. Likewise, combination with other devices (e.g. transcranial pulsed stimulation, transcranial alternate current stimulation and near-infrared transcranial radiation) may have additional benefits, although before going deeper in such effort, it is important to fully understand the effects of tDCS. Moreover, the use of neuronavigation and functional near-infrared spectroscopy associated with neuromodulatory techniques may contribute with the mechanistic understanding of these interventions.
Although the field has developed quickly in the past 10 years, for the next 5 years more robust clinical trials may also help us further understand the clinical effects of treatments for pain conditions.
6. Key issues.
Fibromyalgia is a high burden in patient’s biopsychosocial well-being and interface with morbidity as well as frequent health care demands. Common pharmacological interventions have low effectiveness and potential side effects;
Evidence suggests that the pathophysiology of fibromyalgia is related to the dysfunction in the endogenous pain control system that involves a disruptive perception of peripheral pain signals by the primary sensory areas and limbic system. Regarding this complex condition novel multimodal interventions aimed at specific targets in the central nervous system are needed;
Transcranial direct current stimulation combined with aerobic exercise can act synergistically in the endogenous pain control system to restore the homeostasis and improve pain symptoms in fibromyalgia;
Anodal stimulation of M1 is an ideal target in the pain neuromatrix acting in a top-down fashion (from cortical: primary and sensory motor cortex, cingulate cortex and insula; to subcortical: thalamus) enhancing neural excitability and complemented by a “bottom-up” effect (from subcortical: thalamus, cerebellum to cortical: sensorimotor cortex, insula and dorsolateral prefrontal cortex) elicited by aerobic exercise;
Proper assessment of the interventions effects by neurophysiological markers such as conditioned pain modulation, temporal slow pain summation, transcranial magnetic stimulation and electroencephalography can help understand the pathophysiology of fibromyalgia and the neurobiological changes caused by the intervention;
A clinical trial was designed to test the hypothesis that optimized tDCS with aerobic exercise can maximize the restoration of the pain endogenous control system by modulating activity in a widespread neural network providing pain relief in patients with fibromyalgia.
Acknowledgments
F.F. is funded by a NIH R01 grant (1R01HD082302–01A1).
Footnotes
Disclosure
All authors did not have any conflicts of interest or financial disclosures to report.
References
Papers of special note have been highlighted as:
*of interest
**of considerable interest
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008. January;58(1):26–35. doi: 10.1002/art.23176. PubMed PMID: 18163497; PubMed Central PMCID: PMCPmc3266664. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verbunt JA, Pernot DH, Smeets RJ. Disability and quality of life in patients with fibromyalgia. Health Qual Life Outcomes. 2008. January;6:8. doi: 10.1186/1477-7525-6-8. PubMed PMID: 18211701; PubMed Central PMCID: PMCPMC2265693. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Häuser W, Walitt B, Fitzcharles MA, et al. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther. 2014. January;16(1):201. doi: 10.1186/ar4441. PubMed PMID: 24433463; PubMed Central PMCID: PMCPMC3979124. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Julien N, Goffaux P, Arsenault P, et al. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005. March;114(1–2):295–302. doi: 10.1016/j.pain.2004.12.032. PubMed PMID: 15733656; eng. [DOI] [PubMed] [Google Scholar]
- 5.de la Coba P, Bruehl S, Moreno-Padilla M, et al. Responses to Slowly Repeated Evoked Pain Stimuli in Fibromyalgia Patients: Evidence of Enhanced Pain Sensitization. Pain Med. 2017. September;18(9):1778–1786. doi: 10.1093/pm/pnw361. PubMed PMID: 28371909; eng. [DOI] [PubMed] [Google Scholar]
- 6.*Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014. June;8(2):143–51. doi: 10.1097/SPC.0000000000000055. PubMed PMID: 24752199; PubMed Central PMCID: PMCPMC4301419. eng.A very important paper to understand the mechanisms of chronic pain.
- 7.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. The Journal of clinical investigation. 2010. November;120(11):3779–87. doi: 10.1172/jci43766. PubMed PMID: 21041960; PubMed Central PMCID: PMCPmc2964993. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabino GS, Santos CM, Francischi JN, et al. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J Pain. 2008. February;9(2):157–63. doi: 10.1016/j.jpain.2007.09.003. PubMed PMID: 17988952; eng. [DOI] [PubMed] [Google Scholar]
- 9.Chesterton LS, Barlas P, Foster NE, et al. Sensory stimulation (TENS): effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain. 2002. September;99(1–2):253–62. PubMed PMID: 12237203; eng. [DOI] [PubMed] [Google Scholar]
- 10.Polanía R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. 2012. October;33(10):2499–508. doi: 10.1002/hbm.21380. PubMed PMID: 21922602; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antal A, Terney D, Kühnl S, et al. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage. 2010. May;39(5):890–903. doi: 10.1016/j.jpainsymman.2009.09.023. PubMed PMID: 20471549; eng. [DOI] [PubMed] [Google Scholar]
- 12.Singh AM, Duncan RE, Neva JL, et al. Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci Med Rehabil. 2014;6:23. doi: 10.1186/2052-1847-6-23. PubMed PMID: 25031838; PubMed Central PMCID: PMCPMC4100033. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stagg NJ, Mata HP, Ibrahim MM, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011. April;114(4):940–8. doi: 10.1097/ALN.0b013e318210f880. PubMed PMID: 21386701; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villamar MF, Wivatvongvana P, Patumanond J, et al. Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J Pain. 2013. April;14(4):371–83. doi: 10.1016/j.jpain.2012.12.007. PubMed PMID: 23415877; eng. [DOI] [PubMed] [Google Scholar]
- 15.**Castillo-Saavedra L, Gebodh N, Bikson M, et al. Clinically Effective Treatment of Fibromyalgia Pain With High-Definition Transcranial Direct Current Stimulation: Phase II Open-Label Dose Optimization. J Pain. 2016. January;17(1):14–26. doi: 10.1016/j.jpain.2015.09.009. PubMed PMID: 26456677; PubMed Central PMCID: PMCPMC5777157. eng.Provides important insights in the importance of a greater number of sessions of tDCS for Fibromyalgia
- 16.Garcia-Hermoso A, Saavedra JM, Escalante Y. Effects of exercise on functional aerobic capacity in adults with fibromyalgia syndrome: A systematic review of randomized controlled trials. J Back Musculoskelet Rehabil. 2015;28(4):609–19. doi: 10.3233/BMR-140562. PubMed PMID: 25408119. [DOI] [PubMed] [Google Scholar]
- 17.Ellingson LD, Stegner AJ, Schwabacher IJ, et al. Exercise Strengthens Central Nervous System Modulation of Pain in Fibromyalgia. Brain Sci. 2016. February;6(1). doi: 10.3390/brainsci6010008. PubMed PMID: 26927193; PubMed Central PMCID: PMCPMC4810178. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.*Borckardt JJ, Bikson M, Frohman H, et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012. February;13(2):112–20. doi: 10.1016/j.jpain.2011.07.001. PubMed PMID: 22104190; eng.Relevant work on modelling of electrical fields elicited by tDCS
- 19.Bolognini N, Vallar G, Casati C, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011 2011. Nov-Dec;25(9):819–29. doi: 10.1177/1545968311411056. PubMed PMID: 21803933; eng. [DOI] [PubMed] [Google Scholar]
- 20.Fregni F, Boggio PS, Nitsche M, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005. September;166(1):23–30. doi: 10.1007/s00221-005-2334-6. PubMed PMID: 15999258; eng. [DOI] [PubMed] [Google Scholar]
- 21.O’Connell NE, Marston L, Spencer S, et al. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018. April;4:CD008208. doi: 10.1002/14651858.CD008208.pub5. PubMed PMID: 29652088; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.**Mendonça ME, Simis M, Collange L, et al. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: A randomized placebo-controlled clinical trial [Clinical Trial]. Frontiers in Human Neuroscience. 2016;10. doi: 10.3389/fnhum.2016.00068. English.A pilot trial with promissing results from the combination of tDCS and exercise.
- 23.Yelle MD, Oshiro Y, Kraft RA, et al. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009. August;29(33):10264–71. doi: 10.1523/JNEUROSCI.4648-08.2009. PubMed PMID: 19692600; PubMed Central PMCID: PMCPMC2739444. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.**Staud R Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. 2012. May;12(5):577–85. doi: 10.1586/ern.12.41. PubMed PMID: 22550986; PubMed Central PMCID: PMCPMC3373184. eng. An important summary of pain as a consequence of a disruptive endogenous pain system.
- 25.Taylor BK. Pathophysiologic mechanisms of neuropathic pain. Curr Pain Headache Rep. 2001. April;5(2):151–61. PubMed PMID: 11252149; eng. [DOI] [PubMed] [Google Scholar]
- 26.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007. August;55(3):377–91. doi: 10.1016/j.neuron.2007.07.012. PubMed PMID: 17678852; eng. [DOI] [PubMed] [Google Scholar]
- 27.Mason P Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–77. doi: 10.1146/annurev.neuro.24.1.737. PubMed PMID: 11520917; eng. [DOI] [PubMed] [Google Scholar]
- 28.Kong J, Gollub RL, Rosman IS, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006. January;26(2):381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. PubMed PMID: 16407533; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009. October;139(2):267–84. doi: 10.1016/j.cell.2009.09.028. PubMed PMID: 19837031; PubMed Central PMCID: PMCPMC2852643. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zelenin PV, Lyalka VF, Hsu LJ, et al. Effects of reversible spinalization on individual spinal neurons. J Neurosci. 2013. November;33(48):18987–98. doi: 10.1523/JNEUROSCI.2394-13.2013. PubMed PMID: 24285903; PubMed Central PMCID: PMCPMC3841459. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, Wang S, Stein JF, et al. Reciprocal interactions between the human thalamus and periaqueductal gray may be important for pain perception. Exp Brain Res. 2014. February;232(2):527–34. doi: 10.1007/s00221-013-3761-4. PubMed PMID: 24217977; eng. [DOI] [PubMed] [Google Scholar]
- 32.Zeidan F, Adler-Neal AL, Wells RE, et al. Mindfulness-Meditation-Based Pain Relief Is Not Mediated by Endogenous Opioids. J Neurosci. 2016. March;36(11):3391–7. doi: 10.1523/JNEUROSCI.4328-15.2016. PubMed PMID: 26985045; PubMed Central PMCID: PMCPMC4792946. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DosSantos MF, Ferreira N, Toback RL, et al. Potential Mechanisms Supporting the Value of Motor Cortex Stimulation to Treat Chronic Pain Syndromes. Front Neurosci. 2016;10:18. doi: 10.3389/fnins.2016.00018. PubMed PMID: 26903788; PubMed Central PMCID: PMCPMC4749700. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto CB, Teixeira Costa B, Duarte D, et al. Transcranial Direct Current Stimulation as a Therapeutic Tool for Chronic Pain. J ECT. 2018. June. doi: 10.1097/YCT.0000000000000518. PubMed PMID: 29952860; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.*Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993. March;78(3):393–401. doi: 10.3171/jns.1993.78.3.0393. PubMed PMID: 8433140; eng.A seminal paper about the stimulation of motor cortex to treat pain.
- 36.Kisler LB, Gurion I, Granovsky Y, et al. Can a single pulse transcranial magnetic stimulation targeted to the motor cortex interrupt pain processing? PLoS One. 2018;13(4):e0195739. doi: 10.1371/journal.pone.0195739. PubMed PMID: 29630681; PubMed Central PMCID: PMCPMC5891059. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Larrea L, Peyron R, Mertens P, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotactic and functional neurosurgery. 1997;68(1–4 Pt 1):141–8. PubMed PMID: 9711707; eng. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study [Clinical Trial]. Pain. 1999. November;83(2):259–73. PubMed PMID: 10534598; eng. [DOI] [PubMed] [Google Scholar]
- 39.DaSilva AF, Truong DQ, DosSantos MF, et al. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front Neuroanat. 2015;9:89. doi: 10.3389/fnana.2015.00089. PubMed PMID: 26236199; PubMed Central PMCID: PMCPMC4502355. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams JE, Hosobuchi Y, Fields HL. Stimulation of internal capsule for relief of chronic pain. J Neurosurg. 1974. December;41(6):740–4. doi: 10.3171/jns.1974.41.6.0740. PubMed PMID: 4609304; eng. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: From phenomenology to mechanisms. Neuroimage. 2007;37 Suppl 1:S71–9. doi: 10.1016/j.neuroimage.2007.05.062. PubMed PMID: 17644413; eng. [DOI] [PubMed] [Google Scholar]
- 42.Peyron R, Faillenot I, Mertens P, et al. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage. 2007. January;34(1):310–21. doi: 10.1016/j.neuroimage.2006.08.037. PubMed PMID: 17055297; eng. [DOI] [PubMed] [Google Scholar]
- 43.Boggio PS, Zaghi S, Lopes M, et al. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. European Journal of Neurology. 2008. October;15(10):1124–1130. doi: 10.1111/j.1468-1331.2008.02270.x. PubMed PMID: WOS:000259605800018. [DOI] [PubMed] [Google Scholar]
- 44.Simis M, Reidler JS, Duarte Macea D, et al. Investigation of central nervous system dysfunction in chronic pelvic pain using magnetic resonance spectroscopy and noninvasive brain stimulation. Pain Pract. 2015. June;15(5):423–32. doi: 10.1111/papr.12202. PubMed PMID: 24799153; PubMed Central PMCID: PMCPMC4216781. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mordillo-Mateos L, Turpin-Fenoll L, Millán-Pascual J, et al. Effects of simultaneous bilateral tDCS of the human motor cortex. Brain Stimul. 2012. July;5(3):214–22. doi: 10.1016/j.brs.2011.05.001. PubMed PMID: 21782545; eng. [DOI] [PubMed] [Google Scholar]
- 46.Knotkova H, Nitsche MA, Cruciani RA. Putative physiological mechanisms underlying tDCS analgesic effects. Front Hum Neurosci. 2013. September;7:628. doi: 10.3389/fnhum.2013.00628. PubMed PMID: 24133434; PubMed Central PMCID: PMCPMC3783844. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu CE, Yu B, Zhang W, et al. Effiectiveness and safety of transcranial direct current stimulation in fibromyalgia: A systematic review and meta-analysis. J Rehabil Med. 2017. January;49(1):2–9. doi: 10.2340/16501977-2179. PubMed PMID: 27983739; eng. [DOI] [PubMed] [Google Scholar]
- 48.Hou WH, Wang TY, Kang JH. The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology (Oxford). 2016. August;55(8):1507–17. doi: 10.1093/rheumatology/kew205. PubMed PMID: 27150193; eng. [DOI] [PubMed] [Google Scholar]
- 49.Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 2017. January;128(1):56–92. doi: 10.1016/j.clinph.2016.10.087. PubMed PMID: WOS:000396377300008. [DOI] [PubMed] [Google Scholar]
- 50.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015. January;156(1):62–71. doi: 10.1016/j.pain.0000000000000006. PubMed PMID: WOS:000347658400011. [DOI] [PubMed] [Google Scholar]
- 51.Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise. An update. Sports Med. 1997. July;24(1):8–16. PubMed PMID: 9257407; eng. [DOI] [PubMed] [Google Scholar]
- 52.Kramer AF, Erickson KI. Effects of physical activity on cognition, well-being, and brain: human interventions. Alzheimers Dement. 2007. April;3(2 Suppl):S45–51. doi: 10.1016/j.jalz.2007.01.008. PubMed PMID: 19595974; eng. [DOI] [PubMed] [Google Scholar]
- 53.Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000. February;29(2):85–98. PubMed PMID: 10701712; eng. [DOI] [PubMed] [Google Scholar]
- 54.Holschneider DP, Yang J, Guo Y, et al. Reorganization of functional brain maps after exercise training: Importance of cerebellar-thalamic-cortical pathway. Brain Res. 2007. December;1184:96–107. doi: 10.1016/j.brainres.2007.09.081. PubMed PMID: 17964551; PubMed Central PMCID: PMCPMC2692362. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williamson JW, Nobrega AC, McColl R, et al. Activation of the insular cortex during dynamic exercise in humans. J Physiol. 1997. September;503 ( Pt 2):277–83. PubMed PMID: 9306272; PubMed Central PMCID: PMCPMC1159862. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williamson JW, McColl R, Mathews D, et al. Activation of the insular cortex is affected by the intensity of exercise. J Appl Physiol (1985). 1999. September;87(3):1213–9. doi: 10.1152/jappl.1999.87.3.1213. PubMed PMID: 10484598; eng. [DOI] [PubMed] [Google Scholar]
- 57.Fuentes C JP, Armijo-Olivo S, Magee DJ, et al. Effects of exercise therapy on endogenous pain-relieving peptides in musculoskeletal pain: a systematic review. Clin J Pain. 2011. May;27(4):365–74. doi: 10.1097/AJP.0b013e31820d99c8. PubMed PMID: 21430521; eng. [DOI] [PubMed] [Google Scholar]
- 58.Bütefisch CM, Davis BC, Wise SP, et al. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000. March;97(7):3661–5. doi: 10.1073/pnas.050350297. PubMed PMID: 10716702; PubMed Central PMCID: PMCPMC16296. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Fujiwara T, Liu W, et al. Effects of pedaling exercise on the intracortical inhibition of cortical leg area. Exp Brain Res. 2012. May;218(3):401–6. doi: 10.1007/s00221-012-3026-7. PubMed PMID: 22349500; eng. [DOI] [PubMed] [Google Scholar]
- 60.Sidhu SK, Lauber B, Cresswell AG, et al. Sustained cycling exercise increases intracortical inhibition. Med Sci Sports Exerc. 2013. April;45(4):654–62. doi: 10.1249/MSS.0b013e31827b119c. PubMed PMID: 23190593; eng. [DOI] [PubMed] [Google Scholar]
- 61.Mooney RA, Coxon JP, Cirillo J, et al. Acute aerobic exercise modulates primary motor cortex inhibition. Exp Brain Res. 2016. December;234(12):3669–3676. doi: 10.1007/s00221-016-4767-5. PubMed PMID: 27590480; eng. [DOI] [PubMed] [Google Scholar]
- 62.Smith AE, Goldsworthy MR, Garside T, et al. The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Exp Brain Res. 2014. June;232(6):1875–82. doi: 10.1007/s00221-014-3879-z. PubMed PMID: 24570388; eng. [DOI] [PubMed] [Google Scholar]
- 63.Rainville J, Hartigan C, Martinez E, et al. Exercise as a treatment for chronic low back pain. Spine J. 2004 2004. Jan-Feb;4(1):106–15. PubMed PMID: 14749199; eng. [DOI] [PubMed] [Google Scholar]
- 64.Thibaut A, Carvalho S, Morse LR, et al. Delayed pain decrease following M1 tDCS in spinal cord injury: A randomized controlled clinical trial. Neurosci Lett. 2017. September;658:19–26. doi: 10.1016/j.neulet.2017.08.024. PubMed PMID: 28822837; eng. [DOI] [PubMed] [Google Scholar]
- 65.Marlow NM, Bonilha HS, Short EB. Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: a systematic review. Pain Pract. 2013. February;13(2):131–45. doi: 10.1111/j.1533-2500.2012.00562.x. PubMed PMID: 22631436; eng. [DOI] [PubMed] [Google Scholar]
- 66.Vural M, Berkol TD, Erdogdu Z, et al. Evaluation of the effectiveness of an aerobic exercise program and the personality characteristics of patients with fibromyalgia syndrome: a pilot study. J Phys Ther Sci. 2014. October;26(10):1561–5. doi: 10.1589/jpts.26.1561. PubMed PMID: 25364113; PubMed Central PMCID: PMCPMC4210398. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinto CB, Saleh Velez FG, Bolognini N, et al. Optimizing Rehabilitation for Phantom Limb Pain Using Mirror Therapy and Transcranial Direct Current Stimulation: A Randomized, Double-Blind Clinical Trial Study Protocol. JMIR Res Protoc. 2016. July;5(3):e138. doi: 10.2196/resprot.5645. PubMed PMID: 27383993; PubMed Central PMCID: PMCPMC4954918. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soler MD, Kumru H, Pelayo R, et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010. September;133(9):2565–77. doi: 10.1093/brain/awq184. PubMed PMID: 20685806; PubMed Central PMCID: PMCPMC2929331. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumru H, Soler D, Vidal J, et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. Eur J Pain. 2013. January;17(1):55–66. doi: 10.1002/j.1532-2149.2012.00167.x. PubMed PMID: 22610590; eng. [DOI] [PubMed] [Google Scholar]
- 70.Thieme H, Mehrholz J, Pohl M, et al. Mirror therapy for improving motor function after stroke. Stroke. 2013. January;44(1):e1–2. PubMed PMID: 23390640; eng. [DOI] [PubMed] [Google Scholar]
- 71.Jax SA, Rosa-Leyra DL, Coslett HB. Enhancing the mirror illusion with transcranial direct current stimulation. Neuropsychologia. 2015. May;71:46–51. doi: 10.1016/j.neuropsychologia.2015.03.017. PubMed PMID: 25796410; eng. [DOI] [PubMed] [Google Scholar]
- 72.Hazime FA, Baptista AF, de Freitas DG, et al. Treating low back pain with combined cerebral and peripheral electrical stimulation: A randomized, double-blind, factorial clinical trial. Eur J Pain. 2017. August;21(7):1132–1143. doi: 10.1002/ejp.1037. PubMed PMID: 28440001; eng. [DOI] [PubMed] [Google Scholar]
- 73.Schabrun SM, Burns E, Thapa T, et al. The Response of the Primary Motor Cortex to Neuromodulation is Altered in Chronic Low Back Pain: A Preliminary Study. Pain Med. 2018. June;19(6):1227–1236. doi: 10.1093/pm/pnx168. PubMed PMID: 29016867; eng. [DOI] [PubMed] [Google Scholar]
- 74.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain. 2010. April;14(4):339. doi: 10.1016/j.ejpain.2010.02.004. PubMed PMID: 20227310; eng. [DOI] [PubMed] [Google Scholar]
- 75.Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015. June;9(2):131–7. doi: 10.1097/SPC.0000000000000126. PubMed PMID: 25699686; eng. [DOI] [PubMed] [Google Scholar]
- 76.Yarnitsky D Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010. October;23(5):611–5. doi: 10.1097/ACO.0b013e32833c348b. PubMed PMID: 20543676; eng. [DOI] [PubMed] [Google Scholar]
- 77.Youssef AM, Macefield VG, Henderson LA. Cortical influences on brainstem circuitry responsible for conditioned pain modulation in humans. Hum Brain Mapp. 2016. July;37(7):2630–44. doi: 10.1002/hbm.23199. PubMed PMID: 27104478; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Youssef AM, Macefield VG, Henderson LA. Pain inhibits pain; human brainstem mechanisms. Neuroimage. 2016. January;124(Pt A):54–62. doi: 10.1016/j.neuroimage.2015.08.060. PubMed PMID: 26343321; eng. [DOI] [PubMed] [Google Scholar]
- 79.Bosma RL, Ameli Mojarad E, Leung L, et al. Neural correlates of temporal summation of second pain in the human brainstem and spinal cord. Hum Brain Mapp. 2015. December;36(12):5038–50. doi: 10.1002/hbm.22993. PubMed PMID: 26366748; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran TD, Wang H, Tandon A, et al. Temporal summation of heat pain in humans: Evidence supporting thalamocortical modulation. Pain. 2010. July;150(1):93–102. doi: 10.1016/j.pain.2010.04.001. PubMed PMID: 20494516; PubMed Central PMCID: PMCPMC2916061. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mhalla A, de Andrade DC, Baudic S, et al. Alteration of cortical excitability in patients with fibromyalgia. Pain. 2010. June;149(3):495–500. doi: 10.1016/j.pain.2010.03.009. PubMed PMID: 20356675; eng. [DOI] [PubMed] [Google Scholar]
- 82.Kim GW, Ko MH. Facilitation of corticospinal tract excitability by transcranial direct current stimulation combined with voluntary grip exercise. Neurosci Lett. 2013. August;548:181–4. doi: 10.1016/j.neulet.2013.05.037. PubMed PMID: 23726882; eng. [DOI] [PubMed] [Google Scholar]
- 83.Sarnthein J, Stern J, Aufenberg C, et al. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006. January;129(Pt 1):55–64. doi: 10.1093/brain/awh631. PubMed PMID: 16183660; eng. [DOI] [PubMed] [Google Scholar]
- 84.Llinás RR, Ribary U, Jeanmonod D, et al. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999. December;96(26):15222–7. PubMed PMID: 10611366; PubMed Central PMCID: PMCPMC24801. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim M, Kim JS, Kim DJ, et al. Increased Low- and High-Frequency Oscillatory Activity in the Prefrontal Cortex of Fibromyalgia Patients. Front Hum Neurosci. 2016;10:111. doi: 10.3389/fnhum.2016.00111. PubMed PMID: 27014041; PubMed Central PMCID: PMCPMC4789463. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meeus M, Hermans L, Ickmans K, et al. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: a double-blind randomized controlled trial. Pain Pract. 2015. February;15(2):98–106. doi: 10.1111/papr.12181. PubMed PMID: 24528544; eng. [DOI] [PubMed] [Google Scholar]
- 87.Braulio G, Passos SC, Leite F, et al. Effects of Transcranial Direct Current Stimulation Block Remifentanil-Induced Hyperalgesia: A Randomized, Double-Blind Clinical Trial. Front Pharmacol. 2018;9:94. doi: 10.3389/fphar.2018.00094. PubMed PMID: 29515438; PubMed Central PMCID: PMCPMC5825908. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang WJ, Bennell KL, Hodges PW, et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial. PLoS One. 2017;12(6):e0180328. doi: 10.1371/journal.pone.0180328. PubMed PMID: 28665989; PubMed Central PMCID: PMCPMC5493377. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walton KD, Llinas RR. Frontiers in Neuroscience. Central Pain as a Thalamocortical Dysrhythmia: A Thalamic Efference Disconnection? In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton, FL: CRC Press/Taylor & Francis Llc; 2010. [PubMed] [Google Scholar]
- 90.Alshelh Z, Di Pietro F, Youssef AM, et al. Chronic Neuropathic Pain: It’s about the Rhythm. 2016. January 20;36(3):1008–18. doi: 10.1523/jneurosci.2768-15.2016. PubMed PMID: 26791228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flood A, Waddington G, Cathcart S. High-Definition Transcranial Direct Current Stimulation Enhances Conditioned Pain Modulation in Healthy Volunteers: A Randomized Trial. J Pain. 2016. May;17(5):600–5. doi: 10.1016/j.jpain.2016.01.472. PubMed PMID: 26844419; eng. [DOI] [PubMed] [Google Scholar]
- 92.Vierck CJ, Staud R, Price DD, et al. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001. December;2(6):334–44. doi: 10.1054/jpai.2001.25533. PubMed PMID: 14622813; eng. [DOI] [PubMed] [Google Scholar]
- 93.Henderson LA, Peck CC, Petersen ET, et al. Chronic pain: lost inhibition? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013. April 24;33(17):7574–82. doi: 10.1523/jneurosci.0174-13.2013. PubMed PMID: 23616562; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chapter Mason P. 15 Descending pain modulation as a component of homeostasis. Handbook of clinical neurology. 2006;81:211–8. doi: 10.1016/s0072-9752(06)80019-9. PubMed PMID: 18808837; eng. [DOI] [PubMed] [Google Scholar]
- 95.Wilder-Smith OH, Schreyer T, Scheffer GJ, et al. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. Journal of pain & palliative care pharmacotherapy. 2010. June;24(2):119–28. doi: 10.3109/15360281003706069. PubMed PMID: 20504133; eng. [DOI] [PubMed] [Google Scholar]
- 96.Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clinical rheumatology. 2007. April;26(4):465–73. doi: 10.1007/s10067-006-0433-9. PubMed PMID: 17115100; PubMed Central PMCID: PMCPmc1820749. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Staud R, Price DD, Fillingim RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (windup). J Pain. 2006. August;7(8):575–82. doi: 10.1016/j.jpain.2006.02.005. PubMed PMID: 16885014; eng. [DOI] [PubMed] [Google Scholar]
- 98.Ribeiro H, Sesterhenn RB, de Souza A, et al. Preoperative transcranial direct current stimulation: Exploration of a novel strategy to enhance neuroplasticity before surgery to control postoperative pain. A randomized sham-controlled study. Plos One. 2017. November;12(11). doi: 10.1371/journal.pone.0187013. PubMed PMID: WOS:000416841900011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flood A, Waddington G, Keegan RJ, et al. The effects of elevated pain inhibition on endurance exercise performance. Peerj. 2017. March;5. doi: 10.7717/peerj.3028. PubMed PMID: WOS:000396903800006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.da Silva NRJ, Laste G, Deitos A, et al. Combined neuromodulatory interventions in acute experimental pain: assessment of melatonin and non-invasive brain stimulation. Frontiers in Behavioral Neuroscience. 2015. March;9. doi: 10.3389/fnbeh.2015.00077. PubMed PMID: WOS:000352286800001. [DOI] [PMC free article] [PubMed] [Google Scholar]


